

Danish research ethics committees
- Responsible conduct of research
The research ethics committee system
To ensure that health research projects are conducted in an ethically responsible manner, a research ethics committee system has been set up, consisting of regional committees and The National Research Ethics Committee (DNVK).
The research ethics committee system has been established under the Danish Act on Research Ethics Review of Health Research Projects .
Any health research project should as a general rule be capable of being reported to the research ethics committee system, see Section 14 of the Act.
For the purposes of the Act, a health research project is defined as a project that includes trials involving liveborn human individuals, human gametes intended for fertilisation, fertilised human eggs, embryonic cells and embryos, tissue, cells and genetic material from humans, embryos, etc., or deceased persons. Also included are clinical trials of medicines in humans and clinical trials of medical devices, see Section 2, item 1, of the Act referring to the definitions in items 2 and 3 of the same provision.
Detailed guidelines on notification, mandatory reporting, etc. are available in Danish.
See also the research ethics committee’s website .
About The Author
Clement Salung Petersen
The Danish Code of Conduct for Research Integrity

- Download publication (pdf)
A national Code of Conduct for research integrity in Denmark has been finished. The Code aims to ensure credibility, integrity and thereby quality in Danish research through common principles and standards for responsible conduct of research.
The Code is aimed at both public and private research institutions, including universities, the research council system, foundations and enterprises. It is a common framework meant to be implemented and developed across fields of research.
The Danish Code of Conduct for Research Integrity was drafted by a working group set up by the Ministry of Higher Education and Science and the organisation Danish Universities. A draft Code was sent for public consultation in the spring of 2014 and was also presented and discussed at an open conference in May 2014.
Document Actions
- Print this page
Breadcrumbs Section. Click here to navigate to respective pages.

Research Ethics Committees in Denmark
DOI link for Research Ethics Committees in Denmark
Click here to navigate to parent product.
There is an established scientific ethics committee system in Denmark, with seven regional scientific ethics committees and a Central Scientific Ethical Committee. The purpose of the law is to lay down the legal framework for the scientific ethical evaluation of biomedical research projects. The system of ethical review is regulated by the Act on a Scientific Ethics Committee System and the Processing of Biomedical Research Projects. The Central Scientific Ethical Committee may lay down regulations for the practical organization of the scientific and ethical assessment of projects being carried out at more than one research institution. Research ethics committees (RECs) can reject or advise against activities that are lawful if they judge they are unethical. Unlawful activities will never be approved. The REC must pay attention to the law and all the members at the secretariat have a degree within law.
- Privacy Policy
- Terms & Conditions
- Cookie Policy
- Taylor & Francis Online
- Taylor & Francis Group
- Students/Researchers
- Librarians/Institutions
Connect with us
Registered in England & Wales No. 3099067 5 Howick Place | London | SW1P 1WG © 2024 Informa UK Limited
You are using an outdated browser. Please upgrade your browser to improve your experience.
- Find person Search for contact information on employees
- Directory Finding your way at the University of Southern Denmark
- itslearning log on to SDU's e-learn platform
- bibliotek see your status, your reservations and renew your loans
- DigitalExam Login to DigitalExam
- SDUmail - Webmail service Outlook Web Mail
- mySDU mySDU - For students at SDU
- Reset default page
- Set as default page
Research Ethics and Compliance Support
When applying for external funding or when submitting papers to scientific journals, researchers are sometimes demanded to prove that their research projects comply with ethical standards. SDU provides a research ethics review and approval service. A research ethics approval from SDU is an offer, not a requirement.
Get a Research Ethics Approval
Which projects can be reviewed by sdu’s research ethics committee.
SDU researchers, including PhD students, can apply for a research ethics review and approval of their projects. SDU’s Research Ethics Committee does not review students’ research projects. Projects are eligible for review only before participant recruitment and before data collection has been initiated. SDU’s Research Ethics Committee generally does not review projects that have already obtained an ethics approval from another ethics review body. This also applies to projects that are legally obligated to obtain ethics approval from the Regional or National Committees on Health Research Ethics. However, there may be exceptions. For instance, a clinical research project funded by the National Institutes of Health (NIH) may need approval from the Regional Committee on Health Research Ethics as well as from SDU’s Research Ethics Committee.
Validity should be renewed

GDPR-related issues

- Contact SDU RIO Legal Services
The Research Ethics Committee (REC) checks if a research project meets certain ethical standards. REC reviews projects with SDU researchers as principle investigators. The committee reviews and approves research projects from SDU researchers. This service is not available for student projects. The REC includes a chair person, a lay/community representative, representatives from the administration, and representatives from each of SDU’s five faculties.
The REC receives applications continuously. You will get a confirmation when you submit your application. After you submit your application, it is processed by SDU's Research Ethics Committee and discussed at the next available meeting. Meetings are held once a month. You will receive an answer to your application approximately one week after the case has been discussed at a meeting. The answer can be a direct approval, a rejection, or an approval that is conditional on you answering certain questions or making certain changes to the project.
The REC includes a chair person, a lay/community representative, representatives from the administration, and representatives from each of SDU’s five faculties (primary and alternate members). The REC is supported by a Research Ethics Consultant who serves as the point of contact. The REC-members appointed by the faculties are:
- Chair: Professor Kirsten Kyvik (Fac. of Health Sciences, Dept. of Clinical Research)
- Lay/community representative: Pastor Susanne Haastrup Holst
- Faculty of Science: Professor Lars Porskjær Christensen (FKF, co-chair)
- Faculty of Humanities: Ass. Professor Stig Børsen Hansen (IDK), Professor Anne Gerdes (IDK)
- Faculty of Engineering: Ass. Professor Eva Arnspang Christensen (IGT), Ass. Professor Dylan Cawthorne (MMMI)
- Faculty of Health Sciences: Ass. Professor Søren Fryd Birkeland (OPEN), Ass. Professor Henrik Steen Hansen (KI), Ass. Professor Mikael Thingaard (IST), Professor Cecilie Thøgersen-Ntoumani (IOB)
- Faculty of Business and Social Sciences: Professor Stephan Billinger (IMM), Professor Morten Skovsgaard (Centre for Journalism)
- Corporate Communication: Head of Media Relations Bolette Marie Kjær Jørgensen
- SDU Library: Research Librarian Lone Bredahl Jensen, Research Librarian Mette Brandt Eriksen
Applications are reviewed at the next available meeting. Here are the deadlines for 2024:
- September 11
You will receive a response approximately one week after the committee meeting.
What information do I need to fill out the application form?
What you need to complete the application form.
The information about the project that you need to have ready to complete the application includes:
- Basic information about the project, such as project title, information about the responsible/participating researchers, how the project is funded, when the data collection will take place.
- Short layman's description of the research project, in Danish. Divide it into the sections: Background (max 100 words), Research goals (max 50 words) and Methods (max 100 words).
- Your own reflections on the research ethics issues that may be in the project. Answers to several questions relating to the ethics of the project.
Documents to submit with the application
At the final pages of the questionnaire, you will be asked to upload supplemental documentation. The documents you need to submit are:
- Project description, 5-10 pages*
- Information material for research participants (if applicable)
- Informed consent statement for research participants (if applicable)
- Interview guide, questionnaire or similar (if applicable)
- Other documents relevant to the case
- All documents must be in .pdf format and must have meaningful names (e.g. "Project Description.pdf")
Application form
Apply for a research ethics approval now. Fill out the form in English.
- Application Form for Ethics Approval
Contacts Research Support

Jakob Ousager
Research Ethics Consultant
[email protected] 60 11 38 46
Possibilities of fast track & explanatory document
Have you already collected your data and submitted your research paper? Then it is too late to get an ethics approval but you can get an exemption letter. Such a letter explains that your research project follows the law but is in a situation where it is too late to get the ethics approval. It is not an approval but an explanatory document about Danish rules within this area.
Fast track reviews and approvals
go back to research support hub
Last Updated 27.07.2024
Aarhus BSS School of Business and Social Sciences Aarhus University
Research ethics committees – what and why.
With the addition of an official Research Ethics Committee (IRB) at Aarhus University, there are now several places to get ethics approval for projects. But which one do you need? All of them or none? Read our guide to get a quick overview of the available options.

