
Researchers a step closer to a cure for HIV
By 2030, the World Health Organization (WHO), the Global Fund and UNAIDS are hoping to end the human immunodeficiency virus (HIV) and AIDS epidemic. An international team of researchers led by Eric Arts, professor at the Schulich School of Medicine & Dentistry, and Jamie Mann, senior lecturer at the University of Bristol (U.K.), has brought us another step closer to meeting this goal, by finding an effective and affordable targeted treatment strategy for an HIV cure.
In a first, the study published in the journal Emerging Microbes and Infections demonstrated the team's patented therapeutic candidate, an HIV-virus-like-particle (HLP), is 100 times more effective than other candidate HIV cure therapeutics for people living with chronic HIV on combined antiretroviral therapy (cART). If successful in clinical trials, HLP could be used by millions of people living around the world to free them of HIV. This study was done using blood samples from people living with chronic HIV.
HLPs are dead HIV particles hosting a comprehensive set of HIV proteins that increase immune responses without infecting a person. When compared with other potential cure approaches, HLP is an affordable biotherapeutic and can be administered by intramuscular injection -- similar to the seasonal flu vaccine.
"The development of this HIV cure was ten years in the making but with strong support from our collaborators in the U.S., Canada and Uganda, we have observed a striking ability of HLP to drive out the last remnants of HIV-1, which we hope will provide an affordable cure for all," said Arts, who is a Canada Research Chair in HIV Pathogenesis and Viral Control. "To live HIV-free is a goal for the 39 million infected. It is also the priority of the UN and WHO to end the HIV pandemic by 2030."
HIV is a retrovirus that attacks the body's immune system and if left untreated, can lead to acquired immunodeficiency syndrome (AIDS). The virus weakens a person's immune system by destroying CD4-T white blood cells, which are tasked with helping the immune system fight infections. Approximately 95 per cent of people living with HIV have chronic HIV -- where the virus is slowly causing a slow destruction of the patients' immune systems when they initiated lifelong cART.
While cART is effective at treating HIV, it has been unable to completely eliminate the virus from the body. This is because of the virus' ability to create a "latent reservoir" -- where it hides dormant inside of cells, safe from detection. Using blood samples from 32 participants living with chronic HIV from the U.S., Uganda and Canada, who were on stable cART for a median of approximately 13 years, researchers found that HLP was able to specifically target just the immune cells containing latent HIV reservoir and purge these cells of their HIV, a critical step towards an HIV-1 cure. An HIV cure is typically described as therapy and approach that eliminates all HIV without the need of continuous antiretroviral therapy.
"Over time, the virus grows more diverse within a single individual that is not on treatment which makes it more difficult to target," said co-lead author Ryan Ho, master's student in the department of microbiology and immunology. "This formulation we've crafted covers the theoretical diversity so it can reach the HIV-1 in all those people living with HIV."
Minh Ha Ngo, lead author and postdoctoral scholar in the department of microbiology and immunology, says one concern expressed among people living with HIV for years is that continued use of cART could lead to the virus becoming unreachable and unable to be eliminated. The results of this study, by contrast, demonstrate that combining HLP with cART is still able to trigger the latent reservoir, even in chronic cases. If these dormant latent reservoirs can be awakened, then they can be eliminated from the body.
"Owing to its high mutation rate, HIV exhibits remarkable genetic diversity, resulting in different viral subtypes; some of which predominate in particular regions of the globe," said Mann. "We were excited to see preliminary evidence that our HLP cure therapy reverses latency irrespective of the subtype of the individual's infection. Whilst this needs to be explored further, it hints at the global applicability of our approach."
In the future, researchers plan to test HLP on a larger representative HIV cohort with subtype C infections, which includes people living in South Africa, Ethiopia, Vietnam and India. This would help determine if the treatment strategy is effective for most people living with acute and chronic HIV.
Current studies involve confirming a lack of toxicity in preparation for human clinical trials.
These studies will be made possible with the advanced Pathogen Research Centre at Schulich Medicine & Dentistry. This study was conducted in collaboration with University of Bristol, University of Toronto, Case Western Reserve University, Rakai Health Sciences Program, Johns Hopkins University School of Medicine and the U.S. National Institutes of Health.
The study was funded by the American Foundation for AIDS Research, and by the Canadian Institutes of Health Research, U.S. National Institute of Allergy and Infectious Diseases; part of the National Institutes of Health, and the Canada Research Chairs Program.
- HIV and AIDS
- Infectious Diseases
- Sexual Health
- Diseases and Conditions
- Chronic Illness
- Immune System
- Antiretroviral drug
- Ionizing radiation
Story Source:
Materials provided by University of Western Ontario . Original written by Cynthia Fazio. Note: Content may be edited for style and length.
Journal Reference :
- Minh Ha Ngo, Joshua Pankrac, Ryan C. Y. Ho, Emmanuel Ndashimye, Rahul Pawa, Renata Ceccacci, Tsigereda Biru, Abayomi S. Olabode, Katja Klein, Yue Li, Colin Kovacs, Robert Assad, Jeffrey M. Jacobson, David H. Canaday, Stephen Tomusange, Samiri Jamiru, Aggrey Anok, Taddeo Kityamuweesi, Paul Buule, Ronald M. Galiwango, Steven J. Reynolds, Thomas C. Quinn, Andrew D. Redd, Jessica L. Prodger, Jamie F. S. Mann, Eric J. Arts. Effective and targeted latency reversal in CD4 + T cells from individuals on long term combined antiretroviral therapy initiated during chronic HIV-1 infection . Emerging Microbes & Infections , 2024; 13 (1) DOI: 10.1080/22221751.2024.2327371
Cite This Page :
Explore More
- Treating Cataracts and Other Eye Conditions
- Early Arrival of Palaeolithic People On Cyprus
- Networks Regulating Gene Function in Human Brain
- Birth of Universe's Earliest Galaxies
- Why the Brain Can Robustly Recognize B&W Images
- Birth Control Pill for Men?
- Intriguing World Sized Between Earth, Venus
- Billions of Orphan Stars Revealed
- Massive Catalog of Strange Worlds
- Mental Disorders May Spread Thru Social Networks
Trending Topics
Strange & offbeat.
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
Emily Mullin
There’s New Hope for an HIV Vaccine
Since it was first identified in 1983, HIV has infected more than 85 million people and caused some 40 million deaths worldwide.
While medication known as pre-exposure prophylaxis , or PrEP, can significantly reduce the risk of getting HIV, it has to be taken every day to be effective. A vaccine to provide lasting protection has eluded researchers for decades. Now, there may finally be a viable strategy for making one.
An experimental vaccine developed at Duke University triggered an elusive type of broadly neutralizing antibody in a small group of people enrolled in a 2019 clinical trial. The findings were published today in the scientific journal Cell .
“This is one of the most pivotal studies in the HIV vaccine field to date,” says Glenda Gray, an HIV expert and the president and CEO of the South African Medical Research Council, who was not involved in the study.
A few years ago, a team from Scripps Research and the International AIDS Vaccine Initiative (IAVI) showed that it was possible to stimulate the precursor cells needed to make these rare antibodies in people. The Duke study goes a step further to generate these antibodies, albeit at low levels.
“This is a scientific feat and gives the field great hope that one can construct an HIV vaccine regimen that directs the immune response along a path that is required for protection,” Gray says.
Vaccines work by training the immune system to recognize a virus or other pathogen. They introduce something that looks like the virus—a piece of it, for example, or a weakened version of it—and by doing so, spur the body’s B cells into producing protective antibodies against it. Those antibodies stick around so that when a person later encounters the real virus, the immune system remembers and is poised to attack.
While researchers were able to produce Covid-19 vaccines in a matter of months, creating a vaccine against HIV has proven much more challenging. The problem is the unique nature of the virus. HIV mutates rapidly, meaning it can quickly outmaneuver immune defenses. It also integrates into the human genome within a few days of exposure, hiding out from the immune system.
“Parts of the virus look like our own cells, and we don’t like to make antibodies against our own selves,” says Barton Haynes, director of the Duke Human Vaccine Institute and one of the authors on the paper.
The particular antibodies that researchers are interested in are known as broadly neutralizing antibodies, which can recognize and block different versions of the virus. Because of HIV’s shape-shifting nature, there are two main types of HIV and each has several strains. An effective vaccine will need to target many of them.
Some HIV-infected individuals generate broadly neutralizing antibodies, although it often takes years of living with HIV to do so, Haynes says. Even then, people don’t make enough of them to fight off the virus. These special antibodies are made by unusual B cells that are loaded with mutations they’ve acquired over time in reaction to the virus changing inside the body. “These are weird antibodies,” Haynes says. “The body doesn’t make them easily.”

By Carlton Reid

By Emily Mullin

By Andy Greenberg
By Scott Gilbertson
Haynes and his colleagues aimed to speed up that process in healthy, HIV-negative people. Their vaccine uses synthetic molecules that mimic a part of HIV’s outer coat, or envelope, called the membrane proximal external region. This area remains stable even as the virus mutates. Antibodies against this region can block many circulating strains of HIV.
The trial enrolled 20 healthy participants who were HIV-negative. Of those, 15 people received two of four planned doses of the investigational vaccine, and five received three doses. The trial was halted when one participant experienced an allergic reaction that was not life-threatening. The team found that the reaction was likely due to an additive in the vaccine, which they plan to remove in future testing.
Still, they found that two doses of the vaccine were enough to induce low levels of broadly neutralizing antibodies within a few weeks. Notably, B cells seemed to remain in a state of development to allow them to continue acquiring mutations, so they could evolve along with the virus. Researchers tested the antibodies on HIV samples in the lab and found that they were able to neutralize between 15 and 35 percent of them.
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but that challenges remain. “It outlines a path for vaccine development, but there’s a lot of work that needs to be done,” he says.
For one, he says, a vaccine would need to generate antibody levels that are significantly higher and able to neutralize with greater efficacy. He also says a one-dose vaccine would be ideal. “If you’re ever going to have a vaccine that’s helpful to the world, you’re going to need one dose,” he says.
Targeting more regions of the virus envelope could produce a more robust response. Haynes says the next step is designing a vaccine with at least three components, all aimed at distinct regions of the virus. The goal is to guide the B cells to become much stronger neutralizers, Haynes says. “We’re going to move forward and build on what we have learned.”
You Might Also Like …
In your inbox: Will Knight's Fast Forward explores advances in AI
Indian voters are being bombarded with millions of deepfakes
They bought tablets in prison —and found a broken promise
The one thing that’s holding back the heat pump
It's always sunny: Here are the best sunglasses for every adventure

Carl Zimmer
Matt Reynolds
Beth Mole, Ars Technica
After decades of failures, researchers have renewed hopes for an effective HIV vaccine
The world needs an HIV vaccine if it ever hopes to beat a virus that still infects over 1 million people a year and contributes to hundreds of thousands of deaths.
Despite 20 years of failures in major HIV vaccine trials — four this decade alone — researchers say recent scientific advances have likely, hopefully, put them on the right track to develop a highly effective vaccine against the insidious virus.
But probably not until the 2030s.
“An effective vaccine is really the only way to provide long-term immunity against HIV, and that’s what we need,” Dr. Julie McElrath, the director of the vaccine and infectious disease division at the Fred Hutchinson Cancer Center in Seattle, said Monday at the Conference on Retroviruses and Opportunistic Infections in Denver.
All current HIV vaccine action is in the laboratory, animal studies or very early human trials.
Researchers at the retrovirus conference presented favorable results from two HIV vaccine studies. One found that a modification to the simian version of HIV spurred production of what are known as broadly neutralizing antibodies against the virus in monkeys. Another showed promise in the effort to coax the immune system’s B cells to make the powerful antibodies in humans.
“These trials illustrate as a proof of concept that we can train the immune system. But we need to further optimize it and test it in clinical trials,” Karlijn van der Straten, a Ph.D. student at the Academic Medical Center at Amsterdam University, who presented the human study, said at a news conference Monday.
Still, the scrappy scientists in this field face a towering challenge. HIV is perhaps the most complex pathogen ever known.
“The whole field has learned from the past,” said William Schief, who leads Moderna’s HIV vaccine efforts. “We’ve learned strategies that don’t work.”
The cost has already been immense. Nearly $17 billion was spent worldwide on HIV -vaccine research from 2000 to 2021. Nearly $1 billion more is spent annually, according to the Joint United Nations Program on HIV/AIDS and the nonprofit HIV group AVAC.
“Maintaining the funding for HIV vaccines right now is really important,” said Dr. Nina Russell, who directs HIV research at the Bill & Melinda Gates Foundation. She pointed to the field’s own “progress and the excitement” and to how “HIV vaccine science and scientists continue to drive innovation and science that benefits other infectious diseases and global health in general.”
Case in point: Covid. Thanks to HIV research, the mRNA vaccine technology was already available in 2020 to speed a coronavirus vaccine to market.
Why the HIV vaccine efficacy trials failed
In strong contrast to Covid, the HIV vaccine endeavor has spanned four decades. Only one of the nine HIV vaccine trials have shown efficacy: a trial conducted in Thailand and published in 2009 that reported a modest 31% reduction in HIV risk.
HIV vaccine researchers subsequently spent years seeking to retool and improve that vaccine strategy, leading to a series of trials that launched in the late 2010s — only to fail.
Researchers have concluded those latest trials were doomed because, aside from prompting an anti-HIV response based in immune cells, they only drove the immune system to produce what are known as non-neutralizing antibodies. Those weapons just weren’t strong enough for such a fearsome foe.
Preventing HIV through vaccination remains a daunting challenge because the immune system doesn’t naturally mount an effective defense against the virus, as it does with so many other vaccine-preventable infections, including Covid. An HIV vaccine must coax from the body a supercharged immune response with no natural equivalent.
That path to victory is based on a crucial caveat: A small proportion of people with HIV do produce what are known as broadly neutralizing antibodies against the virus. They attack HIV in multiple ways and can neutralize a swath of variants of the virus.
Those antibodies don’t do much apparent good for people who develop them naturally, because they typically don’t arise until years into infection. HIV establishes a permanent reservoir in the body within about a week after infection, one that their immune response can’t eliminate. So HIV-positive people with such antibodies still require antiretroviral treatment to remain healthy.
Researchers believe that broadly neutralizing antibodies could prevent HIV from ever seeding an infection, provided the defense was ready in advance of exposure. A pair of major efficacy trials, published in 2021 , demonstrated that infusions of cloned versions of one such antibody did, indeed, protect people who were exposed to certain HIV strains that are susceptible to that antibody.
However, globally, those particular strains of the virus comprise only a small subset of all circulating HIV. That means researchers can’t simply prompt a vaccine to produce that one antibody and expect it to be effective. Importantly, from this study they got a sense of what antibody level would be required to prevent infection.
It’s a high benchmark, but at least investigators now have a clearer sense of the challenge before them.
Also frustrating the HIV vaccine quest is that the virus mutates like mad. Whatever spot on the surface of the virus that antibodies target might be prone to change through mutation, thus allowing the virus to evade their attack. Consequently, researchers search for targets on the virus’ surface that aren’t highly subject to mutation.
Experts also believe warding off the mutation threat will require targeting multiple sites on the virus. So researchers are seeking to develop a portfolio of immune system prompts that would spur production of an array of broadly neutralizing antibodies.
Prompting the development of such antibodies requires a complex, step-by step process of coaxing the infection-fighting B cells, getting them to multiply and then guiding their maturation into potent broadly neutralizing antibody-producing factories.
HIV vaccine development ‘in a better place’
Dr. Carl Dieffenbach, the head of the AIDS division at the National Institute of Allergy and Infectious Diseases, said numerous recent technological advances — including mRNA, better animal models of HIV infection and high-tech imaging technology — have improved researchers’ precision in designing, and speed in producing, new proteins to spur anti-HIV immune responses.
Global collaboration among major players is also flourishing, researchers said. There are several early-stage human clinical trials of HIV-vaccine components underway.
Three mRNA- based early human trials of such components have been launched since 2022. Among them, they have been led or otherwise funded by the global vaccine research nonprofit group IAVI, Fred Hutch, Moderna, Scripps Research, the Gates Foundation, the National Institutes of Health, the U.S. Agency for International Development, and university teams. More such trials are in the works.
On Friday, Science magazine reported concerning recent findings that among the three mRNA trials, a substantial proportion of participants — 7% to 18%, IAVI said in a statement — experienced skin-related symptoms following injections, including hives, itching and welts.
IAVI said in its statement that it and partners are investigating the HIV trials’ skin-related outcomes, most of which were “mild or moderate and managed with simple allergy medications.”
Researchers have shown success in one of those mRNA trials in executing a particular step in the B-cell cultivation process.
That vaccine component also generated “helper” CD4 cells primed to combat HIV. The immune cells are expected to operate like an orchestra conductor for the immune system, coordinating a response by sending instructions to B cells and scaling up other facets of an assault on HIV.
A complementary strategy under investigation seeks to promote the development of “killer” CD8 cells that might be primed to kill off any immune cells that the antibodies failed to save from infection.
Crucially, investigators believe they are now much better able to discern top vaccine component candidates from the duds. They plan to spend the coming years developing such components so that when they do assemble the most promising among them into a multi-pronged vaccine, they can be much more confident of ultimate success in a trial.
“An HIV vaccine could end HIV,” McElrath said at the Denver conference. “So I say, ‘Let’s just get on with it.”
Dr. Mark Feinberg, president and CEO of IAVI, suggested that the first trial to test effectiveness of the vaccine might not launch until 2030 or later.
Even so, he was bullish.
“The field of HIV vaccine development is in a better place now than it’s ever been,” he said.
Benjamin Ryan is independent journalist specializing in science and LGBTQ coverage. He contributes to NBC News, The New York Times, The Guardian and Thomson Reuters Foundation and has also written for The Washington Post, The Nation, The Atlantic and New York.
- What Are HIV and AIDS?
- How Is HIV Transmitted?
- Who Is at Risk for HIV?
- Symptoms of HIV
- U.S. Statistics
- Impact on Racial and Ethnic Minorities
- Global Statistics
- HIV and AIDS Timeline
- In Memoriam
- Supporting Someone Living with HIV
- Standing Up to Stigma
- Getting Involved
- HIV Treatment as Prevention
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Preventing Sexual Transmission of HIV
- Alcohol and HIV Risk
- Substance Use and HIV Risk
- Preventing Perinatal Transmission of HIV
- HIV Vaccines
- Long-acting HIV Prevention Tools
- Microbicides
- Who Should Get Tested?
- HIV Testing Locations
- HIV Testing Overview
- Understanding Your HIV Test Results
- Living with HIV
- Talking About Your HIV Status
- Locate an HIV Care Provider
- Types of Providers
- Take Charge of Your Care
- What to Expect at Your First HIV Care Visit
- Making Care Work for You
- Seeing Your Health Care Provider
- HIV Lab Tests and Results
- Returning to Care
- HIV Treatment Overview
- Viral Suppression and Undetectable Viral Load
- Taking Your HIV Medicine as Prescribed
- Tips on Taking Your HIV Medication Every Day
- Paying for HIV Care and Treatment
- Other Health Issues of Special Concern for People Living with HIV
- Alcohol and Drug Use
- Coronavirus (COVID-19) and People with HIV
- Hepatitis B & C
- Vaccines and People with HIV
- Flu and People with HIV
- Mental Health
- Mpox and People with HIV
- Opportunistic Infections
- Sexually Transmitted Infections
- Syphilis and People with HIV
- HIV and Women's Health Issues
- Aging with HIV
- Emergencies and Disasters and HIV
- Employment and Health
- Exercise and Physical Activity
- Food Safety and Nutrition
- Housing and Health
- Traveling Outside the U.S.
- Civil Rights
- Workplace Rights
- Limits on Confidentiality
- National HIV/AIDS Strategy (2022-2025)
- Implementing the National HIV/AIDS Strategy
- Prior National HIV/AIDS Strategies (2010-2021)
- Key Strategies
- Priority Jurisdictions
- HHS Agencies Involved
- Learn More About EHE
- Ready, Set, PrEP
- Ready, Set, PrEP Pharmacies
- Ready, Set, PrEP Resources
- AHEAD: America’s HIV Epidemic Analysis Dashboard
- HIV Prevention Activities
- HIV Testing Activities
- HIV Care and Treatment Activities
- HIV Research Activities
- Activities Combating HIV Stigma and Discrimination
- The Affordable Care Act and HIV/AIDS
- HIV Care Continuum
- Syringe Services Programs
- Finding Federal Funding for HIV Programs
- Fund Activities
- The Fund in Action
- About PACHA
- Members & Staff
- Subcommittees
- Prior PACHA Meetings and Recommendations
- I Am a Work of Art Campaign
- Awareness Campaigns
- Global HIV/AIDS Overview
- U.S. Government Global HIV/AIDS Activities
- U.S. Government Global-Domestic Bidirectional HIV Work
- Global HIV/AIDS Organizations
- National Black HIV/AIDS Awareness Day February 7
- HIV Is Not A Crime Awareness Day February 28
- National Women and Girls HIV/AIDS Awareness Day March 10
- National Native HIV/AIDS Awareness Day March 20
- National Youth HIV & AIDS Awareness Day April 10
- HIV Vaccine Awareness Day May 18
- National Asian & Pacific Islander HIV/AIDS Awareness Day May 19
- HIV Long-Term Survivors Awareness Day June 5
- National HIV Testing Day June 27
- Zero HIV Stigma July 21
- Southern HIV/AIDS Awareness Day August 20
- National Faith HIV/AIDS Awareness Day August 27
- National African Immigrants and Refugee HIV/AIDS and Hepatitis Awareness Day September 9
- National HIV/AIDS and Aging Awareness Day September 18
- National Gay Men's HIV/AIDS Awareness Day September 27
- National Latinx AIDS Awareness Day October 15
- World AIDS Day December 1
- Event Planning Guide
- U.S. Conference on HIV/AIDS (USCHA)
- National Ryan White Conference on HIV Care & Treatment
- AIDS 2020 (23rd International AIDS Conference Virtual)
Want to stay abreast of changes in prevention, care, treatment or research or other public health arenas that affect our collective response to the HIV epidemic? Or are you new to this field?
HIV.gov curates learning opportunities for you, and the people you serve and collaborate with.
Stay up to date with the webinars, Twitter chats, conferences and more in this section.
HIV Treatment Research and Key Takeaways: Dr. Dieffenbach’s Final Update from CROI 2024
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on Email
On Wednesday as the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) was winding down, HIV.gov spoke with NIH’s Dr. Carl Dieffenbach about highlights of long-acting HIV treatment research discussed at the conference. Dr. Dieffenbach is the Director of the Division of AIDS at NIH’s National Institute of Allergy and Infectious Diseases . He spoke with Brian Minalga, MSW, Deputy Director of the NIH-supported Office of HIV/AIDS Network Coordination Exit Disclaimer . Watch our conversation with Dr. Dieffenbach below:
Research Suggests Possible Expanded Options for Long-Acting HIV Treatment
Dr. Dieffenbach highlighted findings from several clinical trials and a plenary session presented at CROI about current and future options for long-acting antiretroviral treatment (ART) for HIV.
First, he discussed a NIAID-supported randomized clinical trial that found that long-acting ART with cabotegravir and rilpivirine was superior in suppressing HIV replication compared to daily oral ART in adults who had been unable to maintain viral suppression through an oral daily regimen. The LATITUDE study Exit Disclaimer enrolled participants in 31 sites in the United States. Last month, the trial’s Data and Safety Monitoring Board conducted a planned review of interim data and recommended halting randomization and offering all eligible study participants long-acting ART based on its observed superior viral suppression of HIV. At CROI, study leaders reported that the interim analysis of data from 294 participants showed that the chance of experiencing unsuppressed HIV was 7% among people taking long-acting ART compared to 25% among those taking daily oral ART . The likelihood of discontinuing the assigned regimen due to adverse events or experiencing unsuppressed HIV was 10% among people taking long-acting ART compared to 26% among those taking daily ART. These findings were statistically significant. Dr. Dieffenbach observed that these results may support expanding the use of long-acting ART among a broader population. Read the study abstract Exit Disclaimer . Read more in this NIAID news release .
Another ongoing clinical trial reported initial findings on the safety of the same long-acting injectable treatment regimen for adolescents with HIV with a suppressed viral load. The NIH-supported MOCHA study Exit Disclaimer enrolled participants aged 12 to 17 who were virally suppressed in Botswana, South Africa, Thailand, Uganda, and the United States. In what he characterized as very encouraging results, Dr. Aditya Gaur of St. Jude Children's Research Hospital, one of the trial’s co-chairs, reported that after the first six months all participants remained virally suppressed, and the level of the ART in their systems was comparable to what has been shown as efficacious in adult studies of the same drug . He also reported that, while about one-third of the participants reported an injection-site reaction, there were no surprising or unanticipated adverse events. These data support the use of cabotegravir and rilpivirine in virally suppressed adolescents, according to Dr. Gaur and colleagues. Dr. Dieffenbach noted that NIH will continue to support safety and dosing studies to determine the proper doses for adolescents and that these studies could eventually expand access to this long-acting HIV treatment to more people. Read the abstract Exit Disclaimer . Read NIAID’s news release about the study .
In addition, Dr. Dieffenbach mentioned an industry-sponsored Phase 2 trial that presented 24-week results of an oral once-weekly investigational combination of two drugs ( islatravir and lenacapavir ). Researchers reported that the investigational combination maintained a high level of viral suppression among study participants and was well tolerated. The study will continue to gather data and suggests that a weekly oral HIV treatment regimen could someday be possible . Read the abstract Exit Disclaimer .
Finally, Dr. Dieffenbach discussed Wednesday’s plenary session by Dr. Charles Flexner of The Johns Hopkins University School of Medicine, which was titled “The End of Oral? How Long-Acting Formulations Are Changing the Management of Infectious Diseases.” In his big picture, future-focused presentation exploring long-acting drug delivery, Dr. Flexner observed that there is a need for HIV products with less frequent dosing, greater convenience, and greater likelihood of viral suppression, as well as for the prevention and treatment of other diseases, including tuberculosis, malaria, and viral hepatitis. He discussed recent advances in formulation science that are going to help make available better replacements for daily oral drugs for HIV and many other infectious diseases . Dr. Dieffenbach underscored Dr. Flexner’s point that these novel products must be developed with access and equity in mind so that people who need them, especially in resource-limited settings, can use them.
Key Takeaways
Reflecting on key takeaways from the entire conference, both Dr. Dieffenbach and Brian pointed to the importance of partnership between the HIV community and scientists in all aspects of HIV research , a theme also discussed in HIV.gov’s conversation with Dr. LaRon Nelson from the conference. In terms of research highlights, Dr. Dieffenbach pointed to the results reported from the IMPAACT P1115 study in which several children who started HIV treatment within hours of birth later surpassed a year of HIV remission after a treatment pause. ( See HIV.gov’s interview with Dr. Deborah Persaud about this study .) He also noted that the additional data accumulating on the effectiveness of Doxy-PEP is encouraging and will hopefully soon be reflected in clinical guidelines that help to reduce the incidence of syphilis, chlamydia, and gonorrhea in men who have sex with men and transgender women.
Catch Up on More HIV Research Updates
HIV.gov has shared other interviews from CROI 2024 with federal HIV leaders, participating researchers, and community members. You can find all of them on HIV.gov’s social media channels and recapped here on the blog this week and next week.
More than 3,600 HIV and infectious disease researchers from 73 countries gathered in Denver and virtually from March 3-6 this year for CROI, an annual scientific meeting on the latest research that can help accelerate global progress in the response to HIV and other infectious diseases, including STIs and viral hepatitis. Over 1,000 summaries of original research were presented. Visit the conference website Exit Disclaimer for more information. Session webcasts and more information will be published there for public access in 30 days.
Related HIV.gov Blogs
- CROI Conference on Retroviruses & Opportunistic Infections
- NIAID National Institute of Allergy & Infectious Diseases
- NIH National Institutes of Health
- Treatment HIV Treatment
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Springer Nature - PMC COVID-19 Collection

