The Ultimate Guide to Writing a Medical Abstract

Other pages on the topic:
We'll deliver straight to your inbox.
Key Learnings contained in this article:
In the world of medical research , a well-written abstract is essential. Serving as a concise summary of your entire study, a medical abstract plays a crucial role in both informing and attracting readers. It provides a snapshot of your research, allowing readers to quickly grasp the significance and key findings of your study without having to read the entire paper. To help you master the art of writing a compelling medical abstract, this ultimate guide will provide valuable insights and practical tips.
Understanding the Importance of a Medical Abstract
Before delving into the intricacies of crafting an effective medical abstract, it's essential to understand its significance. A medical abstract acts as a gateway to your research, enticing readers to explore further. It serves as a representation of the quality and relevance of your study, making it crucial to invest time and effort into perfecting this concise summary.
The Role of a Medical Abstract in Research
A medical abstract serves multiple purposes within the realm of research. Firstly, it enables readers to quickly determine whether your study aligns with their area of interest. By reading the abstract, potential readers, such as fellow researchers or medical professionals, can decide if your findings are relevant to their own work or practice. Additionally, abstracts act as a way of archiving and indexing research studies, ensuring that important findings are easily accessible to the wider scientific community.
Moreover, a well-crafted medical abstract can also contribute to the advancement of knowledge in the field. When abstracts are published in scientific journals or presented at conferences , they allow researchers to gain insights into the latest developments and build upon existing studies. This exchange of information fosters collaboration and encourages the growth of scientific knowledge, ultimately leading to improved healthcare practices and patient outcomes.
Key Components That Make a Medical Abstract Effective
To make your medical abstract compelling and informative, certain key components need to be included . Firstly, your abstract should clearly state the purpose and objectives of your study. It should also provide a concise overview of your methodology , outlining the key research methods employed. Additionally, your abstract should highlight the most significant findings and conclusions of your research. Lastly, including key implications or potential applications of your research can further enhance the impact of your abstract.
Furthermore, it is important to consider the language and writing style used in your medical abstract. Clarity and precision are paramount, as the abstract should be easily understood by readers from various backgrounds. Avoiding jargon and using plain language can help ensure that your abstract is accessible to a wider audience. Additionally, paying attention to the overall structure and coherence of your abstract can make it more engaging and enjoyable to read.
Preparing to Write Your Medical Abstract
Before diving into the actual writing process, some preparatory work is necessary to ensure a well-structured and coherent medical abstract. This section will guide you through the crucial steps of gathering, organizing, and identifying the key components of your research.
When embarking on the journey of crafting a medical abstract, it is essential to delve into the depths of your research with a discerning eye. The meticulous process of selecting the most pertinent information is akin to a skilled surgeon carefully choosing the precise instruments for a delicate operation. Each piece of data, every conclusion drawn, must be scrutinised for its relevance and impact, ensuring that only the most vital elements are included in the abstract.
Gathering and Organizing Your Research
Start by reviewing your research paper and identifying the most relevant and impactful aspects to include in your abstract. Carefully select the key findings, methods, and conclusions that best encapsulate the essence of your study. It's important to prioritize brevity and clarity during this stage, as your abstract should provide a concise summary rather than a detailed account of your entire research.
As you sift through the wealth of information at your disposal, imagine yourself as an archaeologist meticulously excavating a site to uncover hidden treasures. Each piece of data unearthed is like a precious artefact, waiting to be polished and presented in your abstract for the world to marvel at.
Identifying Your Key Findings and Conclusions
Once you have selected the most significant aspects of your research, focus on identifying your key findings and conclusions. These should be the main takeaways from your study – the results that have the most impact and significance. Clearly articulating these key points in your abstract will help readers understand the value of your research and its potential implications.
Just as a skilled detective pieces together clues to solve a complex case, you must weave a narrative in your abstract that leads the reader on a journey of discovery. Each key finding and conclusion acts as a breadcrumb, guiding the reader through the labyrinth of your research towards a greater understanding of the implications and applications of your work.
Structuring Your Medical Abstract
Now that you have gathered and organized your research, it's time to structure your medical abstract. A well-structured abstract will guide readers through your study in a logical and engaging manner, keeping them interested and informed.
Before delving into the specifics of structuring your medical abstract, it's important to understand the significance of this concise summary. The abstract serves as a snapshot of your entire research paper, providing readers with a quick overview of your study's purpose, methods, results, and conclusions. It acts as a gateway to your work, enticing readers to delve deeper into the full paper for a comprehensive understanding.
The Four Main Sections of a Medical Abstract
A typical medical abstract consists of four main sections: introduction, methods, results, and conclusion. The introduction should provide context for your research, explaining the problem or gap in knowledge that your study aims to address. The methods section outlines the approach and techniques used in your research. The results section presents the key findings of your study, and the conclusion summarizes the main takeaways and potential implications of your research.
Each section of the abstract plays a crucial role in conveying the essence of your research. The introduction sets the stage, drawing readers in by highlighting the relevance and importance of your study. The methods section acts as a roadmap, detailing the steps you took to conduct your research, ensuring transparency and reproducibility. The results section showcases your findings, providing readers with valuable insights into the outcomes of your study. Finally, the conclusion ties everything together, offering a concise summary of your key findings and their broader implications.
Tips for Writing a Concise and Clear Abstract
When crafting your medical abstract, it is crucial to keep it concise and clear. Avoid excessive jargon, acronyms, or technical terms that may confuse or alienate readers. Use plain language that can be easily understood by a wide range of individuals, including those outside your specific field of study. Remember, the goal is to present your research in a way that is accessible and engaging to a broader audience.
Furthermore, consider the tone and style of your abstract. Aim for a balance between professionalism and readability, ensuring that your abstract is informative yet engaging. By striking this balance, you can effectively communicate the significance of your research while maintaining the interest of your readers. Remember, the abstract is your research paper's first impression, so make it count!
Writing the Introduction of Your Medical Abstract
The introduction of a medical abstract sets the stage for your research. It should succinctly explain the background and rationale behind your study, highlighting the importance and relevance of your research question.
When crafting the introduction of your medical abstract, it is crucial to strike a balance between providing enough context for readers to understand the significance of your research while avoiding unnecessary details that may overwhelm or confuse your audience. A well-crafted introduction can captivate the interest of readers and compel them to delve deeper into your study.
Setting the Context for Your Research
Begin your introduction by providing a brief overview of the field or topic area in which your study is situated. This helps readers understand the broader context and significance of your research. Clearly articulate the problem or research gap that your study aims to address, providing a concise rationale for the importance of your research.
Moreover, consider incorporating recent advancements or key findings in the field to demonstrate the evolving nature of the subject matter. This not only showcases your awareness of current research trends but also positions your study within the larger landscape of scholarly work.
Stating Your Research Objectives
Following the contextual information, state your research objectives. These objectives should clearly outline what you aim to achieve through your study and provide a roadmap for readers to understand the direction and purpose of your research. By clearly stating your research objectives, you set clear expectations for what readers can expect to find in the subsequent sections of your abstract.
Furthermore, consider highlighting the potential implications of your research findings on clinical practice, policy development, or future research directions. This foresight not only underscores the relevance of your study but also showcases the practical applications of your research within the medical field.
Detailing Your Methods and Results
The methods and results sections of your medical abstract provide readers with insight into your research design, data collection, and analysis. These sections should be clear, concise, and informative, allowing readers to understand the scope and integrity of your study.
Describing Your Research Methods
In the methods section, provide a concise overview of the design, participants, and procedures employed in your research. Clearly explain the steps taken to collect and analyze data, ensuring that readers understand the rigor and validity of your study. Be sure to mention any ethical considerations or limitations that may be relevant to your research.
For instance, if your study involved human participants, it is essential to outline the informed consent process and any measures taken to protect their privacy and confidentiality. Additionally, if your research involved animal subjects, it is crucial to mention the ethical approval obtained from the relevant regulatory bodies.
Furthermore, detailing the sample size and characteristics of your participants can provide valuable context to your study. This information allows readers to assess the generalizability of your findings and understand any potential biases that may have influenced the results.
Presenting Your Results Accurately
In the results section, present the key findings of your study. Avoid going into excessive detail, focusing instead on the most significant and impactful outcomes. Utilize clear and concise language to describe your results, ensuring that readers can easily interpret and understand the implications of your findings. Visual aids such as graphs, tables, or charts can be included if they enhance the clarity and comprehensibility of your results.
Moreover, it is important to discuss any unexpected or contradictory results that may have emerged during your research. This demonstrates your scientific integrity and allows readers to gain a more nuanced understanding of your study's outcomes.
Additionally, consider providing a brief discussion of the limitations of your study. This can include factors such as sample size, potential confounding variables, or any methodological constraints that may have influenced the results. Acknowledging these limitations showcases your awareness of the study's boundaries and encourages future researchers to build upon your work.
By following this ultimate guide, you will be well-equipped to confidently write a compelling and informative medical abstract. Remember to prioritize clarity, brevity, and accessibility while showcasing the significance and value of your research. Mastering the art of writing a medical abstract will not only increase the visibility and impact of your work but also contribute to the advancement of medical knowledge.
Therapeutic
- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
- APA Citation Generator
- MLA Citation Generator
- Chicago Citation Generator
- Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources
How to Write a Medical Abstract for Publication
Preparing Your Study, Review, or Article for Publication in Medical Journals
The majority of social, behavioral, biological, and clinical journals follow the conventional structured abstract form with the following four major headings (or variations of these headings):
OBJECTIVE (Purpose; Aim; Goal) : Tells reader the purpose of your research and the questions it intends to answer
METHODS (Setting; Study Design; Participants) : Explains the methods and process so that other researchers can assess, review, and replicate your study.
RESULTS (Findings; Outcomes) : Summarizes the most important findings of your study
CONCLUSIONS (Discussion; Implications; Further Recommendations) : Summarizes the interpretation and implications of these results and presents recommendations for further research
Sample Health/Medical Abstract

Structured Abstracts Guidelines *
- Total Word Count: ~200-300 words (depending on the journal)
- Content: The abstract should reflect only the contents of the original paper (no cited work)
* Always follow the formatting guidelines of the journal to which you are submitting your paper.
Useful Terms and Phrases by Abstract Section
Objective: state your precise research purpose or question (1-2 sentences).
- Begin with “To”: “We aimed to…” or “The objective of this study was to…” using a verb that accurately captures the action of your study.
- Connect the verb to an object phrase to capture the central elements and purpose of the study, hypothesis , or research problem . Include details about the setting, demographics, and the problem or intervention you are investigating.
METHODS : Explain the tools and steps of your research (1-3 sentences)
- Use the past tense if the study has been conducted; use the present tense if the study is in progress.
- Include details about the study design, sample groups and sizes, variables, procedures, outcome measures, controls, and methods of analysis.
RESULTS : Summarize the data you obtained (3-6 sentences)
- Use the past tense when describing the actions or outcomes of the research.
- Include results that answer the research question and that were derived from the stated methods; examine data by qualitative or quantitative means.
- State whether the research question or hypothesis was proven or disproven.
CONCLUSIONS : Describe the key findings (2-5 sentences)
- Use the present tense to discuss the findings and implications of the study results.
- Explain the implications of these results for medicine, science, or society.
- Discuss any major limitations of the study and suggest further actions or research that should be undertaken.
Before submitting your abstract to medical journals, be sure to receive proofreading services from Wordvice, including journal manuscript editing and paper proofreading , to enhance your writing impact and fix any remaining errors.
Related Resources
- 40 Useful Words and Phrases for Top-Notch Essays (Oxford Royale Academy)
- 100+ Strong Verbs That Will Make Your Research Writing Amazing (Wordvice)
- Essential Academic Writing Words and Phrases (My English Teacher.eu)
- Academic Vocabulary, Useful Phrases for Academic Writing and Research Paper Writing (Research Gate)
- How to Compose a Journal Submission Cover Letter (Wordvice/YouTube)
- How to Write the Best Journal Submission Cover Letter (Wordvice)
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
- How to Write an Abstract

Expedite peer review, increase search-ability, and set the tone for your study
The abstract is your chance to let your readers know what they can expect from your article. Learn how to write a clear, and concise abstract that will keep your audience reading.
How your abstract impacts editorial evaluation and future readership
After the title , the abstract is the second-most-read part of your article. A good abstract can help to expedite peer review and, if your article is accepted for publication, it’s an important tool for readers to find and evaluate your work. Editors use your abstract when they first assess your article. Prospective reviewers see it when they decide whether to accept an invitation to review. Once published, the abstract gets indexed in PubMed and Google Scholar , as well as library systems and other popular databases. Like the title, your abstract influences keyword search results. Readers will use it to decide whether to read the rest of your article. Other researchers will use it to evaluate your work for inclusion in systematic reviews and meta-analysis. It should be a concise standalone piece that accurately represents your research.
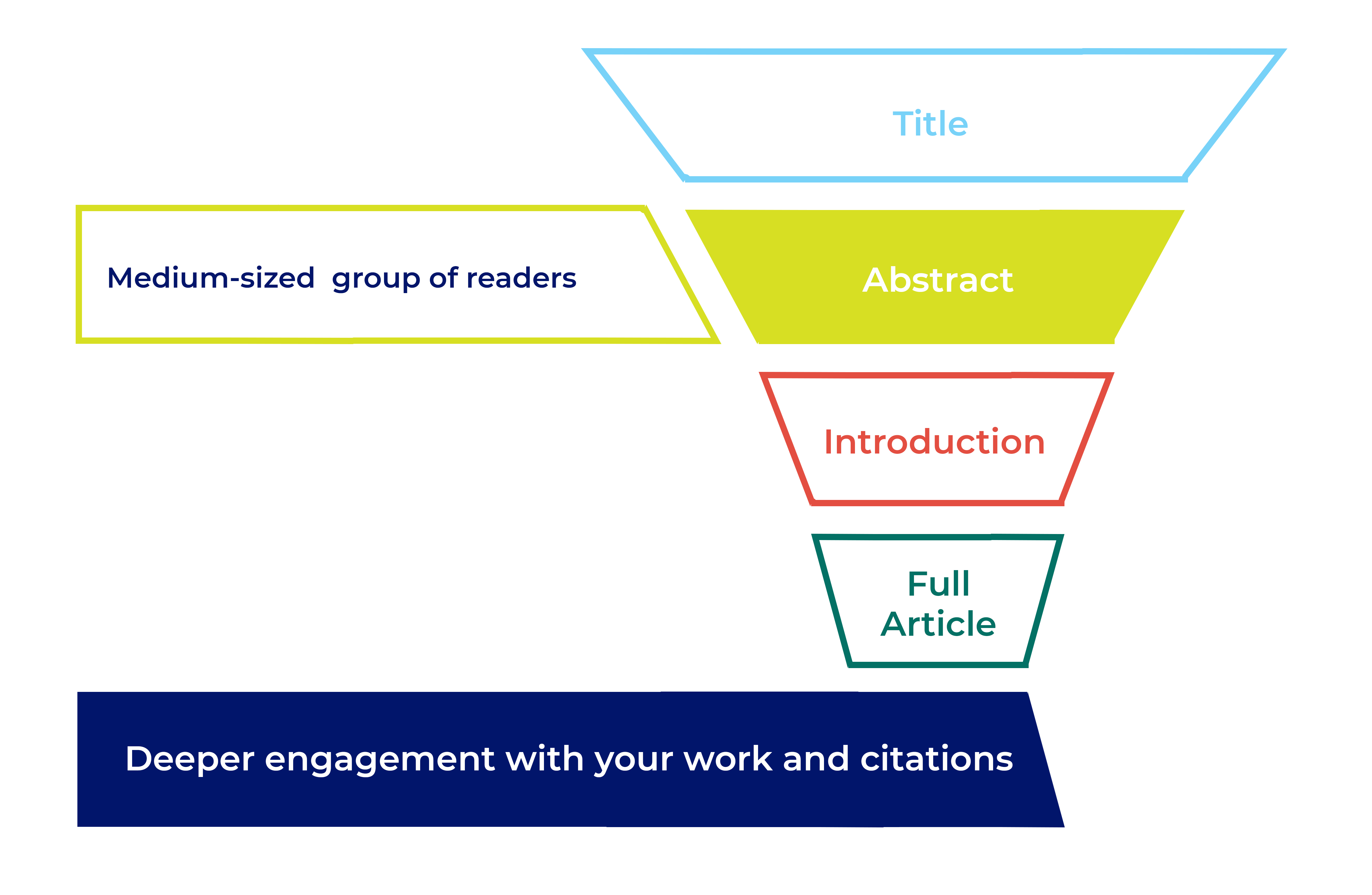
What to include in an abstract
The main challenge you’ll face when writing your abstract is keeping it concise AND fitting in all the information you need. Depending on your subject area the journal may require a structured abstract following specific headings. A structured abstract helps your readers understand your study more easily. If your journal doesn’t require a structured abstract it’s still a good idea to follow a similar format, just present the abstract as one paragraph without headings.
Background or Introduction – What is currently known? Start with a brief, 2 or 3 sentence, introduction to the research area.
Objectives or Aims – What is the study and why did you do it? Clearly state the research question you’re trying to answer.
Methods – What did you do? Explain what you did and how you did it. Include important information about your methods, but avoid the low-level specifics. Some disciplines have specific requirements for abstract methods.
- CONSORT for randomized trials.
- STROBE for observational studies
- PRISMA for systematic reviews and meta-analyses
Results – What did you find? Briefly give the key findings of your study. Include key numeric data (including confidence intervals or p values), where possible.
Conclusions – What did you conclude? Tell the reader why your findings matter, and what this could mean for the ‘bigger picture’ of this area of research.
Writing tips
The main challenge you may find when writing your abstract is keeping it concise AND convering all the information you need to.

- Keep it concise and to the point. Most journals have a maximum word count, so check guidelines before you write the abstract to save time editing it later.
- Write for your audience. Are they specialists in your specific field? Are they cross-disciplinary? Are they non-specialists? If you’re writing for a general audience, or your research could be of interest to the public keep your language as straightforward as possible. If you’re writing in English, do remember that not all of your readers will necessarily be native English speakers.
- Focus on key results, conclusions and take home messages.
- Write your paper first, then create the abstract as a summary.
- Check the journal requirements before you write your abstract, eg. required subheadings.
- Include keywords or phrases to help readers search for your work in indexing databases like PubMed or Google Scholar.
- Double and triple check your abstract for spelling and grammar errors. These kind of errors can give potential reviewers the impression that your research isn’t sound, and can make it easier to find reviewers who accept the invitation to review your manuscript. Your abstract should be a taste of what is to come in the rest of your article.