Please note: This article is intended to offer a basic understanding of how the different committees operate and under which circumstances researchers need approval from one or the other. Researchers will need to seek additional information to determine for themselves what committees they need to submit their projects to for review. Links to more information is provided throughout.
At the moment, there are at least three committees where researchers at Aarhus University can get ethics approval:
1) The Human Subjects Committee (Cognition and Behavior Lab, AU BSS) 2) The Research Ethics Committee (Aarhus University) 3) The Central Jutland Regional Committee on Health Research Ethics, or the National Committee on Health Research Ethics (both are part of the Danish committee system as defined by the Committee Act )
Each have a specific purpose and jurisdiction, detailed below. Currently, researchers should never need approval from all three committees.
The Central Jutland Regional Committee on Health Research Ethics
In short: You need approval from this committee if the law requires it.
The Committee Act is a piece of Danish legislation that exists to ensure that health research projects in Denmark are conducted according to a proper ethical standard. The committee system is made up of a committee at the national level (the National Committee on Health Research Ethics, or in Danish, Den Nationale Videnskabsetiske Komité ), as well as several regional committees. For researchers based in the Aarhus area, the legal body governing health research projects is the Central Jutland Regional Committee on Health Research Ethics ( De Videnskabsetiske Komitéer for Region Midtjylland ). Under normal circumstances, this is the committee that researchers at Aarhus University will submit their projects to for review.
According to the National Committee on Health Research Ethics (NCHRE), all health research projects in Denmark must be notified to a research ethics committee. Quoting from their website, the duty to notify comprises, among others, “ trials involving live-born human individuals, human gametes intended for fertilisation, fertilised human eggs, embryos and foetuses, tissue, cells and genetic material from humans, foetuses etc. or deceased persons” (“ What to notify? ", 03.04.2019). Studies involving clinical trials of medicinal products for human use and clinical testing of medical devices are also covered by the duty to notify.
Research within social sciences often involves minimal intervention, which in many cases means that they don’t need to be submitted to the Central Jutland Regional Committee on Health Research Ethics (RCHRE) for a full review. For example, studies that use questionnaires and interviews, but where no human biological material is involved or collected, do not need to seek approval (“ What to notify? ”, 03.04.2019). However, if there is doubt about whether a research project qualifies as a “health” project, then the project should be submitted to the committee. Based on the Committee Act, an assessment will be made of whether the project should be subject to a full committee review. Researchers will receive a formal reply with an explanation of the decision including references to the relevant legal clauses.
For more information about which projects should be submitted for assessment or full review, as well as examples for clarification and links to relevant legislation, go to “ What to notify? ” Other helpful links: Website for the National Committee on Health Research Ethics The Committee Act (Danish only)
Aarhus University’s Research Ethics Committee
In short: You need approval from this committee if it is necessary for you to have ethics approval, yet your project is not eligible to be reviewed by the regional or national committee under the rules of the Committee Act.
Quoting from the website for Aarhus University’s Research Ethics Committee (AUREC): ” Research projects are increasingly subject to requirements for ethical approval by the relevant university department. This requirement stems mainly from the EU’s framework programmes and international journals, and relates specifically to projects that collect empirical data and are not covered by the Committee Act on Regional Committees for Health Research Ethics [sic] , which only applies to research projects in the field of health sciences. […] The Committee’s responsibility is to ensure a consistent and responsible framework for project approval and make it easier for AU researchers to obtain grants and publish .” (” Ethical approval of research ”, 03.04.2019)
In other words: The committee exists for the benefit of those researcher who, due to requirements from e.g. the EU, journals, etc., need ethics approval, but are not eligible to be reviewed by the RCHRE or NCHRE under the Committee Act. Therefore, researchers will not currently need approval from both committees: An approval by a committee under the Committee Act should make an approval from AUREC redundant.
AUREC acts as an Institutional Review Board in that it grants institutionalised approval of projects. Researchers who receive approval from AUREC may write in their publications: “ the project was approved by the Institutional Review Board at Aarhus University, approval #: [approval number] ”. However, “ The Committee is not responsible for ensuring that individual projects comply with research ethics or data protection regulation. Responsibility for this lies with the researcher/research group in question. ” (” Guidelines for the AU Research Ethics Committee ”, 04.04.2019)
AUREC can advise that research projects which do not comply with AU’s regulation is stopped, but it is not authorised to terminate research projects.
For more information and a guide to the application process as well as the evaluation criteria, see following links: Ethical approval of research projects Guidelines for the AU Research Ethics Committee
Cognition and Behavior Lab’s Human Subjects Committee
In short: You need approval from this committee if you want access to resources in COBE Lab.
The purpose of Cognition and Behavior Lab’s Human Subjects Committee (COBE HSC) is to ensure that the research that takes place in COBE Lab follows the Lab’s ethics policy . This is to protect the participants as well as the Lab’s reputation. The COBE HSC will also comment on any issues with data protection that they find concerning, and can recommend or require that AU’s Technology Transfer Office (TTO) is involved to ensure that personal data is properly handled. Finally, the HSC will at times comment on the study design as a form of peer feedback. Only ethics and data concerns can block a researcher’s access to COBE Lab – not concerns about the research design.
The purpose of the HSC is only to allow or deny access to COBE’s resources based on COBE’s ethics policy. Whether researchers need approval from COBE HSC therefore depends entirely on whether the researcher wants access to COBE Lab’s resources (labs, equipment, and participant pool). Approval from COBE HSC is needed in that case; an approval from either AUREC or RCHRE will not grant researchers access to COBE Lab. This is due to the specific requirements of COBE Lab’s ethics policy, for example the Lab’s no-deception policy, or the requirement to pay participants a set average amount of money for their time.
Conversely, approval from COBE HSC is not useful outside of the Lab. Therefore, if researchers face external requirements to obtain ethics approval for a project, an approval from COBE HSC will not be useful; an approval from AUREC or RCHRE is likely needed instead (depending on the specific case).
For more information about the Lab’s ethics policy, the purpose of the HSC, and the approval procedure, follow the links below: Ethics Human Subjects Committee Procedure guide
In conclusion
Due to the many differences between the available committees, there is not currently a “one size fits all” solution for getting a single ethics approval which is valid everywhere. Neither are there plans to consolidate the different ethics committees into one entity.
It is highly recommended that researchers stay informed about the requirements for ethics approval. This is especially the case in relation to AUREC and the committees under the Danish Committee Act, as an ethics approval from either of these committees have further reach and implications than the ethics approval that is available from Cognition and Behavior Lab.
For those researchers who want access to COBE Lab’s resources, information can be found on the Lab’s website. You can also contact lab management directly for advice about the application procedure.
- Related Networks
About EUREC
- EUREC Board
- EUREC Office
- EUREC Members
- EUREC Statute
- EUREC Projects
National Information
- RECs in Europe
- National Legislation
- EU Legislation
- Relevant Literature
- Event Calendar
- Information for Researchers
- Training Materials
- Login for EUREC members
This network has received funding from the European Union.
Eurecnet - National Information: Denmark
National information: denmark (last update: january 2023), short description of recs system:.
There are 12 regional committees. The National committee (the Danish National Committee on Biomedical Research Ethics (National Videnskabsetisk Komit�, (NVK) ): coordinates the work in the regional committees, lays down guidelines for amongst others researchers, gives opinions on issues of a fundamental nature, if this is not related to the approval of a concrete research project, acts as an appeals committee in connection with findings in the regional committees and decide on matters where members of the regional committees disagree, monitors the development of research within the health sector and further the understanding of the ethical problems resulting from the development in relation to the health services and the biomedical research environments.
The National Committee consists of 13 members. 3 members, including the chairman, are appointed by the Minister of Health, 5 members are appointed by the Minister of Health together with the Minister for Science, Innovation and Higher Education, 5 members are appointed by the Minister of Health after recommendation of the regions The Danish National Committee on Biomedical Research Ethics coordinates the work of the regional committees. The Danish National Committee on Biomedical Research Ethics: Forside | Nationalt Center for Etik
Furthermore 3 Medical Research Ethics Committees are established. These committees work with the Clinical Trials Regulation, the Medical Device Regulation and the In vitro diagnostics Regulation. The committees are not regional but governmental. The Minister of Health appoints the chairman. 5 members are appointed by the Minister of Health after recommendation of the regions. 2 members are appointed by the Minister of Health after recommendation of patient organizations.
Networking between RECs:
A coordination forum with representatives from the secretariats in the National Committee, the Medical Research Ethics Committees and the regional committees is established. There is an annual meeting for all members of the ethics committee system and the staff.There is a current contact on emails and telephone between the secretariat for the national committee and Medical Ethics Committees with the secretariats of RECs with the purpose to coordinate and interpret the laws etc. At the same time there is current contacts between the staff of the RECs. Every four years � when new members of the committees are nominated � the national committee arranges a course of education.
Contact: [email protected]
Staff service at AU » Support for your research practice
Research ethical approval of research studies - the research ethics committee.
Research projects are increasingly subject to requirements for research ethical approval by the university. This requirement stems mainly from the EU’s framework programmes and international journals, and relates specifically to studies that collect empirical data and are not covered by the Committees Act on regional scientific ethical committees , which only applies to research projects in the field of health sciences.
Aarhus University (AU) studies are approved by the Research Ethics Committee. The Committee’s responsibility is to ensure a consistent and responsible framework for study approval and make it easier for AU researchers to obtain grants and publish.
The Committee acts as an Institutional Review Board (IRB) in cases where researchers are required to demonstrate that the university has approved the ethical reserach aspects of their research.
How do I apply for approval?
- You must complete and submit an information form with appendices. The Committee will then assess your application with a view to granting research ethical approval.
- You must attach a study protocol using the template (max 5 pages).
- Please apply early to ensure that you have obtained approval before starting your research study.
- Your data collection must begin only when the Committee has approved your application.
- It is recommended that the final design of a study, including local requirements for the use of equipment, laboratory facilities, etc. be clarified before the application to the Committee.
- Participant information, any questionnaires and a declaration of consent must be drawn up in a language in which the participants have in-depth knowledge, usually their native language or English.
- It is sufficient to attach a Danish-language version of this information for the Committee.
- Participant information must be clear and easily understandable.
- Please note that data protection (GDPR) assessment is outside the Committee’s area and that research ethical requirements are a supplement to the requirements in the data protection rules (GDPR).
What type of studies can be assessed?
- The Committee exclusively assesses studies requiring ethical reaserch approval which fall outside of the existing research ethics committee system, and which are headed by researchers employed at AU.
- The Committee only approves empirical studies that collect data from volunteer study participants, including observation studies and questionnaire-based studies that involve the collection of data from individuals.
The Committee distinguishes between a ‘research project’ and a ‘study’. Only empirical studies can be processed by the Committee. The object of assessment is the research design and the treatment of the people and environments involved in the studies. It is therefore only possible to apply for research ethical approval of an empirical study and not for ethical approval of a research project. Obtaining research ethical approval of an empirical study involves accounting for ethical considerations in the planned research procedures. For this reason, such considerations must be addressed. This means, for example, that it is not sufficient to simply note that a given study contains no ethical challenges. The rationale and explanation for the planned procedures must be described. In certain cases, the application may be returned to the applicant with a request for further explication of the ethical implications of parts of the study. The opportunity to apply for research ethical approval is new for most AU researchers. The Committee strives to clearly establish what this approval requires and entails, and it operates on the principle that the treatment of the people and environments affected by the research should be in focus.
Requirements for consent
Informed participant consent: The participation of human subjects in a research study requires their informed consent. A declaration of consent and any accompanying additional participant information must be formulated in a language that enables persons being asked to provide their consent to understand what they are consenting to. Typically, the declaration of consent or participant information will include the following types of information:
- The name of the principal investigator
- An explanation of the purpose of the research project and the study
- The sources of funding for the research project
- How study participants will be recruited
- The method/procedures to be used in the study
- Expected risks/adverse effects
- Who will benefit from the results of the research
- How the results of the study will be communicated (including feedback to the participants in the study)
- How sensitive personal data will be protected, including how long such data will be stored
- How participants can withdraw from the study, including how participant data will be handled in connection with withdrawal during/after the completion of the study
- Opportunities for participants to review and, where relevant, comment on transcriptions of any interviews and quotes
- Where relevant, debriefing opportunities for participants
As a matter of principle, obtaining informed, active and explicit informed consent from participants in research studies is always necessary (see additional information on deception and debriefing below). If electronic questionnaires are used to document informed consent, participants may provide their consent by filling out a form. In the absence of informed consent, the reasons for this must be substantiated in the application in a satisfactory manner.
Please note that the requirement for informed consent is based on research ethical considerations that supplement, but are not identical to, the requirements for participant information and informed consent according to GDPR rules.
Please also note that it may necessary to reconfirm participant consent; misunderstandings, challenges and difficult situations may arise during the study as it develops. In qualitative studies, the purpose of the study may also develop. It is always the researcher's task and responsibility to handle any challenges that may arise and ensure that the participants know what they are participating in. In the application, considerations in this regard must be elaborated on.
Voluntary participation The duty to protect participants also involves sensitivity to power relationships and different interests and expectations with regard to the research results.
Participating in a research study should be entirely voluntary. This should be stated clearly in the participant information, for example: “Your participation in this survey is entirely voluntary. It is up to you to decide whether or not you wish to participate ."
It should always be possible for participants to withdraw informed consent and delete information up until such time as the results or data from the study are published. Participants must be informed about how to do this and the timeframe for doing so. If at some point during the study the information is anonymised and it is therefore no longer possible to identify which data originates from the individual participants, the participants must be informed of this. If consent is withdrawn while data is being collected, participants must be informed whether or not data collected prior to the withdrawal of consent will be included in the study.
Informed consent regarding children and adolescents While interviews with or observation of children and adolescents should not be conducted without the consent of parents/guardians, their consent alone is not sufficient: if the participants are children/adolescents under the age of 18, consent from both the participants themselves and the parents/guardians is necessary. Participant information must be tailored to relevant age groups, which means that it may be necessary to provide different information to parents and children respectively, and to different age groups. In the case of younger children, one possible approach to obtaining consent is asking them to repeat an oral statement of consent. In research involving young children without language, the researcher must continuously take stock of the situation. The application must explicitly account for how the researcher intends to do so.
There may be studies which cannot be carried out if the consent of the parent/guardian is a prerequisite for their performance. If this is the case, the applicant must carefully explain the reasons for waiving the requirement that the parent/guardian grant consent and describe how the participants will be protected, as well as the specific ways available to children/young people for opting in and opting out.
Deception and debriefing Participant information, informed consent and subsequent debriefing (when relevant) are fundamental to ethical research practices. For research study designs that employ deception, a subsequent debriefing with the possibility to withdraw from the study is often an absolute requirement.
Important regarding data
External panels and recruitment bureaus The person(s) listed as responsible for the study guarantees the legal and research-ethical handling of data collected by the recruitment platform.
Methods for the anonymisation of data and publication of results must be outlined in the study description.
Collection of data in other countries The Committee requires that approval for the study is also sought in other participating countries in which such approval is relevant or required. This is in order to ensure that the study and collection of personal data also meets the relevant national/local research ethical requirements and standards in these countries.

