Advertisement
- Publications
This site uses cookies to enhance your user experience. By continuing to use this site you are agreeing to our COOKIE POLICY .

Grab your lab coat. Let's get started
Create an account below to get 6 c&en articles per month, receive newsletters and more - all free., it seems this is your first time logging in online. please enter the following information to continue., as an acs member you automatically get access to this site. all we need is few more details to create your reading experience., not you sign in with a different account..
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Already have an ACS ID? Log in here
The key to knowledge is in your (nitrile-gloved) hands
Access more articles now. choose the acs option that’s right for you..
Already an ACS Member? Log in here
$0 Community Associate
ACS’s Basic Package keeps you connected with C&EN and ACS.
- Access to 6 digital C&EN articles per month on cen.acs.org
- Weekly delivery of the C&EN Essential newsletter
$80 Regular Members & Society Affiliates
ACS’s Standard Package lets you stay up to date with C&EN, stay active in ACS, and save.
- Access to 10 digital C&EN articles per month on cen.acs.org
- Weekly delivery of the digital C&EN Magazine
- Access to our Chemistry News by C&EN mobile app
$160 Regular Members & Society Affiliates $55 Graduate Students $25 Undergraduate Students
ACS’s Premium Package gives you full access to C&EN and everything the ACS Community has to offer.
- Unlimited access to C&EN’s daily news coverage on cen.acs.org
- Weekly delivery of the C&EN Magazine in print or digital format
- Significant discounts on registration for most ACS-sponsored meetings

Your account has been created successfully, and a confirmation email is on the way.
Your username is now your ACS ID.
Environment
Five green chemistry success stories, the 2017 green chemistry challenge awards recognize chemical innovations that prevent pollution and promote sustainability, by stephen k. ritter, june 26, 2017 | a version of this story appeared in volume 95, issue 26.
- How can companies recycle wind turbine blades?
- Can Europe’s chemical industry survive net zero?
- Are fluorinated drugs PFAS?
- Periodic Graphics: Summer hair color changes
- What is ball lightning, a reality or myth?
The 12 Principles of Green Chemistry are a how-to guide written 20 years ago for chemists and chemical engineers. They provide insight on developing new chemicals and chemical processes and revitalizing existing ones so that they achieve their desired function while being environmentally and economically friendly. It’s a creative challenge to put the 12 principles into action.
Five technologies that have succeeded in meeting that creative challenge have received 2017 green chemistry challenge awards . merck, dow chemical , koehler , amgen, bachem, unienergy technologies, and university of pennsylvania chemistry professor eric j. schelter were honored for their achievements at a ceremony held on june 12 at the national academy of sciences in washington, d.c. the award winners presented details of their technologies the next day at the annual green chemistry & engineering conference ., the environmental protection agency established the awards program in 1995 to help achieve federal goals set by the provisions of the pollution prevention act of 1990. these include reducing toxicity of chemical products, saving water or energy, and reducing waste even if it’s not hazardous. the program is administered by epa’s green chemistry program and is supported by partners including the american chemical society and its green chemistry institute ., “the green chemistry challenge awards highlight the importance of sustainable chemistry and its impact across a range of disciplines,” says princeton university’s paul j. chirik, a 2016 award recipient. “striking features common among many of the winners is that green chemistry often results in an improved product or a cost savings, demonstrating that environmentally responsible science does not have to come with reduced performance or added cost.”, the following vignettes tell the stories of this year’s award-winning technologies., heat-and-serve thermal paper bypasses bisphenol a.
Chemists regularly come up with great products that are later discovered to have a shortcoming. With green chemistry principles as a guide, that’s an opportunity to go back to the drawing board and find a game-changing solution. Thermal paper used for printing cash register receipts, tickets, and labels is one such success story.
In traditional thermal paper, a colorless dye and a chemical developer such as bisphenol A are coated on the paper. When heated, BPA interacts with and protonates the dye to alter the structure, switching its color from white to black. However, concerns over the estrogen-mimicking properties of bisphenols have led chemists to replace them where possible to reduce retail worker and consumer exposures.
Dow Chemical and papermaker Koehler jointly landed the Designing Greener Chemicals Award for a technology that uses a polymer coating on paper to create fade-resistant thermal-printed images stemming from the altered refractive index of the coating. This physical process replaces the chemicals in the thermal paper.
The coating is made from an opaque layer containing Dow’s Ropaque styrene acrylic resin hollow spheres and a colored layer containing a permanent pigment, such as carbon black. Dow originally developed Ropaque as a pigment to replace more expensive titanium dioxide in paint formulations, and it also has come to be used in personal care products such as sunscreens. The spheres function as air voids that scatter light. This is the same effect that makes polystyrene foam and clouds appear white.

When a thermal printhead heats the paper, the air voids collapse and become transparent, revealing the color below without the need for a chemical developer. The added benefit of the new technology is that it works using existing thermal printers. The paper has been tested in a few stores so far and will be in commercial use this year.
Ropaque-based thermal paper “is an amazing innovation,” says A. N. Sreeram, Dow’s chief technology officer. “It takes an entirely new approach by eliminating chemical developers for improved safety, yet it still works in existing equipment. This technology really demonstrates the passion of our people to deliver inventive solutions to customer problems.”
“It’s a classic approach to simply replace molecules that are discovered to have adverse environmental or health effects, such as BPA,” says chemistry professor Audrey Moores of McGill University, an expert in green chemistry who focuses on recyclable nanoparticle catalysts. “However, the replacement can be just as bad. To escape this unproductive cycle, a more innovative approach is to reinvent the material from scratch so that it achieves the same function with less chemistry. This is exactly what Dow and Koehler have done. This is a true example of a material made to be ‘benign by design’ by thinking outside the box.”

The rare-earth metals are a group of 17 elements, lanthanum to lutetium along with scandium and yttrium, whose properties make them useful and often irreplaceable in electronics and lighting applications. Manufacturers often blend rare earths to tune the properties of the needed materials, such as permanent magnets for electronics and phosphors for lighting. But the chemical properties of rare-earth cations are similar, making separating them for recycling difficult.
Ligand separates rare earths for recycling
Eric J. Schelter’s group at the University of Pennsylvania got the nod for this year’s Academic Award for developing a simplified process that uses tailored nitroxide ligands to separate mixtures of rare-earth metals for recycling. The approach is expected to reduce energy use and waste generated during recycling of rare-earth metals from cell phones, magnets used in motors, and other products to help minimize new rare-earth mining—a costly, energy-intensive, and waste-generating process.

The ligand that the researchers designed, tris(2- tert -butylhydroxylaminato)benzylamine, or TriNOx, forms a size-sensitive, tripod-shaped aperture when it binds the metals. For larger diameter metals, a dimeric complex forms. For smaller diameter metals, a monomeric complex forms. For example, neodymium-based permanent magnets (Nd 2 Fe 14 B) contain some dysprosium to improve thermal performance. When TriNOx is added to solutions containing salts of the two metals, neodymium—the larger metal—forms a soluble dimeric complex, whereas dysprosium forms a monomeric complex that precipitates. Schelter’s group developed a complete recycling process to recover the two metals by filtration and reuse the ligand.
The team has also shown the ligand’s separation prowess for phosphor materials that include mixtures of yttrium and europium. Overall, the UPenn researchers have demonstrated the ability to separate more than 50 pairwise combinations of rare earths. The new approach offers an easier, less expensive alternative to redox chemistry, acid-leaching processes, and ionic liquid extraction currently being used and explored for recycling rare earths.

“Our method demonstrates that rare-earth mixtures can be purified by applying the principles of coordination chemistry,” Schelter says. “The work is still in the early stages, but the results are important because they demonstrate a new type of targeted metal separation specifically for recycling.”
“Rare-earth recycling has enormous potential benefits,” says chemistry professor Audrey Moores of McGill University, an expert in green chemistry who focuses on recyclable nanoparticle catalysts. “This discovery has a major impact on greener processes at multiple levels: It means less mining pollution, less e-waste, and better access to key elements for cleantech innovation.”
Peptides have gained increased interest as therapeutic drugs over the past three decades because of their high specificity and safety compared with small-molecule drugs. They are becoming the treatment of choice for some cancers, enzyme and protein disorders, and degenerative and infectious diseases. Their pharmaceutical rise has prompted companies to look at more efficient manufacturing processes for peptide-based therapeutics to reduce the environmental impact and production costs.
Chipping away at the cost of peptide manufacturing
Biotechnology firm Amgen and peptide manufacturer Bachem teamed up to receive the Greener Reaction Conditions Award for improving the manufacturing process for etelcalcetide , the active ingredient in Parsabiv, a calcium inhibitor to help control overactivity of the thyroid gland in patients with kidney disease. The new process produces more peptide in less time while drastically cutting solvent and water use and reducing production costs.

“We’re proud of this award that recognizes how scientific innovation can improve our manufacturing technologies and lead to a green and more efficient process,” says Margaret Faul, Amgen’s executive director of process development. “This new process for solid-phase synthesis leveraged the different areas of expertise across Amgen and Bachem.”
Peptides are synthesized stepwise by coupling the carboxyl group, or C-terminus, of one amino acid to the amino group, or N-terminus, of another using liquid-phase or solid-phase synthesis. In solid-phase synthesis, which is now most common, the peptide backbone is assembled one amino acid at a time while attached to resin beads, which requires washing away residual reagents at each step. Producing 1 kg of peptide typically requires several metric tons of solvent and thousands of liters of water, according to the companies’ environmental analysis.
As Amgen anticipated etelcalcetide approval, Faul and her colleagues realized the original production process would be problematic for commercial-scale manufacturing, given the amount of materials needed and the waste generated. Amgen and Bachem redesigned the process to bypass one of the five production stages and optimize the remaining four.
The process development team, led by Amgen’s Sheng Cui, eliminated an ion-exchange column process requiring more than 3 L of water for every gram of drug and reduced the number of energy-intensive freeze-drying (lyophilization) purifications from 13 per batch of peptides to one. The results are a fivefold increase in manufacturing capacity while cutting manufacturing time by more than half and reducing solvent use by 71%. Overall, the new process cut manufacturing costs by 76%.
“This work constitutes a textbook example of how green chemistry and engineering improvements for a process can result in both clear and tangible environment benefits while making the costs of the process more favorable,” says chemistry professor Audrey Moores of McGill University, an expert in green chemistry who focuses on recyclable nanoparticle catalysts. “The green improvements to all stages of the manufacture of the active species essentially made it possible for the companies to launch this molecule. This is quite remarkable and shows that green chemistry and economics often operate hand in hand.”
As electrical grids become larger and more complex, supplemental energy storage using batteries and other technologies is needed to smooth out supply and demand peaks and troughs. Lithium-ion batteries have the energy density needed for this task, but they are capable of operating for only a couple of hours at a time and have a limited lifetime. In addition, lithium-ion batteries have notable challenges with thermal runaway of their layered materials and with flammability of their organic-based electrolyte.
Better batteries for the electrical grid
Redox flow batteries are a promising technology for long-duration applications for electrical grids and to manage power for commercial and industrial facilities. But scientists and engineers must improve flow battery efficiency and reduce their size and cost.

UniEnergy Technologies , in collaboration with Pacific Northwest National Laboratory (PNNL), garnered the Small Business Award for its design of a next-generation vanadium redox flow battery system that takes a giant step in that direction. The company’s megawatt-scale Uni.System has double the energy density of previous vanadium redox flow batteries even though it’s one-fifth the size and requires smaller amounts of chemicals.
Instead of storing electrical energy in solid electrodes, as most batteries do, a redox flow battery stores chemical energy in a pair of electrolyte solutions. The conversion from electrical energy to chemical energy (charging) and vice versa (discharging) occurs within the flow battery’s electrodes as the electrolytes circulate through the cell.
UniEnergy’s vanadium redox flow battery chemistry originated at PNNL. Liyu Li and Gary Yang, two members of PNNL’s energy storage team, founded UniEnergy, licensed the technology, and recruited a technical and business team. The key innovation for the new battery was replacing a sulfate-based electrolyte with a chloride-based electrolyte.
This seemingly simple switch improves the stability of the battery to increase its lifetime and enables it to function with a broader operating temperature range compared with the prior generation, so it can be deployed just about anywhere, even in extreme hot or cold climate zones. Furthermore, the electrolyte storage tanks act as a heat-exchange system, so the battery stays cool—no thermal runaway. And the aqueous electrolyte is nonflammable and recyclable.
The previous generation of vanadium redox flow batteries took up the space of a tennis court. UniEnergy designed the new battery to fit in standard 20-foot shipping containers, which reduces the amount of vanadium and construction materials needed. The Uni.System is now being used at multiple sites in the U.S. and Europe.

“Advances in chemistry have made this flow battery competitive with lithium-ion batteries for long-duration applications,” says Imre Gyuk, director of energy storage research at the Department of Energy, which funded the original battery development.
“This change in electrolyte chemistry has allowed these inventors to greatly improve the stability of flow batteries to reach unlimited cycles without flammability,” says chemistry professor Audrey Moores of McGill University, an expert in green chemistry who focuses on recyclable nanoparticle catalysts. “The discovery is an example of fundamental electrochemistry research leading to the design of better materials that are necessary to support the transition to renewable energy.”
When pharmaceutical companies have a promising drug candidate that is ready to move forward for clinical testing, process chemists are called upon to develop a synthetic pathway to scale up production of the compound. Often the new synthesis is done in a hurry because time is of the essence to get the drug into the clinic. When the company is ready to move the drug forward for approval, process chemists revisit the synthesis, looking for ways to improve it for manufacturing.
Process chemists find a greener path to antiviral drug
When Merck process chemists investigated ways to streamline the synthesis of the antiviral drug letermovir, they discovered a number of new asymmetric reactions to reduce its environmental footprint and published their initial success story ( Org. Process Res. Dev. 2016, DOI: 10.1021/acs.oprd.6b00076 ). The team’s revised synthesis could have been used as the manufacturing route. However, the new alkaloid-based quaternary ammonium phase-transfer catalyst for the key asymmetric cyclization step was ultimately not recyclable. The team went back to the screening phase and discovered a more stable and effective, fully recyclable catalyst. Merck’s overall achievement has been recognized with this year’s Greener Synthetic Pathways Award.

Letermovir is currently awaiting approval for fighting human cytomegalovirus infections in organ transplant recipients, a condition that currently doesn’t have an effective drug. The award-winning synthesis reduces the process mass intensity for making letermovir, a sustainability measure of raw materials, solvents, and water used per amount of product made, by 73% compared with the original synthesis.
“We’ve had a long-standing commitment to green and sustainable processes,” says Kevin R. Campos, who leads Merck’s process chemistry group. “We are proud of the fact that nearly every atom of every reagent in the commercial process for letermovir is either incorporated into the molecule or recycled—it’s highly atom-economical.”
“Our ultimate goal is ‘zero waste’ pharmaceutical manufacturing,” adds Merck process chemist Guy R. Humphrey, who helped lead the discovery team and development of the manufacturing route.
The original synthesis centered on a procedure involving a guanidine intermediate to obtain the desired letermovir stereoisomer, which had limited the overall product yield. Other inefficiencies were the use of a large amount of palladium catalyst to prepare an earlier intermediate in the synthesis pathway, as well as the use of nine different solvents, including hazardous dioxane and chlorobenzene.
Using high-throughput screening tools, the Merck team explored four alternative asymmetric reactions with hundreds of potential catalysts and reaction conditions. The researchers tested thousands of combinations on a submilligram scale to find the optimal replacement for the procedure to isolate the needed stereoisomer. The outcome was the discovery of a new asymmetric aza-Michael cyclization using a hydrogen-bonding chiral bistriflamide organocatalyst.
The combined improvements increased letermovir’s overall yield by more than 60% and reduced raw material costs by 93%. The researchers estimate that the optimized process will eliminate more than 15,000 metric tons of waste over the lifetime of letermovir.
“Merck really showcases production optimization of letermovir in the context of its ‘zero waste’ goal,” says chemistry professor Audrey Moores of McGill University, an expert in green chemistry who focuses on recyclable nanoparticle catalysts. “Their strategy combining innovative organic synthesis methodology with life-cycle analysis results in an impressive reduction of the carbon footprint and water usage with a direct economic impact.”

Sign up for C&EN's must-read weekly newsletter
Contact us to opt out anytime
You might also like...

- Share on Facebook
- Share on Linkedin
- Share on Reddit
This article has been sent to the following recipient:
Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter
The power is now in your (nitrile gloved) hands
Sign up for a free account to get more articles. or choose the acs option that’s right for you..
Already have an ACS ID? Log in
Create a free account To read 6 articles each month from
Join acs to get even more access to.

- ABOUT GREEN CHEMISTRY
- WHY TO USE THE GREEN CHEMISTRY TOOLKIT?
- WHO CAN USE THE GREEN CHEMISTRY TOOLKIT?
- INTRODUCTION
- ONE-DAY TRAINING
- FOUR-DAYS TRAINING
- CASE STUDIES
- LIST OF TECHNOLOGIES
- USEFUL LINKS
Home Case Studies
Case Studies
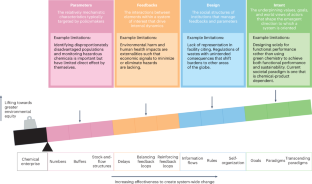
Selected case studies illustrate how the Green Chemistry approach can be applied in different companies and how it contributes to reduce the consumption of hazardous chemicals and enhance their economic and environmental performance
green chemistry, green chemistry toolkit, green chemistry toolkit UN, green chem, current research in green and sustainable chemistry, sustainable chemistry, green chemistry examples, green chemistry is, green analytical chemistry, green chemistry rsc, green chemical engineering, need of green chemistry, examples of green chemistry in everyday life, green chemistry meaning, green chemicals list, the green chemist, green chemistry notes, green chemistry in sustainable development, green chemistry in pharmaceutical industry, green chemical company, trends in green chemistry, green chemistry companies, green sustainable chemistry, green chemistry and technology, green and sustainable chemistry, green chemistry experiments, green chemistry, green chemistry toolkit, green chemistry toolkit UN, green chem, current research in green and sustainable chemistry, sustainable chemistry, green chemistry examples, green chemistry is, green analytical chemistry, green chemistry rsc, green chemical engineering, need of green chemistry, examples of green chemistry in everyday life, green chemistry meaning, green chemicals list, the green chemist, green chemistry notes, green chemistry in sustainable development, green chemistry in pharmaceutical industry, green chemical company, trends in green chemistry, green chemistry companies, green sustainable chemistry, green chemistry and technology, green and sustainable chemistry, green chemistry experiments
Ionic liquids as potential green solvents for purification of manufacturing waste-water Chemical: Ionic liquids as a green solvent Chemicals replaced: Conventional toxic organic solvents Process: Purification of manufacturing waste-water Country: Serbia
Change in the formulation of solvents derived from petroleum for d-limonene considered green solvent in several polikem products Chemical: D – limonene as a green solvent Chemicals replaced: Petroleum-derived solvents Process: Cleaning/degreasing of brakes, motors, and other metal parts Country: Colombia
Production of ethylene from bio-ethanol Chemical: Bio-Ethanol – organic waste as a green raw material Chemicals replaced: Natural gas or petroleum products Process: Utilization of waste / production of chemical product (ethylene) Country: Egypt
Water-base application for wood coating Chemical: Water based coatings Chemicals replaced: Solvent-based coatings Process: Wood coating Country: Sri Lanka
Watch the videos Business Case
This can be useful List of technologies
1.1 Green Chemistry: First, Do No Harm
1.1.1 definition of green chemistry, 1.1.2 principles of green chemistry, 1.1.3 economic driving force, 1.2 better living through chemistry, 1.2.1 predicting harm: evolution of refrigerants, 1.3 environmental pollution, 1.3.1 ddt and silent spring, 1.3.2 times beach and love canal super fund sites, 1.4 risk is a function of hazard and exposure, 1.4.1 hazards: union carbide explosion in bhopal, india, 1.5 toxicology and environmental chemistry, 1.6 life cycle analysis, 1.7 case study: polylactic acid (pla) (natureworks ® ), 1.8 resources: the scientific literature, 1.8.1 databases, 1.8.2 journals, 1.9 implementation of green chemistry, 1.10 summary, 1.11 problems: prevent waste, problem 1.1, problem 1.2, problem 1.3, problem 1.4, problem 1.5, problem 1.6, problem 1.7, problem 1.8, problem 1.9, 1: prevent waste.
- Published: 03 Dec 2019
- Product Type: Textbooks
- Open the Chapter PDF for in another window
- Get permissions
- Cite Icon Cite
Green Chemistry: Principles and Case Studies, The Royal Society of Chemistry, 2019, pp. 1-22.
Download citation file:
- Ris (Zotero)
- Reference Manager
Green chemistry is a conscious change in the way we do chemistry—to do no harm. Chemistry is a powerful tool to improve the quality of our lives. We all reap the benefits of chemists who have improved the quality of modern life. Tragic experiences, such as Silent Spring, Times Beach, Love Canal, and Bhopal, have proven that it is nearly impossible to eliminate exposure to hazardous chemicals that have already been used in the production of these beneficial modern products. So green chemistry seeks to eliminate risk by avoiding the use and production of hazardous chemicals in the first place. Of great use to green chemistry are the tools of toxicology, environmental chemistry, life cycle analysis, and of course, the scientific literature that encompasses all of these areas. Cargill Dow exemplified the use of life cycle analysis in the production of NatureWorks ® polylactic acid from corn. Implementing green chemistry is a question of public policy: should governments attempt to force the change, or will market forces make it happen?