Don’t
- Sensationalize your research.
- Speculate about where this research might lead in the future.
- Use abbreviations or acronyms (unless absolutely necessary or unless they’re widely known, eg. DNA).
- Repeat yourself unnecessarily, eg. “Methods: We used X technique. Results: Using X technique, we found…”
- Contradict anything in the rest of your manuscript.
- Include content that isn’t also covered in the main manuscript.
- Include citations or references.
Tip: How to edit your work
Editing is challenging, especially if you are acting as both a writer and an editor. Read our guidelines for advice on how to refine your work, including useful tips for setting your intentions, re-review, and consultation with colleagues.
- How to Write a Great Title
- How to Write Your Methods
- How to Report Statistics
- How to Write Discussions and Conclusions
- How to Edit Your Work
The contents of the Peer Review Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
The contents of the Writing Center are also available as a live, interactive training session, complete with slides, talking points, and activities. …
There’s a lot to consider when deciding where to submit your work. Learn how to choose a journal that will help your study reach its audience, while reflecting your values as a researcher…
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- How to write an...
How to write an abstract that will be accepted
- Related content
- Peer review
- Mary Higgins , fellow in maternal fetal medicine 1 ,
- Maeve Eogan , consultant obstetrician and gynaecologist 2 ,
- Keelin O’Donoghue , consultant obstetrician and gynaecologist, and senior lecturer 3 ,
- Noirin Russell , consultant obstetrician and gynaecologist 3
- 1 Mount Sinai Hospital, Toronto, Ontario, Canada
- 2 Rotunda Hospital Dublin, Ireland
- 3 Cork University Maternity Hospital, Ireland
- mairenihuigin{at}gmail.com
Researchers do not always appreciate the importance of producing a good abstract or understand the best way of writing one. Mary Higgins and colleagues share some of the lessons they have learnt as both researchers and reviewers of abstracts
Effective abstracts reflect the time, work, and importance of the scientific research performed in the course of a study. A last minute approach and poor writing may not reflect the good quality of a study.
Between the four of us we have written over 150 published papers, as well as having reviewed numerous abstracts for national and international meetings. Nevertheless, we have all had abstracts rejected, and this experience has emphasised a number of teaching points that could help maximise the impact of abstracts and success on the world, or other, stage.
An abstract is the first glimpse an audience has of a study, and it is the ticket to having research accepted for presentation to a wider audience. For a study to receive the respect it deserves, the abstract should be as well written as possible. In practice, this means taking time to write the abstract, keeping it simple, reading the submission guidelines, checking the text, and showing the abstract to colleagues.
It is important to take the necessary time to write the abstract. Several months or years have been spent on this groundbreaking research, so take the time to show this. Five minutes before the call for abstracts closes is not the time to start putting it together.
Keep it simple, and think about the message that needs to be communicated. Some abstracts churn out lots of unrelated results and then have a conclusion that does not relate to the results, and this is just confusing. Plan what points need to be made, and then think about them a little more.
Read the submission guidelines and keep to the instructions provided in the call for abstracts. Don’t submit an unstructured abstract if the guidance has asked for a structured one. Comply with the word or letter count, and do not go over this.
An abstract comprises five parts of equal importance: the title, introduction and aims, methods, results, and conclusion. Allow enough time to write each part well.
The title should go straight to the point of the study. Make the study sound interesting so that it catches people’s attention. The introduction should include a brief background to the research and describe its aims. For every aim presented there needs to be a corresponding result in the results section. There is no need to go into detail in terms of the background to the study, as those who are reviewing the abstract will have some knowledge of the subject. The methods section can be kept simple—it is acceptable to write “retrospective case-control study” or “randomised controlled trial.”
The results section should be concrete and related to the aims. It is distracting and irritating to read results that have no apparent relation to the professed aims of the study. If something is important, highlight it or put it in italics to make it stand out. Include the number of participants, and ensure recognition is given if 10 000 charts have been reviewed. Equally, a percentage without a baseline number is not meaningful.
In the conclusion, state succinctly what can be drawn from the results, but don’t oversell this. Words like “possibly” and “may” can be useful in this part of the abstract but show that some thought has been put into what the results may mean. This is what divides the good from the not so good. Many people are capable of doing research, but the logical formation of a hypothesis and the argument of its proof are what make a real researcher.
Once you have written the abstract, check the spelling and grammar. Poor spelling or grammar can give the impression that the research is also poor. Show the abstract to the supervisor or principal investigator of the study, as this person’s name will go on the abstract as well. Then show the abstract to someone who knows nothing about the particular area of research but who knows something about the subject. Someone detached from the study might point out the one thing that needs to be said but that has been forgotten.
Then let it go; abstracts are not life and death scenarios. Sometimes an abstract will not be accepted no matter how wonderful it is. Perhaps there is a theme to the meeting, into which the research does not fit. Reviewers may also be looking for particular things. For one conference, we limited the number of case reports so that only about 10% were accepted. It may be that your research is in a popular or topical area and not all abstracts in that area can be chosen. On occasions, politics play a part, and individual researchers have little control over that.
Finally, remember that sometimes even the best reviewer may not appreciate the subtleties of your research and another audience may be more appreciative.
Competing interests: We have read and understood the BMJ Group policy on declaration of interests and have no relevant interests to declare.
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Medical Studies
How to Write a Medical Abstract
Last Updated: May 15, 2019 References
This article was co-authored by Chris M. Matsko, MD . Dr. Chris M. Matsko is a retired physician based in Pittsburgh, Pennsylvania. With over 25 years of medical research experience, Dr. Matsko was awarded the Pittsburgh Cornell University Leadership Award for Excellence. He holds a BS in Nutritional Science from Cornell University and an MD from the Temple University School of Medicine in 2007. Dr. Matsko earned a Research Writing Certification from the American Medical Writers Association (AMWA) in 2016 and a Medical Writing & Editing Certification from the University of Chicago in 2017. There are 12 references cited in this article, which can be found at the bottom of the page. This article has been viewed 64,798 times.
The purpose of a medical abstract is to provide a concise and useful summary of a longer medical article or study. A good abstract informs readers briefly of the research and ideas that are presented in the full article. Before writing the abstract, be sure you understand the research you're summarizing. Describe the background to your research, your expectations or hypotheses, the methods you used, and the outcomes of your medical investigation.
Getting Ready to Write the Abstract

- If you have co-authors on the publication, have them look over a draft of the abstract before submitting it.
- If you don’t have co-authors, submit a draft of the abstract to a peer in your field of research, or a trusted mentor knowledgeable about the abstract submission and publication process.
Providing Essential Information

- For instance, you might write, “Livingston (2009) has demonstrated the efficacy of nucleotide reparation in E. Coli UBPs.”

- For instance, you might write, “Our hypothesis was that medication X was superior in treating epilepsy than medication Y.”
- Some medical abstracts do not require a background section. In an abstract without a background section, you will start the body of your abstract with information on the goals and expectations of your research. [7] X Research source

- Setting — Where did you conduct your research?
- Sample size —How many individuals participated in the research? How were they selected? This includes animal populations as well.
- Design — How were measurements and statistics recorded?
- Variables — What were the specific variables you looked at? How did you account for them?
- Interventions — How did you intervene to manipulate the variables?

- Do not provide interpretation of your results in this section. Interpretation and analysis should be saved for the conclusion.
- Do not include tables or charts in your abstract. These should be included in the main body of the paper.

Putting the Finishing Touches on Your Abstract

- For instance, “New Corticosteroids Provide Asthma Relief” is a poor abstract title.
- “Corticosteroid Treatment in Asthmatic Patients,” on the other hand, is a good title.
- Don’t use puns or jokes in your title. This may make your work seem trivial and unimportant.

- Some abstracts expect you to list all authors in alphabetical order according to their last names.
- Other publications might expect you to list authors of increasing seniority toward the end of the author list. In this arrangement, the study’s lead researcher or team mentor would be listed last.
- You might also need to list each author’s credentials. For instance, you might need to write “John Smith MD”
- The title and authors should be listed at the top of the abstract, and before the main information of the abstract.

- Additionally, remember to proofread your work. Spelling errors, typos and grammatical mistakes will discredit your hard work and research.
- It might help to read the abstract out loud to yourself to make sure it sounds right before submitting it. Ask a colleague to read over it for you to ensure it is easy to understand and makes sense.
- After you’ve edited the abstract, submit it to the appropriate journal, professional society, or conference committee for approval.
Expert Q&A
- Don’t reword a previous abstract to describe similar research. Thanks Helpful 0 Not Helpful 0

You Might Also Like

- ↑ http://rc.rcjournal.com/content/49/10/1206.full.pdf
- ↑ https://www.academia.edu/3697187/Good_Abstract_Writing_for_a_Medical_Science_Journal_Article_The_Tits_and_Bits
- ↑ https://www.nlm.nih.gov/bsd/policy/structured_abstracts.html
- ↑ http://www.ruf.rice.edu/~bioslabs/tools/report/reportform.html#form
- ↑ https://www.acponline.org/membership/residents/competitions-awards/acp-national-abstract-competition/guide-to-preparing-for-the-abstract-competition/writing-a-research-abstract
About This Article

Medical Disclaimer
The content of this article is not intended to be a substitute for professional medical advice, examination, diagnosis, or treatment. You should always contact your doctor or other qualified healthcare professional before starting, changing, or stopping any kind of health treatment.
Read More...
The best way to start a medical abstract is to begin with one or two sentences of background about why you did the research. For example, you might write, “Livingston (2009) has demonstrated the efficacy of nucleotide reparation in E. Coli UBPs”. Once you’ve stated the background and inspiration for your research, you should state your own goals and hypotheses while emphasizing your objectivity as a researcher. In the next section of your abstract, provide an outline of your methods that answers the question, “How did you investigate the topic or problem?”. Though you should avoid over-describing your methods, make sure you include things like the research setting, sample size, design, variables, and interventions you made to manipulate the variables. Finally, you’ll want to take 6 to 8 sentences to briefly summarize your findings using specific numbers and statistics. For more help from our Medical co-author, like how to title your abstract, read on! Did this summary help you? Yes No
- Send fan mail to authors
Reader Success Stories
Getty. M Tshala
Apr 2, 2020
Did this article help you?

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
Don’t miss out! Sign up for
wikiHow’s newsletter

Public Health Writing Resource Center
- Public Health Writing
- Public Health Blog
Abstract Writing
- Using and Citing Sources
- Online Writing Resources
- Books and Podcasts
- Videos & Tutorials
- Boston Writing Resources
- On Campus Writing Resources
- Ask a Librarian
An abstract usually has the following sections.
Conference and journal guidelines will tell you the word limit and what format to use. Some will ask you to break the information into sections (as seen below), others will ask you to put the information together in a single paragraph.
- Background / Objective : What is public health problem you are addressing? What is its scope? What is the purpose of your article / presentation?
- Methods : What was your study design? How did you collect data? How did you analyze your data?
- Results : What did you find that is most relevant to the objective stated above?
- Discussion / Implications / Recommendations : What is the significance of your research? What are the implications for addressing the public health challenge? What next steps do you recommend?
Beard, J. (2022, April 13). Writing public health abstracts . Public Health Writing Program. Retrieved July 12, 2022, from https://blogs.bu.edu/jenbeard/2022/04/13/writing-public-health-abstracts/
- SPH Blog: Writing Public Health Abstracts
Useful Resources
Suhasini Nagda. 2013. How to Write Scientific Abstracts . Journal of Indian Prosthdontic Society. 13(3): 382-383
Karen McKee. 2022. How to Write a Scientific Abstract for Your Research Article . Wiley Network.
Purdue Owl. Writing Scientific Abstracts .
For more information about Abstract Writing, head over to SPH's Public Health Writing blog , where you will find countless wonderful articles written by SPH Professor and Director of the Public Health Writing Program, Jen Beard.
- << Previous: Public Health Blog
- Next: Using and Citing Sources >>
- Last Updated: Apr 11, 2024 3:31 PM
- URL: https://medlib-bu.libguides.com/public-health-writing-resource-center
How to Write an Abstract?
- Open Access
- First Online: 24 October 2021
Cite this chapter
You have full access to this open access chapter

- Samiran Nundy 4 ,
- Atul Kakar 5 &
- Zulfiqar A. Bhutta 6
58k Accesses
5 Altmetric
An abstract is a crisp, short, powerful, and self-contained summary of a research manuscript used to help the reader swiftly determine the paper’s purpose. Although the abstract is the first paragraph of the manuscript it should be written last when all the other sections have been addressed.
Research is formalized curiosity. It is poking and prying with a purpose. — Zora Neale Hurston, American Author, Anthropologist and Filmmaker (1891–1960)
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others

Writing the Abstract

Abstract and Keywords

Additional Commentaries
1 what is an abstract.
An abstract is usually a standalone document that informs the reader about the details of the manuscript to follow. It is like a trailer to a movie, if the trailer is good, it stimulates the audience to watch the movie. The abstract should be written from scratch and not ‘cut –and-pasted’ [ 1 ].
2 What is the History of the Abstract?
An abstract, in the form of a single paragraph, was first published in the Canadian Medical Association Journal in 1960 with the idea that the readers may not have enough time to go through the whole paper, and the first abstract with a defined structure was published in 1991 [ 2 ]. The idea sold and now most original articles and reviews are required to have a structured abstract. The abstract attracts the reader to read the full manuscript [ 3 ].
3 What are the Qualities of a Good Abstract?
The quality of information in an abstract can be summarized by four ‘C’s. It should be:
C: Condensed
C: Critical
4 What are the Types of Abstract?
Before writing the abstract, you need to check with the journal website about which type of abstract it requires, with its length and style in the ‘Instructions to Authors’ section.
The abstract types can be divided into:
Descriptive: Usually written for psychology, social science, and humanities papers. It is about 50–100 words long. No conclusions can be drawn from this abstract as it describes the major points in the paper.
Informative: The majority of abstracts for science-related manuscripts are informative and are surrogates for the research done. They are single paragraphs that provide the reader an overview of the research paper and are about 100–150 words in length. Conclusions can be drawn from the abstracts and in the recommendations written in the last line.
Critical: This type of abstract is lengthy and about 400–500 words. In this, the authors’ own research is discussed for reliability, judgement, and validation. A comparison is also made with similar studies done earlier.
Highlighting: This is rarely used in scientific writing. The style of the abstract is to attract more readers. It is not a balanced or complete overview of the article with which it is published.
Structured: A structured abstract contains information under subheadings like background, aims, material and methods, results, conclusion, and recommendations (Fig. 15.1 ). Most leading journals now carry these.
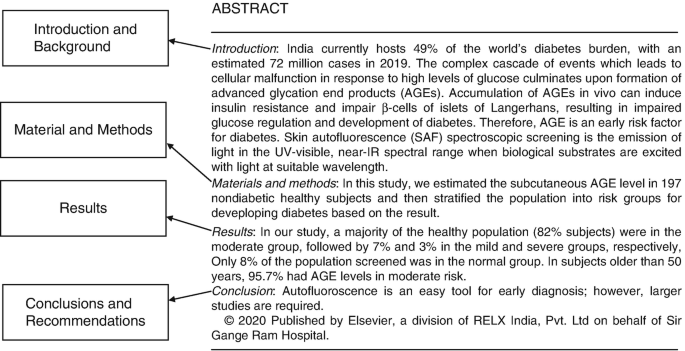
Example of a structured abstract (with permission editor CMRP)
5 What is the Purpose of an Abstract?
An abstract is written to educate the reader about the study that follows and provide an overview of the science behind it. If written well it also attracts more readers to the article. It also helps the article getting indexed. The fate of a paper both before and after publication often depends upon its abstract. Most readers decide if a paper is worth reading on the basis of the abstract. Additionally, the selection of papers in systematic reviews is often dependent upon the abstract.
6 What are the Steps of Writing an Abstract?
An abstract should be written last after all the other sections of an article have been addressed. A poor abstract may turn off the reader and they may cause indexing errors as well. The abstract should state the purpose of the study, the methodology used, and summarize the results and important conclusions. It is usually written in the IMRAD format and is called a structured abstract [ 4 , 5 ].
I: The introduction in the opening line should state the problem you are addressing.
M: Methodology—what method was chosen to finish the experiment?
R: Results—state the important findings of your study.
D: Discussion—discuss why your study is important.
Mention the following information:
Important results with the statistical information ( p values, confidence intervals, standard/mean deviation).
Arrange all information in a chronological order.
Do not repeat any information.
The last line should state the recommendations from your study.
The abstract should be written in the past tense.
7 What are the Things to Be Avoided While Writing an Abstract?
Cut and paste information from the main text
Hold back important information
Use abbreviations
Tables or Figures
Generalized statements
Arguments about the study

8 What are Key Words?
These are important words that are repeated throughout the manuscript and which help in the indexing of a paper. Depending upon the journal 3–10 key words may be required which are indexed with the help of MESH (Medical Subject Heading).
9 How is an Abstract Written for a Conference Different from a Journal Paper?
The basic concept for writing abstracts is the same. However, in a conference abstract occasionally a table or figure is allowed. A word limit is important in both of them. Many of the abstracts which are presented in conferences are never published in fact one study found that only 27% of the abstracts presented in conferences were published in the next five years [ 6 ].
Table 15.1 gives a template for writing an abstract.
10 What are the Important Recommendations of the International Committees of Medical Journal of Editors?
The recommendations are [ 7 ]:
An abstract is required for original articles, metanalysis, and systematic reviews.
A structured abstract is preferred.
The abstract should mention the purpose of the scientific study, how the procedure was carried out, the analysis used, and principal conclusion.
Clinical trials should be reported according to the CONSORT guidelines.
The trials should also mention the funding and the trial number.
The abstract should be accurate as many readers have access only to the abstract.
11 Conclusions
An Abstract should be written last after all the other sections of the manuscript have been completed and with due care and attention to the details.
It should be structured and written in the IMRAD format.
For many readers, the abstract attracts them to go through the complete content of the article.
The abstract is usually followed by key words that help to index the paper.
Andrade C. How to write a good abstract for a scientific paper or conference presentation? Indian J Psychiatry. 2011;53:172–5.
Article Google Scholar
Squires BP. Structured abstracts of original research and review articles. CMAJ. 1990;143:619–22.
CAS PubMed PubMed Central Google Scholar
Pierson DJ. How to write an abstract that will be accepted for presentation at a national meeting. Respir Care. 2004 Oct;49:1206–12.
PubMed Google Scholar
Tenenbein M. The abstract and the academic clinician. Pediatr Emerg Care. 1995;11:40–2.
Article CAS Google Scholar
Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. The principles of biomedical scientific writing: abstract and keywords. Int J Endocrinol Metab. 2020;18:e100159.
PubMed PubMed Central Google Scholar
Grover S, Dalton N. Abstract to publication rate: do all the papers presented in conferences see the light of being a full publication? Indian J Psychiatry. 2020;62:73–9.
Preparing a manuscript for submission to a medical journal. Available on http://www.icmje.org/recommendations/browse/manuscript-preparation/preparing-for-submission.html . Accessed 10 May 2020.
Download references
Author information
Authors and affiliations.
Department of Surgical Gastroenterology and Liver Transplantation, Sir Ganga Ram Hospital, New Delhi, India
Samiran Nundy
Department of Internal Medicine, Sir Ganga Ram Hospital, New Delhi, India
Institute for Global Health and Development, The Aga Khan University, South Central Asia, East Africa and United Kingdom, Karachi, Pakistan
Zulfiqar A. Bhutta
You can also search for this author in PubMed Google Scholar
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions
Copyright information
© 2022 The Author(s)
About this chapter
Nundy, S., Kakar, A., Bhutta, Z.A. (2022). How to Write an Abstract?. In: How to Practice Academic Medicine and Publish from Developing Countries?. Springer, Singapore. https://doi.org/10.1007/978-981-16-5248-6_15
Download citation
DOI : https://doi.org/10.1007/978-981-16-5248-6_15
Published : 24 October 2021
Publisher Name : Springer, Singapore
Print ISBN : 978-981-16-5247-9
Online ISBN : 978-981-16-5248-6
eBook Packages : Medicine Medicine (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