Information form and protocol
Fill out the information form with all appendices (in PDF format). Find the form here.
If you have any questions regarding the form, please send an email to [email protected] .
Please also fill out this protocol template. The protocol is compulsory and must be max. 5 pages.
What appendices do I need to submit?
Submit the appendices relevant to your study. In addition to the protocol (max. five pages in a compulsory template), the following material is usually required:
- Participant information
- Declaration of consent
- Recruitment material
- Study responsible’s CV (max. one page)
Participant information, possible questionnaires and the declaration of consent must be written in a language that the participants have an in-depth knowledge of, which is usually their native language. In case of Danish-language participants, it is only necessary to enclose the Danish-language version of the documents. There is no need to enclose published material, e.g. articles.
Description of purpose and risks: The description of the purpose and risks must demonstrate that the benefit of the study is balanced against the human resources involved in the project and the risks/inconveniences that they may be exposed to in connection with recruitment, study and reporting. The declaration must also demonstrate that the social group represented by the study participants will benefit from the outcome of the study.
Equal processing All participants in a study must be treated equally. If participants receive compensation, it should be the same for everyone.
Subsequent changes The Committee’s approval is based on the specific documents in the application. If any significant changes are made subsequently, a new application must be sent to the Committee. The application for amendments of applications or individual documents can be sent to [email protected] .
The application for changes must include a short description of the change, and the changes in updated documents must be clearly indicated.
Meetings of the Committee
The Committee annually holds 8 meetings in each sub-committee and 8 meetings in the joint committee.
Application deadlines in 2024:
Wednesday 10 January
Wednesday 21 February
Wednesday 3 April
Wednesday 15 May
Wednesday 14 August
Wednesday 18 September
Wednesday 23 October
Wednesday 27 November
- - Google Chrome
Intended for healthcare professionals
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Denmark takes a lead...
Denmark takes a lead on research ethics
- Related content
- Peer review
- Sandra Goldbeck-Wood
Danish medical researchers will soon have to observe tough new regulations to gain ethics committee approval for their research, according to a decision taken by the Danish national research ethics committee.
The measure, agreed by the committee last November and to be implemented shortly, requires applicants for ethical approval to show that they have carried out a full systematic review of the relevant scientific literature before the study will be approved. They must also continue …
Log in using your username and password
BMA Member Log In
If you have a subscription to The BMJ, log in:
- Need to activate
- Log in via institution
- Log in via OpenAthens
Log in through your institution
Subscribe from £184 *.
Subscribe and get access to all BMJ articles, and much more.
* For online subscription
Access this article for 1 day for: £50 / $60/ €56 ( excludes VAT )
You can download a PDF version for your personal record.
Buy this article
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
How does the ethical review process work in Denmark and which are the designated ethics committees?
According to Danish law , all research projects in Denmark involving human beings or any kind of human tissue, cells etc. need permission from an regional ethics committee. In the case of medicinal and medicinal devices trial projects a permission from the Danish Medicines Agency is also required before the project can be initiated.
The investigator and the sponsor of the research project must apply for permission from the regional research ethics committee for the area in which the investigator is operating. The application should conform with the “Guidelines about notification etc. of a biomedical research project to the committee system on biomedical research ethics” .
The investigator and sponsor shall use an electronic application form: www.drvk.dk/anmeldelse and send the application on email to the competent research ethics committee along with the trial protocol and other information required for the committee’s assessment. The email must be send using a digital signature. Further information at the regional research ethical committees.
In the case of multi-center trials, the investigator shall only apply for permission from one regional committee, i.e. the regional committee in the area, where the principal investigator carries out the research project. However, in the case of multi-national trial projects, a permission from a Danish committee is always required.
The review of the application by the regional research ethics committee will take place when a complete and valid application has been submitted. A valid application must include the following elements:
- Application form
- The clinical trial protocol
- Subject information and the informed consent procedure
- A protocol resumé.
The trial protocol and other information can be received in english with a Danish protocol resumé. Further information at guidelines about notification. Applicants whose project is rejected by the regional ethics committee can appeal the decision at The Danish National Committee on Health Research Ethics.
Weblinks to Danish Ethics Committees
EAST Regional Research Ethics Committees for the region of Hovedstaden (6 committees) Kongens Vænge 2 3400 Hillerød Telefon 38 66 63 95 [email protected] Weblink to Region Hovedstadens website
EAST Regional Research Ethics Committees for the region of Sjælland Kvalitet og Udvikling Alléen 15 4180 Sorø Telefon 57 87 52 55 [email protected] Weblink to Region Sjællands website
WEST Regional Research Ethics Committees for the region of Syddanmark (2 committees) Regionshuset Damhaven 12 7100 Vejle Telefon 76 63 82 20 / 76 63 82 21 / 76 63 82 22 [email protected] Weblink to Region Syddanmarks website
NORTH Regional Research Ethics Committees for the region of Midtjylland (2 committees) Regionssekretariatet, Juridisk kontor Skottenborg 26 8800 Viborg Telefon 78 41 01 83 [email protected] Weblink to Region Midtjyllands website
NORTH Regional Research Ethics Committees for the region of Nordjylland Regionssekretariatet Niels Bohrs Vej 30 9220 Aalborg Ø Telefon 97 64 84 41 [email protected] Weblink to Region Nordjyllands website
The National Research Ethics Committee Holbergsgade 6 1057 København K Telefon 72 26 93 70 [email protected] Weblink to The National Research Ethics Committee
OUR MISSION
"Piloting the clinical and regulatory development and market introduction of innovative medical devices in Europe and beyond!"
"Extend the capabilities and expertise of our customers!"
OUR COMMITMENT
"Integrity, quality, partnership and teamwork!"
OUR INSPIRATION
"Making innovative new therapies and diagnostic products available to patients worldwide!"
- Ethics Committees
[The regional ethical committees. Responsibilities and challenges]
- Ugeskrift for Laeger 166(24):2341-2
- 166(24):2341-2
- This person is not on ResearchGate, or hasn't claimed this research yet.

