- Research article
- Open access
- Published: 04 June 2021

Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
17k Accesses
34 Citations
14 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
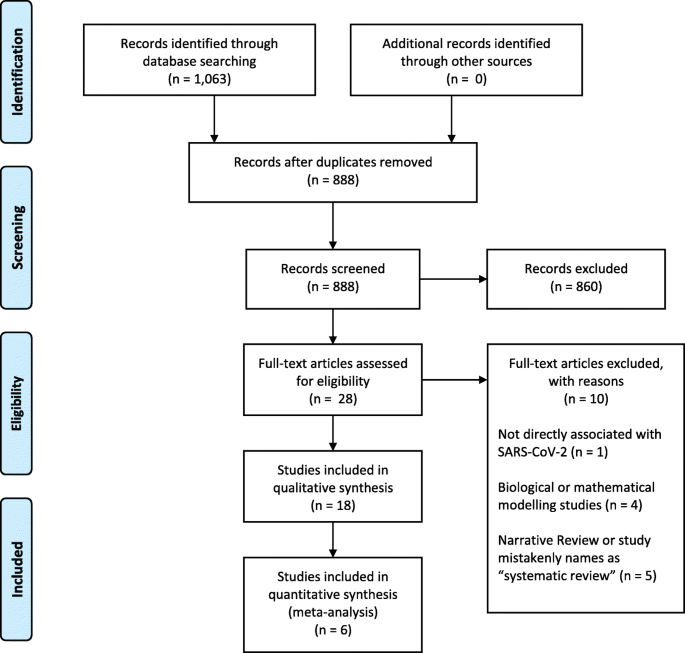
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
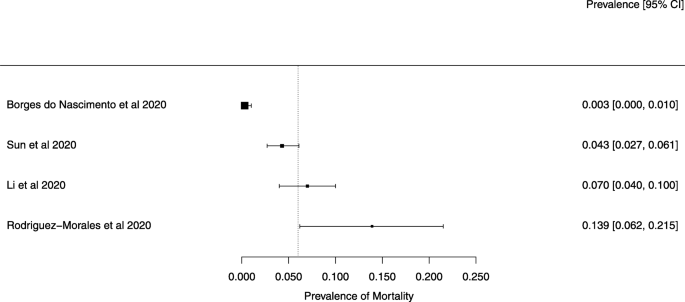
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
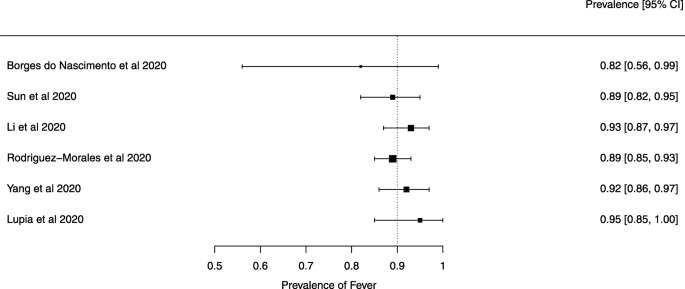
A meta-analysis of the prevalence of fever
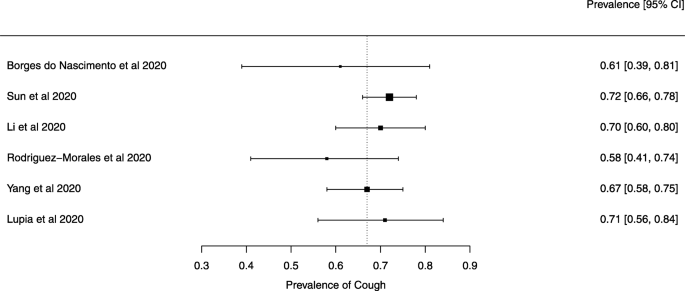
A meta-analysis of the prevalence of cough
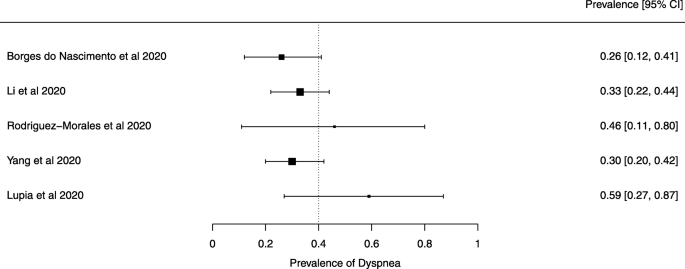
A meta-analysis of the prevalence of dyspnea
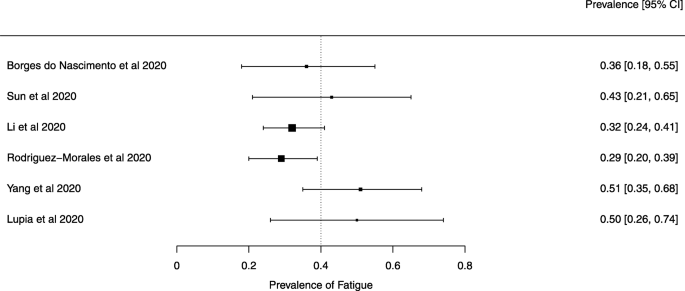
A meta-analysis of the prevalence of fatigue or myalgia
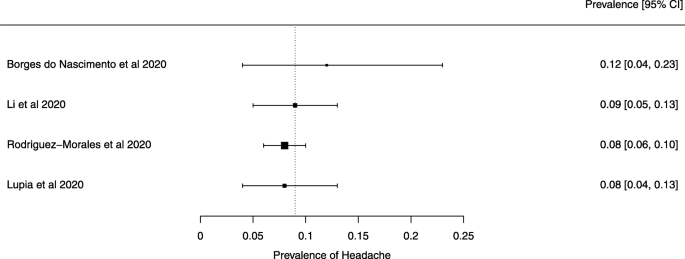
A meta-analysis of the prevalence of headache
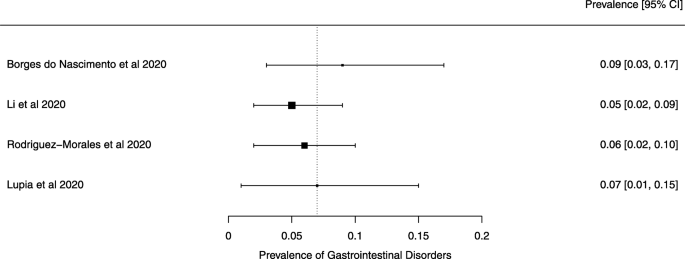
A meta-analysis of the prevalence of gastrointestinal disorders
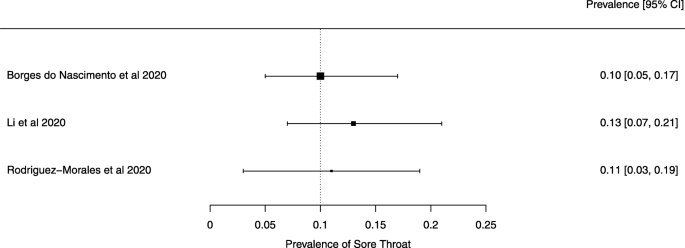
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ther Adv Vaccines Immunother

Comprehensive literature review on COVID-19 vaccines and role of SARS-CoV-2 variants in the pandemic
Charles yap.
School of Medicine, National University of Ireland, Galway, Ireland
Abulhassan Ali
Amogh prabhakar, akul prabhakar, ying yi lim, pramath kakodkar.
School of Medicine, National University of Ireland, Galway, University Road, Galway H91 TK33, Ireland
Since the outbreak of the COVID-19 pandemic, there has been a rapid expansion in vaccine research focusing on exploiting the novel discoveries on the pathophysiology, genomics, and molecular biology of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Although the current preventive measures are primarily socially distancing by maintaining a 1 m distance, it is supplemented using facial masks and other personal hygiene measures. However, the induction of vaccines as primary prevention is crucial to eradicating the disease to attempt restoration to normalcy. This literature review aims to describe the physiology of the vaccines and how the spike protein is used as a target to elicit an antibody-dependent immune response in humans. Furthermore, the overview, dosing strategies, efficacy, and side effects will be discussed for the notable vaccines: BioNTech/Pfizer, Moderna, AstraZeneca, Janssen, Gamaleya, and SinoVac. In addition, the development of other prominent COVID-19 vaccines will be highlighted alongside the sustainability of the vaccine-mediated immune response and current contraindications. As the research is rapidly expanding, we have looked at the association between pregnancy and COVID-19 vaccinations, in addition to the current reviews on the mixing of vaccines. Finally, the prominent emerging variants of concern are described, and the efficacy of the notable vaccines toward these variants has been summarized.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there have been devasting effects in other sectors contributing to our economy. This has plunged the global economies toward deep recession and has racked up a debt of approximately 19.5 trillion USD. 2
Immune protection in COVID-19 infection can be conceptualized as a spectrum wherein sterile immunity is at the end of positive spectrum. This is followed by transient infection (<3 days) and asymptomatic infection (~1 week). The negative spectrum of immune protection includes patients who are symptomatic, or hospitalized, or admitted to the intensive care unit for multiorgan support. The extreme end of the negative spectrum of immune protection is encompassed by case fatality. The vaccine will intervene prior to the viral insult and stabilize the population at the positive end of the spectrum of the immune protection. It will also prevent the perpetuating cycle of infection and reinfection via variants of SARS-CoV-2 virus in those who have achieved prior convalescence. One study by Dan et al. showed that in patients infected with COVID-19, immunological memory to SARS-CoV-2 remained intact for up to 6 months. 3 Unfortunately, there is no long-term data on the duration of protected immunity against SARS-CoV-2 in patients after convalescence. Therefore, these patients may also require vaccination but the current priority for vaccination can be stretched relative to the unaffected population.
While the ideal goal of the COVID-19 vaccine roll-out is to instill a global herd immunity; it is important to remember that this goal may never be reached. Furthermore, additional goals of vaccination may be to reduce mortality and stress on healthcare systems by reducing the cases of admitted patients. Various countries have already approved COVID-19 vaccines for human use, and more are expected to be licensed in the upcoming year. It is important that these vaccines are safe, efficacious, and can be deployed on a large scale. It is also prudent to eliminate the concerns of both the scientific and general community regarding its effectiveness, side-effects, and dosing strategies.
Historically, the process of vaccine manufacturing and clinical trials required approximately 10 years, but due to the burden of this disease, various observational studies were expedited so that all crucial information regarding the vaccine pharmacokinetics, pharmacodynamics, dosing, efficacy, and adverse events can be collected within a short period of time. Furthermore, there is a need to provide a compilation of accredited and appraised scientific literature on each of these approved vaccines with an aim to instill public health knowledge and vaccine literacy to members of the scientific and general community. A section dedicated to COVID-19 vaccines and pregnancy is also included in the penultimate section of this review.
Finally, the emergence of the SARS-CoV-2 viral variants of concern (VOC) has attained increased replication, transmission, and infectivity warranting exploration of these genomic mutations as their phenotypes. Hence, the final section of this review will aim to clarify the jargon, highlight the vaccine efficacy (VE) against VOCs, and eliminate any misinformation regarding these variants.
Vaccine physiology
The global burden of the pandemic requires an efficacious vaccine that elicits a lasting protective immune response against SARS-CoV-2. This will be an essential armament for the prevention and mitigation of the downstream morbidity and mortality caused by SARS-CoV-2 infection. As of July 20, 2021, there are approximately 108 vaccines in clinical development and 184 vaccines in pre-clinical development with several vaccines being distributed globally. 4
The technologies employed in the vaccine synthesis and development aim to trigger the adaptive immune system and elicit memory cells that will protect the body from subsequent infections. These technologies may be mRNA-based vaccines such as the Moderna and Pfizer/BioNTech, inactivated virus vector vaccines, DNA vaccines, and numerous other technologies. 5
Due to the urgent implementation of vaccine development, the most obvious target will be the robust proteins expressed on the surface of the virus. Therefore, these technologies target molecular expression of the trimeric SARS-CoV-2 spike (S) glycoprotein. These targets could include its mRNA, DNA, full S1 subunit, or fusion subunits. The S protein is a major component of the virus envelope, it is vital for viral fusion, receptor binding, and virus-entry through recognition of host-cellular receptor. The S protein comprises of two main functional units, the S1 subunit, which contains the receptor-binding domain (RBD) and the S2 subunit which is responsible for virus fusion with the host-cell membrane. 6 The choice to proceed with S protein as the target was reinforced when a study by Dan et al. confirmed that in 169 patients infected with SARS-CoV-2, spike-specific immunoglobulin G (IgG) remained stable for over 6 months. 3 In addition, both spike-specific CD4+ T-cells (CD137+ and OX40+) and spike-specific CD8+ T-cells (CD69+ and CD137+) were present at the 6-month post-convalescence period, but their subpopulations exhibited a steady decline with a half-life of 139 days and 225 days, respectively. 3
There are subtle differences in the mechanism by which the different vaccine products interact within host cells to induce immunity. Many successful vaccines of the 20 century utilized the target proteins directly such as the tetanus and pertussis vaccine. A summary of the major types of vaccines and their mechanism of action are shown in Figure 1 .

Summary of major vaccine types and their mechanism of action.
DNA, deoxyribonucleic acid; HPV, Human papillomavirus; mRNA, messenger ribonucleic acid; MMR, Measles, Mumps, and Rubella; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Historically, vaccines usually contained adjuvants which are protein sensitizers that heighten the migratory and sampling response of antigen presenting cells (APCs). Interestingly, the current mRNA vaccines are engineered to code for their own sensitizing protein alongside the S-protein epitopes. Therefore, these new mRNA vaccines usually do not contain any adjuvants. In addition, the mRNA vaccines utilize lipid nanoparticles to deliver the genetic material of a viral S-protein. Contrastingly, vaccines such as the AstraZeneca vaccine may employ a chimpanzee adenovirus vector to carry the DNA genome of the S-protein to the host-cell. 7 Once undergoing the processes of transcription and translation into proteins, these are trafficked and expressed on the host cell surface wherein the adaptive immune system mounts a response via the major histocompatibility complex (MHC) molecules ( Figure 2 ).

Mechanism of induction of immunity through vaccination.
APC, antigen presenting cells; DNA, deoxyribonucleic acid; MHC, major histocompatibility complex; mRNA, messenger ribonucleic acid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
There are two types of MHC molecules, the first one that will be discussed is the MHC-II, which is found exclusively on APC: these comprise of B-cells, macrophages, and dendritic cells in the lymph nodes. Once the S-protein antigen is presented at the cell surface of the MHC-II molecules, the naïve helper T-cell’s (Th Cells) T-cell receptor (TCR) complex will interact with this antigen leading to activation of CD4+ Th cells. This activation is perpetuated by a secondary activation signal with B7 on the APC recognizing the CD28 on the Th cell which triggers the proliferation of Th cells that can recognize the S-protein antigen. Activated CD4+ Th cells then secrete numerous cytokines, namely interleukin (IL)-2 which activates CD8+ cytotoxic T-cells (Tc cell) and trigger clonal expansion of B-cells in memory B-cells and plasma cells. The cytokines IL-4 and IL-5 facilitate B-cell isotype switching and maturation to plasma cells; promoting secretion of IgG antibodies against S-protein. 8 Formation of antibodies allows the immune system to direct an immune response against cells expressing the S-protein of the virus. The second process involves MHC-I, which activates CD8+ Naïve Tc cells through TCR complex interaction with processed endogenously synthesized S-protein expressed on MHC-I. MHC-I is expressed in all nucleated cells, APCs, and platelets and require a second activation signal provided by IL-2 from activated CD4+ Th cells. This activates CD8+ Tc cells which can mount a cytotoxic response against SARS-CoV-2-infected cells through two mechanisms of apoptosis. The first mechanism is the secretion of perforin which create pores to allow granzyme to enter the targeted cell, thus activating apoptosis. The second mechanism is via the expression of FasL, which binds Fas on target cells and induces apoptosis. 8 A crucial part of this process is the stimulation of memory T-cells and memory B-cells. Importantly, while the SARS-CoV-2 vaccine’s lasting effect is still being researched in the context of the pandemic, theoretically these should provide lasting immunity and allow the immune system to mount a faster and more effective response should a vaccinated individual encounter the virus in the future.
Current prominent COVID-19 vaccines
Biontech/pfizer.