“Principle 1: It is better to prevent waste than to treat or clean up waste after it has been created.” 1
Green chemistry aspires to give us all of the wonderful products we have come to expect in our lives, without the associated pollution of the past. The job of green chemists is to eliminate hazards completely from our processes and products, so that no accidental release or exposure would be possible. That is the ideal, the lofty goal we aspire to, but the reality is one of more gradual shift and compromise, tempered by economics and regulations. The Green Chemistry Institute was founded in 1995, the same year the US Presidential Green Chemistry Challenge (PGCC) Awards were established by the US Environmental Protection Agency (EPA) under President Bill Clinton. These awards have now inspired industrial and academic chemists to invent new products and processes that are “benign by design,” or sustainable without polluting the environment.
Chemists have serious responsibilities in our profession. Whether a chemist or a company chooses to ignore the consequences of their chosen chemicals and methods, or to take great pains to produce the best products in the least polluting way that one can imagine, chemistry has consequences. Green chemistry holds that each chemist, each company, is responsible for preventing accidents and pollution, and for using the least amount of resources necessary. Green chemistry has as its core value, a chemist's version of the Hippocratic Oath, “First, Do No Harm.” 2 This injunction is particularly apt in the case of chemistry, where the products of our labor may last for years, or even centuries, for better or for worse.
“Green chemistry is the design of chemical products and processes that reduce or eliminate the use and generation of hazardous substances.” †
A course in Green Chemistry might just as well be called Industrial Chemistry, because green chemistry is about making commercial products by clean and efficient methods. In many ways, industry is leading the way in making greener products. Half of the PGCC awards have been to industry. PGCC awards also go to academic chemists with a strong desire that their greener process or product find application in society, so much so that they often start a company to commercialize their chemistry. Many of the chemicals described in the case studies of this book are commodity chemicals, such as starting materials for things like plastics or dyes. Commodity chemicals are defined by the large scale of their production, and the global market for their distribution. Others are fine chemicals, made on smaller scale, but just as crucial, like medicines. Green chemistry is all about the applications, thus this book will be grounded in case studies, primarily from the US Presidential Green Chemistry Challenge (PGCC) Awards.
In 1998, Paul Anastas and John C. Warner published a seminal book, Green Chemistry: Theory and Practice , which established the 12 Principles of Green Chemistry as the foundation for this dramatic shift in the way we do chemistry. 1 The 12 Principles fall into three general categories: (1) use and produce no toxic chemicals, (2) minimize the use of chemical and energy resources, and (3) prevent accidents. The chapters in this book are organized by Principle, but many of the case studies use several of the principles to achieve their goals. These illustrative case studies are designed to inspire the student to find new ways to apply the principles in their future work.
One of the cool things about green chemistry is that greener processes and products are also typically more economical, with renewable feedstocks, fewer steps, lower energy costs, and lower hazardous waste disposal costs.
In 2001, Shaw Industries was bought by Berkshire-Hathaway Inc. headed by Warren Buffett. Shaw developed EcoWorx carpet tiles that were completely recyclable back into carpeting. 4 A number of economic advantages propelled EcoWorx to a market share of 80% of the carpet sold in their product line. The feedstocks were old carpet tiles sent back for remanufacturing. The carpet tiles were made from lighter materials that required less energy in manufacturing and shipping. The EcoWorx product line is highly successful.
Carpet tiles originated with Interface, Inc., whose owner, Ray Anderson said, “Sustainability is proving to be incredibly good for business. What began as the right thing to do quickly became the smart thing to do. Sustainability doesn't cost, it pays.” 5
Unfortunately, we are still in a time of cheap fossil feedstocks—petroleum and coal, and this can doom a greener process. For example, Dupont developed a new method for breaking down polyethylene terephthalate (PET) into the two monomers that could then be repolymerized into virgin PET. They built a pilot plant in North Carolina in 1996 that produced 100 million pounds of PET per year, but a drop in oil prices led to the plant closure and dismantling in 2000. There may come a day when oil prices rise, and recycling PET could again become cost competitive.
There is no doubt that the modern world has benefited enormously from the efforts of chemists, without which we would not have most medicines, fuels, computers, in short, the stuff of modern life. In 1935, Walter Carothers invented a nearly indestructible fabric, nylon. The same year, Dupont asserted in an advertising slogan, “Better Things and Better Living…through Chemistry,” which was later co-opted and shortened to “Better Living Through Chemistry.” One of the earliest organic synthesis industries, the German synthetic color dye industry, spawned the first medicinal chemistry program. The first antibiotic, sulfanilamide (first a red dye), 6 saved countless lives, and was forerunner to the whole class of antibiotic drugs. We are now on the cusp of individualized medicine, in which each patient's unique physiology and disease progression are considered when designing and prescribing medical treatment. The tricky part will be whether we can, at the same time, avoid side effects and the production of toxic pollutants in the process of converting to individualized medicine.
We have to know what the potential harm might be before we can know that we should avoid it. In the early 1900s, refrigerators that used ammonia as the coolant were used commercially, but the acute toxicity of ammonia gas prevented widespread use in homes. The solution came when chemists at General Motors developed Freon ( Figure 1.1 ), patented in 1928 as a refrigerant, which was non-flammable and of very low toxicity. Perhaps this could be considered an early attempt at green chemistry. Fifty years later, chemists Frank S. Rowland and Mario J. Molina, discovered that Freon could destroy the ozone layer of the stratosphere that protects living things from UV radiation. Together with Paul Crutzen, they were awarded the Nobel Prize in Chemistry in 1995 for their work on ozone depletion. The chemists at General Motors who invented CFCs did not anticipate, nor could they even have been expected to anticipate, any larger problems with CFCs.

Refrigerants, L to R: Freon, (HFC)-134a, and (HFO)-1234yf.
The next step in replacements for CFCs contained only fluorine, carbon, and hydrogen, primarily hydrofluorocarbon (HFC)-134a, 1,1,1,2-tetrafluoroethane, which does not destroy the ozone layer ( Figure 1.1 ). Unfortunately, the saturated HFCs are potent greenhouse gases. 7 HFC-134a has a 100-year global warming potential (GWP) that is equivalent to 1430 times that of CO 2 . 8 Currently, hydrofluoroolefin (HFO)-1234yf (2,3,3,3,-tetrafluoropropene) is considered an efficient and safe refrigerant with a 100-year GWP less than 1 ( Figure 1.1 ). US and Japanese automakers are switching to HFO-1234yf, however Mercedes-Benz engineers in Germany found that it ignited on a hot engine when mixed with the compressor oil necessary for air conditioning. 9 Highly corrosive HF gas, given off in the fire, etched the windshield in their test. 9 Under ordinary release conditions, HFO-1234yf degrades by oxidation to trifluoroacetic acid (TFA), 10 a strong, toxic acid for which the environmental safety has not yet been fully assessed. 11
The most benign refrigerant under development is CO 2 . 12 CO 2 has a GWP of 1 by definition, well below the European Union requirement for refrigerants to be below a GWP of 150. CO 2 is cheap, abundant, non-toxic, and all natural; it is not subject to government regulation and can be released to the atmosphere requiring no special recovery methods. Any CO 2 used in refrigeration would either be withdrawn from the atmosphere or recovered from burning fossil fuel for energy. One problem with CO 2 is that it operates as a refrigerant best under transcritical conditions, above the critical pressure of 73 atm. 13 This is essentially an engineering design problem, requiring new systems with stronger containers and special valves. There are also engineering advantages. The volumetric refrigeration capacity is higher for CO 2 , so units can be smaller. 13 The compression ratio is about half that of HFC compressors, increasing the efficiency. 13 The problems have been solved for stationary refrigeration, and German automakers are working on a solution for vehicles. 14
We have yet to discover a truly benign, efficient substitute for HFCs. This illustrates the difficulty of finding truly green chemical solutions for significant societal needs. No one wants to go back to the days of smelly, moldy iceboxes, or worse yet, no refrigeration or air conditioning at all. Replacing HFCs remains a significant opportunity for green chemists.
The chemists who developed Freon, indeed all of us, were unaware of stratospheric ozone destruction by CFCs. Our collective ignorance leads one to wonder if there are other types of environmental or biological harmful effects that we do not yet know, cannot predict, or have missed in the literature. It is wise for chemists to pay attention to the work of toxicologists and environmental chemists. Whatever type of harm is known, we should take care to avoid in our work.
Most people lack interest in, or feel incapable of comprehending, the chemistry used to make the products they use. We just want our things to function well. We want durable goods, like a refrigerator, a house, or a car, to last a long time and to still function. We want a product that is supposed to be temporary, like a plastic bag, to go away; we want to drop it in the trash and have it disappear. And this is where chemists have succeeded heroically, and also failed miserably. Our work is a work in progress.
What most people do care about is a clean environment, at a minimum in their own backyard—hence the term “not in my back yard” or NIMBY, that refers, often derogatively, to people fighting against contamination of their home or community environment. We want clean air to breathe, clean water to drink, clean food to eat, and clean places to live, play and work. The connection between the chemistry used to make products and a clean environment is obscure to many, though it has become more mainstream since 1970, when the US Clean Air Act was passed. 15 The US Federal Water Pollution Control Act Amendments of 1972, now known as the Clean Water Act of 1977, 16 did much the same for water pollution consciousness.
The environmental consciousness of the modern era has been attributed to publication in 1962 of the book, Silent Spring by Rachel Carson. 17,18 In this moving and superbly written account of the dramatic deformities and stillbirths of birds caused by the pesticide 2,2- p,p ′ - dichlorodiphenyl-1,1,1-trichloroethane (DDT, Figure 1.2 ), Carson was first to observe endocrine disruption in birds. It was Carson who first pointed the finger at ourselves, “…no enemy action had silenced the rebirth of new life in this stricken world. The people had done it themselves.” 18 This new attitude found its way into popular culture with Walt Kelly's Pogo comic strip on the newly founded Earth Day in 1971 ( Figure 1.3 ).

The structure of 2,2- p,p ′ - dichlorodiphenyl-1,1,1-trichloroethane (DDT).

We have met the enemy, and he is us. Pogo comic strip by Walt Kelly published on Earth Day, April 22, 1971. Copyright Okefenokee Glee & Perloo, Inc. Used by permission. Contact [email protected].
The environmental movement had a big job cut out for it. Although toxicology is an ancient science, the twist was that chemical manufacturing was producing and selling novel synthetic or “man-made” substances for which there were no health effects data, and none were required by law. Two events galvanized a public now attuned to environmental issues. In 1972, Russell Martin Bliss sprayed waste oil contaminated with dioxin (2,3,7,8-tetrachlorodibenzodioxin, Figure 1.4 ) in Times Beach, Missouri. The dioxin and trichlorophenol caused many birds and horses to die immediately, and caused severe health effects to the people living in the town. Dioxins are among the most potent toxins known.
![case study on green chemistry Structure of the most common dioxin: 2,3,7,8-tetrachlorodibenzo[b,e][1,4]dioxin.19](https://rsc.silverchair-cdn.com/rsc/content_public/books/871/parts/bk9781788017985-00001/5/m_bk9781788017985-00001-f4.jpeg?Expires=1728187174&Signature=DUnFhcvSJiKUTnk69ic3SwNz8beCC0yr2vxLkQckXG7Cn1mxZH~8rkAwIBR4UUJBJGlkj8TC5K0W6IogV2f6zJOZKenbS6j~9P1aoODG-8b12BCijxcUK1TP0DvAUIPLBlIZDhszXKevfagz70eKyNQBTKghxR57-ErEtWsrgEtxOaNJm6~-6CqSXUyKHIg9ygO1OgwuhPnlXRwL9fFhdgAbevQTtFleNnMdrDL4joP6~aacoAOIZVnkU4lkj3MWlh5fSuC44~Pr2GDfcKPUiCAIEBnX-i8D6hk3ORR6Bup7c18i5EbLVJ-LK0ODs0-di-hwX024mHhQ94Qkx5WSOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Structure of the most common dioxin: 2,3,7,8-tetrachlorodibenzo[b,e][1,4]dioxin. 19
There is still much concern about the formation of dioxins in municipal waste incinerators and paper bleaching. In order to completely destroy dioxins during incineration, three conditions are necessary: (1) high combustion temperature, (2) adequate combustion time, and (3) high combustion turbulence. 19 Dioxins form as flue gases are cooling during emissions or flue gas filtration, so incinerator design is vital, especially when chlorine-containing compounds are being incinerated. 19
In 1977, severe health effects due to toxins leaking from the Hooker Chemical Co. waste site at Love Canal, NY came to light by the efforts of two investigative newspaper reporters for the Niagara Falls Gazette, David Pollak and David Russell. The result was the Super Fund, or Comprehensive Environmental Response, Compensation and Liability Act of 1980, which is used to this day to clean up hazardous waste sites.
The sad lesson of Love Canal is that burial of toxic waste in landfills is no guarantee of safety on a human history time scale, or in the case of nuclear waste, on a geological time scale. Thus, the primary goal of green chemistry is neither to use nor produce toxic substances in the first place.
Green chemistry differs from environmental chemistry in that we seek to avoid using and creating chemical products that are in any way hazardous. In my first job out of college, I worked with and synthesized very hazardous substances—radioactive carcinogens—that were used in environmental and biological studies of pollution. Our primary goal was, understandably, to protect ourselves with fume hoods, double-door entries, rubber shoe covers, white paper “zoot” suits worn over our clothes, double gloves, safety glasses, and weekly urine and lab monitoring. This approach epitomizes the exposure-avoidance model of safety that was in vogue in 20th century industry, which is still used in most chemistry laboratories. What we did not much consider was that the hazardous fumes were going out the hood into the community, or the barrels of radioactive, carcinogenic, and flammable waste that were sealed and put into landfills. (Explosives were taken out into a field and shot with a rifle to detonate and inactivate them.) Little consideration was given to what happens when those barrels corrode and begin to leak into the environment, such as what happened at Love Canal in Niagara Falls, NY in 1953. After many other infamous chemical spills, releases and exposures later, including the horrific explosion in Bhopal, India, and the spraying of dirt roads and horse arenas with dioxin-contaminated oil in Times Beach, Missouri (unsettlingly close to my home town), we are finally wising up.
Anastas and Warner set out the principle of risk as an equation. 1 If either hazard or exposure is zero, then zero risk is involved. Zero risk, that is, in a perfect world.
Their point was that we have historically aimed to eliminate risk by eliminating exposure, through the use of barriers, such as gloves, laboratory coats, fume hoods, blast shields, double-walled reactors, double-hulled petroleum tankers, etc. The sad truth is that barriers break, gloves tear, fume hoods emit toxins into the local air, ships are breached, and we are all eventually exposed to toxins that begin leaking from hazardous waste sites once a steel drum begins to rust, like at Love Canal.
Many chemists have a sense of pride in their ability to handle hazardous chemicals safely. I am one of these—I have used 3 H and 14 C (both radioactive), Hg, HF, and many other extremely hazardous chemicals in my work over the years, and I am proud of my skills in their safe handling. There are many chemicals I would rather not ever touch. Derek Lowe, a medicinal chemist well known for his blog “In the Pipeline,” has an entertaining, long, and growing list of “Things I Won't Work With.” 20 A better way, the ideal of green chemistry, is to use non-toxic, non-flammable, and non-explosive chemicals to eliminate the hazard part of the risk equation entirely, so that exposure is no longer an issue, and risk ideally becomes zero.
We will see that green chemists sometimes compromise. In a case study on the BHC synthesis of ibuprofen in Chapter 2, extremely dangerous HF is used as a Lewis acid to replace AlCl 3 in a Friedel–Crafts reaction, which eliminates tons of hazardous waste (AlCl 3 · xH 2 O). The HF is easily recycled because of its low boiling point. This is a compromise because the reaction is more atom economical. Yet HF was the first chemical Derek Lowe posted on his “Things I Won't Work With” blog, a good starting list of chemicals to avoid for prevention.
Toxicity is not the only hazard that green chemistry seeks to avoid. Highly reactive, compressed gas, explosive, flammable, corrosive, strongly acidic, basic, oxidizing or reducing chemicals are also hazard concerns. On December 3, 1984, a terrible explosion at a Union Carbide India plant resulted in the death of 5200 people, and thousands of others were permanently or partially injured in Bhopal, India. 21 A storage unit that contained 42 tons of methyl isocyanate (MIC) had water leak into it through a faulty valve, initiating an exothermic reaction. The pressure built up and the tank exploded, releasing MIC along with other reaction products. MIC is flammable, reactive with water, and very toxic to the respiratory system. 22 MIC has a boiling point of just 39 °C, so that it becomes a gas with minimal temperature elevation. MIC is extremely hazardous—the cause of most of these deaths was pulmonary edema, or excess fluid in the lungs. 23 One of the lessons from Bhopal is that highly reactive chemicals, such as MIC, should be avoided in chemical synthesis if at all possible.
In order to design better processes and products, chemists need to know what is hazardous, and why, in order to avoid it. Toxicology is the discipline that studies the biological effects of toxic chemicals, both the qualitative (severity), and quantitative (potency) aspects of toxicity, as well as the biological mechanism of action. We will see that there are ways to avoid toxicity by design in Chapter 4.
Environmental chemistry is quite distinct from green chemistry, yet environmental chemistry is a critical partner in ensuring that chemistry becomes sustainable, that all chemistry becomes green chemistry. Wikipedia says, “Environmental chemistry can be defined as the study of the sources, reactions, transport, effects, and fates of chemical species in the air, soil, and water environments.” 24 Since all matter is chemical, we should be more specific and say that environmental chemistry is the study of synthetic or mined pollutants in the environment. There are other types of pollution, such as soil loss due to agriculture or construction, but this is typically not the domain of chemists.
Environmental chemists have been analyzing ever-lower concentrations of toxic and hazardous substances in a wide array of environmental and biological samples. For example, three years after the initial dioxin spraying, and after 18 inches of topsoil were removed, soil samples at the Shenandoah Stables in Times Beach were found to contain 30 ppm of dioxin, which is a very high level of this potent toxin. 25 Now, environmental chemists routinely find parts-per-trillion (ppt), equivalent to picomolar (10 −12 M), concentrations of dioxin. 26 This illustrates that there is no such thing as a zero concentration of a pollutant in the environment. It is a question of the detection level of an instrument for a specific substance, by a particular method, in a particular matrix.
Once the type and amount of a substance is found, environmental chemists are charged with figuring out how to clean up the mess, whether to attempt to destroy it, or to gather it up for disposal in a landfill, which of course could leak later. Environmental chemistry has the unenviable job of detecting, analyzing, and cleaning up manmade pollution.
The best tool that has emerged for pollution prevention with green chemistry is the life cycle analysis (LCA), the study of the production of a product from cradle to grave, or better yet, as McDonough and Braungart titled their book, cradle-to-cradle . 27 LCA is an extremely useful method for chemists and engineers to analyze the entire life cycle of a product.
LCA is a 21st century method for analyzing the sustainability of a product or process from the extraction, mining, or growing of a resource to the ultimate fate of the product, whether landfilled, recycled, or composted. LCA is typically both qualitative and quantitative. The new greener product is compared with products currently on the market. Sustainability is measured by the overall efficiency of the process in the use of raw materials or feedstocks, energy, greenhouse gases, water, and the output of pollution. Life cycle thus refers to the entire process to make a product, use it, and dispose of it throughout the product's useful lifetime. Greener products or processes are compared with traditional products or processes that perform the same or similar functions. Each LCA establishes the criteria to be used to assess sustainability.
The first step in an LCA is to decide what will be measured. These are frequent candidates: materials in, materials out, energy used, energy produced, water used, water released. This may sound formidable, yet it is already a necessary part of the economics of designing a chemical production plant, or the process chemistry to synthesize a medicine.
A complete LCA can sometimes deliver surprising results. Hexamethylene diamine (HMDA) is one of the two key monomers used to make nylon-6,6, which is the nylon used in our backpacks, clothing, carpet, car parts, and many others. Solvay (China) Co. discovered that production of HMDA from starch is more polluting and energy-intensive than production from petroleum ( Figure 1.5 ). 28 Although the bio-based route from sugars had greenhouse gas emission advantages over the petroleum-based route, energy-intensive drying of the hydroxymethylfurfural (HMF) intermediate increased marine and freshwater eutrophication. Growing corn and potatoes for the starch feedstock was also found to have negative environmental consequences. 28 These challenges are not insurmountable, however. Improvements in drying technology and agricultural practices could result in flipping the LCA outcome.

Life cycle analysis (LCA) of the production of HMDA from food crop starch vs. petroleum. Reproduced from ref. 28 with permission from the Royal Society of Chemistry.
An early example of a complete LCA was performed by NatureWorks ® , a subsidiary of Cargill Dow LLC, for their polymer, polylactic acid (PLA). 29 Cargill Dow has defined sustainability by a “triple bottom line approach” of economic, social and environmental sustainability. We will only be concerned with the environmental sustainability, although the social sustainability exemplifies some green chemistry principles as well. For example, under the social sustainability criterion, Cargill Dow does not allow the endocrine disrupter (Chapter 3) bis-phenol A (BPA) to be used as a plasticizer in products made with their PLA, and they do not allow PLA to be used for packaging tobacco products. 29
PLA is a biodegradable polymer derived entirely from the renewable resource cornstarch. The carbon source is thus entirely carbon dioxide and water, which plants take up to synthesize polysaccharides using energy from the sun by photosynthesis. 29 The cornstarch is enzymatically converted into natural d -glucose, also called dextrose ( Figure 1.6 ). Dextrose is then fermented at neutral pH to produce lactic acid. A pre-polymerization step is necessary to remove water before the lactic acid can be cyclized to produce the key intermediate, lactide. The lactide is then distilled in one of the higher energy-intensive steps ( Figure 1.6 ). The final step is a solvent-free ring-opening polymerization (ROP) to produce the high molecular weight PLA product with the desired properties.

NatureWorks ® process for manufacturing polylactic acid (PLA) from cornstarch. 29
Cargill Dow chose three indicators for the LCA of PLA: (1) the use of fossil energy in production, (2) net greenhouse gases produced, and (3) water use. 29 The indicators for PLA were compared with nine petrochemical-based polymer products, and cellophane, made from an older renewable feedstock, cellulose. LCA methodology is very complex, with many decisions to be made about inclusion or exclusion of data, weighting of various factors, and approximations. 29 First, the use of petroleum, both as feedstock and as the energy source in the production of plastics, was evaluated ( Figure 1.7 ). Since PLA is derived from a bio-feedstock instead of petroleum, the total amount of fossil energy used in production is less than all of the other plastics. A net use of 54 MJ kg −1 of fossil energy was for agricultural purposes in growing the corn for feedstock, for transportation, and for chemical processing, minus the embodied solar energy in the corn feedstock. With the inclusion of lactide from agricultural waste (stalks, husks and leaves, called “corn stover”) as the biomass feedstock (Bio) and wind power (WP), fossil energy use was decreased to only about 9 MJ kg −1 ( Figure 1.7 ). Improvements in process energy would also lead to a more cost competitive product. Another advantage of using corn stover is that the process would not compete with the food supply. Thus, energy efficiency improves all three components of the triple bottom line: environment, economics, and society. 29

Fossil energy requirement for some petroleum-based polymers and polylactide. The cross-hashed part of the bars represents the fossil energy used as chemical feedstock (the fossil resource to build the polymer chain). The solid part of each bar represents the gross fossil energy use for the fuels and operations supplies used to drive the production processes. PC=polycarbonate; HIPS=high impact polystyrene; GPPS=general purpose polystyrene; LDPE=low density polyethylene; PET SSP=polyethylene terephthalate, solid state polymerization (bottle grade); PP=polypropylene; PET AM=polyethylene terephthalate, amorphous (fibers and film grade); PLA1=polylactide (first generation); PLA B/WP (polylactide, biomass/wind power scenario). Reprinted and reproduced from ref. 29 with permission from Elsevier. Copyright 2003.
Solar energy warms objects on earth, think of a rock in the sun, or a parking lot. Greenhouse gases trap heat radiated from these objects in the atmosphere. The mechanism is simply absorption of electromagnetic radiation, and emission in the infrared region back into the atmosphere. Of course, some heat is radiated out to space, but the effect of higher concentrations of greenhouse gases is net global warming. Not all geographical locations will experience the same warming; northern latitudes are warming at the greatest rate of all, and the eastern US has experienced cooler temperatures, even as the western US is burning, literally. This is why we now call it climate change. The three major greenhouse gases are rated in terms of CO 2 equivalents (C-eq): CO 2 is designated 1 C-eq; CH 4 has 21 C-eq; and N 2 O has 310 C-eq. 30
Cargill Dow included these three greenhouse gases in the LCA of PLA. In this analysis, the first generation PLA1 from feed corn did not fare much better than low density polyethylene or polypropylene, but it was much better than Nylon-6,6 or Nylon-6 ( Figure 1.8 ). The production of nylon is very energy intensive as well, which illustrates the connection of greenhouse gas production to energy use ( Figure 1.7 ). Using corn stover as feedstock and wind energy in the production of PLA B/WP actually removes greenhouse gases from the atmosphere because growing corn uses CO 2 as the most basic feedstock ( Figure 1.8 ).

Contributions to global climate change for some petrochemical polymers and the two polylactide polymers. Reprinted and reproduced from ref. 29 with permission from Elsevier. Copyright 2003.
The final impact assessed in the LCA was the use of water in plastic production. Three uses of water were analyzed: irrigation water, process water, and cooling water ( Figure 1.9 ). Even though the corn feedstock requires irrigation with water to grow it, PLA is competitive with all the major commercial plastics studied, and it beats the nylons and cellophane by a substantial margin.