- Herlev Hospital
Discover the world's research
- 25+ million members
- 160+ million publication pages
- 2.3+ billion citations
No full-text available
To read the full-text of this research, you can request a copy directly from the authors.
- Ugeskr Laeger

- Recruit researchers
- Join for free
- Login Email Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google Welcome back! Please log in. Email · Hint Tip: Most researchers use their institutional email address as their ResearchGate login Password Forgot password? Keep me logged in Log in or Continue with Google No account? Sign up

About the Danish Research Ethics Committee System
The purpose of the scientific ethics committee system is to ensure that health science research projects are conducted in an ethically sound manner. Consideration for the rights, safety and well-being of research subjects takes precedence over scientific and societal interests in creating the possibility of obtaining new knowledge or investigating existing knowledge that may justify the implementation of the research project.
The Regional Research Ethics Committees (RVK), established by the Regions, the Danish National Medical Research Ethics Committee (VMK) , and the Danish National Committee on Health Research Ethics (NVK) , established by the Ministry of Health, deal with all types of research projects in the health sciences involving human subjects or human biological material.
Choose at subject below to read more about the history of the committee system, impartiality in the committee system, etc.

The History of the Committee System

The Regional Research Ethics Committees

Quality Assurance in the Committee System

Avoidance of Bias in the Committee Systems
This website uses third-party cookies for visit statistics. If you choose "Accept All," you consent to third-party services storing information about your visit. Read more about cookies

- Basic Search
- Advanced search
- Individual Login
- Create an account
- Purchase options
- Subscribe to the MJA
- Publish with us
- Current issue
- Online first
- Anatomy and physiology
- Anesthesia, analgesia and pain
- Cardiovascular diseases
- Complementary therapies
- Diagnostic techniques and procedures
- Digestive system diseases
- Emergency medicine
- Endocrine system diseases
- Environment and public health
- Ethics and law
- Eye diseases
- General medicine
- Gerontology
- Global health
- Health occupations
- Health services administration
- Hematologic diseases
- History and humanities
- Immune system diseases
- Indigenous health
- Infectious diseases
- Information science
- Medical education
- Medical genetics
- Men's health
- Mental disorders
- Musculoskeletal diseases
- Nervous system diseases
- Nutritional and metabolic diseases
- Occupational diseases
- Otorhinolaryngologic diseases
- Palliative care
- Pediatric medicine
- Pharmaceutical preparations
- Public and environmental health
- Rehabilitation
- Respiratory tract diseases
- Rheumatology
- Sexual health
- Skin and connective tissue diseases
- Social determinants of health
- Sports medicine
- Statistics, epidemiology and research design
- Substance-related disorders
- Substance‐related disorders
- Surgical procedures, operative
- Urologic diseases
- Vascular diseases
- Women's health
- Wounds and injuries
- Research letters
- Guidelines and statements
- Narrative reviews
- Perspectives
- Reflections
- Competitions
- The Medical Journal of Australia

- Archive keyboard_arrow_down
- search SEARCH -->
Issues by year
Supplements
Article types