The BNT162b2 COVID-19 vaccine developed by BioNTech and Pfizer is a lipid nanoparticle-formulated, nucleoside-modified RNA vaccine that encodes a prefusion membrane-anchored SARS-CoV-2 full-length spike protein. 9 It was the first vaccine approved by the US Food and Drug Association (FDA) and now it has been approved in many other countries. 10 The BNT162b2 COVID-19 vaccine may be stored at standard refrigerator temperatures prior to use, but it requires very cold temperatures for long-term storage and shipping (−70°C) to maintain the stability of the lipid nanoparticle. In a phase-1 trial, it was compared to another vaccine candidate BNT162b1, and it was found to have a milder systemic side-effect profile with a similar antibody response. 11 Therefore, it was pushed forward to a blinded phase-2/3 clinical study. 9 In total, 43,548 participants were randomized to receive either two doses of the BNT162b2 vaccine (n = 21,720) or a placebo (n = 21,728) 21 days apart. The participant ages ranged from 16 to 91 years, 35.1% of participants were classified as having obesity and comorbidities within participants included HIV, malignancy, diabetes, and vascular diseases. 9 Based on the results of the study, 7 days after the second BNT162b2 dose, the VE was 95% (95% confidence interval (CI), 90.3–97.6) with only eight observed cases of COVID-19 in the vaccine recipients and 162 cases in the placebo recipients. 9 The efficacy remained consistent across subgroups characterized by age, sex, race, ethnicity, body mass index (BMI), and comorbidities (generally 90–100%). 9 Although there were 10 cases of severe COVID-19 with onset after the first dose, only one occurred in a vaccine recipient and nine in placebo recipients. Like the phase-1 trial results, the safety profile remained favorable with the most common local reaction being mild-to-moderate pain at the injection site while the most common systemic symptoms were fatigue and headache (reported in ⩾50%). 9 In both the vaccine and placebo group, the incidence of severe adverse events did not differ significantly (0.6% and 0.5%, respectively) and no deaths occurred related to the vaccine. As indicated by the manufacturer’s information, contraindications for use include hypersensitivity to the active substance or any of the excipients. 12 These studies show that the mRNA-vaccine BNT162b2 is safe and effective in protecting against COVID-19. However, further investigations are needed to confirm long-term safety and to establish safety and efficacy for populations not included in this study.
The mRNA-1273 vaccine, developed by Moderna, relies on mRNA technology to encode prefusion stabilized SARS-CoV-2 spike protein. It is the second COVID-19 vaccine to receive emergency use approval by the US FDA, and it is given as two 100-µg doses intramuscularly into the deltoid muscle, 28 days apart. 13 Storage of the vaccine is done at temperatures between −25°C to −15°C for long-term storage, 2°C to 8°C for 30 days, or 8°C to 25°C for up to 12 hours. Results from the COVE phase-3 trial showed that the mRNA-1273 vaccine was effective at preventing COVID-19 illness in persons 18 years of age or older. A total of 30,420 participants aged 18 years or older were randomized 1:1 to receive either two doses of the vaccine or a placebo, 28 days apart. 14 The mean age of the participants was 51.4 years, and enrollment was adjusted for equal representation of racial and ethnic minorities. In the trial, symptomatic COVID-19 illness occurred in 11 participants within the vaccine group versus 185 participants within the placebo group, showing a 94.1% (95% CI, 89.3–96.8%) efficacy of the vaccine. Efficacy was similar across age, sex, race, and ethnicity as well as in patients with and without risk factors for severe disease (e.g. chronic lung disease, cardiac disease, and severe obesity). Importantly, a secondary endpoint for determining the efficacy of the vaccine in preventing severe COVID-19 was also used. All 30 participants with severe COVID-19 were in the placebo group, indicating a 100% efficacy of no hospital admissions. 14 Regarding the side effects of the vaccine, adverse events at the injection site and systemic adverse events occurred more commonly with the mRNA-1273 group compared to the placebo. The most common local reaction was mild to moderate pain at the injection site (75%). The most common systemic symptoms were fatigue, myalgia, arthralgia, and headache (50%). 14 The overall incidence of serious adverse events did not differ significantly between groups and no deaths occurred in relation to the vaccine. While this vaccine is already being administered, further investigations are still necessary to establish safety and efficacy profiles for populations not included in this study as well as to assess its long-term effects. Current contraindications of the mRNA-1273 vaccine include any persons with known allergy to polyethylene glycol (PEG), another mRNA vaccine component or polysorbate. 15
AstraZeneca
The Oxford and AstraZeneca ChAdOx1 COVID-19 vaccine uses a chimpanzee adenovirus vector to deliver the genetic sequence of a full-length spike protein of SARS-CoV-2 into host cells. 16 The storage for the ChAdOx1 vaccine is favorable, as it may be refrigerated at 2°C–8°C for 6 months. Pooled analysis of four ongoing clinical studies was used to assess efficacy, safety, and immunogenicity of the ChAdOx1 vaccine: COV001 (phase 1/2), COV002 (phase 2/3), COV003 (phase 3), and COV005 (phase 1/2). 17 Across the four studies participants over 18 were randomized to receive either the vaccine or a control (meningococcal group A, C, W, or saline). ChAdOx1 vaccine recipients received two standard doses (SDs) of the vaccine (SD/SD cohort) except for a subset in the COV002 trial who received a half lower dose (LD) followed by an SD (LD/SD cohort). 17 In the four studies, there was a total 23,848 participants, all of whom were used for gathering safety data; only 11,636 participants from the COV002 and COV003 trials were included in the primary efficacy analysis. 17 Of the 11,636 participants in the efficacy analysis, 2741 were in the LD/SD cohort, 88% were between 18 and 55 years old, and comorbidities present included cardiovascular disease, respiratory disease, and diabetes. 17 The results show that in the intended dosing regimen (SD/SD cohort), the VE was 62.1% (95% CI, 41.0–75.7) ⩾14 days after the second injection for symptomatic COVID-19 (27 cases vs 71 cases respectively). 17 In the group that received an LD (LD/SD cohort), the VE was 90.0% (95% CI, 67.4–97.0; 3 cases vs 30 cases, respectively) while across the two dosing regimens the overall efficacy was 70.4% (95.8% CI, 54.8–80.6;30 cases vs 101 cases, respectively). 17 The higher efficacy observed in the LD/SD cohort can be attributed to this group having a longer dosing interval between the two doses in comparison to the SD/SD cohort. Regarding safety, most of the adverse events were mild-moderate with the most frequently reported being injection site pain/tenderness, fatigue, headache, malaise, and myalgia. 18 About 175 serious adverse events were noted, only three of which were possibly linked to intervention: transverse myelitis 14 days after second dose, haemolytic anemia in a control recipient and fever >40°C in a participant still masked to group allocation. One contraindication for use of the vaccine is hypersensitivity to any of its components. In very rare cases, AstraZeneca has been associated internationally with venous thromboembolic events with thrombocytopenia with current estimates being 10–15 cases per million vaccinated patients. 19 This adverse event has been termed thrombosis with thrombocytopenia syndrome (TTS). In summary, these studies demonstrate that the AstraZeneca ChAdOx1 vaccine has a good efficacy and side-effect profile. Limitations include that less than 4% of participants were >70, no one over 55 got the mixed-dose regimen (LD/SD cohort), and those with comorbidities were a minority. Additional investigations are required to analyze long-term effects and assess efficacy and safety in populations not included or underrepresented.
Janssen COVID-19 vaccine
The Janssen (Johnson & Johnson) COVID-19 vaccine, developed by Janssen Pharmaceutical in Netherlands. It is a single-dose intramuscular (IM) vaccine that contains a recombinant, replication incompetent human adenovirus (Ad26) vector encoding the spike protein of SARS-CoV-2 in the stabilized conformation. 20 It can be stored between 2°C and 8°C for up to 6 hours or at room temperature for a duration of 2 hours. The ENSEMBLE Phase-3 trial (n = 43,783) is a randomized, double-blind, placebo-controlled study which included participants ⩾18 years. Efficacy assessment was performed at day 14 and 28. The primary outcome only included moderate and severe (hospitalization and death) infection. Overall, the VE in the moderate to severe cohort was 66.9% (95% CI: 59.0–73.4) at 14 days and 66.1% (95% CI: 55.0–74.8) at 28 days. 20 In the severe cohort, the VE was 76.7% (95% CI: 54.6–89.1) and 85.4% (95% CI: 54.2–96.9) at day 14 and 28 days, respectively. 20 At the time of the study, 96.4% of the strains in the United States, 96.4% were identified as the Wuhan-H1 variant D614G. The VE in the United States for the moderate to severe cohort was 74.4% (95% CI: 65.0–81.6) and 72.0% (95% CI: 58.2–81.7) at 14 days and 28 days, respectively. 20 In the US severe cohort, the VE was 78.0% (95% CI: 33.1–94.6) and 85.9% (95% CI: −9.4 to 99.7) at day 14 and 28 days, respectively. 20 Alternatively, 94.5% of the strains in South Africa were identified as beta variant. The VE in South Africa for the moderate to severe cohort was 52.0% (95% CI: 30.3–67.4) and 64.0% (95% CI: 41.2–78.7) at 14 days and 28 days, respectively. 20 In the South African severe cohort, the VE was 73.1% (95% CI: 40.0–89.4) and 81.7% (95% CI: 46.2–95.4) at day 14 and 28 days, respectively. 20 In Brazil, 69.4% of the strains were identified as P.2 lineage variant and 30.6% were identified as Wuhan-H1 variant D614G. The VE in Brazil for the moderate to severe cohort was 66.2% (95% CI: 51.0–77.1) and 68.1% (95% CI: 48.8–80.7) at 14 days and 28 days, respectively. 20 In the Brazilian severe cohort, the VE was 81.9% (95% CI: 17.0–98.1) and 87.6% (95% CI: 7.8–99.7) at day 14 and 28 days, respectively. 20 The most common localized solitary adverse reaction was the injection site pain (48.6%). Conversely, the most common systemic adverse reactions included headache, fatigue, myalgia, and nausea. 20 In the post authorization phase, adverse reaction included anaphylaxis, thrombosis with thrombocytopenia, Guillain Barré syndrome, and capillary leak syndrome. 20 Overall, the data demonstrate that the Janssen vaccine has a good efficacy and side-effect profile.
Sputnik V or Gam-COVID-Vac, developed by the Gamaleya Institute, is a recombinant human adenovirus-based vaccine that uses two different vectors (rAd26 and rAd5) to carry the gene encoding for the spike protein of SARS-CoV-2. Only one vector (rAd26) is given at dose 1 and the other (rAd5) at dose 2. This strategy prevents immunity against the vector. It can be stored as either a liquid at −18°C, or it can be freeze-dried and stored at 2°C to 8°C. 21 Regarding the safety and efficacy of the vaccine, both were evaluated in a randomized, double-blind phase-3 trial performed in Moscow, Russia. In the trial, a total of 21,977 participants aged 18 years or older were randomized in a 3:1 ratio to the vaccine or placebo groups. Two doses of the vaccine or placebo were given 21 days apart to the respective groups. 21 The mean age of the participants was 45.3 years, and the majority of participants were Caucasian (98.5%). 21 From 21 days after the first dose of the vaccine, efficacy against symptomatic COVID-19 illness was 91.6% (95% CI, 85.6–95.2%) with 16 confirmed cases of COVID-19 in the vaccine group and 62 confirmed in the placebo group. 21 There were also 20 cases of moderate to severe COVID-19 infection confirmed in the placebo group at least 21 days after the first dose and 0 in the vaccine group, indicating a VE of 100% against moderate to severe infection. 21 The most common adverse effects in both groups were flu-like illness, injection site reactions, headaches, and asthenia, with the majority being grade 1 (94.0%). 21 Serious adverse events were also reported in both the vaccine group and placebo group, but they were deemed not to be associated with the vaccination. Further investigations are still needed to determine the duration of protection of the vaccine and to determine the safety and efficacy of the vaccine in populations not included in the study (e.g. children, adolescents, and pregnant and lactating women).
CoronaVac is an inactivated vaccine developed by SinoVac Biotech containing inactivated SARS-CoV-2. 22 The vaccine can be stored at 2°C to 8°C for up to 3 years making it an attractive option for development. Two phase-1/2 clinical trials assessed the safety, tolerability, and immunogenicity of the CoronaVac vaccine. 22 , 23 The first study (18–59 years old included only) placed 744 participants in either a vaccine or placebo group where they were further divided based on vaccination schedule and dosage (3 and 6 μg). In the second study (⩾60 years old included only), 422 participants were randomized to receive two doses of CoronaVac or placebo 28 days apart and then further divided based on dosage amount only (3 and 6 μg for phase 1; 1.5, 3, and 6 μg for phase 2). Safety results from both trials show a favorable side-effect profile with most symptoms being transient and of mild severity. The most common adverse effect was injection site pain; others included fatigue and fever. In the 18–59 years old study, one serious adverse event of acute hypersensitivity was possibly related to vaccination. 22 No serious adverse events were associated with the vaccine or placebo in the ⩾60-year-old study. Between the dosage amounts in both studies, the tolerability was consistent and the immunogenicity was also similar for the 3 and 6 μg doses (less in 1.5 μg). 23 Multiple phase-3 trials have also taken place to determine the effectiveness of CoronaVac in countries, such as Brazil, Indonesia, and Turkey. In the Brazil trial, 252 cases of COVID-19 were recorded from roughly 9200 health care workers, with 167 in the placebo group and 85 in the vaccine group. 24 The reported efficacy of the vaccine in preventing mild and severe COVID-19 infection was 50.4%. In comparison, the Turkey trial reported that the vaccine was 83.5% effective at preventing symptomatic infection based on 29 COVID-19 cases among 1,322 volunteers while results from the Indonesia trial found that the vaccine was 65.3% effective at preventing symptomatic infection based on 25 COVID-19 cases among 1,600 people. 24 Some reasons for the lower efficacy of CoronaVac in the Brazil trial may include increased likelihood of exposure to the virus since participants were healthcare workers, and insufficient time for participants to reach peak immunity since the doses were administered only 2 weeks apart. 24 The phase-3 SinoVac study in Chile showed the VE 14 days post second dose to prevent symptomatic COVID-19 (67%, 95% CI: 65–69%), hospital admission (85%, 95% CI: 83–87%), intensive care unit (ICU) admission (89%, 95%CI: 84–92%) and death (80%, 95%CI: 73–86%). 25 The Phase-3 SinoVac trial in Brazil showed an overall VE against symptomatic COVID-19 (50.7%, 95% CI: 35.9–62%), moderate cases requiring hospitalization (83.7%, 95% CI: 58–93.7%), and severe cases requiring hospitalization (100%, 95%CI: 56.4–100%). 26 As with any vaccine, a contraindication for CoronaVac is anaphylaxis to it or to one of its constituents.
Other prominent COVID-19 vaccines
Due to the disease burden of SARS-CoV-2, the development and manufacturing of COVID-19 vaccines has been occurring at a remarkable pace which has not been seen before. There are many emerging vaccines with different mechanisms of actions that will be briefly explored. Bharat Biotech, an Indian company, has designed the inactivated COVID-19 vaccine Covaxin (BBV152). Once inside the body, the inactivated viruses can initiate an immune response through the interaction of surface proteins with APCs. Phase-1/2 trials showed no serious side effects and phase-3 trials are currently underway. 27 The state-owned Chinese company Sinopharm has also made an inactivated COVID-19 vaccine called BBIBP-CorV. The Sinopharm phase-3 trial showed that the VE in symptomatic cases for the WIV04 strain-based vaccine (72.8, 95% CI: 58.1–82.4%) and HB02 strain-based vaccine (78.1 95% CI: 64.8–86.3%). 28 , 29 It is approved in Bahrain, U.A.E, and China. NVX-CoV2373 is another promising vaccine produced by Novavax. It is a protein subunit vaccine made by assembling SARS-CoV-2 spike proteins into nanoparticles. A phase-3 trial in the United Kingdom displayed an efficacy rate of 89.3%; however, a phase-2 trial in South Africa had an efficacy just under 50%. 28 This discrepancy is thought to arise because of a new variant in South Africa. Other emerging vaccines include CoVLP produced by Medicago which uses the plant N. benthamiana to create virus-like particles that mimic SARS-CoV-2, CVnCoV produced by CureVac which is an mRNA vaccine, Convidecia produced by CanSino Biologics which is adenovirus based (Ad5), Ad26.COV2.S produced by Johnson & Johnson which is also adenovirus based (Ad26), and ZF2001 created by Anhui Zhifei Longcom which is a protein subunit vaccine. Even though highly effective, COVID-19 vaccines are already in use, it is still important to have a range of vaccines such as those listed above to bring the pandemic under control. Having a diverse profile ensures that vaccines will work for individuals from all ethnic backgrounds and with various underlying health conditions. 30 Getting the virus under control will also require doses for a large proportion of the world. To meet this requirement as soon as possible, having multiple vaccines will help in maximizing the volume of doses that can be produced. In addition, there are many technical issues such as cold storage and transportation, cost, and dosing of certain vaccines that arise when trying to vaccinate remote populations. For example, both the Pfizer-BioNTech and Moderna vaccines are expensive and transported at temperatures of −70°C and −20°C making it difficult to access many locations all at once. Since most vaccines require two doses spaced a few weeks apart, it can be challenging for individuals without regular access to healthcare as well. 30 Such considerations highlight the importance of having a range of single-dose vaccines and vaccines without the need for cold storage. A summary of efficacy, prominent side effects and storage recommendations for all the notable COVID-19 vaccines are shown in Table 1 .