Gross water use by petrochemical polymers and the two PLA cases. Reprinted and reproduced from ref. 29 with permission from Elsevier. Copyright 2003.
Overall, Cargill Dow's LCA of PLA is a model for the use of LCA for other products. They found that the current production of PLA from feed corn already has a significant environmental advantage over other polymers, and that switching to corn stover feedstock and wind power represents a dramatic improvement even over the current process. Notably, Cargill Dow did not include an analysis of the pollution prevention impact of PLA, which is a compostable product, keeping more permanent polymers, such as polyethylene terephthalate (PET) and nylons out of landfills and the oceans.
As you work through this textbook, many ideas and problems will require the use of the scientific literature. Several databases and journal resources are particularly useful and complete.
The most complete database for chemical research is Scifinder . An important tool in searching databases is the use of applicable key words. Sometimes search words will need to be made more specific to narrow the range of hits, other times search words need to be made more general. For example, in 2015, a search in Scifinder using “green chemistry” as search words turned up thousands of hits; it was too broad. On one hand, “sustainable (or green) synthesis of adderall” turned up no relevant hits; it was too narrow. On the other hand, “synthesis of adderall” garnered 669 hits; it would be extremely difficult to read and assess the green merits of each synthesis. One way to get around this is to select a recent comprehensive review article, and find the most efficient synthesis described therein. To get the best result from a search—a search result of 10 to 100 hits is useful and manageable—one must try different search words, combinations of search words, chemical structures, and reactions. Searching the literature is like a video game in that persistence and experience help you find your way, gather tools, and reach your goals.
Scifinder , chemical literature, by library subscription.
PubMed , biomedical literature, free to the public.
Web of Science , multidisciplinary, by library subscription.
Green chemistry articles are increasingly published in traditional chemical journals. Specific society collections of journals, such as the American Chemical Society (ACS: pubs.acs.org) and the Royal Society of Chemistry (RSC: pubs.rsc.org), have useful search routines that are specific for the chemical literature. The “Advanced” search links are especially useful, because you can narrow your search by years, author, or journal. Specific journals devoted to green chemistry may be useful in identifying current areas of research, and finding solutions to particular problems. You can keep up to date by browsing the table of contents of these on a regular basis.
Green Chemistry , Royal Society of Chemistry, since 1999 http://pubs.rsc.org/en/journals/journalissues/gc#!recentarticles&adv , accessed July 19, 2019.
ACS Sustainable Chemistry and Engineering , American Chemical Society, since 2013 http://pubs.acs.org/journal/ascecg , accessed July 7, 2019.
John Warner and Paul Anastas established the Principles of Green Chemistry 25 years ago. Where are we now? In 2010, Scientific American published an article that said hazardous chemicals had been reduced by about 0.5 billion kg over 15 years, but that is a drop in the bucket compared with the 33.5 billion kg per day that the US produces or imports. 31 There is still a lot of disagreement about how to make all chemistry green chemistry, that is to make the “green” in green chemistry superfluous. 31
Edward Woodhouse, a political scientist at Rensselaer Polytechnic Institute said, “One way to think about it is to ask yourself: ‘What is the purpose of government? Why isn't everything done by voluntary exchange among willing buyers and sellers?’ The answer is, of course, that a lot of important things that need doing won't be done voluntarily.” 31
“[John] Warner favors the ‘build a better mousetrap’ philosophy: Do green chemistry by making alternatives that are not only safer but effective and economical, and chemical companies will eagerly adopt them.” 31
As you learn about this new way of doing chemistry, think about whether you agree with Woodhouse, or with Warner, about the best way to achieve these goals, to make it happen.
Green chemistry is a conscious change in the way we do chemistry—to do no harm. Chemistry is a powerful tool to improve the quality of our lives. We all reap the benefits of chemists who have improved the quality of modern life. Tragic experiences, such as Silent Spring , Times Beach, Love Canal, and Bhopal, have proven that it is nearly impossible to eliminate exposure to hazardous chemicals that have already been used in the production of these beneficial modern products. So green chemistry seeks to eliminate risk by avoiding the use and production of hazardous chemicals in the first place. Of great use to green chemistry are the tools of toxicology, environmental chemistry, LCA, and of course, the scientific literature that encompasses all of these areas. Cargill Dow exemplified the use of LCA in the production of NatureWorks ® polylactic acid from corn. Implementing green chemistry is a question of public policy: should government attempt to force the change, or will market forces make it happen?
(a) Three general categories were given for the 12 Principles of Green Chemistry. List which principles fit into each of the three general categories, using the short title of each principle. Some principles may apply to more than one category.
(b) Explain your choices with as few words as possible. (Ask your instructor whether or not to use complete sentences.)
(a) Terephthalic acid (1,4-benzenedicarboxylic acid) is one of the monomers for PET. Is terephthalic acid considered a fine chemical, or a commodity chemical?
(b) Ibuprofen is an anti-inflammatory over-the-counter drug. Is Ibuprofen a fine chemical, or a commodity chemical?
DDT is used as a potent insecticide against the malaria mosquito in Africa. An article in Scientific American ( http://www.scientificamerican.com/article/ddt-use-to-combat-malaria/ ) lays out the cases for and against using DDT to control malaria.
(a) Read the article, and perhaps find others. Then choose one side or the other and write a brief argument in support.
(b) What can chemists do to prevent malaria and the use of DDT?
(c) Find one scientific paper in the chemical literature (Scifinder or Pubmed) describing a new mosquito insecticide, and assess if it would be more environmentally degradable, less toxic, or less susceptible to insect resistance. Give the full reference (Authors, Title, Journal, Year, Volume, page numbers).
DDT is considered an endocrine disrupter, a substance that interferes with the hormone chemical messenger system, often a mimic of estrogen.
(a) Draw the structure of 17β-estradiol.
(b) Next to it, redraw the structure of DDT.
(c) Circle and label the parts of the two molecules that are of similar type (polar, hydrophobic, charged), and similar arrangement in space.
(a) What are the disadvantages of choosing CO 2 as a refrigerant?
(b) What are the disadvantages of choosing N 2 O as a refrigerant?
(a) Draw the balanced reaction of methyl isocyanate (MIC) with excess water.
(b) What are the physical forms of the products?
(c) Why did the MIC tank in Bhopal explode when water leaked into it?
(a) Dioxin can be destroyed by incineration. What are the three criteria for achieving complete combustion of dioxins?
(b) Is this environmental or green chemistry? Explain briefly.
(c) Terrence Collins invented a catalyst that can be used to accelerate paper bleaching by hydrogen peroxide and avoids the formation of dioxins. Is this environmental or green chemistry? Explain briefly.
Use PubMed and Scifinder to search for “paper bleaching with hydrogen peroxide” and “health effects of dioxin.”
(a) How many hits do you get for each search term in each of the two databases?
(b) Is one database better than the other for certain topics?
(a) Draw a curved arrow mechanism for the ring-opening polymerization (ROP) of lactide into PLA initiated by H 2 O.
(b) Is this a step-growth or a chain-growth polymerization? (Hint: Look up the criteria for step-growth and chain-growth polymerization.)
(c) Why do you think the lactide intermediate must be distilled before the ROP?
From ref. 3 . Copyright © 2017 Yale University. All rights reserved.
- Campaigning and outreach
- News and events
- Awards and funding
- Privacy policy
- Journals and databases
- Locations and contacts
- Membership and professional community
- Teaching and learning
- Help and legal
- Cookie policy
- Terms and conditions
- Get Adobe Acrobat Reader
- Registered charity number: 207890
- © Royal Society of Chemistry 2023
This Feature Is Available To Subscribers Only
Sign In or Create an Account
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Perspective
- Published: 30 January 2023
Green chemistry as just chemistry
- Mary Kate M. Lane ORCID: orcid.org/0000-0001-6208-3903 1 , 2 na1 ,
- Holly E. Rudel ORCID: orcid.org/0000-0003-3535-8697 1 , 2 na1 ,
- Jaye A. Wilson 2 , 3 ,
- Hanno C. Erythropel ORCID: orcid.org/0000-0003-3443-9794 1 , 2 , 3 ,
- Andreas Backhaus ORCID: orcid.org/0000-0002-5314-3799 1 , 2 ,
- Elise B. Gilcher ORCID: orcid.org/0000-0002-0326-5501 2 , 3 ,
- Momoko Ishii 1 , 2 , 3 ,
- Cheldina F. Jean ORCID: orcid.org/0000-0002-5214-874X 1 , 2 ,
- Fang Lin 2 , 4 ,
- Tobias D. Muellers ORCID: orcid.org/0000-0001-8180-6724 2 , 3 ,
- Tong Wang ORCID: orcid.org/0000-0002-9715-9135 1 , 2 ,
- Gerald Torres ORCID: orcid.org/0000-0002-6322-7777 3 , 5 ,
- Dorceta E. Taylor 3 ,
- Paul T. Anastas 2 , 3 , 6 na1 &
- Julie B. Zimmerman ORCID: orcid.org/0000-0002-5392-312X 1 , 2 , 3 na1
Nature Sustainability volume 6 , pages 502–512 ( 2023 ) Cite this article
3590 Accesses
28 Citations
20 Altmetric
Metrics details
- Chemical safety
- Sustainability
Environmental injustices have exposed our current system of reliance on polluting and toxic chemicals and chemistries as untenable and one whose risks and burdens are disproportionately borne by those who are disadvantaged. Aiming for effective interventions to create system-wide change, green chemistry and adjacent approaches are powerful leverage points to deeply address environmental injustices by changing the very nature of the molecular (for example, chemical, material, energy) basis of our economy and our society, obviating the need to rely on procedural systems that can either serve to enable progress or reinforce the status quo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
111,21 € per year
only 9,27 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Scientists’ warning on affluence

Evaluating the efficacy and equity of environmental stopgap measures

Equity, technological innovation and sustainable behaviour in a low-carbon future
Taylor, D. E. Toxic Communities: Environmental Racism, Industrial Pollution, and Residential Mobility (New York Univ. Press, 2014).
Bullard, R. D. Dumping in Dixie: Race, Class, and Environmental Quality (Routledge, 1994).
Bullard, R. D., Mohai, P., Saha, R. & Wright, B. Toxic wastes and race at twenty: why race still matters after all of these years environmental justice: making it a reality. Environ. Law 38 , 371–412 (2008).
Google Scholar
Mohai, P., Pellow, D. & Roberts, J. T. Environmental justice. Annu. Rev. Environ. Resour. 34 , 405–430 (2009).
Article Google Scholar
Taylor, D. E. The Environment and the People in American Cities, 1600s–1900s: Disorder, Inequality, and Social Change (Duke Univ. Press, 2009).
Anastas, P. T. & Warner, J. C. Green Chemistry: Theory and Practice (Oxford Univ. Press, 1998).
Erythropel, H. C. et al. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 20 , 1929–1961 (2018).
Article CAS Google Scholar
Anastas, P. T. & Zimmerman, J. B. Design through the 12 principles of green engineering. Environ. Sci. Technol. 37 , 94A–101A (2003).
Zimmerman, J. B., Anastas, P. T., Erythropel, H. C. & Leitner, W. Designing for a green chemistry future. Science 367 , 397–400 (2020).
Delegates of the First National People of Color Environmental Leadership Summit. The Principles of Environmental Justice. First National People of Color Environmental Leadership Summit (United Christ of Church, New York, 1991).
Commission for Racial Justice. Toxic Wastes and Race in the United States: A National Report on the Racial and Socio-Economic Characteristics of Communities with Hazardous Waste Sites (United Christ of Church, New York, 1987).
Bullard, R. D. Solid waste sites and the black Houston community. Sociol. Inq. 53 , 273–288 (1983).
Anastas, P. T. & Zimmerman, J. B. The molecular basis of sustainability. Chem 1 , 10–12 (2016).
Newman, M. K. et al. New Perspectives on Environmental Justice: Gender, Sexuality, and Activism (Rutgers Univ. Press, 2004).
Pellow, D. Toward a critical environmental justice studies: black lives matter as an environmental justice challenge. Du Bois Rev. 13 , 221–236 (2016).
Al-Kohlani, S. A. & Campbell, H. E. Extending environmental justice research to religious minorities. Rev. Policy Res. 39 , 90–112 (2022).
Dombey, M. How governments are systematically poisoning indigenous communities and the U.N.’s role. Univ. Miami Int. Comp. Law Rev. Environ. Racism 27 , 131–154 (2020).
Jacobs, B. Environmental Racism on Indigenous Lands and Territories (Canadian Political Science Association, 2010).
Goldsmith, L. & Bell, M. L. Queering environmental justice: unequal environmental health burden on the LGBTQ+ Community. Am. J. Public Health 112 , 79–87 (2021).
Pellow, D. & Vazin, J. The intersection of race, immigration status, and environmental justice. Sustainability 11 , 3942 (2019).
Rockström, J. et al. Planetary boundaries: exploring the safe operating space for humanity. Ecol. Soc. 14 , 32 (2009).
Millennium Ecosystem Assessment. Ecosystems and Human Well-being: Synthesis (Island Press, 2005).
Raworth, K. A Safe and Just Space for Humanity: Can We Live Within the Doughnut? Discussion Paper (Oxfam International, 2012).
Taylor, D. E. The rise of the environmental justice paradigm: injustice framing and the social construction of environmental discourses. Am. Behav. Sci. 43 , 508–580 (2000).
The Routledge Handbook of Environmental Justice (Routledge, 2018).
Anastas, P. T. & Zimmerman, J. B. The periodic table of the elements of green and sustainable chemistry. Green Chem. 21 , 6545–6566 (2019).
Anastas, P. T. & Zimmerman, J. B. The United Nations sustainability goals: how can sustainable chemistry contribute? Curr. Opin. Green Sustain. Chem. 13 , 150–153 (2018).
Keijer, T., Bakker, V. & Slootweg, J. C. Circular chemistry to enable a circular economy. Nat. Chem. 11 , 190–195 (2019).
Kümmerer, K., Clark, J. H. & Zuin, V. G. Rethinking chemistry for a circular economy. Science 367 , 369–370 (2020).
Rittel, H. W. J. & Webber, M. M. Dilemmas in a general theory of planning. Policy Sci. 4 , 155–169 (1973).
Ritchey, T. in Wicked Problems – Social Messes: Decision Support Modelling with Morphological Analysis (ed. Ritchey, T.) 19–29 (Springer, 2011).
Meadows, D. H. in Thinking in Systems Ch. 6 (ed. Wright, D.) 145–165 (Chelsea Green Publishing, 2008).
Abson, D. J. et al. Leverage points for sustainability transformation. Ambio 46 , 30–39 (2017).
Aubrecht, K. B., Bourgeois, M., Brush, E. J., MacKellar, J. & Wissinger, J. E. Integrating green chemistry in the curriculum: building student skills in systems thinking, safety, and sustainability. J. Chem. Educ. 96 , 2872–2880 (2019).
Lasker, G. A. & Brush, E. J. Integrating social and environmental justice into the chemistry classroom: a chemist’s toolbox. Green Chem. Lett. Rev. 12 , 168–177 (2019).
Lasker, G. A., Mellor, K. E., Mullins, M. L., Nesmith, S. M. & Simcox, N. J. Social and environmental justice in the chemistry classroom. J. Chem. Educ. 94 , 983–987 (2017).
Matlin, S. A., Mehta, G., Hopf, H. & Krief, A. One-world chemistry and systems thinking. Nat. Chem. 8 , 393–398 (2016).
Kümmerer, K. Sustainable chemistry: a future guiding principle. Angew. Chem. Int. Ed. 56 , 16420–16421 (2017).
Mahaffy, P. G., Matlin, S. A., Holme, T. A. & MacKellar, J. Systems thinking for education about the molecular basis of sustainability. Nat. Sustain. 2 , 362–370 (2019).
Mahaffy, P. G., Ho, F. M., Haack, J. A. & Brush, E. J. Can chemistry be a central science without systems thinking? J. Chem. Educ. 96 , 2679–2681 (2019).
Matlin, S. A., Krief, A., Hopf, H. & Mehta, G. Re-imagining priorities for chemistry: a central science for ‘freedom from fear and want’. Angew. Chem. Int. Ed. 60 , 25610–25623 (2021).
Holifield, R., Porter, M. & Walker, G. Spaces of environmental justice: frameworks for critical engagement. Antipode 41 , 591–612 (2009).
Walker, G. Environmental Justice: Concepts , Evidence and Politics (Routledge, 2012).
Pasgaard, M. & Dawson, N. Looking beyond justice as universal basic needs is essential to progress towards ‘safe and just operating spaces’. Earth Syst. Gov. 2 , 100030 (2019).
Orum, P., Moore, R., Roberts, M. & Sanchez, J. Who’s In Danger? Race, Poverty and Chemical Disasters: A Demographic Analysis of Chemical Disaster Vulnerability Zones (Environmental Justice and Health Alliance for Chemical Policy Reform, 2014).
Lerner, S. Sacrifice Zones: the Front Lines of Toxic Chemical Exposure in the United States (MIT Press, 2010).
Siting of Hazardous Waste Landfills and Their Correlation With Racial and Economic Status of Surrounding Communities (US Government Accountability Office, 1983).
Banzhaf, H. S., Ma, L. & Timmins, C. Environmental justice: establishing causal relationships. Annu. Rev. Resour. Econ. 11 , 377–398 (2019).
Mohai, P. & Saha, R. Which came first, people or pollution? Assessing the disparate siting and post-siting demographic change hypotheses of environmental injustice. Environ. Res. Lett. 10 , 115008 (2015).
James, W., Jia, C. & Kedia, S. Uneven magnitude of disparities in cancer risks from air toxics. Int. J. Environ. Res. Public Health 9 , 4365–4385 (2012).
Johnston, J. & Cushing, L. Chemical exposures, health, and environmental justice in communities living on the fenceline of industry. Curr. Environ. Health Rep. 7 , 48–57 (2020).
Wright, B. H., Bryant, P. & Bullard, R. D. in Unequal Protection: Environmental Justice and Communities of Color (ed. Bullard, R. D.) 110–129 (Sierra Club Books, 1994).
Terrell, K. A. & St Julien, G. Air pollution is linked to higher cancer rates among black or impoverished communities in Louisiana. Environ. Res. Lett. 17 , 14033 (2022).
Landrigan, P. J., Rauh, V. A. & Galvez, M. P. Environmental justice and the health of children. Mt Sinai J. Med. 77 , 178–187 (2010).
Banzhaf, S., Ma, L. & Timmins, C. Environmental justice: the economics of race, place, and pollution. J. Econ. Perspect. 33 , 185–208 (2019).
Elliott, M. R., Wang, Y., Lowe, R. A. & Kleindorfer, P. R. Environmental justice: frequency and severity of US chemical industry accidents and the socioeconomic status of surrounding communities. J. Epidemiol. Community Health 4 , 24–30 (2004).
Friedman-Jiménez, G. Achieving environmental justice: the role of occupational health. Fordham Urban Law J. 21 , 605–632 (1994).
Office of Emergency Management & Office of Land and Emergency Management. Accidental Release Prevention Requirements: Risk Management Programs Under the Clean Air Act, Section 112(r)(7) (US Environmental Protection Agency, 2017); https://www.regulations.gov/document/EPA-HQ-OEM-2015-0725-0734
Chemical Releases Caused By Natural Hazard Events and Disasters - Information for Public Health Authorities (WHO, 2018).
Jafry, T., Helwig, K. & Mikulewicz, M. The Routledge Handbook of Climate Justice (Routledge, 2019).
Wright, B. H. in Race and the Incidence of Environmental Hazards (eds Bryant, B. & Mohai, P.) 114–125 (Routledge, 1992).
Calvert, G. M. et al. Acute pesticide poisoning among agricultural workers in the United States, 1998–2005. Am. J. Ind. Med. 51 , 883–898 (2008).
Moyce, S. C. & Schenker, M. Migrant workers and their occupational health and safety. Annu. Rev. Public Health 39 , 351–365 (2018).
Castillo, F. et al. Environmental health threats to Latino migrant farmworkers. Annu. Rev. Public Health 42 , 257–276 (2021).
Occupational Exposures in Petroleum Refining; Crude Oil and Major Petroleum Fuels (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 1989); https://www.ncbi.nlm.nih.gov/books/NBK531272/
Minority and Female Employment in the Oil & Gas and Petrochemical Industries (IHS Global, 2014).
Bertazzi, P. A., Pesatori, A. C., Zocchetti, C. & Latocca, R. Mortality study of cancer risk among oil refinery workers. Int. Arch. Occup. Environ. Health 61 , 261–270 (1989).
Xin, F., Susiarjo, M. & Bartolomei, M. S. Multigenerational and transgenerational effects of endocrine disrupting chemicals: a role for altered epigenetic regulation? Semin. Cell Dev. Biol. 43 , 66–75 (2015).
Rothstein, M. A., Harrell, H. L. & Marchant, G. E. Transgenerational epigenetics and environmental justice. Environ. Epigenet. 3 , dvx011 (2017).
Lane, M. K. M. et al. What to expect when expecting in lab: a review of unique risks and resources for pregnant researchers in the chemical laboratory. Chem. Res. Toxicol. 35 , 163–198 (2022).
Martin, E. M. & Fry, R. C. Environmental influences on the epigenome: exposure- associated DNA methylation in human populations. Annu. Rev. Public Health 39 , 309–333 (2018).
Reichert, A. K. Impact of a toxic waste superfund site on property values. Appraisal J. LXV , 381–392 (1997).
Currie, J., Davis, L., Greenstone, M. & Walker, R. Environmental health risks and housing values: evidence from 1,600 toxic plant openings and closings. Am. Econ. Rev. 105 , 678–709 (2015).
Messer, K. D., Schulze, W. D., Hackett, K. F., Cameron, T. A. & Mcclelland, G. H. Can stigma explain large property value losses? The psychology and economics of Superfund. Environ. Resour. Econ. 33 , 299–324 (2006).
Boehm, T. P. & Schlottmann, A. M. Housing and Wealth Accumulation: Intergenerational Impacts (Joint Center for Housing Studies of Harvard University, 2001).
Anguelovski, I. in Neighborhood as Refuge: Community Reconstruction, Place Remaking, and Environmental Justice in the City (ed. Gottlieb, R.) 29–54 (MIT Press, 2014).
LaDuke, W. All Our Relations: Native Struggles for Land and Life (South End Press, 1999).
Lewis, J., Hoover, J. & MacKenzie, D. Mining and environmental health disparities in native american communities. Curr. Environ. Health Rep. 4 , 130–141 (2017).
Leonard, L. G. III Sovereignty, self-determination, and environmental justice in the Mescalero Apache’s decision to store nuclear waste. Boston Coll. Environ. Aff. Law Rev. 24 , 651–693 (1996).
Martinez-Alier, J. Mapping ecological distribution conflicts: the EJAtlas. Extr. Ind. Soc. 8 , 100883 (2021).
Castleman, B. The export of hazardous industries in 2015. Environ. Health 15 , 8 (2016).
Bogdal, C. & Scheringer, M. Climate Change and POPs: Predicting the Impacts (UNEP/AMAP, 2011).
Pellow, D. N. Resisting Global Toxics: Transnational Movements for Environmental Justice (MIT Press, 2007).
Friedman, R. S. et al. How just and just how? A systematic review of social equity in conservation research. Environ. Res. Lett. 13 , 53001 (2018).
McDermott, M., Mahanty, S. & Schreckenberg, K. Examining equity: a multidimensional framework for assessing equity in payments for ecosystem services. Environ. Sci. Policy 33 , 416–427 (2013).
Pascual, U., Muradian, R., Rodríguez, L. C. & Duraiappah, A. Exploring the links between equity and efficiency in payments for environmental services: a conceptual approach. Ecol. Econ. 69 , 1237–1244 (2010).
Schulte, P. A. et al. Occupational safety and health, green chemistry, and sustainability: a review of areas of convergence. Environ. Health 12 , 31 (2013).
Anastas, P. T. & Hammond, D. G. Inherent Safety at Chemical Sites: Reducing Vulnerability to Accidents and Terrorism Through Green Chemistry (Elsevier, 2015).
Krings, A. & Thomas, H. in The Routledge Handbook of Green Social Work Ch. 32 (ed. Dominelli, L.) (Routledge, 2018).
Zimmerman, J. B. & Anastas, P. T. When is waste not a waste? Sustain. Sci. Eng. 1 , 201–221 (2006).
Boodhoo, K. & Harvey, A. Process Intensification Technologies for Green Chemistry: Engineering Solutions for Sustainable Chemical Processing (Wiley, 2013).
Costanza, R. et al. The value of the world’s ecosystem services and natural capital. Nature 387 , 253–260 (1997).
Ecosystems and Human Well-Being: Synthesis (Millennium Ecosystem Assessment Panel, 2005); https://www.millenniumassessment.org/documents/document.356.aspx.pdf
Mihelcic, J. R. et al. Sustainability science and engineering: the emergence of a new metadiscipline. Environ. Sci. Technol. 37 , 5314–5324 (2003).
United States Environmental Protection Agency. Environmental Justice Timeline. EPA (accessed 9 Jan 2023); https://www.epa.gov/environmentaljustice/environmental-justice-timeline
Download references
Author information
These authors contributed equally: Mary Kate M. Lane, Holly E. Rudel, Paul T. Anastas, Julie B. Zimmerman.
Authors and Affiliations
Department of Chemical and Environmental Engineering, Yale University, New Haven, CT, USA
Mary Kate M. Lane, Holly E. Rudel, Hanno C. Erythropel, Andreas Backhaus, Momoko Ishii, Cheldina F. Jean, Tong Wang & Julie B. Zimmerman
Center for Green Chemistry and Green Engineering, Yale University, New Haven, CT, USA
Mary Kate M. Lane, Holly E. Rudel, Jaye A. Wilson, Hanno C. Erythropel, Andreas Backhaus, Elise B. Gilcher, Momoko Ishii, Cheldina F. Jean, Fang Lin, Tobias D. Muellers, Tong Wang, Paul T. Anastas & Julie B. Zimmerman
School of the Environment, Yale University, New Haven, CT, USA
Jaye A. Wilson, Hanno C. Erythropel, Elise B. Gilcher, Momoko Ishii, Tobias D. Muellers, Gerald Torres, Dorceta E. Taylor, Paul T. Anastas & Julie B. Zimmerman
Department of Chemistry, Yale University, New Haven, CT, USA
Law School, Yale University, New Haven, CT, USA
Gerald Torres
School of Public Health, Yale University, New Haven, CT, USA
Paul T. Anastas
You can also search for this author in PubMed Google Scholar
Contributions
M.K.M.L. and H.E.R. contributed equally to this work as co-first authors. P.T.A. and J.B.Z. contributed equally to this work as co-last authors. M.K.M.L., H.E.R. and J.B.Z. wrote the first draft and M.K.M.L., H.E.R., J.A.W., H.C.E., A.B., E.B.G., M.I., C.F.J., F.L., T.D.M., T.W., G.T., D.E.T., P.T.A. and J.B.Z. contributed subsequently to its refinement, editing and critical revision. M.K.M.L., A.B., P.T.A. and J.B.Z. were responsible for visualization. M.K.M.L., H.E.R., H.C.E., P.T.A. and J.B.Z. supervised the writing effort. M.K.M.L., H.E.R., J.A.W., H.C.E., A.B., E.B.G., M.I., C.F.J., F.L., T.D.M., T.W., G.T., D.E.T., P.T.A. and J.B.Z. approved the final manuscript.
Corresponding author
Correspondence to Julie B. Zimmerman .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Sustainability thanks Jennifer Burney, Grace A. Lasker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Lane, M.K.M., Rudel, H.E., Wilson, J.A. et al. Green chemistry as just chemistry. Nat Sustain 6 , 502–512 (2023). https://doi.org/10.1038/s41893-022-01050-z
Download citation
Received : 16 June 2022
Accepted : 08 December 2022
Published : 30 January 2023
Issue Date : May 2023
DOI : https://doi.org/10.1038/s41893-022-01050-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Boosting the zinc storage performance of vanadium dioxide by integrated morphology engineering and carbon nanotube conductive networks.
- Xiaolin Wang
- Jianchao Sun
Nano Research (2024)
Electrocatalytic organic transformation reactions in green chemistry: Exploring nanocrystals and single atom catalysts
- Wenxing Chen
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Advertisement
Industrial applications of green chemistry: Status, Challenges and Prospects
- Review Paper
- Published: 23 January 2020
- Volume 2 , article number 263 , ( 2020 )
Cite this article