HIV/AIDS: Current Updates on the Disease, Treatment and Prevention
Praveen kumar gupta.
Department of Biotechnology, R.V College of Engineering, Bangalore, 560059 India
Apoorva Saxena
CCR5-delta 32 homozygous stem cell transplantation for HIV-infected individuals is being treated as a milestone in the global AIDS epidemic. Since 2008, when the second Berlin patient was cured from HIV after undergoing transplantation from a donor with delta-32 mutation, scientists are aiming for a long-term cure for the wider population. In 2019, a London patient became the second person to be free of HIV and came off the antiretroviral drugs completely. CCR5 gene is now being treated as a viable target for HIV treatment. It can be used in the treatment of HIV either through administration of drugs that bind to CCR5 and stop the receptor from working or through gene therapy to alter the CCR5 gene using CRISPR/Cas9 and prevent protein production. This review article aims to identify the obstacles and the need to overcome them in order to bridge the gap between current research and future potential cures for HIV.
Introduction
Human immunodeficiency virus or HIV is the cause of HIV infection that leads to the autoimmune disorder acquired immune deficiency syndrome (AIDS) [ 1 ] (Fig. 1 ). The major cause of spreading of HIV is through unprotected sex, during pregnancy from mother to foetus, through contaminated hypodermic needles and infected blood transfusions [ 1 ]. In the year 2016, an estimated 37 million people were living with HIV and 1 million deaths were reported. HIV/AIDS is a pandemic condition—an epidemic of diseases that spreads across large areas like multiple continents or even worldwide [ 1 ]. The first time AIDS was recognized was in the year 1981 by the United States Center for Disease Control and Prevention (CDC). Since the reported case of an individual who had successfully undergone a stem cell transplant from a person who showed a homozygous CCR5-delta 32 mutation, after receiving extensive high dose chemotherapy, there has been a greater interest in finding a potential cure.

Human Immunodeficiency Virus [ 5 ]
HIV is a type of retrovirus that adversely infects the immune system of a human, mainly targeting the CD4 + T-helper cells, accessory cells and the macrophages [ 2 ]. When it gains entry into the target cell, the viral genomic RNA undergoes a process of the reverse transcription with the help of reverse transcriptase enzyme and forms double stranded DNA (ds-DNA). This ds-DNA then gets integrated into the target cellular DNA with the help of enzyme integrase and other host co-factors [ 3 ]. The virus now can either become dormant or conceal itself and the target cell detection by the host immune system or it can get transcribed into new viral RNA and proteins that are released from the cell and begin the cycle again. HIV can be characterized into 2 major classes—HIV-1 and HIV-2. HIV-1, which is more virulent, infective and the major cause of HIV in humans, was discovered first and was initially referred to as HTLV-III or LAV [ 4 ] (Fig. 2 ). HIV-2 is less infective and far fewer people exposed to it are infected.

Structure of HIV-1 [ 8 ]
The crucial factor in gaining entry into target cell is through binding of HIV to the CD4 receptor present on the T-helper cells and to one of the chemokine receptors- either CCR5 or CXCR4 [ 6 , 7 ]. Binding to the co-receptor depends on the virus’s tropism which is the ability to bind to a specific receptor. Naturally, there are two types of tropic strains—R5 that bind to CCR5 and X4 which bind to CXCR4. Dual tropic strains are capable of binding to both. Of these two co-receptors, CCR5 is the prime receptor for virus’s entry into the target cell. R5-tropic strains prevail during early stages of infection, whereas the X4-tropic strains emerge later with disease progression. The envelope-like glycoprotein structure of HIV-1 is paramount in ensuring the viral entry into a target host cell [ 7 ]. This glycoprotein has 2 protein subunits: the gp41 (transmembrane) subunit and gp120 (external) subunit, which mimics a chemokine [ 6 , 7 ]. It does not manifest the unique structure of the chemokine but somehow manages to bind to both the co-receptors [ 6 ]. It forms a heterotrimeric complex wherein the gp120 subunit binds to the CD4 protein and specific co-receptor present on the target cell [ 6 ]. When this complex is formed, it triggers the release of a peptide which facilitates cell–cell fusion, that causes the viral membrane to fuse with the target cell membrane [ 6 ]. Binding to CD4 alone is not sufficient as it can result in gp120 shedding. So, it has to bind to the specific co-receptor for the fusion to proceed. The V1–V2 region of gp120 is recognized by the co-receptor, that influences which co-receptor will bind to the protein and is determined by degree of N-linked glycosylation and peptide composition. The highly variable V3 loop is the one that determines co-receptor specificity. The binding of gp120 glycoprotein to the CCR5 co-receptor is determined by two essential factors—the tyrosine-sulphated amino terminus of CCR5 receptor and following which there must be reciprocal action between the transmembrane domains of CCR5 and gp120 protein, i.e., inter-communication and synergy.
Antiretroviral Therapy
The usage of a combination of three or more antiretroviral drugs for suppression of the HIV infection is called antiretroviral therapy. Using multiple drugs in combination to increase the effectivity on various viral targets is called highly active antiretroviral therapy (HAART). It helps in maintaining the immune system to function, preventing HIV from developing resistance and other infections that potentially lead to death. The five classes of drugs used in combination to treat HIV infection are: entry inhibitors, nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, integrase inhibitors and protease inhibitors.
Zidovudine/ZVD (also called azidothymidine) is an extensively used antiretroviral medication [ 9 ]. It is a thymidine analogue and is dosed twice daily in combination with other antiretrovirals. Its function is to particularly inhibit the reverse transcriptase enzyme which is necessary for the production of ds-DNA.
Cellular enzymes are used in converting AZT into the 5′-triphosphate form. Research studies suggest that the termination of forming ds-DNA chains is a crucial factor that leads to an inhibitory effect.
Studies have also shown that at very high dosage of this drug, its triphosphate form may inhibit the DNA polymerase enzyme which is used for cell division by the uninfected cells and mitochondria for replication. It may lead to toxic but reversible effects on certain skeletal and the cardiac muscles, causing the condition of myositis [ 10 ]. However, zidovudine also shows greater affinity for the reverse transcriptase enzyme, which is around 100-fold. This selectivity has been proven by the cell's ability to quickly repair its DNA strands if broken by AZT during its formation, whereas the HIV virus will lack this ability (Fig. 3 ).

Structure of zidovudine [ 11 ]
Zidovudine is commonly used in combination with nucleotide reverse transcriptase inhibitor, non-nucleoside reverse transcriptase inhibitor, HIV integrase strand transfer inhibitor and protease inhibitor [ 9 ]. The combination of lamivudine and zidovudine is not recommended for non-pregnant HIV-infected adults and adolescents due to greater toxicity but is used as an alternative, though not a preferred one, in antiretroviral-naive pregnant women as an initial treatment [ 9 ]. However, for paediatric patients (neonates, infants and children of age 12 or less), zidovudine with lamivudine/emtricitabine is a preferred option. For adolescents greater than the age of 12, it is an alternative [ 9 ].
Zidovudine Administration and Pharmacokinetics
Administration and dosage.
It is usually administered orally or by continuous IV infusion, although not rapid infusion and IM injection [ 9 ] (Tables (Tables1, 1 , ,2). 2 ). The dosage for paediatric patients and adult patients depends on their body weight (Tables (Tables3, 3 , ,4 4 ).
Oral administration [ 9 ]
IV administration [ 9 ]
Dosage for paediatric patients [ 9 ]
Dosage for adult patients [ 9 ]
Administration
Zidovudine: 1 mg/kg every 4 h [ 9 ].
Pharmacokinetics
Pharmacokinetics gives a detailed view of the fate of drugs in the human system. It includes various components like absorption, distribution, excretion or elimination and metabolism (Tables (Tables5, 5 , ,6, 6 , ,7). 7 ). The stability of such retroviral drugs should also be taken into account for both oral and parenteral dosage forms (Table (Table8 8 ).
Absorption [ 9 ]
Distribution [ 9 ]
Elimination process [ 9 ]
Stability of antiretrovirals [ 9 ]
Contraindications [ 9 ]
- Zidovudine has a history of life-threatening hypersensitivity reactions like Stevens–Johnson syndrome and anaphylaxis to the drug or maybe due to some ingredient in the formulation.
- Lamivudine/zidovudine: hypersensitivity history.
- Abacavir/zidovudine/lamivudine: history of hypersensitivity to abacavir, zidovudine or lamivudine; hepatic impairments may be mild or severe.
CCR5 Gene Structure
C–C chemokine receptor type 5 (also called CCR5 or CD195) is a receptor for chemokines present on the white blood cells. The CCR5 gene in humans is located on the short arm (p) at position 21 on chromosome number 3 (Fig. 4 ). It is mainly expressed cells like T-cells, macrophages, microglia, dendritic cells and eosinophils and is found within a cluster of genes coding for some other receptors like XCR1, CCBP2, etc. [ 12 , 13 ]. The gene has two promoters, three exons and two introns. Pu or PR2, the upstream promoter, has a 1.9 kb region, 57 bp in length and precedes the exon 1 [ 12 ]. Exon 1, which is the start of the coding region, is followed by the first intron, 501 bp in length. The second exon 2 is intron-less. It is found as exon 2a, 235 bp in length, and exon 2b, 54 bp in length. Pd or PR1, the second promoter, accommodates the intron 1 and exon 2 regions [ 12 ]. A 1.9 kb length intron is located between exon 2 and exon 3. Exon 3 is also intron-less and consists of the full ORF of the CCR5 gene, 11 bp of the 5′ untranslated regions and the complete 3′ untranslated regions [ 12 ].

Location of CCR5 gene on chromosome 21 [ 14 ]
These two promoters are devoid of the consensus TATA and CCAAT sequences, although the Pd promoter has a non-consensus TATA sequence and have an unusually high content of pyrimidine in them [ 12 ]. The upstream Pu promoter was found to be weaker than the downstream Pd promoter which had exhibited up to fivefold greater activity. But these results were established as erroneous [ 13 ]. With the help of RT-PCR technique, it was later identified that the Pu promoter was used in stimulated T-cells and the Pd promoter was used in unstimulated primary T-cells [ 13 ]. The error resulted due to the use of transformed T-cells affecting the overall expression of CCR5 protein via the Pu promoter [ 13 ]. Results also showed that transcription of the CCR5 gene when controlled by the Pu promoter containing exon 1 resulted in CCR5A or B and when controlled by the Pd promoter resulted in truncated isoforms [ 13 ].
CCR5 Gene Expression Regulation
The expression of CCR5 gene is regulated at three levels: 1. genetic factors, 2. factors involved in activation, signalling and trafficking of the receptor which includes desensitization, internalization and recycling and 3. environmental triggers [ 13 ].
CCR5 receptor is part of the G-protein coupled receptor family, which binds to its ligand and releases αi and βγ G-protein subunits. This results in a mediated effector response. Such responses stimulate the release of phospholipase Cβ and adenylyl cyclase. This in turn facilitates the release of intracellular calcium and form inositol triphosphate [ 13 ]. This leads to activation of phosphorylation of the CCR5 receptor which occurs at the serine and C-terminal residues via protein kinase C and G-protein coupled receptor kinases [ 13 ]. The regulatory proteins, β-arrestin 1 and 2, bind to the activated serine and the conserved DRY motif in the intracellular loop [ 13 ]. The β-arrestin proteins have functions like desensitizing the receptor to further stimulation and participating in endocytosis. The CCR5 expression level is controlled by the rates of recycling and endocytosis [ 13 ]. In the endocytosis process, β-arrestin protein facilitates the binding process between clathrin-coated pits and the phosphorylated receptor. Infection and entry of HIV into cells do not require CCR5 signalling, but the chemokine-induced endocytosis decreases the available receptor for HIV entry. This is the process of chemokine-mediated anti-HIV activity [ 13 ].
Environmental factors affecting CCR5 expression are infectious pathogenic agents like Mycobacterium tuberculosis , which increases the CCR5 expression. Studies have shown that CCR5 expression is considerably increased in all leukocyte subset cells during tuberculosis and dual infection with HIV [ 13 ]. However, the level of CCR5 expression on CD4 + T-cells was not increased. Conversely, it was also shown that HIV affects the level of expression of CCR5, due to a correlation with HIV disease progression. Individuals with end stage HIV were shown to have the highest percentages of CCR5 expressing CD4 + T-cells [ 13 ].
The regulation of CCR5 is complex. The introns as well as sequences in the 5′ UTR and 3′ UTR affect CCR5 gene regulation [ 13 ]. Therefore, mutations in these regions should be considered critical in the regulation process.
CCR5-Delta 32 Mutation
The discovery of CCR5-delta 32 mutation in the CCR5 gene in 1996 which exhibited some protection against HIV was a ground breaking one. Studies showed that the CD4 + T-cells when expressing this mutation prevented HIV envelope fusion [ 12 ] (Fig. 5 ). The mutant allele has a length of 215 in comparison to the wild type which contains 352 amino acid residues [ 13 ]. This mutation basically results due to the deletion of 32 base pairs from the position of nucleotides starting from 794 till 825, a frameshift mutation, and seven new amino acids are incorporated between amino acid 174 and stop codon at amino acid 182 [ 13 ] (Fig. 6 ). This mutation affects the region of second extracellular loop where the resultant protein lacked the last three transmembrane domains and also some regions necessary for G-protein interaction and signal transduction.

Comparison of HIV infecting cell with CCR5 and without CCR5 [ 15 ]

Difference between wild type CCR5 and CCR5-delta 32 [ 16 ]
This mutation is majorly restricted to people of European descent. The gene frequencies are found to be around 10% and shows a decline from north to south latitude. A 2–5% gene frequency in Europe, the Middle East and parts of the Indian subcontinent was observed in more than 3000 individuals. The highest frequency, at 20.93%, was discovered in the Ashkenazi Jewish population. The mutant allele is absent in Black populations excluding the African American group who may have acquired the mutation through genetic admixture [ 13 ].
The origin of the delta-32 mutant allele has been dated back to the year 275–1875, which increased over a period of time as a result of selective pressure, mainly the Black plague. However, historical data have shown that Black plague may not in fact be the cause [ 13 ]. The distribution of the delta-32 mutant allele in a north to south gradient does not correlate to the casualties of the plague and instead follows a south to the north gradient. The Black plague has shown the greatest casualties in areas like the Mediterranean region and China, with lowest allele frequencies of the mutation [ 13 ].
Studies suggested that delta-32 arose without a selective event. Tandem repeats found in the coding region of the CCR5 gene could cause unequal homologous recombination, which results in the delta-32 allele. The origins of the delta-32 mutation, however, remain a mystery [ 13 ].
The hype about the delta-32 mutation comes from its ability to protect homozygous individuals from HIV. The protective effect of the delta-32 mutation is a result of eliminating the expression of CCR5 protein on the cell surface, which prevents HIV’s entry into the cell. In the year 1997, however, studies showed that some of them having the homozygous delta-32 mutation were HIV-infected [ 13 ]. Further studies revealed the HIV virus was of the X4 type, which led to very rapid CD4 + T cell decline. Hence, this mutation is limited in its function and does not protect against viral strains which utilize other receptors or show dual-tropism [ 13 ].
In contrast, however, the delta-32 protein product which is localized to the endoplasmic reticulum is an important factor. It is shown to exert a trans-dominant negative effect on the wild-type CCR5 protein, which inhibits its transport to the cell surface. Further analysis in vitro showed the reduction of surface expression of wild type CCR5 and CXCR4 through dimerization by this mutant protein product [ 13 ]. This confers an inhibition to R5, X4 and R5X4 HIV infections [ 13 ]. Homozygous delta-32 individuals with this mutant protein were shown to have suppressed CXCR4 surface protein expression and decreased susceptibility to X4 infection. Experimental proofs also suggested that delta-32 heterozygous individuals with HIV infection do not stably express the mutant protein, are devoid of the molecular mechanism of complete protection and only maybe partially protected [ 13 ].
Stem Cell Transplantation
Stem cells are undifferentiated cells that can differentiate into specialized cells and can also undergo mitosis to produce more stem cells. There are mainly two classes—embryonic stem cells (ECS) and adult stem cells. Stem cells are also taken from the umbilical cord blood just after birth. These act as a repair mechanism for the body, such as skin, blood or intestinal tissues. Adult stem cells are majorly used in medical therapies like bone marrow transplantation. Bone marrow is the spongy tissue present inside the bones which serves as a rich source of adult stem cells. Long-term control of HIV is possible with CCR5-delta 32 stem cell transplantation [ 13 ].
Allogeneic transplantation of stem cells with this mutation in patients with HIV infection and malignancy has been considered as an option since the late 1990s (Fig. 7 ). Human leukocyte antigen (HLA) is a critical factor to be considered during the process of transplantation. The HLA should be a proper match; otherwise, it would lead to rejection by the recipient’s immune system. The limited availability of HLA-matched unrelated donors has made it even more difficult. Only about 1% of Caucasians possess this CCR5 null allele [ 13 ].

Allogeneic hematopoietic stem cell transplant [ 17 ]
Gene Therapy
Zinc finger nuclease technology is a popular tool which can be used for targeting specific DNA sequences in the genome. It falls in the class of restriction enzymes and is artificially made by fusing a zinc finger DNA-binding domain and DNA-cleavage domain. This technique is also engineered to eliminate the CCR5 expression over CD4 + T-cells, and the modified cells have shown to have a half-life of 48 weeks [ 13 ]. But it has its own issues. It is difficult to ensure that the desired repair mechanism is one which is used to repair the double stranded break (DBS) [ 13 ]. It is also challenging to scale it upwards and is an expensive technique.
A breakthrough technique, the CRISPR/Cas9 gene-editing system, is also used to eliminate the CCR5 receptor on the blood stem cells which can give rise to differentiated blood cells that are devoid of this receptor [ 18 ] (Fig. 8 ). These gene-edited stem cells can be established into an HIV-infected patient through bone marrow transplantation and give rise to an HIV-resistant immune system [ 18 ]. This technique, however, can also go sideways which leads to unwanted results that can cause ethical issues to rise. As seen in the highly controversial case of the Chinese scientist, He Jiankui, who with the help of this technology deleted the CCR5 gene in the twins, Lulu and Nana, introduced some unintended mutations in their genetic codes. There is still a lot of research needed to make this technology bioethically a safe tool.