Summary of vaccine efficacy, dosing strategy, and side-effects of different COVID-19 vaccines.
| Company | Phase-III efficacy against non-variant COVID-19 strain % (95% CI) | Injection type | Pooled side effects across doses (%frequency, n) | Storage | Reference |
|---|---|---|---|---|---|
| BioNTech/Pfizer (Germany/USA) | Dual dose: 94.1% (89.8–97.6) at ⩾35 days Single dose: 92.6% (69.0–98.3) between days 14–28 | IM (2 doses) | Phase-II trial results 1. Injection site pain (80.6%, n = 3536) 2. Fatigue (53.1%, n = 2332) 3. Headache (46.6%, n = 2044) 4. Myalgia (28.9%, n = 1270) 5. Arthralgia (16.2%, n = 710) 6. Fever ⩾ 38.0°C (9.5%, n = 416) 7. Vomiting (1.5%, n = 68) * data for 18–55 years old | −70°C | , |
| Moderna (USA) | Dual dose: 94.1% (89.3–96.8) at ⩾42 days Single dose: 92.1% (68.8–99.1) between days 14–28 | IM (2 doses) | Phase-II trial results 1. Pain at the injection site (92.0%, n = 13,970) 2. Fatigue (70.0%, n = 10,630) 3. Headache (64.7%, n = 9825) 4. Myalgia (61.5%, n = 9339) 5. Arthralgia (46.4%, n = 7046) 6. Chills (45.4%, n = 6894) 7. Nausea/vomiting (23.0%, n = 3493) 8. Axillary swelling (19.8%, n = 3007) 9. Fever (15.5%, n = 2354) 10. Injection site swelling (14.7%, n = 2232) 11. Injection site erythema (10.0%, n = 1519) * data for ⩾18 years old | −25°C and −15°C | , |
| AstraZeneca (UK) | Dual dose: 66.7% (57·4–74·0) at 104 days Single dose: 76% (59·3–85·9) between days 22–90 | IM (2 doses) | Phase-II trial results 1. Pain at the injection site (63.7%, n = 7657) 2. Tenderness at the injection site (54.2%, n = 6515) 3. Fatigue (53.1%, n = 6383) 4. Headache (52.6%, n = 6323) 5. Malaise (44.2%, n = 5313) 6. Myalgia (44.0%, n = 5289) 7. Chills (31.9%, n = 3835) 8. Arthralgia (26.4%, n = 3174) 9. Fever ⩾ 38.0°C (7.9%, n = 950) * data for ⩾18 years old with at least one dose | 2°C–8°C | , |
| Janssen/Johnson & Johnson (Netherlands/US) | Single dose: Symptomatic 66.3% (59.9–71.8) Hospitalization 93% (71–98) | IM (1 dose) | Phase-I trial results 1. Injection site pain 2. Fatigue 3. Headache 4. Myalgia 5. Nausea 6. Pyrexia * data for 18–55 years old | 2°C–8°C | , |
| Gamaleya Sputnik V Gam-COVID-Vac (Russia) | Dual dose: 91.6% (85.6–95.2) Single dose: 73.6% from 15–21 days | IM (2 doses) | Pooled phase-I and phase-II trial results 1. Hyperthermia (68%, n = 27) 2. Injection site pain (50%, n = 20) 3. Headache (40%, n = 16) 4. Asthenia (38%, n = 15) 5. Myalgia/arthralgia (28%, n = 11) 6. Rhinorrhoea (10%, n = 4) * data for 18–60 years old | −18°C or 2°C–8°C | , |
| SinoVac (China) | Dual dose: Symptomatic: 50.7% Moderate hospitalization: 83.7% Severe hospitalization: 100% | IM (2 doses) | Phase-II trial results 1. Injection site pain (11.2%, n = 27) 2. Diarrhea (2.5%, n = 6) 3. Fever (2.0%, n = 5) 4. Fatigue (1.7%, n = 4) 5. Myalgia (1.3%, n = 3) 6. Headache (0.8%, n = 2) *data for 18–59 years old, 3-μg dose on days 0 and 14 | 2°C–8°C | , |
| Bharat Biotech COVAXIN BBV152 (India) | Dual dose: Asymptomatic 63.6% (29·0–82·4) Mild: 77.8% (65·2–86·4) Severe: 93.4% (57·1–99·8) | IM (2 dose) | Phase-II trial results 1. Fever (3.2%, n = 12) 2. Injection site pain (2.9%, n = 11) 3. Body ache (1.3%, n = 5) 4. Headache (1.1%, n = 4) 5. Weakness (0.8%, n = 3) * data for 12–65 years old, 6 μg + adjuvant | 2°C–8°C | , |
| Sinopharm BBIBP-CorV (China) | Dual dose: 78.1% (64.9–86.3) | IM (2 doses) | Phase-I trial results 1. Injection site pain (12%, n = 10) 2. Injection site swelling (4%, n = 3) 3. Fever (4%, n = 3) 4. Nausea (2%, n = 2) 5. Headache (1%, n = 1) 6. Fatigue (1%, n = 1) * data for 18–59 years-old, 4 μg on days 0 and 21 | 2°C–8°C | , |
| Novavax (USA) | Dual dose: 89.7% (80.2–94.6) | IM (2 doses) | Phase-I trial results 1. Local tenderness (71.7%, n = 81) 2. Injection site pain (52.2%, n = 59) 3. Myalgia (42.5%, n = 48) 4. Fatigue (39.8%, n = 45) 5. Headache (38.1%, n = 43) 6. Malaise (25.7%, n = 29) * data for 18–59 years old, 5 μg + adjuvant, 25 μg + adjuvant | 2°C–8°C | , |
| Medicago (Canada) | – | IM (2 doses) | Phase-I trial results 1. Injection site pain (97.4%, n = 38) 2. Fatigue (48.7%, n = 19) 3. Headache (43.6%, n = 17) 4. Chills (30.8%, n = 12) 5. Injection site swelling (23.1%, n = 9) 6. Myalgia (20.5%, n = 8) 7. Fever (17.9%, n = 7) 8. Injection site redness (17.9%, n = 7) 9. Arthralgia (7.7%, n = 3) * data for 18–55 years old, 3.75 μg dose + adjuvant | 2°C–8°C | |
| CureVac CVnCoV (Germany) | 47% | IM (2 doses) | Phase-I trial results 1. Fatigue (96.3%, n = 52) 2. Injection site pain (88.9%, n = 48) 3. Headache (87.0%, n = 47) 4. Chills (83.3%, n = 45) 5. Myalgia (75.9%, n = 41) 6. Fever (55.6%, n = 30) 7. Arthralgia (50.0%, n = 27) 8. Nausea/vomiting (33.3%, n = 18) 9. Diarrhea (14.8%, n = 8) * data for 18–60 years old, 12-μg dose | 2°C–8°C | , |
| CanSino (China) | – | IM (1 dose) | Phase-I trial results 1. Injection site pain (56.8%, n = 217) 2. Fatigue (39.2%, n = 150) 3. Headache (28.5%, n = 109) 4. Fever (26.9%, n = 103) 5. Myalgia (16.2%, n = 62) 6. Arthralgia (12.3%, n = 47) * data for 18 years old or older, 1 × 10 viral particle dose, 5 × 10 viral particle dose | 2°C–8°C | |
| Anhui Zhifei Longcom (China) | – | IM (2–3 doses) | Phase-I trial results 1. Injection site itch (19%, n = 29) 2. Injection site redness (16%, n = 24) 3. Injection site swelling (14%, n = 21) 4. Injection site pain (12%, n = 18) 5. Fever (8%, n = 12) 6. Headache (2%, n = 3) * data for 18–59 years old, 25-μg, 3-dose regimen | 2°C–8°C |
CI, confidence interval; COVID-19, coronavirus disease 2019; IM, intramuscular.
Post-vaccination contagion
With the endurance of the COVID-19 vaccine still being heavily researched, a chief concern is the sustainability of the vaccine-mediated immune response. This is important in the consideration of whether vaccinated individuals could still contract, transmit, or be carriers of SARS-CoV-2 virus. Vaccinated individuals currently may not understand the rationale behind why social restriction rules still apply to them. Most COVID-19 mRNA vaccines require at least 3 weeks to mount an immunological response and create the required antibodies and proliferate accessory cells of the adaptive immune system of the appropriate recognition repertoire. 50 This may be particularly relevant in the context of travel, as the World Health Organization (WHO) states that a proof of vaccination should not exempt international travelers from complying with social restrictions and risk-reduction measures. 51
Contraindications for COVID-19 vaccines
All vaccines are contraindicated in cases of documented hypersensitivity to the active substance or any of the excipients. There are a set of general guidelines relative to patients which must be adhered to until further information is provided; predominantly regarding groups such as pregnant or lactating women and immunodeficient patients. The Centers for Disease Control and Prevention (CDC) considers absolute contraindications to patients who have had severe anaphylactic reactions to a previous dose of an mRNA COVID-19 vaccine or PEG, a component of the vaccine. Moreover, immediate allergic reactions of any severity to polysorbate are also a significant contraindication. Importantly, there are many precautions which are not classified as contraindications but must be considered, such as patients who have had allergic reactions to any vaccine or injectable therapy. In the cases of patients with a precaution to the vaccine, they should be counseled on the benefits and risks, but are not contraindicated from vaccination. 15 In the instance of patients with autoimmune diseases, there is currently insubstantial data regarding the efficacy of the vaccine; however, current guidelines suggest that individuals with autoimmune conditions may take the vaccine if they do not have any absolute contraindications. In the case of patients with HIV, limited data from COVID-19 mRNA vaccination trials suggest that they can receive the vaccine barring any contraindications.
COVID-19 vaccines and pregnancy
Prior to discussing the relationship between the current vaccines for COVID-19 and pregnancy, it is crucial to gain an insight of the relationship between pregnancy and COVID-19 itself. Adhikari et al. showed that there was no difference in the frequency of Caesarean section, pre-eclampsia, preterm births, and abnormal fetal cardiotocography in pregnant women with and without SARS-CoV-2 infection. In addition, examination of the placenta revealed were no abnormalities, which were initially suspected due to the cross-matching between the SARS-CoV-2 spike protein and the placental synctyin-1 protein. 52 Similarly, there was no association found between COVID-19 and first-trimester spontaneous abortions. 53 A systematic review and meta-analysis revealed that COVID-19 leads to higher preterm deliveries (odds ratio (OR): 3.01, 95% CI: 1.16–7.85) and an increase in the ICU admission rates (OR: 71.63, 95% CI: 9.81–523.06) in pregnant women. 54
Pregnancy remained an exclusion criterion for all the COVID-19 vaccine trial; therefore, the efficacy of the COVID-19 vaccines in pregnant women is unavailable. However, given the effectiveness of the influenza vaccines elucidated in a meta-analysis conducted by Quach et al ., it can be hypothesized that the effects of pregnancy on the vaccine would be minimal, but more data would be needed for confirmation. 55 Pfizer’s animal studies revealed antibodies in the maternal rats, fetus, and offspring, in addition to no effects on fertility pregnancy or fetal development. 56 A similar study was conducted with the Moderna vaccine which led the US FDA to conclude that the vaccine did not have any adverse effects on female reproduction, fetal development, or postnatal development. 34 Furthermore, the Oxford-AstraZeneca vaccine animal studies are still pending. However, as a precaution, the National Immunization Advisory Committee (NIAC) has recommended for the two-dose schedule to not commence before 14 weeks of gestation and to be completed by week 33 of gestation. This precaution reduces any potential associations with miscarriage or pre-term birth. 57
Despite the exclusion of pregnancy in the preliminary stages of the trials, 23 Pfizer, 13 Moderna, and 21 AstraZeneca subjects became pregnant after enrolment into the trial. Among this cohort, there was one miscarriage part of the Pfizer control group, no miscarriages part of the Pfizer vaccine group, one miscarriage part of the Moderna control group, no miscarriages part of the Moderna vaccine group, three miscarriages part of the AstraZeneca control group, and two miscarriages part of the AstraZeneca vaccine group. While these preliminary numbers support the current guidelines regarding the vaccines being safe in pregnancy, it is crucial to be aware of the ongoing studies as new data emerges.
The CDC v-safe COVID-19 Pregnancy Study explored the effect of mRNA vaccine (Pfizer-BioNTech or Moderna) on the pregnancy. The pregnancy loss within those with a completed pregnancy included a spontaneous abortion (<20 weeks) rate of 12.6% (104 out of 827) and stillbirth (⩾20 weeks) incidence of 0.1% (1 out of 725). 58 The neonatal outcomes within the live birth infant cohort showed preterm birth (<37 weeks) incidence at 9.4% (60 out of 636), small for gestational age incidence of 3.2% (23 out of 724), and congenital anomalies were seen in 2.2% (16 out of 724). 58 No neonatal deaths were observed in this study.
Vaccine dosing strategies
Limited vaccine resources have caused some governments to extend the date of the second dose beyond the recommended manufacturer date. On December 30, NHS England had made the decision to prioritize the administration of the first doses, and to extend the second doses of the vaccine to the end of 12 weeks, rather than the recommended 3–4 weeks as shown in the clinical phase-3 trial. Pfizer-BioNTech at the time had no data to support this decision, and thus stated that the safety and efficacy of the vaccine had not been evaluated on different dosing schedules, and importantly, the second dose should not be administered later than 42 days. 59
Newly accrued evidence might warrant changes in the landscape of this vaccination program. Estimation of the effectiveness of the Pfizer-BioNTech after a single dose from the primary data from Israeli population (n = 500,000) showed that from day 0 to day 8 post–vaccination, the likelihood of contracting COVID-19 infection doubled. 60 This result may appear counterintuitive, but it takes 3 weeks for the vaccine to instill efficacy during which this real-world population could have not maintained the stringent public health measures which lead to the increased incidence in COVID-19 in this time-period. Then from day 8 to day 21 the incidence of COVID-19 declined and at day 21 the vaccine effectiveness was documented at 91%. 60 This efficacy was seen to stabilize at 90% for the duration of the study (9 weeks), and the authors of this study extrapolate this stability up to 6 months. 60 This concludes that the single dose of Pfizer-BioNTech is highly protective from day 21 onwards and supports the NHS England’s vaccination policy for extending gaps between the doses. The data from the Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II) trial in the Scottish population revealed that a single dose of Pfizer (n = 650,000) and Oxford-AstraZeneca (n = 490,000) vaccines resulted in a decline in hospitalization at 4 weeks by 84% and 94%, respectively. 61
However, the trials for the Oxford-AstraZeneca vaccine included varied spacing schedules between doses. The findings from these trials displayed that a greater space between the first and second dose provided a superior immune response. This is supported by a combined trial between a UK and Brazil study, which demonstrated a higher VE 14 days after a second dose in patients who had greater than 6 weeks between their first and second dose than patients who had less than 6 weeks by 53.4%. 17 , 62
It was also proposed that to meet the supply shortage that vaccine dose can be halved. Half-dose of Moderna vaccine (50ug) was in a phase-IIa trial. Immune response in the half-dose group compared to those that received a full dose were the same. Therefore, this dosing strategy is supported from an immunogenicity perspective. It is reasonable to infer that the immunogenicity would translate to immune protection, but unfortunately no clinical trial has validated the immune protection for this dosing strategy.