What are structured abstracts?
A structured abstract is an abstract with distinct, labeled sections (e.g., Introduction, Methods, Results, Discussion) for rapid comprehension (see Figure 1 ).
What kinds of structures are used?
Standardized formats for structured abstracts have been defined for original research studies, review articles and clinical practice guidelines ( 1 , 2 ). The IMRAD format (INTRODUCTION, METHODS, RESULTS, and DISCUSSION), a defacto standard that reflects the process of scientific discovery ( 3 ), is commonly used as a structure for journal abstracts ( 4 , 5 ). The CONSORT (Consolidated Standards of Reporting Trials) Group issued a guideline for how to report randomized controlled trials (RCTs) in journal and conference abstracts using a structured format ( 6 ).
Why use structured abstracts?
Structured abstracts have several advantages for authors and readers. These formats were developed in the late 1980s and early 1990s to assist health professionals in selecting clinically relevant and methodologically valid journal articles. They also guide authors in summarizing the content of their manuscripts precisely, facilitate the peer-review process for manuscripts submitted for publication, and enhance computerized literature searching ( 1 , 2 ).
The International Committee of Medical Journal Editors (ICMJE, of which NLM is a sitting member), whose "Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals" document provides general guidelines for the format of manuscripts submitted to journals, requires the use of structured abstracts for original research articles, systematic reviews, and meta-analyses. ICMJE does acknowledge that the format required for structured abstracts differs from journal to journal and that some journals use more than one structure ( 7 ).
The substantial growth in both the individual number of PubMed records with structured abstracts and in the number of journals that continuously publish structured abstracts demonstrates widespread adoption of structured abstracts over the years ( 8 ). Structured abstracts perform better than unstructured abstracts for the discovery of corresponding MeSH (Medical Subject Headings®) terms using the Medical Text Indexer (MTI) software application ( 9 ). More information about NLM research on structured abstracts including technical details for the NLM implementation of structured abstracts can be found at Structured Abstracts in MEDLINE .
How are structured abstracts formatted in PubMed?
NLM uses all uppercase letters followed by a colon and space for the labels that appear in structured abstracts in MEDLINE/PubMed ® citations (see Figure 1 ).
How can I search for structured abstracts in PubMed?
In a PubMed search box, type:
See the structured abstract search results in PubMed .
References:
1 . Haynes RB, Mulrow CD, Huth EJ, Altman DG, Gardner MJ. More informative abstracts revisited. Ann Intern Med. 1990 Jul 1;113(1):69-76. PubMed PMID: 2190518 . Available from: https://www.acpjournals.org/doi/10.7326/0003-4819-113-1-69 .
2 . Hayward RS, Wilson MC, Tunis SR, Bass EB, Rubin HR, Haynes RB. More informative abstracts of articles describing clinical practice guidelines. Ann Intern Med. 1993 May 1;118(9):731-7. PubMed PMID: 8460861 . Available from: https://www.acpjournals.org/doi/10.7326/0003-4819-118-9-199305010-00012 .
3 . Sollaci LB, Pereira MG. The introduction, methods, results, and discussion (IMRAD) structure: a fifty-year survey. J Med Libr Assoc. 2004 Jul;92(3):364-7. PubMed PMID: 15243643 ; PubMed Central PMCID: PMC442179 .
4 . Nakayama T, Hirai N, Yamazaki S, Naito M. Adoption of structured abstracts by general medical journals and format for a structured abstract. J Med Libr Assoc. 2005 Apr;93(2):237-42. PubMed PMID: 15858627 ; PubMed Central PMCID: PMC1082941 .
5 . Kulkarni H. Structured abstracts: still more. Ann Intern Med. 1996 Apr 1;124(7):695-6. PubMed PMID: 8607606 . Available from: https://www.acpjournals.org/doi/10.7326/0003-4819-124-7-199604010-00020 .
6 . Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, Schulz KF; CONSORT Group. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Med. 2008 Jan 22;5(1):e20. PubMed PMID: 18215107 ; PubMed Central PMCID: PMC2211558 .
7 . International Committee of Medical Journal Editors. Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals (ICMJE Recommendations). 2013 Aug [cited 2013 Aug 21]. Available from: http://www.icmje.org .
8 . Ripple AM, Mork JG, Rozier JM, Knecht LS. Structured abstracts in MEDLINE: twenty-five years later. Bethesda, MD: National Library of Medicine; 2012 [cited 2014 Sep 17]. Available from: https://structuredabstracts.nlm.nih.gov/Structured_Abstracts_in_MEDLINE_Twenty-Years_Later.pdf .
9 . Ripple AM, Mork JG, Thompson HJ, Schmidt SC, Knecht LS. Performance comparison of MEDLINE structured abstracts to unstructured abstracts. Poster session presented at: National Institutes of Health Research Festival; 2014 Sep 22-24; Bethesda, MD. Available from: https://researchfestival.nih.gov/festival14/poster-RSCHSUPP-19.html .
- Ripple AM, Mork JG, Knecht LS, Humphreys BL. A retrospective cohort study of structured abstracts in MEDLINE, 1992-2006. J Med Libr Assoc. 2011 Apr;99(2):160-3. PubMed PMID: 21464855 ; PubMed Central PMCID: PMC3066587 .
Last Reviewed: December 14, 2023
- Log In Username Enter your ACP Online username. Password Enter the password that accompanies your username. Remember me Forget your username or password ?
- Privacy Policy
- Career Connection
- Member Forums
© Copyright 2024 American College of Physicians, Inc. All Rights Reserved. 190 North Independence Mall West, Philadelphia, PA 19106-1572 800-ACP-1915 (800-227-1915) or 215-351-2600
If you are unable to login, please try clearing your cookies . We apologize for the inconvenience.
Writing a Research Abstract
The written abstract is used in making selections for presentations at scientific meetings. Writing a good abstract is a formidable undertaking and many novice researchers wonder how it is possible to condense months of work into 300 to 400 words. Nevertheless, creating a well-written abstract is a skill that can be learned and mastering the skill will increase the probability that your research will be selected for presentation.
The first rule of writing abstracts is to know the rules. Organizers of scientific meetings set explicit limits on the length abstracts.
Authors must pay close attention to the published details of the meeting including deadlines and suggested format. Since reviewers have many abstracts to read and rank; those that don't conform to the stated rules are simply discarded.
The scientific abstract is usually divided into five unique sections: Title and Author Information, Introduction, Methods, Results, and Conclusions. The following paragraphs summarize what is expected in each of these sections.
Title and Author Information: The title should summarize the abstract and convince the reviewers that the topic is important, relevant, and innovative. To create a winning title, write out 6 to 10 key words found in the abstract and string them into various sentences. Once you have a sentence that adequately conveys the meaning of the work, try to condense the title yet still convey the essential message. Some organizations require a special format for the title, such as all uppercase letters, all bolded, or in italics. Be sure to check the instructions.
Following the title, the names of all authors and their institutional affiliations are listed. It is assumed the first author listed will make the oral presentation. Determine if the first author needs to meet any eligibility requirements to make the presentation. For example, the first author may need to be a member of the professional society sponsoring the research meeting. This information is always included with the abstract instructions.
Introduction: This usually consists of several sentences outlining the question addressed by the research. Make the first sentence of the introduction as interesting and dramatic as possible. For example, "100,000 people each year die of…" is more interesting than "An important cause of mortality is…" If space permits, provide a concise review of what is known about the problem addressed by the research, what remains unknown, and how your research project fills the knowledge gaps. The final sentence of the introduction describes the purpose of the study or the study's a priori hypothesis.
Methods: This is the most difficult section of the abstract to write. It must be scaled down sufficiently to allow the entire abstract to fit into the box, but at the same time it must be detailed enough to judge the validity of the work. For most clinical research abstracts, the following areas are specifically mentioned: research design; research setting; number of patients enrolled in the study and how they were selected; a description of the intervention (if appropriate); and a listing of the outcome variables and how they were measured. Finally, the statistical methods used to analyze the data are described.
Results: This section begins with a description of the subjects that were included and excluded from the study. For those excluded, provide the reason for their exclusion. Next, list the frequencies of the most important outcome variables. If possible, present comparisons of the outcome variables between various subgroups within the study (treated vs. untreated, young vs. old, male vs. female, and so forth). This type of data can be efficiently presented in a table, which is an excellent use of space. But before doing this, check the rules to see if tables can be used in the abstract. Numerical results should include standard deviations or 95% confidence limits and the level of statistical significance. If the results are not statistically significant, present the power of your study (beta-error rate) to detect a difference.
Conclusion: State concisely what can be concluded and its implications. The conclusions must be supported by the data presented in the abstract; never present unsubstantiated personal opinion. If there is room, address the generalizability of the results to populations other than that studied and the weaknesses of the study.
Research literature has a special language that concisely and precisely communicates meaning to other researches. Abstracts should contain this special language and be used appropriately. See The Glossary of commonly used research terms.
Avoid the use of medical jargon and excessive reliance on abbreviations. Limit abbreviations to no more than three and favor commonly used abbreviations. Always spell out the abbreviations the first time they are mentioned unless they are commonly recognized (e.g., CBC).
Although short in length, a good abstract typically takes several days to write. Take this into account when budgeting your time. Seek the help of an experienced mentor. Share the abstract with your mentor and make revisions based upon the feedback. Allow others to read your draft for clarity and to check for spelling and grammatical mistakes. Reading the abstract orally is an excellent way to catch grammatical errors and word omissions. Use the Scientific Abstract Checklist to assist your completion of the task. Finally, an example of an abstract previously accepted for presentation at the ACP Resident Research Competition is attached for your review.

- Duke NetID Login
- 919.660.1100
- Directions & Maps
- Floor Plans
- Reserve Spaces
- Reserve a Locker
- Study & Meeting Rooms
- Computers & Equipment
- Wifi Access
Library Updates
- Annual Snapshot
- Conference Presentations
- Contact Information
- Accounts & Access
- Gifts & Donations
- Access From Off Campus
- Building Access
- Pay Fines/Fees
- My Accounts
- Request Articles & Books
- Renew Online
- Course Reserves
- Recommend a Purchase
- Mobile Apps
- Known Access Issues
- Report an Access Issue
- All Databases
- Article Databases
- Basic Sciences
- Clinical Sciences
- Dissertations & Theses
- Drugs, Chemicals & Toxicology
- Grants & Funding
- Interprofessional Education
- Non-Medical Databases
- Search for E-Journals
- Search for Print & E-Journals
- Search for E-Books
- Search for Print & E-Books
- E-Book Collections
- Biostatistics
- Global Health
- MBS Program
- Medical Students
- MMCi Program
- Occupational Therapy
- Path Asst Program
- Physical Therapy
- Researchers
- Community Partners
Conducting Research
- Archival & Historical Research
- Black History at Duke Health
- Data Analytics & Viz Software
- Data: Find and Share
- Evidence-Based Practice
- NIH Public Access Policy Compliance
- Publication Metrics
- Qualitative Research
- Searching Animal Alternatives
- Systematic Reviews
- Test Instruments
Using Databases
- JCR Impact Factors
- Web of Science
Finding & Accessing
- COVID-19: Core Clinical Resources
- Health Literacy
- Health Statistics & Data
- Library Orientation
Writing & Citing
- Creating Links
- Getting Published
- Reference Mgmt
- Scientific Writing

Meet a Librarian
- Request a Consultation
- Find Your Liaisons
- Register for a Class
- Request a Class
- Self-Paced Learning
Search Services
- Literature Search
- Systematic Review
- Animal Alternatives (IACUC)
- Research Impact
Citation Mgmt
- Other Software
Scholarly Communications
- About Scholarly Communications
- Publish Your Work
- Measure Your Research Impact
- Engage in Open Science
- Libraries and Publishers
How to Write an Abstract
When you search PubMed (or most databases), did you know you’re only searching the titles, abstracts, and keywords/subject headings? That’s why it’s so important that you write an effective, concise, and clear abstract! In this session, learn how to refine your abstract writing skills to help users find your paper. Tue. June 11; 12-1p; ONLINE ONLY. This class is FREE but registration is required.
Blog Categories
- Duke Health
- Duke University
- Duke Libraries
- Medical Center Archives
- Duke Directory
- Seeley G. Mudd Building
- 10 Searle Drive
- [email protected]
How to write an abstract that will be accepted for presentation at a national meeting
Affiliation.
- 1 Division of Pulmonary and Critical Care Medicine, Harborview Medical Center, 325 Ninth Avenue, Box 359762, Seattle, WA 98104, USA. [email protected]
- PMID: 15447804
Preparation, submission, and presentation of an abstract are important facets of the research process, which benefit the investigator/author in several ways. Writing an abstract consists primarily of answering the questions, "Why did you start?" "What did you do?" "What did you find?" and "What does it mean?" A few practical steps in preparing to write the abstract can facilitate the process. This article discusses those steps and offers suggestions for writing each of an abstract's components (title, author list, introduction, methods, results, and conclusions); considers the advantages and disadvantages of incorporating a table or figure into the abstract; offers several general writing tips; and provides annotated examples of well-prepared abstracts: one from an original study, one from a method/device evaluation, and one from a case report.
- Abstracting and Indexing / standards*
- Biomedical Research
- Congresses as Topic*
- Societies, Medical
- Writing / standards*
- How it works
- Pay for essays
- Do my homework
- Term Paper Writing Service
- Do my assignment
- Coursework help
- Our Writers

How to Write an Abstract for a Research Paper

A professional writer with ten years of experience and a Ph.D. in Modern History, Catharine Tawil writes engaging and insightful papers for academic exchange. With deep insight into the impact of historical events on the present, she provides a unique perspective in giving students a feel for the past. Her writing educates and stimulates critical thinking, making her a treasure to those wading through the complexities of history.
Do you want to know how to write an abstract like a pro? Composing an abstract for a research paper is a very important stage of your research work. It is a compact and precise description of the main body of your paper, which is intended to help the readers understand the paper quickly. An abstract should be concise, well-organized, and contain all the important issues of your research paper. It enables readers to filter out and choose the rest of the document according to their interests. So, let us explain the details.
What Is an Abstract for a Research Paper?
An abstract is a short, concise summary of a research paper that provides a clear understanding of the paper. It is placed at the beginning of the paper and ranges from 150 to 300 words. It gives a synopsis of the major findings of your study, comprising the research aim, methods used, the most important findings, and the main conclusions.
The abstract gives readers a brief understanding of your paper, and they do not need to read the entire document. It is a complete text that shows why your work is important and summarizes your study's main findings. The quality of the abstract is important because it allows your paper to be picked out in academic databases and, thus, other researchers to read your full paper.
When to Write an Abstract
You should write the abstract and the remaining paper after you have completed your research. This is because the summary is essentially a collation of all the key points of your research, such as the results and conclusions, which are not known until you have conducted the research. An abstract should be written at the end to ensure that it adequately reflects your paper and that no important points are missed.
According to the University of Southern California, your abstract should reflect your research, be clear and concise, and show the main goal of your study and its outcome. Through the final stage of your writing cycle, you can be confident that your abstract has covered all aspects of your article and is consistent with its content.
Types of Abstract
Abstracts can be generally divided into two types, each distinct by purpose. Recognizing the gap in these categories from a researcher's perspective enables the design of an abstract that fits the study's aim and the reader's anticipations. Here is what you need to know about the main abstract types as a student.
Descriptive Abstract
Such an abstract introduces the research's information without giving a detailed account. It explains what the methods and the scope of the research are but won't include the results and conclusions. A descriptive abstract, which is usually very short, less than 100 words, and is often used for short papers or articles, is commonly used for brief papers or articles. Here is its purpose:
- Provides the research's purpose and scope.
- Highlights the methods used.
- Does not include results or conclusions.
Following a descriptive abstract, the reader should have a decent comprehension of the research's purpose and a general idea of the topics covered. However, they should read the full paper to discover the findings and conclusions. It's often not used as much as its informative counterpart, but it can be useful for complex studies that don't require a detailed explanation in the abstract.
Informative Abstract
An informative abstract is a mini-version of the paper. It is a synopsis. It contains not only the study goals and research methods but also the results and conclusions. This type of abstract is more detailed and longer than a descriptive abstract, often going up to 200 to 300 words. The author presents an outline of the study's findings or proofs, the thesis or main arguments, and a brief argument of the implications. Besides, you can always say, "Write my paper." Do not hesitate to ask us for help!
An informative abstract provides enough detail about the content to help the reader decide whether to read the whole paper. We mostly use this type, which is especially convenient for technical or research-intensive documents where the reader does not necessarily need to read the whole paper to get the idea.
Descriptive & Informative Abstracts: Common Differences
The structure of the abstract: step-by-step instructions.
A well-organized abstract provides a concise and summarized overview of your research paper. Each component should be correctly written to provide the key points of your research. The structure typically follows the natural order of the research flow to present the motivation, problem, methods, results, and conclusion in an integrated and consistent manner.
Purpose and Motivation
In your abstract's introduction and purpose section, you explain why you commenced the research and your goals. This part should clearly and unambiguously explain the fact that the research question is crucial and justify the rationale for the study. It should start with giving the context by discussing the wider research field or a particular issue that is the focus of your paper.
The Problem of Research
The purpose statement of the abstract defines the specific problem or gap in understanding your research studies. To be effective, you have to be concise but make sure your message gets the idea across and is clear enough to convey the problem or question you are handling. Here is what you should do:
- Clearly state the research problem.
- Identify the gap in the literature.
- Mention the implications of the problem.
After explaining the problem, you should identify the benefit of solving it for your discipline or the intended group. This defines the importance of your research and shows how your work relates to the ongoing academic debate in the field. By thoroughly identifying the problem, you help your readers comprehend the context and magnitude of the problem being investigated. So, check our research paper abstract guidelines to master your writing.
Researching Approach
The methodology section of your abstract elaborates on the techniques you applied to resolve the research question. A description of the research design, data collection techniques, and analysis methods should be carried out. It is important to summarize why these methods are appropriate for your analysis and how, by using them, you can accomplish your research goals.
Research Results
In the research results section of your abstract, highlight the main conclusions of your investigation. This section is supposed to be straight and concise, indicating major results without getting into an in-depth discussion of the analysis. State whether the results confirm or disprove your hypothesis or answer the research question.
Highlight any commonly occurring trends, relationships, or patterns you have discovered are a must. However, including concrete numbers to clarify the results and impact is important. This brief part should help the reader understand what your research unveiled and how these findings boost the scientific community. Besides, check our latest article on how to polish your reasearch paper format!
The final part of your abstract should stress the practical implications and importance of the findings. The second section of the report summarizes the research findings and describes the broader significance of the results obtained. Outline the study you intend to address, the gap you found in the research question, and what it means for the field. Besides, you need a research paper abstract example.
Tips for Writing an Abstract
A well-written abstract is imperative to stimulate interest in your research and explain it lucidly to readers. It will be your paper's window; it provides the reader with all the necessary information in an easy-to-understand way. Below are some simple tricks to guide you through writing a short abstract that will convey your research and be interesting to read.
Read Other Abstracts
Do you know all the components of a research paper abstract? If not, remember them ASAP! There's nothing wrong with reading abstracts written by other students. Think of it as an opportunity to analyze their thinking processes and approaches to crafting this section of the research paper. Here's what you should pay attention to:
- Observe the language and terminology used.
- Note the balance between conciseness and detail.
- Identify common structures that seem effective.
After familiarizing yourself with each abstract example, write as clearly and concisely as possible. This will help you understand the abstract's nature and how to organize the information.
Reverse Outline
Here is how to craft a research paper abstract like a pro. Try reverse outlining. This technique involves outlining your completed paper and noting the key points from each section: introduction, methods, results, and conclusion. This makes it easier to detect if anything is missing from your abstract and if the content of your abstract accurately reflects the content of your paper.
Cech research paper abstract examples. Begin with the main goal and what you'll be studying, then continue with the methodology, the main findings, and the importance of these findings. The paper is broken down into essential sections so that you can rebuild them into a concise and informative abstract.
Write Clearly and Concisely
Clarity and shortness are vital when writing an abstract. It is the opener, the only part readers will read, so it must briefly tell your research story. Use simple sentences and don't use complex sentence structures to make reading easy for people, especially those outside of English-speaking countries.
Every sentence must accomplish a task, be it articulating the study, summarizing the methods, exhibiting the results, or discussing implications. Avoid telling details and concentrate on what is only important to study your research's range, importance, and consequences. This rigid but essential writing technique will make the abstract for research paper assignments clean and informative.
Check Your Formatting
Writing an abstract for a research paper may seem daunting. Finalizing correctly and adhering to your professor's key instructions is crucial. Ensure that you follow all formatting guidelines and avoid making any careless mistakes. Here's a typical checklist for students:
- Confirm word count limits.
- Check for specific structural requirements.
- Verify if certain sections require bolding or subheadings.
Before submission, cross-check your abstract for consistency in style, like font and spacing, and make sure it meets all submission requirements. Proper formatting not only gives an excellent impression but also makes your work fit into the expected norms of your audience. And check at least one abstract example beforehand!
Things to Avoid in Your Abstract
The abstract is where you need to be as clear and to the point as possible. Do not bother adding unnecessary information or intricate details that will be included in the main body of the paper. So, here is how to write an abstract for a research paper without making mistakes.
Using Jargon
So, research paper abstract writing is not an easy task. Inserting jargon and super technical vocabulary in your abstract will prevent you from reaching readers who are not specialists in your specific area of research. Abstractions should be understandable to a wider audience, including laypersons who may not be literate. Clarify the meaning of the words that are not commonly known and replace the complicated words with those that are easier to understand whenever necessary.
Just check one abstract example! If ambiguous terminology should be avoided, define it as shortly and precisely as possible. Recollect that the main role of an abstract is to convey the main idea of the research briefly and understandably; wordiness can be an obstacle in this way and prevent readers from understanding the significance of your work.
Providing Too Much Detail
When writing an abstract, you should not lose sight of the fact that it is not the main body of the paper but introduces the research. As a summary, the abstract should state the main points and findings without being so detailed as to list all the data or the analysis. The main purpose of the abstract is to give the reader a brief and clear overview of your research and its main points.
Therefore, the abstract should not contain details that confuse readers and prevent them from understanding what you are trying to say. Your research paper abstract structure should be solid. Emphasize concisely addressing the research question, design, key findings, and conclusion. The way of writing this proposal is intended to help the reader keep their interest and motivate them to read the paper.
Introducing New Concepts
Writing an abstract is not an easy task. Here's another red flag you should avoid: introducing new concepts that may not yield the desired results or align with the typical approach to creating research papers. Here is your list of actions as a student:
- Avoid mentioning studies or data not discussed in the paper.
- Do not introduce new frameworks or theories.
- Refrain from including references to literature not cited in the main body.
Ensuring the abstract contains details from the paper will keep it coherent and prevent confusing readers. An abstract summarizes your research, highlighting the major points and providing short and precise information about the research. Now, you know how to write an abstract for a research paper correctly.
Vague or Ambiguous Language
Do you need tips for writing a research paper abstract? Here you go. Being imprecise or ambiguous in an abstract can cause your research to be misinterpreted regarding its relevance and focus. It is significant to utilize accurate and understandable language to specifically convey your research's purpose, methodology, results, and implications. It is advisable not to use general statements that do not provide concrete information.
So, what is an abstract? It is a part of your paper where every sentence should effectively show the importance of your research. Having a clear and concise abstract not only improves readability but also ensures that the audience understands the aim and conclusion of your study without confusion.
Making Unsupported Claims
The argument or claim in an abstract should be supported by evidence presented in the abstract research paper. The lack of evidence backing unsupported claims may lead to the credibility loss of your research and the creation of false perceptions about its validity. It is essential to ensure that all the major paragraphs of the abstract are based on the data you obtained and the findings of your study.
This includes corroborating the results and only drawing conclusions related to them. Don't stretch the implications of your research or suggest a broader application unbacked by evidence. A trustworthy research paper abstract summarizes the research and its outcomes and is integral to the whole assignment and the research process.
Exceeding the Recommended Word Count
So, what is an abstract in a research paper? It is a crucial paper assignment! Sticking to the recommended word count for an abstract is significant. On the other hand, if the text is beyond this limit, it may be overly detailed and too much for your reader to handle. Also, being accepted for print or at conferences can be problematic since most of them have set word count requirements.
Writing an effective research paper abstract can be a game changer. A boxed summary compresses your research into its most important aspects, focusing on the problem, methodology, results, and implications without unnecessary details. Limiting the number of words ensures that your abstract remains clear and concise.
What should be included in a research paper abstract?
The research paper abstract should include a short introduction, the main research question, the methodology applied, the main findings, and finally, the concluding remarks.
How long should a research paper abstract take?
In most cases, a research paper abstract should be 150 words or more and up to 300 words.
What is the difference between a descriptive and informative abstract?
A descriptive abstract describes the paper's topic, providing only general information. On the contrary, an informative abstract describes the research topic in detail, including the purpose, methodology, and other aspects.
How can I make my abstract stand out to readers?
Your abstract should be clear, concise, and direct, focusing on the importance and uniqueness of your research.
Related posts