Indigenous governance, ethics and data collection in Australian clinical registries
- picture_as_pdf Download
Objectives: To examine Indigenous Governance of Data processes in Australian clinical registries.
Design, setting, participants: Audit (via desktop review and interviews) of registries in the Australian Register of Clinical Registries from 17 January 2022 to 30 April 2023.
Main outcome measures: The number of clinical registries collecting ethnicity data, reporting Aboriginal and/or Torres Strait Islander representation on registry governance or steering committees, and reporting human research ethics committee approval.
Results: A total of 107 clinical registries were reviewed. Of these registries, 65 (61%) collected ethnicity data; when these were grouped by geographical coverage, those most likely to collect ethnicity data were binational (24/40 [60%]), national (19/26 [73%]) or state based (19/26 [73%]). Of the registries that collected ethnicity data, 29 (45%) classified their ethnicity item as Aboriginal and/or Torres Strait Islander. Only eight clinical registries (7%) reported Aboriginal and/or Torres Strait Islander representation on their governance or steering committees. Human research ethics approval was reported in 94 registries (88%), with only 11 (12%) having Aboriginal human research ethics committee approval.
Conclusion: Significant variability is evident in clinical registry recording of Indigenous governance of data, meaning that Aboriginal and Torres Strait Islander communities remain invisible in data which is used to inform policy, clinical models of care, health services and initiatives. Radical change is required to facilitate meaningful change in quality indicators for clinical registries nationally.
The known: Clinical registries are data repositories that monitor clinical quality indicators which are used for quality improvement of clinical standards and health care effectiveness in Australia. It is not clear if quality improvement processes from registries include Aboriginal and Torres Strait Islander communities.
The new: Between 17 January 2022 and 30 April 2023, 107 registries were registered with the Australian Register of Clinical Registries, and 39% of these clinical registries did not record any ethnicity data for patients. The vast majority of registries (93%) did not have Aboriginal and/or Torres Strait Islander representation on registry governance or steering committees or approval from an Aboriginal human research ethics committee.
The implications: Variability exists in application of Indigenous governance of data by clinical quality, affecting the visibility of Aboriginal and Torres Strait Islander individuals’ needs for policy, clinical models of care, health services and initiatives. Radical change is required, and clinical registries must work with peak bodies and stakeholders to ensure that quality improvement initiatives target populations in greatest need of this change.
Clinical registries are epidemiological repositories that provide systematic clinical quality indicator monitoring for clinical domains. 1 , 2 , 3 Most clinical registries have nationally accepted quality indicators drawn from clinical models of care, providing feedback on adherence and performance. 1 , 2 Such monitoring enacted through clinical registries is essential for continued quality improvement in clinical standards and effectiveness in health care, through identifying and addressing gaps in clinical quality outcomes. 2 Ongoing monitoring of clinical quality indicators with adaptation for continued improvement can play an important role in improving health outcomes for priority populations. 2 , 4
In Australia, Aboriginal and Torres Strait Islander communities are disproportionately affected with a greater burden of disease and injury compared with the non‐Indigenous Australian population. These inequities include a risk of hospitalisation that is 2–10 times greater for injury or chronic conditions, higher diagnosis and mortality rates for preventable cancers, and a life expectancy that is 10 years lower than that for other Australians, along with significant barriers to accessing affordable and equitable health care. 5 , 6 , 7 These high rates of disease and poor health and social outcomes reflect the ongoing marginalisation of Aboriginal and Torres Strait Islander people in Australia, and systematic racism embedded in the health system — a continued legacy from colonisation. 6
The National Strategy for Clinical Quality Registries and Virtual Registries (2020–2030) and the Framework for Australian clinical quality registries by the Australian Commission on Safety and Quality in Health Care (the Commission) have key priority action areas aimed at addressing health inequities experienced by Aboriginal and Torres Strait Islander people. 2 , 8 One way in which they are implemented is through principles of Indigenous Governance of Data (IG‐Data), which are formal structures for management and governance of any data (procedures, policies, reporting, translation) which relate to or affect Aboriginal and Torres Strait Islander peoples. 9
Currently it is not known how, or if, Australian clinical quality registries contain clinical quality indicators on Aboriginal and Torres Strait Islander identity, if registries have Aboriginal and/or Torres Strait Islander representation on governance or steering committees, or if registries have Aboriginal human research ethics committee (AHREC) approval. In this audit, we sought to understand these unknowns, focusing on how Australian clinical quality registries engage with IG‐Data, through registry items for recording ethnicity, ethics approvals, and Aboriginal and Torres Strait Islander representation on registry governance or steering committees.
Design and participants
The Australian Register of Clinical Registries (ARCR) was established by the Commission, and contains summary information and activity on clinical registries, that have voluntarily registered, to enhance understanding, awareness and collaboration across the health care sector. 10 The ARCR contains standard information on registries: identification, condition, clinical domain, registry name, abbreviation, contact information, data custodians, reporting mode and ethics. It does not contain clinical quality indicators or items recorded and reported by discrete registries.
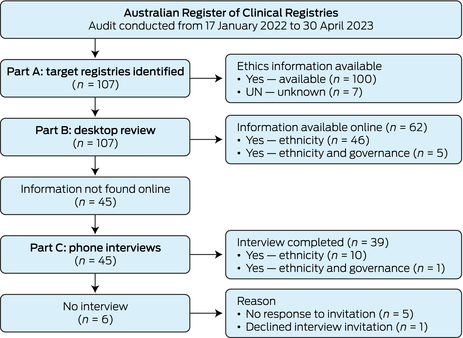
This audit involved a review of all registries in the ARCR from 17 January 2022 to 30 April 2023. General registry demographics were collected from the ARCR: registry establishment year, clinical domain, geographical coverage (international, national, etc), and ethics approval body. Examination of IG‐Data was undertaken by identifying if and how registries collected participant ethnicity, if ethics approval was provided and by whom, and if there was Indigenous representation on registry governance or steering committees. Ethnicity, rather than Indigenous status, as an item was investigated, because Indigenous status is commonly embedded in ethnicity as a data item in registries. The registry audit was conducted over three parts ( Box 1 ): target registries were identified in Part A, identified registries were examined by desktop review in Part B, and registries moved to Part C of the audit (phone interviews) if ethnicity item reporting was not found during the desktop review.
Study governance, ethics approval and analysis
Our investigative team was led by an Aboriginal researcher (CR). Indigenous knowledges (knowing, being and doing) were important for the overall focus and conceptualisation of outcomes. The lead researcher engaged their lived experience in Indigenous Data Governance and registry work, in the context of outcomes, and used yarning with the investigative team to determine the main themes for reporting. Ethics approval was acquired from the Human Research Ethics Committee at UNSW Sydney (HC210907). Ethics approval was not sought from an AHREC as no data were collected from Aboriginal and/or Torres Strait Islander peoples. Data were collected in Microsoft Excel and imported into Stata 14 for calculation of descriptive statistics (frequencies and proportions).
Registry audit
The audit was conducted from 17 January 2022 to 30 April 2023 ( Box 2 ). In Part A, a total of 107 registries were identified in the ARCR ( Supporting Information , Table 1). Part B, the desktop review, provided item information on 62 registries (58%). A total of 45 registries (42%) were marked as unknown for their ethnicity collection and invited to a phone interview in Part C, in which 39 registries participated (87% response rate). One registry declined to participate due to time constraints and five registries did not respond to email requests.
Registry demographics
Of the 107 registries, 40 (37%) were binational (including both Australia and New Zealand), followed by state (26 [24%]) and national (25 [23%]) registries, with smaller numbers of regional (10 [9%]) and international (5 [5%]) registries ( Box 3 ). The majority of registries were young, with 58 registries (54%) having been established for 10 years or less. Only 69 registries (64%) included a prioritised clinical domain area in the ARCR. In terms of broad clinical domain, 29 registries (27%) focused on high burden cancers or cancers, and 11 registries (10%) focused on each of the following: musculoskeletal disorders; critical care; and cardiac, stroke and ischaemic heart disease. Other broad clinical domain areas for registries included: trauma, burns and injury; diabetes; dementia; and maternity.
Ethnicity collection
In total, 65 registries (61%) collected ethnicity data ( Box 4 ). Of these registries, 24 (37%) were binational, 19 (29%) were national and 19 (29%) were state based ( Box 5 ). Indigenous status was embedded in the ethnicity data item ( Box 4 ); of the registries that collected ethnicity data, 62 (95%) collected Indigenous status data, and another five (8%) collected data on other ethnicities. Of the 62 registries collecting Indigenous status, 29 (47%) registries classified this item as Aboriginal and/or Torres Strait Islander ( Box 4 ). None of these 29 registries collected ethnicity data for Aboriginal and Torres Strait Islander individuals at a regional level (eg, Koori, Nunga) or at a language group or community level (eg, Kaurna, Larrakia, Gadigal). For the remaining 36 of those that collected ethnicity data (55%), variability was evident — for example, some binational registries recorded “Māori, Pacific Islander”, and others recorded non‐Indigenous ethnicity.
Governance representation
Many registries had governance or steering committees with a central role in overseeing processes and outputs for each registry. Membership generally consisted of clinicians, allied health specialists, researchers, funding bodies and consumers; however, only eight registries (7%) reported Aboriginal and/or Torres Strait Islander representation on these committees through a specific identified role ( Box 5 ), 87 (81%) did not have such representation, and this information was unknown for the remaining registries.
Registry ethics