- Rajni Ratti 1
29k Accesses
49 Citations
1 Altmetric
Explore all metrics
Green Chemistry is expanding its wings from academic laboratories to industrial units. Sustainable practices include replacement of volatile organic solvents which constitute the bulk of a reaction material, developing recyclable catalysts, developing energy efficient synthesis and encouraging the use of renewable starting material. By following the principles of green chemistry, turn-over of many companies have increased immensely leading to both environmental as well as economic benefits. This review explores various examples wherein green chemistry has enhanced the sustainability factor of industrial processes immensely and suggests the measures which should be taken to promote as well as popularize the green practices in synthesis.
Similar content being viewed by others

Green Catalytic Transformations

Future Trends in Green Synthesis

Atom Economic Green Organic Reactions
Avoid common mistakes on your manuscript.

1 Introduction
The 20th century has seen a phenomenal growth of global economy and a continuous improvement of standard of living in the industrialized countries. The increasingly competitive economic outlook and the shrinking graph of natural resources on the planet pose an urgent need to reduce the energy expenditure as well the production of waste. Sustainability is one of the main drivers for innovations in order to allow the technical industries to work for the well-being of consumers in a safe and healthy environment. The most attractive concept towards achieving sustainability is “Green Chemistry”—a term coined at United States Environmental Protection Agency by Anastas and Warner [ 1 ], and is defined as the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products [ 2 , 3 , 4 ]. The term ‘hazardous’ is used in its broadest context which includes physical (e.g. explosive, flammable), toxicological (e.g. carcinogenic, mutagenic) and global (e.g. ozone depletion, climate change) factors. The tools of green chemistry are alternative feedstock, solvents and reagents, and catalytic versus stoichiometric processes. Developing green methodologies is a challenge that may be viewed through the framework of the “Twelve Principles of Green Chemistry” (Designed by P.T. Anastas and Warner).
2 The twelve guiding principles of green chemistry
Since its inception in early 1990s, green chemistry has grown into a significant, internationally engaged focus area within chemistry [ 5 , 6 , 7 , 8 ]. Green chemistry is basically a proactive approach aimed at designing a synthesis/process in a sustainable way right from the beginning. Preventing waste formation rather than devising methods to cleaning it up, developing atom efficient technologies based on renewable feedstock using minimum energy requirements and inherently safer chemicals, discouraging the use of volatile organic solvents and replacing them by greener alternatives are the main aims of green chemistry. To develop sustainable processes stoichiometric reagents should be replaced by catalytic reagents, end products should be bio-degradable and analytical methodologies should allow real time in-process monitoring. It is not always possible to incorporate all the principles in a particular process, however efforts should be made to follow as many principles as possible.
3 Industrial applications of green chemistry
Green Chemistry is not a lab-curiosity; instead it aims at big objective of creating a sustainable tomorrow. Increasing number of green methodologies developed by academic and industrial researchers enables companies to commercialize these ideas. Industry, from small businesses to large corporations, has already made strategic moves towards sustainability by adopting the principles of green chemistry. The development of less hazardous processes and commercial products, the shift from inefficient chemical routes towards bio-based synthesis, and the replacement of oil-based feed stocks by renewable starting materials are only a few examples of the major decisions taken that will ultimately have vast consequences for the world chemical markets.
As per the analysis of Environmental Protection Agency, the US drug industry has decreased the use of VOCs by 50% between 2004 and 2013 by adopting principles of green chemistry. In the same time span, the amount of chemical waste released to air, land and water decreased by 7% as per Toxics Release Inventory (TRI) of EPA.
Recently four industrial drug units located in Hyderabad region of India has been closed on account of creating pollution [ 9 ]. China, too, has strict environmental concerns and has taken regulatory action on 40% of the industrial units located in thirty provinces [ 10 ]. These changes in policy suggest that it has become imperative to follow green practices.
Plastics, in spite of several uses, have a bad reputation owing to their origin from polymers derived from non-renewable petrochemicals and their non-bio-degradable nature. However, the same can be made from renewable feedstock as shown by a study carried out by Utrecht University [ 11 ]. Studies by Utrecht University also show that the market of bio-plastics will grow by approximately 37% per year till 2013 and at a rate of 6% between 2013 and 2020. Many marketing hubs have joined the initiative to replace plastics with bio-plastics. Wal-Mart has been using bio-plastics in packaging wherever possible [ 12 ]. On similar lines Nokia, a mobile making company, used 50% bio-plastics in Nokia 3111 Evolve phone cover as well as in Nokia C7 phone [ 13 ].
Procter & Gamble replaced most of the PVC based materials with greener alternatives [ 14 ]. Along with other companies P&G have taken the initiative to develop new solvents so as to replace volatile organic carbons in glossy paints.
Greener synthesis of Ibuprofen launched by BASF involves half the number of steps as compared to traditional method. Atom efficiency of new process is almost double than the old synthesis. In pursuit for the development of sustainable methodologies, BASF developed BASIL™ (Bi-phasic Acid scavenging utilizing ionic liquids) process involving the production of generic photo initiator precursor alkoxyphenylphosphine [ 15 ]. Using this technology the yield increased from 50 to 98%.
The Warner Babcock institute for Green Chemistry has developed a green hair-dye “Hairprint” which is a non-toxic, vegetable based product providing an alternative to the toxic, skin irritating and carcinogenic dyes [ 16 ].
USA based Merck & Co., Inc. has successfully applied the principles of green chemistry to the synthesis of antiviral drug (cytomegalovirus infection) Letermovir which is currently in phase III of clinical trials. Cytomegalovirus (CMV) is a common virus whose infections are generally asymptomatic in healthy individuals but can cause severe damage in patients with immuno-depressed systems. The importance of this drug can be judged from the fact that it has been granted Fast-Track status by FDA and Orphan product designation by European Medicine agency for the prevention of CMV viremia in high risk population.
An evaluation of its traditional synthesis scheme revealed several areas for improvements like a very low overall yield of 10% due to a late stage resolution to access a stereogenic center, the use of nine different solvents, high palladium loading in Heck coupling. Moreover, no recycling of solvents and reagents had been there in the scheme.
Greener synthesis, as published by Merck, involves a novel cinchonidine based PTC-catalyzed Aza-Michael reaction for configuring the single stereocenter as shown in Scheme 1 [ 17 ]. Also, there is an increase in overall yield by 60%, reduction in raw material cost by 93% and reduction in water usage by 90%. It has been estimated that, once operational, this optimized process will lead to reduction of more than 15,000 MT of waste over the life time of Letermovir. Life-Cycle Assessment reveals that the green process is expected to decrease the carbon foot-print by 89%. It is quite evident from the green synthesis of Letermovir that the Green Chemistry is not only environmentally friendly but also economically lucrative. This scheme has won the EPA’s Presidential green chemistry award under the category “Greener synthetic pathways” in 2017 [ 18 ].
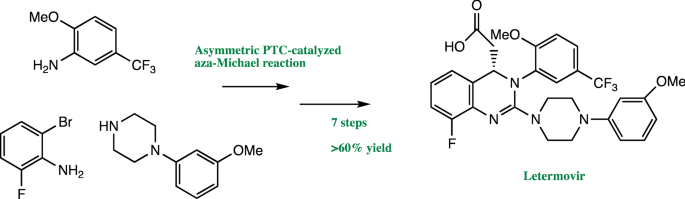
Green synthesis of Letermovir
Of the various technologies used in green chemistry, biocatalysis holds an important place [ 19 ]. Most of the reactions occurring in physiological systems are catalyzed by enzymes which are nature’s catalysts. Enzymes are not only biodegradable but are renewable as well due to the ease of production by fermentation of sugar etc. In order to achieve the aims of sustainability, more and more companies are working in the area of designing and using enzymes as biocatalysts. An impressive case highlighting the impact of biocatalysis on pharmaceutical manufacturing is the greener synthesis of Pregabalin, an active ingredient of neuropathic pain reliever Lyrica ® . In 2008, Pfizer improved the classical route for the synthesis of Pregabalin by adopting biocatalysis as a key step which led to 90% reduction in solvent usage, 50% reduction in the requirement of raw materials besides energy savings [ 20 ]. Solvent and energy saving in the process is equivalent to reducing 3 million tons of CO 2 emissions which is actually equivalent to taking 1 million Indian cars off the road for a year. Schemes 2 and 3 compare the classical and greener route for the synthesis of pregabalin.
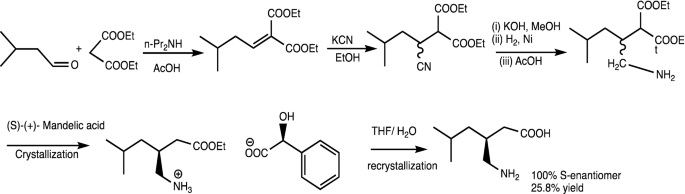
Conventional synthesis of Pregabalin
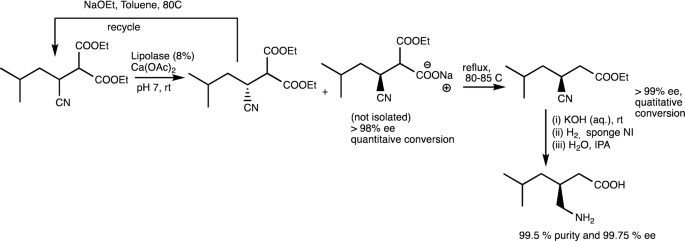
Enzyme catalyzed synthesis of Pregabalin
Not only in drug synthesis, biocatalysts also find important applications in the synthesis of plastics. Now a days, research is mainly targeted towards the synthesis of biodegradable plastics from renewable resources.
California based start-up “Newlight Technologies”, founded in 2003, took a funding of $9.2 million for developing a carbon negative technology that combines air with methane emissions to produce Aircarbon™ a thermoplastic. Aircarbon™ is approximately 40% oxygen from air and 60% carbon and hydrogen from methane emissions. The technology itself was not new but the use of a proprietary biocatalyst by Newlight Technologies made it actually commercially viable by increasing the yield nine times and decreasing the cost by a factor of three thereby making Aircarbon cheaper than oil based plastics. With the commercial scale-up in 2013, Aircarbon™ was adopted by a number of leading brands like Dell, Hewlett-Packard, IKEA, Sprint, The Body Shop and Vinmar for manufacturing their respective products. In recognition of the company’s commercial achievements, Newlight was named “Most innovative company of the year” in 2013 and Aircarbon™ was named “Tech innovation of the year” by The American Business Awards [ 21 ]. For the green attributes of the process involving capturing and using greenhouse gases, Newlight technologies has been awarded the prestigious EPA’s Presidential Green Chemistry Challenge award in 2016 [ 22 ].
Most chemical processes involve solvents in the reaction and separation step to dissolve solids, reduce viscosity, modulate temperature, and recover products by means of extraction or recrystallization as reaction media or for cleaning purposes. Solvents not only dissolve the reactants but they also affect the rates, chemo-, regio- and stereoselectivities of reaction. However, majority of the organic solvents used in industry, despite their inherent advantages, are associated with several ill-effects on human health and environment. Moreover, these solvents are derived from non-renewable resources like petroleum. These parameters are in contradiction to the very basics of Green Chemistry. Due to these reasons, the only alternative available is to substitute these environmentally harmful solvents with some benign solvents. Hungerbuhler et al. [ 23 ] discussed the following four directions towards the development of green solvents
Substitution of hazardous solvents with one that show better EHS (Environment, Health, Safety) properties such as increased biodegradability or reduced ozone depletion potential [ 24 ].
Use of “bio-solvents” i.e. solvents produced from renewable resources such as ethanol produced by fermentation of sugar-containing feeds, starchy feed materials or lignocellulosic materials [ 25 ].
Substitution of organic solvents with supercritical CO 2 in polymer processing avoids the use of chlorofluorocarbons, and reduces the ozone depletion [ 26 ].
With ionic liquids that show low or negligible vapour pressure, and thus fewer emissions to air [ 27 ].
Fabric dyeing consumes a lot of water. About 7 gallons of water is used up to dye a T-shirt and lot of energy is wasted in drying the dyed material. A Dutch start-up recently launched water-free dyeing using supercritical carbon dioxide as a solvent under pressure and at elevated temperature. As no water is used so energy required in drying is also saved [ 28 ].
Elevance Renewable Sciences, Inc., used a nobel prize winning metathesis technology developed by Grubb’s to produce two green solvents
In collaboration with the surfactant manufacturer Stepan, Elevance produced a surfactant called STEPOSOL MET-10U as a replacement for N-methyl pyrrolidone and dichloromethane in adhesive removers and paint strippers. This surfactant can also be used in household and industrial cleaners in place of glycol ethers. STEPOSOL MET-10U is a unique unsaturated di-substituted amide derived from a bio-based feedstock [ 29 ]. With a Kauri-Butanol value greater than 1000, STEPOSOL MET-10U provides superior cleaning performance and is environmentally friendly due to a low vapor pressure, high boiling point, and Biorenewable Carbon Index (BCI) of 75%.
Another heavy-duty green degreasing solvent developed by Elevance Renewable Sciences is Elevance Clean™ 1200 which is a VOC free bio-based solvent [ 30 ]. In 2015, Elevance Clean™ 1200 was awarded bio-based product innovation of the year at WBM bio business awards for its out-standing cleaning performance. Being produced from natural oils this non-flammable solvent meets the various restrictive environmental regulations. Therefore, Elevance Clean™ 1200 is
VOC exempt (Directive 2004/42/CE of the European Parliament and the Council)
REACH registered
Readily biodegradable (by OCED method)
Free of components listed in the EU dangerous substances directive (Regulation No. 1272/2008).
The various advantages of Elevance Clean™ 1200 are enlisted below
Strong solvency characteristics greater than even of d-limonene, dibasic esters, vegetable esters and isoparaffins on the Kauri butanol (Kb) scale.
Excellent performance across a broad range of cleaning applications which includes metal cleaning, industrial and institutional degreasing, transportation and food processing.
Being non-flammable, it is easy to handle. It works very well in the neutral pH range (6–9) thereby eliminating the need of caustic cleaning products.
In 2014, Solberg Company won the first Insight Innovation award at the 3 rd annual THINC for its environmentally-friendly fire-fighting foam concentrate RE-HEALING. Conventional firefighting foams use fluorinated surfactants which are hazardous for the environment. The RE-HEALING firefighting foam concentrate use a blend of non-fluorinated surfactants, sugars, solvent and corrosion inhibitor leading to far less environmental impacts. Control, extinguishing time, and re-ignition resistance are necessary for the safety of fire-fighters and RE-HEALING fulfills all these conditions. The company also won the 2014 EPA Presidential Green Chemistry award for this innovation. [ 31 ].
Using catalytic reagents over stoichiometric reagents is one of the principles of green chemistry. Developing recyclable and recoverable catalyst adds to the green profile of a technology. Exhausts from the automobile engines pose a major threat to the environment. Inside the engine, temperature being very high, oxygen and nitrogen react to form nitric oxide (NO). Conversion of NO to NO 2 is highly desirable for the removal of oxides of nitrogen. However, this reaction is, in general, quite slow. A team of scientists from U.S, China and South Korea developed the catalyst using Mn-Mullite (Sm, Gd) Mn 2 O 5 –manganese–mullite materials containing either Samarium or Gadolinium to convert the toxic diesel engine exhaust product nitric oxide to a more benign nitrous oxide [ 32 ]. Over a range of temperatures, the new catalyst performed better than platinum (around 64% better at 300 °C and 45% better at 120 °C).
RCHEM Pvt. Ltd. Hyderabad, in collaboration with Chaudhuri et al. [ 33 ] developed a green synthesis of anti-ulcer drug Ranitidine. The conventional synthesis generates dimethylsulfide which is a hazardous to human health. Prof. Chaudhuri, from IIT Guwahati, and Prof. Kantam from IICT Hyderabad developed vanadium-titanium and titanium phosphorous based solid supported catalysts. In the presence of these heterogeneous catalysts H 2 O 2 acts as an oxidant to convert the dimethylsulfide to colourless odourless liquid dimethyl sulfoxide (DMSO). The DMSO generated is further used in the manufacturing process of drug thereby reducing the cost of production by 20%.
There are numerous applications where green chemistry has marched beyond the research laboratories and finding commercial applications. However, a lot more efforts are required, particularly in the area of life-cycle analysis so as to evaluate the environmental impact of the various “green” drugs after these traverses the human physiological system. Terry Collins, from the University of Pittsburg, developed a series of tetra-amido macrocyclic ligand based catalysts modelled on peroxidase enzymes [ 34 ]. Collins proposed that addition of these at a late stage in the sewage treatment process could help break down a wide variety of chemical residues from the drugs before they can affect the environment.
4 Challenges
Just being green is not enough for a process to be a commercial success. Regulatory, economic, political and technical challenges often impede the industrial implementation of a green process.
Current regulations are focused on reducing risk through reductions in exposure while green chemistry promotes the reduction of inherent risk by reduction of hazard. In U.S, the regulations require that every time a manufacturer changes the production process, it has to undergo a re-certification process with the FDA. This process is both costly and time- consuming, and hence serves to dissuade firms that would otherwise invest in developing atom efficient chemistries that reduce waste. Changes to more benign processes are inhibited by cost-intensive, control-oriented regulation. Lack of awareness among the different stake-holder groups poses a barrier to the implementation of green processes. Developing a successful green process is not only about green chemistry, it involves the knowledge of green engineering, biotechnology, economics and above all toxicology. The chemists generally lack the training in these disciplines which further hampers the implementation of green chemistry on an industrial scale. Even if all factors are the in the favour of a green process, it can be rejected on a commercial-scale if it fails to be economically attractive. Green industrial processes should be comparable to the traditional processes in terms of costs of the products.
There are a number of examples of technically robust, environmentally-friendly processes that have been started at first but were withdrawn at a later stage due to commercial implications. It does not always pay to be green in the chemicals sector. Thomas Swan and Company in Consett, UK, implemented the work of Martyn Poliakoff (Nottingham University), to start world’s first continuous-flow reactor using supercritical carbon dioxide as a solvent [ 35 ]. Sc-CO 2 system lead to selective hydrogenation of isophorone to 3,3,5-trimethylcyclohexanone without any by-product formation. This lead to elimination of an expensive and energy-intensive separation required by the conventional technique. But due to the lack of government subsidies, the plant could not provide chemicals more cheaply than those made by the traditional non-green methods. Therefore, after commercially running from 2002 to 2009, this plant was taken out of production.
Similar things happen with the process involving isomerization of 3,4-epoxybut-1-ene to 2,5-dihydrofuran in a phosphonium iodide ionic liquid developed by Eastman Chemical Company.
Capital investment also prevents the commercialization of a green technology. IFP (France) used ionic liquids, as solvent as well as co-catalyst, on a large scale for the nickel catalyzed dimerization of alkenes, named as Difasol process [ 36 ]. This is a biphasic process wherein the product forms a separate layer above the ionic liquid layer and thus can be easily separated. Compared to the conventional Dimersol process, this method has many advantages like better catalytic activity, ease of separation of product, better dimer selectivity and higher reactor space time yields. However, the cost of capital equipment posed a hurdle towards its commercial implementation.
The commercialization of green processes also requires many changes in all part of the long and global supply chain. Eden Organic foods developed a BPA-free coating for food packaging which was found to be compatible with some foods like beans but not for highly acidic tomato sauce. Switching to different coating type for different food type implies a smaller market size and change in manufacturing machines and consequently a higher cost. The implementation of such initiatives requires that everyone in the value chain agrees and is willing to accept the changes.
As most of the industries have been driven by monetary profits therefore voluntary adoption of the sustainable practices seems less feasible. A strong, attractive and balanced regulation is required so as to enforce the greener practices. The most promising and significant regulation is the REACH (Registration, Evaluation, Authorization and Restriction of Chemical substances) regulation framed and launched by European Union in 2007 [ 37 ]. On one hand REACH makes it mandatory for the chemical companies to disclose more information on the environmental and health risks of their products; on the other hand it grants potential exemptions on registration for five years for a process which favours new sustainable innovation. This move of European Union has motivated other countries to devise similar regulations so as to create a sustainable chemical industry.
5 Prospects
Green chemistry holds the key to a sustainable society. It has the inherent potential to bridge the gap between society and science. Innovations, backed by sound policies and regulations, will accelerate the large-scale implementation of green processes. Next generation of chemists should be taught the basics of green chemistry at a very early stage so that they can think green and develop safer methodologies. Interdisciplinary and multidisciplinary research can help in solving the various technical hurdles for commercializing this philosophy. Subsidizing the greener initiatives and tax exemptions to the companies adopting green processes will have a positive impact. Industries should realize the fact that getting a new greener process registered and making capital investment is a one-time investment which can have positive impacts on various aspects of society and environment. Collective and sincere efforts by researchers, engineers, corporates and policy- makers can actually make the chemistry Green.
Anastas PT, Warner JC (1998) Green chemistry: theory and practice. Oxford University Press, Oxford
Google Scholar
Anastas PT, Williamson TC (1998) Green chemistry: frontiers in benign chemical syntheses and processes. Oxford University Press, Oxford
Kidwai MM (2006) Pure Appl Chem 78:1983–1992
Article Google Scholar
Kirchhoff M (2005) Resour Conserv Recycl 44:237–243
Clark JH (2006) Green Chem 8:17–21
Manley JB, Anastas PT, Cue BW Jr. (2008) J Clean Prod 16:743–750
Keith LH, Gron LU, Young JL (2007) Chem Rev 107:2695–2708
Mason BP, Price KE, Steinbacher JL, Bogdan AR, McQuade DT (2007) Chem Rev 107:2300–2318
Reddy U (2018) PCB cracks down, shuts four polluting industries. The Times of India. http://www.pharmabiz.com/NewsDetails.aspx?aid=116312&sid=1
Mullin R (2018) Drug chemical makers brace as china cracks down on pollution. Chem Eng News 96:23–25
Shen L, Haufe J, Patel MK (2009) Product overview and market projection of emerging bio-based plastics. PRO-BIP 2009. In: Report commissioned by European Polysaccharide Network of Excellence and European Bioplastics. Copernicus Institute for Sustainable Development and Innovation, Utrecht University, Netherlands
https://www.walmartsustainabilityhub.com
https://www.go-green.ae/greenstory_view.php?storyid=1280
https://en-ae.pg.com/sustainability-reports/ Procter and Gamble sustainability report 2010
BASF SE (2003) Methods for the separation of acids from chemical reaction mixtures by means of ionic fluids. World Patent WO/2003/062251
https://www.warnerbabcock.com/scientific-discovery-reverses-gray-hair-natural-color-invented-warner-babcock-institute/
Humphrey GR, Dalby SM, Andreani T, Xiang B, Luzung MR, Song ZJ, Shelvin M, Christensen M, Belyk KM, Tschaen DM (2016) Org Process Res Dev 20:1097–1103
www.epa.gov/greenchemistry/green-chemistry-challenge-2017-greener-synthetic-pathways-award
Tao J, Xu J-H (2009) Curr Opin Chem Biol 13:43–50
Martinez CA, Hu S, Dumond Y, Tao J, Kelleher P, Tully L (2008) Org Process Res Dev 12:392–398
http://stevieawards.com/aba/newlight-technologies-company-year
https://www.epa.gov/greenchemistry/presidential-green-chemistry-challenge-2016-designing-greener-chemicals-and-specific
Capello C, Fischer U, Hungerbuhler K (2007) Green Chem 9:927–934
Gani R, Gonzalez CJ, Kate A, Crafts PA, Jones M, Powell L, Atherton JH, Cordiner JL (2006) Chem Eng 1:30–41
Savaiko B (2004) A promising future for ethanol. World Ethanol Biofuels Rep 2:20–22
Noyori R (1999) Chem Rev 99:353–354
Petkovic M, Seddon KR, Rebelo LPN, Pereira CS (2011) Chem Soc Rev 40:1383–1403
http://www.dyecoo.com/pdfs/colourist.pdf
http://www.stepan.com/uploadedFiles/Literature_and_Downloads/General_Lit/Household,_Institutional,_and_Industrial_Cleaning/STEPOSOLMET10UGreenChemistryGuide.pdf
G Hategan, C Shaner, R Littich, S Block, M Morie-Bebel (2015) H&PC today 10. https://elevance.com/product/elevance-clean-1200/?prod-id=109&cat-id=cleaning-17
https://www.epa.gov/greenchemistry/presidential-green-chemistry-challenge-2014-designing-greener-chemicals-award
Wang W, McCool G, Kapur N, Yuan G, Shan B, Nguyen M, Graham UM, Davis BH, Jacobs G, Cho K, Hao X (2012) Science 337:832–835
Bora U, Chaudhuri MK, Dehury SK (2002) Curr Sci 82:1427–1436
Ellis WC, Tran CT, Denardo MA, Fischer A, Ryabov AD, Colllins TJ (2004) J Am Chem Soc 6:43–48
Licence P, Ke J, Sokolova M, Ross SK, Poliakoff M (2003) Green Chem 5:99–104
Favre F, Forestiere A, Hugues F, Olivier-Bourbigou H, Chodorge JA (2005) Oil Gas Eur Mag 31:83–87
https://ec.europa.eu/environment/chemicals/reach/reach_en.htm
Download references
No funding has been received by the author for this work.
Author information
Authors and affiliations.
SGGS Khalsa Colllege, Mahilpur, Punjab, India
Rajni Ratti
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Rajni Ratti .
Ethics declarations
Conflict of interest.
The author declares that there is no conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Ratti, R. Industrial applications of green chemistry: Status, Challenges and Prospects. SN Appl. Sci. 2 , 263 (2020). https://doi.org/10.1007/s42452-020-2019-6
Download citation
Received : 13 July 2019
Accepted : 09 January 2020
Published : 23 January 2020
DOI : https://doi.org/10.1007/s42452-020-2019-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sustainable synthesis
- Recyclable catalysts
- Turn-over number
- Bio-catalysis
- Find a journal
- Publish with us
- Track your research
- You are here:
- American Chemical Society
- Green Chemistry
- Design Principles
- 12 Principles of Green Chemistry
- Green Chemistry Examples
- Green Chemistry History
- Green Chemistry News
- Design Principles Booklet
- 12 Principles of Green Engineering
- Sandestin Declaration: 9 Principles of Green Engineering
- Pharmaceutical Roundtable
- Oilfield Chemistry Roundtable
- Natural Polymers Consortium
- Why Join a Roundtable?
- Analytical Chemistry
- Biobased Chemicals
- Endangered Elements
- Green Chemistry and Engineering Metrics
- Process Engineering
- Rational Molecular Design for Reduced Toxicity
- Waste to Chemicals
- Research Tools
- Student Chapters
- Academic Programs
- Textbooks & Printed Resources
- Webinars and Videos
- Green Chemistry Learning Modules
- Green Chemistry Student Awards
- Advisory Board
- ACS GCI in the Press
Developed by Paul Anastas and John Warner in 1998*, the following list outlines a a framework for making a greener chemical, process, or product.
Click on the tabs to reveal articles about each principle. These articles were originally developed for The Nexus Blog .
It is better to prevent waste than to treat or clean up waste after it has been created.
Contributed by Berkeley W. Cue, Jr., Ph.D., BWC Pharma Consulting, LLC.
In their publication “ Green Chemistry, Theory and Practice ” in 1998, Anastas and Warner introduced their 12 principles. My view is the first principle, often referred to as the prevention principle, is the most important and the other principles are the “how to’s” to achieve it
An often-used measure of waste is the E-factor , described by Roger Sheldon, which relates the weight of waste coproduced to the weight of the desired product. More recently, the ACS Green Chemistry Institute Pharmaceutical Roundtable ( ACS GCIPR ) has favored process mass intensity , which expresses a ratio of the weights of all materials (water, organic solvents, raw materials, reagents, process aids) used to the weight of the active drug ingredient (API) produced. This is an important roundtable focus because of the historically large amount of waste coproduced during drug manufacturing—more than 100 kilos per kilo of API in many cases. However, when companies apply green chemistry principles to the design of the API process, dramatic reductions in waste are often achieved, sometimes as much as ten-fold. So, it is important to extend the impressive results achieved by the ACS GCIPR to all parts of the drug industry, especially the biopharma and generic sectors, as well as to other sectors of the chemical enterprise where synthetic chemistry is used to produce their products.
More Resources & Examples:
Process Mass Intensity Tool
2012 PGCCA Winner: Codexis, Inc. and Professor Yi Tang, University of California, Los Angeles “ An Efficient Biocatalytic Process to Manufacture Simvastatin ”
2002 PGCCA Winner: Pfizer, Inc. “ Green Chemistry in the Redesign of the Sertraline Process ”
Pharma Strives for Green Goals , Stephen K. Ritter, Chemical & Engineering News, 90(22), May 28, 2012.
Articles Cited:
The E Factor: fifteen years on ; R.A. Sheldon; Green Chem. 2007, (9), pp 1273-1283, DOI: 10.1039/B713736M
Using the Right Green Yardstick: Why Process Mass Intensity Is Used in the Pharmaceutical Industry to Drive More Sustainable Processes ; Concepcion Jimenez-Gonzalez, Celia S. Ponder, Quirinus B. Broxterman, and Julie B. Manley; Org. Process Res. Dev., 2011, 15 (4), pp 912–917, DOI: 10.1021/op200097d.
- Atom Economy
Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.
Contributed by Michael Cann, Ph.D., Professor of Chemistry, University of Scranton
The second principle of green chemistry can be simply stated as the “atom economy” of a reaction. Atom economy, which was developed by Barry Trost 1 , asks the question “what atoms of the reactants are incorporated into the final desired product(s) and what atoms are wasted?”
Traditionally, the efficiency of a reaction has been measured by calculating the percent yield. Let us assume that the following substitution reaction gives 100% yield. While this is admirable, we can shed more light on the efficiency of a reaction by calculating the “percent atom economy” as follows:
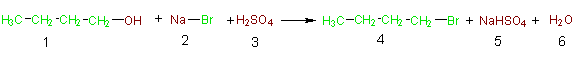
% Atom Economy = (FW of atoms utilized/FW of all reactants) X 100 = (137/275) X 100 = 50%
The percent atom economy is simply the formula weight of the desired product(s) (compound 4, 137 g/mol) divided by the sum of the formula weights of all the reactants (275 g/mol), which gives 50% in this case. Simply put, even if our percent yield is 100%, only half the mass of the reactants atoms are incorporated in the desired product while the other half is wasted in unwanted by-products. Imagine telling your mom you baked a cake and threw away half the ingredients! Thus chemists must not only strive to achieve maximum percent yield, but also design syntheses that maximize the incorporation of the atoms of the reactants into the desired product.
Principle #2 deals with the reactants. However, as those of us who have run a chemical reaction know, we usually use other materials such as solvents and separating agents during a synthesis. These materials usually make up the bulk of the material input, and thus we must also account for the waste that is produced from them. Stay “tuned” as you will see these discussed in subsequent Principles of Green Chemistry.
Atom Economy: A Measure of the Efficiency of a Reaction . Michael C. Cann and Marc E. Connelly; Real-World Cases in Green Chemistry; ACS, Washington, 2000.
1998 PGCCA Winner: Professor Barry M. Trost of Stanford University, " The Development of the Concept of Atom Economy ."
1. The Atom Economy-A Search for Synthetic Efficiency; Barry M. Trost; Science 1991, (254), pp 1471-1477.
- Less Hazardous Chemical Syntheses
Wherever practicable, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
Contributed by David J. C. Constable, Ph.D., Director, ACS Green Chemistry Institute ®
When you think about it, this is a two-part principle divided by the first two words, “wherever practicable.” Saying those two words implies that it may not be practical or possible to avoid using substances that are toxic, and this is, if you will, the get out of jail card most chemists use to try to avoid applying this principle to their work. Let’s face it; chemists use toxic substances all the time because reactive chemicals afford reactions that are kinetically and thermodynamically favorable. And unless—and until—replacement chemicals along with new synthetic protocols are developed, inherently toxic materials will continue to be used. But it’s easier to say that it isn’t practicable and dispense with any thought about the chemical choices that are made.
It’s not that adhering to this principle is particularly difficult to do; it’s more that chemists are disinterested in doing it. For the synthetic organic chemist, effecting a successful chemical transformation in a new way or with a new molecule or in a new order is what matters. I have heard such arguments, as “all the other stuff in the flask is just there to make the transformation possible so it really doesn’t matter,” or “you have to be realistic and focus on the science.” Saying these things implies that the only science that matters is activating a carbon atom to functionalize it, or adding a ligand to a catalyst, etc., etc. This principle is asking chemists to broaden their definition of what constitutes good science.
What many have shown over and over again is that toxicity and the attendant hazard and risk associated with a chemical reaction is directly related to all the other “stuff” in a flask. In fact, the chemistry or chemical transformation in a synthesis generally impacts the overall toxicity profile (and most other measures of sustainability and green) of a product or process the least, except in those cases where we deliberately are producing a molecule that is toxic or biologically active by design. That is certainly the case for many molecules that are synthesized as in the pharmaceutical or agriculture chemical business—the molecules are toxic and/or have other effects on living organisms by design.
The chemicals and materials used in effecting chemical transformations matter and chemists need to pay more attention to the choices they make about what goes into the flask. It’s easy to discount all the other “stuff” and focus all our energy on the synthetic pathway that delivers the desired product. But when we ignore all the other “stuff,” we pay a high price and it’s a price we need to stop paying.
Occasionally, chemists do produce molecules that have toxic or other hazardous effects, and the next principle will have something to say about designing safer molecules.
- Designing Safer Chemicals
Chemical products should be designed to preserve efficacy of function while reducing toxicity.
Contributed by Nicholas D. Anastas, Ph.D., U.S. Environmental Protection Agency- New England
Minimizing toxicity, while simultaneously maintaining function and efficacy, may be one of the most challenging aspects of designing safer products and processes. Achieving this goal requires an understanding of not only chemistry but also of the principles of toxicology and environmental science. Highly reactive chemicals are often used by chemists to manufacture products because they are quite valuable at affecting molecular transformations. However, they are also more likely to react with unintended biological targets, human and ecological, resulting in unwanted adverse effects. Without understanding the fundamental structure hazard relationship, even the most skilled molecular magician enters the challenge lacking a complete toolkit.
Mastering the art and science of toxicology requires innovative approaches to chemical characterization that state that hazard is a design flaw and must be addressed at the genesis of molecular design. The intrinsic hazard of elements and molecules is a fundamental chemical property that must be characterized, evaluated and managed as part of a systems-based strategy for chemical design.
Now is the ideal time to develop a comprehensive and cooperative effort between toxicologists and chemists, focused on training the next generation of scientists to design safer chemicals in a truly holistic and trans-disciplinary manner through innovative curricular advancements. The field of toxicology is evolving rapidly, incorporating and applying the advancements made in molecular biology to reveal the mechanisms of toxicity. Elucidation of these pathways serve as the starting point for articulating design rules that are required by chemists to guide their choices in a quest to make safer chemicals. We are at the dawn of a new sunrise, poised to illuminate the path forward to a safer, healthier and more sustainable world.
More Resources and Examples
Anastas, N. Green Toxicology, 2012 in: Green Techniques for Organic Synthesis and Medicinal Chemistry , W. Zhang and B. Cue, eds., J Wiley.
Anastas, N.D. and J.C. Warner. 2005. Incorporating Hazard Reduction as a Design Criterion in Green Chemistry , Chem. Health. Safety, March/April, 3-15.
Green Chemistry Metrics: Measuring and Monitoring Sustainable Processes , 2009, A. Lapkin and D. Constable eds., J. Wiley.
Green Chemistry Education: Changing the Course of Chemistry , 2009, ACS Symposium Series 1011, P.T. Anastas, I. Levy and K.E. Parent, eds. J. Wiley
Designing Safer Chemicals , 1996, S. DeVito and R. Garrett eds., ACS Symposium Series 640.
US EPA, 2013, http://epa.gov/ncct/Tox21/ (accessed 3/3/13)
Disclaimer:
Although these references are given to provide additional information that may be useful or interesting, EPA is not responsible for, and cannot attest to the accuracy of, the content of these articles.
- Safer Solvents and Auxiliaries
The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and, innocuous when used.
Dr. Concepcíon (Conchita) Jiménez-González, Director, Operational Sustainability, GlaxoSmithKline
It was a green chemistry conference and the very famous synthetic chemist had just received a question about why he had chosen a solvent that was without question a very poor choice. You have to be realistic, chemists know intuitively what's best, and solvents don't matter. It's the chemistry that counts. I've heard this kind of remark repeatedly over many years, despite the fact that it goes against the spirit and letter of Principle 5.
Solvents and mass separation agents of all kinds matter a lot to the chemistry not to mention the chemical process and the overall "greenness" of the reaction. In many cases, reactions wouldn't proceed without solvents and/or mass separation agents. To say that they don't matter, or that it's only the chemistry that counts is not just a logical fallacy, it's chemically incorrect. Solvents and separation agents provide for mass and energy transfer and without this, many reactions will not proceed.
It has also been shown that solvents account for 50 – 80 percent of the mass in a standard batch chemical operation, depending on whether you include water or you don't. Moreover, solvents account for about 75% of the cumulative life cycle environmental impacts of a standard batch chemical operation.
Solvents and mass separation agents also drive most of the energy consumption in a process. Think about it for a moment. Solvents are alternately heated, distilled, cooled, pumped, mixed, distilled under vacuum, filtered, etc. And that's before they may or may not be recycled. If they're not recycled, they are often incinerated.
Solvents are also the major contributors to the overall toxicity profile and because of that, compose the majority of the materials of concern associated with a process. On average, they contribute the greatest concern for process safety issues because they are flammable and volatile, or under the right conditions, explosive. They also generally drive workers to don personal protective equipment of one kind or another.
We will always need solvents, and with many things in chemical processes, it's a matter of impact trading. Optimize a solvent according to one green metric and many times, there are three others that don't look so good. The object is to choose solvents that make sense chemically, reduce the energy requirements, have the least toxicity, have the fewest life cycle environmental impacts and don't have major safety impacts.
Solvents and separation agents do matter and despite one or more famous synthetic organic chemists may think. It is possible to make better choices, and that is what application of this principle should promote.
Design for Energy Efficiency
Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure.
By Dr. David Constable, Director, ACS Green Chemistry Institute ®
In recent years I've begun to talk about the green chemistry and engineering's "forgotten principles," and Design for Energy Efficiency is one of them. Amongst synthetic organic chemists, no consideration is given to temperature or pressure. The chemist just follows a protocol to get a reaction to go to completion and to separate the desired product at as high a yield as possible. Energy, from the chemist’s perspective, is irrelevant and for all intents and purposes, free. Just put the plug in the wall or the heating coil around the flask, or get the liquid nitrogen out of the dewar.
For those that do think about energy, most if not all the attention that energy gets from chemists is devoted to heating, cooling, separations, electrochemistry, pumping and reluctantly, to calculations related to thermodynamics (e.g., Gibbs Free Energy). The attention is not in minimizing or considering where energy comes from or if it matters what form is used, it's just a given that we need to heat or cool or shove electrons into the reaction to make or break bonds. In reflecting on my own training as a chemist, I never was asked to convert any heating, cooling, pumping or electrochemical requirements to a cost for electricity, steam or some other utility. That may be done in chemical engineering, but not in chemistry.
Energy is a key issue for the 21st century. A majority of the energy that is produced is based, and will continue to be based on fossil fuels. And most of the energy that is delivered to the point of use is lost in conversion and transmission. What this means is that if you look at the life cycle of energy production, and you look at how much energy is actually available for useful work at the point of need, it is less than 1 or 2 percent of the energy that was originally available in the fossil fuel. It is also true that most fossil fuel energy is used for transportation services of one kind or another and the second biggest use is in space heating and cooling. There are a tremendous number of opportunities for chemists to change this energy use profile, but it is my experience that very few chemists see themselves as being a part of either transportation or the built environment.
If you think about where most chemists are trained around energy, and certainly chemical engineers are, it's around ∆H in the Gibbs Free Energy equation. Heats of formation, heats of vaporization, enthalpy, exothermic reactions, etc; these are what we think about. The interesting thing is that nature largely works with ∆S and weak forces of interaction. You don’t see a tree doing photosynthesis at reflux using a solvent, or a cell membrane is not extruded at the melt temperature of something like polystyrene.
There is so much more to energy and engaging chemists in thinking about energy than asking them to run reactions at ambient temperature and pressure. Reactions themselves are rarely where a majority of energy is used; most is used in solvent removal to set up for the next reaction, or to remove one solvent and replace it with another, or to isolate the desired product, or to remove impurities. Apart from hydrogenations or reactions that are oxygen or moisture sensitive, most reactions are done at atmospheric pressure. This doesn't mean that energy isn't important, it is just important in areas where most chemists are not focused.
Once again, thinking about more than one part of the reaction or the process during the design of a new molecule is critical not only from the standpoint of energy, but also from many different angles. Energy—like thinking about how to arrange a synthesis to have the fewest number of steps, or use the lowest cost starting materials or any other aspect of interest to the synthetic or process chemist—is just another design parameter. Historically it has not been seen as that, but we can no longer afford to design new molecules in the absence of a detailed and extended consideration of how energy will be used.
- Use of Renewable Feedstocks
A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable.
By Dr. Richard Wool, Professor of Chemical and Biomolecular Engineering and Director of the Affordable Composites from Renewable Materials program, University of Delaware.
The concept of making all our future fuels, chemicals and materials from feedstocks that never deplete is an interesting concept which at first glance seems impracticable. Mankind currently removes fossil fuels, coal, oil and natural gas from the ground and extracts minerals for profit until they are exhausted. In particular, our fossil fuels for carbon-based chemicals and materials are being rapidly depleted in a predictable manner with the expected rise of global populations and expanding energy intensive economies on several continents. The impacts on human health and the environment are significant and present major challenges for our scientists and leaders in the next 50 years.
Can we address these global problems by using Green Chemistry Principal #7? Yes, we will get our feedstock, as if by magic, from “thin air” and it will be renewable. The carbon in the air is in the form of carbon dioxide CO 2 and methane CH 4 and is removed by photosynthetic processes powered by the sun to form plants, trees, crops, algae, etc., which collectively we call “biomass”.
Nature produces about 170 billion tons of plant biomass annually, of which we currently use about 3.5 percent for human needs. It is estimated that about 40 billion tons of biomass, or about 25 percent of the annual production, would be required to completely generate a bio-based economy. The technical challenge in the use of such renewable feedstocks is to develop low energy, non-toxic pathways to convert the biomass to useful chemicals in a manner that does not generate more carbon than is being removed from “thin air”; the difference between C(in) from the air, and C(out) from the energy used, is the carbon footprint ΔC. Ideally, when using Principal #7, all carbon footprints by design should be positive such that C(in) >> C(out). This leads in a natural way to the reduction of global warming gasses impacting our current climate change. We should also insure that the new chemicals and materials derived from renewable resources are non-toxic or injurious to human health and the biosphere.
In 2002, the U.S. Department of Energy in their Vision for Bioenergy and Bio-based Products in the United States stated:
“By 2030, a well-established, economically viable, bioenergy, and bio-based products industry is expected to create new economic opportunities for rural America [globalization through localization], protect and enhance the environment, strengthen the U.S. energy independence, provide economic security, and deliver improved products to consumers.”
In the past 10 years, significant advances have been made in the development of fuels, chemicals and materials from renewable feedstocks. These for example, have included biodiesel from plant oils and algae, bioethanol and butanol from sugars and lignocellulose, plastics, foams and thermosets from lignin and plant oils, and even electronic materials from chicken feathers. In terms of Green Chemistry Principal #7, our future is bright and laced with optimism due to the ongoing fruitful collaborations between several disciplines involving biotechnology, agronomy, toxicology, physics, engineering and others, where new fuels, chemicals and materials are being derived from renewable feedstock from “thin air” with minimal impact on human health and the environment.
Additional Resource
Vision for Bioenergy and Biobased Products in the United States - Updated 2006
- Reduce Derivatives
Unnecessary derivatization (use of blocking groups, protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste.
By Peter J. Dunn, Green Chemistry Lead, Pfizer
One of the key principles of green chemistry is to reduce the use of derivatives and protecting groups in the synthesis of target molecules. One of the best ways of doing this is the use of enzymes. Enzymes are so specific that they can often react with one site of the molecule and leave the rest of the molecule alone and hence protecting groups are often not required.
A great example of the use of enzymes to avoid protecting groups and clean up processes is the industrial synthesis of semi-synthetic antibiotics such as ampicillin and amoxicillin.
In the first industrial synthesis Penicillin G (R=H) is first protected as its silyl ester [R = Si(Me) 3 ] then reacted with phosphorus pentachloride at -40 o C to form the chlorimidate 1 subsequent hydrolysis gives the desired 6-APA from which semi-synthetic penicillins are manufactured.
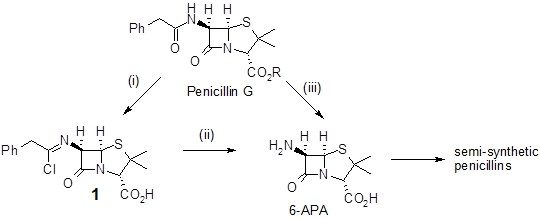
(i) TMSCl then PCl5, PhNMe2, CH2Cl2, -40oC (ii) n-BuOH, -40oC, then H2O, 0oC (iii) Pen-acylase, water
This synthesis has been largely replaced by a newer enzymatic process using pen-acylase. This synthesis occurs in water at just above room temperature. The new synthesis has many advantages from a green perspective one of which is that the silyl protecting group is not required.
More than 10,000 metric tons of 6-APA is made every year and much of it by the greener enzymatic process so this is a fantastic example of Green Chemistry making a real difference.
More Resources
The Importance of Green Chemistry in Process Research and Development
Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
Contributed by Roger A. Sheldon, Ph.D., Emeritus Professor of Biocatalysis and Organic Chemistry, Delft University of Technology and CEO of CLEA Technologies B.V.
A primary goal of green chemistry is the minimization or preferably the elimination of waste in the manufacture of chemicals and allied products: “prevention is better than cure” . This necessitates a paradigm shift in the concept of efficiency in organic synthesis, from one that is focused on chemical yield to one that assigns value to minimization of waste. What is the cause of waste? The key lies in the concept of atom economy: “synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product” . In the reaction scheme we compare, for example, the reduction of a ketone to the corresponding secondary alcohol using sodium borohydride or molecular hydrogen as the reductant. Reduction with the former has an atom economy of 81% while reduction with the latter is 100% atom economic, that is everything ends up in the product and, in principle, there is no waste.
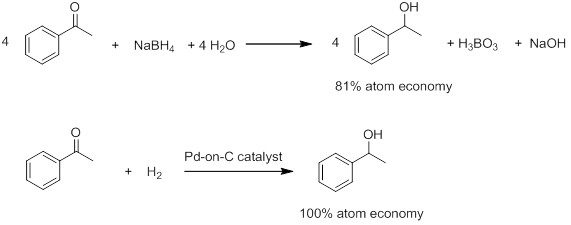
Unfortunately, hydrogen does not react with ketones to any extent under normal conditions. For this, we need a catalyst such as palladium-on-charcoal. A catalyst is defined as “a substance that changes the velocity of a reaction without itself being changed in the process” . It lowers the activation energy of the reaction but in so doing it is not consumed. This means that in principle at least, it can be used in small amounts and be recycled indefinitely, that is it doesn’t generate any waste. Moreover, molecular hydrogen is also the least expensive reductant and, for this reason, catalytic hydrogenations are widely applied in the petrochemical industry, where the use of other reductants is generally not economically viable. It is only in the last two decades, however, following the emergence of green chemistry, that catalysis has been widely applied in the pharmaceutical and fine chemical industries, with the goal of minimizing the enormous amounts of waste generated by the use of stoichiometric inorganic reagents. This involves the use of the full breadth of catalysis: heterogeneous, homogeneous, organocatalysts and, more recently, Nature’s own exquisite catalysts: enzymes. The latter are particularly effective at catalyzing highly selective processes with complex substrates under mild conditions and, hence, are finding broad applications in the pharmaceutical and allied industries. Moreover, they are expected to play an important role in the transition from a chemical industry based on non-renewable fossil resources to a more sustainable bio-based economy utilizing renewable biomass as the raw material, yet another noble goal of green chemistry.
R.A. Sheldon, I. Arends and U. Hanefeld, Green Chemistry and Catalysis , Wiley-VCH, Weinheim, 2007 (ISBN 978-3-527-30715-9)
R.A. Sheldon, Fundamentals of green chemistry: efficiency in reaction design , Chem. Soc. Rev. 41 (2012) 1437-1451.
R.A. Sheldon, E Factors, green chemistry and catalysis: An odyssey Chem. Commun. (2008) 3352-3365.
- Design for Degradation
Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment.
Contributed by Rich Williams, Founder and President at Environmental Science & Green Chemistry Consulting, LLC
Green chemistry practitioners aspire to optimize the commercial function of a chemical while minimizing its hazard and risk. Hazard, the capability to cause harm, is an inherent characteristic arising, like function, from a chemical’s stereochemistry (the content and arrangement of atoms). Green chemistry principles 3, 4, 5, and 12 guide designers to reduce the hazards of chemicals. Principle 10, however, guides the design of products that degrade after their commercial function in order to reduce risk or the probability of harm occurring. Risk is a function of both a molecule’s inherent hazard AND exposure – contact between a chemical and a species. Degradation can eliminate significant exposure, thereby minimizing risk regardless of the hazard of the chemical involved.
Exposure to persistent chemicals can be significant as a result of global dispersion enabled by properties such as volatility or sorption to particles and partitioning into organisms based on properties such as fat solubility. Regulators have established criteria (half-lives in water, soil, air) that define persistence within frameworks used to identify chemicals as PBT (Persistent, Bioaccumulative, Toxic).
A green chemistry objective is to design out molecular features responsible for hazardous characteristics and risk. Trade-offs, or alternative approaches, must be evaluated when the molecular features to be designed in for commercial function overlap with those to be designed out to reduce hazard and risk.
Biodegradation, hydrolysis, and photolysis can be designed into chemical products. In the same way that mechanistic toxicology knowledge is essential to identify and design out molecular features that are the basis for hazards, an understanding of the mechanisms of degradation and persistence are required to design in chemical features that promote degradation and eliminate features that promote persistence. Many persistent compounds are extensively chlorinated. Halogens such as chlorine are electron withdrawing, thereby inhibiting the enzyme systems of microbes because aerobic microbial degradation favors electron rich structures.
Prediction methods that can guide the design of molecular architecture expected to degrade include rules of thumb linking structural features to degradability or persistence, databases of existing knowledge, models that evaluate biodegradability or PBT attributes, and experimental testing. All of these tools can be adapted to individual chemical sectors and specific objectives.
Understanding the anticipated release and transport pathways for a chemical informs the selection of an effective design strategy. Degradation must occur within the relevant environmental compartment(s) and at a meaningful rate. Domestic wastewater typically passes through a vigorous bioreactor within wastewater treatment plants (WWTP). The consumer product industry has designed molecules for removal within these bioreactors. In the early 1960’s, industry transitioned from non-biodegradable branched surfactants, which caused extensive foaming and other health problems in surface waters receiving WWTP effluent, to biodegradable linear alkyl benzene sulfonate based detergents – an approach to innovative design that continues today.
Tools currently exist to enable the implementation of principle 10, but advances in mechanistic understandings linking molecular features to hazards and degradability will enable more comprehensive application of green chemistry to control hazard and risk. Effective communication across disciplines is also essential to provide designers with knowledge they can factor into the complexities of product design. Because of regulatory and business constraints, many product design decisions must be made relatively early. Predictive decision-making tools must provide confidence about hazard and risk in a way that is aligned with the timing and magnitude of development decisions, and most importantly, while there is still flexibility to alter a molecular design or product formulation.
- Real-time analysis for Pollution Prevention
Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances.
Contributed by Douglas Raynie, Assistant Professor, Chemistry & Biochemistry, South Dakota State University
Imagine driving down a busy highway in a car with all of the windows painted an opaque black!!! While that scenario many not seem realistic (or safe), what if you had a 360° camera and the sensors and technology being developed for self-driving cars? Now, the safety of your commute is more ensured.
This description, while applied to automobiles, is illustrative of the 11th principle of green chemistry. Just as we need real-time feedback for driving safety, real-time feedback is essential in proper functioning chemical processes. Most chemists are familiar with laboratory analysis from their undergraduate training. But analysis can also be performed in-line, on-line, or at-line in a chemical plant, a subdiscipline known as process analytical chemistry. Such analysis can detect changes in process temperature or pH prior to a reaction going out of control, poisoning of catalysts can be determined, and other deleterious events can be detected before a major incident occurs.
Process analysis is of such importance that the US Food and Drug Administration encourages such an approach for the manufacture, design, and control of pharmaceutical manufacturing. Since 1984, an industry-academic partnership, the Center for Process Analytical Chemistry, has promoted research into emerging techniques for process analytical chemistry.
While the traditional roles of analytical chemistry also advance green chemistry goals, the effective application of process analytical chemistry directly contributes to the safe and efficient operation of chemical plants worldwide.
Additional resource:
Center for Process Analysis & Control
- Inherently Safer Chemistry for Accident Prevention
Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires.
Contributed by Shelly Bradley, Campus Chemical Compliance Director, Hendrix College; Dr. David C. Finster, Professor of Chemistry, Wittenberg University; and Dr. Tom Goodwin, Elbert L. Fausett Professor of Chemistry, Hendrix College
Safety can be defined as the control of recognized hazards to achieve an acceptable level of risk. Green Chemistry Principle # 12 is known as the “Safety Principle”. It may be the most overlooked of the twelve principles, yet it is the logical outcome of many of the other principles. In fact, it is practically impossible to achieve the goals of Principle 12 without the implementation of at least one of the others. Since the very essence of green chemistry is to “… reduce or eliminate the use or generation of hazardous substances” there is an intrinsic connection to laboratory safety. While there are a few exceptions, the majority of the Green Chemistry Principles will result in a scenario that is also safer.
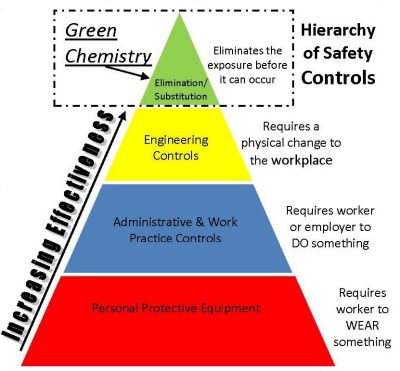
Under the umbrella of the Environmental Protection Agency (EPA), Green Chemistry’s primary focus is clearly to make the environment safer. Materials and processes that are safer for the environment also are likely to be safer for the general public. However, another population that benefits from green chemistry and is not often mentioned is workers. The manufacturing or laboratory worker is often the first in-line person to benefit from hazard reductions.
The health and safety of workers are under the purview of the Occupational Safety and Health Administration (OSHA). In a recent news release, OSHA unveiled a chemical management system designed to increase worker safety. The Hierarchy of Safety Controls as highlighted in OSHA’s new Transitioning to Safer Chemicals Toolkit illustrates the difference between focusing on the control or hazard part of the safety definition. Traditional chemical safety models focus primarily on the control component of that definition. The graphic (adapted from OSHA) shows that the most effective means of increasing safety is eliminating the hazard component. Since the elimination of hazards is the basic tenet of Green Chemistry, this marriage of the ideas of Green Chemistry from both OSHA and EPA should have a synergistic impact on hazard reduction. Combining the forces of these two agencies toward a common goal may lead to conversations and changes that result in safer conditions for workers, a safer environment for the general public, and a safer planet for us all.
References Cited:
Manuele, F. A. Acceptable Risk , Professional Safety , 2010 , 30-38 (accessed 11/22/2013)
Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice ; Oxford University Press; New York, 1998.
US OSHA, OSHA releases new resources to better protect workers from hazardous chemicals , (accessed 11/22/2013)
US OSHA, Transitioning to Safer Chemicals: A Toolkit for Employers and Workers , (accessed 11/22/2013)
US OSHA, Why Transition to Safer Alternatives? , (accessed 11/22/2013)
*Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice, Oxford University Press: New York, 1998, p.30. By permission of Oxford University Press.
Explore the 12 Principles of Green Engineering >>