Cheap Hobbies for Students

How to Write a Response Paper: Step-by-Step Guide to Success

How to Write an Abstract for a Research Paper | A Guide for Students
What are you waiting for?
You are a couple of clicks away from tranquility at an affordable price!
- Introduction
- Article Information
eFigure. Flowchart: nationwide population-matched cohort and sibling-matched cohort, 2001-2018
eTable 1. Codes for identification of premenstrual disorders, psychiatric comorbidities, causes of death
eTable 2. Hazard ratio (HR) and 95% confidence intervals (CI) of all-cause mortality among women with premenstrual disorders, stratified by age and comorbidities: population-based cohort 2001-2018
eTable 3. Incidence rates of death and distribution of underlying causes of death: population-matched cohort 2001-2018
eTable 4. Underlying cause of death by age at diagnosis: population-matched cohort 2001-2018, n=2,325
eTable 5. Distribution of the underlying causes of death for women younger than 25 years old: population-matched cohort 2001-2018, n=196
eTable 6. Sensitivity analysis: hazard ratio (HR) and 95% confidence interval (CI) of mortality for women with premenstrual disorders, overall and by age: nationwide population-matched cohort 2001-2018, PMDs defined with at least 2 diagnoses ≥28 days, N=192,618
eTable 7. Sensitivity analyses: hazard ratio (HR) and 95% confidence interval (CI) of cause-specific mortality: population-matched cohort 2001-2018
eTable 8. Hazard ratio (HR) and 95% confidence intervals (CI) of mortality among women with premenstrual disorders overall, by age at diagnosis/matching and by specific causes: nationwide population-matched cohort, 2001-2018
eTable 9. Hazard ratio (HR) and 95% confidence intervals (CI) of mortality among women with premenstrual disorders by hormone replacement therapy and selective serotonin inhibitor: nationwide population-matched cohort, 2005-2018
eTable 10. The most frequent comorbidities at matching, among individuals with at least one comorbidity, population-matched cohort 2001-2018
eTable 11. Distribution of the underlying causes of death for women diagnosed at age 45 or over, population-matched cohort 2001-2018
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Opatowski M , Valdimarsdóttir UA , Oberg AS , Bertone-Johnson ER , Lu D. Mortality Risk Among Women With Premenstrual Disorders in Sweden. JAMA Netw Open. 2024;7(5):e2413394. doi:10.1001/jamanetworkopen.2024.13394
Manage citations:
© 2024
- Permissions
Mortality Risk Among Women With Premenstrual Disorders in Sweden
- 1 Unit of Integrative Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden
- 2 Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
- 3 Center of Public Health Sciences, Faculty of Medicine, University of Iceland, Reykjavík, Iceland
- 4 Department of Biostatistics and Epidemiology, School of Public Health and Health Sciences, University of Massachusetts Amherst, Amherst
- 5 Department of Health Promotion and Policy, School of Public Health and Health Sciences, University of Massachusetts Amherst, Amherst
Question Do women with premenstrual disorders (PMDs) have higher risks of all-cause and cause-specific mortality compared with women without PMDs?
Findings This nationwide matched cohort study of 406 488 women in Sweden during a mean (SD) follow-up of 6.2 (4.6) years (range, 1-18 years) revealed that overall, women with diagnosed PMDs did not have an increased risk of all-cause premature death. However, there was an increased mortality risk among women with PMDs diagnosed before age 25 years, as well as increased risk of death due to suicide, irrespective of age at diagnosis.
Meaning These findings suggest that women with PMDs are not at increased risk of all-cause mortality, but active surveillance might be needed among young patients and for suicide prevention for all ages.
Importance Premenstrual disorders (PMDs) adversely affect the quality of life of millions of women worldwide, yet research on the long-term consequences of PMDs is limited, and the risk of mortality has not been explored.
Objective To estimate the associations of PMDs with overall and cause-specific mortality.
Design, Setting, and Participants This nationwide, population-based, matched cohort study used data from population and health registers in Sweden. Participants included women of reproductive age with a first diagnosis of PMDs between January 1, 2001, and December 31, 2018. Data analysis was performed from September 2022 to April 2023.
Exposures PMDs were identified through inpatient and outpatient diagnoses and drug dispensing.
Main Outcomes and Measures Dates of death and underlying causes were ascertained from the National Cause of Death Register. Conditional Cox regression was used to estimate the hazard ratios (HRs) of overall and cause-specific death (eg, death due to natural or nonnatural cause, suicide, or cardiovascular events), adjusting for age, socioeconomic status, and somatic and psychiatric comorbidities; in a separate sibling comparison, models were also adjusted for all factors that sisters share.
Results A total of 67 748 women with clinically diagnosed PMDs and 338 740 matched unaffected women were included, for a total of 406 488 women. Women with PMDs received a diagnosis at a mean (SD) age of 35.8 (8.2) years. During a mean (SD) follow-up of 6.2 (4.6) years (range, 1-18 years), 367 deaths were observed among women with PMDs (rate, 8.4 deaths per 10 000 person-years; 95% CI, 7.6-9.3 deaths per 10 000 person-years), and 1958 deaths were observed among women without PMDs (rate, 9.1 deaths per 10 000 person-years; 95% CI, 8.7-9.6 deaths per 10 000 person-years). Compared with unaffected women, women with PMDs had increased risk of death due to nonnatural causes (HR, 1.59; 95% CI, 1.25-2.04), particularly suicide (HR, 1.92; 95% CI, 1.43-2.60), but they did not have increased risk of overall mortality (adjusted HR, 0.91; 95% CI, 0.82-1.02). Notably, women who received a diagnosis before the age of 25 years experienced higher all-cause mortality (HR, 2.51; 95% CI, 1.42-4.42) and death from both suicide (HR, 3.84; 95% CI, 1.18-12.45) and natural causes (HR, 2.59; 95% CI, 1.21-5.54).
Conclusions and Relevance The findings of this matched cohort study suggest that women with PMDs are not at increased risk of early death overall. However, the risk was elevated among young women and for death by suicide. This supports the importance of careful follow-up for young patients and highlights the need to develop suicide prevention strategies for all women with PMDs.
Premenstrual disorders (PMDs) are characterized by a range of mental and physical symptoms occurring during the week before menstruation. 1 These disorders are classified into premenstrual syndrome, which affects 20% to 30% of women of reproductive age, and premenstrual dysphoric disorders, with a prevalence ranging from 2% to 6%. 1 , 2 Numerous studies have highlighted the impairments related to PMDs, which can affect women’s daily activities, relationships, and professional performance. 3 , 4
Despite the dearth of research on long-term consequences of PMDs, there are some indications of an association between PMDs and mortality. For instance, women with PMDs present with elevated blood pressure levels and a 40% higher risk of developing hypertension. 5 , 6 Women with diabetes with PMDs have been found to have a greater risk of uncontrolled blood glucose levels. 7 In addition, PMDs are highly comorbid with psychiatric disorders, 8 , 9 which are associated with elevated risk of both nonnatural and natural-cause mortality. 10 - 12 Furthermore, we recently showed that, even after accounting for psychiatric comorbidities, 13 , 14 women with PMDs were at risk of suicidal behavior and accidents, which are the leading causes of death in young women. 13 To further our understanding of the risk of death associated with PMDs, this study aimed to evaluate the risks of all-cause and cause-specific mortality among women with clinically diagnosed PMDs and the risks stratified by age groups whenever possible, by leveraging the national health registers in Sweden.
This cohort study was approved by the Swedish Ethical Review Authority. Written consent from the participants is not required for register-based studies under Swedish law. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology ( STROBE ) reporting guidelines for cohort studies. 15
The study was based on Swedish national health and population registers 16 : (1) the National Patient Register collects all hospital discharge diagnoses in Sweden from 1987 and 80% of hospital-based outpatient visits from 2001, (2) the National Prescribed Drug Register captures redeemed drug prescriptions from all pharmacies since 2005, (3) the National Cause of Death Register comprises death certificates since 1952, (4) the Longitudinal Integration Database for Health Insurance and Labor Market Studies integrates sociodemographic information for all residents aged 16 years and older since 1990, (5) the Total Population Register includes country of birth and migrations, and (6) the Multi-Generation Register documents parental information on residents since 1961. Registers were linked using the personal identification number assigned to all residents at birth or migration to Sweden.
We conducted a nationwide, population-based matched cohort study. Using incidence density sampling, women of reproductive age with a first diagnosis of PMDs between January 1, 2001, and December 31, 2018, were randomly matched by year of birth to 5 women who were free of PMD at that date. Reproductive age was defined as the period between age 15 years (96% of Swedish women had menarche by then 17 , 18 ) and 52 years (the average age of menopause in Sweden 17 , 18 ). Women who had undergone hysterectomy or bilateral oophorectomy before matching were excluded from the analyses. Individuals were followed-up until death, emigration, or December 31, 2018, whichever came first. If matched unaffected women received a diagnosis of PMD during the study period, their follow-up was censored at that time (eFigure in Supplement 1 ).
We also conducted a sibling comparison to address unmeasured confounding from factors shared by sisters, such as early family environment and genetics. In brief, full siblings were identified as sharing both biological parents recorded in the Multi-Generation Register. Women with PMDs were matched to their full sisters on the basis of age at diagnosis such that the unaffected sister(s) had no PMD diagnosis at the same age when the affected sister received a diagnosis.
As described elsewhere, 13 PMDs were identified through inpatient and outpatient diagnoses, along with treatments. In brief, inpatient and outpatient diagnoses were identified using the Swedish version of International Classification of Diseases, Ninth Revision (ICD-9) or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) . The National Patient Register has nationwide coverage on inpatient care from 1987 onward and includes information on more than 80% of specialist-based outpatient visits from 2001 onward with high validity (positive predictive value of 85%-95% across diseases). 19 To compensate for a lack of information on PMDs diagnosed in primary care, we identified prescriptions for antidepressant and oral contraceptives with a written indication for PMD treatment. Diagnoses and treatment codes are provided in eTable 1 in Supplement 1 .
Date and cause of death were obtained from the National Cause of Death Register. The underlying cause of death was classified according to ICD-10 codes as death due to nonnatural and natural causes (eTable 1 in Supplement 1 ). We further identified deaths due to neoplasms, cardiovascular diseases, and nervous system diseases as deaths from natural causes, whereas suicide and death due to accidents were classified as nonnatural causes. The register has a satisfactory accuracy rate for the studied causes of death (eg, 90% for malignant neoplasms, 87% for ischemic heart disease, and 96% for suicide). 20 - 22 All deaths were considered as premature deaths because they occurred before the age of 70 years. 23
Age at diagnosis or matching was calculated from date of birth, and country of birth was classified as born in Sweden or not. Region of residence, educational level, and income at the time of matching were derived from Longitudinal Integration Database for Health Insurance and Labor Market Studies, with regions grouped into south, center, or north of Sweden. Educational level was classified as 9 years or less, 10 to 12 years, more than 12 years, and unknown; personal income was divided into less than the 20th percentile, 20th to 80th percentile, and greater than the 80th percentile of the income distribution of the study population, and unknown.
Some psychiatric and somatic conditions are associated with both PMDs and premature death 8 , 10 - 12 , 24 - 26 and were considered as confounders in this study. Psychiatric comorbidities were defined as any psychiatric diagnoses recorded for inpatient or outpatient specialist care visits by the time of matching. The Charlson Comorbidity Index, adapted to Swedish ICD-9 or ICD-10 codes, 27 was used to assess somatic comorbidity. Only psychiatric and somatic comorbidities diagnosed before the matching date were included. Codes used to identify covariates are available in eTable 1 in Supplement 1 .
Descriptive statistics were performed to summarize the baseline characteristics between women with and without PMDs. Next, hazard ratios (HRs) of all-cause mortality associated with PMDs and corresponding 95% CIs were estimated using Cox regression models conditional on the matching set for the population-matched cohort and the sibling set for the sibling-matched cohort. The underlying timescales were time since matching for the population-matched cohort and age at follow-up for the sibling-matched cohorts. The Schoenfeld residual-based test was used to confirm that there were no violations to the proportional-hazard assumption. HRs were adjusted for attained age (through matching or underlying timescale), educational level, residence, country of birth, and personal income and were additionally adjusted for somatic and psychiatric comorbidities. Stratification by age at diagnosis was conducted because early onset of PMDs may have a longer impact throughout reproductive ages. We also performed stratification by psychiatric and somatic comorbidities to examine potential risk modification.
Associations with mortality were further estimated for natural and nonnatural causes and for the major causes observed in the population. In corresponding analyses, individuals were censored for deaths due to causes other than the ones under study. Stratification by age at diagnosis was also conducted. Having seen comparable but underpowered results from sibling comparison in the main analysis, these analyses were performed in the population-matched cohort only.
Prospective symptom charting over 2 consecutive menstrual cycles is recommend when diagnosing PMDs. 28 , 29 Because such information is not recorded in registers, we conducted a sensitivity analysis limited to women with a minimum of 2 recorded diagnoses of PMDs 28 days apart or longer. Owing to the challenges of determining intent in clinical practice, our main evaluation of suicide included deaths with undetermined intent, 30 - 32 whereas in a sensitivity analysis we restricted to intentional self-harm ( ICD-10 codes X60-X84). Additional sensitivity analyses for deaths attributed to neoplasms or cardiovascular disease were restricted to individuals without a history of the corresponding disease. Because receiving a diagnosis of PMD can take several years, 33 somatic and psychiatric comorbidities identified in the study could have occurred after PMDs onset and mediated the association with mortality. Allowing this possibility, we repeated the analyses without these comorbidities in the models. Finally, because treatment may influence natural mortality risk, we conducted stratifications by selective serotonin reuptake inhibitor (SSRI; Anatomical Therapeutic Chemical Classification N06AB) and hormonal replacement therapy (HRT; Anatomical Therapeutic Chemical Classification G03C and G03F) prescriptions, assessed as time-varying variables.
For all analyses, we used a 2-sided P < .05 to define statistical significance. Analyses were conducted from September 2022 to April 2023. Data were prepared in SAS statistical software version 9.4 (SAS Institute) and analyzed with Stata statistical software version 17.0 (StataCorp).
A total of 3 700 275 women were identified in the registers (eFigure in Supplement 1 ). Among them, 67 748 received a diagnosis of PMDs between 2001 and 2018 and were free from bilateral oophorectomy or hysterectomy at the time of PMD diagnosis. These women were matched at the time of diagnosis to 5 women fulfilling the same inclusion criteria and free from PMD (338 740 women), for a total population-matched cohort of 406 488 individuals. More than one-third of the women with PMDs had at least 1 full sister, and matching to up to 7 siblings resulted in a sibling-matched cohort of 55 801 individuals.
The mean (SD) age at diagnosis or matching was 35.8 (8.2) years ( Table 1 ). More than one-half of the women in the population-matched cohort resided in the middle of the country, reaching 65.4% (44 285 women) for women with PMDs. Somatic comorbidities were similarly distributed (6718 women with PMD [9.9%] and 30 870 women without PMD [9.1%] had ≥1 somatic comorbidity), whereas psychiatric disorders were more frequent among women with PMDs (16 160 women with PMD [23.8%] vs 47 919 women without PMD [14.1%]). Similar patterns were noted in the sibling-matched cohort.
During a mean (SD) follow-up of 6.2 (4.6) years (range, 1-18 years), 367 deaths were observed among women with PMDs (rate, 8.4 deaths per 10 000 person-years; 95% CI, 7.6-9.3 deaths per 10 000 person-years), and 1958 deaths were observed among women without PMDs (rate, 9.1 deaths per 10 000 person-years; 95% CI, 8.7-9.6 deaths per 10 000 person-years). Overall, women with PMDs did not have a higher risk of all-cause mortality compared with women without PMDs (age-adjusted HR, 0.91; 95% CI, 0.82-1.02) ( Table 2 ). A lower mortality risk was found when accounting for demographics and comorbidities (HR, 0.88; 95% CI, 0.77-0.99). This lower risk was mainly seen among women with PMDs diagnosed at ages 45 to 51 years (HR, 0.79; 95% CI, 0.64-0.97), whereas women with PMDs diagnosed before the age of 25 years had more than doubled risk of death (HR, 2.51; 95% CI, 1.42-4.42). Largely comparable results were found in the sibling comparison (HR, 0.84; 95% CI, 0.67-1.12), although statistical power was limited, particularly in age-stratified analysis.
The association of PMDs with all-cause mortality was comparable between women with and without psychiatric comorbidity ( P for interaction = .58) ( Table 3 ). However, the inverse association was greater among women with somatic comorbidities (HR, 0.47; 95% CI, 0.35-0.62). Again, this finding appeared to be more prevalent among women whose PMD was diagnosed at ages 45 to 51 years (eTable 2 in Supplement 1 ), whereas among women who received a diagnosis before age 25 years, there was no significant interaction with either comorbidity.
The 5 most common causes of death were neoplasms (150 deaths [40.9%]), suicide (100 deaths [27.2%]), cardiovascular diseases (29 deaths [7.9%]), accident (29 deaths [7.9%]), and nervous system disease (18 deaths [4.9%]) (eTable 3 in Supplement 1 ). The PMD-associated risk of death due to natural causes followed the same pattern observed for all-cause mortality, with lower risk overall (HR, 0.73; 95% CI, 0.62-0.84) but elevated risk among women who received a diagnosis before the age of 25 years (HR, 2.59; 95% CI, 1.21-5.54) ( Table 4 ). The lower risk was largely associated with cardiovascular-specific mortality (HR, 0.53; 95% CI, 0.34-0.84). There were 257 deaths attributed to cardiovascular disease, with 133 (51.8%) occurring in women who were included in the cohort at age 45 years or older (eTable 4 in Supplement 1 ). In contrast, mortality due to nonnatural causes was higher among women with PMDs (HR, 1.59; 95% CI, 1.24-2.04) and was primarily explained by suicide (HR, 1.92; 95% CI, 1.42-2.60). Notably, an elevated risk of suicide was observed regardless of the age at diagnosis ( P for interaction = .68) ( Table 5 ), although it was more pronounced among women who received a diagnosis before age 25 years (HR, 3.84; 95% CI, 1.18-12.45). Among women younger than 25 years who died, approximately one-third of the deaths (19 deaths) were due to suicide (eTables 4 and 5 in Supplement 1 ).
Comparable results were observed when the analysis was limited to PMDs with 2 diagnoses 28 days or more apart (eTable 6 in Supplement 1 ). Similarly, applying a stricter definition of suicide resulted in comparable estimates (eTable 7 in Supplement 1 ). Excluding individuals with a history of cancer or cardiovascular disease did not change the HR for the corresponding cause-specific mortality. Removing psychiatric or somatic comorbidities from regression models did not noticeably change the estimates (eTable 8 in Supplement 1 ). In addition, the inverse association between PMDs and natural mortality risk remained among individuals who did not use HRT or SSRI before or during the follow-up (eTable 9 in Supplement 1 ). SSRIs were prescribed to more than 80% of patients with PMD (55 552 women); 43% of women who received a diagnosis of PMDs at age 45 years or older were prescribed HRT (4110 women) compared with 23% of unaffected women (10 829 women). Finally, PMD-free women had a higher prevalence of diabetes or breast cancer (eTables 10 and 11 in Supplement 1 ).
This nationwide, population-based, matched cohort study with follow-up for up to 18 years fills an important gap in the understanding of all-cause and cause-specific mortality among women with PMDs. The use of national registers enabled complete follow-up and comprehensive information on death and its causes. Although no increased risk of all-cause death was observed overall, an elevated risk was noted among women who received a diagnosis before age 25 years. Women with PMDs were also found to have an elevated risk for suicide, regardless of age at diagnosis.
Our study revealed a consistently elevated risk of suicide among women with PMDs across all age groups. This is in line with previous research 14 , 34 showing that individuals with PMDs have a higher prevalence of suicidality. Our recent study 13 showed that women with PMDs were at increased risk of suicide attempt. However, to our knowledge, the current study is the first report to illustrate the increased risk of completed suicide.
Our findings further revealed that young women with diagnosed PMDs had an increased risk of all-cause mortality. Although suicidal behavior is more common in young women, this finding is not completely explained by suicide, which accounted for one-third of the deaths (eTables 4 and 5 in Supplement 1 ). Indeed, we also observed an increased risk of deaths due to natural causes in this group. Future studies with larger sample size are needed to examine the cause-specific mortality for these women.
In contrast, women who received a diagnosis at age 45 years or older had a lower risk of mortality than women without PMDs. These women may present a late-onset PMD or an exacerbation of mild symptoms, with a shorter cumulative impact of PMDs as symptoms are expected to end after menopause. We cannot exclude a potential healthy survivor effect because women who died before receiving a diagnosis or being identified in the registers were not included. Also, we must consider the potential for misclassification, as perimenopausal symptoms and depression may mirror PMDs symptoms, resulting in potential diagnostic errors. Moreover, women with premature menopause are not at risk of PMDs and might be oversampled in the unaffected group. Given that early menopause is associated with elevated risk of cardiovascular events 35 , 36 and premature mortality, 37 this may have influenced and partly explains the inverse associations. Specific information on menopause is lacking from registers; the age of first HRT prescription or diagnosis of early menopause were similar between the studied groups, although diagnoses and treatment rates are low in Sweden. 18
The unexpected finding of a lower risk of death due to natural causes associated with PMDs, particularly from cardiovascular events, warrants further investigation. This association may have been influenced by the reduced mortality in women who received a diagnosis at 45 years or older, because 51.8% of the cardiovascular-specific deaths occurred among this group (eTable 4 in Supplement 1 ). Because PMDs are often underdiagnosed, women identified with PMDs may have a higher level of self-awareness and maintain closer contact with the health care system. They may be more inclined to behavior changes, receiving a diagnosis early, and/or receiving treatment for comorbidities. The lower risk of cardiovascular-specific death could also be related to the use of SSRIs, which were prescribed to more than 80% of the patients with PMD. The cardioprotective effect of SSRI has been reported, although they can confer adverse impact on specific cardiovascular diseases. 38 , 39 A similar hypothesis can be formulated for HRT 40 : 43% of women who received a diagnosis of PMD at age 45 years or older were prescribed HRT compared with 23% for unaffected women. However, the inverse association remained among individuals not exposed to HRT or SSRIs. We also acknowledge the possibility of unexplored biases that could have influenced these unexpected findings, despite our concerted efforts to address them thoroughly.
PMDs are highly comorbid with psychiatric disorders, 8 , 9 , 12 , 41 which are associated with a higher mortality risk. 12 , 40 Although psychiatric comorbidities could have explained our findings among young women, we observed comparable associations in the presence or absence of psychiatric comorbidity. Notably, some women may develop major depression later in life, 9 and such a mediation pathway warrants future research. Regarding somatic comorbidity, we found greater inverse associations between PMDs and mortality in the presence of 1 or more condition. This could be partially attributed a closer contact of affected women with the health care system. Moreover, although the prevalence for 1 or more comorbidity diagnosed before the matching date was comparable between women, the conditions and their severity may differ. For instance, some somatic comorbidities (eg, terminal stage of cancer) or treatments (eg, chemotherapy for breast cancer) can lead to amenorrhea or oligomenorrhea, 7 , 42 - 44 reducing the occurrence of PMD symptoms. Indeed, in our data, PMD-free women had a higher prevalence of diabetes or breast cancer (eTables 10 and 11 in Supplement 1 ). The limited information on disease severity did not allow further investigation. The main strength of this study is the use of the Swedish register data, which allowed us to conduct a nationwide study with complete follow-up and comprehensive information on death and its causes.
This study has several limitations. First, although guidelines suggest diagnosis confirmation by a prospective evaluation of symptoms for 2 menstrual cycles, 29 the registers lacked such information. However, restricting PMDs to 2 diagnoses registered separately yielded comparable results. Moreover, delayed diagnosis is frequent for PMDs, 45 and most are diagnosed in primary care and not treated with medication, leading to potential misclassification of affected women as unaffected. However, such potential misclassification should not be related to the risk of death but may have attenuated the association toward the null.
Second, this study relied on recordings in the National Cause of Death Register, resulting in potential misclassification on the cause of death. 21 A study 20 showed an overall satisfactory accuracy rate of 77%, with variation depending on the disease (90% for malignant neoplasms, 87% for ischemic heart disease) and age (98% and 91% agreement in groups 0-44 and 45-64 years, respectively). We used ICD-9 or ICD-10 chapters to identify and group causes of death, minimizing the potential misclassification. 46
Third, some potential confounding factors were not available in the registers (eg, smoking status 47 , 48 or body mass index 25 , 49 ). Comparable results were observed in the sibling comparison, which allowed us to address factors shared between sisters including familial environment, genetics, and possibly some lifestyle factors.
Fourth, the population under study was relatively young, with a mean (SD) age of 35.8 (8.2) years, and the mean (SD) duration of follow-up was 6.2 (4.6) years. As a result, the study primarily captured deaths due to nonnatural causes, and only short-term mortality could be assessed. The small number of events also limits the generalization of the results. Future studies with longer follow-up are needed to capture long-term consequences.
Our findings suggest that women with clinically diagnosed PMDs were not at elevated risk of all-cause short-term death overall. However, women who received a diagnosis of PMD at an early age showed excess mortality, and the risk of suicide was elevated regardless of age. This supports the importance of careful follow-up for young women with PMDs and highlights the need to develop suicide prevention strategies for all women with PMDs.
Accepted for Publication: March 25, 2024.
Published: May 28, 2024. doi:10.1001/jamanetworkopen.2024.13394
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Opatowski M et al. JAMA Network Open .
Corresponding Author: Marion Opatowski, PhD, Unit of Integrative Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, Nobels väg 13, Stockholm 171 65, Sweden ( [email protected] ).
Author Contributions: Dr Opatowski had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Opatowski, Lu.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Opatowski, Lu.
Critical review of the manuscript for important intellectual content: Valdimarsdóttir, Oberg, Bertone-Johnson, Lu.
Statistical analysis: Opatowski, Valdimarsdóttir.
Obtained funding: Opatowski, Valdimarsdóttir, Lu.
Administrative, technical, or material support: Oberg, Lu.
Supervision: Lu.
Conflict of Interest Disclosures: None reported.
Funding/Support: This work was supported by the Swedish Research Council for Health, Working Life and Welfare (FORTE) (grant No. 2020-00971 to Dr Lu), the Swedish Research Council (Vetenskapsrådet) (grant No. 2020-01003 to Dr Lu), the Karolinska Institutet Strategic Research Area in Epidemiology and Biostatistics (to Dr Lu), the Icelandic Research Fund (grant No. 218274-051 to Dr Valdimarsdóttir), and the Mental Health Foundation (Fonden för Psykisk Hälsa, to Dr Opatowski).
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Meeting Presentation: A poster with preliminary data was presented at the Society for Epidemiologic Research conference; June 15, 2023; Portland, Oregon.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Excess mortality across countries in the Western World since the COVID-19 pandemic: ‘Our World in Data’ estimates of January 2020 to December 2022