CRISPR/Cas9 gene editing [ 19 ]
Researchers have also engineered a molecule called the chimeric antigen receptor (CAR) and introduced a gene for that molecule into blood-forming stem cells [ 18 ]. This molecule has two receptors that will recognize the antigen (HIV) and direct the immune cells to locate and kill the HIV-infected cells [ 18 ]. When transplanted into mice, which would have the CAR-carrying blood stem cells, it would result in reduced levels of HIV by inducing the immune cells to fight effectively against the virus [ 18 ]. An 80% to 95% drop in viral load was observed in the mice [ 18 ]. It was concluded that gene therapy could be a feasible option for treatment in HIV-positive humans.
Immunological Approaches
Studies have shown that vaccine can contribute effectively in viral clearance such as the Rhesus CMV vaccine vector [ 18 ]. A vaccine vector is a kind of vaccine which consists of chemically weakened viruses that are transported in the body to generate an immune response. The genes used in these vaccines are antigen coding surface proteins from that particular pathogen.
SAV001-H is the first and only preventive HIV vaccine which uses killed HIV-1 virus [ 18 ]. It is unique from other vaccines, as it uses genetically engineered whole virus genome, eliminating its pathogenicity and inactivating its virulence through irradiation and chemical treatments, finally approaching to the first “whole-killed virus”-based HIV vaccine [ 18 ]. The results of Phase 1 clinical trial, which were completed in the year 2013, were found to have serious and adverse effects in the 33 participants [ 18 ]. There was also a surprising boost in the antibody production against p24 and gp120. The HIV viral core is mostly made up of the structural protein, p24, which is called the capsid. A crucial factor in the diagnosis of primary HIV-infected individuals is the p24 antigen assay. High levels of p24 are found in the blood serum during the period between infection and seroconversion. The antibody production is found to increase as much as 64-fold [ 18 ]. The antibody production against gp120, which is a glycoprotein, necessary for attachment to a cell receptor and allow HIV entry, is found to increase up to eight-fold [ 18 ].
Another promising vaccine called the Kang's vaccine also uses the “whole-killed HIV-1,” which is similar to vaccines developed for rabies, polio and influenza [ 18 ]. However, HIV-1 is genetically engineered in such vaccines and raises questions about safety and possibility of large quantity production.
Researchers have also tested an immunogen called eOD-GT8 60mer, a protein nanoparticle, which is designed to mimic a crucial part of the HIV envelope protein which will bind to and activate the B cells to produce plasma cells that secrete antibodies needed to fight HIV [ 18 ]. This nanoparticle was developed in the Schief laboratory and tested in mouse models engineered by the Nemazee laboratory [ 18 ]. The researchers showed that immunization with eOD-GT8 60mer produced antibody progenitors with some of the characters crucial to recognize and block the HIV infection, proposing that it could be a promising first step in a series of immunizations against HIV [ 18 ]. The vaccine appears to work well in mouse models. The researchers are now investigating other immunogens that could work in coexistence with eOD-GT8 60mer [ 18 ].
Case Studies
The berlin patient [ 20 ].
The strongest proof available in favour of a HIV cure stems from the case of Timothy Brown who is popularly known as the Berlin patient (Fig. 9 ). He is considered the first person ever to be cured of HIV. The victory was predicated on doctors taking advantage of nature’s own experiment—the genetic mutation of CCR5 gene that produces a protein co-receptor present on the surface of CD4 + T-cells that HIV uses to gain entry. He was attending university in Berlin when was diagnosed HIV positive. His initial treatment include ART, and he was taking low doses of zidovudine and protease inhibitors. He continued to live a normal life for the next 10 years. But one day, he was again feeling extremely exhausted and the doctor had diagnosed it to be anaemia. He had received red blood cell transfusion for nearly a week and was then sent to an oncologist, Dr Huetter, when the previous doctor was unable to resolve the situation. The oncologist performed a painful bone marrow biopsy and after further diagnosis he was informed that he had acute myeloid leukaemia (AML).

Timothy Ray Brown a.k.a. “The Berlin patient” [ 21 ]
He then started receiving treatment at one of the Berlin University hospitals and had to receive four rounds of chemotherapy treatment. During the third round of chemotherapy, he had gotten a fatally dangerous infection and was immediately put into an induced coma. His blood sample was collected and sent to a stem cell donor bank with the German Red Cross to find matches in case he needed transplantation. Luckily, he had 267 matches which sparked an idea to locate donors with a homozygous CCR5 delta-32 mutation on CD4 + T-cells who are almost immune to HIV infection. A donor was found at the 61st attempt and had agreed to donate when necessary (Fig. 10 ).

Adam Castillejo a.k.a “The London patient” [ 23 ]
However, Timothy Brown had been reluctant and had said no to transplantation as the success rate was only 50–50. But at the end of 2006, leukaemia had rebounded and he desperately needed transplantation to survive. He received the stem cell transplant on February 6, 2007 and stopped taking his antiretroviral medication. Nearly 3 months after he underwent transplantation, HIV was no longer found in his body and he had thrived until the end of the year.
Unfortunately, life had other plans for him. After coming back from a trip to the USA, he was diagnosed with pneumonia and the leukaemia was back. The doctors decided to treat him with a second transplantation from the same donor in February 2008. The recovery was a tough one. He was almost paralyzed and went nearly blind. He had, however, eventually learnt to walk again and fully recovered 6 years later. He was continuously tested for HIV with extensive and precise tests. It was finally good news for him! Since 2010, when he decided to go public, he had interviewed for various magazines: POZ Magazine , New York Magazine and Science Magazine among others and decided to devote his life in supporting research for cures against HIV. In July 2012, he started the Timothy Ray Brown Foundation under World AIDS Institute and has worked with many scientists, organizations, research laboratories and universities to work on cures such as vaccination against HIV.
The London Patient [ 22 ]
The London patient may be the second person with HIV to no longer have the virus. In March 2019, in a report published in journal Nature , a group of investigators had announced the cure of a second HIV-positive patient. His success story depicts that CCR5 is a viable target for HIV research and treatment.
The London patient, who had chosen to remain anonymous, came out in public on March 9 th 2020. Adam Castillejo grew up in Caracas, Venezuela, and later shifted to London with his mother, as his parents were divorced. He was first diagnosed with HIV in 2003 and had started taking drugs to control the HIV infection in 2012. He had taken antiretroviral therapy for years before being diagnosed with an advanced form of blood cancer called Hodgkin’s lymphoma. Again, as in the case of the Berlin patient, the cancer was resistant to standard chemotherapy, so his doctors had advised more intensive chemotherapy along with bone marrow stem cell transplant. In 2016, he had agreed to transplantation and received it from a healthy donor who carried the CCR5 mutation. So, when his immune system regrew, it lacked the protein and was impervious to HIV. His virologist, Dr Ravindra Gupta, from the University of Cambridge, thinks it is a cure because a year had passed and they had carried out a few more tests for the viral load. In Adam Castillejo’s own words, “I don’t want people to think, “Oh, you’ve been chosen.” No, it just happened. I was in the right place, probably at the time right time, when it happened.” Adam Castillejo wants to be the “ambassador of hope” for people with this illness.
Although the scientists describe this case as a long-term remission, experts are calling it a potential cure. Such transplants are, however, dangerous and can be fatal. They are also an impractical approach to cure the millions already infected. These are highly risky procedures and can lead to serious complications. There still has to be a lot of research done to extend this type of treatment to a wider population infected with HIV.
A comparative study of the two patients reveals that their cases were in fact quite similar (Table (Table9 9 ).
Summary of the two cases—the Berlin patient and the London patient [ 24 ]
Lifestyle Practices to Prevent HIV Infection
Prevention is better than cure. And with HIV infections, one should practice prevention with utmost care and sincerity. An HIV diagnosis could turn one’s life upside down. So, it’s better to lead a healthy lifestyle by making the correct choices.
Measures for Protection Against HIV Infection
HIV is majorly spread through unprotected vaginal or anal sex. Choose less risky behaviour and be cautious. Not taking medicines to prevent or treat HIV is equally responsible for HIV infection. The number of sexual partners should be limited. One should get tested for sexually transmitted diseases and also know the sexual partner’s status. One can talk about pre-exposure prophylaxis to their respective healthcare provider. It is a preventive option for people who are not infected yet but are exposed to high risks of being HIV positive. HIV is also spread through intravenous injections and blood transfusions. Use of sterile equipment in such cases is a necessity.
Pre-exposure Prophylaxis
This is a preventive method of taking pills by people who are not HIV positive yet but who are at a high risk of getting infected and spreading it to others. A pill, named Truvada, contains two medicinal components, emtricitabine and tenofovir, that are used in combination with other drugs to treat HIV [ 25 ]. These medicines work on keeping the virus from creating a permanent infection.
Post-exposure Prophylaxis
Post-exposure prophylaxis (PEP) is a short course of HIV medicines taken soon after a possible exposure to HIV [ 25 ]. Every hour counts. For the treatment to be effective, the course should begin within 72 h after exposure to HIV; otherwise, it will not have any effect [ 25 ]. This treatment should be used only in cases of emergency. A person prescribed with PEP will need to take the medicines for 28 days at a stretch and then visit their respective healthcare provider for further tests [ 25 ]. Even if taken correctly, it may not be 100% effective. The sooner the medication is started, the better.
Healthy Practices to Follow When Living with HIV
A healthy, well-balanced and nutritious diet can help a person lead a better life by preventing health related issues like malnutrition and stopping the progression from HIV to AIDS. A well-balance diet is rich in whole grains, fresh fruits and vegetables, protein, low fat dairy products and multivitamins like zinc and B12. It also constitutes what should be cut down—fried foods, processed foods and sugary drinks. Smoking should be stopped when diagnosed with HIV. According to CDC, in the USA, the rate of adults with HIV, smoking is two to three times higher in adults infected with HIV than the nearly 18% of uninfected adults who smoke. Researchers at the Syracuse University analysed the data from 212 adults infected with HIV and found that the ones who smoked reported having more symptoms like dizziness and coughing.
Putting a stop to illegal drug use is equally necessary. People should seek treatment for addiction to illegal drugs like heroin, cocaine and methamphetamines. Sharing of needles for drugs can leave one exposed to other infections like hepatitis which might lead to a faster progression from HIV to AIDS. A recent study from the University of Pennsylvania School of Medicine showed a dramatic increase in the ability of HIV to attack healthy cells when methamphetamine is present in the bloodstream. This indicates that illegal drugs are also aiding in the HIV infection.
Being physically fit through a good work-out three to six times a week can help improve a person’s mood, perspective and overall quality of life. A good amount of moderate exercise can help fight HIV symptoms of nerve pain, loss of appetite and reduce the risks of other chronic diseases like heart disease, diabetes and osteoporosis. Taking the prescribed medication on time is known as adherence. This is vital to help reduce the risk of HIV becoming drug resistant and helps the immune system function for a longer time.
Nowadays, with the help of Internet of Things or IoT, patient’s health can be monitored 24/7. The quality of care provided can be increased many-folds with the help of monitoring devices enabled with current technology [ 26 ]. Concept of E-Health and M-Health is currently trending. E-Health makes use of electronic and communication processes with improved cyber security [ 26 ]. Some of the E-Health devices include GPS tracking, pedometer and electronic health records [ 26 ]. M-Health systems provide doctors with the complete medical history of the patient, so the treatment becomes easier and does not delay in case of emergencies. It makes use of mobile phones and other communication systems to help the patients with information about preventive health care services and collects data in real time as well [ 26 ]. The other important applications include chronic disease management, monitoring of diseases and tracking of epidemic outbreaks [ 26 ].
Genomic Diversity and Clinical Implications
Despite billions of dollars being invested, there is currently no HIV vaccine available that can either prevent the disease or treat those who suffer from it. An AIDS patient harbours 100 million genetically distinct variants of HIV [ 27 ]. This high diversity of HIV-1 is due to high replication rates, errors in reverse transcriptase and recombination events that mainly occur during the viral replication process. Reverse transcriptase enzyme has approximately a rate of 10 –4 nucleotide substitutions per replication cycle. Deletions, insertions and duplications are major contributing factors to the genetic variation of the virus [ 27 ]. Genetic recombination also plays an important role in creating genetic diversity. Template switches between two copies of RNA strands occur regularly during reverse transcription [ 27 ]. This generates a lot of mutations with the help of inter- and intra-molecular jumps. These mutations can either be drug resistant or inhibit the viral replication capacity.
HIV-1 can be classified into four main groups: M, N, O and the recently identified P. The M group is further identified into 4 subtypes (A to J). Studies have shown that there is a worldwide spread of non-B subtype viruses, and with the introduction of antiretroviral drugs, more research has to be conducted regarding the responsiveness of the drug resistance in non-B subtypes [ 27 ]. Different types of HIV-1 resistance are observed in different subtypes at varied levels. For example, subtypes B and G have shown to develop resistance against nelfinavir [ 27 ]. Research is also being done in the role of polymorphisms for development of drug resistance, to assess the genotypes before and after the therapy to be able to establish any association between the two [ 27 ].
Variation of Disease Progression Rate
There are 3 phases of the progression of HIV-1 infection- primary infection, chronic asymptomatic phase of infection and finally, AIDS. In the asymptomatic phase, neither signs nor symptoms of the disease are present, and this phase lasts an average of about 10 years. They can be divided as typical progressors, rapid progressors, slow progressors and long-term progressors. Rapid ones (10–20%) develop AIDS within 5 years of infection [ 28 ]. Slow progressors (5–15%) remain free of AIDS 15 years after infection [ 28 ]. Long-term progressors that constitute 1% show no signs and symptoms [ 28 ]. Factors like host genetic make-up, immune responses, co-infection and viral genetics and adaptation are attribute to this huge variation in disease progression [ 28 ]. But there is no solid evidence as such.
Some individuals known as elite controllers are able to manage the viral replication for longer durations, others are shown to rapidly lose CD4 + T-cells after seroconversion in the absence of cART (combination antiretroviral therapy). Scientists have conducted research studies that has led to the conclusion that rapid progression before administration of cART stops the recovery of CD4 + T-cells once the suppressive response to HIV-1 through cART is achieved. These findings have implications in public health policy making, clinical outcomes and science research. Ideally, cART should be initiated as soon the patient is diagnosed with HIV-1 irrespective of the CD4 + T-cell count. However, in clinical settings where cART is not widely available, these results would support strategies that may help in promoting frequent testing to reduce the proportion of patients initiating cART at low CD4 + T-cell counts. For those testing early, frequent CD4 + T-cell count should be monitored close to the time of HIV diagnoses to establish the rapid progressors phenotype in order to avoid unnecessary CD4 + T-cell count decay among rapid progressors. Finally, interpretation of the immunopathological basis of rapid progression can help improve individual clinical outcomes and limit its impact in the global HIV-1 pandemic.
Development of Drug Resistance as a Major Barrier to Treat HIV
HIV-1 has a high mutation rate. An estimated 10 10 virions per day can be produced in untreated patients that may result in variants called quasispecies. The complexity is also increased due to high recombination rate whenever more than one variant infects the same cell. All these are contributing factors that help in invading the host’s immune system and fostering drug resistance. Salvage therapy is also useful in cases when more than one regimen failed or a single regimen failed for a patient. It can be used to suppress the virus levels below the detection level and should have high genetic barrier to resistance to prevent rebound [ 29 ]. Clinicians need to focus on patient’s adherence as well as access to antiretrovirals (ARVs), drug interactions, tolerability, genotypic and phenotypic resistance testing, cross-resistance, genetic barrier and potency of ARVs [ 29 ].
Overcoming Obstacles and Future Prospects
At present, the reason for not being able to achieve a complete cure with the help of ART, in spite of achievement of undetectable viral load, is due to the presence of dormant virus or HIV latency. In a method call shock and kill, immune stimulants shock the latent virus from hidden reservoirs and then attempt to kill reactivated HIV [ 18 ]. An enzyme has been identified which is called histone deacetylase (HDAC) which is responsible for the sustained latency. Some studies show promise but are yet to be confirmed by clinical trials. Flushing these latent CD4 HIV-infected cells from their reservoirs with these HDAC-inhibitors into the blood circulation makes them susceptible to ART. Vorinostat and panobinostat are two such promising drugs [ 18 ].
Histone deacetylase inhibitors seem to have a broad spectrum of epigenetic activities. Vorinostat (also called Zolinza) is a U.S. Food and Drug Administration approved medicine, which has been used for the treatment of cutaneous T-cell lymphoma (CTCL) [ 18 ]. They help in flushing the virus from the reservoirs into the circulation. The dose is 400 mg. Other drugs on the pipeline are Protein kinase C agonist bryostatin-1 and GS-9620—TLR7 agonist [ 18 ].
Romidepsin (also called Istodax) is another HDAC inhibitor drug, which induces HIV-1 transcription to form plasma HIV-1 RNA that can be easily detected with standard assays [ 18 ]. This gives a possibility of reversing the HIV-1 latency in vivo without hindering T cell mediated immune response [ 18 ]. These findings will help the researchers with future clinical trials aiming to eliminate the HIV-1 reservoirs.
Research for curing HIV is at an infant stage but a promising one. Scientists are working on two broad types of HIV cures—a functional cure and a sterilising one.
The approach of the functional cure is to reduce the virus levels in the body to an undetectable stage, where the patient no longer needs to be on HIV medication or has no risk of progression to AIDS nor transferring the virus to others. Unlike the functional cure, however, a sterilising cure aims to get rid of HIV from the body completely by eliminating cells from latent reservoirs. It has proved to be an extremely challenging task for scientists, who believe it may be unachievable in the majority of them living with HIV. However, some findings by researchers at the University of Pittsburgh could lead to a foundation for an HIV vaccine. Clinical trials are in the works.
Abivax, a French company, is developing a drug that binds to some specific sequence of the viral RNA and inhibits its replication. During clinical trials, it has shown that this may have the potential to become a functional cure. The key is that it can target the reservoir of HIV viruses that hide inactive within our cells. It can target the reservoirs where HIV viruses act as inactive, within the infected cells. The result of phase IIa trial was quite promising. Fifteen patients were given the drug in combination with ART, and it was observed after 28 days of treatment that eight patients showed a 25% to almost 50% reduction of their HIV reservoirs compared to those only taking ART. The company is planning a phase IIb clinical trial to confirm the effects of the drug in the long term.
Research and development in HIV and its cure have come a long way since the disease was discovered in the 1980s. ART was a major milestone that has changed the lives of millions for good, but the next ambitious goal is to find an HIV cure before the year 2020. There are several approaches to an HIV cure ranging from shock and kill therapy, immunotherapy, vaccine development to gene editing using zinc finger nucleases and the CRISPR/Cas9 system, but finding the best possible solution is a challenge. One of the biggest challenges around any HIV treatment is the ability of the virus to rapidly mutate and develop resistance. Many of the new approaches do not provide any valuable insights as to whether the virus has the potential to become resistant. As of now, none of these functional cures have reached late-stage clinical trials, and the aim of finding an HIV cure until 2020 seems far-fetched. However, 2020 will likely be marked as an important milestone as the first late-stage trials will be executed. If successful, it could bring the approval of the first functional HIV cure in ten years.
There are two gene therapies undergoing human trials—one is to destroy the CCR5 receptor of the immune cells of people infected with HIV and the other therapy includes the CRISPR technology which is still under early trials. This mutation does not necessarily protect the person against all types of HIV. It was found that in one of the patients who had received the bone marrow treatment, it was found to have the CXCR4-tropic form. It uses a different type of receptor to enter and infect the cells. It was, however, not known whether this virus was acquired after the treatment or if some patients do contract a small amount of CXCR4-tropic virus that starts to multiply when other types are not present.
HIV research continues on many fronts that could provide the same results and only some of which rely on the CCR5 delta 32 mutation, which should be explored extensively. There are many strategies which are in the early stages of development. Scientific process can be slow but if done correctly, advances can be made to find a scalable, cost-effective cure for everyone.
Acknowledgments
The authors listed in this paper wish to express their appreciation to the RSST trust Bangalore for their continuous support and encouragement.
Authors Contribution
All authors have contributed equally with their valuable comments which made the manuscript to this form.
There was no funding provided for the above research and preparation of the manuscript.
Compliance with Ethical Standards
The authors declare that they have no conflict of interest.
All the authors listed hereby confirmed that in the above research, there were no human participants and/or animals involved in any kind of determination, evaluation or research studies.
There is also final confirmation given by all the listed authors for the submission of manuscript in its actual state. The authors listed above also confirm that the above-mentioned manuscript is in its original state and the manuscript is neither submitted anywhere nor in the submission process in any other journals. In addition, all the authors have solely contributed their original work in the preparation of this manuscript. If the copying or similarity have been found, then in all situations the listed authors are solely responsible.
Significance Statement
This article aims to increase awareness among the society about the current scenario of HIV/AIDS. The scientists are working on 2 types of cures—functional and sterilizing. The path to finding a cure is a promising one as late-phase trials begin in 2020.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Praveen Kumar Gupta, Email: ni.ude.ecvr@atpugkneevarp .
Apoorva Saxena, Email: [email protected] .
- Skip to main content
- Keyboard shortcuts for audio player
The FDA has approved a new drug in the fight against AIDS
Jason Beaubien
The Food and Drug Administration has approved the first injectable medication for HIV prevention. Health advocates say it could be a game changer in protecting people against AIDS
Copyright © 2021 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by an NPR contractor. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Research & training, advances in hiv/aids research.
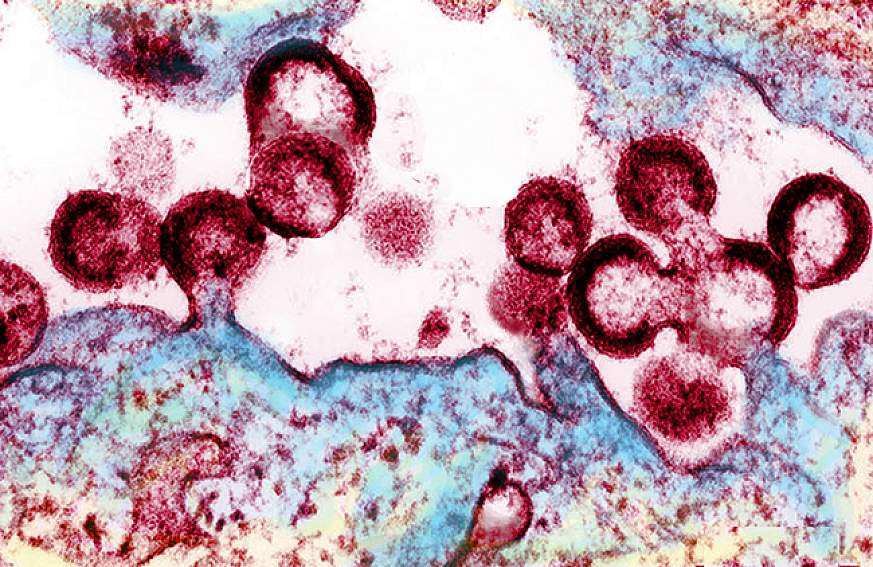
For an update on what medical science is doing to fight the global HIV/AIDS pandemic, read a Parade article by NIH Director Francis S. Collins and NIAID Director Anthony S. Fauci, AIDS in 2010: How We're Living with HIV .
Over the past several decades, researchers have learned a lot about the human immunodeficiency virus (HIV) and the disease it causes, acquired immunodeficiency syndrome (AIDS). But still more research is needed to help the millions of people whose health continues to be threatened by the global HIV/AIDS pandemic.
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as well as to better understand the virus with the goal of finding a cure. For example, in recent months, NIAID and its partners made progress toward finding a vaccine to prevent HIV infection. Check out other promising areas of NIAID-funded research on HIV/AIDS at http://www.niaid.nih.gov/topics/hivaids/Pages/Default.aspx .
Other NIH institutes, including the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute on Alcohol Abuse and Alcoholism, also support research to better control and ultimately end the HIV/AIDS pandemic. Some of these researchers have found a simple, cost-effective way to cut HIV transmission from infected mothers to their breastfed infants. Others have developed an index to help measure the role of alcohol consumption in illness and death of people with HIV/AIDS.
Find out more about these discoveries and what they mean for improving the health of people in the United States and all around the globe.
This page last reviewed on August 20, 2015
Connect with Us
- More Social Media from NIH
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
Last update: May 22, 2024
Raising life expectancy for youth with HIV requires more than just adherence to care regimens, researchers say
- Date 6 hours 12 hours 1 day 3 days all
- Rank Last day 1 week 1 month all
- LiveRank Last day 1 week 1 month all
- Popular Last day 1 week 1 month all
HIV & AIDS news
Life expectancy of youth with HIV is projected to be 10.4 years less in males and 11.8 years less in females compared to individuals without HIV, a study by researchers at Massachusetts General Hospital (MGH), a founding ...
May 22, 2024
A boost for HIV vaccine research: Studies present comprehensive platform for validating next steps
HIV has proven a hard target for vaccine design. The most promising approach, germline-targeting (GT), proposes a series of immunizations: a first shot to activate inexperienced B cells—antibody-producing white blood cells—followed ...
May 21, 2024
US pediatricians reverse decades-old advice against HIV-positive mothers breastfeeding
People with HIV can breastfeed their babies, as long as they are taking medications that effectively suppress the virus that causes AIDS, a top U.S. pediatricians' group said Monday in a sharp policy change.
May 20, 2024