SARS-CoV-2 genome mutations
Mutations are changes in the SARS-CoV-2 viral genome that occur naturally over time. These mutations from the parent SARS-CoV-2 virus create variants. A certain amount of genetic variation is expected as SARS-CoV-2 replicates as such it is important to monitor circulating viral variants to collate key mutations. Fortunately, coronaviruses have a slower rate of mutation of 1 to 2 nucleotides per month. 63 These definitions become complicated when environmental factors apply selective pressures on these variants that enable them to express distinct phenotypes that may facilitate viral fitness. This ability of a variant to express distinct phenotypes is termed as a strain. A compilation of beneficial lineage defining mutations can create a strain that has a higher transmission rate or induce severe disease. This raises the question: will the current vaccines or convalescent immunity from a non-variant SARS-CoV-2 infection provide adequate immunological protection against these new variants?
Coronaviruses mutate spontaneously via antigenic drift. This process typically utilizes the virus-specific transcription regulatory network (TRN) sequence to initiate the change, resulting in a new mRNA sequence virus being formed. Homologous and genetic recombination allows for the virus to gain more ecological features and has been speculated to be the reason why SARS-CoV-2 was zoonotic in origin. 64 A variant of the original SARS-CoV-2 virus with a D614G substitution in the spike protein encoding gene emerged in early February 2020, and by June 2020, D614G became the dominant form of the virus circulating globally. 65 Studies have shown that the D614G mutation resulted in increased infectivity and transmissibility. 66 Since then, there have been many viral lineages to note, most notable VOC include the B.1.1.7/20I/501.Y.V1 variant that was first detected in the United Kingdom in October 2020, the B.1.351/20 H/501Y.V2 variant that was detected in South Africa in December 2020, and the Lineage P.1. (B.1.1.28.1) variant that was detected in Tokyo in January 2021 but is believed to have originated from Brazil.
Currently, there exists two open-source real-time software tools to analyze and assign nomenclature of genetic variations in the SARS-CoV-2 virus: Nextstrain and PANGOLIN. 64 , 67 Both refer to the GISAID (Global Initiative on Sharing All Influenza Data) genomic database but have slight differences with regards to their nomenclature to describe various lineages of the virus. The COVID-19 Genomics UK Consortium has also developed CoV-GLUE, an open-source browser application that allows for easy referral of all sequenced SARS-CoV-2 genetic replacements, insertions, and deletions. 68 Therefore, sequencing every local infection will yield a repository to track the development of new mutations and variants.
Notable mutation drivers in the SARS-CoV-2 genome
Before diving deeper into these variants, it is important to understand the physical alteration in the S-protein at a molecular level and the perceived functional advantages that the SARS-CoV-2 gains. Table 2 highlights some of the notable S-protein mutations as they evolve amid the pandemic.
Summary of the physical and functional alterations of S-protein due to notable amino acid substitutions.
| Mutation | Alterations in S-protein structure | Functional Consequences for VOC | Distribution Earliest/latest (frequency) | Notes/references |
|---|---|---|---|---|
| D614G | • Substitution of aspartate to glycine at site 614. • Open conformity of S1 spike protein | • Increased transmissibility • Increased vulnerability to host immune attack (speculated) • Higher nasal viral loads and correlates with the prevalence of anosmia | 1/3/2020 Switzerland (0.4) UK (0.19) France (0.15) Italy (0.11) | , |
| E484K | • Substitution of glutamate to lysine at site 484 • This change takes place in the receptor-binding motif on RBD | • Increased ACE2 binding • Potential for escaping recognition from S-protein neutralizing antibodies. • Documented case of reinfection • Moderna and Pfizer have shown small but significant reduction in neutralization | 12/10/2020 Brazil (0.08) South Africa (0.06) 08/02/2021 Brazil (0.58) France (0.22) | , |
| N501Y | • Substitution from asparagine to tyrosine at position 501 • This change takes place at the RBD of the S-protein | • Increase ACE2 binding due to increase duration in open conformation. • Potential for escaping recognition from S-protein neutralizing antibodies. • >Moderna and Pfizer have shown small but significant reduction in neutralization. | 01/06/2020 Netherlands (0.01) 08/02/2021 UK (0.87) France (0.53) Australia (0.43) Brazil (0.42) | – |
| HV69-70 del | • Deletion of histidine at site 69 and valine at site 70 in the S1 domain of S-protein. • Predicted altered structure will be a ‘tucked in’ spike N-terminal domain | • Potential for escaping recognition from S-protein neutralizing antibodies. The serum virus neutralization (SVN) assay showed reduced neutralization to human SARS-CoV-2 convalescent plasma. | 10/08/2020 Switzerland (0.04) Denmark (0.01) 08/02/2021 UK (0.87) Australia (0.45) France (0.36) Singapore (0.26) | , |
| P681H | • Substitution of proline to histidine at position 681 is immediately adjacent to the furin cleavage site between S1 and S2 in S-protein | • Enables increased cleavage activity by TMPRSS2. Therefore, increase SARS-CoV-2 entry | 12/10/2020 Nigeria (NA) UK (NA) |
RBD, receptor-binding domain; VOC, variants of concern.
Notable emerging VOC
Newly emerged variants of SARS-CoV-2 have now become VOC which can be attributed to their new ability of increased transmission and infectivity. Therefore, it is important to collate the data on the mutations they acquired, the extend of spread, and the efficacy of different vaccines to create a repository for further analysis ( Table 3 ).
Summary of data on features, acquired cluster of S-protein mutations, and vaccine efficacy studies for the major COVID-19 variants of concern.
| Names (PANGOLIN, Nexstrain, Media) | Features | Notable mutations in S-Protein | Vaccine efficacy reduction | Countries reported (n) as of August 17, 2021 | References |
|---|---|---|---|---|---|
| B.1.1.7, 20I/501.Y.V1, VOC/20201201, UK strain (Alpha Variant) | • Increased binding to ACE2 receptor • 30–70% increased transmissibility • Realistic possibility of increased severity Reproduction rate [range 1.5–1.7] • Higher nasal viral load and increased shedding, prolonged viral shedding, and heighten stability in the current environment • Decreased neutralization | • N501Y • HV69-70 del • P681H • Y144 del, • A570D • E484K • D614G | • Efficacy data • Novavax 86% • Pfizer/BioNTech Single dose 47.5% (95% CI: 41.6–52.8) • Pfizer/BioNTech Dual dose 93.7% (95% CI: 91.6–95.3) • AstraZeneca Single dose 48.7% (95% CI: 45.2–51.9) • AstraZeneca dual dose 74.5% (95% CI: 68.4–79.4) • Mean loss in neutralization: • At day 43 after dual doses at day 28 • Moderna (n = 12): 1.8-fold • Pfizer/BioNTech (n = 10): 2-fold | 190 | , – |
| B.1.351, 20H/501.Y.V2, South African strain (Beta Variant) | • Increased severity • Increased transmission • Reinfection is possible as the convalescent immunity cannot mount a response against this new variant | • E484K • K417N • N501Y • D614G orf1b deletion | • Efficacy data • Janssen Vaccine (moderate to severe at day 28) 64.0% (95% CI: 41.2–78.7) • Janssen Vaccine (Severe at day 28) 81.7% (95% CI: 46.2–95.4) • Novavax Dual dose 60.1% (95%CI: 19.9–80.1) • AstraZeneca Dual dose 10.4% (95% CI: −76.8 to 54.8) • Mean loss in neutralization compared to wild type: • Moderna (n = 12): 8.6-fold • Pfizer/BioNTech (n = 10): 6.5-fold • BBIBP-CorV: 10-fold | 138 | , , , – |
| Lineage P.1, B.1.1.28.1, Brazilian strain (Gamma Variant) | • Increased severity • Increased transmissibility • Documented case of reinfection | • N501Y • E484K • D614G • K417N/T • orf1b deletion | • Pfizer/BioNTech: Significant reduction in neutralization • Moderna: Significant reduction in neutralization • SinoVac: seroconversion and geometric mean titres in the neutralizing antibody assays | 82 | , , , |
| B.1.617.2 Indian Strain (Delta Variant) | • Increase transmission • Decrease neutralization | • L452R • D614R • P681R | • Efficacy data • Pfizer/BioNTech Single dose 35.6% (95% CI: 22.7–46.4) • Pfizer/BioNTech Dual dose 88.0% (95% CI: 85.3–90.1) • AstraZeneca Single dose 30.0% (95% CI: 24.3–35.3) • AstraZeneca Dual dose 67.0% (95% CI: 61.3–71.8) • BBV152 Dual Dose 65·2% (95% CI: 33·1–83·0) • Mean loss in neutralization compared to wild type: • BBIBP-CorV: 1.38- fold | 148 | , , |
CI, confidence interval; COVID-19, coronavirus disease 2019; VOC, variants of concern.
There are more variants emerging as the pandemic progresses, but it is important to note that there is still a myriad of available vaccines in our armamentarium that are adequately efficacious in the performed neutralization assays as well as the real-world data. Furthermore, while vaccines induce the antibody-dependent immunity, they can also stimulate other components of the adaptative immune system such as the Memory B-cells, CD8+ Tc cells, and CD4+ Th cells to mount their own response against the viral variants. This can compensate for the reduction in neutralization rate by the vaccine induced antibodies. Interestingly, the adaptative immune system can proliferate libraries of memory B-cells with mutated antibody repertoires that can predict viral variants. Therefore, it is prudent to commence vaccinations in accordance with the local public health bodies. This combined with the continued implementation of public health measures until target level of herd immunity is acquired can lead toward mitigating the prevalence and incidence of COVID-19 variants.
This review highlighted the current available vaccines and candidates being rolled out amid the ongoing prevention measures and summarized the documented findings with regards to their efficacies, side-effects, and storage requirements. An overview of the physiology of immunogenic responses against the disease provided by the more prominent vaccines were discussed, alongside questions regarding the implementation of vaccines; heterologous prime-boosting, vaccine contraindications, dosing strategies, side effects, and the presence of SARS-CoV-2 mutations and variants.
There are still many unanswered questions that need to be addressed with regards to antibodies produced in individuals including their impact on the clinical course and severity of the disease, how long will they remain in the body to protect from the disease, and if what we have is enough to deal with newly emerging variants. Studies on these topics are rapidly being conducted and published on a global scale, and scientific communities are working on the clock to produce as much information to bring us a better understanding on how to deal with this disease.
For this global pandemic to end, it is imperative that people are vaccinated as quickly as possible until herd immunity can be achieved. One aspect of achieving this, in the face of vaccine hesitancy, is to address the lack of community understanding on how vaccines work, the risks, and the factors that keep this area of research volatile and distribution policies ever-changing. In addition, it is important to remain cautious about the information being released and to trust the accredited sources and experts, rather than the aberrant rumors being spread through social media. Nonetheless, the COVID-19 vaccines have shown to be highly promising and we recommend for everyone that is eligible to take the vaccine at the correct dosing interval when they are given the chance as this would potentiate a positive trend toward pandemic resolution.
Authors’ contributions: CY, AA, Amogh P, Akul P, AP performed acquisition and curation of the data; CY, AA, Amogh P, Akul P, AP, YYL and PK analyzed the data, performed interpretation of the data, and wrote of the original draft; YYL and PK performed the critical revision; All authors have read and approved the final manuscript.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.

Contributor Information
Charles Yap, School of Medicine, National University of Ireland, Galway, Ireland.
Abulhassan Ali, School of Medicine, National University of Ireland, Galway, Ireland.
Amogh Prabhakar, School of Medicine, National University of Ireland, Galway, Ireland.
Akul Prabhakar, School of Medicine, National University of Ireland, Galway, Ireland.
Aman Pal, School of Medicine, National University of Ireland, Galway, Ireland.
Ying Yi Lim, School of Medicine, National University of Ireland, Galway, Ireland.
Pramath Kakodkar, School of Medicine, National University of Ireland, Galway, University Road, Galway H91 TK33, Ireland.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
Collection 29 March 2022
2021 Top 25 COVID-19 Articles
The 25 most downloaded Nature Communications articles* on COVID-19 published in 2021 illustrate the collaborative efforts of the international community to combat the ongoing pandemic. These papers highlight valuable research into the biology of coronavirus infection, its detection, treatment as well as into vaccine development and the epidemiology of the disease.
Browse all Top 25 subject area collections here .
*Data obtained from SN Insights (based on Digital Science's Dimensions) and normalised to account for articles published later in the year.
Research highlights
Anti-spike antibody response to natural SARS-CoV-2 infection in the general population
Most people who are infected with SARS-CoV-2 seroconvert within a few weeks, but the determinants and duration of the antibody response are not known. Here, the authors characterise these features of the immune response using data from a large representative community sample of the UK population.
- Philippa C. Matthews
- the COVID-19 Infection Survey team
Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials
Hydroxychloroquine and chloroquine have been investigated as a potential treatment for Covid-19 in several clinical trials. Here the authors report a meta-analysis of published and unpublished trials, and show that treatment with hydroxychloroquine for patients with Covid-19 was associated with increased mortality, and there was no benefit from chloroquine.
- Cathrine Axfors
- Andreas M. Schmitt
- Lars G. Hemkens
Malignant cerebral infarction after ChAdOx1 nCov-19 vaccination: a catastrophic variant of vaccine-induced immune thrombotic thrombocytopenia
Vaccination is an effective strategy in suppressing COVID-19 pandemic, but rare adverse effects have been reported, including cerebral venous thrombosis. Here the authors report two cases of middle cerebral artery infarct within 9-10 days following ChAdOx1 nCov-19 vaccination that also manifest pulmonary and portal vein thrombosis.
- M. De Michele
- M. Iacobucci
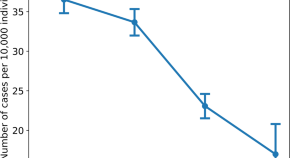
Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine
The duration of effectiveness of SARS-CoV-2 vaccination is not yet known. Here, the authors present preliminary evidence of BNT162b2 vaccine waning across all age groups above 16, with a higher incidence of infection in people who received their second dose early in 2021 compared to later in the year.
- Barak Mizrahi
- Tal Patalon
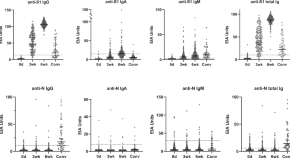
COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants
Emerging SARS-CoV-2 variants contain mutations in the spike protein that may affect vaccine efficacy. Here, Jalkanen et al . show, using sera from 180 BNT162b2-vaccinated health care workers, that neutralization of SARS-CoV2 variant B.1.1.7 is not affected, while neutralization of B.1.351 variant is five-fold reduced.
- Pinja Jalkanen
- Pekka Kolehmainen
- Ilkka Julkunen
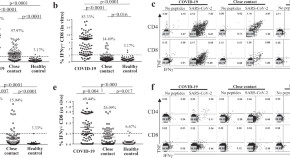

Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection
T cells compose a critical component of the immune response to coronavirus infection with SARS-CoV-2. Here the authors characterise the T cell response to SARS CoV-2 in patients and their close contacts, and show the presence of SARS-CoV-2 specific T cells in the absence of detectable virus infection.
- Zhongfang Wang
- Xiaoyun Yang
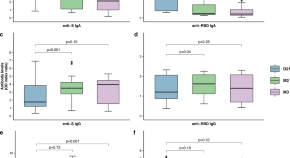
Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers
The humoral immune response to SARS-CoV-2 infection is not yet fully understood. Here, Marot et al. monitor the longitudinal profile and neutralizing activity of IgG, IgA, and IgM among 26 healthcare workers and provide evidence for a short-lasting humoral immune protection due to a decrease of neutralizing antibody titers within 3 months.
- Stéphane Marot
- Isabelle Malet
- Anne-Geneviève Marcelin
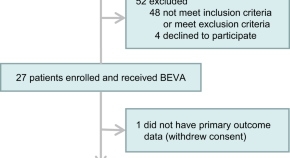
Efficacy and tolerability of bevacizumab in patients with severe Covid-19
In this single-arm clinical trial, the authors show that treatment of COVID-19 patients with bevacizumab, an anti-vascular endothelial growth factor drug, can improve PaO 2 /FiO 2 ratios and oxygen-support status. Relative to an external control group, bevacizumab shows clinical efficacy by improving oxygenation.