Marcel Hoogland ,
Minke Huibers ,
Gertjan Kaspers .
https://doi.org/ 10.1136/bmjph-2023-000282
Introduction Excess mortality during the COVID-19 pandemic has been substantial. Insight into excess death rates in years following WHO’s pandemic declaration is crucial for government leaders and policymakers to evaluate their health crisis policies. This study explores excess mortality in the Western World from 2020 until 2022.
Methods All-cause mortality reports were abstracted for countries using the ‘Our World in Data’ database. Excess mortality is assessed as a deviation between the reported number of deaths in a country during a certain week or month in 2020 until 2022 and the expected number of deaths in a country for that period under normal conditions. For the baseline of expected deaths, Karlinsky and Kobak’s estimate model was used. This model uses historical death data in a country from 2015 until 2019 and accounts for seasonal variation and year-to-year trends in mortality.
Results The total number of excess deaths in 47 countries of the Western World was 3 098 456 from 1 January 2020 until 31 December 2022. Excess mortality was documented in 41 countries (87%) in 2020, 42 countries (89%) in 2021 and 43 countries (91%) in 2022. In 2020, the year of the COVID-19 pandemic onset and implementation of containment measures, records present 1 033 122 excess deaths (P-score 11.4%). In 2021, the year in which both containment measures and COVID-19 vaccines were used to address virus spread and infection, the highest number of excess deaths was reported: 1 256 942 excess deaths (P-score 13.8%). In 2022, when most containment measures were lifted and COVID-19 vaccines were continued, preliminary data present 808 392 excess deaths (P-score 8.8%).
Conclusions Excess mortality has remained high in the Western World for three consecutive years, despite the implementation of containment measures and COVID-19 vaccines. This raises serious concerns. Government leaders and policymakers need to thoroughly investigate underlying causes of persistent excess mortality.
What is already known on this topic
Excess mortality during the COVID-19 pandemic has been substantial. Insight into excess death rates in years following WHO’s pandemic declaration is crucial for government leaders and policymakers to evaluate their health crisis policies.
What this study adds
Excess mortality has remained high in the Western World for three consecutive years, despite the implementation of containment measures and COVID-19 vaccines. This raises serious concerns.
How this study might affect research, practice or policy
Government leaders and policymakers need to thoroughly investigate the underlying causes of persistent excess mortality.
- Introduction
Excess mortality is internationally recognised as an accurate measure for monitoring and comparing health crisis policies across geographic regions. 1–4 Excess mortality concerns the number of deaths from all causes during a humanitarian emergency, such as the COVID-19 pandemic, above the expected number of deaths under normal circumstances. 5–7 Since the outbreak of the COVID-19 pandemic, excess mortality thus includes not only deaths from SARS-CoV-2 infection but also deaths related to the indirect effects of the health strategies to address the virus spread and infection. 1–4 The burden of the COVID-19 pandemic on disease and death has been investigated from its beginning. Numerous studies expressed that SARS-CoV-2 infection was likely a leading cause of death among older patients with pre-existing comorbidities and obesity in the early phase of the pandemic, that various containment measures were effective in reducing viral transmission and that COVID-19 vaccines prevented severe disease, especially among the elderly population. 1 8–14 Although COVID-19 containment measures and COVID-19 vaccines were thus implemented to protect citizens from suffering morbidity and mortality by the COVID-19 virus, they may have detrimental effects that cause inferior outcomes as well. 1 2 15 It is noteworthy that excess mortality during a crisis points to a more extensive underlying burden of disease, disablement and human suffering. 16
On 11 March 2020, WHO declared the COVID-19 pandemic. 17 Countries in the Western World promptly implemented COVID-19 containment measures (such as lockdowns, school closures, physical distancing, travel restrictions, business closures, stay-at-home orders, curfews and quarantine measures with contact tracing) to limit virus spread and shield its residents from morbidity and mortality. 18 These non-pharmaceutical interventions however had adverse indirect effects (such as economic damage, limited access to education, food insecurity, child abuse, limited access to healthcare, disrupted health programmes and mental health challenges) that increased morbidity and mortality from other causes. 19 Vulnerable populations in need of acute or complex medical treatment, such as patients with cardiovascular disease, cerebrovascular conditions, diabetes and cancer, were hurt by these interventions due to the limited access to and delivery of medical services. Shortage of staff, reduced screening, delayed diagnostics, disrupted imaging, limited availability of medicines, postponed surgery, modified radiotherapy and restricted supportive care hindered protocol adherence and worsened the condition and prognosis of patients. 19–26 A recent study investigated excess mortality from some major non-COVID causes across 30 countries in 2020. Significant excess deaths were reported from ischaemic heart diseases (in 10 countries), cerebrovascular diseases (in 10 countries) and diabetes (in 19 countries). 27 On 14 October 2020, Professor Ioannidis from Stanford University published an overall Infection Fatality Rate of COVID-19 of 0.23%, and for people aged <70 years, the Infection Fatality Rate was 0.05%. 28 Governments in the Western World continued to impose lockdowns until the end of 2021.
In December 2020, the UK, the USA and Canada were the first countries in the Western World that started with the roll-out of the COVID-19 vaccines under emergency authorisation. 29–31 At the end of December 2020, a large randomised and placebo-controlled trial with 43 548 participants was published in the New England Journal of Medicine , which showed that a two-dose mRNA COVID-19 vaccine regimen provided an absolute risk reduction of 0.88% and relative risk reduction of 95% against laboratory-confirmed COVID-19 in the vaccinated group (8 COVID-19 cases/17 411 vaccine recipients) versus the placebo group (162 COVID-19 cases/17 511 placebo recipients). 32 33 At the beginning of 2021, most other Western countries followed with rolling out massive vaccination campaigns. 34–36 On 9 April 2021, the overall COVID-19 Infection Fatality Rate was reduced to 0.15% and expected to further decline with the widespread use of vaccinations, prior infections and the evolution of new and milder variants. 37 38
Although COVID-19 vaccines were provided to guard civilians from suffering morbidity and mortality by the COVID-19 virus, suspected adverse events have been documented as well. 15 The secondary analysis of the placebo-controlled, phase III randomised clinical trials of mRNA COVID-19 vaccines showed that the Pfizer trial had a 36% higher risk of serious adverse events in the vaccine group. The risk difference was 18.0 per 10 000 vaccinated (95% CI 1.2 to 34.9), and the risk ratio was 1.36 (95% CI 1.02 to 1.83). The Moderna trial had a 6% higher risk of serious adverse events among vaccine recipients. The risk difference was 7.1 per 10 000 vaccinated (95% CI −23.2 to 37.4), and the risk ratio was 1.06 (95% CI 0.84 to 1.33). 39 By definition, these serious adverse events lead to either death, are life-threatening, require inpatient (prolongation of) hospitalisation, cause persistent/significant disability/incapacity, concern a congenital anomaly/birth defect or include a medically important event according to medical judgement. 39–41 The authors of the secondary analysis point out that most of these serious adverse events concern common clinical conditions, for example, ischaemic stroke, acute coronary syndrome and brain haemorrhage. This commonality hinders clinical suspicion and consequently its detection as adverse vaccine reactions. 39 Both medical professionals and citizens have reported serious injuries and deaths following vaccination to various official databases in the Western World, such as VAERS in the USA, EudraVigilance in the European Union and Yellow Card Scheme in the UK. 42–48 A study comparing adverse event reports to VAERS and EudraVigilance following mRNA COVID-19 vaccines versus influenza vaccines observed a higher risk of serious adverse reactions for COVID-19 vaccines. These reactions included cardiovascular diseases, coagulation, haemorrhages, gastrointestinal events and thromboses. 39 49 Numerous studies reported that COVID-19 vaccination may induce myocarditis, pericarditis and autoimmune diseases. 50–57 Postmortem examinations have also ascribed myocarditis, encephalitis, immune thrombotic thrombocytopenia, intracranial haemorrhage and diffuse thrombosis to COVID-19 vaccinations. 58–67 The Food and Drug Administration noted in July 2021 that the following potentially serious adverse events of Pfizer vaccines deserve further monitoring and investigation: pulmonary embolism, acute myocardial infarction, immune thrombocytopenia and disseminated intravascular coagulation. 39 68
Insight into the excess death rates in the years following the declaration of the pandemic by WHO is crucial for government leaders and policymakers to evaluate their health crisis policies. 1–4 This study therefore explores excess mortality in the Western World from 1 January 2020 until 31 December 2022.
- Materials and methods
The Western World is primarily defined by culture rather than geography. It refers to various countries in Europe and to countries in Australasia (Australia, New Zealand) and North America (the USA, Canada) that are based on European cultural heritage. The latter countries were once British colonies that acquired Christianity and the Latin alphabet and whose populations comprised numerous descendants from European colonists or migrants. 69
Study design
All-cause mortality reports were abstracted for countries of the Western World using the ‘Our World in Data’ database. 12 Only countries that had all-cause mortality reports available for all three consecutive years (2020–2022) were included. If coverage of one of these years was missing, the country was excluded from the analysis.
The ‘Our World in Data’ database retrieves their reported number of deaths from both the Human Mortality Database (HMD) and the World Mortality Dataset (WMD). 5 HMD is sustained by research teams of both the University of California in the USA and the Max Planck Institute for Demographic Research in Germany. HMD recovers its data from Eurostat and national statistical agencies on a weekly basis. 5 70 The ‘Our World in Data’ database used HMD as their only data source until February 2021. 5 WMD is sustained by the researchers Karlinsky and Kobak. WMD recovers its data from HMD, Eurostat and national statistical agencies on a weekly basis. 5 71 The ‘Our World in Data’ database started to use WMD as a data source next to HMD since February 2021. 5
‘Excess mortality’ is assessed as the deviation between the reported number of deaths in a country during a certain week or month in 2020 until 2022 and the expected or projected number of deaths in a country for that period under normal conditions. 5 For the baseline of expected deaths, the estimate model of Karlinsky and Kobak was used. This linear regression model uses historical death data in a country from 2015 until 2019 and accounts for seasonal variation in mortality and year-to-year trends due to changing population structure or socioeconomic factors. 5 7
‘Excess mortality P-score’ concerns the percentage difference between the reported number of deaths and the projected number of deaths in a country. 5 This measure permits comparisons between various countries. Although presenting the raw number of excess deaths provides insight into the scale, it is less useful to compare countries because of their large population size variations. 5 The ‘Our World in Data’ database presents P-scores in a country during a certain week or month in 2020 until 2022. 5 These P-scores are calculated from both the reported number of deaths in HMD and WMD and the projected number of deaths using the estimate model of Karlinsky and Kobak in WMD. 5 7 70 71
For correct interpretation of excess mortality provided by the ‘Our World in Data’ database, the following needs to be taken into consideration: the reported number of deaths may not represent all deaths, as countries may lack the infrastructure and capacity to document and account for all deaths. 5 In addition, death reports may be incomplete due to delays. It may take weeks, months or years before a death is actually reported. The date of a reported death may refer to the actual death date or to its registration date. Sometimes, a death may be recorded but not the date of death. Countries that provide weekly death reports may use different start and end dates of the week. Most countries define the week from Monday until Sunday, but not all countries do. Weekly and monthly reported deaths may not be completely comparable, as excess mortality derived from monthly calculations inclines to be lower. 5 7
For our analysis, weekly all-cause mortality reports from the ‘Our World in Data’ database were converted to monthly reports. Subsequently, the monthly reports were converted to annual reports.
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
The ‘Our World in Data’ database contained all-cause mortality reports of 47 countries (96%) in the Western World for the years 2020, 2021 and 2022. Only Andorra and Gibraltar were excluded. Both countries lacked all-cause mortality reports for the year 2022. Most countries (n=36, 77%) present weekly all-cause mortality reports, whereas 11 countries (23%) report monthly. The latter countries include the following: Albania, Bosnia Herzegovina, Faeroe Islands, Greenland, Kosovo, Liechtenstein, Moldova, Monaco, North Macedonia, San Marino and Serbia.
The all-cause mortality reports were abstracted from the ‘Our World in Data’ database on 20 May 2023. At this date, four countries (9%) still lacked all-cause mortality reports for various periods: Canada (1 month), Liechtenstein (3 months), Monaco (3 months) and Montenegro (4 months). It is noteworthy that all-cause mortality reports are also still being updated for the other countries due to registration delays which may take weeks, months or even years.
Excess mortality
Online supplemental table 1 illustrates that the total number of excess deaths in the 47 countries of the Western World was 3 098 456 from 1 January 2020 until 31 December 2022. Excess mortality was documented in 41 countries (87%) in 2020, in 42 countries (89%) in 2021 and in 43 countries (91%) in 2022.
In 2020, the year of the COVID-19 pandemic and implementation of the containment measures, 1 033 122 excess deaths (P-score 11.4%) were recorded. In 2021, the year in which both COVID-19 containment measures and COVID-19 vaccines were used to address virus spread and infection, a total of 1 256 942 excess deaths (P-score 13.8%) were reported. In 2022, the year in which most containment measures were lifted and COVID-19 vaccines were continued, preliminary available data counts 808 392 excess deaths (P-score 8.8%).
Figure 1 presents the excess mortality and cumulative excess mortality in 47 countries of the Western World over the years 2020, 2021 and 2022. The linear excess mortality trendline is almost horizontal.
Excess mortality and cumulative excess mortality in the Western World (n=47 countries). Preliminary and incomplete all-cause mortality reports are available for 2022.
Excess mortality P-scores
Figure 2 shows the excess mortality P-scores per country in the Western World. Only Greenland had no excess deaths between 2020 and 2022. Among the other 46 countries with reported excess mortality, the percentage difference between the reported and projected number of deaths was highest in 13 countries (28%) during 2020, in 21 countries (46%) during 2021 and in 12 countries (26%) during 2022. Figure 3 exemplifies excess mortality P-score curves of the highest-populated country of North America (the USA), the four highest-populated countries of Europe (Germany, France, the UK and Italy) and the highest-populated country of Australasia (Australia).
Excess mortality P-scores per country in the Western World (n=47 countries). Preliminary and incomplete all-cause mortality reports are available for 2022.
Excess mortality P-score curves of six countries in the Western World. Preliminary and incomplete all-cause mortality reports are available for 2022.
Figure 4 highlights a map of excess mortality P-scores in the Western World over the years 2020, 2021 and 2022. 74 Table 1 presents a classification of excess mortality P-scores in the Western World.
Map of excess mortality P-scores in the Western World (n=47 countries). 74 Preliminary and incomplete all-cause mortality reports are available for 2022.
This study explored the excess all-cause mortality in 47 countries of the Western World from 2020 until 2022. The overall number of excess deaths was 3 098 456. Excess mortality was registered in 87% of countries in 2020, in 89% of countries in 2021 and in 91% of countries in 2022. During 2020, which was marked by the COVID-19 pandemic and the onset of mitigation measures, 1 033 122 excess deaths (P-score 11.4%) were to be regretted. 17 18 A recent analysis of seroprevalence studies in this prevaccination era illustrates that the Infection Fatality Rate estimates in non-elderly populations were even lower than prior calculations suggested. 37 At a global level, the prevaccination Infection Fatality Rate was 0.03% for people aged <60 years and 0.07% for people aged <70 years. 38 For children aged 0–19 years, the Infection Fatality Rate was set at 0.0003%. 38 This implies that children are rarely harmed by the COVID-19 virus. 19 38 During 2021, when not only containment measures but also COVID-19 vaccines were used to tackle virus spread and infection, the highest number of excess deaths was recorded: 1 256 942 excess deaths (P-score 13.8%). 26 37 Scientific consensus regarding the effectiveness of non-pharmaceutical interventions in reducing viral transmission is currently lacking. 75 76 During 2022, when most mitigation measures were negated and COVID-19 vaccines were sustained, preliminary available data count 808 392 excess deaths (P-score 8.8%). 39 The percentage difference between the documented and projected number of deaths was highest in 28% of countries during 2020, in 46% of countries during 2021, and in 26% of countries during 2022.
This insight into the overall all-cause excess mortality since the start of the COVID-19 pandemic is an important first step for future health crisis policy decision-making. 1–4 The next step concerns distinguishing between the various potential contributors to excess mortality, including COVID-19 infection, indirect effects of containment measures and COVID-19 vaccination programmes. Differentiating between the various causes is challenging. 16 National mortality registries not only vary in quality and thoroughness but may also not accurately document the cause of death. 1 19 The usage of different models to investigate cause-specific excess mortality within certain countries or subregions during variable phases of the pandemic complicates elaborate cross-country comparative analysis. 1 2 16 Not all countries provide mortality reports categorised per age group. 2 12 Also testing policies for COVID-19 infection differ between countries. 1 2 Interpretation of a positive COVID-19 test can be intricate. 77 Consensus is lacking in the medical community regarding when a deceased infected with COVID-19 should be registered as a COVID-19 death. 1 77 Indirect effects of containment measures have likely altered the scale and nature of disease burden for numerous causes of death since the pandemic. However, deaths caused by restricted healthcare utilisation and socioeconomic turmoil are difficult to prove. 1 78–81 A study assessing excess mortality in the USA observed a substantial increase in excess mortality attributed to non-COVID causes during the first 2 years of the pandemic. The highest number of excess deaths was caused by heart disease, 6% above baseline during both years. Diabetes mortality was 17% over baseline during the first year and 13% above it during the second year. Alzheimer’s disease mortality was 19% higher in year 1 and 15% higher in year 2. In terms of percentage, large increases were recorded for alcohol-related fatalities (28% over baseline during the first year and 33% during the second year) and drug-related fatalities (33% above baseline in year 1 and 54% in year 2). 82 Previous research confirmed profound under-reporting of adverse events, including deaths, after immunisation. 83 84 Consensus is also lacking in the medical community regarding concerns that mRNA vaccines might cause more harm than initially forecasted. 85 French studies suggest that COVID-19 mRNA vaccines are gene therapy products requiring long-term stringent adverse events monitoring. 85 86 Although the desired immunisation through vaccination occurs in immune cells, some studies report a broad biodistribution and persistence of mRNA in many organs for weeks. 85 87–90 Batch-dependent heterogeneity in the toxicity of mRNA vaccines was found in Denmark. 48 Simultaneous onset of excess mortality and COVID-19 vaccination in Germany provides a safety signal warranting further investigation. 91 Despite these concerns, clinical trial data required to further investigate these associations are not shared with the public. 92 Autopsies to confirm actual death causes are seldom done. 58 60 90 93–95 Governments may be unable to release their death data with detailed stratification by cause, although this information could help indicate whether COVID-19 infection, indirect effects of containment measures, COVID-19 vaccines or other overlooked factors play an underpinning role. 1 8–14 20–25 39–60 68 90 This absence of detailed cause-of-death data for certain Western nations derives from the time-consuming procedure involved, which entails assembling death certificates, coding diagnoses and adjudicating the underlying origin of death. Consequently, some nations with restricted resources assigned to this procedure may encounter delays in rendering prompt and punctual cause-of-death data. This situation existed even prior to the outbreak of the pandemic. 1 5
A critical challenge in excess mortality research is choosing an appropriate statistical method for calculating the projected baseline of expected deaths to which the observed deaths are compared. 96 Although the analyses and estimates in general are similar, the method can vary, for instance, per length of the investigated period, nature of available data, scale of geographic area, inclusion or exclusion of past influenza outbreaks, accounting for changes in population ageing and size and modelling trend over years or not. 7 96 Our analysis of excess mortality using the linear regression model of Karlinsky and Kobak varies thus to some extent from previous attempts to estimate excess deaths. For example, Islam et al conducted an age- and sex-disaggregated time series analysis of weekly mortality data in 29 high-income countries during 2020. 97 They used a more elaborate statistical approach, an overdispersed Poisson regression model, for estimating the baseline of expected deaths on historical death data from 2016 to 2019. In contrast to the model of Karlinsky and Kobak, their baseline is weighing down prior influenza outbreaks so that every novel outbreak evolves in positive excess mortality. 7 97 Islam’s study found that age-standardised excess death rates were higher in men than in women in nearly all nations. 97 Alicandro et al investigated sex- and age-specific excess total mortality in Italy during 2020 and 2021, using an overdispersed Poisson regression model that accounts for temporal trends and seasonal variability. Historical death data from 2011 to 2019 were used for the projected baseline. When comparing 2020 and 2021, an increased share of the total excess mortality was attributed to the working-age population in 2021. Excess deaths were higher in men than in women during both periods. 98 Msemburi et al provided WHO estimates of the global excess mortality for its 194 member states during 2020 and 2021. For most countries, the historical period 2015–2019 was used to determine the expected baseline of excess deaths. In locations missing comprehensive data, the all-cause deaths were forecasted employing an overdispersed Poisson framework that uses Bayesian inference techniques to measure incertitude. This study describes huge differences in excess mortality between the six WHO regions. 99 Paglino et al used a Bayesian hierarchical model trained on historical death data from 2015 to 2019 and provided spatially and temporally granular estimates of monthly excess mortality across counties in the USA during the first 2 years of the pandemic. The authors found that excess mortality decreased in large metropolitan counties but increased in non-metropolitan counties. 100 Ruhm examined the appropriateness of reported excess death estimates in the USA by four previous studies and concluded that these investigations have likely understated the projected baseline of excess deaths and therewith overestimated excess mortality and its attribution to non-COVID causes. Ruhm explains that the overstatement of excess deaths may partially be explained by the fact that the studies did not adequately take population growth and age structure into account. 96 101–104 Although all the above-mentioned studies used more elaborate statistical approaches for estimating baseline mortality, Karlinsky and Kobak argue that their method is a trade-off between suppleness and chasteness. 7 It is the simplest method to captivate seasonal fluctuation and annual trends and more transparent than extensive approaches. 7
This study has various significant limitations. Death reports may be incomplete due to delays. It may take weeks, months or years before a death is registered. 5 Four nations still lack all-cause mortality reports for 1–4 months. Some nations issue complete data with profound arrears, whereas other nations publish prompt, yet incomplete data. 5 7 The presented data, especially for 2022, are thus preliminary and subject to backward revisions. The more recent data are usually more incomplete and therefore can undergo upward revisions over time. This implies that several of the reported excess mortality estimates can be underestimations. 7 The completeness and reliability of death registration data can also differ per nation for other reasons. The recorded number of deaths may not depict all deaths accurately, as the resources, infrastructure and registration capacity may be limited in some nations. 5 7 Most countries report per week, but some per month. Weekly reports generally provide the date of death, whereas monthly reports often provide the date of registration. Weekly and monthly reports may not be entirely comparable. 5 7 Our data are collected at a country level and provide no detailed stratification for sociodemographic characteristics, such as age or gender. 5 7
In conclusion, excess mortality has remained high in the Western World for three consecutive years, despite the implementation of COVID-19 containment measures and COVID-19 vaccines. This is unprecedented and raises serious concerns. During the pandemic, it was emphasised by politicians and the media on a daily basis that every COVID-19 death mattered and every life deserved protection through containment measures and COVID-19 vaccines. In the aftermath of the pandemic, the same morale should apply. Every death needs to be acknowledged and accounted for, irrespective of its origin. Transparency towards potential lethal drivers is warranted. Cause-specific mortality data therefore need to be made available to allow more detailed, direct and robust analyses to determine the underlying contributors. Postmortem examinations need to be facilitated to allot the exact reason for death. Government leaders and policymakers need to thoroughly investigate underlying causes of persistent excess mortality and evaluate their health crisis policies.
Dissemination to participants and related patient and public communities
We will disseminate findings through a press release on publication and contact government leaders and policymakers to raise awareness about the need to investigate the underlying causes of persistent excess mortality.
- Supplementary files
- Publication history
- Open access
- Published: 03 June 2024
Continue nursing education: an action research study on the implementation of a nursing training program using the Holton Learning Transfer System Inventory
- MingYan Shen 1 , 2 &
- ZhiXian Feng 1 , 2
BMC Medical Education volume 24 , Article number: 610 ( 2024 ) Cite this article
25 Accesses
Metrics details
To address the gap in effective nursing training for quality management, this study aims to implement and assess a nursing training program based on the Holton Learning Transfer System Inventory, utilizing action research to enhance the practicality and effectiveness of training outcomes.
The study involved the formation of a dedicated training team, with program development informed by an extensive situation analysis and literature review. Key focus areas included motivation to transfer, learning environment, and transfer design. The program was implemented in a structured four-step process: plan, action, observation, reflection.
Over a 11-month period, 22 nurses completed 14 h of theoretical training and 18 h of practical training with a 100% attendance rate and 97.75% satisfaction rate. The nursing team successfully led and completed 22 quality improvement projects, attaining a practical level of application. Quality management implementation difficulties, literature review, current situation analysis, cause analysis, formulation of plans, implementation plans, and report writing showed significant improvement and statistical significance after training.
The study confirms the efficacy of action research guided by Holton’s model in significantly enhancing the capabilities of nursing staff in executing quality improvement projects, thereby improving the overall quality of nursing training. Future research should focus on refining the training program through long-term observation, developing a multidimensional evaluation index system, exploring training experiences qualitatively, and investigating the personality characteristics of nurses to enhance training transfer effects.
Peer Review reports
Introduction
The “Medical Quality Management Measures“ [ 1 ] and “Accreditation Standards for Tertiary Hospitals (2020 Edition)” [ 2 ] both emphasize the importance of using quality management tools in medical institutions to carry out effective quality management [ 3 ]. However, there is a notable gap in translating theoretical training into effective, practical application in clinical settings [ 4 ]. This gap is further highlighted in the context of healthcare quality management, as evidenced in studies [ 5 ] which demonstrate the universality of these challenges across healthcare systems worldwide.
Addressing this issue, contemporary literature calls for innovative and effective training methods that transition from passive knowledge acquisition to active skill application [ 6 ]. The Holton Learning Transfer System Inventory [ 7 ] provides a framework focusing on key factors such as motivation, learning environment, and transfer design [ 7 , 8 , 9 ]. This study aims to implement a nursing training program based on the Holton model, using an action research methodology to bridge the theoretical-practical gap in nursing education.
Quality management training for clinical nurses has predominantly been characterized by short-term theoretical lectures, a format that often fails to foster deep engagement and lasting awareness among nursing personnel [ 10 ]. The Quality Indicator Project in Taiwan’s nursing sector, operational for over a decade, demonstrates the effective use of collective intelligence and scientific methodologies to address these challenges [ 11 ]. The proposed study responds to the need for training programs that not only impart knowledge but also ensure the practical application of skills in real-world nursing settings, thereby contributing to transformative changes within the healthcare system [ 12 ].
In April 2021, the Nursing Education Department of our hospital launched a quality improvement project training program for nurses. The initiation of this study is underpinned by the evident disconnect between theoretical training and the practical challenges nurses face in implementing quality management initiatives, a gap also identified in the work [ 13 ]. By exploring the efficacy of the Holton Learning Transfer System Inventory, this study seeks to enhance the practical application of training and significantly contribute to the field of nursing education and quality management in healthcare.
Developing a nursing training program with the Holton Learning Transfer System Inventory
Establishing a research team and assigning roles.
There are 10 members in the group who serve as both researchers and participants, aiming to investigate training process issues and solutions. The roles within the group are as follows: the deputy dean in charge of nursing is responsible for program review and organizational support, integrating learning transfer principles in different settings [ 14 ]; the deputy director of the Nursing Education Department handles the design and implementation of the training program, utilizing double-loop learning for training transfer [ 15 ]; the deputy director of the Nursing Department oversees quality control and project evaluation, ensuring integration of evidence-based practices and technology [ 16 ] and the deputy director of the Quality Management Office provides methodological guidance. The remaining members consist of 4 faculty members possessing significant university teaching experience and practical expertise in quality control projects, and 2 additional members who are jointly responsible for educational affairs, data collection, and analysis. Additionally, to ensure comprehensive pedagogical guidance in this training, professors specializing in nursing pedagogy have been specifically invited to provide expertise on educational methodology.
Current situation survey
Based on the Holton Learning Transfer System Inventory (refer to Fig. 1 ), the appropriate levels of Motivation to Improve Work Through Learning (MTIWL), learning environment, and transfer design are crucial in facilitating changes in individual performance, thereby influencing organizational outcomes [ 17 , 18 ]. Motivation to Improve Work Through Learning (MTIWL) is closely linked to expectation theory, fairness theory, and goal-setting theory, significantly impacting the positive transfer of training [ 19 ]. Learning environment encompasses environmental factors that either hinder or promote the application of learned knowledge in actual work settings [ 20 ]. Transfer design, as a pivotal component, includes training program design and organizational planning.