Trial HIV vaccine triggers elusive and essential antibodies, pointing the way toward a successful vaccine
An HIV vaccine candidate developed at the Duke Human Vaccine Institute triggered low levels of an elusive type of broadly neutralizing HIV antibodies among a small group of people enrolled in a 2019 clinical trial.
May 17, 2024

What is PrEP? Will it stop me getting HIV?
HIV prevention was allocated A$43.9 million over three years in this week's federal budget. Some $26m of this is for "PrEP" for people without access to Medicare.
May 16, 2024

Injectable HIV medication is superior to oral medication for patients who frequently miss doses, study finds
When a person is diagnosed with HIV, they are placed on a lifelong HIV treatment regimen, called antiretroviral therapy, to keep the virus under control. But for many people, having to take medicine every day can be a struggle ...
May 14, 2024
Study highlights need for cell-type-specific therapies in treatment of HIV
Researchers from the University of Illinois have demonstrated the importance of cell-type-specific targeting in the treatment of HIV. Their study, published in the Proceedings of the National Academy of Sciences, is one of ...
May 13, 2024
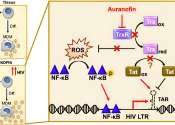
New research traces the spread of HIV in and from Indonesia
The HIV variant dominant in Indonesia was introduced from Thailand over multiple events. A Kobe University study traces where it came from and how it spread from there, offering possible insights into the development of treatments ...
May 10, 2024
GPS-like system shows promise as HIV vaccine strategy to elicit critical antibodies
A team led by the Duke Human Vaccine Institute (DHVI) has developed a vaccine approach that works like a GPS, guiding the immune system through the specific steps to make broadly neutralizing antibodies against HIV.
May 9, 2024
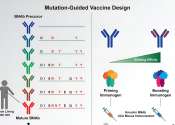
Keto diet boosts lifesaving antifungal drug in mice
For the roughly 150,000 AIDS patients who come down with a life-threatening infection called fungal meningitis each year, there aren't many options.
Social networks provide crucial support for older adults living with HIV, study finds
Having social support and strong social networks is vital to the health and well-being of older adults living with HIV, according to a Rutgers Health study.

US geographic region results in vastly different anal cancer risk for people with HIV
A new study that followed a cohort of more than 110,000 people establishes significant disparities in the risk of anal cancer for people with HIV and for men who have sex with men with HIV, depending on the region of the ...
May 7, 2024

Group-based interventions address HIV stigma
Group-based interventions have the potential to address HIV-related stigma among adolescents living with the virus, finds a recent study from researchers at the Brown School at Washington University in St. Louis and Makerere ...

Study explores coping strategies and self-stigma among people living with HIV in Indonesia
Individuals living with HIV often face significant physical and mental stress, including self-stigma, which can impede their ability to seek treatment and disclose their status.
May 6, 2024

Study shows ChatGPT can be helpful for Black women's self-education about HIV, PrEP
The artificial intelligence (AI) chatbot called ChatGPT is a powerful way for Black women to educate themselves about HIV prevention, as it provides reliable and culturally sensitive information, according to a study in The ...
May 3, 2024

Closing the US/Mexico border during COVID-19 increased HIV transmission, study finds
The border crossing separating San Diego, California, from Tijuana, Mexico, is a dynamic place. When it was closed during the COVID-19 pandemic, drug tourism from San Diego to Tijuana continued. This provided a flow of people ...
May 1, 2024

Study highlights importance of early interventions to combat HIV
A study has compared the development of HIV reservoirs—locations in the body where the virus persists in a latent state—between patients who receive either early or late medical interventions. The findings highlight the ...
Apr 30, 2024
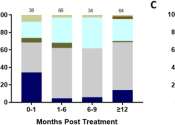
Visa rules jeopardize HIV management, study finds
A Monash University sexual health expert has warned that an unintended consequence of Australia's migration rules could compromise Australia's goal to end the HIV epidemic by 2030.
Semaglutide alleviates metabolic-linked liver disease in people with HIV
For people with HIV (PWH), semaglutide is effective for metabolic dysfunction-associated steatotic liver disease (MASLD), according to a research letter published online April 30 in the Annals of Internal Medicine.
'Vampire facials' were linked to cases of HIV. Here's what to know about the beauty treatment
Three women were diagnosed with HIV after getting "vampire facial" procedures at an unlicensed New Mexico medical spa, the Centers for Disease Control and Prevention said in a report last week, marking the first documented ...
Apr 29, 2024
11 hours ago
13 hours ago

15 hours ago

16 hours ago
18 hours ago
19 hours ago


E-mail newsletter

- About UNAIDS
- Global AIDS Strategy 2021-2026
- United Nations declarations and goals
- UNAIDS governance
- UNAIDS Programme Coordinating Board
- Results and transparency portal
- UNAIDS Cosponsors
- UNAIDS ambassadors and global advocates
- UNAIDS leadership
- UNAIDS evaluation office
- UNAIDS ethics office
- UNAIDS transformation
- Community pandemic response
- Education Plus Initiative
- Global alliance to end AIDS in children
- Equal access to cutting edge HIV technologies
- Save lives: Decriminalize
- Global council on inequality, AIDS and pandemics
- Resources and financing
- War in Ukraine
- Global HIV Prevention Coalition
- Global Partnership to Eliminate Stigma and Discrimination
- COVID-19 and HIV
- 2025 AIDS targets
- AIDS and SDGs
- Community mobilization
- Fast-Track cities
- H6 partnership
- HIV prevention
- HIV treatment
- Human rights
Key populations
- Private sector and the AIDS response
- Security and humanitarian affairs
- Social protection
- Universal health coverage
- Young people
- Press centre
- Publications
- Infographics
- FAQ on HIV and AIDS
- World AIDS Day
- Zero Discrimination Day
- Latest data on HIV
- Data on key populations
- Laws and policies
- HIV financial resources
- Technical Support Mechanism
- Learn about HIV and AIDS
- Take action
- Become a donor
- Investment Book
Global HIV & AIDS statistics — Fact sheet
Global HIV statistics
- 39 million [33.1 million–45.7 million] people globally were living with HIV in 2022.
- 1.3 million [1 million–1.7 million] people became newly infected with HIV in 2022.
- 630 000 [480 000–880 000] people died from AIDS-related illnesses in 2022.
- 29.8 million people were accessing antiretroviral therapy in 2022.
- 85.6 million [64.8 million–113.0 million] people have become infected with HIV and 40.4 million [32.9 million–51.3 million] people have died from AIDS-related illnesses since the start of the epidemic.
People living with HIV
- 37.5 million [31.8 million–43.6 million] adults (15 years or older).
- 1.5 million [1.2 million–2.1 million] children (0–14 years).
- 53% of all people living with HIV were women and girls.
- 86% [73– >98%] of all people living with HIV knew their HIV status in 2022.
People living with HIV accessing antiretroviral therapy
- 77% [65–90%] of adults aged 15 years and older had access to treatment; however, just 57% [44–78%] of children aged 0–14 years had access.
- 82% [69–95%] of women aged 15 years and older had access to treatment; however, just 72% [60–84%] of men aged 15 years and older had access.
- 82% [64–98%] of pregnant women living with HIV had access to antiretroviral medicines to prevent transmission of HIV to their child in 2022.
- 9.2 million people living with HIV did not have access to antiretroviral treatment in 2022.
New HIV infections
- In 2022, 1.3 million [1 million–1.7 million] people were newly infected with HIV, compared to 3.2 million [2.5 million–4.3 million] people in 1995.
- Women and girls accounted for 46% of all new infections in 2022.
- Since 2010, new HIV infections have declined by 38%, from 2.1 million [1.6 million–2.8 million] to 1.3 million [1 million–1.7 million] in 2022.
- Since 2010, new HIV infections among children have declined by 58%, from 310 000 [210 000–490 000] in 2010 to 130 000 [90 000–210 000] in 2022.
AIDS-related deaths
- AIDS-related deaths have been reduced by 69% since the peak in 2004 and by 51% since 2010.
- In 2022, around 630 000 [480 000–880 000] people died from AIDS-related illnesses worldwide, compared to 2.0 million [1.5 million–2.8 million] people in 2004 and 1.3 million [970 000–1.8 million] people in 2010.
- AIDS-related mortality has declined by 55% among women and girls and by 47% among men and boys since 2010.
Women and girls
- Globally 46% of all new HIV infections were among women and girls in 2022.
- In sub-Saharan Africa, adolescent girls and young women accounted for more than 77% of new infections among young people aged 15-24 years in 2022.
- In sub-Saharan Africa adolescent girls and young women (aged 15-24 years) in were more than three times as likely to acquire HIV than their male peers in 2022.
- Every week, 4000 adolescent girls and young women aged 15–24 years became infected with HIV globally in 2022. 3100 of these infections occurred in sub-Saharan Africa.
- Only about 42% of districts with high HIV incidence in sub-Saharan Africa had dedicated HIV prevention programmes for adolescent girls and young women in 2021.
- 2.5% among sex workers
- 7.5% among gay men and other men who have sex with men
- 5.0% among people who inject drugs
- 10.3% among transgender persons
- 1.4% among people in prisons.
Testing and treatment targets (95–95–95)
- In 2022, 86% [73– >98%] of all people living with HIV knew their HIV status. Among people who knew their status, 89% [75– >98%] were accessing treatment. And among people accessing treatment, 93% [79– >98%] were virally suppressed.
- Among all people living with HIV, 86% [73– >98%] knew their status, 76% [65–89%] were accessing treatment and 71% [60–83%] were virally suppressed in 2022.
- Five countries— Botswana, Eswatini, Rwanda, the United Republic of Tanzania, and Zimbabwe had achieved the 95-95-95 targets by 2022.
- A total of US$ 20.8 billion (constant 2019 US$) was available for HIV programmes in low- and middle-income countries in 2022––2.6% less than in 2021 and well short of the US$ 29.3 billion needed by 2025.
- Around 60% of resources available in 2022 were sourced domestically, compared with around 50% in 2010.
- The reduction in resources available for HIV in 2022 is due to declines in both international and domestic funding. The US$ 8.3 billion of external HIV funding in 2022 was 3% lower than in 2021. At the same time, domestic funding is diminishing.
- Bilateral funding from the United States Government constituted 58% of all international assistance for HIV, while disbursements from the Global Fund to Fight AIDS, Tuberculosis and Malaria accounted for about 29%. Other international donors contributed the remainder, but that share has diminished considerably, from approximately US$ 3 billion in 2010 to US$ 1.2 billion in 2022, a 61% decrease.
- In 2022, there was an estimated 90% funding gap for HIV prevention programmes among people from key populations, compared with the funding needed by 2025.
Download the full version to view regional statistics, global and regional data
Download fact sheet

HIV estimates with uncertainty bounds 1990-Present
Download spreadsheet (updated 31 Aug 2023)
Additional resources
Regional and thematic fact sheets
UNAIDS data 2023

Tuberculosis fact sheet
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 01 December 2021
Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021
- Steven G. Deeks 1 ,
- Nancie Archin 2 ,
- Paula Cannon ORCID: orcid.org/0000-0003-0059-354X 3 ,
- Simon Collins 4 ,
- R. Brad Jones 5 ,
- Marein A. W. P. de Jong 6 ,
- Olivier Lambotte 7 ,
- Rosanne Lamplough 8 ,
- Thumbi Ndung’u 9 , 10 , 11 ,
- Jeremy Sugarman ORCID: orcid.org/0000-0001-7022-8332 12 ,
- Caroline T. Tiemessen ORCID: orcid.org/0000-0002-0991-1690 13 ,
- Linos Vandekerckhove ORCID: orcid.org/0000-0002-8600-1631 14 ,
- Sharon R. Lewin ORCID: orcid.org/0000-0002-0330-8241 15 , 16 , 17 &
The International AIDS Society (IAS) Global Scientific Strategy working group
Nature Medicine volume 27 , pages 2085–2098 ( 2021 ) Cite this article
61k Accesses
138 Citations
191 Altmetric
Metrics details
- Translational research
Despite the success of antiretroviral therapy (ART) for people living with HIV, lifelong treatment is required and there is no cure. HIV can integrate in the host genome and persist for the life span of the infected cell. These latently infected cells are not recognized as foreign because they are largely transcriptionally silent, but contain replication-competent virus that drives resurgence of the infection once ART is stopped. With a combination of immune activators, neutralizing antibodies, and therapeutic vaccines, some nonhuman primate models have been cured, providing optimism for these approaches now being evaluated in human clinical trials. In vivo delivery of gene-editing tools to either target the virus, boost immunity or protect cells from infection, also holds promise for future HIV cure strategies. In this Review, we discuss advances related to HIV cure in the last 5 years, highlight remaining knowledge gaps and identify priority areas for research for the next 5 years.
Similar content being viewed by others