- Design for Energy Efficient

Download the Green Chemistry Pocket Guide
ACS GCI's Green Chemistry and Engineering Conference
The 28 th Annual Green Chemistry & Engineering Conference will be held June 2-5, 2024 in Atlanta, Georgia, with the theme AI-Generated Green Chemistry .
Contact the ACS Green Chemistry Institute
gci@acs.org +1 202-872-6102 or 1-800-227-5558, ext. 6102
Follow ACS Green Chemistry Institute
Nexus newsletter.
Get the latest news an updates from the ACS Green Chemistry Institute ® .
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society

Developing green chemistry educational principles by exploring the pedagogical content knowledge of secondary and pre-secondary school teachers

First published on 19th October 2022
Green chemistry developed historically from twelve industrial principles for research chemists. Recently, interest has grown to begin introducing these principles in science classrooms even at the secondary and pre-secondary school levels. However, teachers must do significant work to adapt and translate green chemistry from the industrial or manufacturing perspective into one more appropriate to students at younger ages. This research project explores how a group of current teachers in the US and Canada have been developing their language and understanding of green chemistry through Beyond Benign's Lead Teacher Program. Transcripts from phone interviews with program participants are analyzed to propose a classroom-based definition of green chemistry and its justification as an approach at the secondary and pre-secondary school levels. This pedagogical understanding provides a foundation to solidify green chemistry as a standard practice in science education. Then classroom observations and case studies of four teachers are developed into a framework for green chemistry education at the K-12 level.
Introduction
Green chemistry education: from industrial principles to educational values.
Chemistry education also needed to contend with this negative image. As Haack and Hutchinson note, “the strategies of green chemistry provided a new context for teaching students the concepts and skills of chemistry that cast the discipline in a more positive light while better preparing students to discover and develop sustainable chemistries to meet society's needs” (2016, p. 5890). Green chemistry was both a public relations shift for the field and a call to action for chemists to consider the broader impacts of chemical processes all the way from resource extraction to waste processing.
Historically, chemistry applications to the environment had often been seen as supplementary or even as a less-rigorous approach to chemistry appropriate for non-majors (such as Chemistry in the Community, see Sutman and Bruce, 1992 ; American Chemical Society, 2011 ). A major accomplishment of green chemistry has been to shift this perspective to recognize the challenge and necessity of considering environmental impact as a core part of chemistry ( Sharma and Mudhoo, 2011 ).
It will be helpful to frame this discussion of green chemistry with the educational outcomes expected from this approach. In order to define educational outcomes for green chemistry, several core competencies for the undergraduate chemistry curriculum have been developed by the American Chemical Society Green Chemistry Institute ( MacKellar et al. , 2020 ). These broad competencies hearken back to the optimistic perspective of science as a productive discipline that can actively improve human society and the planet, rather than only minimizing harm by restricting chemical activity.
The lack of K-12 green chemistry resources
However, not many K-12 teachers are using the principles directly in their classrooms ( Anastas and Eghbali, 2010 ). The role of green chemistry in K-12 classrooms has not been well-documented yet, although this lack of published research could reflect the absence of publication culture among K-12 educators rather than the absence of green chemistry. Among the available K-12 perspectives, one secondary school teacher describes the principles of “prevent waste” and “atom economy” as important for instruction, as well as “benign solvents and auxiliaries” and “inherently benign chemistry for accident prevention” as important for choosing chemicals for demonstrations ( Ause, 2018 , pp. 186–187). Additionally, green chemistry education research has largely focused on labs and chemical practices in classrooms rather than content or pedagogy ( Haack and Hutchison, 2016 ). There is a need for further integration of these principles throughout K-12 classrooms.
Since green chemistry helps to address the material dimensions of sustainability, there should be some minimum level of understanding we would want most citizens of an industrialized country to have. † The K-12 level is a particularly important time to teach students about green chemistry because it might be the last chemistry or science course many of the students take. For a scientifically literate society, we need students to learn about sustainability before the college level. These should be foundational topics, not advanced or niche contexts. To achieve that goal, we need better research about how K-12 teachers can begin preparing students for this work and ensure that students who will not pursue science further have a basis for understanding the molecular nature of sustainability.
Finally, a green chemistry teaching approach must be connected to specific student learning outcomes to justify its use. Without a clear pedagogical justification, “green chemistry” risks being (or being seen as) simply a Trojan horse to bring any trendy educational practice into science classrooms, similar to criticisms of environmental education ( Sanera and Shaw, 1997 ). Teachers, administrators, families, and students will want to know if “green chemistry” is simply a marketing tool or another attempt at “green-washing.” Other authors have specified the need “to delineate the differences and overlap between green and sustainable chemistry because not all green chemistry is sustainable chemistry ” ( Green Chemistry Institute, 2020 , p. 4, italics in original). For green chemistry to succeed in shifting scientific literacy towards more sustainable considerations, there must be coherent content and clear benefits to students, not just an ideological framework. Fortunately, there are signs that green chemistry is better equipped to address these concerns than previous environmental movements in chemistry.
Is it really ‘Chemistry?’ The place of traditional content knowledge
Some teachers have justified the use of green and sustainable chemistry as a motivating force for traditional content learning. Aubrecht et al. (2019) write that “though the primary focus in general and organic chemistry courses is instruction of fundamental chemistry concepts, raising student awareness on the potential of the chemistry enterprise to address global issues involving sustainability can both inspire them and challenge them” (p. 2877). These authors also include charts for the general and organic chemistry curricula to show the overlap of traditional content (“enduring understanding”) and green chemistry connections ( Aubrecht et al. , 2019 , pp. 2874–2875). Another study confirmed the positive change in high school students’ interest in chemistry and its perceived relevance to their lives through inquiry-based, life-cycle thinking approaches to sustainable chemistry ( Juntunen and Aksela, 2013 ). When used well, environmental problems can provide a motivating context for student learning of content. Such a benefit might convince skeptical teachers that a sacrifice of some conventional content is worthwhile.
Finally, green chemistry also supports an optimistic and economic perspective of chemistry that can actively solve problems in the world rather than simply describing issues. In this way, it is a clear response to concerns about environmental education that convey a pessimistic image of the state of the world. Green chemistry provides an opportunity and framework for students and industrial chemists to invent new solutions that work better for people, the planet, and a community's economic well-being.
With this overview of environmental curricula, a case has been made that green chemistry can address significant concerns in current science education to motivate traditional content learning and foster skills that prepare students to consider the chemistry involved in environmental issues. The next section introduces the research background for this project designed to develop an actionable understanding of green chemistry principles and classroom practices for K-12 teachers through the lens of the Beyond Benign Lead Teacher Program. This understanding will provide a basis for further research and practice in the development of the field of green chemistry education.
Pedagogical content knowledge: the research of teacher expertise
The perspective of PCK emphasizes that an expert chemistry teacher is distinguished neither by advanced content knowledge nor by skilled use of educational strategies but by an area of expertise specific to teaching chemistry at a certain grade level. An experienced chemist cannot simply learn some educational techniques and become an expert teacher. Similarly, an experienced high school chemistry teacher would likely struggle to transition to teaching English even with deep familiarity with the content. Teachers in any discipline require a rationale for their pedagogy that is not transferable from other subject areas. Shulman gave the analogy that “a professional is capable not only of practicing and understanding his or her craft, but of communicating the reasons for professional decisions and actions to others” (1986, p. 13). A true test of teaching expertise would be this type of reflective reasoning as described by PCK.
Because PCK is linked with content, it must also be studied in content-specific ways that may require unique frameworks. Within science education research, Magnusson et al. (1999) defined the following five components of PCK for science teaching:
1. Orientations toward science teaching,
2. Knowledge and beliefs about science curriculum,
3. Knowledge and beliefs about students’ understanding of specific science topics,
4. Knowledge and beliefs about assessment in science, and
5. Knowledge and beliefs about instructional strategies for teaching science (p. 97).
These five components delineate the connections that Shulman (1986) implied among content, pedagogy, and PCK.
Importantly, PCK does not supplant content knowledge. As other researchers have noted, “the most effective teachers have deep knowledge of the subjects they teach, and when teachers’ knowledge falls below a certain level it is a significant impediment to students’ learning” ( Coe et al. , 2014 , p. 2). As a defining level of teacher expertise, PCK draws from “the three base domains of teacher knowledge: subject matter, pedagogy, and context” ( Magnusson et al. , 1999 ). Expert teachers need deep knowledge in each of these three areas, yet this knowledge alone does not guarantee that a teacher has or will develop the necessary PCK to teach a topic at a certain grade level.
After the concept of PCK was better defined and accepted in the academic community, the practical research interest became: are there ways to teach PCK directly in order to prepare teachers better? Instead of each teacher learning independently, could PCK provide a shortcut to develop expert teachers more quickly within a community of teachers in training? Research began to focus on this practical dimension of PCK for teacher training ( Neumann et al. , 2019 ). For example, Demirdöğen et al. (2016) used PCK to structure and assess a course for pre-service chemistry teachers. As Magnusson et al. , (1999) explained:
The practical value of pedagogical content knowledge as a construct has to do with its potential to define important dimensions of expertise in science teaching that can guide the focus and design of pre-service and in-service teacher education programs. Many science teachers and science teacher educators have a wealth of knowledge about how to help particular students understand ideas such as force, photosynthesis, or heat energy; they know the best analogies to use, the best demonstrations to include, and the best activities in which to involve students (p. 116).
Studying PCK is one way to access and share this wealth of knowledge with wider communities.
More recent research has identified PCK as a major indicator of student learning. In an extensive literature review on teaching effectiveness, Coe et al. (2014) identified PCK as one of six components of great teaching and one that has “strong evidence of impact on student outcomes” (p. 2). They also noted the positive impact of physics teachers’ PCK and motivation on students’ achievement and interest. Therefore, studying a teacher's PCK can be an indirect way of predicting student growth in a course.
However, researchers have been careful to note differences in types of PCK. Aydeniz and Kirbulut (2014) emphasized “that PCK must be understood and explored at two levels: (1) espoused/planned PCK and (2) enacted PCK” (p. 149). Although both areas are important, this subtle distinction clarifies that how a teacher describes their pedagogy might be different from how they enact that pedagogy. Even if a teacher has highly developed espoused PCK, there can still be barriers to integrating that knowledge into instructional practice ( Barendsen and Henze, 2019 ).
Understandably, research on PCK has focused especially on pre-service training or individual administrative interventions ( De Jong et al. , 2005 ; Aydeniz and Kirbulut, 2014 ; Parga Lozano, 2015 ; Demirdöğen et al. , 2016 ). However, more research is needed to understand how PCK is shared between in-service peers beyond one-time interventions and in specific contexts like green chemistry education ( Baxter and Lederman, 1999 ; Loughran et al. , 2012 ). Part of our research involved developing descriptions of pedagogical content knowledge specifically related to green chemistry, inspired by previous research with pre-service teachers. These descriptions will allow future research to more easily track and categorize green chemistry principles and associated teaching practices as a novel and valuable area of chemistry PCK.
The resource folios approach to pedagogical content knowledge
In their pivotal text, Loughran et al. (2012) reviewed the work on PCK and critiqued some developments that have become counterproductive. They highlighted the tension between the generalizing thrust of research and the specificity inherent in the concept of PCK, saying, “It seems as though the more that PCK is refined and/or redefined in a bid to make it more concrete, the less valuable it becomes as a descriptor of specialist or expert knowledge of practice” ( Loughran et al. , 2012 , p. x). Furthermore, an overly prescriptive approach to PCK risks omitting some factors that support good teaching. As they argue, “although it is important to have some routines in teaching, when teaching becomes ‘routinized’, elements of quality teaching ( e.g. engagement, enjoyment and intellectual challenge) can be dramatically diminished; or worse, absent all together” ( Loughran et al. , 2012 , p. 2). Therefore, PCK research needed some clarification to avoid overly concretizing or routinizing teaching practice.
Loughran et al. (2012) also argued that research on PCK has not been tailored to the practical goal of supporting teacher practice. As they explain ( Loughran et al. , 2012 ),
The manner in which research into PCK has been conducted has created an impasse for teachers. The research literature on PCK is certainly extensive; however, the outcomes of such research appear to speak more to educational researchers and other such academics than to teachers who surely are not only the producers of such knowledge, but also important end users… much time and energy was expended evaluating PCK as opposed to exploring concrete examples of how teachers teach particular content topics in particular ways that promote understanding. Therefore, unfortunately, PCK has not been developed through the research literature in ways that necessarily directly correlate with enhancing the practice of science teaching (p. 11).
Some research on PCK became too theoretical and separated from classroom practice in a way that made it less meaningful.
The major value of PCK, then, is its role in clarifying teachers’ and researchers’ language about teacher practice. In other words, for PCK as a practical tool, “the value to teachers was in terms of encouraging reflection on practice, creating a shared language for discussing science teaching and learning, and offering insights into practice, all of which became a springboard for their own professional learning.” ( Loughran et al. , 2012 , p. x). Developing teachers’ PCK does not guarantee good teaching practice, but it does provide one additional touchpoint for teachers to develop with each other. Loughran et al. (2012) refocused PCK research on teacher practice because,
Teachers are in fact producers, not just users, of sophisticated knowledge of teaching and learning. And, the complex ideas associated with exemplary practice are better able to be portrayed and shared in meaningful ways if labels and descriptors such as PCK are better understood and used. Therefore, a language that comprises aspects of professional practice is central to moving knowledge of practice out from the individual and into the professional community at large. For example, in many studies by teacher researchers, language (a shared vocabulary) has been central to the development and sharing of their sophisticated knowledge of practice (p. 12).
This perspective should inform both research and interventions about teacher practice. Importantly, other aspects of language, like content knowledge and pedagogical knowledge as distinct from PCK, can still contribute to teachers’ professional learning ( Loughran et al. , 2012 , p. 5). Similar to other researchers, they noted that individual teachers naturally develop their own PCK. The goal is to use the language of PCK to facilitate the transfer and development of teaching practice for more teachers ( Loughran et al. , 2012 , p. 13).
One challenge in this research is that such a depth of engagement required in developing PCK for individual topics means sacrificing the breadth of topics normally covered in a class. “The dilemma, then, is that although students’ conceptual understanding may well be richer, the amount of content covered is likely (at least, initially) to be much less than that which might normally be achieved” ( Loughran et al. , 2012 , p. 16). Teachers and researchers might need to be convinced of the long-term value of developing PCK to overcome the inertia of existing curricular structures.
The structure of resource folios: CoRes and PaP-eRs
| Grade level and topic for this CoRe: | Big idea or subunit topic |
|---|---|
| Title or description of the big idea. | |
| What you intend students to learn about this idea. | |
| Why it is important for students to know this idea. | |
| What else you know about this idea that you do not intend students to know yet. | |
| Difficulties, limitations, or misconceptions connected with teaching this idea. | |
| Knowledge about students’ thinking which influences your teaching about this idea. | |
| Other factors that influence your teaching of this idea. | |
| Teaching procedures (and particular reasons for using them to engage with this idea). | |
| Specific ways of ascertaining students’ understanding or confusion around this idea. |
The second structure is a Pedagogical and Professional-experience Repertoire (PaP-eR) which provides a contextualized example of teacher practice for a given topic through a range of formats, like a syllabus, annotated lesson plan, or stylized interview ( Loughran et al. , 2012 , p. 17). In contrast to the overarching perspective of CoRes, PaP-eRs present a contextualized story of how one teacher approaches the content. These examples help support teacher development because, “in many ways, teachers’ stories actually carry most of the important information that helps other teachers to identify with, and therefore extract their own meaning from, a given description of a teaching and learning situation” ( Loughran et al. , 2012 , p. 16). It might seem counter-intuitive that a more specific example is more easily transferable, but the contextual information of a real class often helps teachers to imagine the content in their own classroom.
After compiling CoRes and PaP-eRs for a given topic, these Resource Folios can then be used in professional development for teachers, providing the common language to move research into practice ( Loughran et al. , 2012 , p. 20). This structure helps prompt teacher reflection on their often-unstated knowledge about teaching certain content. In these types of professional development experiences, “participants required an opportunity to work with a CoRe to develop a familiarity with the process in order to manage the demands inherent in completing the task” ( Loughran et al. , 2012 , p. 217).
There are significant challenges to developing Resource Folios for any content. For many teachers, “in reflecting upon one's own experiences of teaching and learning in science, it can sometimes be difficult to look back and see the changes in practice (and the reasons for those changes) that led to the manner in which one teaches at the present point in time” ( Loughran et al. , 2012 , p. 223). Researchers and facilitators still have a significant role to play in supporting and scaffolding this type of reflection with teachers, working together to construct accurate representations of PCK that can be shared with a wider community.
Research partner: Beyond Benign
A group of staff and teachers started the Lead Teacher Program (LTP) in 2016 to help train K-12 educators in principles of green chemistry and empower them to share that expertise with other teachers. The program accepts a small group of two to eight teachers each year for a three-year, stipend position. Throughout this project, there were about 13 teachers in the program at a time across the three years. Within the cohorts, teachers had a wide variety of teaching experience, from new teachers (full time in the classroom for one to three years) to veteran teachers (full time in the classroom for more than twenty years). The teachers also varied in their familiarity with green chemistry, with some teachers having just completed an introductory series of two graduate level courses developed through Beyond Benign or with some teachers who had been heavily involved in green chemistry and resource development prior to their involvement with LTP. Because of these variations, the teachers’ years of involvement in LTP does not necessarily reflect their familiarity with green chemistry education. These teachers participate in a wide variety of activities, which include developing curriculum, presenting webinars, and joining monthly phone calls with other program participants. By working at the K-12 level, LTP seeks to integrate green chemistry principles into early levels of science education. They emphasize the importance of starting at this level to influence higher education, industry, and the whole planet.
Research questions
To address these questions, the primary author first conducted twenty-eight semi-structured interviews of teachers and staff from LTP after receiving Institutional Review Board (IRB) approval to work with human subjects (project #3028). Informed consent was obtained from each participant before each interview following the approved protocol. All active teachers from two years of the program as well as the majority of former teachers from LTP were interviewed, for a total of twenty-six teachers from a wide variety of public and private schools throughout the US and Canada. Most of these teachers ( n = 18) teach primarily at the secondary-level, and the rest ( n = 8) teach primarily pre-secondary students, including three teachers from the elementary level. The two staff members directly involved with LTP were also interviewed in order to provide their direct programmatic reflections on the program and better shape research conclusions. These population sampling approaches help ensure that our research is reflective of all program perspectives.
As a research team, we transcribed each interview to collect qualitative data from subsequent coding. Each week, we discussed our initial impressions of the interviews as we would transcribe them and started noticing some commonalities amongst them. Once the interviews were transcribed, we were able to begin the qualitative coding process. This process involved assigning pairs (in rotation) to code each interview transcript. We constantly compared coding decisions and ultimately would come to an agreement on which code would fit a certain part of an interview best. If there was uncertainty, it could be brought up in the weekly team meeting to further discuss. This consensus coding process lessened biases and allowed for collaboration as a team. Once the interviews were coded, they were put into NVivo for further sub-coding.
Classroom observations
Field notes and content representations.
Additionally, the Resource Folio approach provided another method of investigating teacher's espoused PCK ( Loughran et al. , 2012 ). Before the LTP Summit from July 20–22 of 2020, each teacher was asked to work on a Content Representation (CoRe) worksheet, which prompted them to describe their classroom practices for specific content. See Table 1 for an example CoRe worksheet. This method is designed to make explicit the often implicit knowledge that teachers have about PCK. It provides a common structure and language for teachers to share their teaching expertise with each other and with a wider community. At the Summit, one session was reserved for teachers of similar content areas to talk about their initial CoRe. These conversations and the worksheets helped shape interview questions and later research plans.
The field of K-12 green chemistry education
To investigate this area of expertise, the interview script asked participants directly to define green chemistry as they understand it in their classroom. Based on interview data, here is our working definition as a synthesis of participants’ descriptions:
Green chemistry is practicing sustainability; it is a healthier, safer, and more cost-effective approach to studying chemistry. It is a lens that allows for the understanding that sustainability at all levels can change our surroundings. As a worldview, it demonstrates the need to shift towards safer production methods, ultimately reducing waste and toxicity in the environment.
Some teachers described green chemistry with a more industrial or manufacturing understanding, such as one participant who said, “green chemistry is a way of making stuff safely and smartly, a way that improves everyone's lives without messing with your own health or negatively impacting others that you don't necessarily think about. It's the safest and smartest way to make stuff to help improve the world.” Many teachers retained some connection with the manufacturing roots of green chemistry and convey that message to students.
Other teachers described green chemistry in terms of the educational benefits it brings, such as “emphasizing that chemistry doesn't need to be taught using things that make explosions, that uses chemicals that have higher risk, whether it's reactivity, flammability, things like that, but it can be done in a safe way and actually… when you use materials that are more benign, kids [ sic ] are typically more familiar with them and can make better connections to the concepts we're trying to teach anyway.” For many teachers, green chemistry necessarily included these types of connections to students’ lives.
The variety of descriptions from these teachers helps form a basis for an educational understanding of green chemistry. This type of description can help support the program goals of empowering and equipping other teachers to implement green chemistry. However, the diverse ways of defining green chemistry also imply a need for making these teachers’ PCK more explicit to share with wider audiences. The CoRe worksheets will be an additional source of data to understand how green chemistry is incorporated to these teachers’ classrooms and share that information more widely.
Justification
A large set of justifications covered the educational benefits of green chemistry. Teachers noted many observations from their own classrooms involving student motivation and learning which confirmed their teaching approach. One teacher understood their classroom in this way:
And having made that switch [to be ‘more hands-on’] what a teacher always fears are accidents in the classroom and so you tend to do many demonstrations with students. And of course, if the students don't understand you kind of give them the answer. But having the green chemistry part and having students take ownership of it… it actually opens up a whole spectrum for these students.
This teacher highlighted both the paired benefits of allowing students to be “more hands-on” and to take more “ownership” of their work in the classroom, which allowed students more freedom for inquiry in a lab, pursuing questions that interested them within a safe context as guided by their teacher. Green chemistry is not the only educational approach that includes these types of goals. However, for teachers in LTP, green chemistry seemed to answer many of their wider questions about modern science education, which became a powerful justification for taking that approach.
Another benefit of designing labs to be safer and more environmentally friendly is creating more opportunities within special education. As one teacher said:
But with green chemistry, I think there is a market there where we could target… that's my goal for this year: target special ed. If you have a child who can't behaviorally, academically, physically, or if you have a kid that flails, if you have CP or things like that, and there are body motion issues. If they have issues with that, using the harsh chemicals with the goggles, they may not want to wear them. Just because it's a tactile issue. They may not want gloves on. But we might have replacement labs that they can learn the same skill in a safe manner that maybe the special ed teacher could even do with them, because they don't have to worry about the harsh chemicals.
This teacher brings up an important aspect of green chemistry in education which is inclusivity. Students with disabilities can be more safely taught and involved in hands-on experiences through using green chemistry.
Several teachers spoke passionately about their moral commitment to green chemistry and sustainable science education. As one of them explained:
It's the only way that we can kind of save this planet, and I think that it's exciting for kids. It's empowering for kids, and it's a different way of thinking about things. And we have to change the way we think about things.
These teachers viewed science education as part of their broader commitment to the planet and to their students. For them, green chemistry is a vital tool for addressing environmental issues and, therefore, imperative to prepare “conscious citizens and not just scientists.”
Teachers also explained the benefits of green chemistry to foster life skills that extend beyond their classrooms. Collaboration was a key skill that one teacher highlighted by saying, “We want our students to be scientifically literate once they’re out in the community. But if they can't collaborate well together, that's not going to change anything.” Another teacher extended some of the twelve principles of green chemistry into life skills beyond chemistry, saying:
I had students going on and not only going into chemistry, which I was excited for, but if they were going into any other field, they're taking those skills with them and they’re really branching out and again, utilizing those skills that they're learning, whether it be as simple as waste reduction or toxicity, or you name it, any principle that we're really looking at.
The ethical framework embedded in green chemistry offered these teachers something different than other science curricula. In the interviews, these justifications built upon the motivations to explain why participants stayed involved in LTP and continued using green chemistry principles in their classroom beyond their initial interest.
Green chemistry as chemistry
You don't have to be a chemist to implement green chemistry. [Teachers] don’t have to be teaching chemistry to implement green chemistry. Let's think about the sustainable practices or changing the name slightly so you feel more comfortable with that implementation in the classroom, but outside of more teaching pedagogy and just general outreach to some younger grades or younger teachers as well.
These teachers struggled to decide on the best language for their collective classroom approach, but green chemistry served as a common entry point or hook for all of them.
For these teachers, green chemistry added to and supported their existing approach to science education. One of the teachers gave this definition: “Green chemistry really is chemistry but done in a more thoughtful manner with the health and safety of not just your students but the environment at the forefront.” For some teachers, green chemistry became the predominant lens through which they saw and described their own teaching practice, such as one teacher who said, “Green chemistry can be found in every single unit that I do.” By viewing green chemistry more as sustainability rather than chemistry, the term can reduce the intimidation many K-12 students feel towards chemistry as a subject. Another teacher added that they had been using green chemistry even before using the term, explaining, “as an elementary teacher, I’ve always done sustainable science, and we always do green chemistry. Everything in elementary is kitchen science.” Their efforts to translate this commitment to other teachers seemed to require a reconsideration of their language and led to these distinctions around “sustainability” or “green chemistry” simply as “chemistry.”
Summary of green chemistry in education