A total of 94 registries (88%) provided details to the ARCR on governing bodies which had provided human research ethics committee (HREC) approval; the remaining 13 (12%) cited quality improvement initiatives for safety and quality, such as jurisdiction agreements or health care or hospital acts in place of ethics approvals ( Supporting Information , table 1). Of the 94 registries which had sought ethics approval, 11 (12%) had sought approval through a state or territory AHREC for their registry in addition to their primary HREC approval. Of the 83 registries (78%) that did not have documented AHREC approval, 45 (54%) had sought approval from an HREC in a state which does not have a registered AHREC or similar body. In addition, 49 of these 83 registries (59%) recorded ethnicity status in their registries ( Box 5 ).
This study provides a comprehensive review of IG‐Data processes around quality indicator collection, ethics approval, and Indigenous representation on registry governance in Australian clinical registries. Our outcomes suggest that collection of Aboriginal and Torres Strait Islander status is not a priority for clinical registries, with no data items for collection of Indigenous status in nearly 40% of registries. For international registries, this lack of data items may be attributable to legal requirements as it is not legally allowed to collect ethnicity status across Sámi, Ainu, Ryūkyūan and Kanak countries (Denmark, Finland, Norway, Sweden, France–New Caledonia, and Japan); however, this only affects 5% of registries in this study. 11 The lack of appropriate data items may also be indicative of challenges to data quality in collection of ethnicity status for registries. Under‐identification of Aboriginal and Torres Strait Islander status is well documented in administrative data collections (eg, censuses, taxation information, health records), which may influence the decision of a registry as to whether they collect ethnicity status. 12 , 13 , 14 Improvements to Aboriginal and Torres Strait Islander identification in administrative data have occurred through data linkage and application of enhancement or multistage algorithms by industry. 12 , 14 These processes are costly and require specialised data science skills, which are not conceivable for many registries that are constrained by tight resourcing and often rely on the goodwill of staff in registry participation sites to upload this information as part of quality and safety reporting without additional resourcing.
Lack of reporting of Indigenous status in registries may also represent conceptualisation of health and wellbeing models from Western biomedical models of health, where quality indicators and patient care focus on clinical knowledge, and cultural nuances are precluded. These approaches do not recognise diversity or pluralism in health care journeys or relationality in Aboriginal and Torres Strait Islander patient experience. 7 , 11 For these reasons, the Commission has redeveloped principles in their clinical quality registries framework through prioritising consumer engagement, particularly with Aboriginal and Torres Strait Islander communities, in clinical design and outcome translation. 2 In addition, recommendations in the National Clinical Quality Registry and Virtual Registry Strategy 2020–2030 call for prioritisation of identification of Aboriginal and Torres Strait Islander people in datasets. 8 These recommendations support national initiatives to improve accuracy and completeness of ethnicity status in hospital medical records, which are often used for registries, through training of health care staff on the standard Indigenous question. 7 However, further reforms are needed for accurate and reliable Aboriginal and Torres Strait Islander health data analysis through community‐centric approaches, such as strength‐based analysis and community contextualisation to inform policy, clinical models of care, service responses and health services. 15 Time will tell if further uptake of these national principles has long term impacts on recording and reporting of health care quality and effectiveness for Aboriginal and Torres Strait Islander communities.
For the 61% of registries which contained an ethnicity item, variation existed in coding of this item. Nearly 30% of registries that we audited coded their ethnicity item using national phrasing of “Aboriginal and/or Torres Strait Islander”; however, no registries collected data at a regional, community or language group level. Other registries used collective phrasing of “Indigenous”, or even tautologies of “Indigenous Australian or Torres Strait Islander”. This ambiguity is indicative of white possessive logic in registry processes 16 which is reflective of AHREC engagement — only 10% of registries had this approval. In addition, 49 of the registries that had sought ethics approval but not AHREC approval (59%) recorded ethnicity. While some of these registries may have approval in a jurisdiction without an AHREC or may not formally report on Aboriginal and Torres Strait Islander status, it does question integrity around ethics processes when working with Indigenous data. The National Health and Medical Research Council (NHMRC) and Australian Institute of Aboriginal and Torres Strait Islander Studies (AIATSIS) provide six core values for research in this area (reciprocity, respect, equality, responsibility, survival and protection, and spirit and integrity); 17 , 18 this does not preclude Indigenous data or registry work. Registries need to consider the significant role that they play in IG‐Data to work towards Indigenous data sovereignty.
In addition, the National Strategy for Clinical Quality Registries and Virtual Registries (2020–2030) provides priority actions to enhance quality and efficiency of systematic data collection through all registries having a “standard Indigenous status item”. 8 However, no standard methods exist to support registries in developing meaningful and ethically appropriate ethnicity coding for Indigenous communities internationally and for Aboriginal and Torres Strait Islander communities nationally. Our recommendation is for registries to work with Aboriginal organisations to co‐develop ethical data collection approaches, items and coding for ethnicity collection, 7 which should include AHREC approvals. This might involve, for example, peak bodies (ie, the National Aboriginal Community Controlled Health Organisation, AIATSIS or Lowitja Institute) for binational and national registries, with AIATSIS ethics approval and AHREC approval across represented jurisdictions. These organisations are best placed in providing informed, ethical, community‐centric data governance approaches for Indigenous data. This may include cases where it is inappropriate for a clinical registry to collect ethnicity data at a language group level due to individual identification.
Data custodians of clinical registries have a responsibility to establish governance or steering committees who provide input into overarching strategies, policies and procedures, such as data access agreements or annual reporting. 2 , 8 Representation on these governance or steering committees generally includes researchers, clinicians and patients or consumers. Recommendations from the Commission and Australian Government Department of Health and Age Care include identified roles for Aboriginal and/or Torres Strait Islander participants on these committees. 2 , 8 However, despite these national recommendations, less than one in ten clinical registries had Aboriginal and/or Torres Strait Islander representation. The invisibility of Indigenous knowledges at this level contributes and reinforces deficit discourses of Aboriginal and Torres Strait Islander communities against clinical quality indicators and outcome measures. The national recommendations, in part, act to disrupt these approaches and recognise the sovereign rights of Aboriginal and Torres Strait Islander communities to data control, ownership and self‐determination, along with the responsibility of clinical registries to facilitate meaningful engagement and champion change. 7 , 11 , 19 This should be further supported by registries enacting principles from the AIATSIS research code of ethics 17 and the NHMRC Road Map 3 for improving Aboriginal and Torres Strait Islander health through research. 18
Limitations
Registration with the Commission's ARCR is purely voluntary, thus not all clinical quality registries in Australia may presently be registered. Despite this, to our knowledge, our study is the first to undertake a comprehensive audit of ARCR registries, focusing on IG‐Data processes. We hope that current clinical registries, and researchers and clinicians looking to establish registries, will find outcomes from this audit of use to guide their current work. Additional limitations of this study include the targeted focus of this audit on specific IG‐Data processes, in which specific statistical processes and reporting have not been examined. We note that five registries did not respond to interview requests. Since the time of our data collection, both desktop review and interviews, some registries may have added new items (eg, ethnicity status) to their registries or obtained AHREC approval, and more registries may have been added to the ARCR.
Indigenous governance of data is a key priority area for national government frameworks and strategies in Australia. However, there is significant variability across clinical quality registries in how IG‐Data is enacted or addressed. These approaches affect Aboriginal and Torres Strait Islander patients, rendering communities invisible in policy, clinical models of care, health services and initiatives. It is clear that radical change is required to facilitate new meaningful approaches to clinical quality indicator development. This development must engage with peak stakeholders and community organisations with accountability in targeting health inequities for improvement in health outcomes.
Box 1 – Audit process and data collection steps
|
| Sources, considerations, and steps for collecting data | ||||||||||||||
|
|
| ||||||||||||||
| Source | Australian Register of Clinical Registries | ||||||||||||||
| Demographics | Condition, clinical domain, year established, geographical coverage, ethics approval | ||||||||||||||
| AHREC coding | |||||||||||||||
| Broad clinical domains | Review and grouping of Australian Commission on Safety and Quality in Health Care‐defined conditions for each registry | ||||||||||||||
| Clinical domain (Commission defined) | |||||||||||||||
|
|
| ||||||||||||||
| Sources | Registry websites, annual reports, data dictionaries and other relevant registry documents available online | ||||||||||||||
| Ethnicity collection coding | |||||||||||||||
| Ethnicity coding | Outlining how the ethnicity item is coded by registries (eg, “Indigenous Australian”) | ||||||||||||||
| Governance representation | |||||||||||||||
|
|
| ||||||||||||||
| Sources | Registry data custodians and/or administrators | ||||||||||||||
| Recruitment | Email invitation to phone interview, with follow‐up to participate conducted twice if no response | ||||||||||||||
| Ethnicity collection question | Do you collect ethnicity data for patients in your registry? | ||||||||||||||
| Ethnicity coding question | If you do collect this item, how is this item coded? | ||||||||||||||