Prevention, treatment and cure of HIV infection
CD8+ T cells in HIV control, cure and prevention
Therapeutic vaccination following early antiretroviral therapy elicits highly functional T cell responses against conserved HIV-1 regions
Modern antiretroviral regimens can effectively block HIV replication in people with HIV for decades, but these therapies are not curative and must be taken for life. However, there is evidence that a cure can be achieved; initially, this came from a single case study (Timothy Brown, a man living with HIV who became widely known as the ‘Berlin patient’) following bone-marrow transplantation from a donor who was naturally resistant to HIV 1 . On the basis of this inspiring development and the recognition that not everyone can access and/or adhere indefinitely to antiretroviral therapy (ART), a global consensus emerged approximately 10 years ago that a curative intervention was a high priority for people with HIV and would be necessary to bring an end to the HIV pandemic. Since then, there has been a second case report of a cure following bone-marrow transplantation 2 as well as evidence of persistence of only defective forms of the virus in certain patients 3 and enhanced immune control of the virus by others after only a short time on ART 4 —further supporting the notion that a cure for HIV can be achieved.
An HIV cure includes both remission and eradication. Here, we define the term remission as durable control of virus in the absence of any ongoing ART. Eradication is the complete removal of intact and rebound-competent virus. The minimal and optimal criteria for an acceptable target product profile for an HIV cure, including the duration and level of virus control off ART, has recently been developed and published by the International AIDS Society (IAS), following wide consultation with multiple stakeholders 5 .
In 2011 and 2016, the IAS convened expert working groups to outline a strategy for developing an effective and scalable cure 6 , 7 . Since then, significant progress has been made, and the overall agenda has evolved. Here, we assembled a group of experts from academia, industry, and the community (Box 1 ) to evaluate recent progress and to outline cure-related research priorities for the next 5 years. The key recommendations for each component of the strategy are summarized in Box 2 .
Box 1 The Global Cure Strategy—forming a consensus
The Global Cure Strategy was created using a full online process during the COVID-19 pandemic from November 2020 to August 2021. The co-chairs of the initiative identified the major topics which were divided into eight subthemes, each with its own working group, which included a chair, three scientific experts, at least one community member, an IAS Research-for-Cure fellow, and an industry representative. Working groups met at least twice virtually to generate a summary of key advances and recommendations for the next five years. The steering committee consisted of the chairs of each working group, the co-chairs of the cure strategy and a community expert, selected for diversity in geographic background, gender, age, and expertise. We engaged people living with HIV at all levels as well as a wide range of scientific and nonscientific stakeholders.
The Global Cure Strategy was further refined through a broad, online stakeholder consultation, including an online survey, a review by key stakeholders in the field, and interviews with select experts and opinion leaders (more than 25 respondents). The survey received 162 responses, primarily from people working in academia, nongovernmental organizations, and hospitals or research institutions; 11% of respondents were from organizations of people living with HIV, and 4% were from industry. The majority of respondents were working in Africa, followed by Western and Central Europe, North America, and Central and South America. The summary and detailed responses can be found here: https://www.surveymonkey.com/results/SM-7YYFTZ599/.
Box 2 Key research goals to be addressed in the next 5 years
Understanding hiv reservoirs.
Define and characterize the sources of the replication- and rebound-competent viruses during ART
Define the phenotype of cells harboring intact HIV genomes
Define the clinical significance of defective yet inducible proviruses
Define the mechanisms of clonal proliferation
Determine if infected cells that persist on ART are resistant to cell death
Define the impact of sex and other factors on the reservoir and virus-specific therapies
HIV reservoir measurement
Develop and validate a high-throughput assay to quantify the rebound-competent reservoir
Develop assays that quantify integration sites
Develop assays that account for key qualitative differences in viral transcripts
Develop methods to quantify HIV protein expression in cells and tissues
Develop imaging modalities that quantify the size, distribution, and activity of the reservoir in tissues
Define the link between the cellular reservoirs, residual plasma viremia, and the rebounding virus
Develop assays for point-of-care and eventually at-home viral-load monitoring
Mechanisms of virus control
Identify the mechanisms that contribute to SIV/HIV control
Define the role of HIV-specific antibodies, B cells, and the innate immune response in virus elimination or control
Define the viral dynamics and biomarkers associated with post-treatment control
Optimize human organoid models, as well as mouse and nonhuman primate models, for cure- and remission-related studies
Targeting the provirus
Develop improved strategies to reverse latency
Develop strategies to permanently silence the provirus
Determine the impact of targeting the provirus at the time of initiation of ART
Define the role of viral subtype on the effectiveness of interventions that target the provirus
Targeting the immune system
Develop ‘reduce and control’ approaches
Develop immune modulators
Conduct clinical trials to determine whether combination immunotherapies will result in safe and durable HIV remission
Cell and gene therapy
Define the level of antigen expression needed to enable recognition of infected cells by immunotherapies
Develop gene-editing strategies that target the provirus
Develop strategies for sustained production in vivo of antiviral antibodies
Leverage advances in other biomedical fields to develop safer and more scalable approaches
Pediatric remission and cure
Characterize the establishment, persistence, and potential for preventing or reversing HIV latency in infants and children on ART
Develop assays to monitor and identify biomarkers to predict the efficacy of HIV-1 cure therapeutics
Test HIV immunotherapies and other strategies in infants and children
Social, behavioral, and ethical aspects of cure
Expand community/stakeholder engagement and capacity building
Develop HIV cure research with equity, representation, and scalability considerations
Establish standards for the safe conduct of clinical research
Integrate social, behavioral, and ethics research as part of HIV cure trials
Build capacity for basic discovery research and clinical trials in high-burden, resource-limited settings
A shared definition of the HIV reservoir is crucial for researchers, clinicians, and people living with HIV. Here, we use the term ‘HIV reservoir’ in the context of eradication or remission, as a representative term for all cells infected with replication-competent HIV in both the blood and different anatomical sites in individuals on ART—in other words, all potential sources of viral rebound in the context of a treatment interruption. Although the source of virus rebound is still not entirely understood, we now know that virus can persist in multiple forms, in multiple cells and in multiple sites.
Characterization of the complete HIV reservoir
HIV DNA can be detected in CD4 + T cells in blood and lymphoid tissue in nearly all people with HIV on ART. These viral genomes are mainly defective. Only a small proportion (less than 5%) appear to be intact and potentially replication-competent 8 . But the HIV reservoir goes beyond circulating CD4 + T cells; it also includes tissue-resident CD4 + T cells and cells of the monocyte/macrophage lineage, further complicating efforts to characterize and quantify it. In vitro, HIV preferentially integrates into transcriptionally active genes 9 ; however, in people with HIV on ART, many proviruses (defined as virus that is integrated into the host genome), including intact ones, have been identified in genomic regions that are silent (known as ‘gene deserts’), which limits or precludes their reactivation 3 .
Our initial conception of the HIV reservoir as a static viral archive has given way to a more dynamic view in which, over time on ART, certain within-host HIV variants are gradually eliminated while others persist through various mechanisms, including clonal expansion of infected cells 10 , 11 , 12 , 13 , 14 , 15 . Sporadic infection of new cells during ART has been reported 16 , although there has been no convincing demonstration that viral sequences evolve during effective ART 17 , suggesting that the degree of virus spread is minimal. The sources of viral rebound following cessation of ART are incompletely defined. Multiple factors can contribute to viral replication following ART, including anatomical and microanatomical locations, the infected cell type, cellular phenotype, the nature of the provirus, the antigen specificity of the infected cell, the potential for transcriptional activity given the specific integration site, and/or distribution of antiretroviral drugs within tissues (Fig. 1 ).
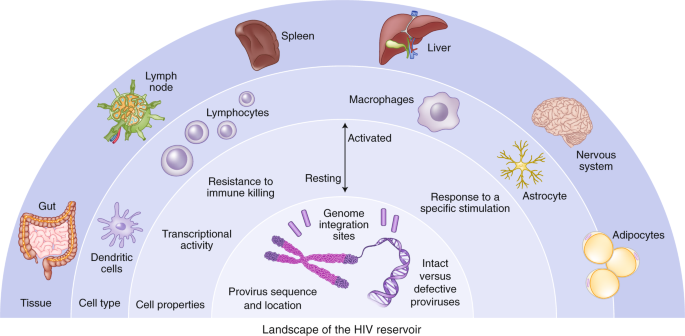
The HIV reservoir can be defined across a number of dimensions, including: (1) anatomical and microanatomical locations, (2) cell type (for example, CD4 + T cell or macrophage), (3) cell functional profile (activated or resting; resistance to killing), (4) pool of proviruses with a particular functional profile (for example, interferon-alpha resistant) or (5) triggering event (for example, response to stimulation with a particular antigen), and (6) integration-site features of the rebounding virus.
We recommend prioritizing efforts to understand integration sites of the virus during long-term ART and to understand the inducibility of a provirus on the basis of its chromosomal context. In addition, large prospective studies incorporating analytical treatment interruptions (ATIs) are still needed to probe clinically relevant sources of viral rebound and to identify a biomarker that predicts this. A favorable cure intervention could either prolong the time to the point when virus is detectable (that is, rebound) in plasma or reduce the viral ‘set point’ (that is, post-treatment control).
One of the most daunting obstacles to designing more effective methods to target persistent HIV infection is the lack of biomarkers to unambiguously identify the cells that harbor the rebound-competent reservoir. Recent work has demonstrated that the viral reservoir is preferentially enriched in cells that express programmed death-1 (PD-1) and other immune checkpoint markers, activation markers such as HLA-DR, and chemokine receptors such as CCR6 and CXCR3, but there is no phenotypic marker specific for the reservoir 18 , 19 , 20 , 21 . Specific biomarkers of the reservoir are needed, particularly to assess the impact of cure interventions. Furthermore, understanding how HIV persists in specific tissue sites and relevant local cell populations, such as those in the brain, gastrointestinal tract liver, or genital tract, will be important, given that the mechanism for persistence in each site may be distinct, and therefore different approaches may be required to eliminate each of these reservoirs.
There is growing evidence that some defective proviruses can produce transcripts and proteins (including novel viral RNAs and chimeric viral proteins) that in turn can elicit immune responses and perhaps contribute to chronic inflammation 22 , 23 , 24 , 25 . This may be of high relevance to end organ complications, such as HIV-associated neurological disease 26 . If the production of RNA and proteins from these defective proviruses proves to have clinical relevance, then their removal may be necessary to ensure long-term health.
A major mechanism of HIV persistence is the proliferation of cells that were infected prior to ART, resulting in large clonal populations of infected cells that arise as a result of the site of HIV integration 27 , 28 , response to antigen 29 , 30 , or homeostatic drivers 31 . Characterization of these presumably physiological expansions might lead to the development of therapies aimed at interrupting proliferation of infected cells. It will be important to determine to what degree these expanded clones are transcriptionally active, whether they are an important of post-ART viral rebound, and whether they have some innate survival advantage that prevents the cells from being effectively cleared by the host.
Recent studies have provided some evidence for preferential survival of infected cells with proliferative advantages or with deeper viral latency. Prosurvival and immune-resistance profiles may be particularly important in infected cells that persist despite expression of viral RNA or proteins 32 , 33 , 34 . Opportunities likely exist for collaboration and cross-fertilization of concepts with the cancer field, where the clonal dynamics of tumors have been extensively studied in relation to prosurvival and immune-resistance advantages, such as the work being done on lung cancer through prospective genetic studies in TRACRx ( https://clinicaltrials.gov/ct2/show/NCT01888601 ).
Biological sex can influence HIV pathogenesis, the immune response to HIV infection, and response to antiviral therapy 35 . Furthermore, in some but not all studies, women’s reservoirs have been shown to be less transcriptionally active and less inducible than those of men 36 , 37 , 38 , 39 , 40 . Sex, therefore, is a critical variable that should be considered as new therapies to target the reservoir are developed.
Quantification of the HIV reservoir
Significant progress toward a cure for HIV depends on having sensitive, specific, and quantitative measures of persistent virus that can be applied to various anatomical compartments 41 . Achieving this has been challenging, however, owing to the many sources and heterogeneous properties of persistent, replication-competent HIV. The reservoir can be quantified using assays that measure viral nucleic acid (total and integrated DNA, intact and defective DNA, or different forms of RNA), virus protein (p24), or viral inducibility (by measuring HIV RNA or virus replication following activation in vitro). Each approach has advantages and limitations, and assay outcomes may not always be interchangeable, comparable, or even correlated 8 .
Several groups have developed droplet digital PCR-based assays that discriminate genetically intact proviruses from a large background of defective proviruses, which are slightly less accurate but more high throughput than full genome sequencing 42 , 43 . The application of these assays to large clinical cohorts has demonstrated that there is a modest decrease in the frequency of cells with intact provirus over years on ART 44 , 45 , 46 . These assays have largely been optimized for subtype B virus, the major HIV subtype found in the United States and Europe. Yet there are over ten subtypes worldwide, some of which have evolved different mechanisms for immune evasion and persistence 47 . Pan-subtype-specific assays will need to be developed, and challenges related to cost and scalability remain. Research in this field should ideally culminate in harmonization across laboratories and crossvalidation of results. Future work will need to expand from quantification of virus in blood to quantification in tissue, particularly the more accessible tissues such as lymph nodes and gut mucosa.
Understanding the proviral landscape (defined as the degree of intactness, its transcriptional activity, and its location) is crucial, as these characteristics almost certainly influence the degree to which a provirus will rebound 48 . Over the last decade, several assays have been developed to analyze the exact location at which the virus integrates and whether the integrated virus is intact or defective. The ability to analyze single cells for integration site, viral sequence, and transcription is a major advance 48 ; however, these assays are expensive and low throughput. Technological advances are required to apply this more broadly to clinical samples, including assessment of interventions that target the reservoir.
Cell-associated viral RNA (CA-RNA) provides a measure of the total transcriptional activity of proviruses within a given sample. Several assays have recently been developed that quantify different RNA species, including total, elongated, unspliced, polyadenylated, and multi-spliced RNA, and these stand to give higher-resolution insights into the impact of therapeutic intervention 49 . An important unmet need is to develop approaches to distinguish transcripts arising from defective versus intact proviruses. Another shortcoming hampering broad use of RNA assays is the fact that they are subtype-sensitive. Overall, our ability to study the biology of transcriptionally active proviruses and the role of transcriptional activity as a potential biomarker needs to be further explored.
Since HIV protein expression is also required for recognition by HIV-specific T cells and other immune-based therapies, measuring and characterizing viral proteins in cells and tissues is an important step to understanding HIV persistence and might prove to be a critical determinant for the efficacy of therapies that target the HIV-infected cells directly (for example, chimeric antigen receptor (CAR) T cells or broadly neutralizing antibodies). Quantification of the p24 protein with ultrasensitive enzyme-linked immunosorbent assays can determine the efficacy of therapies that target the reservoir directly. Ultrasensitive p24 assays have emerged as useful tools 25 , but drawbacks include low levels of sensitivity compared with nucleic acid detection, overestimation of the replication-competent reservoir, and the requirement for specialized instrumentation 25 , 50 . Detection of viral envelope protein (the target of many therapeutic interventions for an HIV cure) also remains a challenge. Future strategies should leverage advances in single-cell techniques and new approaches to imaging tissue using super-resolution or expansion microscopy, together with multi-omics approaches.
Substantial progress in other fields of medicine has been made in using advanced imaging techniques to quantify rare diseased cells in tissues. On the basis of some preliminary success in nonhuman primate models 51 , efforts to use radiolabeled HIV-specific tracers and sensitive imaging modalities (for example, positron emission tomography, PET) have been initiated 52 . Similar efforts aimed at characterizing sites of inflammation or expression of specific surface markers that are associated with HIV persistence should also be a priority.
Several studies have attempted to identify sources of rebound virus by probing phylogenetic linkages with the proviral sequences present in various anatomical and cellular compartments. Success has been limited, however, in part owing to the challenging nature of obtaining full-length sequences from the limited number of infected cells in blood or tissue, as well as from plasma with low level viremia 53 . Strategies that can enhance enrichment of infected cells and/or depth of viral sequencing together with high-throughput low-cost single-cell analyses are likely to advance the field. As the RNA in circulating virions is a well-accepted surrogate marker for untreated HIV disease, this measurement could be an effective tool to characterize the rebound-competent population of HIV-infected cells.
Currently, any impact of a therapeutic intervention on the viral reservoir can only be determined with an ATI. A tool for very early detection of viral rebound post-ART using a nonvirological marker—such as measures of the innate immune response 54 —could be very valuable. In addition, better ways to monitor viral load that do not require frequent healthcare appointments will be needed 5 . This should include the development of home-based tests that may not necessarily require high sensitivity as long as testing is performed frequently 55 . Finally, emerging evidence suggests that virus replication during an ATI may be associated with some long-term adverse events 56 , so careful follow up of participants in ATI studies will be necessary.
Mechanisms and models of virus control
Natural control in people living with hiv.
Individuals who naturally control HIV in the absence of any therapy and can maintain a viral load of <50 copies/ml (known as ‘elite’ controllers) have been the focus of intense investigation for years. Research in this area is increasingly focused on those controllers who exhibit remarkably stringent control (‘exceptional’ controllers) 57 , 58 , some of whom might be considered true cures 48 , 59 , and those who became controllers after ART interruption (post-treatment controllers) 60 , 61 . In exceptional controllers, the frequency of infected cells is extremely low, often below the limit of detection of most standard assays for HIV DNA 57 , 59 , there is no intact virus 48 and the site of HIV integration may be distinct 48 ; an agreed definition for an exceptional controller is needed.
Virus-specific CD8 + T cells targeting particularly vulnerable or conserved epitopes are generally recognized as the key mediator of elite control; such cells are rare in post-treatment controllers and have not yet been characterized in exceptional controllers 4 , 62 . Further characterization of the various controller phenotypes (elite, exceptional, post-treatment) should remain a priority; the identification of unique and potentially informative phenotypes should also be pursued, including individuals on ART who have very small reservoirs 62 . Functional multi-omics studies and emerging single-cell technologies should help to determine the mechanisms involved in exceptional, elite, and post-treatment control. Better animal models of exceptional and post-treatment control would greatly enhance the field, giving access to tissue and the opportunity for longitudinal assessment of virus control 63 .
Virus elimination and control will likely require a coordinated immune response involving more than just T cells. Recent data suggest that autologous antibodies targeting archived viruses as well as interferon sensitivity might influence which virus populations emerge post-ART 54 , 64 , 65 . Studies in simian immunodeficiency virus (SIV)-infected nonhuman primates that naturally control infection have provided indirect evidence that natural killer (NK) cells might be able to effectively control virus in tissues 66 . Better insights into the role of antibodies, natural killer cells, and innate immunity in post-treatment and/or post-intervention control are needed.
The interplay between the virus and immune system during acute infection or immediately after the interruption of ART is largely unknown, at least in humans. During acute infection, those destined to become controllers typically have an initial period of poorly controlled viremia 61 , 67 . For post-treatment controllers, virus control is often achieved more rapidly after cessation of ART than after primary infection 61 , 68 . We need to understand the viral dynamics associated with eventual post-ART control/remission, as this will inform how a treatment interruption should be conducted. It is likely that biomarkers other than the plasma HIV RNA level might allow for the development of safer and more cost-effective strategies for interrupting ART.
Animal models of control
The role of humanized mouse models in cure research is still evolving. Recent studies showing similar effects of latency-reversing strategies in mice and the less scalable nonhuman primate model are encouraging 69 , 70 . Given that access to nonhuman primates for cure studies will likely remain a barrier, ongoing optimization, standardization, and validation of mouse models should be prioritized.
An important discrepancy in translating cure-related findings from SIV-infected nonhuman primates to people with HIV lies in the duration of ART. Although effective ART regimens with integrase inhibitors have been optimized in nonhuman primates, high costs, and treatment-related toxicities necessitate relatively shorter study durations (less than 1–2 years of ART). One possible solution would be for primate research centers to maintain colonies of SIV-infected nonhuman primates receiving very-long-term ART to be directly assigned for studies.
There is ongoing debate about the most appropriate virus to be used in cure-related studies in nonhuman primates. Investigations utilizing broadly neutralizing antibodies or select vaccines directed against the HIV-1 envelope necessitate infection with a virus that expresses HIV envelope proteins (simian-human immunodeficiency virus, SHIV). However, SHIV infections with some strains are characterized by post-treatment control in the absence of any intervention 71 , while others can induce significant disease progression 72 . Therefore, the specific strain used can limit the generalizability of the model. Although SIV infection of nonhuman primates can cause more significant disease progression than HIV infection of people, early ART for SIV infection can limit rapid disease progression and is therefore a useful model for cure studies 73 . Developing immunotherapies that target the SIV envelope in addition to SHIV should also be pursued.
A major recent advance has been the development of genetically barcoded SIV mac239 strains 74 . Because the barcode ‘tags’ are easily quantified and also passed on to progeny virus, this model allows for tracking of clonal dynamics, providing more precise insights into how interventions affect seeding of the reservoir, viral reactivation during ART, or viral recrudescence after ART interruption.
Therapeutic interventions
Since the discovery that HIV can establish a latent infection with minimal HIV transcription, a range of approaches has emerged that specifically target latently infected cells. These include pharmacological modulation of epigenetic or signaling pathways involved in HIV transcription to reactivate latent HIV such that the cells can be targeted and eliminated (‘shock and kill’) or to permanently silence HIV transcription (‘block and lock’) 75 , 76 , 77 . Recent reports have demonstrated that HIV latency is heterogeneous and that latency reactivation is stochastic, implying that a combination of agents targeting various pathways controlling HIV transcription may be necessary to achieve either robust silencing or latency reversal 49 , 78 , 79 , 80 .
A clear limitation of the ‘shock and kill’ approach comes from the discovery that only a fraction of proviruses is intact and among these, only some are inducible by a potent stimulus such as T cell stimulation, let alone by far less potent latency-reversing agents (LRAs) 8 , 81 , 82 , 83 . Furthermore, cells containing reactivated latent HIV may also be relatively resistant to killing by cytotoxic T cells 84 . Complicating the situation even more, CD8 + T cells appear to suppress HIV transcription and can blunt the effect of LRAs 70 .
Although LRAs tested in humans can induce HIV RNA expression and virion production in vivo, they have failed to reduce the size of the reservoir, even when combined with immunotherapeutic strategies designed to enhance clearance of infected cells 85 , 86 , 87 , 88 , 89 , 90 . This could be due to poor antigen induction by LRAs or insufficient clearance of these targets by immunotherapies (Fig. 2 ). Furthermore, many of the tested LRAs have off-target effects. Newer approaches for delivery of LRAs to reduce toxicity, enhance potency, and improve targeting, potentially leveraging advances in nanomedicine, should be explored. Greater potency could potentially be achieved using LRAs in combination, however, care is needed in these clinical trials, given that unexpected toxicities can emerge—as was recently demonstrated in the evaluation of high-dose disulfiram and vorinostat 91 . Finally, LRAs will likely need to be partnered with therapies that enhance the clearance of cells expressing viral proteins, such as immune-enhancing strategies or proapoptotic drugs 92 .
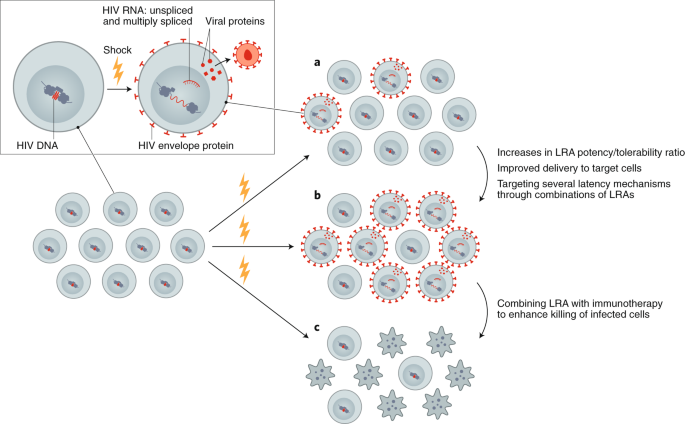
Reversing latency is an important component of revealing HIV-infected cells, allowing for conversion of a latently infected to a productively infected cell. a , Currently available LRAs reverse latency in only a subset of infected cells, and, when used alone, do not sufficiently eliminate these. b , Enhancing the efficacy of an LRA can be achieved with increased potency, targeted delivery or through using combinations of LRAs. c , Ultimately, depletion of the reservoir will require combining an LRA with other interventions, such as immunotherapy or a proapoptotic drug.
Permanently silencing the HIV promoter by suppressing factors that promote HIV transcription has also emerged as a strategy to target the provirus. The concept is to therapeutically drive HIV into a permanently silenced epigenetic state that resists reactivation (‘deep latency’). The Tat inhibitor didehydro-cortistatin A (dCA) blocks HIV reactivation from human CD4 + T cells in vitro through epigenetic repression; treatment with dCA in ART-suppressed humanized mouse latency models induces a measurable delay in virus rebound 76 , 93 . Gene therapy can also play an important part in permanent silencing of the provirus using short interfering RNA or other modalities 94 . Thus far, these approaches have yet to be successfully translated into human trials.