To conduct the survey, the research team retrieved 26 quality improvement reports from the nursing quality information management system, which were generated by nursing units in 2020. A checklist was formulated, and a retrospective evaluation was conducted across eight aspects, namely, team participation, topic selection feasibility, method accuracy, indicator scientificity, program implementation rate, effect maintenance, and promotion and application. Methods employed in the evaluation process included report analysis, on-site tracking, personnel interviews, and data review within the quality information management system [ 21 ]. From the perspective of motivation [ 22 ], learning environment [ 23 ], and transfer design, a total of 14 influencing factors were identified. These factors serve as a reference for designing the training plan and encompass the following aspects: lack of awareness regarding importance, low willingness to participate in training, unclear understanding of individual career development, absence of incentive mechanisms, absence of a scientific training organization model, lack of a training quality management model, inadequate literature retrieval skills and support, insufficient availability of practical training materials and resources, incomplete mastery of post-training methods, lack of cultural construction plans, suboptimal communication methods and venues, weak internal organizational atmosphere, inadequate leadership support, and absence of platforms and mechanisms for promoting and applying learned knowledge.
Learning Transfer System Inventory
Development of the training program using the 4W1H approach
Drawing upon Holton’s Learning Transfer System Inventory and the hospital training transfer model diagram, a comprehensive training outline was formulated for the training program [ 24 , 25 ]. The following components were considered:
(1) Training Participants (Who): The training is open for voluntary registration to individuals with an undergraduate degree or above, specifically targeting head nurses, responsible team leaders, and core members of the hospital-level nursing quality control team. Former members who have participated in quality improvement projects such as Plan-Do-Check-Act Circle (PDCA) or Quality control circle (QCC) are also eligible.
(2) Training Objectives (Why): At the individual level, the objectives include enhancing the understanding of quality management concepts, improving the cognitive level and application abilities of project improvement methods, and acquiring the necessary skills for nursing quality improvement project. At the team level, the aim is to enhance effective communication among team members and elevate the overall quality of communication. Moreover, the training seeks to facilitate collaborative efforts in improving the existing nursing quality management system and processes. At the operational level, participants are expected to gain the competence to design, implement, and manage nursing quality improvement project initiatives. Following the training, participants will lead and successfully complete a nursing quality improvement project, which will undergo a rigorous audit.
(3) Training Duration (When): The training program spans a duration of 11 months.
(4) Training Content (What): The program consists of 14 h of theoretical courses and 18 h of practical training sessions, as detailed in Table 1 .
(5) Quality Management Approach (How): To ensure quality throughout the training process, two team members are assigned to monitor the entire training journey. This encompasses evaluating whether quality awareness education, quality management knowledge, and professional skills training are adequately covered. Additionally, attention is given to participants’ learning motivation, the emphasis placed on active participation in training methods, support from hospital management and relevant departments, as well as participants’ satisfaction and assessment results. Please refer to Fig. 2 for a visual representation.
In-house training model from Holton Learning Transfer
Implementation of the nursing project training program using the action research method
The first cycle (april 2021).
In the initial cycle, a total of 22 nurses were included as training participants after a self-registration process and qualification review. The criteria used to select these participants, elaborated in Section Development of the training program using the 4W1H approach, ‘Development of the Training Program,’ were meticulously crafted to capture a broad spectrum of experience, expertise, and functional roles within our hospital’s nursing staff. The primary focus was to investigate their learning motivation. The cycle comprised the following key activities:
(1) Training Objectives: The focus was on understanding the learning motivation of the participating nurses.
(2) Theoretical Training Sessions: A total of 7 theoretical training sessions, spanning 14 class hours, were completed. The contents covered various aspects, including an overview of nursing quality improvement projects, methods for selecting project topics, common tools used in nursing quality improvement projects, effective leadership strategies to promote project practices, literature retrieval and evaluation methods, formulation and promotion of project plans, and writing project reports. Detailed course information, including the title, content, and class hours, is listed in Table 1 . At the end of each training session, a course satisfaction survey was conducted.
(3) Assessment and Reporting: Following the completion of the 7 training sessions, a theoretical assessment on quality management knowledge was conducted. Additionally, nurses were organized to present their plans for special projects to be carried out during the training. Several issues were identified during this cycle:
Incomplete Literature Review Skills: Compared to other quality control tools, nursing quality improvement project places more emphasis on the scientific construction of project plans. The theoretical evaluation and interviews with nurses highlighted the incomplete and challenging nature of their literature review skills.
Insufficient Leadership: Among the participants, 6 individuals were not head nurses, which resulted in a lack of adequate leadership for their respective projects.
Learning environment and Support: The learning environment, as well as the support from hospital management and relevant departments, needed to be strengthened.
Second cycle (may-october 2021)
In response to the issues identified during the first cycle, our approach in the second cycle was both corrective and adaptive, focusing on immediate issues while also setting the stage for addressing any emerging challenges. The team members actively implemented improvements during the second cycle. The key actions taken were as follows:
(1) Establishing an Enabling Organizational Environment: The quality management department took the lead, and multiple departments collaborated in conducting the “Hospital Safety and Quality Red May” activity. This initiative aimed to enhance the overall quality improvement atmosphere within the hospital. Themed articles were also shared through the hospital’s WeChat public account.
(2) Salon-style Training Format: The training sessions were conducted in the form of salons, held in a meeting room specifically prepared for this purpose. The room was arranged with a round table, warm yellow lighting, green plants, and a coffee bar, creating a conducive environment for free, democratic, and equal communication among the participants. The salon topics included revising project topic selection, conducting current situation investigations, facilitating communication and guidance for literature reviews, formulating improvement plans, implementing those plans, and writing project reports. After the projects were presented, quality management experts provided comments and analysis, promoting the transformation of training outcomes from mere memory and understanding to higher-level abilities such as application, analysis, and creativity.
(3) Continuous Support Services: Various support services were provided to ensure ongoing assistance. This included assigning nursing postgraduates to aid in literature retrieval and evaluation. Project team members also provided on-site guidance and support, actively engaging in the project improvement process to facilitate training transfer.
(4) Emphasis on Spiritual Encouragement: The Vice President of Nursing Department actively participated in the salons and provided feedback on each occasion. Moreover, the President of the hospital consistently commended the training efforts during the weekly hospital meetings.
Issues identified in this cycle
(1) Inconsistent Ability to Write Project Documents: The proficiency in writing project documents for project improvement varied among participants, and there was a lack of standardized evaluation criteria. This issue had the potential to impact the quality of project dissemination.
(2) Lack of Clarity Regarding the Platform and Mechanism for Training Result Transfer: The platform and mechanisms for transferring training results were not clearly defined, posing a challenge in effectively sharing and disseminating the outcomes of the training.
The third cycle (November 2021-march 2022)
During the third cycle, the following initiatives were undertaken.
(1) Utilizing the “Reporting Standards for Quality Improvement Research (SQUIRE)”, as issued by the US Health Care Promotion Research, to provide guidance for students in writing nursing project improvement reports.
(2) Organizing a hospital-level nursing quality improvement project report meeting to acknowledge and commend outstanding projects.
(3) Compiling the “Compilation of Nursing Quality Improvement Projects” for dissemination and exchange among nurses both within and outside the hospital.
(4) Addressing the issue of inadequate management of indicator monitoring data, a hospital-level quality index management platform was developed. The main evaluation data from the 22 projects were entered into this platform, allowing for continuous monitoring and timely intervention.
Effect evaluation
To assess the efficacy of the training, a diverse set of evaluation metrics, encompassing both outcome and process measures [ 26 ]. These measures can be structured around the four-level training evaluation framework proposed by Donald Kirkpatrick [ 27 ].
Process evaluation
Evaluation method.
To assess the commitment and support within the organization, the process evaluation involved recording the proportion of nurses’ classroom participation time and the presence of leaders during each training session. Additionally, a satisfaction survey was conducted after the training to assess various aspects such as venue layout, time arrangement, training methods, lecturer professionalism, content practicality, and interaction. On-site recycling statistics were also collected for project evaluation purposes.
Evaluation results the results of the process evaluation are as follows
Nurse training participation rate: 100%.
Training satisfaction rate (average): 97.75%.
Proportion of nurses’ participation time in theoretical training sessions (average): 36.88%.
Proportion of nurses’ participation time in salon training sessions (average): 74.23%.
Attendance rate of school-level leaders: 100%.
Results evaluation
Assessment of theoretical knowledge of quality management.
To evaluate the effectiveness in enhancing the trainees’ theoretical knowledge of quality management, the research team conducted assessments before the training, after the first round of implementation, and after the third round of implementation. Assessments to evaluate the effectiveness of the training program were conducted immediately following the first round of implementation, and after the third round of implementation. This dual-timing approach was designed to evaluate both the immediate impact of the training and its sustained effects over time, addressing potential influences of memory decay on the study results. The assessment consisted of a 60-minute examination with different question types, including 30 multiple-choice questions (2 points each), 2 short-answer questions (10 points each), and 1 comprehensive analysis question (20 points). The maximum score achievable was 100 points.
The assessment results are as follows:
Before training (average): 75.05 points.
After the first round of implementation (average): 82.18 points.
After the third round of implementation (average): 90.82 points.
Assessment of difficulty in quality management project implementation
To assess the difficulty of implementing quality management projects, the trainees completed the “Quality Management Project Implementation Difficulty Assessment Form” before and after the training. They self-evaluated 10 aspects using a 5-point scale, with 5 indicating the most difficult and 1 indicating no difficulty. The evaluation results before and after implementation are presented in Table 2 .
Statistically significant differences were found in the following items: literature review, current situation analysis, cause analysis, plan formulation, implementation plan, and report writing. This indicates that the training significantly enhanced the nurses’ confidence and ability to tackle practical challenges.
Evaluation of transfer effect
To assess how effectively the training translated into practical applications. The implementation of the 22 quality improvement projects was evaluated using the application hierarchy analysis table. The specific results are presented in Table 3 .
In addition, the “Nursing Project Guidance Manual” and “Compilation of Nursing Project Improvement Projects” were compiled and distributed to the hospital’s management staff, nurses, and four collaborating hospitals, receiving positive feedback. The lecture titled “Improving Nurses’ Project Improvement Ability Based on the Training Transfer Theory Model” shared experiences with colleagues both within and outside the province in national and provincial teaching sessions in 2022. Furthermore, four papers were published on the subject.
The effectiveness of the training program based on the Holton Learning transfer System Inventory
The level of refined management in hospitals is closely tied to the quality management awareness and skills of frontline medical staff. Quality management training plays a crucial role in improving patient safety management and fostering a culture of quality and safety. Continuous quality improvement is an integral part of nursing management, ensuring that patients receive high-quality and safe nursing care. Compared to the focus of existing literature on the individual performance improvements following nursing training programs [ 28 , 29 , 30 ], our study expands the evaluation framework to include organizational performance metrics. Our research underscores a significantly higher level of organizational engagement as evidenced by the 100% attendance rate of school-level leaders. The publication of four papers related to this study highlights not only individual performance achievements but also significantly broadens the hospital organization’s impact on quality management, leading to meaningful organizational outcomes.
Moreover, our initiative to incorporate indicators of quality projects into a hospital-level evaluation index system post-training signifies a pivotal move towards integrating quality improvement practices into the very fabric of organizational operations. In training programs, it is essential not only to achieve near-transfer, but also to ensure that nurses continuously apply the acquired management skills to their clinical work, thereby enhancing quality, developing their professional value, and improving organizational performance. The Holton learning Transfer System Inventory provides valuable guidance on how to implement training programs and evaluate their training effect.
This study adopts the training transfer model as a framework to explore the mechanisms of “how training works” rather than simply assessing “whether training works [ 31 ].” By examining factors such as Motivation to Improve Work Through Learning (MTIWL), learning environment, and transfer design, the current situation is analyzed, underlying reasons are identified, and relevant literature is reviewed to develop and implement training programs based on the results of a needs survey. While individual transfer motivation originates from within the individual, it is influenced by the transfer atmosphere and design. By revising the nurse promotion system and performance management system and aligning them with career development, nurses’ motivation to participate and engage in active learning has significantly increased [ 32 ]. At the learning environment level, enhancing the training effect involves improving factors such as stimulation and response that correspond to the actual work environment [ 33 ]. This project has garnered attention and support from hospital-level leaders, particularly the nursing dean who regularly visits the training site to provide guidance, which serves as invaluable recognition. Timely publicity and recognition of exemplary project improvement initiatives have also increased awareness and understanding of project knowledge among doctors and nurses, fostering a stronger quality improvement atmosphere within the team.
Transfer design, the most critical component for systematic learning and mastery of quality management tools, is achieved through theoretical lectures, salon exchanges, and project-based training. These approaches allow nurses to gain hands-on experience in project improvement under the guidance of instructors. Throughout the project, nurses connect project management knowledge and skills with practical application, enabling personal growth and organizational development through problem-solving in real work scenarios. Finally, a comprehensive evaluation of the training program was conducted, including assessments of theoretical knowledge, perception of management challenges, and project quality. The results showed high satisfaction among nurses, with a satisfaction rate of 97.75%. The proportion of nurses’ participation time in theoretical and practical training classes was 36.88% and 74.23%, respectively. The average score for theoretical knowledge of quality management increased from 75.05 to 90.82. There was also a significant improvement in the evaluation of the implementation difficulties of quality management projects. Moreover, 22 nurses successfully led the completion of one project improvement project, with six projects focusing on preventing the COVID-19 pandemic, demonstrating valuable crisis response practices.
Action research helps to ensure the quality of organizational management of training
Well-organized training is the basis for ensuring the scientific and standardized development of nursing project improvement activities. According to the survey results of the current situation, there is a lot of room for improvement in the training quality; since it is the first time to apply the Holton training transfer model to the improvement training process of nurses in the hospital, in order to allow the nurses to have sufficient time to implement and evaluate the improvement project, the total training time Set at 11 months, a strong methodology is required to ensure training management during this period. Action research is a research method that closely combines research with solving practical problems in work. It is a research method aimed at solving practical problems through self-reflective exploration in realistic situations, emphasizing the participation of researchers and researchees. Practice, find problems in practice, and adjust the plan in a timely manner. According to the implementation of the first round, it was found that nurses had insufficient literature review skills, insufficient leadership, and lack of support from hospital management and related departments [ 32 ]. In the second round, the training courses were carried out in the form of salons. The project team members went deep into the project to improve on-site guidance, arranged graduate students to assist in document retrieval and evaluation, and promoted the transfer of training; the “Hospital Safety and Quality Red May” activity was carried out, and the vice president of nursing Regularly participate in the salon and make comments. The problems exposed after this round of implementation are the low quality of the project improvement project document, and the unclear platform and mechanism for the transfer of training results. In the third round, the “Reporting Standards for Quality Improvement Research (SQUIRE)” was used to standardize the writing of the report [ 33 ], and the “Compilation of Nursing Project Improvement Projects” was completed, and the main evaluation data of 22 projects were entered into the hospital-level quality index management platform for continuous monitoring and intervention. As of May 2022, the effect maintenance data of each project has reached the target value. It can not only produce useful improvement projects, but also help to promote the dissemination and penetration of quality awareness.
Future research directions
Drawing on the Holton training evaluation model, this study implemented nurse quality improvement project training using action research methodology, resulting in a successful exploration practice, and achieving positive transfer effects. To further advance this research area, the following future research directions are recommended:
Summarize the experiences gained from this action research training and continue to refine and enhance the training program. Through ongoing practice, reflection, and refinement in subsequent training sessions, long-term observation of the transfer effects can be conducted to establish an effective experiential model that can serve as a reference for future initiatives.
Develop a multidimensional evaluation index system for assessing transfer effects. A comprehensive framework that captures various dimensions of transfer, such as knowledge application, skill utilization, and behavior change, should be established. This will enable a more holistic and accurate assessment of the training program’s impact on the participants and the organization.
Conduct qualitative research to explore the training experiences of nurses. By gathering in-depth insights through interviews or focus group discussions, a deeper understanding of the nurses’ perceptions, challenges, and facilitators of training transfer can be obtained. This qualitative exploration will provide valuable information to further refine and tailor the training program to meet the specific needs and preferences of the nurses.
Investigate the personality characteristics of nurses who actively engage in training transfer and consider developing them as internal trainers. By identifying and cultivating nurses with a proactive attitude and a strong inclination towards knowledge application and skill development, the organization can enhance employee participation and initiative. These internal trainers can play a crucial role in motivating their colleagues and driving the transfer of training outcomes into daily practice.
By pursuing these future research directions, the field of healthcare and nursing care can continue to advance in optimizing training programs, enhancing transfer effects, and ultimately improving the quality of care and patient outcomes.
Limitations
The research was conducted with a cohort of 22 nurses and a 10-member research team from Grade 3, Class A hospitals in China Southeast. This specific composition and the relatively small sample size may affect the generalizability of our findings. The experiences and outcomes observed in this study might not fully encapsulate the diverse challenges and environments encountered by nursing professionals in varying healthcare settings. The significant improvements noted in the capabilities of the participating nursing staff underscore the potential impact of the training program. However, the study’s focus on a specific demographic—nurses from high-grade hospitals in a developed urban center—may limit the external validity of the findings.
Conclusions
This study affirms the efficacy of the Holton Learning Transfer System Inventory-based training program, coupled with action research, in significantly advancing nursing quality management practices. The strategic incorporation of motivation to improve work through learning, an enriched learning environment, and thoughtful transfer design significantly boosted the nurses’ engagement, knowledge acquisition, and practical application of quality management tools in their clinical work.
It highlights the importance of continuous learning, organizational support, and methodological flexibility in achieving sustainable improvements in healthcare quality and safety. Future endeavors should aim to expand the scope of this training model to diverse nursing contexts and evaluate its long-term impact on organizational performance and patient care outcomes.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to hospital policy but are available from the corresponding author on reasonable request.
National Health and Family Planning Commission. Medical Quality Management Measures. Published 2016. Accessed September 19, 2018. http://www.nhc.gov.cn/fzs/s3576/201610/ae125f28eef24ca7aac57c8ec530c6d2.shtml .
National Health Commission of the People’s Republic of China. Notice of the National Health Commission on Printing and Distributing the Standards for the Review of Third-level Hospitals (2020 Edition) [EB/OL]. (2021-10-21) [2022-07-17].
Grossu-Leibovica D, Kalkis H. Total quality management tools and techniques for improving service quality and client satisfaction in the healthcare environment: a qualitative systematic review. Manage Sci Lett. 2023;13(2):118–23. https://doi.org/10.1051/shsconf/202213102009 .
Article Google Scholar
Shade L, Reeves K, Rees J, Hendrickson L, Halladay J, Dolor RJ, Bray P, Tapp H. Research nurses as practice facilitators to disseminate an asthma shared decision making intervention. BMC Nurs. 2020;19:40. https://doi.org/10.1186/s12912-020-00414-0 .
Ali J, Jusoh A, Abbas A, Nor K. Global trends of Service Quality in Healthcare: a bibliometric analysis of Scopus Database. J Contemp Issues Bus Government. 2021;27:2917.
Google Scholar
Ahmed FA, Choudhary RA, Khan H, Ayub F, Hassan SSU, Munir T, Asif F, Ajani K, Jaffer M, Tharani Z, Aboumatar HJ, Haider A, Latif A. Incorporating Patient Safety and Quality Course into the nursing curriculum: an Assessment of Student gains. J Patient Saf. 2023;19(6):408–14. https://doi.org/10.1097/PTS.0000000000001146 .
Holton EF. Holton’s evaluation model: new evidence and construct elaborations. Adv Developing Hum Resour. 2005;7(1):37–54. https://doi.org/10.1177/1523422304272080 .
Rigot SK, DiGiovine KM, Boninger ML, Hibbs R, Smith I, Worobey LA, Connerton C, Mason J, Bonhotal S. (2023). Peer-Led Functional Mobility and Transfer Training. Nurse Educator, 48(5), 286.
Wang C, Liu A, Xu J, et al. Work Method and Effect of Hospital Quality Management Circle Stage Management model [J]. Chin Hosp. 2016;20(12):23–5.
Elshama S. Quality Management in Medical Education between Theory and Application: paradigm shift or falsification of. J Clin Case Rep Stud. 2022;3:1–5. https://doi.org/10.31579/2690-8808/109 .
Chang S-J, Huang HH-C, Li-Hua, Chang H. Taiwan quality indicator project and hospital productivity growth. Omega. 2011;39(1):14–22.
Arnold AP, Laurene Finley [email protected], Roberta G, Sands, Joretha Bourjolly & Victoria Stanhope. (2012) Training Mental Health Providers in Cultural Competence: A Transformative Learning Process, American Journal of Psychiatric Rehabilitation, 15:4, 334–356, https://doi.org/10.1080/15487768.2012.733287 .
Chang S-J, Hsiao H-C, Huang L-H, Chang H. 2011. Taiwan quality indicator project and hospital productivity growth, Omega, Elsevier, vol. 39(1), pages 14–22, January. 2010.01.006.
Finn F, Chesser-Smyth P. Promoting learning transfer in Preceptor Preparation. J Nurses Prof Dev. 2013;29:309–15. https://doi.org/10.1097/NND.0000000000000014 .
Guzman G, Fitzgerald J, Fulop L, Hayes K, Poropat A, Avery M, Campbell S, Fisher R, Gapp R, Herington C, McPhail R, Vecchio N. How best practices are copied, transferred, or translated between health care facilities: a conceptual framework. Health Care Manage Rev. 2015;40:193–202. https://doi.org/10.1097/HMR.0000000000000023 .
Billings D, Connors H, Skiba D. Benchmarking Best practices in web-based nursing courses. Adv Nurs Sci. 2001;23:41–52. https://doi.org/10.1097/00012272-200103000-00005 .
Devos C, Dumay X, Bonami M, Bates R, Holton E. The learning transfer system inventory (LTSI) translated into French: Internal structure and predictive validity. Eur Economics: Labor Social Conditions eJournal. 2007. https://doi.org/10.1111/j.1468-2419.2007.00280.x .
Mongkolsirikiet K, Akaraborworn C, Research Model. (2019). A Revisit of Holton’s HRD Evaluation and (2005) for Learning Transfer., 12, 15–34. https://doi.org/10.14456/JCDR-HS.2019.12 .
Yaqub Y, Singh A. Impact of training design on trainees’ motivation: an empirical study. Industrial Commercial Train. 2021. https://doi.org/10.1108/ict-05-2021-0038 .
Dewettinck K, Dijk H. Linking Belgian employee performance management system characteristics with performance management system effectiveness: exploring the mediating role of fairness. Int J Hum Resource Manage. 2013;24:806–25. https://doi.org/10.1080/09585192.2012.700169 .
Duprez V, Vandecasteele T, Verhaeghe S, Beeckman D, Hecke A. The effectiveness of interventions to enhance self-management support competencies in the nursing profession: a systematic review. J Adv Nurs. 2017;73:1807–24. https://doi.org/10.1111/jan.13249 .
Norouzi S, Mogadam F. Experiences of nursing Student\‘s clinical evaluation: a qualitative content analysis. J Med Educ Dev. 2016;11:134–45.
Niskala J, Kanste O, Tomietto M, Miettunen J, Tuomikoski A, Kyngäs H, Mikkonen K. Interventions to improve nurses’ job satisfaction: a systematic review and meta-analysis. J Adv Nurs. 2020. https://doi.org/10.1111/jan.14342 .
Gkioka M, Schneider J, Kruse A, Tsolaki M, Moraitou D, Teichmann B. Evaluation and Effectiveness of Dementia Staff Training Programs in General Hospital settings: a narrative synthesis with Holton’s three-level Model Applied. J Alzheimers Dis. 2020;78:1089–108. https://doi.org/10.3233/JAD-200741 .
Ghazvini A, Shukur Z. Awareness training transfer and Information Security Content Development for Healthcare Industry. Int J Adv Comput Sci Appl. 2016. https://doi.org/10.14569/IJACSA.2016.070549 . 7.
Ragsdale J, Berry A, Gibson J, Herber-Valdez C, Germain L, Engle D. Evaluating the effectiveness of undergraduate clinical education programs. Med Educ Online. 2020;25. https://doi.org/10.1080/10872981.2020.1757883 .
Kirkpatrick DL. Techniques for evaluation training programs. J Am Soc Train Dir. 1959;13:21–6.
Kirkman TR. High Fidelity Simulation Effectiveness in Nursing Students’ Transfer of Learning International Journal of Nursing Education Scholarship, vol. 10, no. 1, 2013, pp. 171–176. https://doi.org/10.1515/ijnes-2012-0009 .
Chen SL, Huang TW, Liao IC, Liu C. Development and validation of the simulation learning effectiveness inventory. J Adv Nurs. 2015;71(10):2444–53. https://doi.org/10.1111/jan.12707 .
Ayed A, Khalaf I, Fashafsheh I, Saleh A, Bawadi H, Abuidhail J, Thultheen I, Joudallah H. Effect of High-Fidelity Simulation on Clinical Judgment among nursing students. Inquiry: J Med Care Organ Provis Financing. 2022;59. https://doi.org/10.1177/00469580221081997 .
Yun J, Kim D, Park Y. The influence of informal learning and learning transfer on nurses’ clinical performance: a descriptive cross-sectional study. Nurse Educ Today. 2019. https://doi.org/10.1016/J.NEDT.2019.05.027 .
Bhatti M, Ali S, Isa M, Battour M. Training transfer and transfer motivation: the influence of individual, environmental, situational, Training Design, and affective reaction factors. Perform Improv Q. 2014;27:51–82. https://doi.org/10.1002/PIQ.21165 .
Kontoghiorghes C. Factors affecting training effectiveness in the context of the introduction of a New Technology–A U.S. Case Study. Int J Train Dev. 2001;5:248–60. https://doi.org/10.1111/1468-2419.00137 .
Download references
This study was funded by Department of Education of Zhejiang Province, Grant Number jg20220475.
Author information
Authors and affiliations.
School of Nursing, Zhejiang Shuren University, 8 Shuren Road, 310015, Hangzhou, ZheJiang, China
MingYan Shen & ZhiXian Feng
Department of Nursing, Shulan (Hangzhou) Hospital, Shulan International Medical College, Zhejiang Shuren University, 310022, Hangzhou, China
You can also search for this author in PubMed Google Scholar
Contributions
The following statements specify the individual contributions of each author to the manuscript titled “Continue Nursing Education: An Action Research Study on the Implementation of a Nursing Training Program Using the Holton Learning Transfer System Inventory”:ZhiXian Feng conceived and designed the analysis; led the research team and coordinated the project; critically reviewed and revised the manuscript for important intellectual content; oversaw the implementation of the training program; MingYan Shen conducted the research; collected and organized the data; analyzed and interpreted the data; contributed to the statistical analysis; wrote the initial draft of the manuscript; managed logistics and operational aspects of the study.
Corresponding author
Correspondence to ZhiXian Feng .
Ethics declarations
Ethics approval and consent to participate.
Approval of this study was granted by the Research Ethics Committee of Shulan Hospital (Approval no. KY2021042).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Prior to participation, all subjects (or their legal guardians) were informed about the nature, objectives, potential benefits, and risks of the study. Written informed consent was obtained from all subjects and/or their legal guardians. All data were collected and processed maintaining strict confidentiality and anonymity, safeguarding the privacy and rights of all participants.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shen, M., Feng, Z. Continue nursing education: an action research study on the implementation of a nursing training program using the Holton Learning Transfer System Inventory. BMC Med Educ 24 , 610 (2024). https://doi.org/10.1186/s12909-024-05552-6
Download citation
Received : 16 December 2023
Accepted : 13 May 2024
Published : 03 June 2024
DOI : https://doi.org/10.1186/s12909-024-05552-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Nursing education
- Quality improvement
- Action research
- Holton Learning Transfer System Inventory
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.15(8); 2023 Aug
- PMC10492634