| A mind map illustrating the overarching outcomes of teaching green chemistry in K-12 classrooms (blue boxes) as developed from interview transcripts. The red boxes reflect common ideas that branch out from these outcomes, while green boxes represent less frequent or more specific topics mentioned in one or more interviews. | ||
Health and safety were described by many teachers as a way to develop safe and effective solutions to problems in the classroom. With an emphasis on the word “safe,” labs can be designed to foster a safe environment for the instructor and the students. A direct result of this is waste prevention, specifically regarding the labs done in the classroom. A teacher said “green chemistry is not just looking at the immediate effect, it's looking at the long-term effect and how do we make it better and safer for all of us.” It is important to think about the entire chemical processes when it comes to green chemistry in education, not just the end products and cleaning up in response. By teaching green chemistry in the classroom, students can begin to think about the risks that are involved with every decision they make. One teacher described this as “looking at the very beginning of the creation of those molecules all the way through their end of life.” Then asking ourselves, “how can we make things greener and make them so that they have the least amount of impact on the environment and have the lowest risk involved to people?” The goal is to move forward with sustainability enforcing the prevention of harm to human health and the environment.
Through designing and problem solving in the classroom, students learn to think creatively. This requires the use of critical thinking and logic to arrive to the best possible solutions with green chemistry as the basis. One teacher said that “green chemistry is an important way to advance chemistry education by increasing sustainability and also increasing creativity for students.” As a direct result of designing and problem-solving, students can develop a more sustainable mindset. They can also become more thoughtful in the decisions they make both in and outside of the classroom. Teachers say this can further develop with students as they are made aware of the classroom and lab decisions being made behind the scenes. A teacher described an example where students “learn how to read an MSDS sheet because they need to do that in order to evaluate whether they're going to use iron or they're going to use nickel.” The students also “have to figure out which one is the safer option they’re going to use, and which one produces a safer byproduct.” This way of teaching chemistry was described as “the green chemistry way”. For similar reasons, another teacher promotes “students to look at the toxicity as well as different chemicals [they] are using in the classroom.” Rather than designing a safer, more environmentally friendly lab for the students and having them do it, the teachers mentioned involving or explaining to the students the decisions that were made and why.
In addition to students becoming more aware and involved in classroom lab decisions, students can be impacted by green chemistry beyond the classroom setting in both short and long-term ways. They can become more conscious of their daily life decisions beyond their K-12 education, as echoed by one teacher who named the goal of “preparing conscious citizens and not just scientists.”
Pedagogical and professional experience repertoires as classroom case studies
To accomplish these goals, four case studies are presented below with further commentary developed into a Content Representation (CoRe) for green chemistry in K-12 classrooms. We chose to present these PaP-eRs first to build our grounded theory starting from the classroom experiences of teachers before generalizing an overall CoRe. As Loughran et al. (2012) state:
PaP-eRs bring the CoRe to life and shed new light on the complex nature of PCK. They help create ways to better understand and value the specialist knowledge, skills and ability of teachers thus making that which is so often tacit, explicit for others (p. 25).
These case studies highlight the expertise of four exceptional teachers of green chemistry at the K-12 level.
In one of the interviews, a teacher described a full lesson to illustrate how green chemistry works on different levels between teachers and students to encourage ownership of safety in the classroom.
One of my favorite things to do with Beyond Benign is… a types of reactions lab. Ultimately my curriculum said students have to be able to identify different types of reactions, and they should conduct experimentation with those different reactions… They’re told they have to evaluate both of them using the 12 principles of green chemistry.
So one of those synthesis reactions will maybe be you have to heat things using the Bunsen burner. The other one you don't have to heat it, and so those two reactions they evaluate which one they're going to do, and they have to explain based on the 12 principles, which activity adheres best, or it's either safest or occurs at an ambient temperature, or, you know, whichever one of the 12 principles it hits. Explain why, and then they do that for reaction type one, and then reaction type two, which is a decomposition reaction and then a single displacement. Then a double displacement. So, there are eight potential reactions, and they gotta pick which four they're going to do.
And they do that by researching the toxicity of the chemicals by looking at, you know, the waste products that may be produced by looking at the safety aspect of which ones do I have to use PPE for? Which ones do I only need safety glasses for? Which ones will I need gloves for? And also, which ones you’ve done at ambient temperature? Which ones have to be heated?
And they go through, and they pick the four that they're going to do, and then they justify them, and all of that is done in pre-lab, so that might be done in the period before the lab and then the next day they actually conduct the experiment where they do the four different experiments, they make their observations, they go through, they write the chemical reaction, they balance the chemical reaction, they classify the reaction.
So that is them meeting the curriculum expectations. But the pre-work is where they actually had to evaluate the safety of the molecules, of materials, safety of the process as well as the waste stream. That's the green chemistry part. That's the sustainability piece, that's where the Lead Teacher Program leads them through. So where in my class students have to evaluate which reaction they do, another class in another school the teacher might prescribe them the four reactions, but those four reactions may not have even been the four safe ones, and even if they were the safe ones, that's the teacher doing green chemistry, not the students doing the green chemistry.
If the teacher does the green chemistry. That's good. You save the materials you save the cost, you are doing it safe for the students, but the students aren't getting the opportunity to evaluate the different procedures, and so that's where that topic goes from being just a very cursory cookbook lesson: mix these chemicals, write the reactions… which still they should hit the expectation.
But by doing it the green chemistry way, by doing it through the lesson plan developed through the Lead Teacher Program, then you actually have students being thoughtful about choosing them. They learn how to read an MSDS sheet because they need to do that in order to evaluate whether they're going to use iron or they're going to use nickel. And they have to figure out which one is the safer options they’re going to use and which one produces a more safe byproduct.
This teacher integrated an understanding of green chemistry that includes teachers making design decisions for their students while also enabling their students to consider chemical safety throughout a lab. This approach to safety represents the first component of PCK from Magnusson et al. (1999) : “orientations toward science teaching.” For this teacher, science education involves students actively in the process of planning and carrying out investigations safely.
As exemplified in this case study, a green chemistry approach enables teachers to make decisions that minimize risks and include students actively in the safety process with appropriate levels of support. Students’ understandings and abilities differ across grade levels, but even younger students can be guided to think through potential chemical or physical risks involved in an experiment. Therefore, green chemistry is not only a behind-the-scenes method for teachers. It can also support a direct approach to involve students in planning and carrying out investigations.
The teacher first reminded students that they had previously talked about green chemistry. As a reminder, he summarized three “criteria” of green chemistry: materials that are safer to make and clean, just as effective, and, on an industrial level, cheaper. He then told the students that they would be making a green glue, which refers “not to the color” but to it being “made of household chemicals.” As further emphasis, he added that “you could eat it. It would taste nasty, but it wouldn’t hurt you.” Finally, he noted that other glues can contain many harmful chemicals, in contrast to the green glue which the students will make.
Throughout the procedure, the teacher guided students to work in pairs by talking through each step. First, he asked them to pick up materials from lab benches including baking soda, vinegar, and powdered milk which were already measured out into plastic cups. Then he had students label the cups on their own, noting that they can easily “find the vinegar with your nose, even with a mask on.” He reminded them of several safety rules when they are in the lab like being aware of the space around them and cleaning up any spills immediately. Next, he had them begin adding ingredients together. The teacher brought hot water to each table to mix in with the powdered milk and dissolve it. Then students needed to add the vinegar to the milk and mix until a curd formed, which they removed and put on a paper towel. The teacher collected the remaining liquid whey. Then students broke up the curd with a fork in the plastic cup and added some hot water as needed. Finally, they added some pinches of baking soda to neutralize the remaining solution and finish their green glue.
After finishing the procedure and cleaning up briefly, the teacher returned to the green chemistry criteria to decide if this glue fit. He emphasized that the materials were safer going in, since the students did not wear aprons or goggles, and going out, since they are able to pour everything down the drain at the end. He connected the importance of waste that goes down the drain because it can impact well water and aquifers in the local area. Then he suggested that they could calculate how cost-effective the glue was by adding up the ingredients. Finally, he asked: “does it work?” He continued by saying, “If it's not effective, then there's no point in doing it.” So, he suggested that students use their green glue to create a collage out of torn up construction paper in the last ten minutes of class. Then they could check the next day to see how their glue worked. Several students were proud to show me their collages as they finished.
Overall, this lesson exemplifies the second component of PCK from Magnusson et al. (1999) : “knowledge and beliefs about science curriculum.” For elementary students, making glue from ingredients that they knew was an experience of green chemistry as a creative activity. The curriculum for this lesson and this teacher's specific approach guided students through the process safely and effectively. Students of any age being able to hold something in their hands that they helped make is an empowering tool for learning. These students might not be able to restate the three criteria of green chemistry, but they could certainly show off the collages they created and know they had a hands-on role in creating something useful.
In one of the classroom observations for a high school chemistry class, the teacher introduced the twelve principles as part of a unit on plastics. This case study provides an annotated outline of the principles as presented in this class, including the examples the teacher used for each, to provide a basis for other teachers to consider how or if they can integrate the principles directly ( Table 2 ).
| Green chemistry principle | Formal description and teacher connections |
|---|---|
| 1. Waste prevention | Formal description: prioritize the prevention of waste, rather than cleaning up and treating waste after it has been created. Plan ahead to minimize waste at every step. |
| Simplified description: “Whenever you’re doing a chemical reaction, it ends up producing some waste… maybe not everything you have in that reaction actually gets used, so the whole point is to limit that waste.” | |
| 2. Atom economy | Formal description: reduce waste at the molecular level by maximizing the number of atoms from all reagents that are incorporated into the final product. Use atom economy to evaluate efficiency. |
| Simplified description: “Whatever atoms you start with and put into the reaction should be what you’re taking out of the reaction.” | |
| Connection to previous material: “If we remember working with our molymods [molecular modeling kits], we were making all of those different chemicals. We added a bunch of chemicals together, and those atoms are what ended up in our product… Basically: do you have atoms that are hanging out, or are they all present in your products?” | |
| 3. Less hazardous chemical synthesis | Formal description: design chemical reactions and synthetic routes to be as safe as possible. Consider the hazards of all substances during the reaction including waste. |
| Simplified description: “Basically that means that we’re designing safe reactions.” | |
| Real-life examples: “If I’m talking about a safe reaction, what are we not going to be doing during a safe reaction? What would that not look like?… We wouldn’t be lighting things on fire. We wouldn’t be causing things to explode… This actually happened to me when I was in undergraduate. I was working in a research lab, and someone was working with a reaction that was very sensitive to water. And they actually ended up having a bunch of glassware explode on them that day… They were doing everything correct; it was just humid that day.” | |
| 4. Designing safer chemicals | Formal description: minimize toxicity directly by molecular design. Predict and evaluate aspects such as physical properties, toxicity, and environmental fate throughout the design process. |
| Simplified description: “This is going more into the toxicity of it. So how toxic is that chemical compared to others?” | |
| Real-life examples: “A big area you can kind of think about designing safer chemicals are actually with your skincare and healthcare products. So are there any skin or healthcare things that you try to avoid?… Botox might be one of them. That's quite literally putting something poisonous in it so that it freezes that area… Lead, yeah, so pencils are now made of graphite, so we’re not ingesting a lot of lead… Asbestos, that is pretty common with a lot of household materials.” | |
| 5. Safe solvents and auxiliaries | Formal description: choose the safest solvent available for any step. Minimize the total amount of solvents and auxiliary substances used, as these make up a large percentage of the total waste created. |
| Real-life examples: “This one is a little bit tricky to understand… One of the safest solvents you can actually use is water. Water is a nice, safe solvent that we use in most chemical reactions to mix a lot of those powders and things.” | |
| 6. Designed for energy and efficiency | Formal description: choose the least energy-invasive chemical route. Avoid heating and cooling, as well as pressurized and vacuum conditions (i.e., ambient temperature and pressure are optimal). |
| Real-life examples: “What would it look like to not be energy-efficient? If we’re not energy-efficient, what are we using?… Fossil fuels, yeah, so that's going into more the renewability of it. But in terms of energy, maybe we’re using fossil fuels to power up a gas generator that we’re pulling energy from. Anytime you have to heat up a reaction, you’re using electricity. That is not energy efficient. Anytime you have to cool it down. Anytime you have to add pressure to it. All of those situations require energy that you don’t need. If you could do a reaction just on your tabletop without doing anything, that is a nice, energy-efficient reaction.” | |
| 7. Use of renewable feedstock | Formal description: use of chemicals which are made from renewable (i.e., plant based) sources, rather than other, equivalent chemicals originating from petrochemical sources. |
| Real-life examples: “That's basically anything that's not oil-based, so like corn, potato, tapioca. All of those feedstocks we can grow again and again, so those are all considered renewable resources.” | |
| 8. Reduce derivatives | Formal description: minimize the use of temporary derivatives such as protecting groups. Avoid derivatives to reduce reactive steps, resources required, and waste created. |
| Simplified description: “We’re trying to minimize how many side products that get made.” | |
| Connection to previous material: “A lot of the time, the way that I’ve taught you a reaction,” teacher says while drawing an example reaction on the board, “is you start with your reactants, and all of that goes together to make your product. In real life, that's not what happens. You get some side products. You get a whole lot of different things happening. Maybe you get like part of A mixed with part of B. So you might be able to get a lot of derivatives from that reaction, and this is all about how efficient your reaction is. The whole goal is that you don’t get a lot of these derivatives.” | |
| 9. Catalysis | Formal description: use catalytic instead of stoichiometric reagents in reactions. Choose catalysts to help increase selectivity, minimize waste, and reduce reaction times and energy demands. |
| Simplified description: “We’re using a catalyst to help speed up that reaction, and it also reduces waste and reduces the reaction time. Basically, we’re speeding it up.” | |
| Real-life example: “What is a catalyst that got you to school today?… Your car, okay, so your car got you to school. Is your car getting the education it needs right now? No, so your car helped get you here. It got you here quicker so that you can learn and then you can go home. That is like a catalyst. It's not part of the reaction. It's not part of your learning, but it's helping you make that learning possible.” | |
| 10. Design for degradation | Formal description: design chemicals that degrade and can be discarded easily. Ensure that both chemicals and their degradation products are not toxic, bio accumulative, or environmentally persistent. |
| Simplified description: “That basically means: can it degrade and dissolve in whatever material? Is it going to break down and not just make a little microplastic? Ideally when it degrades, it degrades into things that are not toxic, because a lot of times things degrade into things that are toxic. So how can we prevent that toxicity?” | |
| 11. Real-time prevention | Formal description: monitor chemical reactions in real-time as they occur to prevent the formation and release of potentially hazardous and polluting substances. |
| Simplified description: “That's all about preventing hazardous pollutants. That one's pretty self-explanatory.” | |
| 12. Safer chemistry for accident prevention | Formal description: choose and develop chemical procedures that are safer and inherently minimize the risk of accidents. Know the possible risks and assess them beforehand. |
| Simplified description: “You don’t want to have accidents, especially if we want to bring this to a mass scale. So that's really taking in all of the risk factors and seeing ‘are those risk factors going to affect us?’” | |
In the first part of this observation, the class watched videos about recycling and ocean plastic clean-up as initial context to motivate the lesson. The teacher then asked students to consider examples, benefits, and drawbacks of plastics from their experience. To introduce green chemistry, the teacher explained that creating a biodegradable plastic would be one solution to the recycling issues. She gave a brief history of the development of green chemistry and stated this initial definition: “green chemistry is the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances.” Then students were asked to match up formal descriptions of each of the twelve principles with the titles, as shown below. They had time in pairs to work on that matching exercise on their computers. After about ten minutes, the teacher went through each principle and gave examples or annotations to make the ideas more relatable to the students. Connections to previous material, simplified descriptions, and real-life examples were used for different principles to explain them more fully. The examples serve as initial entry points and providing them here can allow teachers to consider their own examples or descriptions more easily.
These initial examples were intended as an overview of the principles before continuing the unit on plastics where the students would apply the principles more directly. After these examples, the teacher also acknowledged that “if you didn’t get all of these, that's okay. A lot of these definitions are very technical. We’re going to continue working with these throughout the year as we’re learning these different principles.” Finally, the teacher assigned one principle each to pairs of students for them to explore more deeply by watching a video and answering a series of prompts. In subsequent days of this unit, students created their own plastics based on polylactic acid with a variety of starting materials and additives.
Overall, this case study helps to address one of the central issues of green chemistry education at the K-12 level, which is how to adapt a system that was developed for an industrial and research context into educationally appropriate and valuable content. Teachers often adapt green chemistry by providing examples that make sense developmentally and culturally to their students. The expertise needed to make these kinds of connections was one of the main ways that PCK was initially described by Shulman (1986, p. 9) . This expertise will necessarily differ at other levels of education like in university classrooms ( Grieger et al. , 2022 ). This classroom provides just one glimpse into that process from a high school teacher's perspective.
The supplemental examples that teachers used for green chemistry represent another subset of expertise that varies depending on local context and how familiar the teacher is with the material. Knowing appropriate examples would fit into the third component of PCK from Magnusson et al. (1999) : “knowledge and beliefs about students’ understanding of specific science topics.” Naturally these examples would differ based on classrooms, local regions, cultural background of students, and age range. These types of connections are similar to the approach of place-based education ( Sobel, 2013 ), and they help to capture the PCK that teachers develop for their individual classrooms.
The teacher's role in this classroom was largely to propose questions for the students and ask them to explain their reasoning or to give evidence for their conclusions, a method heavily reflective of the NGSS Lead States (2013) . The types of questions varied based on the abilities of the students and where they were in the experiment. At one point, the teacher warned a student to think through their experiment thoroughly, saying, “I’m going to ask you a bunch of questions before you finish, so make sure you understand.” In fact, questions were the vast majority of ways that the teacher spoke with students and provide insight into how this teacher views the experimental process.
Some of the questions that the teacher asked included:
(1) “What are you going to do? How are you going to do that?”
(2) “Is this making sense?”
(3) “What is a rule of good chemistry? What is important to remember when you are doing chemistry?”
(4) “What do you mean by ‘precautions?’ What would be an example of a safety precaution?”
(5) “How will you be safe? What kinds of things will you do to stay safe?”
(6) “What are you going to do next? You get to decide.”
(7) “What are you doing for your second iteration?”
(8) “What did we learn?”
(9) “How is [the soap] going to take away the germs? And how is homemade soap different from other types, like Dove?”
(10) “What scents are you using [for your soap]? What additives are you using? What are you putting in yours?”
(11) “What did you notice about the pictures you took before and after washing your hands?”
(12) “Other groups had pictures that looked like this, but yours looked like that. Why do you think that is?”
The teacher rarely gave direct answers or instructions to students and instead focused on prompting their own reflection on the experiment and their conclusions. At times, the teacher gave advice to students about the experimental procedure to limit the range of options, such as adding essential oils one drop at a time. The procedural scaffolding supported students to focus on the scientific process of investigation and explaining their reasoning.
Because of the inherent safety of green chemistry, this teacher was better able to construct the classroom in a way that supported this type of open and self-paced inquiry. This approach exemplifies the fourth component of PCK from Magnusson et al. (1999) : “knowledge and beliefs about assessment in science.” Verbal assessments gave this teacher direct information about students’ understanding in addition to the written assignments included in the unit. For a green chemistry approach that can tend to be more variable and student-directed, this type of assessment works well. Labs and activities are built around scientific explanations more than accurate results or correct answers. Green chemistry does not require this assessment approach, but it does allow for significant flexibility and explicit scaffolding from the twelve principles to enable students to test and defend their ideas safely and thoughtfully.
Green chemistry content representation
The CoRe outlines some of the aspects of PCK “most attached to that content,” but it is not the only representation. It is a necessary, but incomplete, generalization that helps to make the complexity accessible and manageable; it is neither complete nor absolute (p. 25).
The descriptions in Table 3 explain the main components of this CoRe connected with the relevant components of PCK from Magnusson et al. (1999) : (1) orientations toward science teaching, (2) knowledge and beliefs about science curriculum, (3) knowledge and beliefs about students’ understanding of specific science topics, (4) knowledge and beliefs about assessment in science, and (5) knowledge and beliefs about instructional strategies for teaching science (p. 97).
| CoRe area | Description | Relevant PCK components(s) from (1999) |
|---|---|---|
| Chemical safety | Green chemistry is inherently safer, smaller scale, and more cost effective compared to traditional curricula. It encourages teacher and student ownership of chemical safety throughout the classroom. | 1 |
| Practice of chemistry | Green chemistry provides an empowering experience of making and using chemicals, helping students to imagine creative possibilities of the future. | 1, 2 |
| Teacher-facilitated connections | Teachers support their students’ engagement with green chemistry by making connections with industrial, community-based, or real-life examples. | 3, 5 |
| Experimentation mindset | Green chemistry supports classrooms to be exploratory, design-oriented, and based in problems and solutions. Students are supported to make claims and cite evidence to support their conclusions. | 4, 5 |
The four CoRe areas reflect the four case studies above but are generalized to be applicable to more classrooms. Overall, the CoRe summarizes the expertise of teachers in LTP beyond traditional chemistry content and classroom management strategies. These four areas speak to the unique contribution that green chemistry makes to K-12 science education and can serve as a basis for further reflection and development by teachers and researchers. Some of the areas overlap, but each one highlights an emphasis that green chemistry brings compared to more traditional chemistry curricula.
First, green chemistry makes chemical safety more explicit for teachers and students. By using less hazardous chemicals and smaller scales, experiments are inherently safer for students and for the environment. Everyone in the classroom can take ownership for safety through open conversations and applying the twelve principles to their experiments. Teachers who choose to incorporate green chemistry view learning as a shared process with students in their classroom. An added benefit of chemical safety also includes cost savings from materials, waste disposal, and addressing risks of injuries.
Second, green chemistry supports an empowering experience of chemistry as a creative science of solutions. K-12 green chemistry curricula should be designed to encourage engagement with this practice of chemistry rather than simply a science of following procedures. Through green chemistry, teachers and students can develop a perspective of science that is capable of confronting future issues in ways that protect people and the planet.
Third, green chemistry requires teachers to develop a wide range of connections to real-life and their local region in order to connect students with the content. A contextual approach to chemistry engages students and motivates their learning far beyond the classroom. Simplified descriptions and connections to previous material also serve to weave green chemistry into existing curricula.
Finally, green chemistry includes an experimentation mindset where students are assessed based on their ability to cite evidence from experience to justify their claims. These classrooms are exploratory, design-oriented, and based in finding solutions to real world problems. Both instruction and assessment must be adapted to this type of environment.
Limitations
Conclusions, implications for k-12 green chemistry education.
The value of teaching green chemistry to K-12 students was also very prevalent throughout this research. There are many benefits for the students to learn green chemistry regardless of their intent to pursue a science education or not. Teachers described using green chemistry to engage their students and develop a sustainable mindset. Students can think about the consequences of their daily life decisions and how they can impact human health and the environment.
Teachers often highlighted the idea that all chemistry should be seen through the lens of green chemistry. However, from a research standpoint, they are separate for a reason ( MacKellar et al. , 2020 ). Yes, it is beneficial for educators to teach chemistry through a green chemistry lens across many topics. However, the point of green chemistry is ultimately to make our current and new chemistry practices safer for the environment and human health ( Mahaffy et al. , 2019 ). If all chemistry was green chemistry, chemists may be less likely to use this sustainable mindset in re-evaluating current practices or in designing new ones.
One important take-away from this research is the need for more K-12 green chemistry resources. K-12 teachers have difficulty integrating the twelve principles of green chemistry directly because they are not as applicable to the curriculum of younger students ( Ause, 2018 ). However, given the demands of their job and work-load as teachers, the green chemistry educational community could benefit from having access to more resources to allow greater integration of green chemistry in their classrooms.
Future research directions
A critical component of any educational program is its effects on students in the short and long term. With a more well-defined foundation for green chemistry in K-12 classrooms, further work will be needed to study how this approach affects student learning ( Bransford et al. , 1999 ; Grieger et al. , 2022 ). With a basis for defining PCK for green chemistry, connections could be investigated more thoroughly between teachers’ levels of expertise and student outcomes ( Coe et al. , 2014 ). Future studies can consider the longer term impact of learning about green chemistry for students in later university classes or careers in industry. These types of connections would be critical to advancing green chemistry education.
Finally, we return to the purpose for this project. The future cultivation of teacher expertise in the current landscape of green chemistry can benefit from the results and discussion covered here. Developing pedagogical content knowledge among K-12 teachers in the Lead Teacher Program has served to advance to prominence and understanding of green chemistry. And if continued to be developed in ways that are sustainable for participants and staff, LTP and other work by Beyond Benign can continue to bear fruit for many years to come.
The reaction equation of exceptional chemistry teaching
What is needed is a green approach to K-12 education that more directly supports exceptional teaching and learning. The Pedagogical Content Knowledge developed with teachers in LTP through this project can act as a catalyst to provide alternate kinetic patterns and help teachers to identify the most sustainable and productive educational pathways for their own classroom. The approach of green chemistry more broadly can act as a renewable energy source to drive the reaction thermodynamically. Together, the knowledge and practices explored in this project can make exceptional chemistry teaching more sustainable and attainable for K-12 teachers. As one Lead Teacher shared, that vision will truly be “something that's valuable to humankind at the end of the day.”
Berkeley Haas Case Series
The Berkeley Haas Case Series is a collection of business case studies created by UC Berkeley faculty