- Jiaojiao Pang
Evidence for SARS-CoV-2 related coronaviruses circulating in bats and pangolins in Southeast Asia
A bat origin for SARS-CoV-2 has been proposed. Here, by sampling wild Rhinolophus acuminatus bats from Thailand, the authors identified a SARS-CoV-2-related coronavirus (SC2r-CoV), designated as RacCS203, with 91.5% genome similarity to SARS-CoV-2, and show that sera obtained from bats and Malayan pangolin neutralize SARS-CoV-2.
- Supaporn Wacharapluesadee
- Chee Wah Tan
- Lin-Fa Wang
SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes
The SARS-CoV-2 gene set remains unresolved, hindering dissection of COVID-19 biology. Comparing 44 Sarbecovirus genomes provides a high-confidence protein-coding gene set. The study characterizes protein-level and nucleotide-level evolutionary constraints, and prioritizes functional mutations from the ongoing COVID-19 pandemic.
- Irwin Jungreis
- Rachel Sealfon
- Manolis Kellis
Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival
Antibody responses are critical for protection from developing severe COVID-19 following SARS-CoV-2 infection. Here the authors show that antibody responses against SARS-CoV-2 spike protein correlate with neutralizing capacity and protection, are not affected by heterologous boosting of influenza or common cold immunity, and can last up to 8 months.
- Stefania Dispinseri
- Massimiliano Secchi
- Gabriella Scarlatti

New-onset IgG autoantibodies in hospitalized patients with COVID-19
Infection with SARS-CoV2 and the development of Coronavirus disease 2019 (COVID-19) has been linked to induction of autoimmunity and autoantibody production. Here the authors characterise the new-onset IgG autoantibody response in hospitalised patients with COVID-19 which they correlate to the magnitude of the SARS-CoV2 response.
- Sarah Esther Chang
- Paul J. Utz
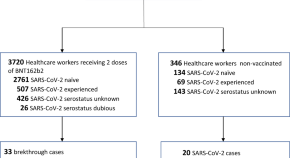
SARS-CoV-2 vaccine breakthrough infections with the alpha variant are asymptomatic or mildly symptomatic among health care workers
Several COVID-19 vaccines have shown good efficacy in clinical trials. Here, the authors provide real world effectiveness data in a group of BNT162b2 vaccinated health care workers and find that breakthrough infections are asymptomatic or mild.
- Francesca Rovida
- Irene Cassaniti
- Fausto Baldanti
Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19)
Duration of infectious SARS-CoV-2 shedding is an important measure for improved disease control. Here, the authors use virus cultures of respiratory tract samples from COVID-19 patients and observe a median shedding duration of 8 days and a drop below 5% after 15,2 days post onset of symptoms.
- Jeroen J. A. van Kampen
- David A. M. C. van de Vijver
- Annemiek A. van der Eijk
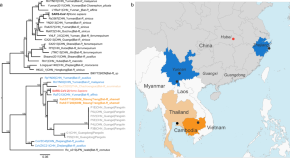
A novel SARS-CoV-2 related coronavirus in bats from Cambodia
In this study, Delaune et al., isolate and characterise a SARS-CoV-2-related coronavirus from two bats sampled in Cambodia. Their findings suggest that the geographic distribution of SARS-CoV-2-related viruses is wider than previously reported.
- Deborah Delaune
- Veasna Duong
Neutralizing antibody titres in SARS-CoV-2 infections
Here, the authors perform plaque reduction neutralization (PRNT) assays quantitating SARS-CoV-2 specific neutralizing antibodies from 195 patients in different disease states and find that patients with severe disease exhibit higher peaks of neutralizing antibody titres than patients with mild or asymptomatic infections and that serum neutralizing antibody persists for over 6 months in most people.
- Eric H. Y. Lau
- Owen T. Y. Tsang
- Malik Peiris
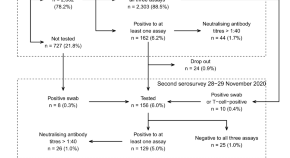
SARS-CoV-2 antibody dynamics and transmission from community-wide serological testing in the Italian municipality of Vo’
Vo’, Italy, is a unique setting for studying SARS-CoV-2 antibody dynamics because mass testing was conducted there early in the pandemic. Here, the authors perform two follow-up serological surveys and estimate seroprevalence, the extent of within-household transmission, and the impact of contact tracing.
- Ilaria Dorigatti
- Enrico Lavezzo
- Andrea Crisanti
Discrete SARS-CoV-2 antibody titers track with functional humoral stability
The extent of antibody protection against SARS-CoV-2 remains unclear. Here, using a cohort of 120 seroconverted individuals, the authors longitudinally characterize neutralization, Fc-function, and SARS-CoV-2 specific T cell responses, which they show to be prominent only in those subjects that elicited receptor-binding domain (RBD)-specific antibody titers above a certain threshold, suggesting that development of T cell responses to be related to anti-RBD Ab production.
- Yannic C. Bartsch
- Stephanie Fischinger
- Galit Alter
Mechanisms of SARS-CoV-2 neutralization by shark variable new antigen receptors elucidated through X-ray crystallography
Shark antibodies (Variable New Antigen Receptors, VNARs) are the smallest naturally occurring antibody fragments. Here, the authors screen a VNAR phage display library against the SARS-CoV2 receptor binding domain (RBD) and identify VNARs that neutralize the SARSCoV-2 virus and discuss their mechanisms of viral neutralization.
- Obinna C. Ubah
- Eric W. Lake
- Caroline J. Barelle
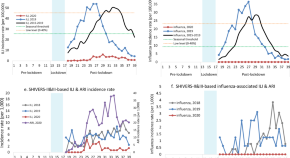
Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand
New Zealand has been relatively successful in controlling COVID-19 due to implementation of strict non-pharmaceutical interventions. Here, the authors demonstrate a striking decline in reports of influenza and other non-influenza respiratory pathogens over winter months in which the interventions have been in place.
- Q. Sue Huang
- Richard J. Webby
A potent SARS-CoV-2 neutralising nanobody shows therapeutic efficacy in the Syrian golden hamster model of COVID-19
Neutralizing nanobodies (Nb) are of considerable interest as therapeutic agents for COVID-19 treatment. Here, the authors functionally and structurally characterize Nbs that bind with high affinity to the receptor binding domain of the SARS-CoV-2 spike protein and show that an engineered homotrimeric Nb prevents disease progression in a Syrian hamster model of COVID-19 when administered intranasally.
- Jiandong Huo
- Halina Mikolajek
- Raymond J. Owens
Reprogrammed CRISPR-Cas13b suppresses SARS-CoV-2 replication and circumvents its mutational escape through mismatch tolerance
Cas13b can be harnessed to target and degrade RNA transcripts inside a cellular environment. Here the authors reprogram Cas13b to target SARSCoV-2 transcripts in infected mammalian cells and reveal its resilience to variants thanks to single mismatch tolerance.
- Mohamed Fareh
- Joseph A. Trapani
SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells
T cells are instrumental to protective immune responses against SARS-CoV-2, the pathogen responsible for the COVID-19 pandemic. Here the authors show that, in convalescent COVID-19 patients, memory T cell responses are detectable up to 317 days post-symptom onset, in which the presence of stem cell-like memory T cells further hints long-lasting immunity.
- Jae Hyung Jung
- Min-Seok Rha
- Eui-Cheol Shin
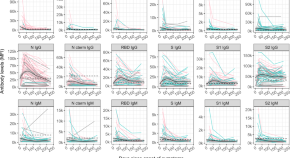
Seven-month kinetics of SARS-CoV-2 antibodies and role of pre-existing antibodies to human coronaviruses
Long-term characterisation of SARS-CoV-2 antibody kinetics is needed to understand the protective role of the immune response. Here the authors describe antibody levels and neutralisation activity in healthcare workers over seven months and investigate the role of immunity to endemic human coronaviruses.
- Natalia Ortega
- Marta Ribes
- Carlota Dobaño
Mechanism of SARS-CoV-2 polymerase stalling by remdesivir
Remdesivir is a nucleoside analog that inhibits the SARS-CoV-2 RNA dependent RNA polymerase (RdRp) and is used as a drug to treat COVID19 patients. Here, the authors provide insights into the mechanism of remdesivir-induced RdRp stalling by determining the cryo-EM structures of SARS-CoV-2 RdRp with bound RNA molecules that contain remdesivir at defined positions and observe that addition of the fourth nucleotide following remdesivir incorporation into the RNA product is impaired by a barrier to further RNA translocation.
- Goran Kokic
- Hauke S. Hillen
- Patrick Cramer
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Contact Tracing
- Pandemic Data Initiative
- Webcasts & Videos
- 30-Minute COVID-19 Briefing
Research Papers
Jhu has stopped collecting data as of.
After three years of around-the-clock tracking of COVID-19 data from...
The Johns Hopkins Coronavirus Resource Center has collected, verified, and published local, regional, national, and international pandemic data since it launched in March 2020. From the beginning, the information has been freely available to all — researchers, institutions, the media, the public, and policymakers. As a result, the CRC and its data have been cited in many published research papers and reports. Here we have gathered publications authored by CRC team members that focus on the CRC or its data.
July 14, 2022
Misaligned Federal and State Covid data limits demographic insights
CDC underreports cases and deaths among African American and Hispanic or Latino individuals.
February 17, 2022
Experts Call for Open Public Health Data
Johns Hopkins team highlighted the urgent need for better COVID data collection.
Unifying Epidemiologists and Economists
Researchers from disparate fields join to chart a new path for formulating policies in response to future pandemics.
Mobility Data Supported Social Distancing
Study found that physical distancing was an effective COVID mitigation strategy.
Johns Hopkins Engineers Build COVID Dashboard
Lancet Infectious Diseases published first paper detailing how the global map was built.
Researchers Identify Disparities in COVID Testing
Johns Hopkins team conducted an analysis of state-published demographic data
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Coronavirus disease 2019 (COVID-19): A literature review
Affiliations.
- 1 Medical Research Unit, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Tropical Disease Centre, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Department of Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- 2 Division of Infectious Diseases, AichiCancer Center Hospital, Chikusa-ku Nagoya, Japan. Electronic address: [email protected].
- 3 Department of Family Medicine, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- 4 Department of Pulmonology and Respiratory Medicine, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- 5 School of Medicine, The University of Western Australia, Perth, Australia. Electronic address: [email protected].
- 6 Siem Reap Provincial Health Department, Ministry of Health, Siem Reap, Cambodia. Electronic address: [email protected].
- 7 Department of Microbiology and Parasitology, Faculty of Medicine and Health Sciences, Warmadewa University, Denpasar, Indonesia; Department of Medical Microbiology and Immunology, University of California, Davis, CA, USA. Electronic address: [email protected].
- 8 Medical Research Unit, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Tropical Disease Centre, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Department of Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Department of Clinical Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- 9 Department of Epidemiology, University of Michigan, Ann Arbor, Michigan, MI 48109, USA. Electronic address: [email protected].
- 10 Medical Research Unit, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Tropical Disease Centre, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia; Department of Microbiology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Electronic address: [email protected].
- PMID: 32340833
- PMCID: PMC7142680
- DOI: 10.1016/j.jiph.2020.03.019
In early December 2019, an outbreak of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan City, Hubei Province, China. On January 30, 2020 the World Health Organization declared the outbreak as a Public Health Emergency of International Concern. As of February 14, 2020, 49,053 laboratory-confirmed and 1,381 deaths have been reported globally. Perceived risk of acquiring disease has led many governments to institute a variety of control measures. We conducted a literature review of publicly available information to summarize knowledge about the pathogen and the current epidemic. In this literature review, the causative agent, pathogenesis and immune responses, epidemiology, diagnosis, treatment and management of the disease, control and preventions strategies are all reviewed.
Keywords: 2019-nCoV; COVID-19; Novel coronavirus; Outbreak; SARS-CoV-2.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
PubMed Disclaimer
- COVID-19 pandemic and Internal Medicine Units in Italy: a precious effort on the front line. Montagnani A, Pieralli F, Gnerre P, Vertulli C, Manfellotto D; FADOI COVID-19 Observatory Group. Montagnani A, et al. Intern Emerg Med. 2020 Nov;15(8):1595-1597. doi: 10.1007/s11739-020-02454-5. Epub 2020 Jul 31. Intern Emerg Med. 2020. PMID: 32737837 Free PMC article. No abstract available.
Similar articles
- Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak - United States, December 31, 2019-February 4, 2020. Patel A, Jernigan DB; 2019-nCoV CDC Response Team. Patel A, et al. MMWR Morb Mortal Wkly Rep. 2020 Feb 7;69(5):140-146. doi: 10.15585/mmwr.mm6905e1. MMWR Morb Mortal Wkly Rep. 2020. PMID: 32027631 Free PMC article.
- The First 75 Days of Novel Coronavirus (SARS-CoV-2) Outbreak: Recent Advances, Prevention, and Treatment. Yan Y, Shin WI, Pang YX, Meng Y, Lai J, You C, Zhao H, Lester E, Wu T, Pang CH. Yan Y, et al. Int J Environ Res Public Health. 2020 Mar 30;17(7):2323. doi: 10.3390/ijerph17072323. Int J Environ Res Public Health. 2020. PMID: 32235575 Free PMC article. Review.
- COVID-19: The outbreak caused by a new coronavirus. Sifuentes-Rodríguez E, Palacios-Reyes D. Sifuentes-Rodríguez E, et al. Bol Med Hosp Infant Mex. 2020;77(2):47-53. doi: 10.24875/BMHIM.20000039. Bol Med Hosp Infant Mex. 2020. PMID: 32226003 Review. English.
- The Novel Coronavirus: A Bird's Eye View. Habibzadeh P, Stoneman EK. Habibzadeh P, et al. Int J Occup Environ Med. 2020 Apr;11(2):65-71. doi: 10.15171/ijoem.2020.1921. Epub 2020 Feb 5. Int J Occup Environ Med. 2020. PMID: 32020915 Free PMC article. Review.
- Occupational health responses to COVID-19: What lessons can we learn from SARS? Koh D, Goh HP. Koh D, et al. J Occup Health. 2020 Jan;62(1):e12128. doi: 10.1002/1348-9585.12128. J Occup Health. 2020. PMID: 32515882 Free PMC article.
- Evaluating the resilience of residential buildings during a pandemic with a sustainable construction approach. Heydari A, Abbasianjahromi H. Heydari A, et al. Heliyon. 2024 May 14;10(10):e31006. doi: 10.1016/j.heliyon.2024.e31006. eCollection 2024 May 30. Heliyon. 2024. PMID: 38803988 Free PMC article.
- Network pharmacology, molecular docking, and dynamics analyses to predict the antiviral activity of ginger constituents against coronavirus infection. Samy A, Hassan A, Hegazi NM, Farid M, Elshafei M. Samy A, et al. Sci Rep. 2024 May 27;14(1):12059. doi: 10.1038/s41598-024-60721-3. Sci Rep. 2024. PMID: 38802394 Free PMC article.
- Inflammatory Biomarkers for Assessing In-Hospital Mortality Risk in Severe COVID-19-A Retrospective Study. Bimbo-Szuhai E, Botea MO, Romanescu DD, Beiusanu C, Gavrilas GM, Popa GM, Antal D, Bontea MG, Sachelarie L, Macovei IC. Bimbo-Szuhai E, et al. J Pers Med. 2024 May 10;14(5):503. doi: 10.3390/jpm14050503. J Pers Med. 2024. PMID: 38793085 Free PMC article.
- Vascular Alterations Following COVID-19 Infection: A Comprehensive Literature Review. Karakasis P, Nasoufidou A, Sagris M, Fragakis N, Tsioufis K. Karakasis P, et al. Life (Basel). 2024 Apr 24;14(5):545. doi: 10.3390/life14050545. Life (Basel). 2024. PMID: 38792566 Free PMC article. Review.
- Job burnout and its influencing factors among village doctors during the COVID-19 pandemic: a cross-sectional study. Zhao Z, Li Q, Yang C, Zhang Z, Chen Z, Yin W. Zhao Z, et al. Front Public Health. 2024 Apr 18;12:1388831. doi: 10.3389/fpubh.2024.1388831. eCollection 2024. Front Public Health. 2024. PMID: 38699414 Free PMC article.
- Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan China: the mystery and the miracle. J Med Virol. 2020 - PMC - PubMed
- Hui D.S., E I.A., Madani T.A., Ntoumi F., Kock R., Dar O. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. - PMC - PubMed
- Gorbalenya A.E.A. Severe acute respiratory syndrome-related coronavirus: the species and its viruses – a statement of the Coronavirus Study Group. BioRxiv. 2020 doi: 10.1101/2020.02.07.937862. - DOI
- Burki T.K. Coronavirus in China. Lancet Respir Med. 2020 - PMC - PubMed
- NHS press conference, February 4, 2020. Beijing, China. National Health Commission (NHC) of the People's Republic of China. http://www.nhc.gov.cn/xcs/xwbd/202002/235990d202056cfcb202043f202004a202... .
Publication types
- Search in MeSH
LinkOut - more resources
Full text sources.
- Elsevier Science
- Europe PubMed Central
- PubMed Central
Other Literature Sources
- The Lens - Patent Citations
Research Materials
- NCI CPTC Antibody Characterization Program
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.

COVID-19 Research Articles Downloadable Database
March 19, 2020
Updated January 12, 2024
COVID-19 Research Guide Home
- Research Articles Downloadable Database
- COVID-19 Science Updates
- Databases and Journals
- Secondary Data and Statistics
Important announcement:
The CDC Database of COVID-19 Research Articles became a collaboration with the WHO to create the WHO COVID-19 database during the pandemic to make it easier for results to be searched, downloaded, and used by researchers worldwide.
The last version of the CDC COVID-19 database was archived and remain available on this website. Please note that it has stopped updating as of October 9, 2020 and all new articles were integrated into the WHO COVID-19 database . The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
If you have any questions, concerns, or problems accessing the WHO COVID-19 Database please email the CDC Library for assistance.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
Below are options to download the archive of COVID-19 research articles. You can search the database of citations by author, keyword (in title, author, abstract, subject headings fields), journal, or abstract when available. DOI, PMID, and URL links are included when available.
This database was last updated on October 9, 2020 .
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide. The WHO Covid-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). Its content remains searchable and spans the time period March 2020 to June 2023. Since June 2023, manual updates to the database have been discontinued.
- To help inform CDC’s COVID-19 Response, as well as to help CDC staff stay up to date on the latest COVID-19 research, the Response’s Office of the Chief Medical Officer has collaborated with the CDC Office of Library Science to create a series called COVID-19 Science Update . This series, the first of its kind for a CDC emergency response, provides brief summaries of new COVID-19-related studies on many topics, including epidemiology, clinical treatment and management, laboratory science, and modeling. As of December 18, 2021, CDC has stopped production of the weekly COVID-19 Science Update.
Excel download:
- Articles from August until October 9 2020 [XLS – 29 MB]
- Articles from December 2019 through July 2020 [XLS – 45 MB]
- The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database . Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers worldwide.
- October 8 in Excel [XLS – 1 MB]
- October 7 in Excel [XLS – 1 MB]
- October 6 in Excel [XLS – 1 MB]
- Note the main Excel file can also be sorted by date added.
Citation Management Software (EndNote, Mendeley, Zotero, Refman, etc.) download:
- Part 1 [ZIP – 38 MB]
- Part 2 [ZIP – 43 MB]
- October 8 in citation management software format [RIS – 2 MB]
- October 7 in citation management software format [RIS – 2 MB]
- October 6 in citation management software format [RIS – 2 MB]
- Note the main RIS file can also be sorted by date added.
The COVID-19 pandemic is a rapidly changing situation. Some of the research included above is preliminary. Materials listed in this database are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings.
To access the full text, click on the DOI, PMID, or URL links. While most publishers are making their COVID-19 content Open Access, some articles are accessible only to those with a CDC user id and password. Find a library near you that may be able to help you get access to articles by clicking the following links: https://www.worldcat.org/libraries OR https://www.usa.gov/libraries .
CDC users can use EndNote’s Find Full Text feature to attach the full text PDFs within their EndNote Library. CDC users, please email Martha Knuth for an EndNote file of all citations. Once you have your EndNote file downloaded, to get the full-text of journal articles listed in the search results you can do the following steps:
- First, try using EndNote’s “Find Full-Text” feature to attach full-text articles to your EndNote Library.
- Next, check for full-text availability, via the E-Journals list, at: http://sfxhosted.exlibrisgroup.com/cdc/az .
- If you can’t find full-text online, you can request articles via DocExpress, at: https://docexpress.cdc.gov/illiad/
The following databases were searched from Dec. 2019-Oct. 9 2020 for articles related to COVID-19: Medline (Ovid and PubMed), PubMed Central, Embase, CAB Abstracts, Global Health, PsycInfo, Cochrane Library, Scopus, Academic Search Complete, Africa Wide Information, CINAHL, ProQuest Central, SciFinder, the Virtual Health Library, and LitCovid. Selected grey literature sources were searched as well, including the WHO COVID-19 website, CDC COVID-19 website, Eurosurveillance, China CDC Weekly, Homeland Security Digital Library, ClinicalTrials.gov, bioRxiv (preprints), medRxiv (preprints), chemRxiv (preprints), and SSRN (preprints).
Detailed search strings with synonyms used for COVID-19 are below.
Detailed search strategy for gathering COVID-19 articles, updated October 9, 2020 [PDF – 135 KB]
Note on preprints: Preprints have not been peer-reviewed. They should not be regarded as conclusive, guide clinical practice/health-related behavior, or be reported in news media as established information.
Materials listed in these guides are selected to provide awareness of quality public health literature and resources. A material’s inclusion does not necessarily represent the views of the U.S. Department of Health and Human Services (HHS), the Public Health Service (PHS), or the Centers for Disease Control and Prevention (CDC), nor does it imply endorsement of the material’s methods or findings. HHS, PHS, and CDC assume no responsibility for the factual accuracy of the items presented. The selection, omission, or content of items does not imply any endorsement or other position taken by HHS, PHS, and CDC. Opinion, findings, and conclusions expressed by the original authors of items included in these materials, or persons quoted therein, are strictly their own and are in no way meant to represent the opinion or views of HHS, PHS, or CDC. References to publications, news sources, and non-CDC Websites are provided solely for informational purposes and do not imply endorsement by HHS, PHS, or CDC.
To receive the COVID-19 Science Update, please enter your email address to subscribe today.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
The impact of the COVID-19 pandemic on scientific research in the life sciences
Roles Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing
Affiliation AXES, IMT School for Advanced Studies Lucca, Lucca, Italy
Roles Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Chair of Systems Design D-MTEC, ETH Zürich, Zurich, Switzerland
- Massimo Riccaboni,
- Luca Verginer

- Published: February 9, 2022
- https://doi.org/10.1371/journal.pone.0263001
- Reader Comments
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on world scientific production in the life sciences and find indications that the usage of medical subject headings (MeSH) has changed following the outbreak. We estimate through a difference-in-differences approach the impact of the start of the COVID-19 pandemic on scientific production using the PubMed database (3.6 Million research papers). We find that COVID-19-related MeSH terms have experienced a 6.5 fold increase in output on average, while publications on unrelated MeSH terms dropped by 10 to 12%. The publication weighted impact has an even more pronounced negative effect (-16% to -19%). Moreover, COVID-19 has displaced clinical trial publications (-24%) and diverted grants from research areas not closely related to COVID-19. Note that since COVID-19 publications may have been fast-tracked, the sudden surge in COVID-19 publications might be driven by editorial policy.
Citation: Riccaboni M, Verginer L (2022) The impact of the COVID-19 pandemic on scientific research in the life sciences. PLoS ONE 17(2): e0263001. https://doi.org/10.1371/journal.pone.0263001
Editor: Florian Naudet, University of Rennes 1, FRANCE
Received: April 28, 2021; Accepted: January 10, 2022; Published: February 9, 2022
Copyright: © 2022 Riccaboni, Verginer. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The processed data, instructions on how to process the raw PubMed dataset as well as all code are available via Zenodo at https://doi.org/10.5281/zenodo.5121216 .
Funding: The author(s) received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The COVID-19 pandemic has mobilized the world scientific community in 2020, especially in the life sciences [ 1 , 2 ]. In the first three months after the pandemic, the number of scientific papers about COVID-19 was fivefold the number of articles on H1N1 swine influenza [ 3 ]. Similarly, the number of clinical trials related to COVID-19 prophylaxis and treatments skyrocketed [ 4 ]. Thanks to the rapid mobilization of the world scientific community, COVID-19 vaccines have been developed in record time. Despite this undeniable success, there is a rising concern about the negative consequences of COVID-19 on clinical trial research, with many projects being postponed [ 5 – 7 ]. According to Evaluate Pharma, clinical trials were one of the pandemic’s first casualties, with a record number of 160 studies suspended for reasons related to COVID-19 in April 2020 [ 8 , 9 ] reporting a total of 1,200 trials suspended as of July 2020. As a consequence, clinical researchers have been impaired by reduced access to healthcare research infrastructures. Particularly, the COVID-19 outbreak took a tall on women and early-career scientists [ 10 – 13 ]. On a different ground, Shan and colleagues found that non-COVID-19-related articles decreased as COVID-19-related articles increased in top clinical research journals [ 14 ]. Fraser and coworker found that COVID-19 preprints received more attention and citations than non-COVID-19 preprints [ 1 ]. More recently, Hook and Porter have found some early evidence of ‘covidisation’ of academic research, with research grants and output diverted to COVID-19 research in 2020 [ 15 ]. How much should scientists switch their efforts toward SARS-CoV-2 prevention, treatment, or mitigation? There is a growing consensus that the current level of ‘covidisation’ of research can be wasteful [ 4 , 5 , 16 ].
Against this background, in this paper, we investigate if the COVID-19 pandemic has induced a shift in biomedical publications toward COVID-19-related scientific production. The objective of the study is to show that scientific articles listing covid-related Medical Subject Headings (MeSH) when compared against covid-unrelated MeSH have been partially displaced. Specifically, we look at several indicators of scientific production in the life sciences before and after the start of the COVID-19 pandemic: (1) number of papers published, (2) impact factor weighted number of papers, (3) opens access, (4) number of publications related to clinical trials, (5) number of papers listing grants, (6) number of papers listing grants existing before the pandemic. Through a natural experiment approach, we analyze the impact of the pandemic on scientific production in the life sciences. We consider COVID-19 an unexpected and unprecedented exogenous source of variation with heterogeneous effects across biomedical research fields (i.e., MeSH terms).
Based on the difference in difference results, we document the displacement effect that the pandemic has had on several aspects of scientific publishing. The overall picture that emerges from this analysis is that there has been a profound realignment of priorities and research efforts. This shift has displaced biomedical research in fields not related to COVID-19.
The rest of the paper is structured as follows. First, we describe the data and our measure of relatedness to COVID-19. Next, we illustrate the difference-in-differences specification we rely on to identify the impact of the pandemic on scientific output. In the results section, we present the results of the difference-in-differences and network analyses. We document the sudden shift in publications, grants and trials towards COVID-19-related MeSH terms. Finally, we discuss the findings and highlight several policy implications.
Materials and methods
The present analysis is based primarily on PubMed and the Medical Subject Headings (MeSH) terminology. This data is used to estimate the effect of the start of the COVID 19 pandemic via a difference in difference approach. This section is structured as follows. We first introduce the data and then the econometric methodology. This analysis is not based on a pre-registered protocol.
Selection of biomedical publications.
We rely on PubMed, a repository with more than 34 million biomedical citations, for the analysis. Specifically, we analyze the daily updated files up to 31/06/2021, extracting all publications of type ‘Journal Article’. For the principal analysis, we consider 3,638,584 papers published from January 2019 to December 2020. We also analyze 11,122,017 papers published from 2010 onwards to identify the earliest usage of a grant and infer if it was new in 2020. We use the SCImago journal ranking statistics to compute the impact factor weighted number (IFWN) of papers in a given field of research. To assign the publication date, we use the ‘electronically published’ dates and, if missing, the ‘print published’ dates.
Medical subject headings.
We rely on the Medical Subject Headings (MeSH) terminology to approximate narrowly defined biomedical research fields. This terminology is a curated medical vocabulary, which is manually added to papers in the PubMed corpus. The fact that MeSH terms are manually annotated makes this terminology ideal for classification purposes. However, there is a delay between publication and annotation, on the order of several months. To address this delay and have the most recent classification, we search for all 28 425 MeSH terms using PubMed’s ESearch utility and classify paper by the results. The specific API endpoint is https://eutils.ncbi.nlm.nih.gov/entrez/eutils/esearch.fcgi , the relevant scripts are available with the code. For example, we assign the term ‘Ageusia’ (MeSH ID D000370) to all papers listed in the results of the ESearch API. We apply this method to the whole period (January 2019—December 2020) and obtain a mapping from papers to the MeSH terms. For every MeSH term, we keep track of the year they have been established. For instance, COVID-19 terms were established in 2020 (see Table 1 ): in January 2020, the WHO recommended 2019-nCoV and 2019-nCoV acute respiratory disease as provisional names for the virus and disease. The WHO issued the official terms COVID-19 and SARS-CoV-2 at the beginning of February 2020. By manually annotating publications, all publications referring to COVID-19 and SARS-CoV-2 since January 2020 have been labelled with the related MeSH terms. Other MeSH terms related to COVID-19, such as coronavirus, for instance, have been established years before the pandemic (see Table 2 ). We proxy MeSH term usage via search terms using the PubMed EUtilities API; this means that we are not using the hand-labelled MeSH terms but rather the PubMed search results. This means that the accuracy of the MeSH term we assign to a given paper is not perfect. In practice, this means that we have assigned more MeSH terms to a given term than a human annotator would have.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0263001.t001
The list contains only terms with at least 100 publications in 2020.
https://doi.org/10.1371/journal.pone.0263001.t002
Clinical trials and publication types.
We classify publications using PubMed’s ‘PublicationType’ field in the XML baseline files (There are 187 publication types, see https://www.nlm.nih.gov/mesh/pubtypes.html ). We consider a publication to be related to a clinical trial if it lists any of the following descriptors:
- D016430: Clinical Trial
- D017426: Clinical Trial, Phase I
- D017427: Clinical Trial, Phase II
- D017428: Clinical Trial, Phase III
- D017429: Clinical Trial, Phase IV
- D018848: Controlled Clinical Trial
- D065007: Pragmatic Clinical Trial
- D000076362: Adaptive Clinical Trial
- D000077522: Clinical Trial, Veterinary
In our analysis of the impact of COVID-19 on publications related to clinical trials, we only consider MeSH terms that are associated at least once with a clinical trial publication over the two years. We apply this restriction to filter out MeSH terms that are very unlikely to be relevant for clinical trial types of research.
Open access.
We proxy the availability of a journal article to the public, i.e., open access, if it is available from PubMed Central. PubMed Central archives full-text journal articles and provides free access to the public. Note that the copyright license may vary across participating publishers. However, the text of the paper is for all effects and purposes freely available without requiring subscriptions or special affiliation.
We infer if a publication has been funded by checking if it lists any grants. We classify grants as either ‘old’, i.e. existed before 2019, or ‘new’, i.e. first observed afterwards. To do so, we collect all grant IDs for 11,122,017 papers from 2010 on-wards and record their first appearance. This procedure is an indirect inference of the year the grant has been granted. The basic assumption is that if a grant number has not been listed in any publication since 2010, it is very likely a new grant. Specifically, an old grant is a grant listed since 2019 observed at least once from 2010 to 2018.
Note that this procedure is only approximate and has a few shortcomings. Mistyped grant numbers (e.g. ‘1234-M JPN’ and ‘1234-M-JPN’) could appear as new grants, even though they existed before, or new grants might be classified as old grants if they have a common ID (e.g. ‘Grant 1’). Unfortunately, there is no central repository of grant numbers and the associated metadata; however, there are plans to assign DOI numbers to grants to alleviate this problem (See https://gitlab.com/crossref/open_funder_registry for the project).
Impact factor weighted publication numbers (IFWN).
In our analysis, we consider two measures of scientific output. First, we simply count the number of publications by MeSH term. However, since journals vary considerably in terms of impact factor, we also weigh the number of publications by the impact factor of the venue (e.g., journal) where it was published. Specifically, we use the SCImago journal ranking statistics to weigh a paper by the impact factor of the journal it appears in. We use the ‘citation per document in the past two years’ for 45,230 ISSNs. Note that a journal may and often has more than one ISSN, i.e., one for the printed edition and one for the online edition. SCImago applies the same score for a venue across linked ISSNs.