ChatGPT and Artificial Intelligence in Medical Writing: Concerns and Ethical Considerations
Alexander s doyal.
1 Anesthesiology, University of North Carolina at Chapel Hill School of Medicine, Chapel Hill, USA
David Sender
Monika nanda, ricardo a serrano.
Artificial intelligence (AI) language generation models, such as ChatGPT, have the potential to revolutionize the field of medical writing and other natural language processing (NLP) tasks. It is crucial to consider the ethical concerns that come with their use. These include bias, misinformation, privacy, lack of transparency, job displacement, stifling creativity, plagiarism, authorship, and dependence. Therefore, it is essential to develop strategies to understand and address these concerns. Important techniques include common bias and misinformation detection, ensuring privacy, providing transparency, and being mindful of the impact on employment. The AI-generated text must be critically reviewed by medical experts to validate the output generated by these models before being used in any clinical or medical context. By considering these ethical concerns and taking appropriate measures, we can ensure that the benefits of these powerful tools are maximized while minimizing any potential harm. This article focuses on the implications of AI assistants in medical writing and hopes to provide insight into the perceived rapid rate of technological progression from a historical and ethical perspective.
Introduction
The use of technological advances goes through cycles of both disruptive change and gradual transitions. Communication in particular has seen many iterations of technology, from the printing press to more recent changes such as spell check and algorithms that predict what you might like to say in an email or text. Historically, slow and gradual technological changes have been more easily accepted and well utilized. In contrast, rapid changes in new technologies are often met with resistance. Time, experience, and thoughtful policies are needed in order for society to accept and safely utilize advances in technology. Unfortunately, none of these key elements necessary to utilize new technology are present in our current artificial intelligence (AI) environment.
Recently, AI reached a critical stage of development where a non-expert could utilize the technology with little or no computer coding background or specialized medical knowledge. Given how rapidly the field of adaptive AI is evolving, this raises serious concerns. We will discuss the role of generative models in medical writing, with a focus on the historical roots of AI and the ethical implications of its current trajectory.
The evolution of word processing AI, also known as AI-assisted writing, has been driven by advances in natural language processing (NLP) and machine learning (ML) technologies. NLP refers to the branch of computer science that involves understanding written text by combining computational linguistics (rule-based language modeling, e.g., grammar) with statistical/machine-learning/deep learning models. Ideally, NLP allows computers to ‘understand’ human written or spoken language. The history of word processing AI can be broken down into several key phases [ 1 - 3 ].
In the history of word processing programs and AI integration, several distinct phases have shaped the evolution of these technologies. During the early phase of the 1980s-1990s, programs like Microsoft Word and WordPerfect primarily focused on basic editing and formatting functionalities.
In the 1990s-2000s, the introduction of grammar checkers marked a significant step forward. Word processing programs started incorporating rule-based algorithms to identify and correct grammar and spelling errors. This reduced the effort needed for basic editing, thereby freeing up the writer to focus on his/her ideas.
As technology progressed further, the 2000s-2010s witnessed the integration of predictive text capabilities into word processing programs. By utilizing statistical models like n-gram models, these systems suggested words and phrases to users as they typed, improving writing efficiency. This marked a departure from grammar and spelling basics. Now, communication technology has begun to anticipate and predict basic phraseology, leaving the overall structure and development of the topic to the writer.
The 2010s-2020s brought about groundbreaking advancements in deep learning, a subset of machine learning. Word processing AI benefited significantly from this technology, employing neural networks with multiple layers, mimicking the human brain's learning mechanism. Large datasets trained language models like GPT-3 and GPT-4 to generate highly coherent and natural-sounding text. These models found use in various tasks, including grammar checking, text summarization, and question answering.
As AI technology continued to advance, the 2020s to the present day saw the rise of AI-assisted writing tools. Based on deep learning models, these tools provide valuable suggestions on grammar and writing style and even assist with plot and character development for creative writing. Many popular writing software programs, such as Grammarly, Hemingway, and ProWritingAid, have integrated these AI-assisted features, making them widely accessible to users seeking enhanced writing support.
Overall, the evolution of word processing AI has been marked by a gradual increase in the sophistication and intelligence of the underlying technology, leading to more powerful and versatile tools for writers and other users [ 4 - 6 ]. The three major movements towards our current moment with AI can be described as word-based editing, sentence- or phrase-based editing, and idea synthesis. This last leap forward represents a qualitative difference in the kind of work we are asking of AI and ourselves. At this juncture, the author is only responsible for the initial idea. This reality distills the power of the old tech adage, "garbage in, garbage out."
Several forms of AI have been used for writing, each with its own strengths and weaknesses. One form is Natural Language Generation (NLG) systems, which use AI to generate written text in a human-like style automatically. These systems are beneficial for tasks such as generating reports and summaries. Another form is ML-based text generation models that use statistical techniques to generate text based on patterns in a training dataset. These models are commonly used to create news articles, product descriptions, and other types of written content [ 3 ].
Neural network-based models, such as GPT, BERT, and others, are also popular for text generation. These models use deep learning to generate text that is often indistinguishable from human-written text. They have been used in many applications, such as chatbots, language translation, and more. Rule-based systems, on the other hand, use a set of predefined rules to generate text based on a specific set of inputs. These systems are typically used in applications with highly structured output, such as generating code or legal documents [ 7 ].
Hybrid models, which combine the above methods to generate text with a high degree of accuracy and naturalness, are also being increasingly used. These models combine the ability of rule-based systems to generate structured text with the power of neural network-based models to generate natural text. Finally, AI-assisted writing tools are software that helps writers write better by providing suggestions, grammar checking, and more. These tools are particularly useful for writers who want to improve their writing skills or for people who are not native speakers of the language they are writing in [ 8 ].
ChatGPT is the newest generation of artificial intelligence assisting the writing process. ChatGPT is a language generation model developed by OpenAI. It is based on the GPT (generative pre-training transformer) architecture, which uses deep learning to generate human-like text. ChatGPT is trained on a large dataset of conversational text and can create responses to user input in a conversational context. It can be used for various natural language processing tasks such as language translation, text summarization, and question answering [ 9 ].
Citation issues
It is clear that artificial intelligence writing systems such as ChatGPT are here to stay, and different platforms are likely to be developed in the future. One must ask, should these artificial intelligence writing systems be used for medical writing? [ 5 , 6 ]. And if so, how should they be cited in the medical literature [ 10 , 11 ]?
Regarding authorship, it may be appropriate to credit ChatGPT as a tool or resource used in the research process rather than listing it as an author. Authorship is generally reserved for individuals who have made a significant intellectual contribution to the work, such as designing and conducting the study, analyzing the data, and writing the manuscript. However, it is always good practice to acknowledge the tools and resources that were used in the research and writing process, such as the use of AI and machine learning tools [ 11 , 12 ].
It is essential to be transparent about the use of language generation models in research, as this allows others to understand the potential limitations and biases of the generated text and to replicate the research if needed. Additionally, proper citation of the model also gives credit to the model's creators and the training data's contributors [ 10 ].
Medical writing
ChatGPT and other language generation models based on deep learning techniques, such as GPT-3, can be used for various natural language processing tasks, including medical writing. However, it is essential to note that using AI-generated text in the medical field requires careful consideration and review by medical experts to ensure the accuracy and reliability of the generated text [ 13 ].
Some suggested uses of ChatGPT and other language generation models in medical writing include generating reports and summaries of medical research papers and clinical trials, creating patient-specific medical information like discharge summaries and patient education materials, assisting in the writing of medical textbooks and guidelines, generating product labels and package inserts for medical devices and drugs, creating a chatbot or virtual assistant capable of answering medical-related questions, and assisting in highly protocolized letter writing, such as preauthorization letters to insurance companies, work excuses, or letters of recommendation.
Although useful in these contexts, it is necessary for clinicians to critically review and validate any computer-generated text before it is used in any clinical setting or research. AI-generated text has the potential to perpetuate bias, misinformation, and plagiarism. Additionally, as the field of medicine is constantly evolving, computer models should be retrained regularly to ensure they stay up-to-date with the latest knowledge.
Ethical and other considerations
ChatGPT, a language generation model developed by OpenAI, is a powerful tool that can be used for various natural language processing tasks, including medical writing. However, its use also raises significant ethical concerns that must be carefully considered, including bias, misinformation, privacy, a lack of transparency, and plagiarism [ 13 - 15 ].
One primary point of interest is bias. Language generation models are trained on large datasets of text, and any biases present in the training data may be reflected in the generated text. This can lead to discriminatory or offensive language, perpetuating harmful stereotypes. For example, if a model is trained on a dataset that contains a disproportionate amount of text written by men, it may generate text that reflects a male-centric perspective. If a model is trained on a dataset containing "fake news," it will produce consistently inaccurate text [ 7 ].
Therefore, measures to prevent bias in generative AI models should be put in place in a prospective manner instead of a retrospective manner. ChatGPT's initial development stage consisted of scraping hundreds of billions of words from the internet with insufficient attention to filtering out toxic themes and bias. It is very difficult for a deployed model to correct biased outputs once it has been trained. Paradoxically, any attempts to improve data by limiting sources that the AI is incorporating will, in fact, produce their own set of biases [ 16 ].
Another problem is misinformation. Language generation models can generate text that is not factually accurate, which can be a concern when the generated text is used in sensitive domains such as medicine or finance. For example, if a model generates text that provides incorrect medical information, it could potentially harm patients [ 15 ]. Further, these models often present information in an authoritative tone of voice without having actual expertise. Although efficient in producing vague general knowledge, it is insufficient when generating information at the subspecialist level.
Privacy is also a significant concern. Language generation models can be used to generate highly personalized text, such as patient-specific medical information. This patient-specific information requires the AI to have access to a patient’s protected medical record or medical data. There exists a high potential to harm patient privacy rights and erode the faith that patients and clinicians may have entrusted in AI language generation models. Mistrust in these systems may hinder their successful integration into clinical practice.
Lack of transparency is also problematic. Language generation models can be challenging to understand, and it can be hard to know how a specific output is generated. This can make it difficult to determine the quality of the generated text or to identify and correct any errors. Additionally, the sources used by the AI writers are not readily apparent, and it is possible that non-peer-reviewed medical literature is being used to create content [ 5 ].
Even more malicious is the use of made-up scientific references containing misinformation, which could contaminate our existing biomedical knowledge databases at scale. Open AI is attempting to implement a watermark feature that labels content created by ChatGPT [ 17 ]. Other detection tools, such as DetectGPT, are in development. DetectGPT has been reported to correctly determine authorship in 95% of test cases [ 18 ].
Besides ethical considerations, another concern when using ChatGPT and other language generation models for creative writing is the potential to stifle creativity and originality. Similarly, spell checkers have trivialized our educational efforts to learn proper spelling. The generated text may be highly polished and grammatically correct, but it may lack the individuality and creativity that are often associated with human-written text. This could lead to a homogenization of written content, where all text starts to sound the same [ 19 ].
Yet another matter is the potential for the model to generate text that plagiarizes existing work. Language generation models are trained on a vast amount of text, and the model may unintentionally generate text similar to or identical to existing work. This could lead to legal issues and ethical concerns [ 20 , 21 ].
Moreover, the creative process is about the final product and the journey of creating something new, utilizing the writer's personal experience and perspective. Using AI-generated text may take away from the personal and emotional investment of the writer in the writing process, which can be an important aspect of the creative process [ 22 ].
Job displacement is another worry. Automated text generation can be used to automate tasks that were previously done by human medical writers, editors, and others. This could lead to job displacement and economic disruption. Furthermore, using AI-generated text can also lead to a dependence on the technology, which can be harmful if the models are unavailable or fail [ 23 ].
In conclusion, while ChatGPT and other language generation models have the potential to revolutionize the field of medical writing and other natural language processing tasks, it is crucial to consider the ethical concerns that come with their use. These include bias, misinformation, privacy, lack of transparency, job displacement, stifling creativity, plagiarism, authorship, and dependence. Therefore, it is essential to develop strategies to address these concerns, such as common bias and misinformation detection, ensuring privacy, providing transparency, and being mindful of the impact on employment. Experts should review and validate the output generated by these models before they are used in any clinical or medical context. By considering these ethical concerns and taking appropriate measures, we can ensure that the benefits of these powerful tools are maximized while minimizing any potential harm [ 1 , 14 ].
In conclusion, while AI-generated text can offer numerous benefits and enhance various aspects of medical writing, we must approach its use with great caution and mindfulness. The advantages of efficiency, productivity, and support in generating content must be weighed against potential downsides like bias, misinformation, plagiarism, and privacy concerns. As AI technologies continue to advance rapidly, it is essential for the medical community, policymakers, and society as a whole to continually grapple with the ethical implications and challenges posed by AI-generated text.
The responsible use of AI in medical writing necessitates clear guidelines, robust validation processes, and close collaboration between AI systems and human expertise. Transparency and acknowledgment of the role of AI in generating text are vital to ensuring that human authors remain accountable for the final output. Additionally, ongoing research and development are required to address bias detection, misinformation prevention, and privacy protection in AI-generated text.
Ultimately, the question of whether we should use AI-generated text in medical writing will persist. The answer lies in our ability to strike a delicate balance between leveraging AI's potential while respecting the importance of human creativity, critical thinking, and ethical considerations. As we navigate this evolving landscape, it is crucial to maintain a thoughtful approach and prioritize the well-being of patients, the integrity of medical knowledge, and the overall advancement of healthcare practices. By doing so, we can harness the power of AI while upholding the highest standards of medical writing and patient care.
Acknowledgments
This article was edited using artificial intelligence software. This includes Grammarly (editing and plagiarism checker), Microsoft Word (spellchecking and grammar editing), ChatGPT (editing), Google (spellchecking grammar editing).
The authors have declared that no competing interests exist.