Further exploration of the therapeutic potential of permanently silencing the reservoir (‘block and lock’), presumably as part of a combinatorial cure approach, is a high research priority. Some pathways that might be targeted include mTOR, HSF1, and others 95 , 96 , 97 . Efforts to screen for drugs that suppress HIV transcription are encouraged, 96 , 98 , 99 with the goal to rapidly move into preclinical and clinical studies 100 , 101 .
With a recent report indicating that the HIV reservoir is stabilized at the start of ART initiation, efforts should be devised to inhibit this stabilizing effect and/or to enhance reservoir turnover during ART, where such interventions are ideally delivered at ART initiation 102 , 103 , 104 .
Most methods to target the provirus have been developed using subtype B. Thus, while conserved mechanisms govern latency across the different virus subtypes, differences at the level of the promoter may impact responsiveness to various stimuli. Therapies targeting the provirus should be evaluated across multiple HIV subtypes including recombinants.
There is a robust and growing toolbox of immune therapies that might be advanced to proof-of-concept testing. Arguably, the most impactful innovation to date is the isolation and development of broadly neutralizing antibodies for clinical use, but advances have also been made in the development of therapeutic vaccines, vaccine adjuvants, and other immunotherapies. When used in combination in nonhuman primates, these immune therapies have resulted in sustained post-ART control 71 , 105 . When used alone, most of these approaches have had limited effectiveness in people, although some promising results are emerging 88 , 106 , 107 . Combination clinical trials have recently started and are ongoing. Although the combination of either vorinostat or romidepsin (HDAC inhibitors that can increase viral transcription through epigenetic modification) together with different HIV vaccines showed no or minimal reduction in the HIV reservoir 107 , 108 , 109 , results from other studies including a combination of Toll-like receptor agonists, LRAs and broadly neutralizing antibodies are eagerly awaited ( NCT03837756 ; NCT04319367 ; NCT03041012 ).
As it may be challenging to reactivate and eliminate all latently infected cells, or to induce deep irreversible latency in all cells, it seems unlikely that these approaches will be curative by themselves. By reducing the reservoir, however, they might make strategies aimed at controlling the virus long term post-ART more effective. This overall approach of 'reduce and control’ is supported by observations in elite and post-treatment controllers, and theoretical modeling 110 . Multiple approaches that might result in control of a small reservoir are being developed. Assessment of therapeutic vaccines including live vector vaccines such as adenovirus 26, modified vaccine Ankara, and also a cytomegalovirus in nonhuman primate models have been particularly promising, with a subset of animals achieving eradication of virus 71 , 111 . Such studies have not yet been performed in people. Research to develop and test novel immunogen and vaccine designs with broad, potent and durable immunity should be prioritized. Given the recognition that autologous neutralizing antibodies might contribute to reservoir control 64 , novel vaccine approaches aimed at the induction of broadly neutralizing antibodies—including germline targeting 112 —should also be prioritized.
Immune stimulators, immunomodulators, and novel immunotherapies (such as cytokine formulations, Toll-like receptor agonists, immune checkpoint inhibitors or agonists, and novel vaccine adjuvants), used alone or more likely in combination with other approaches, hold promise but have undergone relatively limited testing in HIV-cure studies in people so far 106 , 113 , 114 , 115 .
With the exception of a few anecdotal cases 116 , immunotherapy in people with HIV has yet to recapitulate the promising advances made in nonhuman primates. Combination of various therapies will almost certainly be needed (Fig. 3 ). Conducting such studies is feasible 108 , 117 ; it is expected that initial clinical research will be intensive in nature and designed to identify strategies that might then be tested in well-powered, controlled clinical trials. Defining the mechanisms and potential biomarkers associated with remissions/cures in the preclinical and clinical setting should remain a priority. Determining which combinations to study, and how to define the optimal doses and strategies, poses a significant challenge from a methodological and regulatory perspective. As immunotherapies for HIV move into the clinic, careful attention will have to be paid to immune-related adverse events, including cytokine-release syndrome and autoimmunity.
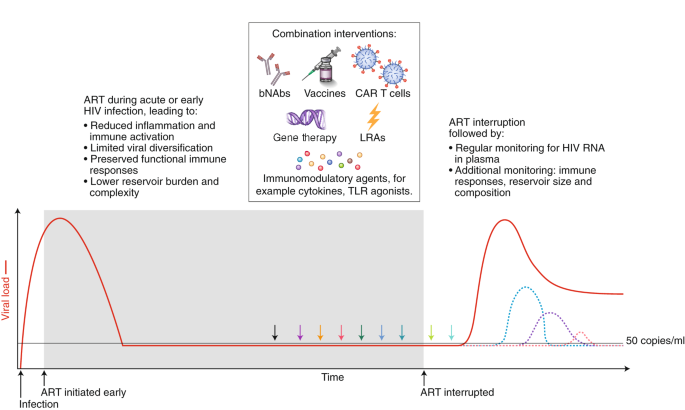
Strategies that will enhance immune-mediated clearance of latently infected cells include early initiation of ART and the administration of combined interventions at the time of suppressive ART (colored arrows) or during the treatment interruption phase, which will allow for increased antigen presentation. Given that there is no biomarker that can predict viral rebound, analytical treatment interruptions are used to determine whether the intervention has had a clinically meaningful impact. The overarching goal is to either delay viral rebound by at least months or years or reduce the set point of virus replication (that is, the stable level of viral load that the body settles at), preferably to a level of <200 copies/ml. The dashed colored lines represent different potential favorable outcomes from a cure intervention. bNAbs, broadly neutralizing antibodies; LRA, latency reversing agent; TLR, Toll-like receptor.
Cell and gene therapy clinical trials for people with HIV, although safe so far, have been small in scale and with no clear demonstrations of efficacy. The interest in gene therapy for an HIV cure was inspired by the elimination of intact virus in Timothy Brown (also known as the Berlin patient) and Adam Casteljo (also known as the London patient), who both received stem-cell transplants from a CCR5-negative donor 1 , 2 to treat their underlying malignancies. CCR5 is a co-receptor that is needed by most strains of HIV to enter a cell; a reduction in the size of the reservoir has also been reported following stem-cell transplantation to people with HIV from donors who are CCR5-positive 118 , 119 , but the HIV reservoir can’t be completely eliminated, irrespective of the CCR5 status of the donor. In the case of CCR5-negative stem-cell transplantation, the absence of CCR5 in the donor cells is thought to protect the newly transplanted cells from infection, at least with CCR5-dependent HIV strains. Interestingly, in both cases of cure following stem-cell transplantation of CCR5-negative cells, defective virus has been detected, but not intact or replication-competent virus 120 , 121 . These reports have prompted researchers to evaluate CCR5-targeted gene editing as a potentially safer path to cure in people living with HIV on ART, given the high mortality rate and significant morbidity associated with stem-cell transplantation. Timothy Brown unfortunately died in early 2020 owing to recurrence of his leukemia, but remained HIV-free until his death.
Ex vivo gene editing of CCR5 using zinc finger nucleases and re-infusion of CCR5-modified T cells has not yet prevented viral rebound following ATI 122 , 123 , possibly because insufficient cell numbers were engineered and/or engrafted with first-generation editing tools and cell culture protocols and/or because CCR5 disruption alone cannot shift the balance in favor of post-treatment control in the presence of persistently infected cells. More recently, gene therapies have shifted to creating effectors, including chimeric antigen receptor (CAR) T cells, which can recognize and eliminate HIV-infected cells (Fig. 4 ). Other approaches include the use of novel delivery systems to deliver genes to local tissues, resulting in the sustained production of systemically acting antivirals such as broadly neutralizing antibodies 124 , 125 and CD4 mimetics 126 . Finally, attempts are being made to directly target integrated proviruses with technologies such as CRISPR–Cas9 and recombinases 127 , 128 . This approach remains conceptually challenging in view of the disparate locations of latently infected cells, the absence of specific markers to target delivery, the heterogeneity of proviral sequences (the majority of which are defective), and the risk of off-target effects.
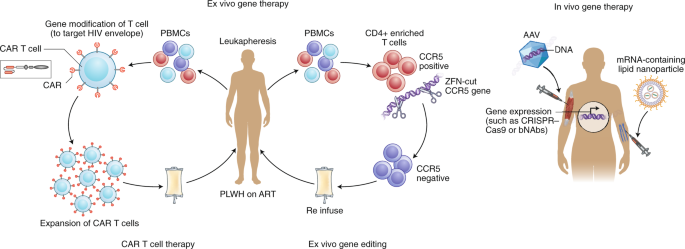
Examples of ex vivo (left) and in vivo (right) gene therapy approaches that have been tested in people with HIV on ART. Ex vivo strategies include gene editing to either delete or inactivate CCR5 or HIV provirus in CD4 + -enriched T cells using gene-editing tools such as zinc finger nucelases (ZFN) or CRISPR–Cas9. Alternatively, autologous T cells can be modified to express a CAR that can recognize HIV envelope, and this can then be reinfused into the participant. In vivo strategies, on the other hand, do not require external manipulation of cells; nanoparticles or viral vectors (such as adeno-associated virus (AAV)), which encapsulate mRNA or DNA, respectively, for the relevant gene to be expressed are administered directly to the patient. These approaches have recently been successful using lipid nanoparticles that contain mRNA encoding CRISPR–Cas9 135 or for expression of anti-HIV broadly neutralizing antibodies such as PG9 or VRC07 (ref. 125 ). PBMCs, peripheral blood mononuclear cells; PLWH, person living with HIV.
Many emerging cell and gene therapies are designed to target viral proteins/epitopes that are expressed in abundance on the surface of tumor cells, for example CD19 for the treatment of lymphoma 129 . Various forms of the HIV viral envelope protein (gp120 trimers and monomers, gp41) are expressed on the surface of infected cells, while multiple peptides are presented via HLA molecules. These antigens are expressed at levels well below that of many cancer antigens now being successfully targeted in the clinic. Cell-based therapies such as CAR T cells, once infused into the patient, will only persist and differentiate if there is sufficient antigenic exposure; however, the levels of antigen during ART may be too low 130 . Removal of ART after infusion of CAR T cells (or similar products) could be used to expand these cells in vivo, or more potent latency-reversing agents could be used to enhance envelope protein expression. In addition, novel adjuvants could expand CAR T cells even when the antigen burden is low, as was recently demonstrated in the nonhuman primate model 131 .
The challenges here are primarily those of delivery to relevant cells. In addition to developing methods to target specific cells, which are common problems faced by all potential in vivo gene therapies, targeting latent proviruses also presents the problem of a lack of robust cell surface markers to identify cells harboring such proviruses. Progress in both of these areas will be needed to develop strategies to deliver gene-editing reagents to latently infected cells. Some promising in vivo delivery strategies for CRISPR–Cas9 have included adeno-associated virus to target the SIV virus in nonhuman primates on ART 127 , as well as using engineered CD4 + cell-homing messenger RNA (mRNA)-containing lipid nanoparticles in mouse models of HIV infection 132 .
Long-term in vivo secretion of antibodies or antibody-like molecules can be achieved following gene therapy vector delivery of antibody cassettes to tissues such as muscle and liver, where enhanced production of antibodies is needed, rather than specific delivery to infected CD4 + T cells. This can be achieved through direct intramuscular injection leading to uptake in the muscle or, alternatively, intravenous injection, which will allow for uptake in the liver. Ectopic expression of these antibodies in liver or muscle cells fails to recapitulate aspects of natural antibody production, such as responsiveness to antigen and ongoing somatic hypermutation. Therefore, editing the B cell Ig locus itself to express antibodies presents an alternative and attractive gene-editing strategy 133 , 134 .
Sustained production of these antivirals could result in sustained (perhaps lifelong) control of the virus. Many barriers to success exist. Antidrug antibodies that target and clear the vectors often form rapidly 125 , limiting the ability to deliver multiple doses. Advances in mRNA encapsulation within lipid nanoparticles may potentially revolutionize delivery of gene therapy, allowing for delivery of mRNA encoding CRISPR–Cas9 and related guide RNAs in vivo, as has recently been successfully demonstrated in the treatment of transthyretin amyloidosis 135 . Also, antibodies targeting multiple antigens will likely need to be produced at high levels to prevent virus replication and escape.
Advances in T cell manufacturing are expected, driven by cancer CAR T cell therapies, which will also benefit HIV therapies. Similarly, advances occurring in gene therapy treatments for genetic diseases, such as hemoglobinopathies, are catalyzing safer and nongenotoxic conditioning for HSPC transplants, for example based on drug–antibody conjugates. Practicality will also be enhanced by moving toward using allogeneic off-the-shelf products.
Gene and cell therapies now require a shift towards a practical focus, identifying ways to expand use, reduce costs, and allow deployment in resource-limited settings. This could be achieved through abbreviated ex vivo cell manufacturing, including automated closed-system devices (‘gene therapy in a box’), to produce product in a place-of-care setting 136 . While still in the early stages of development, in vivo gene therapy also presents exciting possibilities to significantly expand access by eliminating the need for external manipulation of cells and associated technological requirements.
The unique context of perinatal HIV infection necessitates pediatric-specific strategies to achieve ART-free remission in children. The case of the Mississippi child, who started therapy ~30 hours after birth and achieved remission off ART for 27 months before virus rebounded 137 , 138 , raised the possibility that remission for children can be attained. Subsequent reports of early-treated pediatric cases with long-term (>12 years) virological control off ART have provided examples of post-treatment control in children 139 , 140 .
The nature of the reservoir in children is unique from that in adults. For example, naive CD4 + T cells are a more important reservoir for the virus in children 141 , 142 . Further development of infant nonhuman primate models for evaluating ART and cure strategies will contribute to our understanding of the HIV reservoir and how to target it in the unique setting of infancy and immune development, but an understanding of the limitations of this model is also crucially important 141 , 142 , 143 , 144 , 145 .
Many of the recent advances in understanding HIV persistence during ART in adults, including frequency and transcriptional activity of intact virus, clonal expansion, sites of proviral integration, and inducibility, need to be applied to studies of children. Optimizing methods that can be adapted to small blood volumes are also needed.
In the context of childhood infection, clarity is needed on how latency is established in naive T cells, susceptibility of these cells to latency reversal, propensity for T cells to clonally expand, and the relative contribution of clonally expanded cells to viral rebound following cessation of ART. It is still unclear whether integration sites and reactivation potential are different in children, and whether these change with age. Given that initial studies suggest a less-inducible reservoir in cases of perinatal infection 146 , it is especially important to determine how to maximize latency reversal in children. The optimal timing of these interventions (for example, at the time of early ART initiation) could potentially limit the pool of infected cells that persist on ART; such approaches can be explored in a nonhuman primate model.
As in adults, better tools are needed to assess the impact of cure interventions in children, including quantification of HIV persistence and in-depth cellular immune profiling. There is a particular need for noninvasive tools, such as total body imaging, to assess central nervous system and other tissue-based reservoirs. It will also be important to identify biomarkers for post-treatment control, including the degree of reduction or alteration in the composition of the latent reservoir that may be predictive of pediatric remission or cure 147 . Finally, preclinical studies in infant nonhuman primates that test new interventions to reduce or eliminate persistent HIV and/or induce viral remission after ART interruption are needed to inform the development of HIV remission and cure intervention strategies. Early therapy alone is insufficient to reliably achieve a cure or long-term remission in children. Novel approaches, including earlier administration and use of more potent antiretroviral drugs, therapeutic vaccines, or other immunotherapeutics, such as broadly neutralizing antibodies and/or innate-immune-enhancing agents, will be necessary.
Research directed toward an HIV cure intertwines critical social, behavioral, and ethical aspects that must be incorporated in the scientific agenda. This research takes place within particular social contexts and communities that shape its permissibility and appropriateness. Accordingly, affected communities must be meaningfully engaged throughout the research process; social and behavioral factors must be interrogated and taken into account because they affect research feasibility, community support for the research, and the well-being of participants and other stakeholders. Research must also address the many ethical issues associated with developing a therapy, particularly since viable options for treatment are already available. Sufficient funding for research toward social, behavioral, and ethical aspects of a cure and for community involvement is therefore essential.
Substantial progress using more conceptual and normative approaches has also been made regarding the ethical issues associated with the interruption of ART 148 , 149 . Similarly, there has been attention focused on acceptable risk thresholds for research 150 . Finally, given the important role of treatment as prevention, efforts have focused on the ethics of partner-protection measures 151 .
Community engagement in HIV cure research is still suboptimal in many settings, being mostly been limited to advisory boards typically comprised of scientifically literate individuals. Capacity to discuss HIV cure research and to evaluate its potential implications for local and global communities must be built within diverse community groups. Communities should be empowered and supported through education and engagement at all levels of the research process to help shape the HIV cure research agenda and allow for potential study participants to have a voice in trial design 152 .
Since HIV cure research is highly complex and nuanced, there is also a need to ensure understanding of it among other key stakeholders, including Institutional Review Boards (IRBs) and clinicians. For example, IRBs need to appreciate the implications of ATIs for partners who they may not see as within their remit, and clinicians need to understand the rationale for ATIs in the research setting.
Attention must focus on broad representation (for example, age, race and ethnicity, gender and sexuality, geographic location, risk behaviors) in research. Diversity in participation is essential during the development of interventions aimed at complete HIV elimination or durable ART-free control. This necessitates research directed at understanding the reasons for under-representation of certain groups of people in HIV cure research. For example, cisgender and transgender women, as well as individuals of some racial and ethnic backgrounds, are less likely to participate in HIV-cure-focused clinical trials 153 . This highlights the need for more nuanced and theoretically engaged research to understand how gender, race, and other characteristics shape engagement with HIV cure research 154 . At the same time, legal and social considerations unique to each context must be identified and addressed. For example, local laws, stigma, and access to healthcare affect research involving the interruption of ART.
There is also a need to better define ethical considerations involved in the selection of populations of interest in which promising cure strategies will be tested. For example, should priority be given to testing new interventions in individuals who initiated treatment during acute infection over those who began treatment during chronic infection? What are the best means to identify and manage the ethical considerations in pediatric HIV cure research? In addition, what measures ought to be taken to ensure that recruitment is not skewed toward people with HIV in resource-limited settings? Further, ethical questions of equity and justice related to the distribution of safe and effective cure interventions must consider acceptability, scalability, and cost-effectiveness. The COVID-19 pandemic has raised unique considerations for research participants, staff, and communities 155 . Given the rapidly changing nature of the pandemic and the availability of COVID-19 vaccines and other treatments, there is a need to continually revise and assess the safety and feasibility of HIV cure research efforts.
During the study design phase, early engagement is needed in communities where research is being considered in order to determine the nature and acceptability of research-related risks. Similarly, stakeholder perceptions should be elicited to guide the development of target product profiles (the minimal and optimal characteristics of a new therapeutic intervention), as recently done for an HIV cure 5 . In especially complex clinical studies, formative research should be used to help develop a robust, informed consent process. Furthermore, nested social and behavioral research (basic, elemental, supportive, integrative) is needed to enhance understanding of the actual experiences of trial participants as well as of sexual partners of participants. These data will help provide a check on current practices, as well as provide a foundation for future efforts aimed at improving them.
Several of the key topics addressed in the previous sections are prerequisites for the development of successful cure strategies and interventions. To date, most HIV cure research has been restricted to high-income countries with relatively low HIV burden and has most often engaged men who have sex with men. HIV strains are genetically and biologically diverse, and host mechanisms of antiviral immunity required for durable control may differ by sex, geography, and ethnicity. Basic discovery research and clinical trials in resource-limited settings must be strengthened and will require enabling infrastructure development and capacity building.
In the next decade, we expect to see a greater understanding of HIV reservoirs, an increasing number of clinical trials and hopefully reports of individuals who achieved long-term remission with less intensive and more widely applicable strategies. On the basis of the current understanding and lessons from ART, it is likely that combinations of these approaches may be the first approach to be implemented. Inclusion of knowledge from fields such as oncology and COVID-19 could also greatly facilitate progress. Finally, open and responsible communication about trials and realistic expectations will remain important. Although safety is the highest priority, with increasing number of clinical trials, there is an increase in the possibility of adverse events which will need to be appropriately managed while allowing the field to advance.
This global scientific strategy, in combination with the recently developed target product profile 5 , will assist with guiding the field toward a widely applicable, acceptable, and affordable cure. The establishment of the HIV Cure Africa Acceleration Partnership 152 will hopefully enable broader engagement and facilitate rapid implementation of any successes into low- and middle-income settings. Fortunately, the resources for such work remain available, and the field is highly committed to making the long-term commitments necessary to develop an effective and scalable remission or cure strategy.
Hutter, G. et al. Long-term control of HIV by CCR5 ∆32/∆32 stem-cell transplantation. N. Engl. J. Med. 360 , 692–698 (2009).
Article PubMed Google Scholar
Gupta, R. K. et al. HIV-1 remission following CCR5∆32/∆32 haematopoietic stem-cell transplantation. Nature 568 , 244–248 (2019).
Article CAS PubMed PubMed Central Google Scholar
Jiang, C. et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 585 , 261–267 (2020).
Saez-Cirion, A. et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 9 , e1003211 (2013).
Lewin, S. R. et al. Multi-stakeholder consensus on a target product profile for an HIV cure. Lancet HIV 8 , e42–e50 (2021).
International, A. S. S. W. G. O. H. I. V. C. et al. Towards an HIV cure: a global scientific strategy. Nat. Rev. Immunol. 12 , 607–614 (2012).
Article CAS Google Scholar
Deeks, S. G. et al. International AIDS Society global scientific strategy: towards an HIV cure 2016. Nat. Med. 22 , 839–850 (2016).
Eriksson, S. et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 9 , e1003174 (2013).
Schaller, T. et al. HIV-1 capsid–cyclophilin interactions determine nuclear import pathway, integration targeting and replication efficiency. PLoS Pathog. 7 , e1002439 (2011).
Cohn, L. B. et al. HIV-1 integration landscape during latent and active infection. Cell 160 , 420–432 (2015).
Lee, G. Q. et al. Clonal expansion of genome-intact HIV-1 in functionally polarized Th1 CD4 + T cells. J. Clin. Investig. 127 , 2689–2696 (2017).
Article PubMed PubMed Central Google Scholar
Simonetti, F. R. et al. Clonally expanded CD4 + T cells can produce infectious HIV-1 in vivo. Proc. Natl Acad. Sci. USA 113 , 1883–1888 (2016).
Lorenzi, J. C. et al. Paired quantitative and qualitative assessment of the replication-competent HIV-1 reservoir and comparison with integrated proviral DNA. Proc. Natl Acad. Sci. USA 113 , E7908–E7916 (2016).
Bui, J. K. et al. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 13 , e1006283 (2017).
Article PubMed PubMed Central CAS Google Scholar
Halvas, E. K. et al. HIV-1 viremia not suppressible by antiretroviral therapy can originate from large T cell clones producing infectious virus. J. Clin. Invest. 130 , 5847–5857 (2020).
Fletcher, C. V. et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl Acad. Sci. USA 111 , 2307–2312 (2014).
McManus, W. R. et al. HIV-1 in lymph nodes is maintained by cellular proliferation during antiretroviral therapy. J. Clin. Invest. 129 , 4629–4642 (2019).
Neidleman, J. et al. Phenotypic analysis of the unstimulated in vivo HIV CD4 T cell reservoir. eLife 9 , e60933 (2020).
Gosselin, A. et al. HIV persists in CCR6 + CD4 + T cells from colon and blood during antiretroviral therapy. AIDS 31 , 35–48 (2017).
Article CAS PubMed Google Scholar
Fromentin, R. et al. CD4 + T cells expressing PD-1, TIGIT and LAG-3 contribute to HIV persistence during ART. PLoS Pathog. 12 , e1005761 (2016).
Anderson, J. L. et al. Human immunodeficiency virus (HIV)-Infected CCR6 + rectal CD4 + T cells and HIV persistence on antiretroviral therapy. J. Infect. Dis. 221 , 744–755 (2020).
Imamichi, H. et al. Defective HIV-1 proviruses produce novel protein-coding RNA species in HIV-infected patients on combination antiretroviral therapy. Proc. Natl Acad. Sci. USA 113 , 8783–8788 (2016).
Imamichi, H. et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl Acad. Sci. USA 117 , 3704–3710 (2020).
Pollack, R. A. et al. Defective HIV-1 proviruses are expressed and can be recognized by cytotoxic T lymphocytes, which shape the proviral landscape. Cell Host Microbe 21 , 494–506(2017).
Wu, G. et al. Gag p24 is a marker of HIV expression in tissues and correlates with immune response. J. Infect. Dis. 224 , 1593–1598 (2021).
Article PubMed CAS PubMed Central Google Scholar
Spudich, S. et al. Persistent HIV-infected cells in cerebrospinal fluid are associated with poorer neurocognitive performance. J. Clin. Invest. 129 , 3339–3346 (2019).
Maldarelli, F. et al. HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345 , 179–183 (2014).
Wagner, T. A. et al. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345 , 570–573 (2014).
Mendoza, P. et al. Antigen-responsive CD4 + T cell clones contribute to the HIV-1 latent reservoir. J. Exp. Med. 217 , e20200051 (2020).
Simonetti, F. R. et al. Antigen-driven clonal selection shapes the persistence of HIV-1-infected CD4 + T cells in vivo. J. Clin. Invest. 131 , 145254 (2021).
Chomont, N. et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 15 , 893–900 (2009).
Ren, Y. et al. BCL-2 antagonism sensitizes cytotoxic T cell-resistant HIV reservoirs to elimination ex vivo. J. Clin. Investig. 130 , 2542–2559 (2020).
Kuo, H. H. et al. Anti-apoptotic protein BIRC5 maintains survival of HIV-1-infected CD4 + T cells. Immunity 48 , 1183–1194(2018).
Cummins, N. W. et al. Maintenance of the HIV reservoir is antagonized by selective BCL2 inhibition. J. Virol. 91 , e00012-17 (2017).
Scully, E. P. Sex differences in HIV infection. Curr. HIV/AIDS Rep. 15 , 136–146 (2018).
Das, B. et al. Estrogen receptor-1 is a key regulator of HIV-1 latency that imparts gender-specific restrictions on the latent reservoir. Proc. Natl Acad. Sci. USA 115 , E7795–E7804 (2018).
Prodger, J. L. et al. Reduced HIV-1 latent reservoir outgrowth and distinct immune correlates among women in Rakai, Uganda. JCI Insight 5 , e139287 (2020).
Falcinelli, S. D. et al. Impact of biological sex on immune activation and frequency of the latent HIV reservoir during suppressive antiretroviral therapy. J. Infect. Dis. 222 , 1843–1852 (2020).