| Governance representation question | Do you have Aboriginal and/or Torres Strait Islander representation on your registry's governance or steering committee? | ||||||||||||||
| AHREC = Aboriginal human research ethics committee; HREC = human research ethics committee. | |||||||||||||||
Box 2 – Registry audit flow diagram
Box 3 – Registry demographics
| Type of registry | Number (%) | ||||||||||||||
| Geographical coverage |
| ||||||||||||||
| International | 5 (5%) | ||||||||||||||
| Binational between Australia and New Zealand | 40 (37%) | ||||||||||||||
| National | 25 (23%) | ||||||||||||||
| State | 26 (24%) | ||||||||||||||
| Regional | 10 (9%) | ||||||||||||||
| Unknown | 1 (1%) | ||||||||||||||
| Time since registry first started |
| ||||||||||||||
| < 5 years | 29 (27%) | ||||||||||||||
| 5–10 years | 29 (27%) | ||||||||||||||
| 11–20 years | 26 (24%) | ||||||||||||||
| > 20 years | 18 (17%) | ||||||||||||||
| Unknown | 5 (5%) | ||||||||||||||
| Broad clinical domain |
| ||||||||||||||
| Asthma | 2 (2%) | ||||||||||||||
| Blood, liver conditions | 8 (7%) | ||||||||||||||
| Cancer (high burden cancers or cancers) | 29 (27%) | ||||||||||||||
| Cardiac, stroke, ischaemic heart disease | 11 (10%) | ||||||||||||||
| Critical care | 11 (10%) | ||||||||||||||
| Dementia | 2 (2%) | ||||||||||||||
| Diabetes | 3 (3%) | ||||||||||||||
| Maternity | 2 (2%) | ||||||||||||||
| Musculoskeletal disorders | 11 (10%) | ||||||||||||||
| Other congenital or autoimmune condition | 7 (7%) | ||||||||||||||
| Other specialised conditions or service | 4 (4%) | ||||||||||||||
| Other surgery, anaesthetics or implant | 7 (7%) | ||||||||||||||
| Pain and rehabilitation | 2 (2%) | ||||||||||||||
| Pelvic floor procedures, devices, endometriosis | 2 (2%) | ||||||||||||||
| Trauma, burns, injury | 6 (6%) | ||||||||||||||
|
| |||||||||||||||
Box 4 – Variation in ethnicity data item definition
|
| Number (%) | ||||||||||||||
|
|
| ||||||||||||||
| Yes | 65 (61%) | ||||||||||||||
| Indigenous status | 62 (58%) | ||||||||||||||
| Other ethnicities | 5 (5%) | ||||||||||||||
| No | 33 (31%) | ||||||||||||||
| Unknown | 10 (9%) | ||||||||||||||
|
|
| ||||||||||||||
| Aboriginal Islander | 1 (2%) | ||||||||||||||
| Aboriginal and/or Torres Strait Islander | 29 (47%) | ||||||||||||||
| Aboriginal and/or Torres Strait Islander; Indigenous | 1 (2%) | ||||||||||||||
| Aboriginal and/or Torres Strait Islander; Caucasian; Pacific Islander; Native American; Hispanic; Indian; Asian; African American; other | 1 (2%) | ||||||||||||||
| Aboriginal and/or Torres Strait Islander; Oceanic First Peoples; culturally and linguistically diverse | 1 (2%) | ||||||||||||||
| Ethnicity (generic) | 3 (5%) | ||||||||||||||
| Ethnicity, Māori (NZ), Aboriginal and/or Torres Strait Islander | 1 (2%) | ||||||||||||||
| Indigenous | 8 (13%) | ||||||||||||||
| Indigenous; culturally and linguistically diverse | 1 (2%) | ||||||||||||||
| Indigenous Australian or Torres Strait Islander; Māori, Pacific Islander | 1 (2%) | ||||||||||||||
| Indigenous or Torres Strait Islander; Aboriginal and/or Torres Strait Islander | 1 (2%) | ||||||||||||||
| Māori (NZ), Aboriginal and/or Torres Strait Islander | 9 (15%) | ||||||||||||||
| Māori (NZ), Aboriginal and/or Torres Strait Islander; Caucasian; Pacific Islander; Native American; Hispanic; Indian; Asian; African American; other | 2 (3%) | ||||||||||||||
| Māori (NZ), Aboriginal and/or Torres Strait Islander; First Nations; Oceanian (specified with Indigenous peoples [eg, Cook Island Māori, NZ Māori, Aboriginal Australian, Torres Strait Islander, Samoan]) | 1 (2%) | ||||||||||||||
| Māori, Pacific Islander; Aboriginal and/or Torres Strait Islander | 3 (5%) | ||||||||||||||
| Aboriginal and/or Torres Strait Islander; Ethnicity–skin type: Australian Aboriginal; Anglo‐Saxon/Celtic; Arab/North African/Middle Eastern/Central Asian (eg, Lebanese, Turkish); Black African/Caribbean Islander; Māori/Pacific Islander; mixed skin type; Southern/South Eastern European (eg, Italian, Spanish, Maltese, Greek, Croatian); Western/Northern/Eastern European (eg, German, Danish, Polish); North East Asian (eg, Chinese, Japanese, Korean); South East Asian (eg, Vietnamese, Filipino); South Asian (eg, Indian, Pakistani); South American; other | 1 (2%) | ||||||||||||||
| Unknown | 1 (2%) | ||||||||||||||
| NZ = New Zealand. * Denominator for calculation of percentages is 107 (total number of registries reviewed in the study). † Denominator for calculation of percentages is 62 (number of registries that collected and/or reported Indigenous status). | |||||||||||||||
Box 5 – Inclusion of ethnicity analysed by geographical coverage, Indigenous representation and AHREC approval
|
| Number of registries | Number (%) of registries that included ethnicity | |||||||||||||
| Geographical coverage |
|
| |||||||||||||
| International | 5 | 1 (20%) | |||||||||||||
| Binational | 40 | 24 (60%) | |||||||||||||
| National | 26 | 19 (73%) | |||||||||||||
| State | 26 | 19 (73%) | |||||||||||||
| Regional | 9 | 2 (22%) | |||||||||||||
| Indigenous representation on governance or steering committee | 8 | 8 (100%) | |||||||||||||
| AHREC approval |
|
| |||||||||||||
| Yes | 8 | 4 (50%) | |||||||||||||
| No | 33 | 16 (48%) | |||||||||||||
| No — no AHREC* | 50 | 34 (68%) | |||||||||||||
| No — other | 7 | 6 (86%) | |||||||||||||
| Unknown | 8 | 4 (50%) | |||||||||||||
| AHREC = Aboriginal human research ethics committee; HREC = human research ethics committee. * HREC approval in Victoria, Queensland or Tasmania, where there is no state AHREC or similar. † Health care and/or hospital acts or other jurisdiction agreements precluded HREC approval requirements. | |||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
Received 8 September 2023, accepted 18 December 2023
- Courtney Ryder 1 , 2
- Sadia Hossain 1 , 3
- Leanne Howard 4
- Julia Severin 1
- Rebecca Ivers 1 , 4
- 1 Flinders University, Adelaide, SA
- 2 Flinders Health and Medical Research Institute, Flinders University, Adelaide, SA
- 3 Western Sydney University, Sydney, NSW
- 4 UNSW Sydney, Sydney, NSW
Open access:
Open access publishing facilitated by Flinders University, as part of the Wiley ‐ Flinders University agreement via the Council of Australian University Librarians.
We acknowledge the traditional lands in which this manuscript was created and shaped (Eora, Kaurna Yerta, Bedegal, Gadigal and Darug) and pay respect to their Elders past, present and emerging.
No relevant disclosures.
- 1. Australian Commission on Safety and Quality in Health Care. Prioritised list of clinical domains for clinical quality registry development: final report. November 2016. https://www.safetyandquality.gov.au/publications‐and‐resources/resource‐library/prioritised‐list‐clinical‐domains‐clinical‐quality‐registry‐development‐final‐report (viewed Aug 2023).
- 2. Australian Commission on Safety and Quality in Health Care. National arrangements for clinical quality registries. https://www.safetyandquality.gov.au/our‐work/health‐and‐human‐research/national‐arrangements‐clinical‐quality‐registries (viewed Aug 2023).
- 3. Gong J, Singer Y, Cleland H, et al. Driving improved burns care and patient outcomes through clinical registry data: a review of quality indicators in the Burns Registry of Australia and New Zealand. Burns 2021; 47: 14‐24.
- 4. Ryder C, Mackean T, Hunter K, et al. Equity in functional and health related quality of life outcomes following injury in children — a systematic review. Crit Public Health 2020; 30: 352‐366.
- 5. Australian Institute of Health and Welfare. Aboriginal and Torres Strait Islander Health Performance Framework summary report January 2023. Canberra: AIHW, 2023.
- 6. Ryder C, Mackean T, Hunter K, et al. Yarning up about out‐of‐pocket healthcare expenditure in burns with Aboriginal families. Aust N Z J Public Health 2021; 45: 138‐142.
- 7. Diaz A, Soerjomataram I, Moore S, et al. Collection and reporting of Indigenous status information in cancer registries around the world. JCO Glob Oncol 2020; 6: 133‐142.
- 8. Australian Government Department of Health and Age Care. Maximising the value of Australia's clinical quality outcomes data: a national strategy for clinical quality registries and virtual registries 2020–2030. https://www.health.gov.au/resources/publications/a‐national‐strategy‐for‐clinical‐quality‐registries‐and‐virtual‐registries‐2020‐2030?language=en (viewed Aug 2023).
- 9. Maiam nayri Wingara; Indigenous Data Sovereignty Summit 2023. Data for governance: governance of data [briefing paper]. Proceedings of the 2nd Indigenous Data Sovereignty Summit; Cairns, 13 August 2023.
- 10. Australian Commission on Safety and Quality in Health Care. Australian Register of Clinical Registries. https://www.safetyandquality.gov.au/publications‐and‐resources/australian‐register‐clinical‐registries (viewed Jan 2021).
- 11. Balestra C, Fleischer L. Diversity statistics in the OECD: how do OECD countries collect data on ethnic, racial and indigenous identity? (OECD Statistics Working Paper No. 2018/09). Paris: OECD Publishing, 2018. https://www.oecd‐ilibrary.org/economics/diversity‐statistics‐in‐the‐oecd_89bae654‐en (viewed Aug 2023).
- 12. Christensen D, Davis G, Draper G, et al. Evidence for the use of an algorithm in resolving inconsistent and missing Indigenous status in administrative data collections. Aust J Soc Issues 2014; 49: 423‐443.
- 13. Griffiths K, Coleman C, Al‐Yaman F, et al. The identification of Aboriginal and Torres Strait Islander people in official statistics and other data: critical issues of international significance. Stat J IAOS 2019; 35: 91‐106.
- 14. Randall DA, Lujic S, Leyland AH, Jorm LR. Statistical methods to enhance reporting of Aboriginal Australians in routine hospital records using data linkage affect estimates of health disparities. Aust N Z J Public Health 2013; 37: 442‐449.
- 15. Anderson I, Robson B, Connolly M, et al. Indigenous and tribal peoples’ health (The Lancet –Lowitja Institute Global Collaboration): a population study. Lancet 2016; 388: 131‐157.
- 16. Ryder C, Holland AJA, Mackean T, et al. In response to “Driving improved burns care and patient outcomes through clinical registry data: a review of quality indicators in the Burns Registry of Australia and New Zealand”. Burns 2022; 48: 477‐479.
- 17. Australian Institute of Aboriginal and Torres Strait Islander Studies. AIATSIS Code of Ethics for Aboriginal and Torres Strait Islander Research. Canberra: AIATSIS, 2020. https://aiatsis.gov.au/research/ethical‐research/code‐ethics (viewed Jan 2022).
- 18. National Health and Medical Research Council. Road Map 3: a strategic framework for improving Aboriginal and Torres Strait Islander health through research. June 2018. https://www.nhmrc.gov.au/about‐us/publications/road‐map‐3‐strategic‐framework (viewed Jan 2022).
- 19. Ryder C, Wilson R, D'Angelo S, et al. Indigenous data sovereignty and governance: the Australian Traumatic Brain Injury National Data Project. Nat Med 2022; 28: 888‐889.
Publication of your online response is subject to the Medical Journal of Australia 's editorial discretion. You will be notified by email within five working days should your response be accepted.
Do you have any competing interests to declare? *