For the impact factor weighted number (IFWN) of publication per MeSH terms, this means that all publications are replaced by the impact score of the journal they appear in and summed up.
COVID-19-relatedness.
To measure how closely related to COVID-19 is a MeSH term, we introduce an index of relatedness to COVID-19. First, we identify the focal COVID-19 terms, which appeared in the literature in 2020 (see Table 1 ). Next, for all other pre-existing MeSH terms, we measure how closely related to COVID-19 they end up being.
Our aim is to show that MeSH terms that existed before and are related have experienced a sudden increase in the number of (impact factor weighted) papers.
Intuitively we can read this measure as: what is the probability in 2020 that a COVID-19 MeSH term is present given that we chose a paper with MeSH term i ? For example, given that in 2020 we choose a paper dealing with “Ageusia” (i.e., Complete or severe loss of the subjective sense of taste), there is a 96% probability that this paper also lists COVID-19, see Table 1 .
Note that a paper listing a related MeSH term does not imply that that paper is doing COVID-19 research, but it implies that one of the MeSH terms listed is often used in COVID-19 research.
In sum, in our analysis, we use the following variables:
- Papers: Number of papers by MeSH term;
- Impact: Impact factor weighted number of papers by MeSH term;
- PMC: Papers listed in PubMed central by MeSH term, as a measure of Open Access publications;
- Trials: number of publications of type “Clinical Trial” by MeSH term;
- Grants: number of papers with at least one grant by MeSH term;
- Old Grants: number of papers listing a grant that has been observed between 2010 and 2018, by MeSH term;
Difference-in-differences
The difference-in-differences (DiD) method is an econometric technique to imitate an experimental research design from observation data, sometimes referred to as a quasi-experimental setup. In a randomized controlled trial, subjects are randomly assigned either to the treated or the control group. Analogously, in this natural experiment, we assume that medical subject headings (MeSH) have been randomly assigned to be either treated (related) or not treated (unrelated) by the pandemic crisis.
Before the COVID, for a future health crisis, the set of potentially impacted medical knowledge was not predictable since it depended on the specifics of the emergency. For instance, ageusia (loss of taste), a medical concept existing since 1991, became known to be a specific symptom of COVID-19 only after the pandemic.
Specifically, we exploit the COVID-19 as an unpredictable and exogenous shock that has deeply affected the publication priorities for biomedical scientific production, as compared to the situation before the pandemic. In this setting, COVID-19 is the treatment, and the identification of this new human coronavirus is the event. We claim that treated MeSH terms, i.e., MeSH terms related to COVID-19, have experienced a sudden increase in terms of scientific production and attention. In contrast, research on untreated MeSH terms, i.e., MeSH terms not related to COVID-19, has been displaced by COVID-19. Our analysis compares the scientific output of COVID-19 related and unrelated MeSH terms before and after January 2020.
In our case, some of the terms turn out to be related to COVID-19 in 2020, whereas most of the MeSH terms are not closely related to COVID-19.
Thus β 1 identifies the overall effect on the control group after the event, β 2 the difference across treated and control groups before the event (i.e. the first difference in DiD) and finally the effect on the treated group after the event, net of the first difference, β 3 . This last parameter identifies the treatment effect on the treated group netting out the pre-treatment difference.
For the DiD to have a causal interpretation, it must be noted that pre-event, the trends of the two groups should be parallel, i.e., the common trend assumption (CTA) must be satisfied. We will show that the CTA holds in the results section.
To specify the DiD model, we need to define a period before and after the event and assign a treatment status or level of exposure to each term.
Before and after.
The pre-treatment period is defined as January 2019 to December 2019. The post-treatment period is defined as the months from January 2020 to December 2020. We argue that the state of biomedical research was similar in those two years, apart from the effect of the pandemic.
Treatment status and exposure.
The treatment is determined by the COVID-19 relatedness index σ i introduced earlier. Specifically, this number indicates the likelihood that COVID-19 will be a listed MeSH term, given that we observe the focal MeSH term i . To show that the effect becomes even stronger the closer related the subject is, and for ease of interpretation, we also discretize the relatedness value into three levels of treatment. Namely, we group MeSH terms with a σ between, 0% to 20%, 20% to 80% and 80% to 100%. The choice of alternative grouping strategies does not significantly affect our results. Results for alternative thresholds of relatedness can be computed using the available source code. We complement the dichotomized analysis by using the treatment intensity (relatedness measure σ ) to show that the result persists.
Panel regression.
In this work, we estimate a random effects panel regression where the units of analysis are 28 318 biomedical research fields (i.e. MeSH terms) observed over time before and after the COVID-19 pandemic. The time resolution is at the monthly level, meaning that for each MeSH term, we have 24 observations from January 2019 to December 2020.
The outcome variable Y it identifies the outcome at time t (i.e., month), for MeSH term i . As before, P t identifies the period with P t = 0 if the month is before January 2020 and P t = 1 if it is on or after this date. In (3) , the treatment level is measure by the relatedness to COVID-19 ( σ i ), where again the γ 1 identifies pre-trend (constant) differences and δ 1 the overall effect.
In total, we estimate six coefficients. As before, the δ l coefficient identifies the DiD effect.
Verifying the Common Trend Assumption (CTA).
We show that the CTA holds for this model by comparing the pre-event trends of the control group to the treated groups (COVID-19 related MeSH terms). Namely, we show that the pre-event trends of the control group are the same as the pre-event trends of the treated group.
Co-occurrence analysis
To investigate if the pandemic has caused a reconfiguration of research priorities, we look at the MeSH term co-occurrence network. Precisely, we extract the co-occurrence network of all 28,318 MeSH terms as they appear in the 3.3 million papers. We considered the co-occurrence networks of 2018, 2019 and 2020. Each node represents a MeSH term in these networks, and a link between them indicates that they have been observed at least once together. The weight of the edge between the MeSH terms is given by the number of times those terms have been jointly observed in the same publications.
Medical language is hugely complicated, and this simple representation does not capture the intricacies, subtle nuances and, in fact, meaning of the terms. Therefore, we do not claim that we can identify how the actual usage of MeSH terms has changed from this object, but rather that it has. Nevertheless, the co-occurrence graph captures rudimentary relations between concepts. We argue that absent a shock to the system, their basic usage patterns, change in importance (within the network) would essentially be the same from year to year. However, if we find that the importance of terms changes more than expected in 2020, it stands to reason that there have been some significant changes.
To show that that MeSH usage has been affected, we compute for each term in the years 2018, 2019 and 2020 their PageRank centrality [ 17 ]. The PageRank centrality tells us how likely a random walker traversing a network would be found at a given node if she follows the weights of the empirical edges (i.e., co-usage probability). Specifically, for the case of the MeSH co-occurrence network, this number represents how often an annotator at the National Library of Medicine would assign that MeSH term following the observed general usage patterns. It is a simplistic measure to capture the complexities of biomedical research. Nevertheless, it captures far-reaching interdependence across MeSH terms as the measure uses the whole network to determine the centrality of every MeSH term. A sudden change in the rankings and thus the position of MeSH terms in this network suggests that a given research subject has risen as it is used more often with other important MeSH terms (or vice versa).
We then compare the growth for each MeSH i term in g i (2019), i.e. before the the COVID-19 pandemic, with the growth after the event ( g i (2020)).
Publication growth
Changes in output and COVID-19 relatedness
Before we show the regression results, we provide descriptive evidence that publications from 2019 to 2020 have drastically increased. By showing that this growth correlates strongly with a MeSH term’s COVID-19 relatedness ( σ ), we demonstrate that (1) σ captures an essential aspect of the growth dynamics and (2) highlight the meteoric rise of highly related terms.
We look at the year over year growth in the number of the impact weighted number of publications per MeSH term from 2018 to 2019 and 2019 to 2020 as defined in the methods section.
Fig 1 shows the yearly growth of the impact weighted number of publications per MeSH term. By comparing the growth of the number of publications from the years 2018, 2019 and 2020, we find that the impact factor weighted number of publications has increased by up to a factor of 100 compared to the previous year for Betacoronavirus, one of the most closely related to COVID-19 MeSH term.
Each dot represents, a MeSH term. The y axis (growth) is in symmetric log scale. The x axis shows the COVID-19 relatedness, σ . Note that the position of the dots on the x-axis is the same in the two plots. Below: MeSH term importance gain (PageRank) and their COVID-19 relatedness.
https://doi.org/10.1371/journal.pone.0263001.g001
Fig 1 , first row, reveals how strongly correlated the growth in the IFWN of publication is to the term’s COVID-19 relatedness. For instance, we see that the term ‘Betacoronavirus’ skyrocketed from 2019 to 2020, which is expected given that SARS-CoV-2 is a species of the genus. Conversely, the term ‘Alphacoronavirus’ has not experienced any growth given that it is twin a genus of the Coronaviridae family, but SARS-CoV-2 is not one of its species. Note also the fast growth in the number of publications dealing with ‘Quarantine’. Moreover, MeSH terms that grew significantly from 2018 to 2019 and were not closely related to COVID-19, like ‘Vaping’, slowed down in 2020. From the graph, the picture emerges that publication growth is correlated with COVID-19 relatedness σ and that the growth for less related terms slowed down.
To show that the usage pattern of MeSH terms has changed following the pandemic, we compute the PageRank centrality using graph-tool [ 18 ] as discussed in the Methods section.
Fig 1 , second row, shows the change in the PageRank centrality of the MeSH terms after the pandemic (2019 to 2020, right plot) and before (2018 to 2019, left plot). If there were no change in the general usage pattern, we would expect the variance in PageRank changes to be narrow across the two periods, see (left plot). However, PageRank scores changed significantly more from 2019 to 2020 than from 2018 to 2019, suggesting that there has been a reconfiguration of the network.
To further support this argument, we carry out a DiD regression analysis.
Common trends assumption
As discussed in the Methods section, we need to show that the CTA assumption holds for the DiD to be defined appropriately. We do this by estimating for each month the number of publications and comparing it across treatment groups. This exercise also serves the purpose of a placebo test. By assuming that each month could have potentially been the event’s timing (i.e., the outbreak), we show that January 2020 is the most likely timing of the event. The regression table, as noted earlier, contains over 70 estimated coefficients, hence for ease of reading, we will only show the predicted outcome per month by group (see Fig 2 ). The full regression table with all coefficients is available in the S1 Table .
The y axis is in log scale. The dashed vertical line identifies January 2020. The dashed horizontal line shows the publications in January 2019 for the 0–20% group before the event. This line highlights that the drop happens after the event. The bands around the lines indicate the 95% confidence interval of the predicted values. The results are the output of the Stata margins command.
https://doi.org/10.1371/journal.pone.0263001.g002
Fig 2 shows the predicted number per outcome variable obtained from the panel regression model. These predictions correspond to the predicted value per relatedness group using the regression parameters estimated via the linear panel regression. The bands around the curves are the 95% confidence intervals.
All outcome measures depict a similar trend per month. Before the event (i.e., January 2020), there is a common trend across all groups. In contrast, after the event, we observe a sudden rise for the outcomes of the COVID-19 related treated groups (green and red lines) and a decline in the outcomes for the unrelated group (blue line). Therefore, we can conclude that the CTA assumption holds.
Regression results
Table 3 shows the DiD regression results (see Eq (3) ) for the selected outcome measures: number of publications (Papers), impact factor weighted number of publications (Impact), open access (OA) publications, clinical trial related publications, and publications with existing grants.
https://doi.org/10.1371/journal.pone.0263001.t003
Table 3 shows results for the discrete treatment level version of the DiD model (see Eq (4) ).
Note that the outcome variable is in natural log scale; hence to get the effect of the independent variable, we need to exponentiate the coefficient. For values close to 0, the effect is well approximated by the percentage change of that magnitude.
In both specifications we see that the least related group, drops in the number of publications between 10% and 13%, respectively (first row of Tables 3 and 4 , exp(−0.102) ≈ 0.87). In line with our expectations, the increase in the number of papers published by MeSH term is positively affected by the relatedness to COVID-19. In the discrete model (row 2), we note that the number of documents with MeSH terms with a COVID-19 relatedness between 20 and 80% grows by 18% and highly related terms by a factor of approximately 6.6 (exp(1.88)). The same general pattern can be observed for the impact weighted publication number, i.e., Model (2). Note, however, that the drop in the impact factor weighted output is more significant, reaching -19% for COVID-19 unrelated publications, and related publications growing by a factor of 8.7. This difference suggests that there might be a bias to publish papers on COVID-19 related subjects in high impact factor journals.
https://doi.org/10.1371/journal.pone.0263001.t004
By looking at the number of open access publications (PMC), we note that the least related group has not been affected negatively by the pandemic. However, the number of COVID-19 related publications has drastically increased for the most COVID-19 related group by a factor of 6.2. Note that the substantial increase in the number of papers available through open access is in large part due to journal and editorial policies to make preferentially COVID research immediately available to the public.
Regarding the number of clinical trial publications, we note that the least related group has been affected negatively, with the number of publications on clinical trials dropping by a staggering 24%. At the same time, publications on clinical trials for COVID-19-related MeSH have increased by a factor of 2.1. Note, however, that the effect on clinical trials is not significant in the continuous regression. The discrepancy across Tables 3 and 4 highlights that, especially for trials, the effect is not linear, where only the publications on clinical trials closely related to COVID-19 experiencing a boost.
It has been reported [ 19 ] that while the number of clinical trials registered to treat or prevent COVID-19 has surged with 179 new registrations in the second week of April 2020 alone. Only a few of these have led to publishable results in the 12 months since [ 20 ]. On the other hand, we find that clinical trial publications, considering related MeSH (but not COVID-19 directly), have had significant growth from the beginning of the pandemic. These results are not contradictory. Indeed counting the number of clinical trial publications listing the exact COVID-19 MeSH term (D000086382), we find 212 publications. While this might seem like a small number, consider that in 2020 only 8,485 publications were classified as clinical trials; thus, targeted trials still made up 2.5% of all clinical trials in 2020 . So while one might doubt the effectiveness of these research efforts, it is still the case that by sheer number, they represent a significant proportion of all publications on clinical trials in 2020. Moreover, COVID-19 specific Clinical trial publications in 2020, being a delayed signal of the actual trials, are a lower bound estimate on the true number of such clinical trials being conducted. This is because COVID-19 studies could only have commenced in 2020, whereas other studies had a head start. Thus our reported estimates are conservative, meaning that the true effect on actual clinical trials is likely larger, not smaller.
Research funding, as proxied by the number of publications with grants, follows a similar pattern, but notably, COVID-19-related MeSH terms list the same proportion of grants established before 2019 as other unrelated MeSH terms, suggesting that grants which were not designated for COVID-19 research have been used to support COVID-19 related research. Overall, the number of publications listing a grant has dropped. Note that this should be because the number of publications overall in the unrelated group has dropped. However, we note that the drop in publications is 10% while the decline in publications with at least one grant is 15%. This difference suggests that publications listing grants, which should have more funding, are disproportionately COVID-19 related papers. To further investigate this aspect, we look at whether the grant was old (pre-2019) or appeared for the first time in or after 2019. It stands to reason that an old grant (pre-2019) would not have been granted for a project dealing with the pandemic. Hence we would expect that COVID-19 related MeSH terms to have a lower proportion of old grants than the unrelated group. In models (6) in Table 4 we show that the number of old grants for the unrelated group drops by 13%. At the same time, the number of papers listing old grants (i.e., pre-2019) among the most related group increased by a factor of 3.1. Overall, these results suggest that COVID-19 related research has been funded largely by pre-existing grants, even though a specific mandate tied to the grants for this use is unlikely.
The scientific community has swiftly reallocated research efforts to cope with the COVID-19 pandemic, mobilizing knowledge across disciplines to find innovative solutions in record time. We document this both in terms of changing trends in the biomedical scientific output and the usage of MeSH terms by the scientific community. The flip side of this sudden and energetic prioritization of effort to fight COVID-19 has been a sudden contraction of scientific production in other relevant research areas. All in all, we find strong support to the hypotheses that the COVID-19 crisis has induced a sudden increase of research output in COVID-19 related areas of biomedical research. Conversely, research in areas not related to COVID-19 has experienced a significant drop in overall publishing rates and funding.