- Sustainability
Levi Strauss & Co.: Driving Adoption of Green Chemistry
Learning objectives.
Pub Date: Jun 30, 2016
Revision Date: Jul 15, 2016
Discipline: Operations Management
Subjects: Manufacturing, Collaboration, Social responsibility, Sustainability, Supply chain management, Green business
Product #: B5867-PDF-ENG
Industry: Chemicals, Apparel, Manufacturing
Geography: United States, Asia, Europe
Length: 21 page(s)
Recommended
A new collection of business case studies from Berkeley Haas
The aim of the Berkeley Haas Case Series is to incite business innovation by clarifying disruptive trends and questioning the status quo.
Breadcrumbs Section. Click here to navigate to respective pages.

Scalable Green Chemistry
DOI link for Scalable Green Chemistry
Get Citation
Packed with real-world examples, this book illustrates the 12 principles of green chemistry. These diverse case studies demonstrate to scientists and students that beyond the theory, the challenges of green chemistry in pharmaceutical discovery and development remain an ongoing endeavor. By informing and welcoming additional practitioners to this m
TABLE OF CONTENTS
Chapter 1 | 24 pages, introduction to green pharmaceutical science: fact, fiction, and future, chapter 2 | 18 pages, green chemistry in drug development, chapter 3 | 32 pages, development of green-by-design, practical biocatalytic processes, chapter 4 | 30 pages, application of green metrics to scalable industrial synthesis plans: approaches to oseltamivir phosphate (tamiflu r© ), chapter 5 | 36 pages, the road to becoming green: process development of ar-a2, an active pharmaceutical ingredient with, chapter 6 | 16 pages, improved and greener process for pioglitazone and its pharmaceutically acceptable salts and lokeswara rao madivada, chapter 7 | 10 pages, the development of a convergent green synthesis of linezolid, an oxazolidinone antibacterial agent, chapter 8 | 18 pages, development of a nonaqueous process for the synthesis of amino-pentan-1,5-diol, chapter 9 | 22 pages, development of a robust, environmentally responsible process for the manufacture of tofacitinib citrate, chapter 10 | 28 pages, selective nitration under cgmp conditions, chapter 11 | 66 pages, going green using combined real-time analytics and process automation, chapter 12 | 38 pages, approaches to the scale-up of organic chemistry using microwave heating, chapter 13 | 18 pages, challenges faced and future directions.
- Privacy Policy
- Terms & Conditions
- Cookie Policy
- Taylor & Francis Online
- Taylor & Francis Group
- Students/Researchers
- Librarians/Institutions
Connect with us
Registered in England & Wales No. 3099067 5 Howick Place | London | SW1P 1WG © 2024 Informa UK Limited

IMAGES
VIDEO
COMMENTS
Green chemistry as a discipline is gaining increasing attention globally, with environmentally conscious students keen to learn how they can contribute to a safer and more sustainable world. Many universities now offer courses or modules specifically on green chemistry - Green Chemistry: Principles and Case Studies is an essential learning ...
Five technologies that have succeeded in meeting that creative challenge have received 2017 Green Chemistry Challenge Awards. Merck, Dow Chemical, Koehler, Amgen, Bachem, UniEnergy Technologies ...
Case Studies. Selected case studies illustrate how the Green Chemistry approach can be applied in different companies and how it contributes to reduce the consumption of hazardous chemicals and enhance their economic and environmental performance. green chemistry, green chemistry toolkit, green chemistry toolkit UN, green chem, current research ...
solving case studies lend themselves very e ffectively to the teaching of green chemistry. Two examples of this approach have previously been published elsewhere 9, 10 . A Case Study in Green ...
In 1998, Paul Anastas and John C. Warner published a seminal book, Green Chemistry: Theory and Practice, which established the 12 Principles of Green Chemistry as the foundation for this dramatic shift in the way we do chemistry. 1 The 12 Principles fall into three general categories: (1) use and produce no toxic chemicals, (2) minimize the use of chemical and energy resources, and (3) prevent ...
Table 1 Selected case studies of green chemistry efforts advancing environmental justice in the areas of toxicity, renewability, degradability and circularity. Full size table.
CASE STuDIES. Green Chemistry: A Strong Driver of innovAtion, growth, AnD BuSineSS opportunity 39 Apple's Green chemistry leadership role Apple has three priorities when it comes to sustainability: climate change, resources (i.e., finite resources), and smarter chemistry. The last one is a big priority, but so is the goal of becoming
Table 1 | Selected case studies of green chemistry efforts advancing environmental justice in the areas of toxicity, renewability, degradability and circularity Conventional chemistry
The 12 Principles of Green Chemistry act as a guide for chemists for the design and synthesis of sustainable products. Adoption by chemists is essential for the transition. We chose the use of solvents (Principle #3) as an example of our commitment towards improved sustainabilty in chemical synthesis. Herein Green Chemistry Reviews Sustainable Laboratories
Green chemistry is the chemistry for "pollution prevention.". Green chemistry is a step toward the sustainable delivery of services and goods to growing population without compromising environmental quality. According to an estimate by United Nations, the world population will cross 10.7 billion by 2050.
The 12 Principles of Green Chemistry act as a guide for chemists for the design and synthesis of sustainable products. Adoption by chemists is essential for the transition. We chose the use of solvents (Principle #3) as an example of our commitment towards improved sustainabilty in chemical synthesis. Herein Green Chemistry Reviews Sustainable Laboratories
Systems thinking also helps one to manage the complexity that is inherent to sustainability and the implementation of green and sustainable chemistry (Constable, et al., 2019).Figure 1 shows a systems-level view of chemical evaluation. An important point to be made about thinking in systems within the chemistry context is that this should be accompanied by life cycle thinking, i.e., a ...
Royal Society of Chemistry, Cambridge 2020. 447 pp., softcover, € 78.00.—ISBN 978-1-78801-798-5 Skip to Article Content ... Green Chemistry: Principles and Case Studies. By Felicia A. Etzkorn. Roger A. Sheldon, Roger A. Sheldon. Molecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Johannesburg, South Africa ...
Green Chemistry is expanding its wings from academic laboratories to industrial units. Sustainable practices include replacement of volatile organic solvents which constitute the bulk of a reaction material, developing recyclable catalysts, developing energy efficient synthesis and encouraging the use of renewable starting material. By following the principles of green chemistry, turn-over of ...
Green Chemistry Principle # 12 is known as the "Safety Principle". It may be the most overlooked of the twelve principles, yet it is the logical outcome of many of the other principles. In fact, it is practically impossible to achieve the goals of Principle 12 without the implementation of at least one of the others.
As an illustrative example, we demonstrate the process of hacking a case related to Green Chemistry in the pharmaceutical industry, highlighting specific challenges for chemistry and chemical engineering education. ... A complex case: using the case study method to explore uncertainty and ambiguity in undergraduate business education. Teach ...
A case study in green chemistry: ... We hope that our example on transitioning and shifting mindsets towards a complete adoption of green chemistry principles will inspire others as well. A two-year collective effort towards the reduction by 50% of the usage of 7 hazardous solvents (Green Chemistry Principle #5) within a large-scale industrial ...
Royal Society of Chemistry, Cambridge 2020. 447 pp., softcover, € 78.00.—ISBN 978‐1‐78801‐798‐5 Skip to Article Content ... Green Chemistry: Principles and Case Studies. By Felicia A. Etzkorn. Roger A. Sheldon. Molecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Johannesburg, South Africa ...
Introduction Green chemistry education: from industrial principles to educational values Green chemistry originally developed from an industrial perspective and includes twelve principles to conceptualize and use chemicals in a more sustainable way (Anastas and Warner, 1998; Anastas and Eghbali, 2010).A list of the twelve principles is featured in Table 2 along with a classroom case study.
Green chemistry as a discipline is gaining increasing attention globally, with environmentally conscious students keen to learn how they can contribute to a safer and more sustainable world. Many universities now offer courses or modules specifically on green chemistry - Green Chemistry: Principles and Case Studies is an essential learning resource for those interested in mastering the subject.
This case examines the challenges and opportunities faced by Levi Strauss & Co. (LS&Co.) as it attempts to help establish a cross-industry sustainability initiative to eliminate hazardous chemicals in the apparel supply chain. LS&Co.'s Screened Chemistry Program screened chemical formulations against human and environmental health hazard endpoints before they chemicals entered the supply chain ...
Packed with real-world examples, this book illustrates the 12 principles of green chemistry. These diverse case studies demonstrate to scientists and students that beyond the theory, the challenges of green chemistry in pharmaceutical discovery and development remain an ongoing endeavor. By informing and welcoming additional practitioners to this m
Lesson Plan 13: Real-World Cases in Green Chemistry. PowerPoint Presentation 13: Real-World Cases in Green Chemistry ... PGCCA Case Studies: 2016: Newlight Technologies, AirCarbon: Greenhouse Gas Transformed into High-Performance Thermoplastic. 2012: Buckman International, Inc.: Enzymes Reduce the Energy and Wood Fiber Required to Manufacture ...
Description. Green Chemistry in Practice: Greener Material and Chemical Innovation Through Collaboration collects a unique set of case studies based on researchers' experiences in developing practical, green chemistry-driven solutions to industry problems as part of the Greener Solutions Program at the Berkeley Center for Green Chemistry.