IMAGES
VIDEO
COMMENTS
The Danish National Committee on Health Research Ethics (NVK) and Danish National Medical Research Ethics Committee (VMK) process applications for ethical approval for trials throughout Denmark. Submitting for Ethics Approval Submitting Addendums. Useful Shortcuts. Current Interactive Application Guide . We have developed an interactive ...
The research ethics committee system has been established under the Danish Act on Research Ethics Review of Health Research Projects. Any health research project should as a general rule be capable of being reported to the research ethics committee system, see Section 14 of the Act. For the purposes of the Act, a health research project is ...
Guidelines for Research on Deceased Persons; Guidance on Genomics and Research in Sensitive Bioinformatics Data; Guidelines on the Remuneration of Trial Participants; Guidance on Hypothesis-Generating Research; About the Danish National Committee on Health Research Ethics (NVK) About the Medical Research Ethics Committees (MREC)
The Faroe Islands Scientific Ethics Committee. Chairperson: Jens Andreassen, department doctor, researcher. Eirargarður 2 Fo-100 Tórshavn, Faroe Islands. Phone: +29 82 20 815. Email: [email protected]. Website: Scientific Ethics Committee - Faroe Islands. Last updated 01-02-2024. Here you can find information and contact details for the Regional ...
The authority and work of the regional health research ethics committees are within the region (of the 5 Danish regions) where the regional committee is established. You must therefore submit your research project to the regional committee for the region where you work as a investigator. In addition to the committees in Region Southern Denmark ...
The Regional Committees on Health Research Ethics for Southern Denmark. The purpose of the health research ethics committee system is to ensure that from a research ethical point of view, health projects are carried out in a responsible manner. Consideration of trial subjects' rights, safety and wellbeing takes precedence over scientific and ...
Internet ISBN : 978-87-93151-35-2. A national Code of Conduct for research integrity in Denmark has been finished. The Code aims to ensure credibility, integrity and thereby quality in Danish research through common principles and standards for responsible conduct of research. The Code is aimed at both public and private research institutions ...
There is an established scientific ethics committee system in Denmark, with seven regional scientific ethics committees and a Central Scientific Ethical Committee. The purpose of the law is to lay down the legal framework for the scientific ethical evaluation of biomedical research projects. The system of ethical review is regulated by the Act ...
SDU's Research Ethics Committee generally does not review projects that have already obtained an ethics approval from another ethics review body. This also applies to projects that are legally obligated to obtain ethics approval from the Regional or National Committees on Health Research Ethics. However, there may be exceptions.
The purpose of Cognition and Behavior Lab's Human Subjects Committee (COBE HSC) is to ensure that the research that takes place in COBE Lab follows the Lab's ethics policy. This is to protect the participants as well as the Lab's reputation. The COBE HSC will also comment on any issues with data protection that they find concerning, and ...
The Pro-rector may choose to consult the Research Ethics Committee during the approval phase. Researchers who are unsure how to ensure compliance with the integrity guidelines are advised to consult the Research Ethics Committee. Danish Guidelines. The main document providing guidance is "The Danish Code of Conduct for Research Integrity" from ...
National Information: Denmark (Last Update: January 2023) Short description of RECs system: There are 12 regional committees. The National committee (the Danish National Committee on Biomedical Research Ethics (National Videnskabsetisk Komité, (NVK) ): coordinates the work in the regional committees, lays down guidelines for amongst others ...
Aarhus University (AU) studies are approved by the Research Ethics Committee. The Committee's responsibility is to ensure a consistent and responsible framework for study approval and make it easier for AU researchers to obtain grants and publish. The Committee acts as an Institutional Review Board (IRB) in cases where researchers are ...
Information for Researchers. Here we have gathered all content that is relevant for you if you need to apply for approval from a research ethics committee. Not sure how and to whom you should send your application for ethical approval? Find the answers here: Overview of Mandatory Reporting. Interactive Application Guide. Submitting for Ethics ...
Other research projects should be notified to one of the 11 existing regional scientific ethical committees. Notification must be made to the scientific ethical committee in the region where the person responsible for clinical trials in the project works. Cross-border multi-centre-trials must always be notified in Denmark.
The Capital Region of Denmark / Research and Innovation / Research / Ethical Approval of Research. Ethical Approval of Research. 9. May 2019 Fees and Charges. 9. May 2019 New Act 1. January 2012. ... May 2019 The Committees Case Processing and Members (Webid:8380617f-45c8-4f24-a87c-3a7b16c7fc09) ( ContentTypeId ...
The new ethics committee regulations follow a change in Danish national law governing another aspect of research ethics in October 1996. The new law compels researchers to declare their sources of research funding and any conflicts of interest to participating patients, as well as to the research ethics committee and journal editors.
According to Danish law, all research projects in Denmark involving human beings or any kind of human tissue, cells etc. need permission from an regional ethics committee. In the case of medicinal and medicinal devices trial projects a permission from the Danish Medicines Agency is also required before the project can be initiated. The ...
The contents and implications of the EU Directive on good clinical (research) practice (GCP) regarding drug trials are described. As of May 2003, clinical researchers in Denmark must have standard ...
The purpose of the scientific ethics committee system is to ensure that health science research projects are conducted in an ethically sound manner. Consideration for the rights, safety and well-being of research subjects takes precedence over scientific and societal interests in creating the possibility of obtaining new knowledge or ...
h Parliament and to create public debate about developments in biotechnology. The Danish Centre for Bioethics and Risk Assessment aims to assess questions regarding ethics and ris. as well as broader societal concerns in connection with biological research. The Council for Animal Ethics follows general development.
society. Denmark has always been at the forefront of developing the necessary infrastructure to evaluate research ethics in clinical trials, which is underscored by the establishment of a national ethics committee system already in 1980. In the more than 40 years that have passed since, the scope and complexity of clinical research have changed
This led to a situation in which two models of research ethics committees organisation systems exist, being the model in which the "single opinion" is considered to be the decision made by a single ethics committee more effective and simpler in terms of administrative and logistic workload. KW - Clinical Trials as Topic. KW - Conflict of Interest
Human research ethics approval was reported in 94 registries (88%), with only 11 (12%) having Aboriginal human research ethics committee approval. Conclusion: Significant variability is evident in clinical registry recording of Indigenous governance of data, meaning that Aboriginal and Torres Strait Islander communities remain invisible in data ...