Scully, E. P. et al. Sex-based differences in human immunodeficiency virus type 1 reservoir activity and residual immune activation. J. Infect. Dis. 219 , 1084–1094 (2019).
Szotek, E. L., Narasipura, S. D. & Al-Harthi, L. 17β-Estradiol inhibits HIV-1 by inducing a complex formation between β-catenin and estrogen receptor alpha on the HIV promoter to suppress HIV transcription. Virology 443 , 375–383 (2013).
Abdel-Mohsen, M. et al. Recommendations for measuring HIV reservoir size in cure-directed clinical trials. Nat. Med. 26 , 1339–1350 (2020).
Gaebler, C. et al. Sequence evaluation and comparative analysis of novel assays for intact proviral HIV-1 DNA. J. Virol. 95 , e01986-20 (2021).
Bruner, K. M. et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat. Med. 22 , 1043–1049 (2016).
Peluso, M. J. et al. Differential decay of intact and defective proviral DNA in HIV-1-infected individuals on suppressive antiretroviral therapy. JCI Insight 5 , 132997 (2020).
Gandhi, R. T. et al. Selective decay of intact HIV-1 proviral DNA on antiretroviral therapy. J. Infect. Dis. 223 , 225–233 (2020).
Anderson, E. M. et al. Dynamic shifts in the HIV proviral landscape during long term combination antiretroviral therapy: implications for persistence and control of HIV infections. Viruses 12 , E136 (2020).
Article PubMed CAS Google Scholar
Sarabia, I. & Bosque, A. HIV-1 latency and latency reversal: does subtype matter? Viruses 11 , 1104 (2019).
Yukl, S. A. et al. HIV latency in isolated patient CD4 + T cells may be due to blocks in HIV transcriptional elongation, completion, and splicing. Sci. Transl. Med. 10 , eaap9927 (2018).
Pardons, M. et al. Single-cell characterization and quantification of translation-competent viral reservoirs in treated and untreated HIV infection. PLoS Pathog. 15 , e1007619 (2019).
Santangelo, P. J. et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat. Methods 12 , 427–432 (2015).
McMahon, J. H. et al. A clinical trial of non-invasive imaging with an anti-HIV antibody labelled with copper-64 in people living with HIV and uninfected controls. EBioMedicine 65 , 103252 (2021).
De Scheerder, M. A. et al. HIV rebound is predominantly fueled by genetically identical viral expansions from diverse reservoirs. Cell Host Microbe 26 , 347–358(2019).
Mitchell, J. L. et al. Plasmacytoid dendritic cells sense HIV replication before detectable viremia following treatment interruption. J. Clin. Invest. 130 , 2845–2858 (2020).
Draz, M. S. et al. DNA engineered micromotors powered by metal nanoparticles for motion based cellphone diagnostics. Nat. Commun. 9 , 4282 (2018).
Richart, V. et al. High rate of long-term clinical events after ART resumption in HIV-positive patients exposed to antiretroviral therapy interruption. AIDS https://doi.org/10.1097/QAD.0000000000003058 (2021).
Mendoza, D. et al. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood 119 , 4645–4655 (2012).
Canoui, E. et al. A subset of extreme human immunodeficiency virus (HIV) controllers is characterized by a small HIV blood reservoir and a weak T-cell activation level. Open Forum Infect. Dis. 4 , ofx064 (2017).
Casado, C. et al. Permanent control of HIV-1 pathogenesis in exceptional elite controllers: a model of spontaneous cure. Sci. Rep. 10 , 1902 (2020).
Namazi, G. et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) Study: posttreatment controllers identified from 14 clinical studies. J. Infect. Dis. 218 , 1954–1963 (2018).
Galvez, C. et al. Extremely low viral reservoir in treated chronically HIV-1-infected individuals. EBioMedicine 57 , 102830 (2020).
Passaes, C. et al. Optimal maturation of the SIV-specific CD8 + T cell response after primary infection is associated with natural control of SIV: ANRS SIC Study. Cell Rep. 32 , 108174 (2020).
Bertagnolli, L. N. et al. Autologous IgG antibodies block outgrowth of a substantial but variable fraction of viruses in the latent reservoir for HIV-1. Proc. Natl Acad. Sci. USA 117 , 32066–32077 (2020).
Gondim, M. V. P. et al. Heightened resistance to host type 1 interferons characterizes HIV-1 at transmission and after antiretroviral therapy interruption. Sci. Transl. Med. 13 , eabd8179 (2021).
Huot, N. et al. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat. Med. 23 , 1277–1286 (2017).
Madec, Y. et al. Natural history of HIV-control since seroconversion. AIDS 27 , 2451–2460 (2013).
Chun, T. W. et al. Effect of antiretroviral therapy on HIV reservoirs in elite controllers. J. Infect. Dis. 208 , 1443–1447 (2013).
Nixon, C. C. et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 578 , 160–165 (2020).
McBrien, J. B. et al. Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8 + cells. Nature 578 , 154–159 (2020).
Borducchi, E. N. et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature 540 , 284–287 (2016).
Gautam, R. et al. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. J. Virol. 86 , 8516–8526 (2012).
Okoye, A. A. et al. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound. Nat. Med. 24 , 1430–1440 (2018).
Khanal, S. et al. In vivo validation of the viral barcoding of simian immunodeficiency virus SIV mac239 and the development of new barcoded SIV and subtype B and C simian–human immunodeficiency viruses. J. Virol. 94 , e01420-19 (2019).
Elsheikh, M. M., Tang, Y., Li, D. & Jiang, G. Deep latency: a new insight into a functional HIV cure. EBioMedicine 45 , 624–629 (2019).
Kessing, C. F. et al. In vivo suppression of HIV rebound by didehydro-cortistatin A, a ‘block-and-lock’ strategy for HIV-1 treatment. Cell Rep. 21 , 600–611 (2017).
Margolis, D. M. et al. Curing HIV: seeking to target and clear persistent infection. Cell 181 , 189–206 (2020).
Hosmane, N. N. et al. Proliferation of latently infected CD4 + T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J. Exp. Med. 214 , 959–972 (2017).
Kok, Y. L. et al. Spontaneous reactivation of latent HIV-1 promoters is linked to the cell cycle as revealed by a genetic-insulators-containing dual-fluorescence HIV-1-based vector. Sci. Rep. 8 , 10204 (2018).
Singh, A., Razooky, B., Cox, C. D., Simpson, M. L. & Weinberger, L. S. Transcriptional bursting from the HIV-1 promoter is a significant source of stochastic noise in HIV-1 gene expression. Biophys. J. 98 , L32–L34 (2010).
Cillo, A. R. et al. Quantification of HIV-1 latency reversal in resting CD4 + T cells from patients on suppressive antiretroviral therapy. Proc. Natl Acad. Sci. USA 111 , 7078–7083 (2014).
Laird, G. M. et al. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J. Clin. Invest. 125 , 1901–1912 (2015).
Zerbato, J. M. et al. Multiply spliced HIV RNA is a predictive measure of virus production ex vivo and in vivo following reversal of HIV latency. EBioMedicine 65 , 103241 (2021).
Huang, S. H. et al. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8 + T cells. J. Clin. Investig. 128 , 876–889 (2018).
Gay, C. L. et al. Assessing the impact of AGS-004, a dendritic cell-based immunotherapy, and vorinostat on persistent HIV-1 Infection. Sci. Rep. 10 , 5134 (2020).
Fidler, S. et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): a phase 2, randomised trial. Lancet 395 , 888–898 (2020).
Gutiérrez, C. et al. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 30 , 1385–1392 (2016).
Vibholm, L. et al. Short-course Toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin. Infect. Dis. 64 , 1686–1695 (2017).
Riddler, S. A. et al. Vesatolimod, a toll-like receptor 7 agonist, induces immune activation in virally suppressed adults with HIV-1. Clin. Infect. Dis. 72 , e815–e824 (2020).
Elliott, J. H. et al. Short-term administration of disulfiram for reversal of latent HIV infection: a phase 2 dose-escalation study. Lancet HIV 2 , 17 (2015).
Article Google Scholar
McMahon, J. H. et al. Neurotoxicity with high dose disulfiram and vorinostat used for HIV latency reversal. AIDS https://doi.org/10.1097/qad.0000000000003091 (2021).
Kim, Y., Anderson, J. L. & Lewin, S. R. Getting the ‘kill’ into ‘shock and kill’: strategies to eliminate latent HIV. Cell Host Microbe 23 , 14–26 (2018).
Mousseau, G. et al. The tat inhibitor didehydro-cortistatin A prevents HIV-1 reactivation from latency. mBio 6 , e00465 (2015).
Ahlenstiel, C. et al. Novel RNA duplex locks HIV-1 in a latent state via chromatin-mediated transcriptional silencing. Mol. Ther. Nucleic Acids 4 , e261 (2015).
Besnard, E. et al. The mTOR complex controls HIV latency. Cell Host Microbe 20 , 785–797 (2016).
Mori, L. et al. The XPB subunit of the TFIIH complex plays a critical role in HIV-1 transcription and XPB inhibition by spironolactone prevents HIV-1 reactivation from latency. J Virol. 95 , e01247-20 (2020).
Timmons, A. et al. HSF1 inhibition attenuates HIV-1 latency reversal mediated by several candidate LRAs in vitro and ex vivo. Proc. Natl Acad. Sci. USA 117 , 15763–15771 (2020).
Yeh, Y. J. et al. Filgotinib suppresses HIV-1-driven gene transcription by inhibiting HIV-1 splicing and T cell activation. J. Clin. Invest. 130 , 4969–4984 (2020).
Gavegnano, C. et al. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob. Agents Chemother. 58 , 1977–1986 (2014).
Marconi, V. C. et al. Randomized trial of ruxolitinib in antiretroviral-treated adults with HIV. Clin. Infect. Dis. ciab212 (2021).
Henrich, T. J. et al. Everolimus, an mTORC1/2 inhibitor, in ART-suppressed individuals who received solid organ transplantation: A prospective study. Am. J. Transplant 21 , 1765–1779 (2020).
Goonetilleke, N., Clutton, G., Swanstrom, R. & Joseph, S. B. Blocking formation of the stable HIV reservoir: a new perspective for HIV-1 cure. Front. Immunol. 10 , 1966 (2019).
Abrahams, M. R. et al. The replication-competent HIV-1 latent reservoir is primarily established near the time of therapy initiation. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaw5589 (2019).
Brodin, J. et al. Establishment and stability of the latent HIV-1 DNA reservoir. eLlfe 5 , e18889 (2016).
Google Scholar
Borducchi, E. N. et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563 , 360–364 (2018).
SenGupta, D. et al. The TLR7 agonist vesatolimod induced a modest delay in viral rebound in HIV controllers after cessation of antiretroviral therapy. Sci. Transl. Med. 13 , eabg3071 (2021).
Mothe, B. et al. HIVconsv vaccines and romidepsin in early-treated HIV-1-infected individuals: safety, immunogenicity and effect on the viral reservoir (Study BCN02). Front. Immunol. 11 , 823 (2020).
Leth, S. et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV 3 , e463–e472 (2016).
Conway, J. M. & Perelson, A. S. Post-treatment control of HIV infection. Proc. Natl Acad. Sci. USA 112 , 5467–5472 (2015).
Hansen, S. G. et al. Immune clearance of highly pathogenic SIV infection. Nature 502 , 100–104 (2013).
Steichen, J. M. et al. A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses. Science 366 , eaax4380 (2019).
Rasmussen, T. et al. Impact of anti-PD-1 and anti-CTLA-4 on the HIV reservoir in people living with HIV with cancer on antiretroviral therapy: The AIDS Malignancy Consortium-095 study. Clin. Infect. Dis. 73 , e1973–e1981 (2020).
Riddler, S. A. et al. Vesatolimod, a toll-like Receptor 7 agonist, induces immune activation in virally suppressed adults living with human immunodeficiency virus-1. Clin. Infect. Dis. 72 , e815–e824 (2021).
Papasavvas, E. et al. Safety, immune, and antiviral effects of pegylated interferon alpha 2b administration in antiretroviral therapy-suppressed individuals: results of pilot clinical trial. AIDS Res Hum. Retroviruses 37 , 433–443 (2021).
Mendoza, P. et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561 , 479–484 (2018).
Colby, D. J. et al. Safety and immunogenicity of Ad26 and MVA vaccines in acutely treated HIV and effect on viral rebound after antiretroviral therapy interruption. Nat. Med. 26 , 498–501 (2020).
Salgado, M. et al. Mechanisms that contribute to a profound reduction of the hiv-1 reservoir after allogeneic stem cell transplant. Ann. Intern. Med. 169 , 674–683 (2018).
Koelsch, K. K. et al. Impact of allogeneic hematopoietic stem cell transplantation on the HIV Reservoir and immune response in 3 hiv-infected individuals. J. Acquired Immune Defic. Syndromes 75 , 328–337 (2017).
Yukl, S. A. et al. Challenges in detecting HIV persistence during potentially curative interventions: a study of the Berlin patient. PLoS Pathog. 9 , e1003347 (2013).
Gupta, R. K. et al. Evidence for HIV-1 cure after CCR5∆32/∆32 allogeneic haemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV 7 , e340–e347 (2020).
Tebas, P. et al. CCR5-edited CD4 + T cells augment HIV-specific immunity to enable post-rebound control of HIV replication. J. Clin. Invest. 131 , e144486 (2021).
Article CAS PubMed Central Google Scholar
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 370 , 901–910 (2014).
Martinez-Navio, J. M. et al. Adeno-associated virus delivery of anti-HIV monoclonal antibodies can drive long-term virologic suppression. Immunity 50 , 567–575(2019).
Priddy, F. H. et al. Adeno-associated virus vectored immunoprophylaxis to prevent HIV in healthy adults: a phase 1 randomised controlled trial. Lancet HIV 6 , e230–e239 (2019).
Gardner, M. R. et al. AAV-delivered eCD4-Ig protects rhesus macaques from high-dose SIV mac239 challenges. Sci. Transl. Med. 11 , eaau5409 (2019).
Mancuso, P. et al. CRISPR based editing of SIV proviral DNA in ART treated non-human primates. Nat. Commun. 11 , 6065 (2020).
Karpinski, J. et al. Directed evolution of a recombinase that excises the provirus of most HIV-1 primary isolates with high specificity. Nat. Biotechnol. 34 , 401–409 (2016).
Schuster, S. J. et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N. Engl. J. Med. 377 , 2545–2554 (2017).
Herzig, E. et al. Attacking Latent HIV with convertible CAR-T Cells, a highly adaptable killing platform. Cell 179 , 880–894 e810 (2019).
Rust, B. J. et al. Robust expansion of HIV CAR T cells following antigen boosting in ART-suppressed nonhuman primates. Blood 136 , 1722–1734 (2020).
Tombacz, I. et al. Highly efficient CD4 + T cell targeting and genetic recombination using engineered CD4 + cell-homing mRNA-LNPs. Mol. Ther. (2021).
Nahmad, A. D. et al. Engineered B cells expressing an anti-HIV antibody enable memory retention, isotype switching and clonal expansion. Nat. Commun. 11 , 5851 (2020).
Huang, D. et al. Vaccine elicitation of HIV broadly neutralizing antibodies from engineered B cells. Nat. Commun. 11 , 5850 (2020).
Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385 , 493–502 (2021).
Adair, J. E. et al. Towards access for all: 1st Working Group Report for the Global Gene Therapy Initiative (GGTI). https://doi.org/10.1038/s41434-021-00284-4 (2021).
Persaud, D. et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N. Engl. J. Med. 369 , 1828–1835 (2013).
Luzuriaga, K. et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N. Engl. J. Med. 372 , 786–788 (2015).
Frange, P. et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 3 , e49–e54 (2016).
Violari, A. et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat. Commun. 10 , 412 (2019).
Mavigner, M. et al. Simian immunodeficiency virus persistence in cellular and anatomic reservoirs in antiretroviral therapy-suppressed infant rhesus macaques. J. Virol. 92 , e00562-18 (2018).
Obregon-Perko, V. et al. Simian–human immunodeficiency virus SHIV.C.CH505 persistence in ART-suppressed infant macaques is characterized by elevated SHIV RNA in the gut and a high abundance of intact SHIV DNA in naive CD4 + T cells. J. Virol. 95 , e01669-20 (2020).
Bricker, K. M. et al. Therapeutic vaccination of SIV-infected, ART-treated infant rhesus macaques using Ad48/MVA in combination with TLR-7 stimulation. PLoS Pathog. 16 , e1008954 (2020).
Hessell, A. J. et al. Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat. Med. 22 , 362–368 (2016).
Shapiro, M. B. et al. Single-dose bNAb cocktail or abbreviated ART post-exposure regimens achieve tight SHIV control without adaptive immunity. Nat. Commun. 11 , 70 (2020).
Dhummakupt, A. et al. Differences in inducibility of the latent HIV reservoir in perinatal and adult infection. JCI Insight 5 , e134105 (2020).
Article PubMed Central Google Scholar
Hill, A. L. et al. Real-time predictions of reservoir size and rebound time during antiretroviral therapy interruption trials for HIV. PLoS Pathog. 12 , e1005535 (2016).
Julg, B. et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials-report of a consensus meeting. Lancet HIV 6 , e259–e268 (2019).
Garner, S. A. et al. Interrupting antiretroviral treatment in HIV cure research: scientific and ethical considerations. J. Virus Erad. 3 , 82–84 (2017).
Protiere, C. et al. Differences in HIV cure clinical trial preferences of French people living with HIV and physicians in the ANRS-APSEC study: a discrete choice experiment. J. Int AIDS Soc. 23 , e25443 (2020).
Peluso, M. J. et al. A collaborative, multidisciplinary approach to HIV transmission risk mitigation during analytic treatment interruption. J. Virus Erad. 6 , 34–37 (2020).
Dybul, M. et al. The case for an HIV cure and how to get there. Lancet HIV 8 , e51–e58 (2021).
Johnston, R. E. & Heitzeg, M. M. Sex, age, race and intervention type in clinical studies of HIV cure: a systematic review. AIDS Res Hum. Retroviruses 31 , 85–97 (2015).
Dube, K. et al . Considerations for increasing racial, ethnic, gender, and sexual diversity in HIV cure-related research with analytical treatment interruptions: a qualitative inquiry. https://doi.org/10.1089/AID.2021.0023 (2021).
Fidler, S. et al. HIV cure research in the time of COVID-19 — antiretroviral therapy treatment interruption trials: a discussion paper. J. Virus Erad. 7 , 100025 (2021).
Download references
Acknowledgements
We acknowledge the generous contribution of all the participants in the working groups, the key opinion leaders who read and provided feedback on the strategy, participants in the online survey and secretarial support from the International AIDS Society. S.R.L. and S.G.D. are funded by National Institutes of Health Delaney AIDS Research Enterprise (DARE) Collaboratory (UM1AI126611 and UM1AI164560). S.R.L. is also funded by the National Health and Medical Research Council (NHMRC; grant number GNT1149990) of Australia and the Australian Centre for HIV and Hepatitis. R.B.J. is funded by the NIH UM1AI64565. C.T.T. is funded by the South African Research Chairs Initiative of the Department of Science and Innovation and National Research Foundation of South Africa (grant 84177). O.L. is funded by the ANRS, Sidaction, University Paris Saclay, Inserm, and CEA (Commissariat à l’Energie Atomique). P.C. is funded by the NIH (HL156247 and AI164561); N.A. is funded by the NIH Delaney CARE Collaboratory 1UM1AI126619 and from R01AI134363; T.N. is funded by the South African Research Chairs Initiative of the Department of Science and Innovation and National Research Foundation of South Africa (grant 64809), The Bill and Melinda Gates Foundation (INV-033558), the International AIDS Vaccine Initiative (UKZNRSA1001) and DFG German-African Network grant (grant number AL 1043/6-1).
Author information
Authors and affiliations.
University of California San Francisco, San Fransisco, CA, USA
Steven G. Deeks & Steven Deeks
UNC HIV Cure Center, Department of Medicine, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, NC, USA
- Nancie Archin
University of Southern California, Los Angeles, CA, USA
- Paula Cannon
HIV i-Base, London, UK
Simon Collins
Weill Cornell Medicine, Cornell University, New York, NY, USA
- R. Brad Jones
Aidsfonds, Amsterdam, the Netherlands
Marein A. W. P. de Jong & Marein de Jong
University Paris Saclay, AP-HP, Bicêtre Hospital, UMR1184 INSERM CEA, Le Kremlin Bicêtre, Paris, France
- Olivier Lambotte
International AIDS Society, Geneva, Switzerland
Rosanne Lamplough
Africa Health Research Institute and University of KwaZulu-Natal, Durban, South Africa
- Thumbi Ndung’u
University College London, London, UK
Ragon Institute of MGH, MIT and Harvard University, Cambridge, MA, USA
Thumbi Ndung’u & Krista Dong
Berman Institute of Bioethics and Department of Medicine, Johns Hopkins University, Baltimore, MD, USA
- Jeremy Sugarman
National Institute for Communicable Diseases and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- Caroline T. Tiemessen
UZ Ghent, Ghent, Belgium
- Linos Vandekerckhove
Victorian Infectious Diseases Service, The Royal Melbourne Hospital at the Peter Doherty Institute for Infection and Immunity, Melbourne, Australia
Sharon R. Lewin
Department of Infectious Diseases, Alfred Hospital and Monash University, Melbourne, Australia
Department of Infectious Diseases, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity, Melbourne, Australia
Sharon R. Lewin & Sharon Lewin
UKZN, Durban, South Africa
Zaza Ndhlovu
Centre de Recherche du CHUM and Université de Montréal, Montreal, Canada
Nicolas Chomont
BC Centre for Excellence in HIV/AIDS, Faculty of Health Sciences, Simon Fraser University, Vancouver, Canada
Zabrina Brumme
Sun Yat-sen University, Guangzhou, China
ViiV Healthcare, Branford, CT, USA
Luke Jasenosky
Treatment Action Group, New York, NY, USA
Richard Jefferys
Institut Pasteur, Université de Paris, Unité HIV, Inflammation et Persistance, Paris, France
Aurelio Orta-Resendiz
National Cancer Institute, Center for Cancer Research, Bethesda, MD, USA
Frank Mardarelli
UMC Utrecht, Utrecht, the Netherlands
Monique Nijhuis
Perelmann School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
Katharine Bar & Pablo Tebas
Merck & Co., Inc., Department of Infectious Disease & Vaccines, Kenilworth, NJ, USA
Bonnie Howell
European AIDS treatment group (EATG), Zurich, Switzerland
Alex Schneider
1CONICET – Universidad de Buenos Aires. Instituto de Investigaciones, Biomédicas en Retrovirus y SIDA (INBIRS), Buenos Aires, Argentina
Gabriela Turk
Facultad de Medicina, Departamento de Microbiología, Parasitología e Inmunología, Buenos Aires, Argentina
Makerere University, Makerere, Uganda
Rose Nabatanzi
John Hopkins School of Medicine, Baltimore, MD, USA
Joel Blankson
ICATS, UNC School of Medicine, Chapel Hill, NC, USA
J. Victor Garcia
Emory University School of Medicine, Yerkes National Primate Research Center, Atlanta, GA, USA
Mirko Paiardini
ViiV Healthcare, London, UK
Jan van Lunzen
Chelsea and Westminster Hospital NHS Foundation Trust, London, UK
Christina Antoniadi
Laboratório de AIDS e Imunologia Molecular, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil
Fernanda Heloise Côrtes
Scripps Research Institute, Jupiter, FL, USA
Susana Valente
Aarhus University Hospital, Aarhus, Denmark
Ole S. Søgaard
Universidade Federal de Sao Paulo, Sao Paulo, Brazil
Ricardo Sobhie Diaz
Gladstone Institute of Virology, University of California San Francisco, San Francisco, CA, USA
Melannie Ott
USAHIV Drug Discovery, ViiV Healthcare, Qura Therapeutics, and UNC HIV Cure Center, University of North Carolina at Chapel Hill, Research Triangle Park, NC, USA
Richard (Rick) Dunham
EATG, Berlin, Germany
Siegfried Schwarze
Queen’s University, Kingston, Ontario, Canada
Santiago Perez Patrigeon
MUJHU Care limited, Kampala, Uganda
Josephine Nabukenya
The Rockefeller University, New York, NY, USA
Marina Caskey
IrsiCaixa AIDS Research Institute, HUGTIP, Badalona, Barcelona, Spain
Beatriz Mothe
Chinese Academy of Sciences, National Clinical Research Center for Infectious Diseases, Division of Treatment and Care, National Center for AIDS/STD Control and Prevention, Beijing, China
Fu Sheng Wang
Imperial College London, Department of Infectious Disease, Faculty of Medicine, London, UK
Sarah Fidler
Gilead Sciences, Foster City, CA, USA
Devi SenGupta
European AIDS Treatment Group (EATG), Brussels, Belgium
Stephan Dressler
University of North Carolina Project Malawi, Lilongwe, Malawi
Mitch Matoga
Fred Hutchinson Cancer Research Center, Seattle, WA, USA
Hans-Peter Kiem
Joint Clinical Research Centre, Kampala, Uganda
Cissy Kityo
Caring Cross, Gaithersburg, MD, USA
Boro Dropulic
University of Washington, Seattle, WA, USA
Michael Louella
Advanced Medical and Dental Institute, Universiti Sains Malaysia, Pulau Pinang, Malaysia
Kumitaa Theva Das
Johns Hopkins University School of Medicine, Baltimore, MD, USA
Deborah Persaud
Emory University School of Medicine and Children’s Healthcare of Atlanta, Atlanta, GA, USA
Ann Chahroudi
University of Massachusetts, Worcester, MA, USA
Katherine Luzuriaga
Chulalongkorn University, Bangkok, Thailand
Thanyawee Puthanakit
ImmunityBio, Inc, Culver City, CA, USA
Jeffrey Safrit
Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana
Gaerolwe Masheto
UNC Gillings School of Global Public Health, Chapel Hill, NC, USA
Karine Dubé
La Trobe University, Melbourne, Australia
Jennifer Power
AVAC, New York, NY, USA
Jessica Salzwedel
VARG, Chiang Mai, Thailand
Udom Likhitwonnawut
UCSD AntiViral Research Center, Delaney AIDS Research Enterprise/UCSF, Palm Springs, CA, USA
Jeff Taylor
Social Policy, Gender Identity, and Sexual Orientation Studies Association (SPoD), University of Lucerne MSc Health Sciences, Istanbul, Turkey
Oguzhan Latif Nuh
Rakai Health Sciences Program, Rakai, Uganda
Edward Nelson Kankaka
You can also search for this author in PubMed Google Scholar
Core Leadership Group
- Steven Deeks
- , Sharon Lewin
- , Marein de Jong
- , Rosanne Lamplough
- & Simon Collins
Working Group 1 (Understanding HIV reservoirs)
- , Zaza Ndhlovu
- , Nicolas Chomont
- , Zabrina Brumme
- , Luke Jasenosky
- , Richard Jefferys
- & Aurelio Orta-Resendiz
Working Group 2 (HIV reservoir measurement)
- , Frank Mardarelli
- , Monique Nijhuis
- , Katharine Bar
- , Bonnie Howell
- , Alex Schneider
- , Gabriela Turk
- & Rose Nabatanzi
Working Group 3 (Mechanisms of virus control)
- , Joel Blankson
- , J. Victor Garcia
- , Mirko Paiardini
- , Jan van Lunzen
- , Christina Antoniadi
- & Fernanda Heloise Côrtes
Working Group 4 (Targeting the provirus)
- , Susana Valente
- , Ole S. Søgaard
- , Ricardo Sobhie Diaz
- , Melannie Ott
- , Richard (Rick) Dunham
- , Siegfried Schwarze
- , Santiago Perez Patrigeon
- & Josephine Nabukenya
Working Group 5 (Targeting the immune system)
- , Marina Caskey
- , Beatriz Mothe
- , Fu Sheng Wang
- , Sarah Fidler
- , Devi SenGupta
- , Stephan Dressler
- & Mitch Matoga
Working Group 6 (Cell and gene therapy)
- , Hans-Peter Kiem
- , Pablo Tebas
- , Cissy Kityo
- , Boro Dropulic
- , Michael Louella
- & Kumitaa Theva Das
Working Group 7 (Paediatric remission and cure)
- , Deborah Persaud
- , Ann Chahroudi
- , Katherine Luzuriaga
- , Thanyawee Puthanakit
- , Jeffrey Safrit
- & Gaerolwe Masheto
Working Group 8: (Social, behavioral and ethical aspects of cure)
- , Karine Dubé
- , Jennifer Power
- , Jessica Salzwedel
- , Udom Likhitwonnawut
- , Jeff Taylor
- , Oguzhan Latif Nuh
- , Krista Dong
- & Edward Nelson Kankaka
Contributions
S.G.D., S.R.L., M.D.J. and R.L. developed the method for generating the strategy and oversaw the governance and establishment of the working groups. All authors on the masthead were members of the steering group. All authors of the IAS Global Scientific Strategy writing group contributed to the writing and approved the submitted version of the manuscript. Members of the IAS Global Scientific Strategy working groups are identified in the list at the end of the manuscript.
Corresponding authors
Correspondence to Steven G. Deeks or Sharon R. Lewin .
Ethics declarations
Competing interests.
S.G.D. receives research support from Gilead and Merck. He is a member of the scientific advisory boards for BryoLogyx, Enochian Biosciences and Tendel. He has consulted for AbbVie, Biotron, Eli Lilly, GSK/ViiV and Immunocore; J.S. is a member of Merck KGaA’s Ethics Advisory Panel and Stem Cell Research Oversight Committee; a member of IQVIA’s Ethics Advisory Panel; a member of Aspen Neurosciences Clinical Advisory Panel; a member of a Merck Data Monitoring Committee; a consultant to Biogen; and a consultant to Portola Pharmaceuticals Inc. None of these activities are related to the issues discussed in this manuscript; T.N. has received research funding from Gilead Sciences; O.L. has been paid expert testimony and consultancy fees from BMS France, MSD, Astra Zeneca; consultancy fees from Incyte, Sobi, grants from ViiV and Gilead; L.V. receives research grants from J&J, ViiV Healthcare and Gilead Sciences; P.C. is a member of Gilead’s HIV Cure Advisory Board; S.R.L.’s institution receives funding for investigator initiated research from Gilead, Merck and Viiv. She has research collaborations with BMS, Abbvie and Merck. She has received honoraria paid to her for membership of advisory boards to Gilead, Merck, Viiv, Immunocore, Vaxxinity, Biotron, Esfam and Abivax; R.L. is an employee of the International AIDS Society; M.d.J. was paid as a consultant by the International AIDS Society. S.C., R.B.J., C.T. and N.A. have no interests to declare.
Additional information
Peer review information Nature Medicine thanks Ravindra Gupta and the other, anonymous, reviewers for their contribution to the peer review of this work. Karen O’Leary was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Deeks, S.G., Archin, N., Cannon, P. et al. Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat Med 27 , 2085–2098 (2021). https://doi.org/10.1038/s41591-021-01590-5
Download citation
Received : 12 September 2021
Accepted : 27 October 2021
Published : 01 December 2021
Issue Date : December 2021
DOI : https://doi.org/10.1038/s41591-021-01590-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Balancing public health and privacy rights: a mixed-methods study on disclosure obligations of people living with hiv to their partners in china.
- Zhizhuang Duan
Harm Reduction Journal (2024)
Ripretinib inhibits HIV-1 transcription through modulation of PI3K-AKT-mTOR
- Jin-feng Cai
- Jia-sheng Zhou
Acta Pharmacologica Sinica (2024)
Combating antimicrobial resistance in malaria, HIV and tuberculosis
- Maëlle Duffey
- Robert W. Shafer
- Didier Leroy
Nature Reviews Drug Discovery (2024)
- Raphael J. Landovitz
- Hyman Scott
- Steven G. Deeks
Nature Reviews Microbiology (2023)
Inhibition of the TRIM24 bromodomain reactivates latent HIV-1
- Riley M. Horvath
- Zabrina L. Brumme
- Ivan Sadowski
Scientific Reports (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

New Studies Advance HIV Cure Science

Rejuvenating the Field of AIDS Research

Curing HIV— How Far Have We Come?

Join the Fight in 2024
Amfar, the foundation for aids research, is dedicated to ending the global aids epidemic through innovative research..

Timing Is Everything
Study shows how timing of treatment initiation can significantly affect the HIV reservoir.

Findings from the RISE study underscore the importance of community engagement in Global Fund grantmaking processes.

Accelerating Hepatitis B Vaccination
A rapid vaccine schedule could help individuals at risk in the Asia-Pacific region.
Leading the Way to a Cure
amfAR, The Foundation for AIDS Research, is one of the world’s leading nonprofit organizations dedicated to the support of AIDS research, HIV prevention, treatment education, and advocacy. Since 1985, amfAR has raised nearly $900 million in support of its programs and has awarded more than 3,800 grants to research teams worldwide.

Newest Edition of amfAR Magazine Out Now
Featuring a Q&A with Nobel laureate Dr. Drew Weissman , an interview with amfAR grantee Dr. Ya-Chi Ho , reports on our 2023 research grants , a tribute to AIDS activist Dionne Warwick —and much more.
READ LATEST ISSUE
Research Grants
amfAR utilizes formal requests for proposals to solicit grant applications for both targeted and general HIV/AIDS research. Proposals are then peer reviewed by the Scientific Advisory Committee on the basis of their relevance, scientific merit, and promise. To date, amfAR has awarded more than 3,800 grants to research teams worldwide.

Research Grants Awarded

Request for Proposals: Target Grants REGISTRATION CLOSED
Donate to amfar: fuel lifesaving research.
Make your contribution today and join the fight to #CureAIDS . Your support contributes to the advancement of amfAR’s cutting-edge HIV/AIDS research. Show the world that a cure isn’t just possible— it’s within reach .
Stay Informed
Join our community of supporters and sign up today to receive our monthly e-newsletter Insights and our biannual magazine Innovations .
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- About HIV Surveillance and Monitoring
- National HIV Surveillance System (NHSS)
- National HIV Behavioral Surveillance (NHBS)
- National HIV Prevention Program Monitoring and Evaluation Data
- National HIV Initiatives Data
- Medical Monitoring Project (MMP)
- Surveillance of HIV-Related Service Barriers Among Individuals with Early or Late HIV Diagnoses (SHIELD)
- Tools and Resources
- HIV Public Health Partners
- Ending the HIV Epidemic in the U.S. (EHE)
- Let's Stop HIV Together

Initiatives and Program Monitoring and Evaluation Data

Data Table: Monitoring National HIV Prevention Goals

Select Data Releases

HIV Diagnoses, Deaths, and Prevalence
Estimated HIV Incidence and Prevalence
National HIV Prevention and Care Outcomes
Behavioral and Clinical Characteristics of Persons with Diagnosed HIV
HIV Risk, Prevention, and Testing Behaviors Among Men Who Have Sex with Men
HIV Data Resources
Learn about HIV data systems, various HIV data trends in the United States, as well as how data is used to analyze and prevent further HIV transmission.

- Newsletters
Entertainment
Demi moore, cher and more stars raise money for aids research at amfar gala near cannes.
Louise Dixon
Associated Press
2024 Invision
Demi Moore poses for photographers upon arrival at the amfAR Cinema Against AIDS benefit at the Hotel du Cap-Eden-Roc, during the 77th Cannes international film festival, Cap d'Antibes, southern France, Thursday, May 23, 2024. (Photo by Scott A Garfitt/Invision/AP)
ANTIBES – Some of the biggest stars in the French Rivera for the Cannes Film Festival made appearances at the 30th annual amfAR gala to raise money for AIDS research.
Demi Moore, whose film “The Substance” caused a stir at Cannes, hosted this year's gala, a role launched by Elizabeth Taylor in 1993.
Recommended Videos
The red carpet at the exclusive Hôtel du Cap, Eden Roc, was awash with models, actors, singers and fashion designers as well as plenty of festival movers and shakers, who paid thousands of euros for a table at the hottest event in town.
Michelle Yeoh, Heidi Klum, Kelly Rowland, Andie MacDowell, Diane Kruger, Colman Domingo, Michelle Rodriguez, Winnie Harlow, Robin Thicke, Diplo, Paris Jackson, Petra Nemcova, Karolina Kurkova, Natasha Poly and Sarah Ferguson, the Duchess of York, all attended the fundraiser.
Rowland addressed her Cannes controversy from earlier in the week where she appeared to have an altercation with security on the red carpet.
“The woman knows what happened. I know what happened. And, I have a boundary, and I stand by those boundaries, and that is it. And there were other women that attended that carpet who did not quite look like me, and they didn’t get scolded, or pushed off or told to get off,” Rowland told The Associated Press . "And, I stood my ground and she felt like she had to stand hers but I stood my ground and that was it.”
As always the night began with champagne and cocktails under the stars. The sweeping hotel walkway was transformed into a carpet of red glitter down to the ocean, although this year the flashy super yachts were not blocking the horizon.
Guests drank champagne, margaritas, palomas and vodka martinis and posed for multiple selfies and photos at the black tie charity event.
Many of the works of art and sculptures from the auction were on display around the grounds including an iconic hand signed Andy Warhol lithograph of Taylor, which later raised 350,000 euros ($378,289).
A specially commissioned hand drawn picture of the Queen with Swarovski crystals on an aluminum frame, from royal artist Chris Levine, reached 475,000 euros ($513,392) for the charity. Sarah Ferguson took to the podium to help auction this lot with a plea of “this was my mother-in-law” and “stop being so bored, Stop looking at your phone” to the diners.
Guests were served a starter of truffled zucchini flower with cream of mushroom to the live soundtrack of Jess Glynne’s opening performance, followed by beef brulee with a potato emulsion and a strawberry and elderflower profiterole dessert while auctioneers called for bids.
Part way through the dinner guests paused and took to their feet to line the temporary catwalk through the tables for the fashion runway show curated annually by Carine Roitfeld and auctioned off to the highest bidder. This year’s collection, entitled “Fairy Tales” included 26 looks from Chanel, Dior, Louis Vuitton, Saint Laurent, Vivienne Westwood and Prada to name a few. After the models gathered on stage to help the auctioneer up the bids, the collection finally went for 500,000 euros ($540,412).
As part of the experiences on offer, a walk on part in “Emily in Paris“ series 5, plus an invite to the season 4 LA premiere went for 250,000 euros ($270,262).
Nick Jonas brought brother Joe on stage for a medley that got the room dancing and then Cher closed the show with a tribute to Taylor and a high-octane performance of “Believe” complete with gyrating dancers. The afterparty carried on by the hotel’s swimming pool with Diplo on the decks and more drinks as guests danced outdoors until the early hours.
AmfAR, The Foundation for AIDS Research is dedicated to the support of AIDS research, HIV prevention, treatment education and advocacy, raising nearly $900 million in support of it’s programs since 1985.
Copyright 2024 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed without permission.
Click here to take a moment and familiarize yourself with our Community Guidelines.
Hearing aids linked to slower cognitive decline and longer life
by BARBARA MORSE, NBC 10 NEWS

National Institute of Health funded research last year looked at people who had severe hearing loss who were also at risk for developing dementia.
Researchers found over a three year span that those who received hearing aids saw a nearly 50% reduction in the rate of cognitive decline than those who did not get them.
This year, another study , out of the Keck School of Medicine at USC, showed those who needed, and wore hearing aids, tended to live longer.
"One in 10 people who need hearing aids actually get help," said Samantha Elkas, a hearing care practitioner at The Beltone Hearing Aid Center in South Attleboro, Massachusetts.
That used to be Renee Lerohl, of Cumberland.
About 10 years ago, she began noticing a difference in her hearing.
Over time she had to turn up her TV.
Holding conversations became more challenging.
"I couldn't quite understand what they were saying, what the word was that they were saying," recalled Lerohl.
Last year, she came to Beltone where they offer free hearing testing.
"You know what you are hearing but you don't know what you're not hearing," said Elkas.
Until hearing aids open that door.
- HEALTH CHECK: Doctor provides tips to avoid dangerous drug interactions
"Everything is so much sharper, more clear. I understand words," said Lerohl who admits initially, cost was a hurdle. Then she found out her insurance (United Health Care, which has a partnership with Beltone) would cover a portion of it.
For others, another obstacle is what they look like, or at least used to look like.
"Hearing aids have come a long way so they have gotten a lot smaller," said Elkas.
Some wonder do they really work?
"We have hearing aids that are 150% times better in noise situations than the ones that we had even three years ago," said Elkas.
Lerohl can now connect her hearing aids, via Bluetooth, to her phone and tune out the background noise. She loves that.
Highlighting what she used to be missing.
"When you have one of your senses taken away it affects your mind," said Lerohl.
Which is perhaps why research out of USC this year showed a life-extending benefit for regular hearing aid users.
"It affects your quality of life. just how you're hearing your loved ones, relationships," said Elkas.
Medicare doesn't cover prescription hearing aids but some supplemental plans partially cover the cost.
The National Institute on Aging has an information page on hearing loss.
- My View My View
- Following Following
- Saved Saved
Cher, Demi Moore entertain guests at glamorous Cannes fundraiser for AIDS
- Medium Text

Sign up here.
Reporting by Miranda Murray; Editing by Stephen Coates
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Thomson Reuters
Speed editor on the Berlin hub who provides general coverage on everything from politics to energy in Germany, Austria and Switzerland, with the goal of getting the news out as quickly as possible. Miranda previously worked at the German press agency dpa and Chicago Tribune

Lifestyle Chevron
This airline wants all dogs to fly first class.
With this airline, dogs are ditching the kennels and flying first class.

The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

IMAGES
VIDEO
COMMENTS
A new study shows virus-like particle can effectively 'shock and kill' latent HIV reservoir in those living with chronic HIV. Skip to main content Your source for the latest research news
Since its inception, PEPFAR, which one of us (J. N.) now leads, has ensured crucial access to life-saving treatment for more than 20 million people in at least 50 countries. Twenty years after the ...
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but ...
Nearly $17 billion was spent worldwide on HIV -vaccine research from 2000 to 2021. Nearly $1 billion more is spent annually, according to the Joint United Nations Program on HIV/AIDS and the ...
Blockbuster obesity drug leads to better health in people with HIV. Semaglutide reduces weight and fat accumulation associated with the antiretroviral regimen that keeps HIV at bay. Mariana ...
Center for Clinical AIDS Research and Education, David Geffen School of Medicine, University of California, Los Angeles, CA, USA Raphael J. Landovitz Bridge HIV, San Francisco Department of Public ...
This latest research and guidance are being presented at a time when progress towards ending the global AIDS epidemic has lagged, after the COVID-19 pandemic; but the response is rapidly catching up, with some countries now charting a path to end AIDS, including Australia, Botswana, Eswatini, Rwanda, United Republic of Tanzania, and Zimbabwe ...
Read about The National HIV/AIDS Strategy, our country's whole-of-society approach to end the HIV epidemic in the United States. ... 73 countries gathered in Denver and virtually from March 3-6 this year for CROI, an annual scientific meeting on the latest research that can help accelerate global progress in the response to HIV and other ...
R.T. Gandhi and OthersN Engl J Med 2023;389:2468-2476. A 70-year-old woman with advanced HIV infection was evaluated because of cough, shortness of breath, and malaise. Eleven months earlier, she ...
Interview with Dr. Anthony Fauci on progress made during the past four decades of the HIV/AIDS pandemic and ongoing efforts to end this threat. 18m 44s Download. The dramatic saga of the acquired ...
Since 2010, when he decided to go public, he had interviewed for various magazines: POZ Magazine, New York Magazine and Science Magazine among others and decided to devote his life in supporting research for cures against HIV. In July 2012, he started the Timothy Ray Brown Foundation under World AIDS Institute and has worked with many ...
JASON BEAUBIEN, BYLINE: HIV/AIDS has gotten pushed out of the headlines recently. And while the annual number of new HIV infections has declined in recent years, the disease is still a significant ...
At the National Institutes of Health, the HIV/AIDS research effort is led by the National Institute of Allergy and Infectious Diseases (NIAID). A vast network of NIAID-supported scientists, located on the NIH campus in Bethesda, Maryland, and at research centers around the globe, are exploring new ways to prevent and treat HIV infection, as ...
The latest news on HIV, Aids, HIV research, Aids Research, Aids Studies and HIV medicine. Topics. Conditions. Week's top; Latest news; ... New research traces the spread of HIV in and from Indonesia.
Latest AIDS data, HIV data. Preliminary UNAIDS 2021 epidemiological estimates: 37.6 million [30.2 million-45. million] people globally were living with HIV in 2020. 1.5 million [1.1 million-2.1 million] people became newly infected with HIV in 2020. 690 000 [480 000-1 million] people died from AIDS-related illnesses in 2020. 27.4 million [26.5 million-27.7 million] people
Publishing the very latest ground breaking research on HIV and AIDS. Read by all the top clinicians and researchers, AIDS has the highest impact of all AIDS-related journals. With 18 issues per year, AIDS guarantees the authoritative presentation of significant advances. The Editors, themselves noted international experts who know the demands of your work, are committed to making AIDS the most ...
At the same time, NIAID continues to support research to develop new drugs with unique mechanisms of action for daily antiretroviral therapy. Such drugs likely would be effective against HIV strains with resistance to other drug types. ... see the AIDSinfo Drug Database. Content last reviewed on August 26, 2019. Website Policies & Notices ...
An effective and scalable cure strategy is a top priority for the HIV research field; this Review discusses recent advances, knowledge gaps, and priority research areas for the next 5 years.
New Studies Advance HIV Cure Science. Researchers at CROI report on post-treatment control, HIV reservoirs, and early treatment in children. ... The Foundation for AIDS Research, is one of the world's leading nonprofit organizations dedicated to the support of AIDS research, HIV prevention, treatment education, and advocacy. Since 1985, amfAR ...
One small survey of people living with HIV shows that more people prefer long-acting shots than pills you take every day. Most (73%) who responded to the survey said they were definitely or ...
The HIV/AIDS Prevention Research Synthesis (PRS) Project identifies evidence-based HIV behavioral interventions (EBIs) listed in the Compendium of Evidence-Based HIV Behavioral Interventions to help HIV prevention planners and providers in the United States choose the interventions most appropriate for their communities. On January 1, 2012, CDC ...
Research teams are using discovery medicine trials and new vaccine technologies to identify and stimulate the types of immune responses that hold the most promise for preventing HIV. People with HIV have made priceless contributions to HIV vaccine science by participating in research that teaches us how the human immune system responds to HIV.
Estimated HIV Incidence and Prevalence. Data on estimated HIV incidence, prevalence, and diagnosed infections in the US. National HIV Prevention and Care Outcomes. Behavioral and Clinical Characteristics of Persons with Diagnosed HIV. HIV Risk, Prevention, and Testing Behaviors Among Men Who Have Sex with Men.
AmfAR, The Foundation for AIDS Research is dedicated to the support of AIDS research, HIV prevention, treatment education and advocacy, raising nearly $900 million in support of it's programs ...
With over $1.4 million from the National Institute on Alcohol Abuse and Alcoholism, the UNM group analyzed clinical trials of continuing care for CUD. "One of the conditions examined in these ...
"Hearing aids have come a long way so they have gotten a lot smaller," said Elkas. ... Which is perhaps why research out of USC this year showed a life-extending benefit for regular hearing aid users.
Demi Moore will host the 30th edition of the glitzy charity gala organised by amfAR, The Foundation for AIDS Research, that is held annually alongside the nearby Cannes Film Festival. Following a ...
The Department of Health and Human Services (HHS) published a system of records notice in the Federal Register on May 16, 2024, for new system of records "OCSS Research Platform" maintained by HHS' Administration for Children and Families (ACF), Office of Child Support Services (OCSS). The notice contained Start Printed Page 44991 an ...