IMAGES
VIDEO
COMMENTS
A typical medical abstract consists of four main sections: introduction, methods, results, and conclusion. The introduction should provide context for your research, explaining the problem or gap in knowledge that your study aims to address. The methods section outlines the approach and techniques used in your research.
OBJECTIVE: State your precise research purpose or question (1-2 sentences) Begin with "To": "We aimed to…" or "The objective of this study was to…" using a verb that accurately captures the action of your study. Connect the verb to an object phrase to capture the central elements and purpose of the study, hypothesis, or research ...
Step 2: Methods. Next, indicate the research methods that you used to answer your question. This part should be a straightforward description of what you did in one or two sentences. It is usually written in the past simple tense, as it refers to completed actions.
An abstract is a self-contained, short, powerful statement that describes a larger body of work. It may be incorporated as part of a published paper, book, grant proposal, thesis, research report, or a conference paper. An abstract of a scientific paper will be published online independently, so it should make sense when it is read alone.
Definition and Purpose of Abstracts An abstract is a short summary of your (published or unpublished) research paper, usually about a paragraph (c. 6-7 sentences, 150-250 words) long. A well-written abstract serves multiple purposes: an abstract lets readers get the gist or essence of your paper or article quickly, in order to decide whether to….
17. Content of an abstract: Orienting information: Defined as "bits of information, explanation, summary that orient the reader". Motive: the "intellectual context" that's established at the beginning of a paper to suggest why the thesis is original or worthwhile. Thesis: A paper's central claim or promise.
Focus on key results, conclusions and take home messages. Write your paper first, then create the abstract as a summary. Check the journal requirements before you write your abstract, eg. required subheadings. Include keywords or phrases to help readers search for your work in indexing databases like PubMed or Google Scholar.
Report results fully & honestly, as pre-specified. Text (story), Tables (evidence), Figures (highlights) Report primary outcomes first. Give confidence intervals for main results. Report essential summary statistics. Leave out non-essential tables and figures; these can be included as supplementary files. Don't start discussion here.
abstract is the ability to develop a concise, formal academic writing st yle, which takes practice. Sp ecific journals and. conferences may have their own rules on the st yle of abstract needed ...
language of the abstract is designed to be read by the general medical reader, the content must reflect what is most important for patients. Cochrane Abstracts should use the type and level of language that a general medical reader (physician, allied health professional, clinical researcher) is comfortable with. 2.
An abstract comprises five parts of equal importance: the title, introduction and aims, methods, results, and conclusion. Allow enough time to write each part well. The title should go straight to the point of the study. Make the study sound interesting so that it catches people's attention. The introduction should include a brief background ...
A good abstract informs readers briefly of the research and ideas that are presented in the full article. Before writing the abstract, be sure you understand the research you're summarizing. Describe the background to your research, your expectations or hypotheses, the methods you used, and the outcomes of your medical investigation. Method 1.
Most abstracts have a word limit of around 250 to 300 words. Omit needless words, redundant modifiers, over-the-top diction, and excessive detail. An abstract should have the same structure a research article: Introduction, Methods, Results, and Conclusions. Depending on the required format you may be required to use these or similar headings ...
Top Tips from Radiology for Writing a Perfect Abstract. 1. Be concise and specific. The word limit for abstracts in Radiology is 300 words. This includes five sections: Background, Purpose, Materials and Methods, Results, and Conclusion. To meet this word count, be concise but use specific, clear writing.
How to Write a Scientific Abstract for Your Research Article. Wiley Network. Purdue Owl. Writing Scientific Abstracts. For more information about Abstract Writing, head over to SPH's Public Health Writing blog, where you will find countless wonderful articles written by SPH Professor and Director of the Public Health Writing Program, Jen Beard.
Nature Abstract https://www.genomemed.org/ecrresources/2018/9/25/how-to-write-a-great-abstractsummaryMy Abstract https://pubmed.ncbi.nlm.nih.gov/25017211/?fr...
1. Be concise and specific. The word limit for abstracts in Radiology is 300 words. This includes five sections: Background, Purpose, Materials and Methods, Results, and Conclusion. To meet this word count, be concise but use specific, clear writing. Avoid vague wording.
Abstract. An abstract is a crisp, short, powerful, and self-contained summary of a research manuscript used to help the reader swiftly determine the paper's purpose. Although the abstract is the first paragraph of the manuscript it should be written last when all the other sections have been addressed. Research is formalized curiosity.
Structured abstracts have several advantages for authors and readers. These formats were developed in the late 1980s and early 1990s to assist health professionals in selecting clinically relevant and methodologically valid journal articles. They also guide authors in summarizing the content of their manuscripts precisely, facilitate the peer ...
Writing a Research Abstract. The written abstract is used in making selections for presentations at scientific meetings. Writing a good abstract is a formidable undertaking and many novice researchers wonder how it is possible to condense months of work into 300 to 400 words. Nevertheless, creating a well-written abstract is a skill that can be ...
When you search PubMed (or most databases), did you know you're only searching the titles, abstracts, and keywords/subject headings? That's why it's so important that you write an effective, concise, and clear abstract! In this session, learn how to refine your abstract writing skills to help users find your paper. Tue.
A few practical steps in preparing to write the abstract can facilitate the process. This article discusses those steps and offers suggestions for writing each of an abstract's components (title, author list, introduction, methods, results, and conclusions); considers the advantages and disadvantages of incorporating a table or figure into the ...
The deadline for abstract submissions for the AMA Research Challenge—the largest national, multi-specialty research event for medical students, residents and fellows, and international medical graduates—is July 21. David M. Harris, MD, a member of the AMA Research Challenge Advisory Committee offers insight on the key steps to preparing and ...
2 Steps to Writing the Dissertation Abstract. 2.1 #1 Describe the purpose of your research and its value for the community. 2.2 #2 Briefly outline a methodology for your research. 2.3 #3 Present the key findings of your academic research. 2.4 #4 Finalize with discussion to describe the implications. 3 More Tips for Writing an Abstract.
An abstract is a short, concise summary of a research paper that provides a clear understanding of the paper. It is placed at the beginning of the paper and ranges from 150 to 300 words. It gives a synopsis of the major findings of your study, comprising the research aim, methods used, the most important findings, and the main conclusions.
Key Points. Question Do women with premenstrual disorders (PMDs) have higher risks of all-cause and cause-specific mortality compared with women without PMDs?. Findings This nationwide matched cohort study of 406 488 women in Sweden during a mean (SD) follow-up of 6.2 (4.6) years (range, 1-18 years) revealed that overall, women with diagnosed PMDs did not have an increased risk of all-cause ...
A critical challenge in excess mortality research is choosing an appropriate statistical method for calculating the projected baseline of expected deaths to which the observed deaths are compared.96 Although the analyses and estimates in general are similar, the method can vary, for instance, per length of the investigated period, nature of ...
To address the gap in effective nursing training for quality management, this study aims to implement and assess a nursing training program based on the Holton Learning Transfer System Inventory, utilizing action research to enhance the practicality and effectiveness of training outcomes. The study involved the formation of a dedicated training team, with program development informed by an ...
Neural network-based models, such as GPT, BERT, and others, are also popular for text generation. These models use deep learning to generate text that is often indistinguishable from human-written text. They have been used in many applications, such as chatbots, language translation, and more.