Our paper contributes to the literature on the impact of COVID-19 on scientific research: we corroborate previous findings about the surge of COVID-19 related publications [ 1 – 3 ], partially displacing research in COVID-19 unrelated fields of research [ 4 , 14 ], particularly research related to clinical trials [ 5 – 7 ]. The drop in trial research might have severe consequences for patients affected by life-threatening diseases since it will delay access to new and better treatments. We also confirm the impact of COVID-19 on open access publication output [ 1 ]; also, this is milder than traditional outlets. On top of this, we provide more robust evidence on the impact weighted effect of COVID-19 and grant financed research, highlighting the strong displacement effect of COVID-19 on the allocation of financial resources [ 15 ]. We document a substantial change in the usage patterns of MeSH terms, suggesting that there has been a reconfiguration in the way research terms are being combined. MeSH terms highly related to COVID-19 were peripheral in the MeSH usage networks before the pandemic but have become central since 2020. We conclude that the usage patterns have changed, with COVID-19 related MeSH terms occupying a much more prominent role in 2020 than they did in the previous years.
We also contribute to the literature by estimating the effect of COVID-19 on biomedical research in a natural experiment framework, isolating the specific effects of the COVID-19 pandemic on the biomedical scientific landscape. This is crucial to identify areas of public intervention to sustain areas of biomedical research which have been neglected during the COVID-19 crisis. Moreover, the exploratory analysis on the changes in usage patterns of MeSH terms, points to an increase in the importance of covid-related topics in the broader biomedical research landscape.
Our results provide compelling evidence that research related to COVID-19 has indeed displaced scientific production in other biomedical fields of research not related to COVID-19, with a significant drop in (impact weighted) scientific output related to non-COVID-19 and a marked reduction of financial support for publications not related to COVID-19 [ 4 , 5 , 16 ]. The displacement effect is persistent to the end of 2020. As vaccination progresses, we highlight the urgent need for science policy to re-balance support for research activity that was put on pause because of the COVID-19 pandemic.
We find that COVID-19 dramatically impacted clinical research. Reactivation of clinical trials activities that have been postponed or suspended for reasons related to COVID-19 is a priority that should be considered in the national vaccination plans. Moreover, since grants have been diverted and financial incentives have been targeted to sustain COVID-19 research leading to an excessive entry in COVID-19-related clinical trials and the ‘covidisation’ of research, there is a need to reorient incentives to basic research and otherwise neglected or temporally abandoned areas of biomedical research. Without dedicated support in the recovery plans for neglected research of the COVID-19 era, there is a risk that more medical needs will be unmet in the future, possibly exacerbating the shortage of scientific research for orphan and neglected diseases, which do not belong to COVID-19-related research areas.
Limitations
Our empirical approach has some limits. First, we proxy MeSH term usage via search terms using the PubMed EUtilities API. This means that the accuracy of the MeSH term we assign to a given paper is not fully validated. More time is needed for the completion of manually annotated MeSH terms. Second, the timing of publication is not the moment the research has been carried out. There is a lead time between inception, analysis, write-up, review, revision, and final publication. This delay varies across disciplines. Nevertheless, given that the surge in publications happens around the alleged event date, January 2020, we are confident that the publication date is a reasonable yet imperfect estimate of the timing of the research. Third, several journals have publicly declared to fast-track COVID-19 research. This discrepancy in the speed of publication of COVID-19 related research and other research could affect our results. Specifically, a surge or displacement could be overestimated due to a lag in the publication of COVID-19 unrelated research. We alleviate this bias by estimating the effect considering a considerable time after the event (January 2020 to December 2020). Forth, on the one hand, clinical Trials may lead to multiple publications. Therefore we might overestimate the impact of COVID-19 on the number of clinical trials. On the other hand, COVID-19 publications on clinical trials lag behind, so the number of papers related COVID-19 trials is likely underestimated. Therefore, we note that the focus of this paper is scientific publications on clinical trials rather than on actual clinical trials. Fifth, regarding grants, unfortunately, there is no unique centralized repository mapping grant numbers to years, so we have to proxy old grants with grants that appeared in publications from 2010 to 2018. Besides, grant numbers are free-form entries, meaning that PubMed has no validation step to disambiguate or verify that the grant number has been entered correctly. This has the effect of classifying a grant as new even though it has appeared under a different name. We mitigate this problem by using a long period to collect grant numbers and catch many spellings of the same grant, thereby reducing the likelihood of miss-identifying a grant as new when it existed before. Still, unless unique identifiers are widely used, there is no way to verify this.
So far, there is no conclusive evidence on whether entry into COVID-19 has been excessive. However, there is a growing consensus that COVID-19 has displaced, at least temporally, scientific research in COVID-19 unrelated biomedical research areas. Even though it is certainly expected that more attention will be devoted to the emergency during a pandemic, the displacement of biomedical research in other fields is concerning. Future research is needed to investigate the long-run structural consequences of the COVID-19 crisis on biomedical research.
Supporting information
S1 table. common trend assumption (cta) regression table..
Full regression table with all controls and interactions.
https://doi.org/10.1371/journal.pone.0263001.s001
- View Article
- Google Scholar
- PubMed/NCBI
- 8. Brown A, Edwin E, Fagg J. Evaluate Pharma 2021 Preview; 2020. https://www.evaluate.com/thought-leadership/vantage/evaluate-vantage-2021-preview .
- 15. Hook D, Porter S. The COVID Brain Drain; 2020. https://www.natureindex.com/news-blog/covid-brain-drain-scientific-research .
- 17. Page L, Brin S, Motwani R, Winograd T. The PageRank citation ranking: Bringing order to the web. Stanford InfoLab; 1999.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- News & Events
- FDA Newsroom
- Press Announcements
FDA Approves and Authorizes Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants
FDA News Release
Today, the U.S. Food and Drug Administration approved and granted emergency use authorization (EUA) for updated mRNA COVID-19 vaccines (2024-2025 formula) to include a monovalent (single) component that corresponds to the Omicron variant KP.2 strain of SARS-CoV-2. The mRNA COVID-19 vaccines have been updated with this formula to more closely target currently circulating variants and provide better protection against serious consequences of COVID-19, including hospitalization and death. Today’s actions relate to updated mRNA COVID-19 vaccines manufactured by ModernaTX Inc. and Pfizer Inc.
In early June, the FDA advised manufacturers of licensed and authorized COVID-19 vaccines that the COVID-19 vaccines (2024-2025 formula) should be monovalent JN.1 vaccines. Based on the further evolution of SARS-CoV-2 and a rise in cases of COVID-19, the agency subsequently determined and advised manufacturers that the preferred JN.1-lineage for the COVID-19 vaccines (2024-2025 formula) is the KP.2 strain, if feasible.
“Vaccination continues to be the cornerstone of COVID-19 prevention,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research. “These updated vaccines meet the agency’s rigorous, scientific standards for safety, effectiveness, and manufacturing quality. Given waning immunity of the population from previous exposure to the virus and from prior vaccination, we strongly encourage those who are eligible to consider receiving an updated COVID-19 vaccine to provide better protection against currently circulating variants.”
The updated mRNA COVID-19 vaccines include Comirnaty and Spikevax, both of which are approved for individuals 12 years of age and older, and the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine, both of which are authorized for emergency use for individuals 6 months through 11 years of age.
What You Need to Know
- Unvaccinated individuals 6 months through 4 years of age are eligible to receive three doses of the updated, authorized Pfizer-BioNTech COVID-19 Vaccine or two doses of the updated, authorized Moderna COVID-19 Vaccine.
- Individuals 6 months through 4 years of age who have previously been vaccinated against COVID-19 are eligible to receive one or two doses of the updated, authorized Moderna or Pfizer-BioNTech COVID-19 vaccines (timing and number of doses to administer depends on the previous COVID-19 vaccine received).
- Individuals 5 years through 11 years of age regardless of previous vaccination are eligible to receive a single dose of the updated, authorized Moderna or Pfizer-BioNTech COVID-19 vaccines; if previously vaccinated, the dose is administered at least 2 months after the last dose of any COVID-19 vaccine.
- Individuals 12 years of age and older are eligible to receive a single dose of the updated, approved Comirnaty or the updated, approved Spikevax; if previously vaccinated, the dose is administered at least 2 months since the last dose of any COVID-19 vaccine.
- Additional doses are authorized for certain immunocompromised individuals ages 6 months through 11 years of age as described in the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine fact sheets.
Individuals who receive an updated mRNA COVID-19 vaccine may experience similar side effects as those reported by individuals who previously received mRNA COVID-19 vaccines and as described in the respective prescribing information or fact sheets. The updated vaccines are expected to provide protection against COVID-19 caused by the currently circulating variants. Barring the emergence of a markedly more infectious variant of SARS-CoV-2, the FDA anticipates that the composition of COVID-19 vaccines will need to be assessed annually, as occurs for seasonal influenza vaccines.
For today’s approvals and authorizations of the mRNA COVID-19 vaccines, the FDA assessed manufacturing and nonclinical data to support the change to include the 2024-2025 formula in the mRNA COVID-19 vaccines. The updated mRNA vaccines are manufactured using a similar process as previous formulas of these vaccines. The mRNA COVID-19 vaccines have been administered to hundreds of millions of people in the U.S., and the benefits of these vaccines continue to outweigh their risks.
On an ongoing basis, the FDA will review any additional COVID-19 vaccine applications submitted to the agency and take appropriate regulatory action.
The approval of Comirnaty (COVID-19 Vaccine, mRNA) (2024-2025 Formula) was granted to BioNTech Manufacturing GmbH. The EUA amendment for the Pfizer-BioNTech COVID-19 Vaccine (2024-2025 Formula) was issued to Pfizer Inc.
The approval of Spikevax (COVID-19 Vaccine, mRNA) (2024-2025 Formula) was granted to ModernaTX Inc. and the EUA amendment for the Moderna COVID-19 Vaccine (2024-2025 Formula) was issued to ModernaTX Inc.
Related Information
- Comirnaty (COVID-19 Vaccine, mRNA) (2024-2025 Formula)
- Spikevax (COVID-19 Vaccine, mRNA) (2024-2025 Formula)
- Moderna COVID-19 Vaccine (2024-2025 Formula)
- Pfizer-BioNTech COVID-19 Vaccine (2024-2025 Formula)
- FDA Resources for the Fall Respiratory Illness Season
- Updated COVID-19 Vaccines for Use in the United States Beginning in Fall 2024
- June 5, 2024, Meeting of the Vaccines and Related Biological Products Advisory Committee
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, radiation-emitting electronic products, and for regulating tobacco products.

IMAGES
COMMENTS
of COVID-19 can explain 40% of the delayed graduation gap (as well as a substantial part of the gap for other outcomes) between lower- and higher-income students. To our knowledge, this is the rst paper to shed light on the e ects of COVID-19 on college students' experiences. The treatment e ects that we nd are large in economic terms.
The spread of the "Severe Acute Respiratory Coronavirus 2" (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [].The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [], causing massive economic strain ...
Transmission. The role of the Huanan Seafood Wholesale Market in propagating disease is unclear. Many initial COVID-19 cases were linked to this market suggesting that SARS-CoV-2 was transmitted from animals to humans .However, a genomic study has provided evidence that the virus was introduced from another, yet unknown location, into the market where it spread more rapidly, although human-to ...
There have been around 96,000 reported cases of coronavirus disease 2019 (COVID-2019) and 3300 reported deaths to date (05/03/2020). The disease is transmitted by inhalation or contact with infected droplets and the incubation period ranges from 2 to 14 d. The symptoms are usually fever, cough, sore throat, breathlessness, fatigue, malaise ...
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
A two-dose regimen of BNT162b2 (30 μg per dose, given 21 days apart) was found to be safe and 95% effective against Covid-19. The vaccine met both primary efficacy end points, with more than a 99 ...
Introduction. The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in over 192 million cases and 4.1 million deaths as of July 22, 2021. 1 This pandemic has brought along a massive burden in morbidity and mortality in the healthcare systems. Despite the implementation of stringent public health measures, there ...
in the wake of COVID-19, including unemployment, mental illness, and addiction; and, (3) the importance of moderating factors (e.g., age, race and ethnicity, gender, personality, family status, and culture) for which there are likely to be disparate COVID-19 impacts.
In this rapid living systematic evidence synthesis and meta-analysis, we searched EMBASE and the US National Institutes of Health's iSearch COVID-19 Portfolio, supplemented by manual searches of COVID-19-specific sources, until Dec 1, 2022, for studies that reported vaccine effectiveness immediately and at least 112 days after a primary vaccine series or at least 84 days after a booster dose.
The 25 most downloaded Nature Communications articles* on COVID-19 published in 2021 illustrate the collaborative efforts of the international community to combat the ongoing pandemic.These papers ...
The Johns Hopkins Coronavirus Resource Center has collected, verified, and published local, regional, national and international pandemic data since it launched in March 2020. From the beginning, the information has been freely available to all — researchers, institutions, the media, the public, and policymakers. As a result, the CRC and its data have been cited in many published research ...
The costs of COVID-19 in 3 stages The COVID-19 shock can be interpreted as a combination of supply and demand shocks (Baqaee and Farhi, 2020; Caballero and Simsek, 2020; Guerrieri et al., 2020).
rly-stage effects of COVID-19 on small business owners from April 2020 CPS microdata. I find that the number of working business owners plummeted from 15.0 million in Febru. ry 2020 to 11.7 million in April 2020 because of COVID-19 mandates and demand shifts. T. e loss of 3.3 million business owners (or 22 percent) was the largest drop on ...
Abstract. In early December 2019, an outbreak of coronavirus disease 2019 (COVID-19), caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), occurred in Wuhan City, Hubei Province, China. On January 30, 2020 the World Health Organization declared the outbreak as a Public Health Emergency of International Concern.
According to a study published in 2019, An-. Corona virus causes respiratory infection including pneumonia, cold, sneezing and coughing while in animal it causes. diarrhea and upper respiratory ...
1. INTRODUCTION. The COVID-19 pandemic has caused an unprecedented policy response from public health. officials and governments. As of September 16, 2021, approximately 4.7 million deaths. worldwide and 669,000 deaths in the United States were attributed to COVID-19 (Johns Hopkins. Coronavirus Resource Center 2022).
Detailed search strategy for gathering COVID-19 articles, updated October 9, 2020 [PDF - 135 KB] The CDC Database of COVID-19 Research Articles is now a part of the WHO COVID-19 database. Our new search results are now being sent to the WHO COVID-19 Database to make it easier for them to be searched, downloaded, and used by researchers ...
We designed a time-use survey to study whether and how the transition towards "work-. from-home" arrangements (WFH), and away from the office, caused by the COVID-19. pandemic affected the use of time of knowledge workers. Specifically, this study. addresses the following research questions:
DRAFT Prepared by the SAGE Working Group on COVID-19 Vaccines 22 December 2020 5 . even death. Several occupational risks for health workers emerged or were amplified by the COVID-19 response, including (1) occupational infections with COVID-19, (2) skin disorders and heat stress
WHO has described four levels of COVID-19 transmission with varying public health and social measures depending on the local evolution of the COVID-19 pandemic. For more details, please see 'Subject in Focus'. Figure 1. Countries, territories or areas with reported confirmed cases of COVID-19, 1 April 2020 SITUATION IN NUMBERS Globally
The COVID-19 outbreak has posed an unprecedented challenge to humanity and science. On the one side, public and private incentives have been put in place to promptly allocate resources toward research areas strictly related to the COVID-19 emergency. However, research in many fields not directly related to the pandemic has been displaced. In this paper, we assess the impact of COVID-19 on ...
The novel coronavirus disease 2019 (COVID-19, caused by SARS-CoV-2) has spread globally since its first report in Wuhan, China on December 31, 2019. On January 30, the Philippines reported its first two imported cases of COVID-19 in a couple from Wuhan. One of them died on February 1st, becoming the first COVID-19 death outside China.
Abstract: The study of online consumer behavior is especially pertinent today given the development of the COVID-19 epidemic and the growing significance of e-commerce. The goal of this study was to provide a systematic framework for evaluating the linkages and degree of influence of the elements influencing online consumers' purchase behavior against the backdrop of the COVID-19 epidemic. The ...
The updated mRNA COVID-19 vaccines include Comirnaty and Spikevax, both of which are approved for individuals 12 years of age and older, and the Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID ...