- Resources Home 🏠
- Try SciSpace Copilot
- Search research papers
- Add Copilot Extension
- Try AI Detector
- Try Paraphraser
- Try Citation Generator
- April Papers
- June Papers
- July Papers


How To Write A Research Summary

It’s a common perception that writing a research summary is a quick and easy task. After all, how hard can jotting down 300 words be? But when you consider the weight those 300 words carry, writing a research summary as a part of your dissertation, essay or compelling draft for your paper instantly becomes daunting task.
A research summary requires you to synthesize a complex research paper into an informative, self-explanatory snapshot. It needs to portray what your article contains. Thus, writing it often comes at the end of the task list.
Regardless of when you’re planning to write, it is no less of a challenge, particularly if you’re doing it for the first time. This blog will take you through everything you need to know about research summary so that you have an easier time with it.
What is a Research Summary?
A research summary is the part of your research paper that describes its findings to the audience in a brief yet concise manner. A well-curated research summary represents you and your knowledge about the information written in the research paper.
While writing a quality research summary, you need to discover and identify the significant points in the research and condense it in a more straightforward form. A research summary is like a doorway that provides access to the structure of a research paper's sections.
Since the purpose of a summary is to give an overview of the topic, methodology, and conclusions employed in a paper, it requires an objective approach. No analysis or criticism.
Research summary or Abstract. What’s the Difference?
They’re both brief, concise, and give an overview of an aspect of the research paper. So, it’s easy to understand why many new researchers get the two confused. However, a research summary and abstract are two very different things with individual purpose. To start with, a research summary is written at the end while the abstract comes at the beginning of a research paper.
A research summary captures the essence of the paper at the end of your document. It focuses on your topic, methods, and findings. More like a TL;DR, if you will. An abstract, on the other hand, is a description of what your research paper is about. It tells your reader what your topic or hypothesis is, and sets a context around why you have embarked on your research.
Getting Started with a Research Summary
Before you start writing, you need to get insights into your research’s content, style, and organization. There are three fundamental areas of a research summary that you should focus on.
- While deciding the contents of your research summary, you must include a section on its importance as a whole, the techniques, and the tools that were used to formulate the conclusion. Additionally, there needs to be a short but thorough explanation of how the findings of the research paper have a significance.
- To keep the summary well-organized, try to cover the various sections of the research paper in separate paragraphs. Besides, how the idea of particular factual research came up first must be explained in a separate paragraph.
- As a general practice worldwide, research summaries are restricted to 300-400 words. However, if you have chosen a lengthy research paper, try not to exceed the word limit of 10% of the entire research paper.
How to Structure Your Research Summary
The research summary is nothing but a concise form of the entire research paper. Therefore, the structure of a summary stays the same as the paper. So, include all the section titles and write a little about them. The structural elements that a research summary must consist of are:
It represents the topic of the research. Try to phrase it so that it includes the key findings or conclusion of the task.
The abstract gives a context of the research paper. Unlike the abstract at the beginning of a paper, the abstract here, should be very short since you’ll be working with a limited word count.
Introduction
This is the most crucial section of a research summary as it helps readers get familiarized with the topic. You should include the definition of your topic, the current state of the investigation, and practical relevance in this part. Additionally, you should present the problem statement, investigative measures, and any hypothesis in this section.
Methodology
This section provides details about the methodology and the methods adopted to conduct the study. You should write a brief description of the surveys, sampling, type of experiments, statistical analysis, and the rationality behind choosing those particular methods.
Create a list of evidence obtained from the various experiments with a primary analysis, conclusions, and interpretations made upon that. In the paper research paper, you will find the results section as the most detailed and lengthy part. Therefore, you must pick up the key elements and wisely decide which elements are worth including and which are worth skipping.
This is where you present the interpretation of results in the context of their application. Discussion usually covers results, inferences, and theoretical models explaining the obtained values, key strengths, and limitations. All of these are vital elements that you must include in the summary.
Most research papers merge conclusion with discussions. However, depending upon the instructions, you may have to prepare this as a separate section in your research summary. Usually, conclusion revisits the hypothesis and provides the details about the validation or denial about the arguments made in the research paper, based upon how convincing the results were obtained.
The structure of a research summary closely resembles the anatomy of a scholarly article . Additionally, you should keep your research and references limited to authentic and scholarly sources only.
Tips for Writing a Research Summary
The core concept behind undertaking a research summary is to present a simple and clear understanding of your research paper to the reader. The biggest hurdle while doing that is the number of words you have at your disposal. So, follow the steps below to write a research summary that sticks.
1. Read the parent paper thoroughly
You should go through the research paper thoroughly multiple times to ensure that you have a complete understanding of its contents. A 3-stage reading process helps.
a. Scan: In the first read, go through it to get an understanding of its basic concept and methodologies.
b. Read: For the second step, read the article attentively by going through each section, highlighting the key elements, and subsequently listing the topics that you will include in your research summary.
c. Skim: Flip through the article a few more times to study the interpretation of various experimental results, statistical analysis, and application in different contexts.
Sincerely go through different headings and subheadings as it will allow you to understand the underlying concept of each section. You can try reading the introduction and conclusion simultaneously to understand the motive of the task and how obtained results stay fit to the expected outcome.
2. Identify the key elements in different sections
While exploring different sections of an article, you can try finding answers to simple what, why, and how. Below are a few pointers to give you an idea:
- What is the research question and how is it addressed?
- Is there a hypothesis in the introductory part?
- What type of methods are being adopted?
- What is the sample size for data collection and how is it being analyzed?
- What are the most vital findings?
- Do the results support the hypothesis?
Discussion/Conclusion
- What is the final solution to the problem statement?
- What is the explanation for the obtained results?
- What is the drawn inference?
- What are the various limitations of the study?
3. Prepare the first draft
Now that you’ve listed the key points that the paper tries to demonstrate, you can start writing the summary following the standard structure of a research summary. Just make sure you’re not writing statements from the parent research paper verbatim.
Instead, try writing down each section in your own words. This will not only help in avoiding plagiarism but will also show your complete understanding of the subject. Alternatively, you can use a summarizing tool (AI-based summary generators) to shorten the content or summarize the content without disrupting the actual meaning of the article.
SciSpace Copilot is one such helpful feature! You can easily upload your research paper and ask Copilot to summarize it. You will get an AI-generated, condensed research summary. SciSpace Copilot also enables you to highlight text, clip math and tables, and ask any question relevant to the research paper; it will give you instant answers with deeper context of the article..
4. Include visuals
One of the best ways to summarize and consolidate a research paper is to provide visuals like graphs, charts, pie diagrams, etc.. Visuals make getting across the facts, the past trends, and the probabilistic figures around a concept much more engaging.
5. Double check for plagiarism
It can be very tempting to copy-paste a few statements or the entire paragraphs depending upon the clarity of those sections. But it’s best to stay away from the practice. Even paraphrasing should be done with utmost care and attention.
Also: QuillBot vs SciSpace: Choose the best AI-paraphrasing tool
6. Religiously follow the word count limit
You need to have strict control while writing different sections of a research summary. In many cases, it has been observed that the research summary and the parent research paper become the same length. If that happens, it can lead to discrediting of your efforts and research summary itself. Whatever the standard word limit has been imposed, you must observe that carefully.
7. Proofread your research summary multiple times
The process of writing the research summary can be exhausting and tiring. However, you shouldn’t allow this to become a reason to skip checking your academic writing several times for mistakes like misspellings, grammar, wordiness, and formatting issues. Proofread and edit until you think your research summary can stand out from the others, provided it is drafted perfectly on both technicality and comprehension parameters. You can also seek assistance from editing and proofreading services , and other free tools that help you keep these annoying grammatical errors at bay.
8. Watch while you write
Keep a keen observation of your writing style. You should use the words very precisely, and in any situation, it should not represent your personal opinions on the topic. You should write the entire research summary in utmost impersonal, precise, factually correct, and evidence-based writing.
9. Ask a friend/colleague to help
Once you are done with the final copy of your research summary, you must ask a friend or colleague to read it. You must test whether your friend or colleague could grasp everything without referring to the parent paper. This will help you in ensuring the clarity of the article.
Once you become familiar with the research paper summary concept and understand how to apply the tips discussed above in your current task, summarizing a research summary won’t be that challenging. While traversing the different stages of your academic career, you will face different scenarios where you may have to create several research summaries.
In such cases, you just need to look for answers to simple questions like “Why this study is necessary,” “what were the methods,” “who were the participants,” “what conclusions were drawn from the research,” and “how it is relevant to the wider world.” Once you find out the answers to these questions, you can easily create a good research summary following the standard structure and a precise writing style.
You might also like

Consensus GPT vs. SciSpace GPT: Choose the Best GPT for Research

Literature Review and Theoretical Framework: Understanding the Differences

Types of Essays in Academic Writing - Quick Guide (2024)
- Privacy Policy

Home » Research Results Section – Writing Guide and Examples
Research Results Section – Writing Guide and Examples
Table of Contents

Research Results
Research results refer to the findings and conclusions derived from a systematic investigation or study conducted to answer a specific question or hypothesis. These results are typically presented in a written report or paper and can include various forms of data such as numerical data, qualitative data, statistics, charts, graphs, and visual aids.
Results Section in Research
The results section of the research paper presents the findings of the study. It is the part of the paper where the researcher reports the data collected during the study and analyzes it to draw conclusions.
In the results section, the researcher should describe the data that was collected, the statistical analysis performed, and the findings of the study. It is important to be objective and not interpret the data in this section. Instead, the researcher should report the data as accurately and objectively as possible.
Structure of Research Results Section
The structure of the research results section can vary depending on the type of research conducted, but in general, it should contain the following components:
- Introduction: The introduction should provide an overview of the study, its aims, and its research questions. It should also briefly explain the methodology used to conduct the study.
- Data presentation : This section presents the data collected during the study. It may include tables, graphs, or other visual aids to help readers better understand the data. The data presented should be organized in a logical and coherent way, with headings and subheadings used to help guide the reader.
- Data analysis: In this section, the data presented in the previous section are analyzed and interpreted. The statistical tests used to analyze the data should be clearly explained, and the results of the tests should be presented in a way that is easy to understand.
- Discussion of results : This section should provide an interpretation of the results of the study, including a discussion of any unexpected findings. The discussion should also address the study’s research questions and explain how the results contribute to the field of study.
- Limitations: This section should acknowledge any limitations of the study, such as sample size, data collection methods, or other factors that may have influenced the results.
- Conclusions: The conclusions should summarize the main findings of the study and provide a final interpretation of the results. The conclusions should also address the study’s research questions and explain how the results contribute to the field of study.
- Recommendations : This section may provide recommendations for future research based on the study’s findings. It may also suggest practical applications for the study’s results in real-world settings.
Outline of Research Results Section
The following is an outline of the key components typically included in the Results section:
I. Introduction
- A brief overview of the research objectives and hypotheses
- A statement of the research question
II. Descriptive statistics
- Summary statistics (e.g., mean, standard deviation) for each variable analyzed
- Frequencies and percentages for categorical variables
III. Inferential statistics
- Results of statistical analyses, including tests of hypotheses
- Tables or figures to display statistical results
IV. Effect sizes and confidence intervals
- Effect sizes (e.g., Cohen’s d, odds ratio) to quantify the strength of the relationship between variables
- Confidence intervals to estimate the range of plausible values for the effect size
V. Subgroup analyses
- Results of analyses that examined differences between subgroups (e.g., by gender, age, treatment group)
VI. Limitations and assumptions
- Discussion of any limitations of the study and potential sources of bias
- Assumptions made in the statistical analyses
VII. Conclusions
- A summary of the key findings and their implications
- A statement of whether the hypotheses were supported or not
- Suggestions for future research
Example of Research Results Section
An Example of a Research Results Section could be:
- This study sought to examine the relationship between sleep quality and academic performance in college students.
- Hypothesis : College students who report better sleep quality will have higher GPAs than those who report poor sleep quality.
- Methodology : Participants completed a survey about their sleep habits and academic performance.
II. Participants
- Participants were college students (N=200) from a mid-sized public university in the United States.
- The sample was evenly split by gender (50% female, 50% male) and predominantly white (85%).
- Participants were recruited through flyers and online advertisements.
III. Results
- Participants who reported better sleep quality had significantly higher GPAs (M=3.5, SD=0.5) than those who reported poor sleep quality (M=2.9, SD=0.6).
- See Table 1 for a summary of the results.
- Participants who reported consistent sleep schedules had higher GPAs than those with irregular sleep schedules.
IV. Discussion
- The results support the hypothesis that better sleep quality is associated with higher academic performance in college students.
- These findings have implications for college students, as prioritizing sleep could lead to better academic outcomes.
- Limitations of the study include self-reported data and the lack of control for other variables that could impact academic performance.
V. Conclusion
- College students who prioritize sleep may see a positive impact on their academic performance.
- These findings highlight the importance of sleep in academic success.
- Future research could explore interventions to improve sleep quality in college students.
Example of Research Results in Research Paper :
Our study aimed to compare the performance of three different machine learning algorithms (Random Forest, Support Vector Machine, and Neural Network) in predicting customer churn in a telecommunications company. We collected a dataset of 10,000 customer records, with 20 predictor variables and a binary churn outcome variable.
Our analysis revealed that all three algorithms performed well in predicting customer churn, with an overall accuracy of 85%. However, the Random Forest algorithm showed the highest accuracy (88%), followed by the Support Vector Machine (86%) and the Neural Network (84%).
Furthermore, we found that the most important predictor variables for customer churn were monthly charges, contract type, and tenure. Random Forest identified monthly charges as the most important variable, while Support Vector Machine and Neural Network identified contract type as the most important.
Overall, our results suggest that machine learning algorithms can be effective in predicting customer churn in a telecommunications company, and that Random Forest is the most accurate algorithm for this task.
Example 3 :
Title : The Impact of Social Media on Body Image and Self-Esteem
Abstract : This study aimed to investigate the relationship between social media use, body image, and self-esteem among young adults. A total of 200 participants were recruited from a university and completed self-report measures of social media use, body image satisfaction, and self-esteem.
Results: The results showed that social media use was significantly associated with body image dissatisfaction and lower self-esteem. Specifically, participants who reported spending more time on social media platforms had lower levels of body image satisfaction and self-esteem compared to those who reported less social media use. Moreover, the study found that comparing oneself to others on social media was a significant predictor of body image dissatisfaction and lower self-esteem.
Conclusion : These results suggest that social media use can have negative effects on body image satisfaction and self-esteem among young adults. It is important for individuals to be mindful of their social media use and to recognize the potential negative impact it can have on their mental health. Furthermore, interventions aimed at promoting positive body image and self-esteem should take into account the role of social media in shaping these attitudes and behaviors.
Importance of Research Results
Research results are important for several reasons, including:
- Advancing knowledge: Research results can contribute to the advancement of knowledge in a particular field, whether it be in science, technology, medicine, social sciences, or humanities.
- Developing theories: Research results can help to develop or modify existing theories and create new ones.
- Improving practices: Research results can inform and improve practices in various fields, such as education, healthcare, business, and public policy.
- Identifying problems and solutions: Research results can identify problems and provide solutions to complex issues in society, including issues related to health, environment, social justice, and economics.
- Validating claims : Research results can validate or refute claims made by individuals or groups in society, such as politicians, corporations, or activists.
- Providing evidence: Research results can provide evidence to support decision-making, policy-making, and resource allocation in various fields.
How to Write Results in A Research Paper
Here are some general guidelines on how to write results in a research paper:
- Organize the results section: Start by organizing the results section in a logical and coherent manner. Divide the section into subsections if necessary, based on the research questions or hypotheses.
- Present the findings: Present the findings in a clear and concise manner. Use tables, graphs, and figures to illustrate the data and make the presentation more engaging.
- Describe the data: Describe the data in detail, including the sample size, response rate, and any missing data. Provide relevant descriptive statistics such as means, standard deviations, and ranges.
- Interpret the findings: Interpret the findings in light of the research questions or hypotheses. Discuss the implications of the findings and the extent to which they support or contradict existing theories or previous research.
- Discuss the limitations : Discuss the limitations of the study, including any potential sources of bias or confounding factors that may have affected the results.
- Compare the results : Compare the results with those of previous studies or theoretical predictions. Discuss any similarities, differences, or inconsistencies.
- Avoid redundancy: Avoid repeating information that has already been presented in the introduction or methods sections. Instead, focus on presenting new and relevant information.
- Be objective: Be objective in presenting the results, avoiding any personal biases or interpretations.
When to Write Research Results
Here are situations When to Write Research Results”
- After conducting research on the chosen topic and obtaining relevant data, organize the findings in a structured format that accurately represents the information gathered.
- Once the data has been analyzed and interpreted, and conclusions have been drawn, begin the writing process.
- Before starting to write, ensure that the research results adhere to the guidelines and requirements of the intended audience, such as a scientific journal or academic conference.
- Begin by writing an abstract that briefly summarizes the research question, methodology, findings, and conclusions.
- Follow the abstract with an introduction that provides context for the research, explains its significance, and outlines the research question and objectives.
- The next section should be a literature review that provides an overview of existing research on the topic and highlights the gaps in knowledge that the current research seeks to address.
- The methodology section should provide a detailed explanation of the research design, including the sample size, data collection methods, and analytical techniques used.
- Present the research results in a clear and concise manner, using graphs, tables, and figures to illustrate the findings.
- Discuss the implications of the research results, including how they contribute to the existing body of knowledge on the topic and what further research is needed.
- Conclude the paper by summarizing the main findings, reiterating the significance of the research, and offering suggestions for future research.
Purpose of Research Results
The purposes of Research Results are as follows:
- Informing policy and practice: Research results can provide evidence-based information to inform policy decisions, such as in the fields of healthcare, education, and environmental regulation. They can also inform best practices in fields such as business, engineering, and social work.
- Addressing societal problems : Research results can be used to help address societal problems, such as reducing poverty, improving public health, and promoting social justice.
- Generating economic benefits : Research results can lead to the development of new products, services, and technologies that can create economic value and improve quality of life.
- Supporting academic and professional development : Research results can be used to support academic and professional development by providing opportunities for students, researchers, and practitioners to learn about new findings and methodologies in their field.
- Enhancing public understanding: Research results can help to educate the public about important issues and promote scientific literacy, leading to more informed decision-making and better public policy.
- Evaluating interventions: Research results can be used to evaluate the effectiveness of interventions, such as treatments, educational programs, and social policies. This can help to identify areas where improvements are needed and guide future interventions.
- Contributing to scientific progress: Research results can contribute to the advancement of science by providing new insights and discoveries that can lead to new theories, methods, and techniques.
- Informing decision-making : Research results can provide decision-makers with the information they need to make informed decisions. This can include decision-making at the individual, organizational, or governmental levels.
- Fostering collaboration : Research results can facilitate collaboration between researchers and practitioners, leading to new partnerships, interdisciplinary approaches, and innovative solutions to complex problems.
Advantages of Research Results
Some Advantages of Research Results are as follows:
- Improved decision-making: Research results can help inform decision-making in various fields, including medicine, business, and government. For example, research on the effectiveness of different treatments for a particular disease can help doctors make informed decisions about the best course of treatment for their patients.
- Innovation : Research results can lead to the development of new technologies, products, and services. For example, research on renewable energy sources can lead to the development of new and more efficient ways to harness renewable energy.
- Economic benefits: Research results can stimulate economic growth by providing new opportunities for businesses and entrepreneurs. For example, research on new materials or manufacturing techniques can lead to the development of new products and processes that can create new jobs and boost economic activity.
- Improved quality of life: Research results can contribute to improving the quality of life for individuals and society as a whole. For example, research on the causes of a particular disease can lead to the development of new treatments and cures, improving the health and well-being of millions of people.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Research Design – Types, Methods and Examples

Research Approach – Types Methods and Examples

Appendices – Writing Guide, Types and Examples

Background of The Study – Examples and Writing...

Thesis Statement – Examples, Writing Guide

What is a Hypothesis – Types, Examples and...
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Working with sources
- How to Write a Summary | Guide & Examples
How to Write a Summary | Guide & Examples
Published on November 23, 2020 by Shona McCombes . Revised on May 31, 2023.
Summarizing , or writing a summary, means giving a concise overview of a text’s main points in your own words. A summary is always much shorter than the original text.
There are five key steps that can help you to write a summary:
- Read the text
- Break it down into sections
- Identify the key points in each section
- Write the summary
- Check the summary against the article
Writing a summary does not involve critiquing or evaluating the source . You should simply provide an accurate account of the most important information and ideas (without copying any text from the original).
Table of contents
When to write a summary, step 1: read the text, step 2: break the text down into sections, step 3: identify the key points in each section, step 4: write the summary, step 5: check the summary against the article, other interesting articles, frequently asked questions about summarizing.
There are many situations in which you might have to summarize an article or other source:
- As a stand-alone assignment to show you’ve understood the material
- To keep notes that will help you remember what you’ve read
- To give an overview of other researchers’ work in a literature review
When you’re writing an academic text like an essay , research paper , or dissertation , you’ll integrate sources in a variety of ways. You might use a brief quote to support your point, or paraphrase a few sentences or paragraphs.
But it’s often appropriate to summarize a whole article or chapter if it is especially relevant to your own research, or to provide an overview of a source before you analyze or critique it.
In any case, the goal of summarizing is to give your reader a clear understanding of the original source. Follow the five steps outlined below to write a good summary.
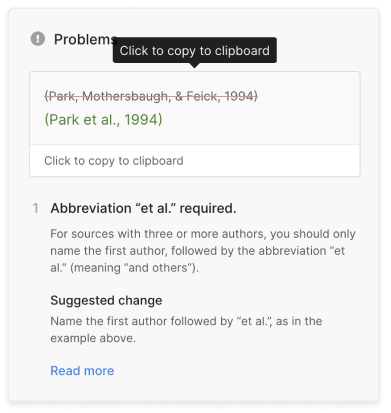
Scribbr Citation Checker New
The AI-powered Citation Checker helps you avoid common mistakes such as:
- Missing commas and periods
- Incorrect usage of “et al.”
- Ampersands (&) in narrative citations
- Missing reference entries
You should read the article more than once to make sure you’ve thoroughly understood it. It’s often effective to read in three stages:
- Scan the article quickly to get a sense of its topic and overall shape.
- Read the article carefully, highlighting important points and taking notes as you read.
- Skim the article again to confirm you’ve understood the key points, and reread any particularly important or difficult passages.
There are some tricks you can use to identify the key points as you read:
- Start by reading the abstract . This already contains the author’s own summary of their work, and it tells you what to expect from the article.
- Pay attention to headings and subheadings . These should give you a good sense of what each part is about.
- Read the introduction and the conclusion together and compare them: What did the author set out to do, and what was the outcome?
To make the text more manageable and understand its sub-points, break it down into smaller sections.
If the text is a scientific paper that follows a standard empirical structure, it is probably already organized into clearly marked sections, usually including an introduction , methods , results , and discussion .
Other types of articles may not be explicitly divided into sections. But most articles and essays will be structured around a series of sub-points or themes.
Now it’s time go through each section and pick out its most important points. What does your reader need to know to understand the overall argument or conclusion of the article?
Keep in mind that a summary does not involve paraphrasing every single paragraph of the article. Your goal is to extract the essential points, leaving out anything that can be considered background information or supplementary detail.
In a scientific article, there are some easy questions you can ask to identify the key points in each part.
| Introduction | or problem was addressed? |
|---|---|
| Methods | |
| Results | supported? |
| Discussion/conclusion |
If the article takes a different form, you might have to think more carefully about what points are most important for the reader to understand its argument.
In that case, pay particular attention to the thesis statement —the central claim that the author wants us to accept, which usually appears in the introduction—and the topic sentences that signal the main idea of each paragraph.
Prevent plagiarism. Run a free check.
Now that you know the key points that the article aims to communicate, you need to put them in your own words.
To avoid plagiarism and show you’ve understood the article, it’s essential to properly paraphrase the author’s ideas. Do not copy and paste parts of the article, not even just a sentence or two.
The best way to do this is to put the article aside and write out your own understanding of the author’s key points.
Examples of article summaries
Let’s take a look at an example. Below, we summarize this article , which scientifically investigates the old saying “an apple a day keeps the doctor away.”
Davis et al. (2015) set out to empirically test the popular saying “an apple a day keeps the doctor away.” Apples are often used to represent a healthy lifestyle, and research has shown their nutritional properties could be beneficial for various aspects of health. The authors’ unique approach is to take the saying literally and ask: do people who eat apples use healthcare services less frequently? If there is indeed such a relationship, they suggest, promoting apple consumption could help reduce healthcare costs.
The study used publicly available cross-sectional data from the National Health and Nutrition Examination Survey. Participants were categorized as either apple eaters or non-apple eaters based on their self-reported apple consumption in an average 24-hour period. They were also categorized as either avoiding or not avoiding the use of healthcare services in the past year. The data was statistically analyzed to test whether there was an association between apple consumption and several dependent variables: physician visits, hospital stays, use of mental health services, and use of prescription medication.
Although apple eaters were slightly more likely to have avoided physician visits, this relationship was not statistically significant after adjusting for various relevant factors. No association was found between apple consumption and hospital stays or mental health service use. However, apple eaters were found to be slightly more likely to have avoided using prescription medication. Based on these results, the authors conclude that an apple a day does not keep the doctor away, but it may keep the pharmacist away. They suggest that this finding could have implications for reducing healthcare costs, considering the high annual costs of prescription medication and the inexpensiveness of apples.
However, the authors also note several limitations of the study: most importantly, that apple eaters are likely to differ from non-apple eaters in ways that may have confounded the results (for example, apple eaters may be more likely to be health-conscious). To establish any causal relationship between apple consumption and avoidance of medication, they recommend experimental research.
An article summary like the above would be appropriate for a stand-alone summary assignment. However, you’ll often want to give an even more concise summary of an article.
For example, in a literature review or meta analysis you may want to briefly summarize this study as part of a wider discussion of various sources. In this case, we can boil our summary down even further to include only the most relevant information.
Using national survey data, Davis et al. (2015) tested the assertion that “an apple a day keeps the doctor away” and did not find statistically significant evidence to support this hypothesis. While people who consumed apples were slightly less likely to use prescription medications, the study was unable to demonstrate a causal relationship between these variables.
Citing the source you’re summarizing
When including a summary as part of a larger text, it’s essential to properly cite the source you’re summarizing. The exact format depends on your citation style , but it usually includes an in-text citation and a full reference at the end of your paper.
You can easily create your citations and references in APA or MLA using our free citation generators.
APA Citation Generator MLA Citation Generator
Finally, read through the article once more to ensure that:
- You’ve accurately represented the author’s work
- You haven’t missed any essential information
- The phrasing is not too similar to any sentences in the original.
If you’re summarizing many articles as part of your own work, it may be a good idea to use a plagiarism checker to double-check that your text is completely original and properly cited. Just be sure to use one that’s safe and reliable.
If you want to know more about ChatGPT, AI tools , citation , and plagiarism , make sure to check out some of our other articles with explanations and examples.
- ChatGPT vs human editor
- ChatGPT citations
- Is ChatGPT trustworthy?
- Using ChatGPT for your studies
- What is ChatGPT?
- Chicago style
- Paraphrasing
Plagiarism
- Types of plagiarism
- Self-plagiarism
- Avoiding plagiarism
- Academic integrity
- Consequences of plagiarism
- Common knowledge
A summary is a short overview of the main points of an article or other source, written entirely in your own words. Want to make your life super easy? Try our free text summarizer today!
A summary is always much shorter than the original text. The length of a summary can range from just a few sentences to several paragraphs; it depends on the length of the article you’re summarizing, and on the purpose of the summary.
You might have to write a summary of a source:
- As a stand-alone assignment to prove you understand the material
- For your own use, to keep notes on your reading
- To provide an overview of other researchers’ work in a literature review
- In a paper , to summarize or introduce a relevant study
To avoid plagiarism when summarizing an article or other source, follow these two rules:
- Write the summary entirely in your own words by paraphrasing the author’s ideas.
- Cite the source with an in-text citation and a full reference so your reader can easily find the original text.
An abstract concisely explains all the key points of an academic text such as a thesis , dissertation or journal article. It should summarize the whole text, not just introduce it.
An abstract is a type of summary , but summaries are also written elsewhere in academic writing . For example, you might summarize a source in a paper , in a literature review , or as a standalone assignment.
All can be done within seconds with our free text summarizer .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
McCombes, S. (2023, May 31). How to Write a Summary | Guide & Examples. Scribbr. Retrieved August 24, 2024, from https://www.scribbr.com/working-with-sources/how-to-summarize/
Is this article helpful?
Shona McCombes
Other students also liked, how to paraphrase | step-by-step guide & examples, how to quote | citing quotes in apa, mla & chicago, the basics of in-text citation | apa & mla examples, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- QuestionPro

- Solutions Industries Gaming Automotive Sports and events Education Government Travel & Hospitality Financial Services Healthcare Cannabis Technology Use Case AskWhy Communities Audience Contactless surveys Mobile LivePolls Member Experience GDPR Positive People Science 360 Feedback Surveys
- Resources Blog eBooks Survey Templates Case Studies Training Help center
Home Surveys Academic Research
Research Summary: What is it & how to write one

The Research Summary is used to report facts about a study clearly. You will almost certainly be required to prepare a research summary during your academic research or while on a research project for your organization.
If it is the first time you have to write one, the writing requirements may confuse you. The instructors generally assign someone to write a summary of the research work. Research summaries require the writer to have a thorough understanding of the issue.
This article will discuss the definition of a research summary and how to write one.
What is a research summary?
A research summary is a piece of writing that summarizes your research on a specific topic. Its primary goal is to offer the reader a detailed overview of the study with the key findings. A research summary generally contains the article’s structure in which it is written.
You must know the goal of your analysis before you launch a project. A research overview summarizes the detailed response and highlights particular issues raised in it. Writing it might be somewhat troublesome. To write a good overview, you want to start with a structure in mind. Read on for our guide.
Why is an analysis recap so important?
Your summary or analysis is going to tell readers everything about your research project. This is the critical piece that your stakeholders will read to identify your findings and valuable insights. Having a good and concise research summary that presents facts and comes with no research biases is the critical deliverable of any research project.
We’ve put together a cheat sheet to help you write a good research summary below.
Research Summary Guide
- Why was this research done? – You want to give a clear description of why this research study was done. What hypothesis was being tested?
- Who was surveyed? – The what and why or your research decides who you’re going to interview/survey. Your research summary has a detailed note on who participated in the study and why they were selected.
- What was the methodology? – Talk about the methodology. Did you do face-to-face interviews? Was it a short or long survey or a focus group setting? Your research methodology is key to the results you’re going to get.
- What were the key findings? – This can be the most critical part of the process. What did we find out after testing the hypothesis? This section, like all others, should be just facts, facts facts. You’re not sharing how you feel about the findings. Keep it bias-free.
- Conclusion – What are the conclusions that were drawn from the findings. A good example of a conclusion. Surprisingly, most people interviewed did not watch the lunar eclipse in 2022, which is unexpected given that 100% of those interviewed knew about it before it happened.
- Takeaways and action points – This is where you bring in your suggestion. Given the data you now have from the research, what are the takeaways and action points? If you’re a researcher running this research project for your company, you’ll use this part to shed light on your recommended action plans for the business.
LEARN ABOUT: Action Research
If you’re doing any research, you will write a summary, which will be the most viewed and more important part of the project. So keep a guideline in mind before you start. Focus on the content first and then worry about the length. Use the cheat sheet/checklist in this article to organize your summary, and that’s all you need to write a great research summary!
But once your summary is ready, where is it stored? Most teams have multiple documents in their google drives, and it’s a nightmare to find projects that were done in the past. Your research data should be democratized and easy to use.
We at QuestionPro launched a research repository for research teams, and our clients love it. All your data is in one place, and everything is searchable, including your research summaries!
Authors: Prachi, Anas
MORE LIKE THIS

Age Gating: Effective Strategies for Online Content Control
Aug 23, 2024

Customer Experience Lessons from 13,000 Feet — Tuesday CX Thoughts
Aug 20, 2024

Insight: Definition & meaning, types and examples
Aug 19, 2024

Employee Loyalty: Strategies for Long-Term Business Success
Other categories.
- Academic Research
- Artificial Intelligence
- Assessments
- Brand Awareness
- Case Studies
- Communities
- Consumer Insights
- Customer effort score
- Customer Engagement
- Customer Experience
- Customer Loyalty
- Customer Research
- Customer Satisfaction
- Employee Benefits
- Employee Engagement
- Employee Retention
- Friday Five
- General Data Protection Regulation
- Insights Hub
- Life@QuestionPro
- Market Research
- Mobile diaries
- Mobile Surveys
- New Features
- Online Communities
- Question Types
- Questionnaire
- QuestionPro Products
- Release Notes
- Research Tools and Apps
- Revenue at Risk
- Survey Templates
- Training Tips
- Tuesday CX Thoughts (TCXT)
- Uncategorized
- What’s Coming Up
- Workforce Intelligence
- USC Libraries
- Research Guides
Organizing Your Social Sciences Research Paper
- 7. The Results
- Purpose of Guide
- Design Flaws to Avoid
- Independent and Dependent Variables
- Glossary of Research Terms
- Reading Research Effectively
- Narrowing a Topic Idea
- Broadening a Topic Idea
- Extending the Timeliness of a Topic Idea
- Academic Writing Style
- Applying Critical Thinking
- Choosing a Title
- Making an Outline
- Paragraph Development
- Research Process Video Series
- Executive Summary
- The C.A.R.S. Model
- Background Information
- The Research Problem/Question
- Theoretical Framework
- Citation Tracking
- Content Alert Services
- Evaluating Sources
- Primary Sources
- Secondary Sources
- Tiertiary Sources
- Scholarly vs. Popular Publications
- Qualitative Methods
- Quantitative Methods
- Insiderness
- Using Non-Textual Elements
- Limitations of the Study
- Common Grammar Mistakes
- Writing Concisely
- Avoiding Plagiarism
- Footnotes or Endnotes?
- Further Readings
- Generative AI and Writing
- USC Libraries Tutorials and Other Guides
- Bibliography
The results section is where you report the findings of your study based upon the methodology [or methodologies] you applied to gather information. The results section should state the findings of the research arranged in a logical sequence without bias or interpretation. A section describing results should be particularly detailed if your paper includes data generated from your own research.
Annesley, Thomas M. "Show Your Cards: The Results Section and the Poker Game." Clinical Chemistry 56 (July 2010): 1066-1070.
Importance of a Good Results Section
When formulating the results section, it's important to remember that the results of a study do not prove anything . Findings can only confirm or reject the hypothesis underpinning your study. However, the act of articulating the results helps you to understand the problem from within, to break it into pieces, and to view the research problem from various perspectives.
The page length of this section is set by the amount and types of data to be reported . Be concise. Use non-textual elements appropriately, such as figures and tables, to present findings more effectively. In deciding what data to describe in your results section, you must clearly distinguish information that would normally be included in a research paper from any raw data or other content that could be included as an appendix. In general, raw data that has not been summarized should not be included in the main text of your paper unless requested to do so by your professor.
Avoid providing data that is not critical to answering the research question . The background information you described in the introduction section should provide the reader with any additional context or explanation needed to understand the results. A good strategy is to always re-read the background section of your paper after you have written up your results to ensure that the reader has enough context to understand the results [and, later, how you interpreted the results in the discussion section of your paper that follows].
Bavdekar, Sandeep B. and Sneha Chandak. "Results: Unraveling the Findings." Journal of the Association of Physicians of India 63 (September 2015): 44-46; Brett, Paul. "A Genre Analysis of the Results Section of Sociology Articles." English for Specific Speakers 13 (1994): 47-59; Go to English for Specific Purposes on ScienceDirect;Burton, Neil et al. Doing Your Education Research Project . Los Angeles, CA: SAGE, 2008; Results. The Structure, Format, Content, and Style of a Journal-Style Scientific Paper. Department of Biology. Bates College; Kretchmer, Paul. Twelve Steps to Writing an Effective Results Section. San Francisco Edit; "Reporting Findings." In Making Sense of Social Research Malcolm Williams, editor. (London;: SAGE Publications, 2003) pp. 188-207.
Structure and Writing Style
I. Organization and Approach
For most research papers in the social and behavioral sciences, there are two possible ways of organizing the results . Both approaches are appropriate in how you report your findings, but use only one approach.
- Present a synopsis of the results followed by an explanation of key findings . This approach can be used to highlight important findings. For example, you may have noticed an unusual correlation between two variables during the analysis of your findings. It is appropriate to highlight this finding in the results section. However, speculating as to why this correlation exists and offering a hypothesis about what may be happening belongs in the discussion section of your paper.
- Present a result and then explain it, before presenting the next result then explaining it, and so on, then end with an overall synopsis . This is the preferred approach if you have multiple results of equal significance. It is more common in longer papers because it helps the reader to better understand each finding. In this model, it is helpful to provide a brief conclusion that ties each of the findings together and provides a narrative bridge to the discussion section of the your paper.
NOTE: Just as the literature review should be arranged under conceptual categories rather than systematically describing each source, you should also organize your findings under key themes related to addressing the research problem. This can be done under either format noted above [i.e., a thorough explanation of the key results or a sequential, thematic description and explanation of each finding].
II. Content
In general, the content of your results section should include the following:
- Introductory context for understanding the results by restating the research problem underpinning your study . This is useful in re-orientating the reader's focus back to the research problem after having read a review of the literature and your explanation of the methods used for gathering and analyzing information.
- Inclusion of non-textual elements, such as, figures, charts, photos, maps, tables, etc. to further illustrate key findings, if appropriate . Rather than relying entirely on descriptive text, consider how your findings can be presented visually. This is a helpful way of condensing a lot of data into one place that can then be referred to in the text. Consider referring to appendices if there is a lot of non-textual elements.
- A systematic description of your results, highlighting for the reader observations that are most relevant to the topic under investigation . Not all results that emerge from the methodology used to gather information may be related to answering the " So What? " question. Do not confuse observations with interpretations; observations in this context refers to highlighting important findings you discovered through a process of reviewing prior literature and gathering data.
- The page length of your results section is guided by the amount and types of data to be reported . However, focus on findings that are important and related to addressing the research problem. It is not uncommon to have unanticipated results that are not relevant to answering the research question. This is not to say that you don't acknowledge tangential findings and, in fact, can be referred to as areas for further research in the conclusion of your paper. However, spending time in the results section describing tangential findings clutters your overall results section and distracts the reader.
- A short paragraph that concludes the results section by synthesizing the key findings of the study . Highlight the most important findings you want readers to remember as they transition into the discussion section. This is particularly important if, for example, there are many results to report, the findings are complicated or unanticipated, or they are impactful or actionable in some way [i.e., able to be pursued in a feasible way applied to practice].
NOTE: Always use the past tense when referring to your study's findings. Reference to findings should always be described as having already happened because the method used to gather the information has been completed.
III. Problems to Avoid
When writing the results section, avoid doing the following :
- Discussing or interpreting your results . Save this for the discussion section of your paper, although where appropriate, you should compare or contrast specific results to those found in other studies [e.g., "Similar to the work of Smith [1990], one of the findings of this study is the strong correlation between motivation and academic achievement...."].
- Reporting background information or attempting to explain your findings. This should have been done in your introduction section, but don't panic! Often the results of a study point to the need for additional background information or to explain the topic further, so don't think you did something wrong. Writing up research is rarely a linear process. Always revise your introduction as needed.
- Ignoring negative results . A negative result generally refers to a finding that does not support the underlying assumptions of your study. Do not ignore them. Document these findings and then state in your discussion section why you believe a negative result emerged from your study. Note that negative results, and how you handle them, can give you an opportunity to write a more engaging discussion section, therefore, don't be hesitant to highlight them.
- Including raw data or intermediate calculations . Ask your professor if you need to include any raw data generated by your study, such as transcripts from interviews or data files. If raw data is to be included, place it in an appendix or set of appendices that are referred to in the text.
- Be as factual and concise as possible in reporting your findings . Do not use phrases that are vague or non-specific, such as, "appeared to be greater than other variables..." or "demonstrates promising trends that...." Subjective modifiers should be explained in the discussion section of the paper [i.e., why did one variable appear greater? Or, how does the finding demonstrate a promising trend?].
- Presenting the same data or repeating the same information more than once . If you want to highlight a particular finding, it is appropriate to do so in the results section. However, you should emphasize its significance in relation to addressing the research problem in the discussion section. Do not repeat it in your results section because you can do that in the conclusion of your paper.
- Confusing figures with tables . Be sure to properly label any non-textual elements in your paper. Don't call a chart an illustration or a figure a table. If you are not sure, go here .
Annesley, Thomas M. "Show Your Cards: The Results Section and the Poker Game." Clinical Chemistry 56 (July 2010): 1066-1070; Bavdekar, Sandeep B. and Sneha Chandak. "Results: Unraveling the Findings." Journal of the Association of Physicians of India 63 (September 2015): 44-46; Burton, Neil et al. Doing Your Education Research Project . Los Angeles, CA: SAGE, 2008; Caprette, David R. Writing Research Papers. Experimental Biosciences Resources. Rice University; Hancock, Dawson R. and Bob Algozzine. Doing Case Study Research: A Practical Guide for Beginning Researchers . 2nd ed. New York: Teachers College Press, 2011; Introduction to Nursing Research: Reporting Research Findings. Nursing Research: Open Access Nursing Research and Review Articles. (January 4, 2012); Kretchmer, Paul. Twelve Steps to Writing an Effective Results Section. San Francisco Edit ; Ng, K. H. and W. C. Peh. "Writing the Results." Singapore Medical Journal 49 (2008): 967-968; Reporting Research Findings. Wilder Research, in partnership with the Minnesota Department of Human Services. (February 2009); Results. The Structure, Format, Content, and Style of a Journal-Style Scientific Paper. Department of Biology. Bates College; Schafer, Mickey S. Writing the Results. Thesis Writing in the Sciences. Course Syllabus. University of Florida.
Writing Tip
Why Don't I Just Combine the Results Section with the Discussion Section?
It's not unusual to find articles in scholarly social science journals where the author(s) have combined a description of the findings with a discussion about their significance and implications. You could do this. However, if you are inexperienced writing research papers, consider creating two distinct sections for each section in your paper as a way to better organize your thoughts and, by extension, your paper. Think of the results section as the place where you report what your study found; think of the discussion section as the place where you interpret the information and answer the "So What?" question. As you become more skilled writing research papers, you can consider melding the results of your study with a discussion of its implications.
Driscoll, Dana Lynn and Aleksandra Kasztalska. Writing the Experimental Report: Methods, Results, and Discussion. The Writing Lab and The OWL. Purdue University.
- << Previous: Insiderness
- Next: Using Non-Textual Elements >>
- Last Updated: Aug 21, 2024 8:54 AM
- URL: https://libguides.usc.edu/writingguide
- Bipolar Disorder
- Therapy Center
- When To See a Therapist
- Types of Therapy
- Best Online Therapy
- Best Couples Therapy
- Managing Stress
- Sleep and Dreaming
- Understanding Emotions
- Self-Improvement
- Healthy Relationships
- Student Resources
- Personality Types
- Sweepstakes
- Guided Meditations
- Verywell Mind Insights
- 2024 Verywell Mind 25
- Mental Health in the Classroom
- Editorial Process
- Meet Our Review Board
- Crisis Support
How to Write an APA Results Section
Verywell / Nusha Ashjaee
What to Include in an APA Results Section
- Justify Claims
- Summarize Results
Report All Relevant Results
- Report Statistical Findings
Include Tables and Figures
What not to include in an apa results section.
Psychology papers generally follow a specific structure. One important section of a paper is known as the results section. An APA results section of a psychology paper summarizes the data that was collected and the statistical analyses that were performed. The goal of this section is to report the results of your study or experiment without any type of subjective interpretation.
At a Glance
The results section is a vital part of an APA paper that summarizes a study's findings and statistical analysis. This section often includes descriptive text, tables, and figures to help summarize the findings.
The focus is purely on summarizing and presenting the findings and should not include any interpretation, since you'll cover that in the subsequent discussion section.
This article covers how to write an APA results section, including what to include and what to avoid.
The results section is the third section of a psychology paper. It will appear after the introduction and methods sections and before the discussion section.
The results section should include:
- A summary of the research findings.
- Information about participant flow, recruitment , retention, and attrition. If some participants started the study and later left or failed to complete the study, then this should be described.
- Information about any reasons why some data might have been excluded from the study.
- Statistical information including samples sizes and statistical tests that were used. It should report standard deviations, p-values, and other measures of interest.
Results Should Justify Your Claims
Report data in order to sufficiently justify your conclusions. Since you'll be talking about your own interpretation of the results in the discussion section, you need to be sure that the information reported in the results section justifies your claims.
When you start writing your discussion section, you can then look back on your results to ensure that all the data you need are there to fully support your conclusions. Be sure not to make claims in your discussion section that are not supported by the findings described in your results section.
Summarize Your Results
Remember, you are summarizing the results of your psychological study, not reporting them in full detail. The results section should be a relatively brief overview of your findings, not a complete presentation of every single number and calculation.
If you choose, you can create a supplemental online archive where other researchers can access the raw data if they choose.
How long should a results section be?
The length of your results section will vary depending on the nature of your paper and the complexity of your research. In most cases, this will be the shortest section of your paper.
Just as the results section of your psychology paper should sufficiently justify your claims, it should also provide an accurate look at what you found in your study. Be sure to mention all relevant information.
Don't omit findings simply because they failed to support your predictions.
Your hypothesis may have expected more statistically significant results or your study didn't support your hypothesis , but that doesn't mean that the conclusions you reach are not useful. Provide data about what you found in your results section, then save your interpretation for what the results might mean in the discussion section.
While your study might not have supported your original predictions, your finding can provide important inspiration for future explorations into a topic.
How is the results section different from the discussion section?
The results section provides the results of your study or experiment . The goal of the section is to report what happened and the statistical analyses you performed. The discussion section is where you will examine what these results mean and whether they support or fail to support your hypothesis.
Report Your Statistical Findings
Always assume that your readers have a solid understanding of statistical concepts. There's no need to explain what a t-test is or how a one-way ANOVA works. Your responsibility is to report the results of your study, not to teach your readers how to analyze or interpret statistics.
Include Effect Sizes
The Publication Manual of the American Psychological Association recommends including effect sizes in your results section so that readers can appreciate the importance of your study's findings.
Your results section should include both text and illustrations. Presenting data in this way makes it easier for readers to quickly look at your results.
Structure your results section around tables or figures that summarize the results of your statistical analysis. In many cases, the easiest way to accomplish this is to first create your tables and figures and then organize them in a logical way. Next, write the summary text to support your illustrative materials.
Only include tables and figures if you are going to talk about them in the body text of your results section.
In addition to knowing what you should include in the results section of your psychology paper, it's also important to be aware of things that you should avoid putting in this section:
Cause-and-Effect Conclusions
Don't draw cause-effect conclusions. Avoid making any claims suggesting that your result "proves" that something is true.
Interpretations
Present the data without editorializing it. Save your comments and interpretations for the discussion section of your paper.
Statistics Without Context
Don't include statistics without narration. The results section should not be a numbers dump. Instead, you should sequentially narrate what these numbers mean.
Don't include the raw data in the results section. The results section should be a concise presentation of the results. If there is raw data that would be useful, include it in the appendix .
Don't only rely on descriptive text. Use tables and figures to present these findings when appropriate. This makes the results section easier to read and can convey a great deal of information quickly.
Repeated Data
Don't present the same data twice in your illustrative materials. If you have already presented some data in a table, don't present it again in a figure. If you have presented data in a figure, don't present it again in a table.
All of Your Findings
Don't feel like you have to include everything. If data is irrelevant to the research question, don't include it in the results section.
But Don't Skip Relevant Data
Don't leave out results because they don't support your claims. Even if your data does not support your hypothesis, including it in your findings is essential if it's relevant.
More Tips for Writing a Results Section
If you are struggling, there are a few things to remember that might help:
- Use the past tense . The results section should be written in the past tense.
- Be concise and objective . You will have the opportunity to give your own interpretations of the results in the discussion section.
- Use APA format . As you are writing your results section, keep a style guide on hand. The Publication Manual of the American Psychological Association is the official source for APA style .
- Visit your library . Read some journal articles that are on your topic. Pay attention to how the authors present the results of their research.
- Get a second opinion . If possible, take your paper to your school's writing lab for additional assistance.
What This Means For You
Remember, the results section of your paper is all about providing the data from your study. This section is often the shortest part of your paper, and in most cases, the most clinical.
Be sure not to include any subjective interpretation of the results. Simply relay the data in the most objective and straightforward way possible. You can then provide your own analysis of what these results mean in the discussion section of your paper.
Bavdekar SB, Chandak S. Results: Unraveling the findings . J Assoc Physicians India . 2015 Sep;63(9):44-6. PMID:27608866.
Snyder N, Foltz C, Lendner M, Vaccaro AR. How to write an effective results section . Clin Spine Surg . 2019;32(7):295-296. doi:10.1097/BSD.0000000000000845
American Psychological Association. Publication Manual of the American Psychological Association (7th ed.). Washington DC: The American Psychological Association; 2019.
Purdue Online Writing Lab. APA sample paper: Experimental psychology .
Berkeley University. Reviewing test results .
Tuncel A, Atan A. How to clearly articulate results and construct tables and figures in a scientific paper ? Turk J Urol . 2013;39(Suppl 1):16-19. doi:10.5152/tud.2013.048
By Kendra Cherry, MSEd Kendra Cherry, MS, is a psychosocial rehabilitation specialist, psychology educator, and author of the "Everything Psychology Book."
- Research Process
- Manuscript Preparation
- Manuscript Review
- Publication Process
- Publication Recognition
- Language Editing Services
- Translation Services

How to write the results section of a research paper
- 3 minute read
- 76.6K views
Table of Contents
At its core, a research paper aims to fill a gap in the research on a given topic. As a result, the results section of the paper, which describes the key findings of the study, is often considered the core of the paper. This is the section that gets the most attention from reviewers, peers, students, and any news organization reporting on your findings. Writing a clear, concise, and logical results section is, therefore, one of the most important parts of preparing your manuscript.
Difference between results and discussion
Before delving into how to write the results section, it is important to first understand the difference between the results and discussion sections. The results section needs to detail the findings of the study. The aim of this section is not to draw connections between the different findings or to compare it to previous findings in literature—that is the purview of the discussion section. Unlike the discussion section, which can touch upon the hypothetical, the results section needs to focus on the purely factual. In some cases, it may even be preferable to club these two sections together into a single section. For example, while writing a review article, it can be worthwhile to club these two sections together, as the main results in this case are the conclusions that can be drawn from the literature.
Structure of the results section
Although the main purpose of the results section in a research paper is to report the findings, it is necessary to present an introduction and repeat the research question. This establishes a connection to the previous section of the paper and creates a smooth flow of information.
Next, the results section needs to communicate the findings of your research in a systematic manner. The section needs to be organized such that the primary research question is addressed first, then the secondary research questions. If the research addresses multiple questions, the results section must individually connect with each of the questions. This ensures clarity and minimizes confusion while reading.
Consider representing your results visually. For example, graphs, tables, and other figures can help illustrate the findings of your paper, especially if there is a large amount of data in the results.
Remember, an appealing results section can help peer reviewers better understand the merits of your research, thereby increasing your chances of publication.
Practical guidance for writing an effective results section for a research paper
- Always use simple and clear language. Avoid the use of uncertain or out-of-focus expressions.
- The findings of the study must be expressed in an objective and unbiased manner. While it is acceptable to correlate certain findings in the discussion section, it is best to avoid overinterpreting the results.
- If the research addresses more than one hypothesis, use sub-sections to describe the results. This prevents confusion and promotes understanding.
- Ensure that negative results are included in this section, even if they do not support the research hypothesis.
- Wherever possible, use illustrations like tables, figures, charts, or other visual representations to showcase the results of your research paper. Mention these illustrations in the text, but do not repeat the information that they convey.
- For statistical data, it is adequate to highlight the tests and explain their results. The initial or raw data should not be mentioned in the results section of a research paper.
The results section of a research paper is usually the most impactful section because it draws the greatest attention. Regardless of the subject of your research paper, a well-written results section is capable of generating interest in your research.
For detailed information and assistance on writing the results of a research paper, refer to Elsevier Author Services.

Writing a good review article

Why is data validation important in research?
You may also like.

Submission 101: What format should be used for academic papers?

Page-Turner Articles are More Than Just Good Arguments: Be Mindful of Tone and Structure!

A Must-see for Researchers! How to Ensure Inclusivity in Your Scientific Writing

Make Hook, Line, and Sinker: The Art of Crafting Engaging Introductions

Can Describing Study Limitations Improve the Quality of Your Paper?

A Guide to Crafting Shorter, Impactful Sentences in Academic Writing

6 Steps to Write an Excellent Discussion in Your Manuscript

How to Write Clear and Crisp Civil Engineering Papers? Here are 5 Key Tips to Consider
Input your search keywords and press Enter.
- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
- APA Citation Generator
- MLA Citation Generator
- Chicago Citation Generator
- Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources
How to Write the Results/Findings Section in Research
What is the research paper Results section and what does it do?
The Results section of a scientific research paper represents the core findings of a study derived from the methods applied to gather and analyze information. It presents these findings in a logical sequence without bias or interpretation from the author, setting up the reader for later interpretation and evaluation in the Discussion section. A major purpose of the Results section is to break down the data into sentences that show its significance to the research question(s).
The Results section appears third in the section sequence in most scientific papers. It follows the presentation of the Methods and Materials and is presented before the Discussion section —although the Results and Discussion are presented together in many journals. This section answers the basic question “What did you find in your research?”
What is included in the Results section?
The Results section should include the findings of your study and ONLY the findings of your study. The findings include:
- Data presented in tables, charts, graphs, and other figures (may be placed into the text or on separate pages at the end of the manuscript)
- A contextual analysis of this data explaining its meaning in sentence form
- All data that corresponds to the central research question(s)
- All secondary findings (secondary outcomes, subgroup analyses, etc.)
If the scope of the study is broad, or if you studied a variety of variables, or if the methodology used yields a wide range of different results, the author should present only those results that are most relevant to the research question stated in the Introduction section .
As a general rule, any information that does not present the direct findings or outcome of the study should be left out of this section. Unless the journal requests that authors combine the Results and Discussion sections, explanations and interpretations should be omitted from the Results.
How are the results organized?
The best way to organize your Results section is “logically.” One logical and clear method of organizing research results is to provide them alongside the research questions—within each research question, present the type of data that addresses that research question.
Let’s look at an example. Your research question is based on a survey among patients who were treated at a hospital and received postoperative care. Let’s say your first research question is:
“What do hospital patients over age 55 think about postoperative care?”
This can actually be represented as a heading within your Results section, though it might be presented as a statement rather than a question:
Attitudes towards postoperative care in patients over the age of 55
Now present the results that address this specific research question first. In this case, perhaps a table illustrating data from a survey. Likert items can be included in this example. Tables can also present standard deviations, probabilities, correlation matrices, etc.
Following this, present a content analysis, in words, of one end of the spectrum of the survey or data table. In our example case, start with the POSITIVE survey responses regarding postoperative care, using descriptive phrases. For example:
“Sixty-five percent of patients over 55 responded positively to the question “ Are you satisfied with your hospital’s postoperative care ?” (Fig. 2)
Include other results such as subcategory analyses. The amount of textual description used will depend on how much interpretation of tables and figures is necessary and how many examples the reader needs in order to understand the significance of your research findings.
Next, present a content analysis of another part of the spectrum of the same research question, perhaps the NEGATIVE or NEUTRAL responses to the survey. For instance:
“As Figure 1 shows, 15 out of 60 patients in Group A responded negatively to Question 2.”
After you have assessed the data in one figure and explained it sufficiently, move on to your next research question. For example:
“How does patient satisfaction correspond to in-hospital improvements made to postoperative care?”
This kind of data may be presented through a figure or set of figures (for instance, a paired T-test table).
Explain the data you present, here in a table, with a concise content analysis:
“The p-value for the comparison between the before and after groups of patients was .03% (Fig. 2), indicating that the greater the dissatisfaction among patients, the more frequent the improvements that were made to postoperative care.”
Let’s examine another example of a Results section from a study on plant tolerance to heavy metal stress . In the Introduction section, the aims of the study are presented as “determining the physiological and morphological responses of Allium cepa L. towards increased cadmium toxicity” and “evaluating its potential to accumulate the metal and its associated environmental consequences.” The Results section presents data showing how these aims are achieved in tables alongside a content analysis, beginning with an overview of the findings:
“Cadmium caused inhibition of root and leave elongation, with increasing effects at higher exposure doses (Fig. 1a-c).”
The figure containing this data is cited in parentheses. Note that this author has combined three graphs into one single figure. Separating the data into separate graphs focusing on specific aspects makes it easier for the reader to assess the findings, and consolidating this information into one figure saves space and makes it easy to locate the most relevant results.
Following this overall summary, the relevant data in the tables is broken down into greater detail in text form in the Results section.
- “Results on the bio-accumulation of cadmium were found to be the highest (17.5 mg kgG1) in the bulb, when the concentration of cadmium in the solution was 1×10G2 M and lowest (0.11 mg kgG1) in the leaves when the concentration was 1×10G3 M.”
Captioning and Referencing Tables and Figures
Tables and figures are central components of your Results section and you need to carefully think about the most effective way to use graphs and tables to present your findings . Therefore, it is crucial to know how to write strong figure captions and to refer to them within the text of the Results section.
The most important advice one can give here as well as throughout the paper is to check the requirements and standards of the journal to which you are submitting your work. Every journal has its own design and layout standards, which you can find in the author instructions on the target journal’s website. Perusing a journal’s published articles will also give you an idea of the proper number, size, and complexity of your figures.
Regardless of which format you use, the figures should be placed in the order they are referenced in the Results section and be as clear and easy to understand as possible. If there are multiple variables being considered (within one or more research questions), it can be a good idea to split these up into separate figures. Subsequently, these can be referenced and analyzed under separate headings and paragraphs in the text.
To create a caption, consider the research question being asked and change it into a phrase. For instance, if one question is “Which color did participants choose?”, the caption might be “Color choice by participant group.” Or in our last research paper example, where the question was “What is the concentration of cadmium in different parts of the onion after 14 days?” the caption reads:
“Fig. 1(a-c): Mean concentration of Cd determined in (a) bulbs, (b) leaves, and (c) roots of onions after a 14-day period.”
Steps for Composing the Results Section
Because each study is unique, there is no one-size-fits-all approach when it comes to designing a strategy for structuring and writing the section of a research paper where findings are presented. The content and layout of this section will be determined by the specific area of research, the design of the study and its particular methodologies, and the guidelines of the target journal and its editors. However, the following steps can be used to compose the results of most scientific research studies and are essential for researchers who are new to preparing a manuscript for publication or who need a reminder of how to construct the Results section.
Step 1 : Consult the guidelines or instructions that the target journal or publisher provides authors and read research papers it has published, especially those with similar topics, methods, or results to your study.
- The guidelines will generally outline specific requirements for the results or findings section, and the published articles will provide sound examples of successful approaches.
- Note length limitations on restrictions on content. For instance, while many journals require the Results and Discussion sections to be separate, others do not—qualitative research papers often include results and interpretations in the same section (“Results and Discussion”).
- Reading the aims and scope in the journal’s “ guide for authors ” section and understanding the interests of its readers will be invaluable in preparing to write the Results section.
Step 2 : Consider your research results in relation to the journal’s requirements and catalogue your results.
- Focus on experimental results and other findings that are especially relevant to your research questions and objectives and include them even if they are unexpected or do not support your ideas and hypotheses.
- Catalogue your findings—use subheadings to streamline and clarify your report. This will help you avoid excessive and peripheral details as you write and also help your reader understand and remember your findings. Create appendices that might interest specialists but prove too long or distracting for other readers.
- Decide how you will structure of your results. You might match the order of the research questions and hypotheses to your results, or you could arrange them according to the order presented in the Methods section. A chronological order or even a hierarchy of importance or meaningful grouping of main themes or categories might prove effective. Consider your audience, evidence, and most importantly, the objectives of your research when choosing a structure for presenting your findings.
Step 3 : Design figures and tables to present and illustrate your data.
- Tables and figures should be numbered according to the order in which they are mentioned in the main text of the paper.
- Information in figures should be relatively self-explanatory (with the aid of captions), and their design should include all definitions and other information necessary for readers to understand the findings without reading all of the text.
- Use tables and figures as a focal point to tell a clear and informative story about your research and avoid repeating information. But remember that while figures clarify and enhance the text, they cannot replace it.
Step 4 : Draft your Results section using the findings and figures you have organized.
- The goal is to communicate this complex information as clearly and precisely as possible; precise and compact phrases and sentences are most effective.
- In the opening paragraph of this section, restate your research questions or aims to focus the reader’s attention to what the results are trying to show. It is also a good idea to summarize key findings at the end of this section to create a logical transition to the interpretation and discussion that follows.
- Try to write in the past tense and the active voice to relay the findings since the research has already been done and the agent is usually clear. This will ensure that your explanations are also clear and logical.
- Make sure that any specialized terminology or abbreviation you have used here has been defined and clarified in the Introduction section .
Step 5 : Review your draft; edit and revise until it reports results exactly as you would like to have them reported to your readers.
- Double-check the accuracy and consistency of all the data, as well as all of the visual elements included.
- Read your draft aloud to catch language errors (grammar, spelling, and mechanics), awkward phrases, and missing transitions.
- Ensure that your results are presented in the best order to focus on objectives and prepare readers for interpretations, valuations, and recommendations in the Discussion section . Look back over the paper’s Introduction and background while anticipating the Discussion and Conclusion sections to ensure that the presentation of your results is consistent and effective.
- Consider seeking additional guidance on your paper. Find additional readers to look over your Results section and see if it can be improved in any way. Peers, professors, or qualified experts can provide valuable insights.
One excellent option is to use a professional English proofreading and editing service such as Wordvice, including our paper editing service . With hundreds of qualified editors from dozens of scientific fields, Wordvice has helped thousands of authors revise their manuscripts and get accepted into their target journals. Read more about the proofreading and editing process before proceeding with getting academic editing services and manuscript editing services for your manuscript.
As the representation of your study’s data output, the Results section presents the core information in your research paper. By writing with clarity and conciseness and by highlighting and explaining the crucial findings of their study, authors increase the impact and effectiveness of their research manuscripts.
For more articles and videos on writing your research manuscript, visit Wordvice’s Resources page.
Wordvice Resources
- How to Write a Research Paper Introduction
- Which Verb Tenses to Use in a Research Paper
- How to Write an Abstract for a Research Paper
- How to Write a Research Paper Title
- Useful Phrases for Academic Writing
- Common Transition Terms in Academic Papers
- Active and Passive Voice in Research Papers
- 100+ Verbs That Will Make Your Research Writing Amazing
- Tips for Paraphrasing in Research Papers

- Langson Library
- Science Library
- Grunigen Medical Library
- Law Library
- Connect From Off-Campus
- Accessibility
- Gateway Study Center

Email this link
Writing a scientific paper.
- Writing a lab report
- INTRODUCTION
Writing a "good" results section
Figures and Captions in Lab Reports
"Results Checklist" from: How to Write a Good Scientific Paper. Chris A. Mack. SPIE. 2018.
Additional tips for results sections.
- LITERATURE CITED
- Bibliography of guides to scientific writing and presenting
- Peer Review
- Presentations
- Lab Report Writing Guides on the Web
This is the core of the paper. Don't start the results sections with methods you left out of the Materials and Methods section. You need to give an overall description of the experiments and present the data you found.
- Factual statements supported by evidence. Short and sweet without excess words
- Present representative data rather than endlessly repetitive data
- Discuss variables only if they had an effect (positive or negative)
- Use meaningful statistics
- Avoid redundancy. If it is in the tables or captions you may not need to repeat it
A short article by Dr. Brett Couch and Dr. Deena Wassenberg, Biology Program, University of Minnesota
- Present the results of the paper, in logical order, using tables and graphs as necessary.
- Explain the results and show how they help to answer the research questions posed in the Introduction. Evidence does not explain itself; the results must be presented and then explained.
- Avoid: presenting results that are never discussed; presenting results in chronological order rather than logical order; ignoring results that do not support the conclusions;
- Number tables and figures separately beginning with 1 (i.e. Table 1, Table 2, Figure 1, etc.).
- Do not attempt to evaluate the results in this section. Report only what you found; hold all discussion of the significance of the results for the Discussion section.
- It is not necessary to describe every step of your statistical analyses. Scientists understand all about null hypotheses, rejection rules, and so forth and do not need to be reminded of them. Just say something like, "Honeybees did not use the flowers in proportion to their availability (X2 = 7.9, p<0.05, d.f.= 4, chi-square test)." Likewise, cite tables and figures without describing in detail how the data were manipulated. Explanations of this sort should appear in a legend or caption written on the same page as the figure or table.
- You must refer in the text to each figure or table you include in your paper.
- Tables generally should report summary-level data, such as means ± standard deviations, rather than all your raw data. A long list of all your individual observations will mean much less than a few concise, easy-to-read tables or figures that bring out the main findings of your study.
- Only use a figure (graph) when the data lend themselves to a good visual representation. Avoid using figures that show too many variables or trends at once, because they can be hard to understand.
From: https://writingcenter.gmu.edu/guides/imrad-results-discussion
- << Previous: METHODS
- Next: DISCUSSION >>
- Last Updated: Aug 4, 2023 9:33 AM
- URL: https://guides.lib.uci.edu/scientificwriting
Off-campus? Please use the Software VPN and choose the group UCIFull to access licensed content. For more information, please Click here
Software VPN is not available for guests, so they may not have access to some content when connecting from off-campus.

Research Voyage
Research Tips and Infromation
How to Write Results Section of Your Research Paper

Introduction
How to summarize the data preprocessing steps in the results section, how to summarize the research findings in the results section, common phrasal verbs used in results section, what are common mistakes observed in the results section, how long should a results section be of a research paper, should the results of a research paper be given in the introduction or in another section.
- What is the difference between the "discussion" and the "results" section of a research paper?
Does the summary be part of the result section in the research article?
Why do some scientific papers not include a ‘methods and results’ section, how do you introduce a results section, why do researchers need to avoid making speculations in the results section of a research paper.
The result section is the third major part of the research paper and it’s probably the most important part because it contains actual outcomes about your experiment. The other sections contain a plan, hope and interpretations but the result section is the actual truth of your study.
In the result section, one should aim to narrate his/her finding without trying to interpret or evaluate them. Basically, the result section explains any issues you faced during your data collection, the main results of the experiment and any other interesting trends in the data.
With the results, we want to convey our data in the most accessible way, so we usually use visual elements like graphs and tables to make it easier to understand. The facts, figures, and findings are to be presented in a logical manner leading to the hypothesis and following the sequence of the method section. Mention must be made for the negative results as it would substantiate the discussion section later on. Interpretation of the meaning of the results section is done in the discussion section .
How Results Section is Structured?
When structuring the results section, it is important that your information is presented in a logical order.
Now, when it comes to the organization of the result section, as a generic rule
- Always start with textual content, not a Table or Figure
- Make sure you show the Tables and Figures after they are mentioned in the text
- Explain any missing data or problems you had while collecting the data.
The results section gives you the opportunity to:
- Summarize the Data Preprocessing Steps
2. Report on the Findings
3. Summarize the Research Findings
At the beginning of the result section, you can discuss how you have collected, transformed and analyzed your data. This step is usually known as data preprocessing.
The data collection step may involve collecting data from various hardware, software or internet sources.
If your research requires data cleaning, then explain the steps and procedures used for data cleaning. Here, the researchers can describe how they transformed data to facilitate analysis (e.g. converting data from one format to another format). If there was missing data, explain how you have substituted missing values and with what techniques you have substituted your data.
You can mention what software or statistical procedures you have used to analyze and interpret the data. Demonstrate with the help of charts or tables the cleansed data ready to be used for getting results. In a few research papers, you may find these steps appearing at the end of the method section.
How to Present your Research Findings in Research Section?
Second, present your findings in a structured way (such as thematically or chronologically), bringing the readers’ attention to any important, interesting, or significant findings.
Be sure to include a combination of text and visuals. Data illustrations should not be used to substitute or replace text, but to enhance the narrative of your findings.
Resultant data are to be presented either through text, figures, graphs or tables or in a combination of all of the best suited for leading to the hypothesis. Care should be taken to prevent any duplication of the text, figures, graphs, and tables. If any result is presented in figures or graphs, it need not be explained through text. Similarly, any data presented through the graph should not be repeated in the table.
Each table and graph should be clearly labelled and titled. Each different finding should be made in a separate sub-section under the proper sub-heading following the sequence adopted in Method Section.
If you are not comfortable with data analysis then you can take professional services for research data analysis .
Figures
Identify and list the figures which are relevant to your results. For example, if you are working on the problem statement of ” Identifying the pathological issues with pomegranate fruits”, then you can add the figures of pomegranate fruits with good quality and bad quality along with their stage of infection. If you are working on pomegranate cultivar-related issues, put the figures of pomegranate fruits belonging to different cultivars.
The key takeaway here is not to add any figures which may not directly contribute to results. These diagrams may include generic block diagrams, and images conveying generic information like farm fields, plantations etc.
While putting the figures, as much as possible use grayscale images as many users take the photocopies in black and white mode. In certain scenarios you are
In the case of figures, the captions should come below, called Fig. 1, Fig. 2 and so on.
You can visit my article on The Power of Images in Research Papers: How They Enhance the Quality of Your Paper? . This article will help you how images or figures enhances the possibility of selection of your paper to top quality journals and conferences.
Tables are good for showing the exact values or showing much different information in one place. Graphs are good for showing overall trends and are much easier to understand quickly. It also depends on your data.
Tables are labelled at the top as Table 1, Table 2 and so on. Every table must have a caption. It’s good if one can put independent variable conditions on the left side vertically, and the things you have measured horizontally so one can easily compare the measurements across the categories. But you need to decide for each table you make, what is easiest to understand, and what fits on the paper.
Visit article on Best Practices for Designing and Formatting Tables in Research Papers for further details on proper representation of tables at proper places.
You can use various types of graphs in your results like a line graph, bar graph, scatter plot, a line graph with colours, a box with whiskers plot and a histogram.
In general, continuous variables like temperature, growth, age, and time can be better displayed in a line graph on a scatter plot or maybe on histograms.
If you have comparative data that you would like to represent through a chart then a bar chart would be the best option. This type of chart is one of the more familiar options as it is easy to interpret.
These charts are useful for displaying data that is classified into nominal or ordinal categories. In any case, you need to decide which is the best option for each particular example you have, but never put a graph and a table with the same data in your paper.
In the case of graphs, the captions should come below, called Fig. 1, Fig. 2 and so on.
A limited number of professional tools provide you the chance to add some life to your graphs, charts, and figures and present your data in a way that will astound your audience as much as your astounding results.
My article on Maximizing the Impact of Your Research Paper with Graphs and Charts will help you in drawing eye catching and informative graphs and charts for your research paper.
The results section should include a closing paragraph that clearly summarizes the key findings of the study. This paves the way for the discussion section of the research paper, wherein the results are interpreted and put in conversation with existing literature.
Any unusual correlation observed between variables should be noted in the result section. But any speculation about the reason for such an unusual correlation should be avoided. Such speculations are the domains of the discussion section.
Comparisons between samples or controls are to be clearly defined by specifically mentioning the common quality and the degree of difference between the comparable samples or controls. Results should always be presented in the past tense.
Common academic phrases that can be used in the results section of a paper or research article. I have included a table with examples to illustrate how these phrases might be used:
| Phrase | Example |
|---|---|
| This phrase is used to describe the basic statistical properties of the data, such as mean, median, and standard deviation. | “The mean accuracy of the machine learning model was 0.85, with a standard deviation of 0.05.” |
| This phrase is used to describe statistical tests used to infer relationships or differences between groups. | “A one-way ANOVA showed a significant difference in performance between the three groups, F(2, 57) = 4.67, p < 0.05.” |
| This phrase is used to describe any graphs, charts, or other visual representations of the data. | “Figure 1 shows a scatter plot of the relationship between the number of hidden layers in a neural network and its accuracy on the test dataset.” |
| This phrase is used to compare the performance of different machine learning models. | “The random forest classifier outperformed the logistic regression model, achieving an AUC of 0.95 compared to 0.83.” |
| This phrase is used to test specific hypotheses about the data or the system being evaluated. | “The null hypothesis that there is no difference in accuracy between the two machine learning models was rejected, t(98) = -3.56, p < 0.01.” |
| : This phrase is used to describe any non-numerical analysis of the data, such as text analysis or content analysis. | “The open-ended survey responses were analyzed using a grounded theory approach to identify key themes and patterns in the data.” |
| This phrase is used to analyze errors or mistakes in the system or the data. | “The confusion matrix shows that the system had high false negative rates for some classes, indicating a potential bias in the data or the model.” |

Let’s look at some of the common mistakes which can be observed in the result section.
- One should not include raw data which are not directly related to your objectives. Readers will not be able to interpret your intentions and may unnecessarily collect unwanted data while replicating your experiments.
- Do not just tell the readers to look at the Table and Figure and figure it out by themselves, e.g “The results are shown in the following Tables and Graphs”.
- Do not give too much explanation about Figures and Tables.
“An Optimized Fuzzy Based Short Term Object Motion Prediction for Real-Life Robot Navigation Environment” ( Paper Link )
Object motions with different motion patterns are generated by a simulator in different directions to generate the initial rule base. The rules generated are clustered based on the direction of the motion pattern into the directional space clusters. Table 1 shows the number of rules that remained in each directional space after removing inconsistencies and redundancies.
| D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 |
| 143 | 178 | 146 | 152 | 141 | 172 | 144 | 183 |
Our predictor algorithm is tested for a real-life benchmark dataset (EC Funded CAVIAR project/IST 2001 37540) to check for relative error. The data set consists of different human motion patterns observed at INRIA Lab at Grenoble, France and Shop Centre. These motion patterns consist of frames captured at 25 frames/second. A typical scenario of the INRIA Lab and the Shop Centre is shown in the Figure below.

Fig.1: A typical scenario of the INRIA Lab and the Shop Centre
For each test case, the average response time is calculated to find its suitability for a real-life environment. The prediction algorithm is tested by processing the frame data of moving human patterns stored in the database at intervals of 50 frames (02 Seconds).
The navigation environment is presented in the form of a Prediction graph where the x-axis represents the Range parameter and the y-axis represents the Angle parameter. The predicted Angle and Range values are compared with actual values obtained from the real-life environment.

The performance of the predictor is tested when more than one object is sensed by the sensor. The tests are carried out assuming at most 6-8 objects can be visible and can affect the decisions to be made regarding robot traversal.
The results section is an essential component of any research paper, as it provides readers with a detailed understanding of the study’s findings. In this blog post, we discussed three important steps for writing a results section: summarizing the data preprocessing steps, reporting on the findings, and summarizing the research findings.
Firstly, summarizing the data preprocessing steps is crucial in the results section, as it provides readers with an understanding of how the raw data was processed and transformed. This step includes data cleaning, data transformation, and data reduction techniques. By summarizing the data preprocessing steps, readers can understand how the data was prepared for analysis, which is critical for interpreting the study’s findings accurately.
Secondly, reporting on the findings is an important step in the results section. It involves presenting the study’s results in a clear and concise manner, using tables, graphs, and statistical analyses where necessary. This step should be focused on answering the research question or hypothesis and should present the findings in a way that is easily understood by the reader. Reporting on the findings can also include providing detailed interpretations of the results, as well as any potential limitations of the study.
Finally, summarizing the research findings is crucial in the results section, as it provides readers with a concise summary of the study’s main results and conclusions. This step should be written in a clear and straightforward manner, highlighting the most important findings and explaining their significance. Additionally, it should relate the study’s findings to the research question or hypothesis and provide a conclusion that is well-supported by the results.
Overall, the results section of a research paper is a critical component that requires careful attention to detail. By following the guidelines discussed in this blog post, researchers can present their findings in a clear and concise manner, helping readers to understand the research process and the resulting conclusions.
Frequently Asked Questions
An IMRaD paper format suggests around 35% of the text should be dedicated to the results and discussion section. For a research paper of length 10 pages, the results and discussion section should occupy 3-4 pages.
The results of a research paper should be given in a separate section. However, the highlights of the results can be discussed in the introduction section.
What is the difference between the “discussion” and the “results” section of a research paper?
The results section only depicts the results obtained by implementing the methodology used. The results will be in the form of figures, tables, charts or graphs. The discussion section elaborates the analysis of the results obtained in the results section.
The summary can be part of the results section of a research paper. However, the results obtained can be summarized in the form of a table in results section of a research paper.
Survey papers and papers which are focussed on theoretical proofs do not involve separate methods and results sections.
The results section is introduced by the data collection steps and the setting up of equipment in different scenarios for obtaining the results.
Making speculations in the results section may lead to wrong interpretations by the researcher who is planning to replicate the methodology used for obtaining the results. This may further lead to wrong comparative analysis.

Upcoming Events
- Visit the Upcoming International Conferences at Exotic Travel Destinations with Travel Plan
- Visit for Research Internships Worldwide

Leave a Reply Cancel reply
You must be logged in to post a comment.
Recent Posts
- Best 5 Journals for Quick Review and High Impact in August 2024
- 05 Quick Review, High Impact, Best Research Journals for Submissions for July 2024
- Top Mistakes to Avoid When Writing a Research Paper
- Average Stipend for Research/Academic Internships
- These Institutes Offer Remote Research/Academic Internships
- All Blog Posts
- Research Career
- Research Conference
- Research Internship
- Research Journal
- Research Tools
- Uncategorized
- Research Conferences
- Research Journals
- Research Grants
- Internships
- Research Internships
- Email Templates
- Conferences
- Blog Partners
- Privacy Policy
Copyright © 2024 Research Voyage
Design by ThemesDNA.com

Please enable JavaScript in your browser to enjoy a better experience.
A Complete Guide to Writing a Research Summary
A summary is a key part of any research. So, how should you go about writing one?
You will find many guides on the Internet about writing research. But, any article seldom covers the prospect of writing a research summary. While many things are shortened versions of the original article, there’s much more to research summaries.
From descriptive statistics to writing scientific research, a summary plays a vital role in describing the key ideas within. So, it begs a few questions, such as:
- What exactly is a research summary?
- How do you write one?
- What are some of the tips for writing a good research summary ?
In this guide, we’ll answer all of these questions and explore a few essential factors about research writing. So, let’s jump right into it.
What is a Research Summary?
A research summary is a short, concise summary of an academic research paper. It is often used to summarize the results of an experiment, summarize the major findings and conclusions, and provide a brief overview of the methods and procedures used in the study.
The purpose of a research summary is to provide readers with enough information about an article to decide whether they want to read it in its entirety. It should be no more than two paragraphs long and should include:
- A brief introduction summarizing why the article was written
- The main idea of the article
- The major findings and conclusions
- An overview of how the study was conducted
In order to write effective research summaries, it is important that you can capture the essential points of the research and provide a concise overview. The key step in writing a good summary is to read through the article and make notes of the key points.
This can be done by underlining or highlighting key phrases in the article. One essential thing is to organize these points into an outline format, which includes an introduction and conclusion paragraph.
Another best and quick way to generate a precise summary of your research paper is to take assistance from the online text summarizer, like Summarizer.org .
The online summarizing tool gets the research paper and creates a precise summary of it by taking the important points.
Finally, you must edit your work for grammar and spelling errors before submitting it for grading.
The purpose of the research summary is to provide a comprehensive sum of everything that’s in the research. This includes a summarization of scientific/literal research, as well as of the writer’s aim and personal thoughts.
As for the summary length, it shouldn’t be more than 10% of the entire content. So, if your research is around 1000-words or so, then your summary should be 100-words. But, considering how most research papers are around 3000-4000 words, it should be 300-400 words.
Key pillars of a Research Summary
The summary of any research doesn’t just include the summarized text of the entire research paper. It includes a few other key things, which we’ll explore later on in this article. But, the purpose of a summary is to give proper insights to the reader, such as:
- The writer’s intention
- sources and bases of research
- the purpose & result.
That’s why it’s important to understand that the summary should tell your reader all these elements. So, the fundamentals of any summary include:
- Write a section and state the importance of the research paper from your perspective. In this section, you will have to describe the techniques, tools, and sources you employed to get the conclusion.
- Besides that, it’s also meant to provide a brief and descriptive explanation of the actionable aspect of your research. In other words, how it can be implemented in real life.
- Treat your research summary like a smaller article or blog. So, each important section of your research should be written within a subheading. However, this is highly optional to keep things organized.
- As mentioned before, the research summary shouldn’t exceed 300-400 words. But, some research summaries are known to surpass 10000-words. So, try to employ the 10% formula and write one-tenth of the entire length of your research paper.
These four main points allow you to understand how a research summary is different from the research itself. So, it’s like a documentary where research and other key factors are left to the science (research paper), while the narration explains the key points (research summary)
How do you write a Research Summary?
Writing a research summary is a straightforward affair. Yet, it requires some understanding, as it’s not a lengthy process but rather a tricky and technical one. In a research summary, a few boxes must be checked. To help you do just that, here are 6 things you should tend to separately:
A summary’s title can be the same as the title of your primary research. However, putting separate titles in both has a few benefits. Such as:
- A separate title shifts attention towards the conclusion.
- A different title can focus on the main point of your research.
- Using two different titles can provide a better abstract.
Speaking of an abstract, a summary is the abstract of your research. Therefore, a title representing that very thought is going to do a lot of good too. That’s why it’s better if the title of your summary differs from the title of your research paper.
2. Abstract
The abstract is the summarization of scientific or research methods used in your primary paper. This allows the reader to understand the pillars of the study conducted. For instance, there has been an array of astrological research since James Webb Space Telescope started sending images and data.
So, many research papers explain this Telescope’s technological evolution in their abstracts. This allows the reader to differentiate from the astrological research made by previous space crafts, such as Hubble or Chandra .
The point of providing this abstract is to ensure that the reader grasps the standards or boundaries within which the research was held.
3. Introduction
This is the part where you introduce your topic. In your main research, you’d dive right into the technicalities in this part. However, you’ll try to keep things mild in a research summary. Simply because it needs to summarize the key points in your main introduction.
So, a lot of introductions you’ll find as an example will be extensive in length. But, a research summary needs to be as concise as possible. Usually, in this part, a writer includes the basics and standards of investigation.
For instance, if your research is about James Webb’s latest findings , then you’ll identify how the studies conducted by this Telescope’s infrared and other technology made this study possible. That’s when your introduction will hook the reader into the main premise of your research.
4. Methodology / Study
This section needs to describe the methodology used by you in your research. Or the methodology you relied on when conducting this particular research or study. This allows the reader to grasp the fundamentals of your research, and it’s extremely important.
Because if the reader doesn’t understand your methods, then they will have no response to your studies. How should you tend to this? Include things such as:
- The surveys or reviews you used;
- include the samplings and experiment types you researched;
- provide a brief statistical analysis;
- give a primary reason to pick these particular methods.
Once again, leave the scientific intricacies for your primary research. But, describe the key methods that you employed. So, when the reader is perusing your final research, they’ll have your methods and study techniques in mind.
5. Results / Discussion
This section of your research needs to describe the results that you’ve achieved. Granted, some researchers will rely on results achieved by others. So, this part needs to explain how that happened – but not in detail.
The other section in this part will be a discussion. This is your interpretation of the results you’ve found. Thus, in the context of the results’ application, this section needs to dive into the theoretical understanding of your research. What will this section entail exactly? Here’s what:
- Things that you covered, including results;
- inferences you provided, given the context of your research;
- the theory archetype that you’ve tried to explain in the light of the methodology you employed;
- essential points or any limitations of the research.
These factors will help the reader grasp the final idea of your research. But, it’s not full circle yet, as the pulp will still be left for the actual research.
6. Conclusion
The final section of your summary is the conclusion. The key thing about the conclusion in your research summary, compared to your actual research, is that they could be different. For instance, the actual conclusion in your research should bring around the study.
However, the research in this summary should bring your own ideas and affirmations to full circle. Thus, this conclusion could and should be different from the ending of your research.
5 Tips for writing a Research Summary
Writing a research summary is easy once you tend to the technicalities. But, there are some tips and tricks that could make it easier. Remember, a research summary is the sum of your entire research. So, it doesn’t need to be as technical or in-depth as your primary work.
Thus, to make it easier for you, here are four tips you can follow:
1. Read & read again
Reading your own work repeatedly has many benefits. First, it’ll help you understand any mistakes or problems your research might have. After that, you’ll find a few key points that stand out from the others – that’s what you need to use in your summary.
So, the best advice anyone can give you is to read your research again and again. This will etch the idea in your mind and allow you to summarize it better.
2. Focus on key essentials in each section
As we discussed earlier, each section of your research has a key part. To write a thoroughly encapsulating summary, you need to focus on and find each such element in your research.
Doing so will give you enough leverage to write a summary that thoroughly condenses your research idea and gives you enough to write a summary out of it.
3. Write the research using a summarizing tool
The best advice you can get is to write a summary using a tool. Condensing each section might be a troublesome experience for some – as it can be time-consuming.
To avoid all that, you can simply take help from an online summarizer. It gets the lengthy content and creates a precise summary of it by using advanced AI technology.
As you can see, the tool condenses this particular section perfectly while the details are light.
Bringing that down to 10% or 20% will help you write each section accordingly. Thus, saving precious time and effort.
4. Word count limit
As mentioned earlier, word count is something you need to follow thoroughly. So, if your section is around 200-word, then read it again. And describe it to yourself in 20-words or so. Doing this to every section will help you write exactly a 10% summary of your research.
5. Get a second opinion
If you’re unsure about quality or quantity, get a second opinion. At times, ideas are in our minds, but we cannot find words to explain them. In research or any sort of creative process, getting a second opinion can save a lot of trouble.
There’s your guide to writing a research summary, folks. While it’s not different from condensing the entire premise of your research, writing it in simpler words will do wonders. So, try to follow the tips, tools, and ideas provided in this article, and write outstanding summaries for your research.

How To Write The Results/Findings Chapter
For qualitative studies (dissertations & theses).
By: Jenna Crossley (PhD). Expert Reviewed By: Dr. Eunice Rautenbach | August 2021
So, you’ve collected and analysed your qualitative data, and it’s time to write up your results chapter. But where do you start? In this post, we’ll guide you through the qualitative results chapter (also called the findings chapter), step by step.
Overview: Qualitative Results Chapter
- What (exactly) the qualitative results chapter is
- What to include in your results chapter
- How to write up your results chapter
- A few tips and tricks to help you along the way
- Free results chapter template
What exactly is the results chapter?
The results chapter in a dissertation or thesis (or any formal academic research piece) is where you objectively and neutrally present the findings of your qualitative analysis (or analyses if you used multiple qualitative analysis methods ). This chapter can sometimes be combined with the discussion chapter (where you interpret the data and discuss its meaning), depending on your university’s preference. We’ll treat the two chapters as separate, as that’s the most common approach.
In contrast to a quantitative results chapter that presents numbers and statistics, a qualitative results chapter presents data primarily in the form of words . But this doesn’t mean that a qualitative study can’t have quantitative elements – you could, for example, present the number of times a theme or topic pops up in your data, depending on the analysis method(s) you adopt.
Adding a quantitative element to your study can add some rigour, which strengthens your results by providing more evidence for your claims. This is particularly common when using qualitative content analysis. Keep in mind though that qualitative research aims to achieve depth, richness and identify nuances , so don’t get tunnel vision by focusing on the numbers. They’re just cream on top in a qualitative analysis.
So, to recap, the results chapter is where you objectively present the findings of your analysis, without interpreting them (you’ll save that for the discussion chapter). With that out the way, let’s take a look at what you should include in your results chapter.

What should you include in the results chapter?
As we’ve mentioned, your qualitative results chapter should purely present and describe your results , not interpret them in relation to the existing literature or your research questions . Any speculations or discussion about the implications of your findings should be reserved for your discussion chapter.
In your results chapter, you’ll want to talk about your analysis findings and whether or not they support your hypotheses (if you have any). Naturally, the exact contents of your results chapter will depend on which qualitative analysis method (or methods) you use. For example, if you were to use thematic analysis, you’d detail the themes identified in your analysis, using extracts from the transcripts or text to support your claims.
While you do need to present your analysis findings in some detail, you should avoid dumping large amounts of raw data in this chapter. Instead, focus on presenting the key findings and using a handful of select quotes or text extracts to support each finding . The reams of data and analysis can be relegated to your appendices.
While it’s tempting to include every last detail you found in your qualitative analysis, it is important to make sure that you report only that which is relevant to your research aims, objectives and research questions . Always keep these three components, as well as your hypotheses (if you have any) front of mind when writing the chapter and use them as a filter to decide what’s relevant and what’s not.
Need a helping hand?
How do I write the results chapter?
Now that we’ve covered the basics, it’s time to look at how to structure your chapter. Broadly speaking, the results chapter needs to contain three core components – the introduction, the body and the concluding summary. Let’s take a look at each of these.
Section 1: Introduction
The first step is to craft a brief introduction to the chapter. This intro is vital as it provides some context for your findings. In your introduction, you should begin by reiterating your problem statement and research questions and highlight the purpose of your research . Make sure that you spell this out for the reader so that the rest of your chapter is well contextualised.
The next step is to briefly outline the structure of your results chapter. In other words, explain what’s included in the chapter and what the reader can expect. In the results chapter, you want to tell a story that is coherent, flows logically, and is easy to follow , so make sure that you plan your structure out well and convey that structure (at a high level), so that your reader is well oriented.
The introduction section shouldn’t be lengthy. Two or three short paragraphs should be more than adequate. It is merely an introduction and overview, not a summary of the chapter.
Pro Tip – To help you structure your chapter, it can be useful to set up an initial draft with (sub)section headings so that you’re able to easily (re)arrange parts of your chapter. This will also help your reader to follow your results and give your chapter some coherence. Be sure to use level-based heading styles (e.g. Heading 1, 2, 3 styles) to help the reader differentiate between levels visually. You can find these options in Word (example below).
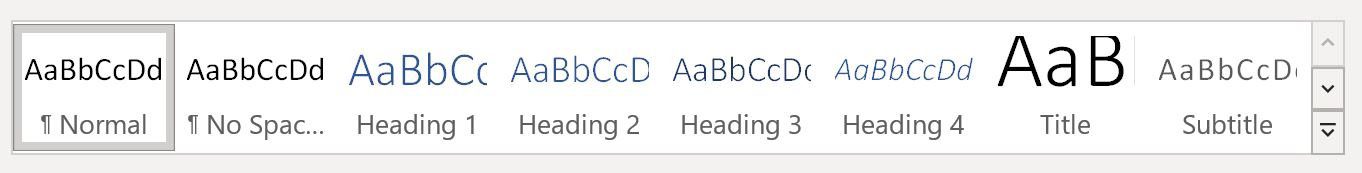
Section 2: Body
Before we get started on what to include in the body of your chapter, it’s vital to remember that a results section should be completely objective and descriptive, not interpretive . So, be careful not to use words such as, “suggests” or “implies”, as these usually accompany some form of interpretation – that’s reserved for your discussion chapter.
The structure of your body section is very important , so make sure that you plan it out well. When planning out your qualitative results chapter, create sections and subsections so that you can maintain the flow of the story you’re trying to tell. Be sure to systematically and consistently describe each portion of results. Try to adopt a standardised structure for each portion so that you achieve a high level of consistency throughout the chapter.
For qualitative studies, results chapters tend to be structured according to themes , which makes it easier for readers to follow. However, keep in mind that not all results chapters have to be structured in this manner. For example, if you’re conducting a longitudinal study, you may want to structure your chapter chronologically. Similarly, you might structure this chapter based on your theoretical framework . The exact structure of your chapter will depend on the nature of your study , especially your research questions.
As you work through the body of your chapter, make sure that you use quotes to substantiate every one of your claims . You can present these quotes in italics to differentiate them from your own words. A general rule of thumb is to use at least two pieces of evidence per claim, and these should be linked directly to your data. Also, remember that you need to include all relevant results , not just the ones that support your assumptions or initial leanings.
In addition to including quotes, you can also link your claims to the data by using appendices , which you should reference throughout your text. When you reference, make sure that you include both the name/number of the appendix , as well as the line(s) from which you drew your data.
As referencing styles can vary greatly, be sure to look up the appendix referencing conventions of your university’s prescribed style (e.g. APA , Harvard, etc) and keep this consistent throughout your chapter.
Section 3: Concluding summary
The concluding summary is very important because it summarises your key findings and lays the foundation for the discussion chapter . Keep in mind that some readers may skip directly to this section (from the introduction section), so make sure that it can be read and understood well in isolation.
In this section, you need to remind the reader of the key findings. That is, the results that directly relate to your research questions and that you will build upon in your discussion chapter. Remember, your reader has digested a lot of information in this chapter, so you need to use this section to remind them of the most important takeaways.
Importantly, the concluding summary should not present any new information and should only describe what you’ve already presented in your chapter. Keep it concise – you’re not summarising the whole chapter, just the essentials.
Tips for writing an A-grade results chapter
Now that you’ve got a clear picture of what the qualitative results chapter is all about, here are some quick tips and reminders to help you craft a high-quality chapter:
- Your results chapter should be written in the past tense . You’ve done the work already, so you want to tell the reader what you found , not what you are currently finding .
- Make sure that you review your work multiple times and check that every claim is adequately backed up by evidence . Aim for at least two examples per claim, and make use of an appendix to reference these.
- When writing up your results, make sure that you stick to only what is relevant . Don’t waste time on data that are not relevant to your research objectives and research questions.
- Use headings and subheadings to create an intuitive, easy to follow piece of writing. Make use of Microsoft Word’s “heading styles” and be sure to use them consistently.
- When referring to numerical data, tables and figures can provide a useful visual aid. When using these, make sure that they can be read and understood independent of your body text (i.e. that they can stand-alone). To this end, use clear, concise labels for each of your tables or figures and make use of colours to code indicate differences or hierarchy.
- Similarly, when you’re writing up your chapter, it can be useful to highlight topics and themes in different colours . This can help you to differentiate between your data if you get a bit overwhelmed and will also help you to ensure that your results flow logically and coherently.
If you have any questions, leave a comment below and we’ll do our best to help. If you’d like 1-on-1 help with your results chapter (or any chapter of your dissertation or thesis), check out our private dissertation coaching service here or book a free initial consultation to discuss how we can help you.

Psst... there’s more!
This post was based on one of our popular Research Bootcamps . If you're working on a research project, you'll definitely want to check this out ...
22 Comments
This was extremely helpful. Thanks a lot guys
Hi, thanks for the great research support platform created by the gradcoach team!
I wanted to ask- While “suggests” or “implies” are interpretive terms, what terms could we use for the results chapter? Could you share some examples of descriptive terms?
I think that instead of saying, ‘The data suggested, or The data implied,’ you can say, ‘The Data showed or revealed, or illustrated or outlined’…If interview data, you may say Jane Doe illuminated or elaborated, or Jane Doe described… or Jane Doe expressed or stated.
I found this article very useful. Thank you very much for the outstanding work you are doing.
What if i have 3 different interviewees answering the same interview questions? Should i then present the results in form of the table with the division on the 3 perspectives or rather give a results in form of the text and highlight who said what?
I think this tabular representation of results is a great idea. I am doing it too along with the text. Thanks
That was helpful was struggling to separate the discussion from the findings
this was very useful, Thank you.
Very helpful, I am confident to write my results chapter now.
It is so helpful! It is a good job. Thank you very much!
Very useful, well explained. Many thanks.
Hello, I appreciate the way you provided a supportive comments about qualitative results presenting tips
I loved this! It explains everything needed, and it has helped me better organize my thoughts. What words should I not use while writing my results section, other than subjective ones.
Thanks a lot, it is really helpful
Thank you so much dear, i really appropriate your nice explanations about this.
Thank you so much for this! I was wondering if anyone could help with how to prproperly integrate quotations (Excerpts) from interviews in the finding chapter in a qualitative research. Please GradCoach, address this issue and provide examples.
what if I’m not doing any interviews myself and all the information is coming from case studies that have already done the research.
Very helpful thank you.
This was very helpful as I was wondering how to structure this part of my dissertation, to include the quotes… Thanks for this explanation
This is very helpful, thanks! I am required to write up my results chapters with the discussion in each of them – any tips and tricks for this strategy?
For qualitative studies, can the findings be structured according to the Research questions? Thank you.
Do I need to include literature/references in my findings chapter?
Submit a Comment Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
- Print Friendly

- Chapter Seven: Presenting Your Results
This chapter serves as the culmination of the previous chapters, in that it focuses on how to present the results of one's study, regardless of the choice made among the three methods. Writing in academics has a form and style that you will want to apply not only to report your own research, but also to enhance your skills at reading original research published in academic journals. Beyond the basic academic style of report writing, there are specific, often unwritten assumptions about how quantitative, qualitative, and critical/rhetorical studies should be organized and the information they should contain. This chapter discusses how to present your results in writing, how to write accessibly, how to visualize data, and how to present your results in person.
- Chapter One: Introduction
- Chapter Two: Understanding the distinctions among research methods
- Chapter Three: Ethical research, writing, and creative work
- Chapter Four: Quantitative Methods (Part 1)
- Chapter Four: Quantitative Methods (Part 2 - Doing Your Study)
- Chapter Four: Quantitative Methods (Part 3 - Making Sense of Your Study)
- Chapter Five: Qualitative Methods (Part 1)
- Chapter Five: Qualitative Data (Part 2)
- Chapter Six: Critical / Rhetorical Methods (Part 1)
- Chapter Six: Critical / Rhetorical Methods (Part 2)
Written Presentation of Results
Once you've gone through the process of doing communication research – using a quantitative, qualitative, or critical/rhetorical methodological approach – the final step is to communicate it.
The major style manuals (the APA Manual, the MLA Handbook, and Turabian) are very helpful in documenting the structure of writing a study, and are highly recommended for consultation. But, no matter what style manual you may use, there are some common elements to the structure of an academic communication research paper.
Title Page :
This is simple: Your Paper's Title, Your Name, Your Institutional Affiliation (e.g., University), and the Date, each on separate lines, centered on the page. Try to make your title both descriptive (i.e., it gives the reader an idea what the study is about) and interesting (i.e., it is catchy enough to get one's attention).
For example, the title, "The uncritical idealization of a compensated psychopath character in a popular book series," would not be an inaccurate title for a published study, but it is rather vague and exceedingly boring. That study's author fortunately chose the title, "A boyfriend to die for: Edward Cullen as compensated psychopath in Stephanie Meyer's Twilight ," which is more precisely descriptive, and much more interesting (Merskin, 2011). The use of the colon in academic titles can help authors accomplish both objectives: a catchy but relevant phrase, followed by a more clear explanation of the article's topic.
In some instances, you might be asked to write an abstract, which is a summary of your paper that can range in length from 75 to 250 words. If it is a published paper, it is useful to include key search terms in this brief description of the paper (the title may already have a few of these terms as well). Although this may be the last thing your write, make it one of the best things you write, because this may be the first thing your audience reads about the paper (and may be the only thing read if it is written badly). Summarize the problem/research question, your methodological approach, your results and conclusions, and the significance of the paper in the abstract.
Quantitative and qualitative studies will most typically use the rest of the section titles noted below. Critical/rhetorical studies will include many of the same steps, but will often have different headings. For example, a critical/rhetorical paper will have an introduction, definition of terms, and literature review, followed by an analysis (often divided into sections by areas of investigation) and ending with a conclusion/implications section. Because critical/rhetorical research is much more descriptive, the subheadings in such a paper are often times not generic subheads like "literature review," but instead descriptive subheadings that apply to the topic at hand, as seen in the schematic below. Because many journals expect the article to follow typical research paper headings of introduction, literature review, methods, results, and discussion, we discuss these sections briefly next.
Introduction:
As you read social scientific journals (see chapter 1 for examples), you will find that they tend to get into the research question quickly and succinctly. Journal articles from the humanities tradition tend to be more descriptive in the introduction. But, in either case, it is good to begin with some kind of brief anecdote that gets the reader engaged in your work and lets the reader understand why this is an interesting topic. From that point, state your research question, define the problem (see Chapter One) with an overview of what we do and don't know, and finally state what you will do, or what you want to find out. The introduction thus builds the case for your topic, and is the beginning of building your argument, as we noted in chapter 1.
By the end of the Introduction, the reader should know what your topic is, why it is a significant communication topic, and why it is necessary that you investigate it (e.g., it could be there is gap in literature, you will conduct valuable exploratory research, or you will provide a new model for solving some professional or social problem).
Literature Review:
The literature review summarizes and organizes the relevant books, articles, and other research in this area. It sets up both quantitative and qualitative studies, showing the need for the study. For critical/rhetorical research, the literature review often incorporates the description of the historical context and heuristic vocabulary, with key terms defined in this section of the paper. For more detail on writing a literature review, see Appendix 1.
The methods of your paper are the processes that govern your research, where the researcher explains what s/he did to solve the problem. As you have seen throughout this book, in communication studies, there are a number of different types of research methods. For example, in quantitative research, one might conduct surveys, experiments, or content analysis. In qualitative research, one might instead use interviews and observations. Critical/rhetorical studies methods are more about the interpretation of texts or the study of popular culture as communication. In creative communication research, the method may be an interpretive performance studies or filmmaking. Other methods used sometimes alone, or in combination with other methods, include legal research, historical research, and political economy research.
In quantitative and qualitative research papers, the methods will be most likely described according to the APA manual standards. At the very least, the methods will include a description of participants, data collection, and data analysis, with specific details on each of these elements. For example, in an experiment, the researcher will describe the number of participants, the materials used, the design of the experiment, the procedure of the experiment, and what statistics will be used to address the hypotheses/research questions.
Critical/rhetorical researchers rarely have a specific section called "methods," as opposed to quantitative and qualitative researchers, but rather demonstrate the method they use for analysis throughout the writing of their piece.
Helping your reader understand the methods you used for your study is important not only for your own study's credibility, but also for possible replication of your study by other researchers. A good guideline to keep in mind is transparency . You want to be as clear as possible in describing the decisions you made in designing your study, gathering and analyzing your data so that the reader can retrace your steps and understand how you came to the conclusions you formed. A research study can be very good, but if it is not clearly described so that others can see how the results were determined or obtained, then the quality of the study and its potential contributions are lost.
After you completed your study, your findings will be listed in the results section. Particularly in a quantitative study, the results section is for revisiting your hypotheses and reporting whether or not your results supported them, and the statistical significance of the results. Whether your study supported or contradicted your hypotheses, it's always helpful to fully report what your results were. The researcher usually organizes the results of his/her results section by research question or hypothesis, stating the results for each one, using statistics to show how the research question or hypothesis was answered in the study.
The qualitative results section also may be organized by research question, but usually is organized by themes which emerged from the data collected. The researcher provides rich details from her/his observations and interviews, with detailed quotations provided to illustrate the themes identified. Sometimes the results section is combined with the discussion section.
Critical/rhetorical researchers would include their analysis often with different subheadings in what would be considered a "results" section, yet not labeled specifically this way.
Discussion:
In the discussion section, the researcher gives an appraisal of the results. Here is where the researcher considers the results, particularly in light of the literature review, and explains what the findings mean. If the results confirmed or corresponded with the findings of other literature, then that should be stated. If the results didn't support the findings of previous studies, then the researcher should develop an explanation of why the study turned out this way. Sometimes, this section is called a "conclusion" by researchers.
References:
In this section, all of the literature cited in the text should have full references in alphabetical order. Appendices: Appendix material includes items like questionnaires used in the study, photographs, documents, etc. An alphabetical letter is assigned for each piece (e.g. Appendix A, Appendix B), with a second line of title describing what the appendix contains (e.g. Participant Informed Consent, or New York Times Speech Coverage). They should be organized consistently with the order in which they are referenced in the text of the paper. The page numbers for appendices are consecutive with the paper and reference list.
Tables/Figures:
Tables and figures are referenced in the text, but included at the end of the study and numbered consecutively. (Check with your professor; some like to have tables and figures inserted within the paper's main text.) Tables generally are data in a table format, whereas figures are diagrams (such as a pie chart) and drawings (such as a flow chart).
Accessible Writing
As you may have noticed, academic writing does have a language (e.g., words like heuristic vocabulary and hypotheses) and style (e.g., literature reviews) all its own. It is important to engage in that language and style, and understand how to use it to communicate effectively in an academic context . Yet, it is also important to remember that your analyses and findings should also be written to be accessible. Writers should avoid excessive jargon, or—even worse—deploying jargon to mask an incomplete understanding of a topic.
The scourge of excessive jargon in academic writing was the target of a famous hoax in 1996. A New York University physics professor submitted an article, " Transgressing the Boundaries: Toward a Transformative Hermeneutics of Quantum Gravity ," to a special issue of the academic journal Social Text devoted to science and postmodernism. The article was designed to point out how dense academic jargon can sometimes mask sloppy thinking. As the professor, Alan Sokal, had expected, the article was published. One sample sentence from the article reads:
It has thus become increasingly apparent that physical "reality", no less than social "reality", is at bottom a social and linguistic construct; that scientific "knowledge", far from being objective, reflects and encodes the dominant ideologies and power relations of the culture that produced it; that the truth claims of science are inherently theory-laden and self-referential; and consequently, that the discourse of the scientific community, for all its undeniable value, cannot assert a privileged epistemological status with respect to counter-hegemonic narratives emanating from dissident or marginalized communities. (Sokal, 1996. pp. 217-218)
According to the journal's editor, about six reviewers had read the article but didn't suspect that it was phony. A public debate ensued after Sokal revealed his hoax. Sokal said he worried that jargon and intellectual fads cause academics to lose contact with the real world and "undermine the prospect for progressive social critique" ( Scott, 1996 ). The APA Manual recommends to avoid using technical vocabulary where it is not needed or relevant or if the technical language is overused, thus becoming jargon. In short, the APA argues that "scientific jargon...grates on the reader, encumbers the communication of information, and wastes space" (American Psychological Association, 2010, p. 68).
Data Visualization
Images and words have long existed on the printed page of manuscripts, yet, until recently, relatively few researchers possessed the resources to effectively combine images combined with words (Tufte, 1990, 1983). Communication scholars are only now becoming aware of this dimension in research as computer technologies have made it possible for many people to produce and publish multimedia presentations.
Although visuals may seem to be anathema to the primacy of the written word in research, they are a legitimate way, and at times the best way, to present ideas. Visual scholar Lester Faigley et al. (2004) explains how data visualizations have become part of our daily lives:
Visualizations can shed light on research as well. London-based David McCandless specializes in visualizing interesting research questions, or in his words "the questions I wanted answering" (2009, p. 7). His images include a graph of the peak times of the year for breakups (based on Facebook status updates), a radiation dosage chart , and some experiments with the Google Ngram Viewer , which charts the appearance of keywords in millions of books over hundreds of years.
The public domain image below creatively maps U.S. Census data of the outflow of people from California to other states between 1995 and 2000.
Visualizing one's research is possible in multiple ways. A simple technology, for example, is to enter data into a spreadsheet such as Excel, and select Charts or SmartArt to generate graphics. A number of free web tools can also transform raw data into useful charts and graphs. Many Eyes , an open source data visualization tool (sponsored by IBM Research), says its goal "is to 'democratize' visualization and to enable a new social kind of data analysis" (IBM, 2011). Another tool, Soundslides , enables users to import images and audio to create a photographic slideshow, while the program handles all of the background code. Other tools, often open source and free, can help visual academic research into interactive maps; interactive, image-based timelines; interactive charts; and simple 2-D and 3-D animations. Adobe Creative Suite (which includes popular software like Photoshop) is available on most computers at universities, but open source alternatives exist as well. Gimp is comparable to Photoshop, and it is free and relatively easy to use.
One online performance studies journal, Liminalities , is an excellent example of how "research" can be more than just printed words. In each issue, traditional academic essays and book reviews are often supported photographs, while other parts of an issue can include video, audio, and multimedia contributions. The journal, founded in 2005, treats performance itself as a methodology, and accepts contribution in html, mp3, Quicktime, and Flash formats.
For communication researchers, there is also a vast array of visual digital archives available online. Many of these archives are located at colleges and universities around the world, where digital librarians are spearheading a massive effort to make information—print, audio, visual, and graphic—available to the public as part of a global information commons. For example, the University of Iowa has a considerable digital archive including historical photos documenting American railroads and a database of images related to geoscience. The University of Northern Iowa has a growing Special Collections Unit that includes digital images of every UNI Yearbook between 1905 and 1923 and audio files of UNI jazz band performances. Researchers at he University of Michigan developed OAIster , a rich database that has joined thousands of digital archives in one searchable interface. Indeed, virtually every academic library is now digitizing all types of media, not just texts, and making them available for public viewing and, when possible, for use in presenting research. In addition to academic collections, the Library of Congress and the National Archives offer an ever-expanding range of downloadable media; commercial, user-generated databases such as Flickr, Buzznet, YouTube and Google Video offer a rich resource of images that are often free of copyright constraints (see Chapter 3 about Creative Commons licenses) and nonprofit endeavors, such as the Internet Archive , contain a formidable collection of moving images, still photographs, audio files (including concert recordings), and open source software.
Presenting your Work in Person
As Communication students, it's expected that you are not only able to communicate your research project in written form but also in person.
Before you do any oral presentation, it's good to have a brief "pitch" ready for anyone who asks you about your research. The pitch is routine in Hollywood: a screenwriter has just a few minutes to present an idea to a producer. Although your pitch will be more sophisticated than, say, " Snakes on a Plane " (which unfortunately was made into a movie), you should in just a few lines be able to explain the gist of your research to anyone who asks. Developing this concise description, you will have some practice in distilling what might be a complicated topic into one others can quickly grasp.
Oral presentation
In most oral presentations of research, whether at the end of a semester, or at a research symposium or conference, you will likely have just 10 to 20 minutes. This is probably not enough time to read the entire paper aloud, which is not what you should do anyway if you want people to really listen (although, unfortunately some make this mistake). Instead, the point of the presentation should be to present your research in an interesting manner so the listeners will want to read the whole thing. In the presentation, spend the least amount of time on the literature review (a very brief summary will suffice) and the most on your own original contribution. In fact, you may tell your audience that you are only presenting on one portion of the paper, and that you would be happy to talk more about your research and findings in the question and answer session that typically follows. Consider your presentation the beginning of a dialogue between you and the audience. Your tone shouldn't be "I have found everything important there is to find, and I will cram as much as I can into this presentation," but instead "I found some things you will find interesting, but I realize there is more to find."
Turabian (2007) has a helpful chapter on presenting research. Most important, she emphasizes, is to remember that your audience members are listeners, not readers. Thus, recall the lessons on speech making in your college oral communication class. Give an introduction, tell them what the problem is, and map out what you will present to them. Organize your findings into a few points, and don't get bogged down in minutiae. (The minutiae are for readers to find if they wish, not for listeners to struggle through.) PowerPoint slides are acceptable, but don't read them. Instead, create an outline of a few main points, and practice your presentation.
Turabian suggests an introduction of not more than three minutes, which should include these elements:
- The research topic you will address (not more than a minute).
- Your research question (30 seconds or less)
- An answer to "so what?" – explaining the relevance of your research (30 seconds)
- Your claim, or argument (30 seconds or less)
- The map of your presentation structure (30 seconds or less)
As Turabian (2007) suggests, "Rehearse your introduction, not only to get it right, but to be able to look your audience in the eye as you give it. You can look down at notes later" (p. 125).
Poster presentation
In some symposiums and conferences, you may be asked to present at a "poster" session. Instead of presenting on a panel of 4-5 people to an audience, a poster presenter is with others in a large hall or room, and talks one-on-one with visitors who look at the visual poster display of the research. As in an oral presentation, a poster highlights just the main point of the paper. Then, if visitors have questions, the author can informally discuss her/his findings.
To attract attention, poster presentations need to be nicely designed, or in the words of an advertising professor who schedules poster sessions at conferences, "be big, bold, and brief" ( Broyles , 2011). Large type (at least 18 pt.), graphics, tables, and photos are recommended.
A poster presentation session at a conference, by David Eppstein (Own work) [CC-BY-SA-3.0 ( www.creativecommons.org/licenses/by-sa/3.0 )], via Wikimedia Commons]
The Association for Education in Journalism and Mass Communication (AEJMC) has a template for making an effective poster presentation . Many universities, copy shops, and Internet services also have large-scale printers, to print full-color research poster designs that can be rolled up and transported in a tube.
Judging Others' Research
After taking this course, you should have a basic knowledge of research methods. There will still be some things that may mystify you as a reader of other's research. For example, you may not be able to interpret the coefficients for statistical significance, or make sense of a complex structural equation. Some specialized vocabulary may still be difficult.
But, you should understand how to critically review research. For example, imagine you have been asked to do a blind (i.e., the author's identity is concealed) "peer review" of communication research for acceptance to a conference, or publication in an academic journal. For most conferences and journals , submissions are made online, where editors can manage the flow and assign reviews to papers. The evaluations reviewers make are based on the same things that we have covered in this book. For example, the conference for the AEJMC ask reviewers to consider (on a five-point scale, from Excellent to Poor) a number of familiar research dimensions, including the paper's clarity of purpose, literature review, clarity of research method, appropriateness of research method, evidence presented clearly, evidence supportive of conclusions, general writing and organization, and the significance of the contribution to the field.
Beyond academia, it is likely you will more frequently apply the lessons of research methods as a critical consumer of news, politics, and everyday life. Just because some expert cites a number or presents a conclusion doesn't mean it's automatically true. John Allen Paulos, in his book A Mathematician reads the newspaper , suggests some basic questions we can ask. "If statistics were presented, how were they obtained? How confident can we be of them? Were they derived from a random sample or from a collection of anecdotes? Does the correlation suggest a causal relationship, or is it merely a coincidence?" (1997, p. 201).
Through the study of research methods, we have begun to build a critical vocabulary and understanding to ask good questions when others present "knowledge." For example, if Candidate X won a straw poll in Iowa, does that mean she'll get her party's nomination? If Candidate Y wins an open primary in New Hampshire, does that mean he'll be the next president? If Candidate Z sheds a tear, does it matter what the context is, or whether that candidate is a man or a woman? What we learn in research methods about validity, reliability, sampling, variables, research participants, epistemology, grounded theory, and rhetoric, we can consider whether the "knowledge" that is presented in the news is a verifiable fact, a sound argument, or just conjecture.
American Psychological Association (2010). Publication manual of the American Psychological Association (6th ed.). Washington, DC: Author.
Broyles, S. (2011). "About poster sessions." AEJMC. http://www.aejmc.org/home/2013/01/about-poster-sessions/ .
Faigley, L., George, D., Palchik, A., Selfe, C. (2004). Picturing texts . New York: W.W. Norton & Company.
IBM (2011). Overview of Many Eyes. http://www.research.ibm.com/social/projects_manyeyes.shtml .
McCandless, D. (2009). The visual miscellaneum . New York: Collins Design.
Merskin, D. (2011). A boyfriend to die for: Edward Cullen as compensated psychopath in Stephanie Meyer's Twilight. Journal of Communication Inquiry 35: 157-178. doi:10.1177/0196859911402992
Paulos, J. A. (1997). A mathematician reads the newspaper . New York: Anchor.
Scott, J. (1996, May 18). Postmodern gravity deconstructed, slyly. New York Times , http://www.nytimes.com/books/98/11/15/specials/sokal-text.html .
Sokal, A. (1996). Transgressing the boundaries: towards a transformative hermeneutics of quantum gravity. Social Text 46/47, 217-252.
Tufte, E. R. (1990). Envisioning information . Cheshire, CT: Graphics Press.
Tufte, E. R. (1983). The visual display of quantitative information . Cheshire, CT: Graphics Press.
Turabian, Kate L. (2007). A manual for writers of research papers, theses, and dissertations: Chicago style guide for students and researchers (7th ed.). Chicago: University of Chicago Press.
Jump to navigation

Cochrane Training
Chapter 15: interpreting results and drawing conclusions.
Holger J Schünemann, Gunn E Vist, Julian PT Higgins, Nancy Santesso, Jonathan J Deeks, Paul Glasziou, Elie A Akl, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group
Key Points:
- This chapter provides guidance on interpreting the results of synthesis in order to communicate the conclusions of the review effectively.
- Methods are presented for computing, presenting and interpreting relative and absolute effects for dichotomous outcome data, including the number needed to treat (NNT).
- For continuous outcome measures, review authors can present summary results for studies using natural units of measurement or as minimal important differences when all studies use the same scale. When studies measure the same construct but with different scales, review authors will need to find a way to interpret the standardized mean difference, or to use an alternative effect measure for the meta-analysis such as the ratio of means.
- Review authors should not describe results as ‘statistically significant’, ‘not statistically significant’ or ‘non-significant’ or unduly rely on thresholds for P values, but report the confidence interval together with the exact P value.
- Review authors should not make recommendations about healthcare decisions, but they can – after describing the certainty of evidence and the balance of benefits and harms – highlight different actions that might be consistent with particular patterns of values and preferences and other factors that determine a decision such as cost.
Cite this chapter as: Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, Akl EA, Guyatt GH. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
15.1 Introduction
The purpose of Cochrane Reviews is to facilitate healthcare decisions by patients and the general public, clinicians, guideline developers, administrators and policy makers. They also inform future research. A clear statement of findings, a considered discussion and a clear presentation of the authors’ conclusions are, therefore, important parts of the review. In particular, the following issues can help people make better informed decisions and increase the usability of Cochrane Reviews:
- information on all important outcomes, including adverse outcomes;
- the certainty of the evidence for each of these outcomes, as it applies to specific populations and specific interventions; and
- clarification of the manner in which particular values and preferences may bear on the desirable and undesirable consequences of the intervention.
A ‘Summary of findings’ table, described in Chapter 14 , Section 14.1 , provides key pieces of information about health benefits and harms in a quick and accessible format. It is highly desirable that review authors include a ‘Summary of findings’ table in Cochrane Reviews alongside a sufficient description of the studies and meta-analyses to support its contents. This description includes the rating of the certainty of evidence, also called the quality of the evidence or confidence in the estimates of the effects, which is expected in all Cochrane Reviews.
‘Summary of findings’ tables are usually supported by full evidence profiles which include the detailed ratings of the evidence (Guyatt et al 2011a, Guyatt et al 2013a, Guyatt et al 2013b, Santesso et al 2016). The Discussion section of the text of the review provides space to reflect and consider the implications of these aspects of the review’s findings. Cochrane Reviews include five standard subheadings to ensure the Discussion section places the review in an appropriate context: ‘Summary of main results (benefits and harms)’; ‘Potential biases in the review process’; ‘Overall completeness and applicability of evidence’; ‘Certainty of the evidence’; and ‘Agreements and disagreements with other studies or reviews’. Following the Discussion, the Authors’ conclusions section is divided into two standard subsections: ‘Implications for practice’ and ‘Implications for research’. The assessment of the certainty of evidence facilitates a structured description of the implications for practice and research.
Because Cochrane Reviews have an international audience, the Discussion and Authors’ conclusions should, so far as possible, assume a broad international perspective and provide guidance for how the results could be applied in different settings, rather than being restricted to specific national or local circumstances. Cultural differences and economic differences may both play an important role in determining the best course of action based on the results of a Cochrane Review. Furthermore, individuals within societies have widely varying values and preferences regarding health states, and use of societal resources to achieve particular health states. For all these reasons, and because information that goes beyond that included in a Cochrane Review is required to make fully informed decisions, different people will often make different decisions based on the same evidence presented in a review.
Thus, review authors should avoid specific recommendations that inevitably depend on assumptions about available resources, values and preferences, and other factors such as equity considerations, feasibility and acceptability of an intervention. The purpose of the review should be to present information and aid interpretation rather than to offer recommendations. The discussion and conclusions should help people understand the implications of the evidence in relation to practical decisions and apply the results to their specific situation. Review authors can aid this understanding of the implications by laying out different scenarios that describe certain value structures.
In this chapter, we address first one of the key aspects of interpreting findings that is also fundamental in completing a ‘Summary of findings’ table: the certainty of evidence related to each of the outcomes. We then provide a more detailed consideration of issues around applicability and around interpretation of numerical results, and provide suggestions for presenting authors’ conclusions.
15.2 Issues of indirectness and applicability
15.2.1 the role of the review author.
“A leap of faith is always required when applying any study findings to the population at large” or to a specific person. “In making that jump, one must always strike a balance between making justifiable broad generalizations and being too conservative in one’s conclusions” (Friedman et al 1985). In addition to issues about risk of bias and other domains determining the certainty of evidence, this leap of faith is related to how well the identified body of evidence matches the posed PICO ( Population, Intervention, Comparator(s) and Outcome ) question. As to the population, no individual can be entirely matched to the population included in research studies. At the time of decision, there will always be differences between the study population and the person or population to whom the evidence is applied; sometimes these differences are slight, sometimes large.
The terms applicability, generalizability, external validity and transferability are related, sometimes used interchangeably and have in common that they lack a clear and consistent definition in the classic epidemiological literature (Schünemann et al 2013). However, all of the terms describe one overarching theme: whether or not available research evidence can be directly used to answer the health and healthcare question at hand, ideally supported by a judgement about the degree of confidence in this use (Schünemann et al 2013). GRADE’s certainty domains include a judgement about ‘indirectness’ to describe all of these aspects including the concept of direct versus indirect comparisons of different interventions (Atkins et al 2004, Guyatt et al 2008, Guyatt et al 2011b).
To address adequately the extent to which a review is relevant for the purpose to which it is being put, there are certain things the review author must do, and certain things the user of the review must do to assess the degree of indirectness. Cochrane and the GRADE Working Group suggest using a very structured framework to address indirectness. We discuss here and in Chapter 14 what the review author can do to help the user. Cochrane Review authors must be extremely clear on the population, intervention and outcomes that they intend to address. Chapter 14, Section 14.1.2 , also emphasizes a crucial step: the specification of all patient-important outcomes relevant to the intervention strategies under comparison.
In considering whether the effect of an intervention applies equally to all participants, and whether different variations on the intervention have similar effects, review authors need to make a priori hypotheses about possible effect modifiers, and then examine those hypotheses (see Chapter 10, Section 10.10 and Section 10.11 ). If they find apparent subgroup effects, they must ultimately decide whether or not these effects are credible (Sun et al 2012). Differences between subgroups, particularly those that correspond to differences between studies, should be interpreted cautiously. Some chance variation between subgroups is inevitable so, unless there is good reason to believe that there is an interaction, review authors should not assume that the subgroup effect exists. If, despite due caution, review authors judge subgroup effects in terms of relative effect estimates as credible (i.e. the effects differ credibly), they should conduct separate meta-analyses for the relevant subgroups, and produce separate ‘Summary of findings’ tables for those subgroups.
The user of the review will be challenged with ‘individualization’ of the findings, whether they seek to apply the findings to an individual patient or a policy decision in a specific context. For example, even if relative effects are similar across subgroups, absolute effects will differ according to baseline risk. Review authors can help provide this information by identifying identifiable groups of people with varying baseline risks in the ‘Summary of findings’ tables, as discussed in Chapter 14, Section 14.1.3 . Users can then identify their specific case or population as belonging to a particular risk group, if relevant, and assess their likely magnitude of benefit or harm accordingly. A description of the identifying prognostic or baseline risk factors in a brief scenario (e.g. age or gender) will help users of a review further.
Another decision users must make is whether their individual case or population of interest is so different from those included in the studies that they cannot use the results of the systematic review and meta-analysis at all. Rather than rigidly applying the inclusion and exclusion criteria of studies, it is better to ask whether or not there are compelling reasons why the evidence should not be applied to a particular patient. Review authors can sometimes help decision makers by identifying important variation where divergence might limit the applicability of results (Rothwell 2005, Schünemann et al 2006, Guyatt et al 2011b, Schünemann et al 2013), including biologic and cultural variation, and variation in adherence to an intervention.
In addressing these issues, review authors cannot be aware of, or address, the myriad of differences in circumstances around the world. They can, however, address differences of known importance to many people and, importantly, they should avoid assuming that other people’s circumstances are the same as their own in discussing the results and drawing conclusions.
15.2.2 Biological variation
Issues of biological variation that may affect the applicability of a result to a reader or population include divergence in pathophysiology (e.g. biological differences between women and men that may affect responsiveness to an intervention) and divergence in a causative agent (e.g. for infectious diseases such as malaria, which may be caused by several different parasites). The discussion of the results in the review should make clear whether the included studies addressed all or only some of these groups, and whether any important subgroup effects were found.
15.2.3 Variation in context
Some interventions, particularly non-pharmacological interventions, may work in some contexts but not in others; the situation has been described as program by context interaction (Hawe et al 2004). Contextual factors might pertain to the host organization in which an intervention is offered, such as the expertise, experience and morale of the staff expected to carry out the intervention, the competing priorities for the clinician’s or staff’s attention, the local resources such as service and facilities made available to the program and the status or importance given to the program by the host organization. Broader context issues might include aspects of the system within which the host organization operates, such as the fee or payment structure for healthcare providers and the local insurance system. Some interventions, in particular complex interventions (see Chapter 17 ), can be only partially implemented in some contexts, and this requires judgements about indirectness of the intervention and its components for readers in that context (Schünemann 2013).
Contextual factors may also pertain to the characteristics of the target group or population, such as cultural and linguistic diversity, socio-economic position, rural/urban setting. These factors may mean that a particular style of care or relationship evolves between service providers and consumers that may or may not match the values and technology of the program.
For many years these aspects have been acknowledged when decision makers have argued that results of evidence reviews from other countries do not apply in their own country or setting. Whilst some programmes/interventions have been successfully transferred from one context to another, others have not (Resnicow et al 1993, Lumley et al 2004, Coleman et al 2015). Review authors should be cautious when making generalizations from one context to another. They should report on the presence (or otherwise) of context-related information in intervention studies, where this information is available.
15.2.4 Variation in adherence
Variation in the adherence of the recipients and providers of care can limit the certainty in the applicability of results. Predictable differences in adherence can be due to divergence in how recipients of care perceive the intervention (e.g. the importance of side effects), economic conditions or attitudes that make some forms of care inaccessible in some settings, such as in low-income countries (Dans et al 2007). It should not be assumed that high levels of adherence in closely monitored randomized trials will translate into similar levels of adherence in normal practice.
15.2.5 Variation in values and preferences
Decisions about healthcare management strategies and options involve trading off health benefits and harms. The right choice may differ for people with different values and preferences (i.e. the importance people place on the outcomes and interventions), and it is important that decision makers ensure that decisions are consistent with a patient or population’s values and preferences. The importance placed on outcomes, together with other factors, will influence whether the recipients of care will or will not accept an option that is offered (Alonso-Coello et al 2016) and, thus, can be one factor influencing adherence. In Section 15.6 , we describe how the review author can help this process and the limits of supporting decision making based on intervention reviews.
15.3 Interpreting results of statistical analyses
15.3.1 confidence intervals.
Results for both individual studies and meta-analyses are reported with a point estimate together with an associated confidence interval. For example, ‘The odds ratio was 0.75 with a 95% confidence interval of 0.70 to 0.80’. The point estimate (0.75) is the best estimate of the magnitude and direction of the experimental intervention’s effect compared with the comparator intervention. The confidence interval describes the uncertainty inherent in any estimate, and describes a range of values within which we can be reasonably sure that the true effect actually lies. If the confidence interval is relatively narrow (e.g. 0.70 to 0.80), the effect size is known precisely. If the interval is wider (e.g. 0.60 to 0.93) the uncertainty is greater, although there may still be enough precision to make decisions about the utility of the intervention. Intervals that are very wide (e.g. 0.50 to 1.10) indicate that we have little knowledge about the effect and this imprecision affects our certainty in the evidence, and that further information would be needed before we could draw a more certain conclusion.
A 95% confidence interval is often interpreted as indicating a range within which we can be 95% certain that the true effect lies. This statement is a loose interpretation, but is useful as a rough guide. The strictly correct interpretation of a confidence interval is based on the hypothetical notion of considering the results that would be obtained if the study were repeated many times. If a study were repeated infinitely often, and on each occasion a 95% confidence interval calculated, then 95% of these intervals would contain the true effect (see Section 15.3.3 for further explanation).
The width of the confidence interval for an individual study depends to a large extent on the sample size. Larger studies tend to give more precise estimates of effects (and hence have narrower confidence intervals) than smaller studies. For continuous outcomes, precision depends also on the variability in the outcome measurements (i.e. how widely individual results vary between people in the study, measured as the standard deviation); for dichotomous outcomes it depends on the risk of the event (more frequent events allow more precision, and narrower confidence intervals), and for time-to-event outcomes it also depends on the number of events observed. All these quantities are used in computation of the standard errors of effect estimates from which the confidence interval is derived.
The width of a confidence interval for a meta-analysis depends on the precision of the individual study estimates and on the number of studies combined. In addition, for random-effects models, precision will decrease with increasing heterogeneity and confidence intervals will widen correspondingly (see Chapter 10, Section 10.10.4 ). As more studies are added to a meta-analysis the width of the confidence interval usually decreases. However, if the additional studies increase the heterogeneity in the meta-analysis and a random-effects model is used, it is possible that the confidence interval width will increase.
Confidence intervals and point estimates have different interpretations in fixed-effect and random-effects models. While the fixed-effect estimate and its confidence interval address the question ‘what is the best (single) estimate of the effect?’, the random-effects estimate assumes there to be a distribution of effects, and the estimate and its confidence interval address the question ‘what is the best estimate of the average effect?’ A confidence interval may be reported for any level of confidence (although they are most commonly reported for 95%, and sometimes 90% or 99%). For example, the odds ratio of 0.80 could be reported with an 80% confidence interval of 0.73 to 0.88; a 90% interval of 0.72 to 0.89; and a 95% interval of 0.70 to 0.92. As the confidence level increases, the confidence interval widens.
There is logical correspondence between the confidence interval and the P value (see Section 15.3.3 ). The 95% confidence interval for an effect will exclude the null value (such as an odds ratio of 1.0 or a risk difference of 0) if and only if the test of significance yields a P value of less than 0.05. If the P value is exactly 0.05, then either the upper or lower limit of the 95% confidence interval will be at the null value. Similarly, the 99% confidence interval will exclude the null if and only if the test of significance yields a P value of less than 0.01.
Together, the point estimate and confidence interval provide information to assess the effects of the intervention on the outcome. For example, suppose that we are evaluating an intervention that reduces the risk of an event and we decide that it would be useful only if it reduced the risk of an event from 30% by at least 5 percentage points to 25% (these values will depend on the specific clinical scenario and outcomes, including the anticipated harms). If the meta-analysis yielded an effect estimate of a reduction of 10 percentage points with a tight 95% confidence interval, say, from 7% to 13%, we would be able to conclude that the intervention was useful since both the point estimate and the entire range of the interval exceed our criterion of a reduction of 5% for net health benefit. However, if the meta-analysis reported the same risk reduction of 10% but with a wider interval, say, from 2% to 18%, although we would still conclude that our best estimate of the intervention effect is that it provides net benefit, we could not be so confident as we still entertain the possibility that the effect could be between 2% and 5%. If the confidence interval was wider still, and included the null value of a difference of 0%, we would still consider the possibility that the intervention has no effect on the outcome whatsoever, and would need to be even more sceptical in our conclusions.
Review authors may use the same general approach to conclude that an intervention is not useful. Continuing with the above example where the criterion for an important difference that should be achieved to provide more benefit than harm is a 5% risk difference, an effect estimate of 2% with a 95% confidence interval of 1% to 4% suggests that the intervention does not provide net health benefit.
15.3.2 P values and statistical significance
A P value is the standard result of a statistical test, and is the probability of obtaining the observed effect (or larger) under a ‘null hypothesis’. In the context of Cochrane Reviews there are two commonly used statistical tests. The first is a test of overall effect (a Z-test), and its null hypothesis is that there is no overall effect of the experimental intervention compared with the comparator on the outcome of interest. The second is the (Chi 2 ) test for heterogeneity, and its null hypothesis is that there are no differences in the intervention effects across studies.
A P value that is very small indicates that the observed effect is very unlikely to have arisen purely by chance, and therefore provides evidence against the null hypothesis. It has been common practice to interpret a P value by examining whether it is smaller than particular threshold values. In particular, P values less than 0.05 are often reported as ‘statistically significant’, and interpreted as being small enough to justify rejection of the null hypothesis. However, the 0.05 threshold is an arbitrary one that became commonly used in medical and psychological research largely because P values were determined by comparing the test statistic against tabulations of specific percentage points of statistical distributions. If review authors decide to present a P value with the results of a meta-analysis, they should report a precise P value (as calculated by most statistical software), together with the 95% confidence interval. Review authors should not describe results as ‘statistically significant’, ‘not statistically significant’ or ‘non-significant’ or unduly rely on thresholds for P values , but report the confidence interval together with the exact P value (see MECIR Box 15.3.a ).
We discuss interpretation of the test for heterogeneity in Chapter 10, Section 10.10.2 ; the remainder of this section refers mainly to tests for an overall effect. For tests of an overall effect, the computation of P involves both the effect estimate and precision of the effect estimate (driven largely by sample size). As precision increases, the range of plausible effects that could occur by chance is reduced. Correspondingly, the statistical significance of an effect of a particular magnitude will usually be greater (the P value will be smaller) in a larger study than in a smaller study.
P values are commonly misinterpreted in two ways. First, a moderate or large P value (e.g. greater than 0.05) may be misinterpreted as evidence that the intervention has no effect on the outcome. There is an important difference between this statement and the correct interpretation that there is a high probability that the observed effect on the outcome is due to chance alone. To avoid such a misinterpretation, review authors should always examine the effect estimate and its 95% confidence interval.
The second misinterpretation is to assume that a result with a small P value for the summary effect estimate implies that an experimental intervention has an important benefit. Such a misinterpretation is more likely to occur in large studies and meta-analyses that accumulate data over dozens of studies and thousands of participants. The P value addresses the question of whether the experimental intervention effect is precisely nil; it does not examine whether the effect is of a magnitude of importance to potential recipients of the intervention. In a large study, a small P value may represent the detection of a trivial effect that may not lead to net health benefit when compared with the potential harms (i.e. harmful effects on other important outcomes). Again, inspection of the point estimate and confidence interval helps correct interpretations (see Section 15.3.1 ).
MECIR Box 15.3.a Relevant expectations for conduct of intervention reviews
| Interpreting results ( ) | |
| . | Authors commonly mistake a lack of evidence of effect as evidence of a lack of effect. |
15.3.3 Relation between confidence intervals, statistical significance and certainty of evidence
The confidence interval (and imprecision) is only one domain that influences overall uncertainty about effect estimates. Uncertainty resulting from imprecision (i.e. statistical uncertainty) may be no less important than uncertainty from indirectness, or any other GRADE domain, in the context of decision making (Schünemann 2016). Thus, the extent to which interpretations of the confidence interval described in Sections 15.3.1 and 15.3.2 correspond to conclusions about overall certainty of the evidence for the outcome of interest depends on these other domains. If there are no concerns about other domains that determine the certainty of the evidence (i.e. risk of bias, inconsistency, indirectness or publication bias), then the interpretation in Sections 15.3.1 and 15.3.2 . about the relation of the confidence interval to the true effect may be carried forward to the overall certainty. However, if there are concerns about the other domains that affect the certainty of the evidence, the interpretation about the true effect needs to be seen in the context of further uncertainty resulting from those concerns.
For example, nine randomized controlled trials in almost 6000 cancer patients indicated that the administration of heparin reduces the risk of venous thromboembolism (VTE), with a risk ratio of 43% (95% CI 19% to 60%) (Akl et al 2011a). For patients with a plausible baseline risk of approximately 4.6% per year, this relative effect suggests that heparin leads to an absolute risk reduction of 20 fewer VTEs (95% CI 9 fewer to 27 fewer) per 1000 people per year (Akl et al 2011a). Now consider that the review authors or those applying the evidence in a guideline have lowered the certainty in the evidence as a result of indirectness. While the confidence intervals would remain unchanged, the certainty in that confidence interval and in the point estimate as reflecting the truth for the question of interest will be lowered. In fact, the certainty range will have unknown width so there will be unknown likelihood of a result within that range because of this indirectness. The lower the certainty in the evidence, the less we know about the width of the certainty range, although methods for quantifying risk of bias and understanding potential direction of bias may offer insight when lowered certainty is due to risk of bias. Nevertheless, decision makers must consider this uncertainty, and must do so in relation to the effect measure that is being evaluated (e.g. a relative or absolute measure). We will describe the impact on interpretations for dichotomous outcomes in Section 15.4 .
15.4 Interpreting results from dichotomous outcomes (including numbers needed to treat)
15.4.1 relative and absolute risk reductions.
Clinicians may be more inclined to prescribe an intervention that reduces the relative risk of death by 25% than one that reduces the risk of death by 1 percentage point, although both presentations of the evidence may relate to the same benefit (i.e. a reduction in risk from 4% to 3%). The former refers to the relative reduction in risk and the latter to the absolute reduction in risk. As described in Chapter 6, Section 6.4.1 , there are several measures for comparing dichotomous outcomes in two groups. Meta-analyses are usually undertaken using risk ratios (RR), odds ratios (OR) or risk differences (RD), but there are several alternative ways of expressing results.
Relative risk reduction (RRR) is a convenient way of re-expressing a risk ratio as a percentage reduction:
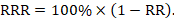
For example, a risk ratio of 0.75 translates to a relative risk reduction of 25%, as in the example above.
The risk difference is often referred to as the absolute risk reduction (ARR) or absolute risk increase (ARI), and may be presented as a percentage (e.g. 1%), as a decimal (e.g. 0.01), or as account (e.g. 10 out of 1000). We consider different choices for presenting absolute effects in Section 15.4.3 . We then describe computations for obtaining these numbers from the results of individual studies and of meta-analyses in Section 15.4.4 .
15.4.2 Number needed to treat (NNT)
The number needed to treat (NNT) is a common alternative way of presenting information on the effect of an intervention. The NNT is defined as the expected number of people who need to receive the experimental rather than the comparator intervention for one additional person to either incur or avoid an event (depending on the direction of the result) in a given time frame. Thus, for example, an NNT of 10 can be interpreted as ‘it is expected that one additional (or less) person will incur an event for every 10 participants receiving the experimental intervention rather than comparator over a given time frame’. It is important to be clear that:
- since the NNT is derived from the risk difference, it is still a comparative measure of effect (experimental versus a specific comparator) and not a general property of a single intervention; and
- the NNT gives an ‘expected value’. For example, NNT = 10 does not imply that one additional event will occur in each and every group of 10 people.
NNTs can be computed for both beneficial and detrimental events, and for interventions that cause both improvements and deteriorations in outcomes. In all instances NNTs are expressed as positive whole numbers. Some authors use the term ‘number needed to harm’ (NNH) when an intervention leads to an adverse outcome, or a decrease in a positive outcome, rather than improvement. However, this phrase can be misleading (most notably, it can easily be read to imply the number of people who will experience a harmful outcome if given the intervention), and it is strongly recommended that ‘number needed to harm’ and ‘NNH’ are avoided. The preferred alternative is to use phrases such as ‘number needed to treat for an additional beneficial outcome’ (NNTB) and ‘number needed to treat for an additional harmful outcome’ (NNTH) to indicate direction of effect.
As NNTs refer to events, their interpretation needs to be worded carefully when the binary outcome is a dichotomization of a scale-based outcome. For example, if the outcome is pain measured on a ‘none, mild, moderate or severe’ scale it may have been dichotomized as ‘none or mild’ versus ‘moderate or severe’. It would be inappropriate for an NNT from these data to be referred to as an ‘NNT for pain’. It is an ‘NNT for moderate or severe pain’.
We consider different choices for presenting absolute effects in Section 15.4.3 . We then describe computations for obtaining these numbers from the results of individual studies and of meta-analyses in Section 15.4.4 .
15.4.3 Expressing risk differences
Users of reviews are liable to be influenced by the choice of statistical presentations of the evidence. Hoffrage and colleagues suggest that physicians’ inferences about statistical outcomes are more appropriate when they deal with ‘natural frequencies’ – whole numbers of people, both treated and untreated (e.g. treatment results in a drop from 20 out of 1000 to 10 out of 1000 women having breast cancer) – than when effects are presented as percentages (e.g. 1% absolute reduction in breast cancer risk) (Hoffrage et al 2000). Probabilities may be more difficult to understand than frequencies, particularly when events are rare. While standardization may be important in improving the presentation of research evidence (and participation in healthcare decisions), current evidence suggests that the presentation of natural frequencies for expressing differences in absolute risk is best understood by consumers of healthcare information (Akl et al 2011b). This evidence provides the rationale for presenting absolute risks in ‘Summary of findings’ tables as numbers of people with events per 1000 people receiving the intervention (see Chapter 14 ).
RRs and RRRs remain crucial because relative effects tend to be substantially more stable across risk groups than absolute effects (see Chapter 10, Section 10.4.3 ). Review authors can use their own data to study this consistency (Cates 1999, Smeeth et al 1999). Risk differences from studies are least likely to be consistent across baseline event rates; thus, they are rarely appropriate for computing numbers needed to treat in systematic reviews. If a relative effect measure (OR or RR) is chosen for meta-analysis, then a comparator group risk needs to be specified as part of the calculation of an RD or NNT. In addition, if there are several different groups of participants with different levels of risk, it is crucial to express absolute benefit for each clinically identifiable risk group, clarifying the time period to which this applies. Studies in patients with differing severity of disease, or studies with different lengths of follow-up will almost certainly have different comparator group risks. In these cases, different comparator group risks lead to different RDs and NNTs (except when the intervention has no effect). A recommended approach is to re-express an odds ratio or a risk ratio as a variety of RD or NNTs across a range of assumed comparator risks (ACRs) (McQuay and Moore 1997, Smeeth et al 1999). Review authors should bear these considerations in mind not only when constructing their ‘Summary of findings’ table, but also in the text of their review.
For example, a review of oral anticoagulants to prevent stroke presented information to users by describing absolute benefits for various baseline risks (Aguilar and Hart 2005, Aguilar et al 2007). They presented their principal findings as “The inherent risk of stroke should be considered in the decision to use oral anticoagulants in atrial fibrillation patients, selecting those who stand to benefit most for this therapy” (Aguilar and Hart 2005). Among high-risk atrial fibrillation patients with prior stroke or transient ischaemic attack who have stroke rates of about 12% (120 per 1000) per year, warfarin prevents about 70 strokes yearly per 1000 patients, whereas for low-risk atrial fibrillation patients (with a stroke rate of about 2% per year or 20 per 1000), warfarin prevents only 12 strokes. This presentation helps users to understand the important impact that typical baseline risks have on the absolute benefit that they can expect.
15.4.4 Computations
Direct computation of risk difference (RD) or a number needed to treat (NNT) depends on the summary statistic (odds ratio, risk ratio or risk differences) available from the study or meta-analysis. When expressing results of meta-analyses, review authors should use, in the computations, whatever statistic they determined to be the most appropriate summary for meta-analysis (see Chapter 10, Section 10.4.3 ). Here we present calculations to obtain RD as a reduction in the number of participants per 1000. For example, a risk difference of –0.133 corresponds to 133 fewer participants with the event per 1000.
RDs and NNTs should not be computed from the aggregated total numbers of participants and events across the trials. This approach ignores the randomization within studies, and may produce seriously misleading results if there is unbalanced randomization in any of the studies. Using the pooled result of a meta-analysis is more appropriate. When computing NNTs, the values obtained are by convention always rounded up to the next whole number.
15.4.4.1 Computing NNT from a risk difference (RD)
A NNT may be computed from a risk difference as
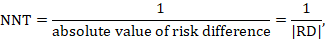
where the vertical bars (‘absolute value of’) in the denominator indicate that any minus sign should be ignored. It is convention to round the NNT up to the nearest whole number. For example, if the risk difference is –0.12 the NNT is 9; if the risk difference is –0.22 the NNT is 5. Cochrane Review authors should qualify the NNT as referring to benefit (improvement) or harm by denoting the NNT as NNTB or NNTH. Note that this approach, although feasible, should be used only for the results of a meta-analysis of risk differences. In most cases meta-analyses will be undertaken using a relative measure of effect (RR or OR), and those statistics should be used to calculate the NNT (see Section 15.4.4.2 and 15.4.4.3 ).
15.4.4.2 Computing risk differences or NNT from a risk ratio
To aid interpretation of the results of a meta-analysis of risk ratios, review authors may compute an absolute risk reduction or NNT. In order to do this, an assumed comparator risk (ACR) (otherwise known as a baseline risk, or risk that the outcome of interest would occur with the comparator intervention) is required. It will usually be appropriate to do this for a range of different ACRs. The computation proceeds as follows:
As an example, suppose the risk ratio is RR = 0.92, and an ACR = 0.3 (300 per 1000) is assumed. Then the effect on risk is 24 fewer per 1000:
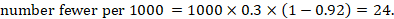
The NNT is 42:
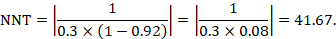
15.4.4.3 Computing risk differences or NNT from an odds ratio
Review authors may wish to compute a risk difference or NNT from the results of a meta-analysis of odds ratios. In order to do this, an ACR is required. It will usually be appropriate to do this for a range of different ACRs. The computation proceeds as follows:
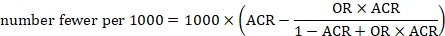
As an example, suppose the odds ratio is OR = 0.73, and a comparator risk of ACR = 0.3 is assumed. Then the effect on risk is 62 fewer per 1000:
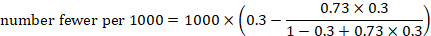
The NNT is 17:
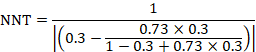
15.4.4.4 Computing risk ratio from an odds ratio
Because risk ratios are easier to interpret than odds ratios, but odds ratios have favourable mathematical properties, a review author may decide to undertake a meta-analysis based on odds ratios, but to express the result as a summary risk ratio (or relative risk reduction). This requires an ACR. Then
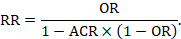
It will often be reasonable to perform this transformation using the median comparator group risk from the studies in the meta-analysis.
15.4.4.5 Computing confidence limits
Confidence limits for RDs and NNTs may be calculated by applying the above formulae to the upper and lower confidence limits for the summary statistic (RD, RR or OR) (Altman 1998). Note that this confidence interval does not incorporate uncertainty around the ACR.
If the 95% confidence interval of OR or RR includes the value 1, one of the confidence limits will indicate benefit and the other harm. Thus, appropriate use of the words ‘fewer’ and ‘more’ is required for each limit when presenting results in terms of events. For NNTs, the two confidence limits should be labelled as NNTB and NNTH to indicate the direction of effect in each case. The confidence interval for the NNT will include a ‘discontinuity’, because increasingly smaller risk differences that approach zero will lead to NNTs approaching infinity. Thus, the confidence interval will include both an infinitely large NNTB and an infinitely large NNTH.
15.5 Interpreting results from continuous outcomes (including standardized mean differences)
15.5.1 meta-analyses with continuous outcomes.
Review authors should describe in the study protocol how they plan to interpret results for continuous outcomes. When outcomes are continuous, review authors have a number of options to present summary results. These options differ if studies report the same measure that is familiar to the target audiences, studies report the same or very similar measures that are less familiar to the target audiences, or studies report different measures.
15.5.2 Meta-analyses with continuous outcomes using the same measure
If all studies have used the same familiar units, for instance, results are expressed as durations of events, such as symptoms for conditions including diarrhoea, sore throat, otitis media, influenza or duration of hospitalization, a meta-analysis may generate a summary estimate in those units, as a difference in mean response (see, for instance, the row summarizing results for duration of diarrhoea in Chapter 14, Figure 14.1.b and the row summarizing oedema in Chapter 14, Figure 14.1.a ). For such outcomes, the ‘Summary of findings’ table should include a difference of means between the two interventions. However, when units of such outcomes may be difficult to interpret, particularly when they relate to rating scales (again, see the oedema row of Chapter 14, Figure 14.1.a ). ‘Summary of findings’ tables should include the minimum and maximum of the scale of measurement, and the direction. Knowledge of the smallest change in instrument score that patients perceive is important – the minimal important difference (MID) – and can greatly facilitate the interpretation of results (Guyatt et al 1998, Schünemann and Guyatt 2005). Knowing the MID allows review authors and users to place results in context. Review authors should state the MID – if known – in the Comments column of their ‘Summary of findings’ table. For example, the chronic respiratory questionnaire has possible scores in health-related quality of life ranging from 1 to 7 and 0.5 represents a well-established MID (Jaeschke et al 1989, Schünemann et al 2005).
15.5.3 Meta-analyses with continuous outcomes using different measures
When studies have used different instruments to measure the same construct, a standardized mean difference (SMD) may be used in meta-analysis for combining continuous data. Without guidance, clinicians and patients may have little idea how to interpret results presented as SMDs. Review authors should therefore consider issues of interpretability when planning their analysis at the protocol stage and should consider whether there will be suitable ways to re-express the SMD or whether alternative effect measures, such as a ratio of means, or possibly as minimal important difference units (Guyatt et al 2013b) should be used. Table 15.5.a and the following sections describe these options.
Table 15.5.a Approaches and their implications to presenting results of continuous variables when primary studies have used different instruments to measure the same construct. Adapted from Guyatt et al (2013b)
|
|
|
|
|
| ||
| 1a. Generic standard deviation (SD) units and guiding rules | It is widely used, but the interpretation is challenging. It can be misleading depending on whether the population is very homogenous or heterogeneous (i.e. how variable the outcome was in the population of each included study, and therefore how applicable a standard SD is likely to be). See Section . | Use together with other approaches below. |
| 1b. Re-express and present as units of a familiar measure | Presenting data with this approach may be viewed by users as closer to the primary data. However, few instruments are sufficiently used in clinical practice to make many of the presented units easily interpretable. See Section . | When the units and measures are familiar to the decision makers (e.g. healthcare providers and patients), this presentation should be seriously considered. Conversion to natural units is also an option for expressing results using the MID approach below (row 3). |
| 1c. Re-express as result for a dichotomous outcome | Dichotomous outcomes are very familiar to clinical audiences and may facilitate understanding. However, this approach involves assumptions that may not always be valid (e.g. it assumes that distributions in intervention and comparator group are roughly normally distributed and variances are similar). It allows applying GRADE guidance for large and very large effects. See Section . | Consider this approach if the assumptions appear reasonable. If the minimal important difference for an instrument is known describing the probability of individuals achieving this difference may be more intuitive. Review authors should always seriously consider this option. Re-expressing SMDs is not the only way of expressing results as dichotomous outcomes. For example, the actual outcomes in the studies can be dichotomized, either directly or using assumptions, prior to meta-analysis. |
|
| ||
| 2. Ratio of means | This approach may be easily interpretable to clinical audiences and involves fewer assumptions than some other approaches. It allows applying GRADE guidance for large and very large effects. It cannot be applied when measure is a change from baseline and therefore negative values possible and the interpretation requires knowledge and interpretation of comparator group mean. See Section | Consider as complementing other approaches, particularly the presentation of relative and absolute effects. |
| 3. Minimal important difference units | This approach may be easily interpretable for audiences but is applicable only when minimal important differences are known. See Section . | Consider as complementing other approaches, particularly the presentation of relative and absolute effects. |
15.5.3.1 Presenting and interpreting SMDs using generic effect size estimates
The SMD expresses the intervention effect in standard units rather than the original units of measurement. The SMD is the difference in mean effects between the experimental and comparator groups divided by the pooled standard deviation of participants’ outcomes, or external SDs when studies are very small (see Chapter 6, Section 6.5.1.2 ). The value of a SMD thus depends on both the size of the effect (the difference between means) and the standard deviation of the outcomes (the inherent variability among participants or based on an external SD).
If review authors use the SMD, they might choose to present the results directly as SMDs (row 1a, Table 15.5.a and Table 15.5.b ). However, absolute values of the intervention and comparison groups are typically not useful because studies have used different measurement instruments with different units. Guiding rules for interpreting SMDs (or ‘Cohen’s effect sizes’) exist, and have arisen mainly from researchers in the social sciences (Cohen 1988). One example is as follows: 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988). Variations exist (e.g. <0.40=small, 0.40 to 0.70=moderate, >0.70=large). Review authors might consider including such a guiding rule in interpreting the SMD in the text of the review, and in summary versions such as the Comments column of a ‘Summary of findings’ table. However, some methodologists believe that such interpretations are problematic because patient importance of a finding is context-dependent and not amenable to generic statements.
15.5.3.2 Re-expressing SMDs using a familiar instrument
The second possibility for interpreting the SMD is to express it in the units of one or more of the specific measurement instruments used by the included studies (row 1b, Table 15.5.a and Table 15.5.b ). The approach is to calculate an absolute difference in means by multiplying the SMD by an estimate of the SD associated with the most familiar instrument. To obtain this SD, a reasonable option is to calculate a weighted average across all intervention groups of all studies that used the selected instrument (preferably a pre-intervention or post-intervention SD as discussed in Chapter 10, Section 10.5.2 ). To better reflect among-person variation in practice, or to use an instrument not represented in the meta-analysis, it may be preferable to use a standard deviation from a representative observational study. The summary effect is thus re-expressed in the original units of that particular instrument and the clinical relevance and impact of the intervention effect can be interpreted using that familiar instrument.
The same approach of re-expressing the results for a familiar instrument can also be used for other standardized effect measures such as when standardizing by MIDs (Guyatt et al 2013b): see Section 15.5.3.5 .
Table 15.5.b Application of approaches when studies have used different measures: effects of dexamethasone for pain after laparoscopic cholecystectomy (Karanicolas et al 2008). Reproduced with permission of Wolters Kluwer
|
|
|
|
|
|
| |
|
|
| |||||
|
|
| |||||
| 1a. Post-operative pain, standard deviation units Investigators measured pain using different instruments. Lower scores mean less pain. | The pain score in the dexamethasone groups was on average than in the placebo groups). | – | 539 (5) | OO Low |
As a rule of thumb, 0.2 SD represents a small difference, 0.5 a moderate and 0.8 a large. | |
| 1b. Post-operative pain Measured on a scale from 0, no pain, to 100, worst pain imaginable. | The mean post-operative pain scores with placebo ranged from 43 to 54. | The mean pain score in the intervention groups was on average
| – | 539 (5) |
OO Low | Scores calculated based on an SMD of 0.79 (95% CI –1.41 to –0.17) and rescaled to a 0 to 100 pain scale. The minimal important difference on the 0 to 100 pain scale is approximately 10. |
| 1c. Substantial post-operative pain, dichotomized Investigators measured pain using different instruments. | 20 per 100 | 15 more (4 more to 18 more) per 100 patients in dexamethasone group achieved important improvement in the pain score. | RR = 0.25 (95% CI 0.05 to 0.75) | 539 (5) | OO Low | Scores estimated based on an SMD of 0.79 (95% CI –1.41 to –0.17).
|
| 2. Post-operative pain Investigators measured pain using different instruments. Lower scores mean less pain. | The mean post-operative pain scores with placebo was 28.1. | On average a 3.7 lower pain score (0.6 to 6.1 lower) | Ratio of means 0.87 (0.78 to 0.98) | 539 (5) | OO Low | Weighted average of the mean pain score in dexamethasone group divided by mean pain score in placebo. |
| 3. Post-operative pain Investigators measured pain using different instruments. | The pain score in the dexamethasone groups was on average less than the control group. | – | 539 (5) | OO Low | An effect less than half the minimal important difference suggests a small or very small effect. | |
1 Certainty rated according to GRADE from very low to high certainty. 2 Substantial unexplained heterogeneity in study results. 3 Imprecision due to wide confidence intervals. 4 The 20% comes from the proportion in the control group requiring rescue analgesia. 5 Crude (arithmetic) means of the post-operative pain mean responses across all five trials when transformed to a 100-point scale.
15.5.3.3 Re-expressing SMDs through dichotomization and transformation to relative and absolute measures
A third approach (row 1c, Table 15.5.a and Table 15.5.b ) relies on converting the continuous measure into a dichotomy and thus allows calculation of relative and absolute effects on a binary scale. A transformation of a SMD to a (log) odds ratio is available, based on the assumption that an underlying continuous variable has a logistic distribution with equal standard deviation in the two intervention groups, as discussed in Chapter 10, Section 10.6 (Furukawa 1999, Guyatt et al 2013b). The assumption is unlikely to hold exactly and the results must be regarded as an approximation. The log odds ratio is estimated as
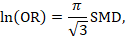
(or approximately 1.81✕SMD). The resulting odds ratio can then be presented as normal, and in a ‘Summary of findings’ table, combined with an assumed comparator group risk to be expressed as an absolute risk difference. The comparator group risk in this case would refer to the proportion of people who have achieved a specific value of the continuous outcome. In randomized trials this can be interpreted as the proportion who have improved by some (specified) amount (responders), for instance by 5 points on a 0 to 100 scale. Table 15.5.c shows some illustrative results from this method. The risk differences can then be converted to NNTs or to people per thousand using methods described in Section 15.4.4 .
Table 15.5.c Risk difference derived for specific SMDs for various given ‘proportions improved’ in the comparator group (Furukawa 1999, Guyatt et al 2013b). Reproduced with permission of Elsevier
|
|
|
|
|
|
|
|
|
|
| ||||||||
| Situations in which the event is undesirable, reduction (or increase if intervention harmful) in adverse events with the intervention | |||||||||||||||||
|
| −3% | −5% | −7% | −8% | −8% | −8% | −7% | −6% | −4% | ||||||||
|
| −6% | −11% | −15% | −17% | −19% | −20% | −20% | −17% | −12% | ||||||||
|
| −8% | −15% | −21% | −25% | −29% | −31% | −31% | −28% | −22% | ||||||||
|
| −9% | −17% | −24% | −23% | −34% | −37% | −38% | −36% | −29% | ||||||||
| Situations in which the event is desirable, increase (or decrease if intervention harmful) in positive responses to the intervention | |||||||||||||||||
|
| 4% | 6% | 7% | 8% | 8% | 8% | 7% | 5% | 3% | ||||||||
|
| 12% | 17% | 19% | 20% | 19% | 17% | 15% | 11% | 6% | ||||||||
|
| 22% | 28% | 31% | 31% | 29% | 25% | 21% | 15% | 8% | ||||||||
|
| 29% | 36% | 38% | 38% | 34% | 30% | 24% | 17% | 9% | ||||||||
15.5.3.4 Ratio of means
A more frequently used approach is based on calculation of a ratio of means between the intervention and comparator groups (Friedrich et al 2008) as discussed in Chapter 6, Section 6.5.1.3 . Interpretational advantages of this approach include the ability to pool studies with outcomes expressed in different units directly, to avoid the vulnerability of heterogeneous populations that limits approaches that rely on SD units, and for ease of clinical interpretation (row 2, Table 15.5.a and Table 15.5.b ). This method is currently designed for post-intervention scores only. However, it is possible to calculate a ratio of change scores if both intervention and comparator groups change in the same direction in each relevant study, and this ratio may sometimes be informative.
Limitations to this approach include its limited applicability to change scores (since it is unlikely that both intervention and comparator group changes are in the same direction in all studies) and the possibility of misleading results if the comparator group mean is very small, in which case even a modest difference from the intervention group will yield a large and therefore misleading ratio of means. It also requires that separate ratios of means be calculated for each included study, and then entered into a generic inverse variance meta-analysis (see Chapter 10, Section 10.3 ).
The ratio of means approach illustrated in Table 15.5.b suggests a relative reduction in pain of only 13%, meaning that those receiving steroids have a pain severity 87% of those in the comparator group, an effect that might be considered modest.
15.5.3.5 Presenting continuous results as minimally important difference units
To express results in MID units, review authors have two options. First, they can be combined across studies in the same way as the SMD, but instead of dividing the mean difference of each study by its SD, review authors divide by the MID associated with that outcome (Johnston et al 2010, Guyatt et al 2013b). Instead of SD units, the pooled results represent MID units (row 3, Table 15.5.a and Table 15.5.b ), and may be more easily interpretable. This approach avoids the problem of varying SDs across studies that may distort estimates of effect in approaches that rely on the SMD. The approach, however, relies on having well-established MIDs. The approach is also risky in that a difference less than the MID may be interpreted as trivial when a substantial proportion of patients may have achieved an important benefit.
The other approach makes a simple conversion (not shown in Table 15.5.b ), before undertaking the meta-analysis, of the means and SDs from each study to means and SDs on the scale of a particular familiar instrument whose MID is known. For example, one can rescale the mean and SD of other chronic respiratory disease instruments (e.g. rescaling a 0 to 100 score of an instrument) to a the 1 to 7 score in Chronic Respiratory Disease Questionnaire (CRQ) units (by assuming 0 equals 1 and 100 equals 7 on the CRQ). Given the MID of the CRQ of 0.5, a mean difference in change of 0.71 after rescaling of all studies suggests a substantial effect of the intervention (Guyatt et al 2013b). This approach, presenting in units of the most familiar instrument, may be the most desirable when the target audiences have extensive experience with that instrument, particularly if the MID is well established.
15.6 Drawing conclusions
15.6.1 conclusions sections of a cochrane review.
Authors’ conclusions in a Cochrane Review are divided into implications for practice and implications for research. While Cochrane Reviews about interventions can provide meaningful information and guidance for practice, decisions about the desirable and undesirable consequences of healthcare options require evidence and judgements for criteria that most Cochrane Reviews do not provide (Alonso-Coello et al 2016). In describing the implications for practice and the development of recommendations, however, review authors may consider the certainty of the evidence, the balance of benefits and harms, and assumed values and preferences.
15.6.2 Implications for practice
Drawing conclusions about the practical usefulness of an intervention entails making trade-offs, either implicitly or explicitly, between the estimated benefits, harms and the values and preferences. Making such trade-offs, and thus making specific recommendations for an action in a specific context, goes beyond a Cochrane Review and requires additional evidence and informed judgements that most Cochrane Reviews do not provide (Alonso-Coello et al 2016). Such judgements are typically the domain of clinical practice guideline developers for which Cochrane Reviews will provide crucial information (Graham et al 2011, Schünemann et al 2014, Zhang et al 2018a). Thus, authors of Cochrane Reviews should not make recommendations.
If review authors feel compelled to lay out actions that clinicians and patients could take, they should – after describing the certainty of evidence and the balance of benefits and harms – highlight different actions that might be consistent with particular patterns of values and preferences. Other factors that might influence a decision should also be highlighted, including any known factors that would be expected to modify the effects of the intervention, the baseline risk or status of the patient, costs and who bears those costs, and the availability of resources. Review authors should ensure they consider all patient-important outcomes, including those for which limited data may be available. In the context of public health reviews the focus may be on population-important outcomes as the target may be an entire (non-diseased) population and include outcomes that are not measured in the population receiving an intervention (e.g. a reduction of transmission of infections from those receiving an intervention). This process implies a high level of explicitness in judgements about values or preferences attached to different outcomes and the certainty of the related evidence (Zhang et al 2018b, Zhang et al 2018c); this and a full cost-effectiveness analysis is beyond the scope of most Cochrane Reviews (although they might well be used for such analyses; see Chapter 20 ).
A review on the use of anticoagulation in cancer patients to increase survival (Akl et al 2011a) provides an example for laying out clinical implications for situations where there are important trade-offs between desirable and undesirable effects of the intervention: “The decision for a patient with cancer to start heparin therapy for survival benefit should balance the benefits and downsides and integrate the patient’s values and preferences. Patients with a high preference for a potential survival prolongation, limited aversion to potential bleeding, and who do not consider heparin (both UFH or LMWH) therapy a burden may opt to use heparin, while those with aversion to bleeding may not.”
15.6.3 Implications for research
The second category for authors’ conclusions in a Cochrane Review is implications for research. To help people make well-informed decisions about future healthcare research, the ‘Implications for research’ section should comment on the need for further research, and the nature of the further research that would be most desirable. It is helpful to consider the population, intervention, comparison and outcomes that could be addressed, or addressed more effectively in the future, in the context of the certainty of the evidence in the current review (Brown et al 2006):
- P (Population): diagnosis, disease stage, comorbidity, risk factor, sex, age, ethnic group, specific inclusion or exclusion criteria, clinical setting;
- I (Intervention): type, frequency, dose, duration, prognostic factor;
- C (Comparison): placebo, routine care, alternative treatment/management;
- O (Outcome): which clinical or patient-related outcomes will the researcher need to measure, improve, influence or accomplish? Which methods of measurement should be used?
While Cochrane Review authors will find the PICO domains helpful, the domains of the GRADE certainty framework further support understanding and describing what additional research will improve the certainty in the available evidence. Note that as the certainty of the evidence is likely to vary by outcome, these implications will be specific to certain outcomes in the review. Table 15.6.a shows how review authors may be aided in their interpretation of the body of evidence and drawing conclusions about future research and practice.
Table 15.6.a Implications for research and practice suggested by individual GRADE domains
| Domain | Implications for research | Examples for research statements | Implications for practice |
| Risk of bias | Need for methodologically better designed and executed studies. | All studies suffered from lack of blinding of outcome assessors. Trials of this type are required. | The estimates of effect may be biased because of a lack of blinding of the assessors of the outcome. |
| Inconsistency | Unexplained inconsistency: need for individual participant data meta-analysis; need for studies in relevant subgroups. | Studies in patients with small cell lung cancer are needed to understand if the effects differ from those in patients with pancreatic cancer. | Unexplained inconsistency: consider and interpret overall effect estimates as for the overall certainty of a body of evidence. Explained inconsistency (if results are not presented in strata): consider and interpret effects estimates by subgroup. |
| Indirectness | Need for studies that better fit the PICO question of interest. | Studies in patients with early cancer are needed because the evidence is from studies in patients with advanced cancer. | It is uncertain if the results directly apply to the patients or the way that the intervention is applied in a particular setting. |
| Imprecision | Need for more studies with more participants to reach optimal information size. | Studies with approximately 200 more events in the experimental intervention group and the comparator intervention group are required. | Same uncertainty interpretation as for certainty of a body of evidence: e.g. the true effect may be substantially different. |
| Publication bias | Need to investigate and identify unpublished data; large studies might help resolve this issue. | Large studies are required. | Same uncertainty interpretation as for certainty of a body of evidence (e.g. the true effect may be substantially different). |
| Large effects | No direct implications. | Not applicable. | The effect is large in the populations that were included in the studies and the true effect is likely going to cross important thresholds. |
| Dose effects | No direct implications. | Not applicable. | The greater the reduction in the exposure the larger is the expected harm (or benefit). |
| Opposing bias and confounding | Studies controlling for the residual bias and confounding are needed. | Studies controlling for possible confounders such as smoking and degree of education are required. | The effect could be even larger or smaller (depending on the direction of the results) than the one that is observed in the studies presented here. |
The review of compression stockings for prevention of deep vein thrombosis (DVT) in airline passengers described in Chapter 14 provides an example where there is some convincing evidence of a benefit of the intervention: “This review shows that the question of the effects on symptomless DVT of wearing versus not wearing compression stockings in the types of people studied in these trials should now be regarded as answered. Further research may be justified to investigate the relative effects of different strengths of stockings or of stockings compared to other preventative strategies. Further randomised trials to address the remaining uncertainty about the effects of wearing versus not wearing compression stockings on outcomes such as death, pulmonary embolism and symptomatic DVT would need to be large.” (Clarke et al 2016).
A review of therapeutic touch for anxiety disorder provides an example of the implications for research when no eligible studies had been found: “This review highlights the need for randomized controlled trials to evaluate the effectiveness of therapeutic touch in reducing anxiety symptoms in people diagnosed with anxiety disorders. Future trials need to be rigorous in design and delivery, with subsequent reporting to include high quality descriptions of all aspects of methodology to enable appraisal and interpretation of results.” (Robinson et al 2007).
15.6.4 Reaching conclusions
A common mistake is to confuse ‘no evidence of an effect’ with ‘evidence of no effect’. When the confidence intervals are too wide (e.g. including no effect), it is wrong to claim that the experimental intervention has ‘no effect’ or is ‘no different’ from the comparator intervention. Review authors may also incorrectly ‘positively’ frame results for some effects but not others. For example, when the effect estimate is positive for a beneficial outcome but confidence intervals are wide, review authors may describe the effect as promising. However, when the effect estimate is negative for an outcome that is considered harmful but the confidence intervals include no effect, review authors report no effect. Another mistake is to frame the conclusion in wishful terms. For example, review authors might write, “there were too few people in the analysis to detect a reduction in mortality” when the included studies showed a reduction or even increase in mortality that was not ‘statistically significant’. One way of avoiding errors such as these is to consider the results blinded; that is, consider how the results would be presented and framed in the conclusions if the direction of the results was reversed. If the confidence interval for the estimate of the difference in the effects of the interventions overlaps with no effect, the analysis is compatible with both a true beneficial effect and a true harmful effect. If one of the possibilities is mentioned in the conclusion, the other possibility should be mentioned as well. Table 15.6.b suggests narrative statements for drawing conclusions based on the effect estimate from the meta-analysis and the certainty of the evidence.
Table 15.6.b Suggested narrative statements for phrasing conclusions
|
|
|
| High certainty of the evidence | |
| Large effect | X results in a large reduction/increase in outcome |
| Moderate effect | X reduces/increases outcome X results in a reduction/increase in outcome |
| Small important effect | X reduces/increases outcome slightly X results in a slight reduction/increase in outcome |
| Trivial, small unimportant effect or no effect | X results in little to no difference in outcome X does not reduce/increase outcome |
| Moderate certainty of the evidence | |
| Large effect | X likely results in a large reduction/increase in outcome X probably results in a large reduction/increase in outcome |
| Moderate effect | X likely reduces/increases outcome X probably reduces/increases outcome X likely results in a reduction/increase in outcome X probably results in a reduction/increase in outcome |
| Small important effect | X probably reduces/increases outcome slightly X likely reduces/increases outcome slightly X probably results in a slight reduction/increase in outcome X likely results in a slight reduction/increase in outcome |
| Trivial, small unimportant effect or no effect | X likely results in little to no difference in outcome X probably results in little to no difference in outcome X likely does not reduce/increase outcome X probably does not reduce/increase outcome |
| Low certainty of the evidence | |
| Large effect | X may result in a large reduction/increase in outcome The evidence suggests X results in a large reduction/increase in outcome |
| Moderate effect | X may reduce/increase outcome The evidence suggests X reduces/increases outcome X may result in a reduction/increase in outcome The evidence suggests X results in a reduction/increase in outcome |
| Small important effect | X may reduce/increase outcome slightly The evidence suggests X reduces/increases outcome slightly X may result in a slight reduction/increase in outcome The evidence suggests X results in a slight reduction/increase in outcome |
| Trivial, small unimportant effect or no effect | X may result in little to no difference in outcome The evidence suggests that X results in little to no difference in outcome X may not reduce/increase outcome The evidence suggests that X does not reduce/increase outcome |
| Very low certainty of the evidence | |
| Any effect | The evidence is very uncertain about the effect of X on outcome X may reduce/increase/have little to no effect on outcome but the evidence is very uncertain |
Another common mistake is to reach conclusions that go beyond the evidence. Often this is done implicitly, without referring to the additional information or judgements that are used in reaching conclusions about the implications of a review for practice. Even when additional information and explicit judgements support conclusions about the implications of a review for practice, review authors rarely conduct systematic reviews of the additional information. Furthermore, implications for practice are often dependent on specific circumstances and values that must be taken into consideration. As we have noted, review authors should always be cautious when drawing conclusions about implications for practice and they should not make recommendations.
15.7 Chapter information
Authors: Holger J Schünemann, Gunn E Vist, Julian PT Higgins, Nancy Santesso, Jonathan J Deeks, Paul Glasziou, Elie Akl, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group
Acknowledgements: Andrew Oxman, Jonathan Sterne, Michael Borenstein and Rob Scholten contributed text to earlier versions of this chapter.
Funding: This work was in part supported by funding from the Michael G DeGroote Cochrane Canada Centre and the Ontario Ministry of Health. JJD receives support from the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham. JPTH receives support from the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
15.8 References
Aguilar MI, Hart R. Oral anticoagulants for preventing stroke in patients with non-valvular atrial fibrillation and no previous history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews 2005; 3 : CD001927.
Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews 2007; 3 : CD006186.
Akl EA, Gunukula S, Barba M, Yosuico VE, van Doormaal FF, Kuipers S, Middeldorp S, Dickinson HO, Bryant A, Schünemann H. Parenteral anticoagulation in patients with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database of Systematic Reviews 2011a; 1 : CD006652.
Akl EA, Oxman AD, Herrin J, Vist GE, Terrenato I, Sperati F, Costiniuk C, Blank D, Schünemann H. Using alternative statistical formats for presenting risks and risk reductions. Cochrane Database of Systematic Reviews 2011b; 3 : CD006776.
Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, Treweek S, Mustafa RA, Rada G, Rosenbaum S, Morelli A, Guyatt GH, Oxman AD, Group GW. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ 2016; 353 : i2016.
Altman DG. Confidence intervals for the number needed to treat. BMJ 1998; 317 : 1309-1312.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr., Zaza S. Grading quality of evidence and strength of recommendations. BMJ 2004; 328 : 1490.
Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, Forbes C, Glanville J, Hicks NJ, Moody J, Twaddle S, Timimi H, Young P. How to formulate research recommendations. BMJ 2006; 333 : 804-806.
Cates C. Confidence intervals for the number needed to treat: Pooling numbers needed to treat may not be reliable. BMJ 1999; 318 : 1764-1765.
Clarke MJ, Broderick C, Hopewell S, Juszczak E, Eisinga A. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database of Systematic Reviews 2016; 9 : CD004002.
Cohen J. Statistical Power Analysis in the Behavioral Sciences . 2nd edition ed. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc.; 1988.
Coleman T, Chamberlain C, Davey MA, Cooper SE, Leonardi-Bee J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2015; 12 : CD010078.
Dans AM, Dans L, Oxman AD, Robinson V, Acuin J, Tugwell P, Dennis R, Kang D. Assessing equity in clinical practice guidelines. Journal of Clinical Epidemiology 2007; 60 : 540-546.
Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials . 2nd edition ed. Littleton (MA): John Wright PSG, Inc.; 1985.
Friedrich JO, Adhikari NK, Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Medical Research Methodology 2008; 8 : 32.
Furukawa T. From effect size into number needed to treat. Lancet 1999; 353 : 1680.
Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E. Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Board on Health Care Services: Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011a; 64 : 383-394.
Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ 1998; 316 : 690-693.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336 : 924-926.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, Akl EA, Post PN, Norris S, Meerpohl J, Shukla VK, Nasser M, Schünemann HJ. GRADE guidelines: 8. Rating the quality of evidence--indirectness. Journal of Clinical Epidemiology 2011b; 64 : 1303-1310.
Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbegovic B, Alonso-Coello P, Post PN, Busse JW, Glasziou P, Christensen R, Schünemann HJ. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. Journal of Clinical Epidemiology 2013a; 66 : 158-172.
Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, Johnston BC, Karanicolas P, Akl EA, Vist G, Kunz R, Brozek J, Kupper LL, Martin SL, Meerpohl JJ, Alonso-Coello P, Christensen R, Schünemann HJ. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. Journal of Clinical Epidemiology 2013b; 66 : 173-183.
Hawe P, Shiell A, Riley T, Gold L. Methods for exploring implementation variation and local context within a cluster randomised community intervention trial. Journal of Epidemiology and Community Health 2004; 58 : 788-793.
Hoffrage U, Lindsey S, Hertwig R, Gigerenzer G. Medicine. Communicating statistical information. Science 2000; 290 : 2261-2262.
Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled Clinical Trials 1989; 10 : 407-415.
Johnston B, Thorlund K, Schünemann H, Xie F, Murad M, Montori V, Guyatt G. Improving the interpretation of health-related quality of life evidence in meta-analysis: The application of minimal important difference units. . Health Outcomes and Qualithy of Life 2010; 11 : 116.
Karanicolas PJ, Smith SE, Kanbur B, Davies E, Guyatt GH. The impact of prophylactic dexamethasone on nausea and vomiting after laparoscopic cholecystectomy: a systematic review and meta-analysis. Annals of Surgery 2008; 248 : 751-762.
Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews 2004; 4 : CD001055.
McQuay HJ, Moore RA. Using numerical results from systematic reviews in clinical practice. Annals of Internal Medicine 1997; 126 : 712-720.
Resnicow K, Cross D, Wynder E. The Know Your Body program: a review of evaluation studies. Bulletin of the New York Academy of Medicine 1993; 70 : 188-207.
Robinson J, Biley FC, Dolk H. Therapeutic touch for anxiety disorders. Cochrane Database of Systematic Reviews 2007; 3 : CD006240.
Rothwell PM. External validity of randomised controlled trials: "to whom do the results of this trial apply?". Lancet 2005; 365 : 82-93.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, Lasserson T, Opiyo N, Kunnamo I, Sinclair D, Garner P, Treweek S, Tovey D, Akl EA, Tugwell P, Brozek JL, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016; 74 : 28-39.
Schünemann HJ, Puhan M, Goldstein R, Jaeschke R, Guyatt GH. Measurement properties and interpretability of the Chronic respiratory disease questionnaire (CRQ). COPD: Journal of Chronic Obstructive Pulmonary Disease 2005; 2 : 81-89.
Schünemann HJ, Guyatt GH. Commentary--goodbye M(C)ID! Hello MID, where do you come from? Health Services Research 2005; 40 : 593-597.
Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 13. Applicability, transferability and adaptation. Health Research Policy and Systems 2006; 4 : 25.
Schünemann HJ. Methodological idiosyncracies, frameworks and challenges of non-pharmaceutical and non-technical treatment interventions. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2013; 107 : 214-220.
Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, Shea B, Wells G, Helfand M. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013; 4 : 49-62.
Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, Ventresca M, Brignardello-Petersen R, Laisaar KT, Kowalski S, Baldeh T, Zhang Y, Raid U, Neumann I, Norris SL, Thornton J, Harbour R, Treweek S, Guyatt G, Alonso-Coello P, Reinap M, Brozek J, Oxman A, Akl EA. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ: Canadian Medical Association Journal 2014; 186 : E123-142.
Schünemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? Journal of Clinical Epidemiology 2016; 75 : 6-15.
Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta-analyses--sometimes informative, usually misleading. BMJ 1999; 318 : 1548-1551.
Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, Bala MM, Bassler D, Mertz D, Diaz-Granados N, Vandvik PO, Malaga G, Srinathan SK, Dahm P, Johnston BC, Alonso-Coello P, Hassouneh B, Walter SD, Heels-Ansdell D, Bhatnagar N, Altman DG, Guyatt GH. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ 2012; 344 : e1553.
Zhang Y, Akl EA, Schünemann HJ. Using systematic reviews in guideline development: the GRADE approach. Research Synthesis Methods 2018a: doi: 10.1002/jrsm.1313.
Zhang Y, Alonso-Coello P, Guyatt GH, Yepes-Nunez JJ, Akl EA, Hazlewood G, Pardo-Hernandez H, Etxeandia-Ikobaltzeta I, Qaseem A, Williams JW, Jr., Tugwell P, Flottorp S, Chang Y, Zhang Y, Mustafa RA, Rojas MX, Schünemann HJ. GRADE Guidelines: 19. Assessing the certainty of evidence in the importance of outcomes or values and preferences-Risk of bias and indirectness. Journal of Clinical Epidemiology 2018b: doi: 10.1016/j.jclinepi.2018.1001.1013.
Zhang Y, Alonso Coello P, Guyatt G, Yepes-Nunez JJ, Akl EA, Hazlewood G, Pardo-Hernandez H, Etxeandia-Ikobaltzeta I, Qaseem A, Williams JW, Jr., Tugwell P, Flottorp S, Chang Y, Zhang Y, Mustafa RA, Rojas MX, Xie F, Schünemann HJ. GRADE Guidelines: 20. Assessing the certainty of evidence in the importance of outcomes or values and preferences - Inconsistency, Imprecision, and other Domains. Journal of Clinical Epidemiology 2018c: doi: 10.1016/j.jclinepi.2018.1005.1011.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.
Loading metrics
Open Access
Peer-reviewed
Research Article
The assembly of neutrophil inflammasomes during COVID-19 is mediated by type I interferons
Roles Conceptualization, Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft
* E-mail: [email protected]
Affiliation Viral Zoonosis Research Unit, Medicum, Department of Virology, University of Helsinki, Helsinki, Finland
Roles Investigation, Writing – review & editing
Affiliations Department of Bacteriology and Immunology, University of Helsinki, Helsinki, Finland, Translational Immunology Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland
Roles Investigation
Affiliations Viral Zoonosis Research Unit, Medicum, Department of Virology, University of Helsinki, Helsinki, Finland, Department of Veterinary Biosciences, University of Helsinki, Helsinki, Finland
Roles Resources
Affiliations Viral Zoonosis Research Unit, Medicum, Department of Virology, University of Helsinki, Helsinki, Finland, Department of Veterinary Biosciences, University of Helsinki, Helsinki, Finland, Department of Tropical Parasitology, Institute of Maritime and Tropical Medicine, Medical University of Gdansk, Gdynia, Poland
Affiliations Human Microbiome Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland, Meilahti Vaccine Research Center MeVac, Department of Infectious Diseases, Inflammation Center, Helsinki University Hospital and University of Helsinki, Helsinki, Finland
Roles Funding acquisition, Resources, Writing – review & editing
Roles Supervision, Writing – review & editing
Affiliations Department of Bacteriology and Immunology, University of Helsinki, Helsinki, Finland, Translational Immunology Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland, Division of Virology and Immunology, HUSLAB Clinical Microbiology, HUS Diagnostic Center, Helsinki University Hospital, Helsinki, Finland
Affiliation Institute for Molecular Medicine Finland (FIMM), HiLIFE, University of Helsinki, Helsinki, Finland
Roles Resources, Writing – review & editing
Roles Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing
Affiliations Department of Veterinary Biosciences, University of Helsinki, Helsinki, Finland, Laboratory for Animal Model Pathology, Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland, Department of Infection Biology & Microbiomes, Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom
Roles Funding acquisition, Resources
Affiliations Viral Zoonosis Research Unit, Medicum, Department of Virology, University of Helsinki, Helsinki, Finland, Department of Veterinary Biosciences, University of Helsinki, Helsinki, Finland, Division of Virology and Immunology, HUSLAB Clinical Microbiology, HUS Diagnostic Center, Helsinki University Hospital, Helsinki, Finland
Roles Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft
- Luz E. Cabrera,
- Suvi T. Jokiranta,
- Sanna Mäki,
- Simo Miettinen,
- Ravi Kant,
- Lauri Kareinen,
- Tarja Sironen,
- Jukka-Pekka Pietilä,
- Anu Kantele,

- Published: August 22, 2024
- https://doi.org/10.1371/journal.ppat.1012368
- Peer Review
- Reader Comments
The severity of COVID-19 is linked to excessive inflammation. Neutrophils represent a critical arm of the innate immune response and are major mediators of inflammation, but their role in COVID-19 pathophysiology remains poorly understood. We conducted transcriptomic profiling of neutrophils obtained from patients with mild and severe COVID-19, as well as from SARS-CoV-2 infected mice, in comparison to non-infected healthy controls. In addition, we investigated the inflammasome formation potential in neutrophils from patients and mice upon SARS-CoV-2 infection. Transcriptomic analysis of polymorphonuclear cells (PMNs), consisting mainly of mature neutrophils, revealed a striking type I interferon (IFN-I) gene signature in severe COVID-19 patients, contrasting with mild COVID-19 and healthy controls. Notably, low-density granulocytes (LDGs) from severe COVID-19 patients exhibited an immature neutrophil phenotype and lacked this IFN-I signature. Moreover, PMNs from severe COVID-19 patients showed heightened nigericin-induced caspase1 activation, but reduced responsiveness to exogenous inflammasome priming. Furthermore, IFN-I emerged as a priming stimulus for neutrophil inflammasomes. These findings suggest a potential role for neutrophil inflammasomes in driving inflammation during severe COVID-19. Altogether, these findings open promising avenues for targeted therapeutic interventions to mitigate the pathological processes associated with the disease.
Author summary
COVID-19, caused by the SARS-CoV-2, ranges from mild “flu”-like symptoms to severe respiratory distress or even death. Neutrophils are important cells of our immune system which are strongly involved in inflammatory responses, including those occurring in COVID-19. However, despite extensive research, the precise contribution of neutrophils to the pathogenesis of COVID-19 remains elusive, and further clarification on their role is still needed. In this study, we isolated neutrophils from COVID-19 patients and healthy controls to analyze changes in their gene expression profile and inflammatory functions. These analyses revealed a distinct type I interferon (IFN-I) gene signature expressed by mature, but not immature, neutrophils from severe COVID-19 patients, which was absent in mild cases and healthy controls. Additionally, neutrophils from severe COVID-19 showed signs of increased inflammasome activation, a protein complex that contributes to inflammation by releasing inflammatory cytokines. Notably, IFN-I alone was able to promote neutrophil inflammasome formation in vitro suggesting a direct link between IFN-I response and inflammasome formation during COVID-19. Furthermore, increased neutrophil inflammasome activity was detected also in a mouse model of COVID-19. These findings suggest a potential role for neutrophils in driving excessive inflammation during severe COVID-19, and a role for IFN-I in priming the assembly of inflammasomes in these cells.
Citation: Cabrera LE, Jokiranta ST, Mäki S, Miettinen S, Kant R, Kareinen L, et al. (2024) The assembly of neutrophil inflammasomes during COVID-19 is mediated by type I interferons. PLoS Pathog 20(8): e1012368. https://doi.org/10.1371/journal.ppat.1012368
Editor: Tom Gallagher, Loyola University Chicago Stritch School of Medicine, UNITED STATES OF AMERICA
Received: February 8, 2024; Accepted: June 24, 2024; Published: August 22, 2024
Copyright: © 2024 Cabrera et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its supporting information files. RNA-seq data are deposited at the GEO (GSE272381 and GSE271808).
Funding: This work was financed by grants by the Academy of Finland to T.S. (321809), A.K. (336439 and 335527); grants by the Helsinki University Hospital funds to O.V. (TYH 2021343); EU Horizon 2020 programme VEO (874735) to O.V.; Finnish governmental subsidy for Health Science Research (TYH 2021315) to A.K.; Paulon Säätiö to L.E.C.; Suomen Lääketieteen Säätiö to L.E.C.; Jane and Aatos Erkko foundation to O.V. The funders had no role in study design, data collection and analysis, nor decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Severe COVID-19 is characterized by a dysregulated immune response with an excessive production of pro-inflammatory cytokines and chemokines. Type I interferons (IFN-I) are critical antiviral cytokines in the innate immune responses against viral infections, drawing particular attention amidst the COVID-19 pandemic [ 1 – 3 ]. While the IFN-I response helps to limit virus replication [ 3 ], its prolonged and uncontrolled activation is detrimental to the overall health of the patient [ 4 ]. As part of the pro-inflammatory response, neutrophils are rapidly recruited to the site of infection in response to SARS-CoV-2 infection [ 5 , 6 ]. Prominent neutrophil recruitment in severe COVID-19 is associated with an increased number of immature low-density granulocytes (LDGs) in the circulation [ 7 – 9 ]. The increased production and subsequent early release of immature cells from the bone marrow occurs in response to emergency myelopoiesis [ 9 ]. This process is initiated by the body to enable the recruitment of innate immune cells into the tissues and to replenish the depleted leukocyte pool, in an effort to combat viral infections including SARS-CoV-2 [ 10 ]. However, the premature release of these cells could be associated with the increased degranulation and formation of neutrophil extracellular traps (NETs) reported during SARS-CoV-2 infection, to which LDGs have a higher propensity than polymorphonuclear cells (PMN) [ 5 , 6 , 11 ].
Neutrophils are involved in several aspects of inflammatory processes, including the release of reactive oxygen species (ROS) and other pro-inflammatory mediators such as Interleukin-6 (IL-6) and IL-8. In addition, recent reports on COVID-19 highlight that neutrophils could be a major source of inflammasome derived IL-1β, which has been implicated as a substantial contributor to COVID-19 pneumonia [ 12 ]. Inflammasomes are intracellular multiprotein complexes involved in the inflammatory response. In the presence of a pathogen, antigen recognition by the immune system triggers the assembly of the inflammasome, a step known as the first signal. This is followed by the recruitment of adaptor molecules that activate NOD-like receptor (NLR) family members and the binding of the apoptosis-associated speck-like protein (ASC), finally activating the inflammasome complex [ 13 ]. The triggered assembly of this complex is known as the second signal. Studies have shown that SARS-CoV-2 infection induces significant inflammasome activation in circulating and lung-infiltrating myeloid cells, such as monocytes and neutrophils [ 14 – 17 ]. However, while the precise mechanism by which inflammasomes are activated in monocytes/macrophages is well established, less is known about molecular mechanisms of inflammasome formation in neutrophils. Thus, this study investigates the inflammasome formation in neutrophils during COVID-19 in more detail, also focusing on the different developmental stages of these cells. In addition, a recently established COVID-19 mouse model served to further explore the role of IFN-I in neutrophil inflammasome assembly.
Materials and methods
Ethics statement.
The study was approved by the Ethics Committee of the Hospital District of Helsinki and Uusimaa (HUS/853/2020, HUS/1238/2020). All volunteers gave a written informed consent, in accordance with the Declaration of Helsinki. For animal experiments, experimental procedures were approved by the Animal Experimental Board of Finland (license number ESAVI/28687/2020).
Patient population
Adult clinical patients with confirmed COVID-19 (RT-PCR positive for SARS-CoV-2) at Helsinki University Hospital (HUH) (hospitalized: n = 34; outpatients: n = 8) were enrolled in the present study. Blood samples were collected during hospitalization for the severe COVID-19 group, and after confirmation of diagnosis for the mild COVID-19 outpatient group. Samples for RNA sequencing were collected in 2020 and representing infections by the original and early SARS-CoV-2 variants, whereas samples for ex vivo culture experiments were collected in 2021–2022 likely representing infections by omicron subvariants of SARS-CoV-2. As controls, healthy blood donors were included for RNA sequencing (n = 7, age 57 ± 7, male/female 3/4) and ex vivo culturing experiments (n = 9, age 38 ± 14, male/female 4/5). For clinical correlation analysis, severe COVID-19 patients were further categorized by severity based on their need for hospitalization and oxygen supplementation, as described previously [ 7 ]. For each patient, medical history and clinical data were collected through retrospective patient record review and are presented for the severe COVID-19, hospitalized patients in Table 1 and as previously described [ 7 ]. Calprotectin was measured from serum (diluted 1:1000) by ELISA, according to the manufacturer’s protocol (calprotectin/S100A8 DuoSet kit, R&D systems).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.ppat.1012368.t001
The World Health Organization (WHO) Ordinal Scale for clinical improvement is a tool designed specifically to assess and measure the progression and clinical improvement of patients [ 67 ]. COVID-19 scoring: 1 = no limitations of activity, 2 = limitations of activity, 3 = no oxygen therapy, 4 = oxygen by mask or nasal cannulae, 5 = non-invasive ventilation or high-flow oxygen, 6 = invasive mechanical ventilation without other organ support, 7 = invasive mechanical ventilation with other organ support, 8 = dead. The baseline score represents the timepoint of the first laboratory sample taken, serving as a reference point for measuring improvement and establishing a starting point for comparison. In contrast, the worst score represents the most severe or critical state of the disease. None of the patients whose samples were used for RNA-seq underwent corticosteroid treatment.
Isolation of granulocytes from human blood
Blood samples from COVID-19 patients and healthy controls (HC) were collected in EDTA vacutainer tubes and transported to the laboratory. Peripheral blood mononuclear cells (PBMCs) or polymorphonuclear cells (PMNs) were isolated from whole blood by density gradient centrifugation using either Ficoll-Paque Plus (GE Healthcare) or Polymorphprep (Axis-Shield) respectively, following standard procedures including the use of 2 mM EDTA in PBS and red blood cell lysis with ACK lysis buffer (Lonza by Thermo Fisher). Subsequently, isolation of CD66 + granulocytes (low-density granulocytes, LDGs) from the PBMC fraction was performed using the CD66abce MicroBead Kit (Miltenyi Biotec, Germany) with an MS column, according to the manufacturer’s instructions. Both the positively selected CD66 + LDGs and the isolated PMNs were then washed and counted, using a TC20 Automated Cell Counter (Bio-Rad Laboratories, Inc.) with trypan blue staining for dead cell exclusion. All described procedures in this section were done at room temperature. An aliquot of cells was lysed in Trizol reagent (Thermo Fisher Scientific, USA) and stored at –80°C for later extraction of total RNA and subsequent RNA sequencing (RNA-seq) analysis.
Caspase1 activity
Caspase1 activity was assessed in isolated cells after 2 h of culture (1 million cells/ml) using the caspase-Glo1 inflammasome assay (Promega) according to the manufacturer’s protocol, with 2.5 μM nigericin (Invivogen) treatment as the activator. The resulting luminescence was measured by a Hidex Sense microplate reader (Hidex).
As another approach, caspase1 activity in isolated cells (1 million cells/ml) was measured by the fluorescent dye FAM-FLICA (Bio-Rad Laboratories). Cells were incubated with FAM-FLICA according to manufacturer’s recommendations for 30 min in culture medium at 37°C, after which cells were washed with PBS and analyzed by LSRII cytometer (BD Biosciences). Data was acquired with BD FACSDiva version 8.0.1 (BD Biosciences) software and further analysis was performed with the FlowJo software v10 (BD Biosciences).
Soluble factor stimulation assays
Isolated granulocytes from HC and COVID-19 patients were cultured at 2 million cells/ml in RPMI 1640 supplemented with 10% fetal bovine serum (R10) at 37°C. Cells were primed (1 st signal) with either LPS (20 ng/ml, Sigma Aldrich) or IFN-I (combination of 2.7*10 4 IU/ml IFN-α and IFN-β, Immunotools) for 4 h, followed by activation (2 nd signal) by 2.5 μM nigericin or monosodium urate crystals (MSU, 100 μg/ml, Invivogen) for an additional 4 h. For the 24 h stimulation experiments, nigericin was added to the cultured cells, in the presence or absence of inflammasome inhibitors MCC995 (2 μg/ml) and Ac-YVAD-FMK (20 μg/ml, both from Invivogen). Cells were pelleted by centrifugation at 400 G for 5 min and stored in Trizol at –80°C for later RNA extraction whereas supernatants were used to measure IL-1β, IL-18, myeloperoxidase (MPO) and IL-8 by ELISAs according to the manufacturer’s protocols (DuoSet kits from R&D systems). LDH was measured in supernatants using Cyquant LDH cytotoxicity assay (ThermoFisher). Where indicated, priming and activation was performed in the presence of 100 μg/ml of anti-human IFNAR1 (Anifrolumab Biosimilar) or mouse IgG1 as control (both from Bio-X-Cell, New Hampshire, USA).
HL-60 cells (ATCC #CCL-240) were activated similarly to neutrophils after a 5-day differentiation period induced by 1% DMSO.
Virus propagation
The SARS-CoV-2 hCoV-19/Finland/THL-202117309/2021 (delta strain B.1.617.2) and the mouse-adapted strain MaVie [ 18 ] were propagated in VeroE6-TMPRSS2 cells (kidney epithelial cells expressing the transmembrane protease serine 2) [ 19 ] grown in DMEM supplemented with 10% inactivated FCS, 100 IU/mL Penicillin, 100 μg/mL Streptomycin and 2 mM L-glutamine at 37°C. The virus was purified from supernatants by ultracentrifugation (SW28 rotor, 27,000 rpm, 90 min, +4°C) through a 0.22 μm-filtered 30% ultra-pure sucrose cushion (in PBS), to obtain virus preparations free of cell culture contaminants. Virus titers were calculated by the median tissue culture infectious dose (TCID50) after assessing cytopathic effects by crystal violet staining of cell cultures infected for 5 days with serially diluted virus.
RNA sequencing
Neutrophils isolated from different cohorts comprised three PMN groups (severe COVID-19, mild COVID-19, and healthy controls), and one LDG group (given that these cells were rare in mild COVID-19 patients and HC, only LDGs from patients with severe COVID-19 were included).
cDNA synthesis from total RNA was performed according to Takara SMARTseq v4 Ultra-low input RNA kit for Sequencing user manual (Takara Bio, Mountain View, CA, USA) followed by Illumina Nextera XT Library preparation according to Illumina Nextera XT Reference Guide (Illumina, San Diego, CA, USA). UDI index setup was used for the Nextera XT libraries. Library quality check was performed using LabChip GX Touch HT High Sensitivity assay (PerkinElmer, USA) and libraries were pooled based on the concentrations acquired from the assay. The pooled libraries were quantified for sequencing using KAPA Library Quantification Kit (KAPA Biosystems, Wilmington, MA, USA) and sequenced on the Illumina NovaSeq6000 system for 200 cycles using S1 flow cell (Illumina, San Diego, CA, USA). Read length for the paired-end run was 2x101 bp. The human RNA-seq data are deposited at the GEO (accession number GSE#272381).
RNA data analysis
Principal Component Analysis (PCA) and enrichment analyses were obtained using ExpressAnalyst [ 20 ]. Briefly, PCA was performed to identify patterns in the data and reduce the dimensionality of the dataset, where the top principal components were selected based on the percentage of variance explained. For enrichment analyses, Gene Set Enrichment Analysis (GSEA) and Over-Representation Analysis (ORA) were performed on the top 5000 DE genes identified by DESeq2 (adjusted P value < 0.05, log2FC >1) [ 20 ]. GSEA was used to identify enriched signaling pathways using the Reactome database, while ORA was used to identify enriched pathways using the KEGG database. The resulting p-values were corrected for multiple testing using the Benjamini-Hochberg method, and pathways with a corrected p-value <0.05 were considered significant.
To visualize the expression patterns of the DE genes, the data was analyzed using the AltAnalyze software [ 21 ], which selected the top 118 genes based on correlation and determined the heatmap clustering, using the Euclidean distance metric and the complete linkage method. Then, the obtained heatmap was re-generated using heatmapper.ca [ 22 ] for better visualization.
CIBERSORTx, a machine learning algorithm that infers cell type proportions using a reference gene expression matrix of known cell types was used to perform RNA-seq deconvolution on the gene expression data to estimate the abundance of immune cell types in the samples [ 23 ]. The signature matrix used was taken from Lasalle et al . [ 8 ]. This reference matrix made use of a published whole-blood single-cell dataset [ 9 ], and included the main immune cell types: monocytes, NK cells, T lymphocytes, B lymphocytes, plasmablasts and neutrophils, the latter subclassified into mature and immature. The smaller subsets of granulocytes (eosinophils and basophils) are not considered separately and are most likely categorized as neutrophils in the bulk data deconvolution. Nonetheless, the resulting cell type proportions were used to compare the immune cell composition between groups.
Additionally, the determination of sample purity (>65% identified as neutrophils) served as a limiting parameter for the visualization of differentially expressed inflammasome related genes from the RNA sequencing results, which were selected and graphed in a heatmap using heatmapper.ca [ 22 ], clustered by complete linkage and ordered by Spearman’s rank.
For the GSEA of the reanalyzed RNA-seq data from LaSalle et al . [ 8 ], we used the fgsea R package using MSigDB pathway sets, as specified in S1 Table .
Volcano plots
To visualize differentially expressed (DE) genes between groups from human and mice RNA-seq results previously identified by DESeq2, a volcano plot was generated using GraphPad Prism. Genes with a P-adjusted value (padj or FDR) <0.05 were considered significant. Similarly, RNA sequencing data from GSE93996 [ 24 ] was reanalyzed, and all DE genes in ATRA-differentiated HL-60 cells were visualized in a volcano plot.
Single cell transcriptomics data analysis
This study made use of the “COVID-19 Immune Atlas: integration of 5 public COVID-19 PBMC single-cell datasets” available online [ 25 ]. This standardized data collection contains cells from different assays (10x 3’ v2, 10x 3’ v3, 10x technology and Seq-Well) and consists of a total of 239,696 cells from the peripheral blood, 3,693 of which are neutrophils. These neutrophils were further subclassified as mature (59%) and immature (41%), based on the immune atlas predetermined cell classes. This was confirmed by a CD16b expression in mature neutrophils, and a higher CD66b expression in the immature population. This data was obtained from and analyzed in the Chan Zuckerberg CELLxGENE platform [ 25 ].
Reverse transcription and quantitative PCR (RT-qPCR) for human selected human genes
Total RNA was extracted from unstimulated or ex vivo stimulated PMNs using the Trizol reagent (Invitrogen, USA) according to the manufacturer’s protocol. Subsequently, cDNA synthesis was performed using the RevertAid RT Reverse Transcription Kit (Thermo Scientific, USA) as per the manufacturer’s instructions. Quantitative PCR (qPCR) was performed using the Stratagene model (Agilent Technologies) and SYBR Green/ROX master mix (Thermo Scientific, USA). The primer sequences for qPCR are presented in S2 Table .
Primer specificity was confirmed using melting curve analysis and dissociation curves. The relative expression levels of the genes of interest were calculated using the 2-ΔΔCT method and normalized to the expression of the housekeeping gene GAPDH. Baseline gene expressions of unstimulated samples were statistically assessed using the Mann-Whitney test, while the two-way ANOVA Tukey’s multiple comparisons test was performed for the ex vivo stimulated samples.
Mouse infections
Female BALB/c mice (Envigo, Indianapolis, IN, USA; 7 to 8 weeks, n = 36 in total) were transferred to the University of Helsinki biosafety level-3 (BSL-3) facility and acclimatized to individually ventilated biocontainment cages (ISOcage; Scanbur, Karl Sloanestran, Denmark) for 7 days with ad libitum water and food (rodent pellets). For subsequent experimental infection, the mice were placed under isoflurane anesthesia and inoculated intranasally with 50 μL of SARS-CoV-2 MaVie strain (5*10 5 TCID50/animal) or PBS (mock-infected control). Daily weighting of all mice was performed, and their well-being was carefully monitored for signs of illness (e.g., changes in posture or behavior, rough coat, apathy, ataxia). Euthanasia was performed by cervical dislocation under terminal isoflurane anesthesia. All animals were dissected immediately after euthanasia, and the lungs were sampled for multiple downstream analyses. The infections were performed as 4 separate experiments (exp): 1) Exp 1 included 8 mice infected with MaVie and 4 mock infected mice. At 2 days post infection (dpi), 4 infected and the mock infected mice were euthanized; the remaining infected mice were euthanized at 4 dpi. The right lung was sampled for virus-specific RT-qPCR (1/5) and neutrophil isolation (4/5), the left lung was fixed for histological and immunohistochemical examination. 2) Exp 2 included 8 infected and 4 mock infected mice of which half were euthanized at 2 dpi and 4 dpi, respectively. From these mice, both lung lobes were subjected to neutrophil isolation. 3) Exp 3 included 8 mice that were infected and immediately inoculated intraperitoneally with 250 μg of anti-mouse IFNAR-1 (n = 4) or mouse IgG1 control (n = 4) (Bio-X-Cell, New Hampshire, USA), and 4 mock-infected animals. All mice were euthanized at 2 dpi. Each 1/5 of the left lobe was processed for virus-specific RT-qPCR and histology/immunohistochemistry, respectively. The remaining 4/5 of the lungs served for neutrophil isolation. 4) Exp 4 included 4 animals in the following 5 groups: PBS-inoculated controls, infected animals euthanized at 2 dpi and at 4 dpi respectively, infected animals euthanized at 2 dpi with intraperitoneal injection of control IgG or anti-mouse IFNAR-1. Each 1/5 of the left lobe was processed for virus-specific RT-qPCR and histology/immunohistochemistry, respectively. The remaining lung tissue served to prepare single cell suspensions. Each 1 million cells were subjected to neutrophil quantification or caspase1 activity measurement by flow cytometry, the rest (approx. 8 million cells) to neutrophil isolation.
Virus titration from mouse lungs
Supernatants of single cell suspensions from Exp 4 were used for virus titration by fluorescent focus forming unit (FFU)-based assay, in which Vero E6 cells were incubated with serially diluted supernatants for 24 hours at 37°C in growth medium. Cells were fixed with 4% PFA for 10 min, blocked and permeabilized with PBS containing 3% BSA and 0.2% TritonX-100 for 15 min and incubated with rabbit anti- SARS-CoV-2 receptor binding domain (RBD) [ 19 ] for 1 hr, followed by anti-rabbit AlexaFluor488 conjugated secondary antibody (Thermo Scientific) for 1 hr. Fluorescence was observed with Zoe fluorescence imager (Bio-Rad laboratories) and the highest dilution not showing any RBD positive cells considered as the virus titer.
Neutrophil quantification and caspase1 activity measurement from mouse lungs by flow cytometry
Single cell suspensions from mouse lungs of Exp 4 were subjected to flow cytometric quantification of Ly6G+ neutrophils. Cells were initially incubated with BV605-conjugated yellow live/dead dye (Thermo Scientific) for 15 min before addition of 1% FBS and a cocktail of antibodies recognizing CD3+ and CD19+ lymphocytes (FITC-conjugated clones 145-2C11 and 1D3, respectively, from Immunotools), Ly6G+ neutrophils (PE-Cy7-conjugated clone 1A8 from BD biosciences) and CD11b (APC-conjugated clone M1/70.15 from Immunotools). After incubation for 30 min at RT, cells were fixed with 2% paraformaldehyde for 30 min and washed with PBS. In parallel, single cell suspensions were stained with FAM-FLICA together with Ly6G antibody and 30 min incubations were performed in R10 at 37°C before fixation. Finally, all cells were subjected to flow cytometric analysis with a three-laser (Blue/Red/Violet lasers) 14-color Fortessa LSRII cytometer (BD Biosciences). Data was acquired with BD FACSDiva version 8.0.1 (BD Biosciences) software and further analysis was performed with the FlowJo software v10 (BD Biosciences).
Neutrophil isolation from mouse lungs
Neutrophil isolation was performed from the lungs of all mice. The dissected lung tissue was chopped into small pieces using scissors and enzymatically digested with a cocktail of Liberase (50 ug/ml; Roche #05401020001 from Merck) and DnaseI (100 ug/ml; Roche #11284932001 from Merck) in RPMI-1640 for 30 min at 37°C. The resulting homogenate was diluted 10-fold in R10 and passed through a 70 μm Cell strainer (Pluriselect) to obtain a single-cell suspension. Neutrophils were isolated by positive selection using Ly6G-binding magnetic beads and MS columns according to the manufacturer’s recommendations (Miltenyi Biotec). Neutrophils were isolated with a purity exceeding 95% based on flow cytometry analysis of Ly6G expression. Where indicated, isolated neutrophils were attached to glass slides through cytospin (800 g, 5 min), fixed with 4% PFA for 10 min and the nuclei stained with Hoechst33342.
RNA sequencing of mouse neutrophils
Mouse neutrophils from Exp 1 were isolated, lysed in Trizol (Thermo Scientific) and the RNA extracted in the liquid phase using chloroform. RNA isolation was carried out using the Rneasy micro kit (Qiagen). Isolated RNA (1 ng) underwent whole transcriptome sequencing with ribodepletion. Briefly, RNA sequencing was performed using the Illumina Stranded with RiboZero library preparation method. Sample quality and integrity were assessed using TapeStation RNA analysis. Sequencing was conducted on the Illumina NextSeq platform, followed by standard bioinformatics analysis for gene expression quantification.
The service was provided by the Biomedicum Functional Genomics Unit at the Helsinki Institute of Life Science and Biocenter Finland at the University of Helsinki. The mouse RNA-seq data are deposited at the GEO (GSE271808).
RT-qPCR of mouse samples
RNA was extracted from dissected lung samples (1/10 of the whole lung) as well as isolated neutrophils of mice in Exp 1, 3 and 4 using Trizol (Thermo Scientific) following the manufacturers’ instructions. The isolated RNA was directly subjected to one-step RT-qPCR analysis based on a previously described protocol using primer-probe sets detecting the viral genome encoding for the RNA-dependent RNA polymerase (RdRp [ 26 ], subgenomic E [ 27 ] as well as mouse OasL2, caspase1, IL1b and GAPDH (Applied biosystems #Mm01336189_m1, #Mm00438023_m1, #Mm00434228_m1 and #Mm99999915_g1 respectively, Thermo Scientific). The PCRs were performed with TaqPath 1-step master mix (Thermo Scientific) using AriaMx instrumentation (Agilent, Santa Clara, CA, USA).
Histology and immunohistochemistry
From animals in Exp 1, 3 and 4 the whole left lung (Exp 1) or 1/5 of the left lung (Exp 3 and 4) were trimmed for histological examination and routinely paraffin wax embedded. Consecutive sections (3 μm) were prepared and routinely stained with hematoxylin-eosin (HE) or subjected to immunohistochemistry (IHC) for the detection of SARS-CoV-2 nucleoprotein (NP) [ 28 ] and Ly6G (neutrophil marker); for Exp 3, a further section of the infected lungs was stained for histone H3 (NET marker) [ 29 ]. All stains followed previously published protocols [ 30 ].
Morphometric analyses
For quantification of SARS-CoV-2 antigen expression and the extent of neutrophil influx into the lungs, a morphometric analysis was undertaken on the slides stained for SARS-CoV-2 NP and Ly6G, respectively. The stained slides were scanned using NanoZoomer 2.0-HT (Hamamatsu, Hamamatsu City, Japan), and several sections of the lung of each animal were quantitatively analysed using the Visiopharm 2022.01.3.12053 software (Visiopharm, Hoersholm, Denmark). The average total tissue area used for quantification was 19.5 ± 6 mm 2 . The morphometric analysis served to quantify the area, in all lung sections of an animal, that showed immunostaining for viral NP and Ly6G, respectively. In Visiopharm, for each section, the lung was manually outlined and annotated as a Region Of Interest (ROI), manually excluding artifactually altered areas. The manual tissue selection was further refined with an Analysis Protocol Package (APP) based on a Decision Forest classifier, with the pixels from the ROI being ultimately classified as either “Tissue” or “Background”. This new “Tissue” ROI, regrouping the different lung samples analysed for each animal, was further quantified by executing two APPs successively. The first APP was based on a Threshold classifier and served to detect and outline areas with immunostaining. The second APP then measured both the surface of the immunostained area (μm 2 ) and the surface of the “Tissue” ROI (μm 2 ). The percentage of immunostained area (%), expressed as the ratio between the immunostained area and the total area, was obtained for each animal in Excel (Microsoft Office 2019; Microsoft, Redmond, Washington, United States), according to the following formula: ([positive area (μm2)]/ [total area (μm2)]) x 100.
Statistical analyses
Statistical analysis was performed using GraphPad Prism 8.3 software (GraphPad Software, San Diego, CA, USA) and R software v3.6.3 (R core team). Statistically significant correlations between parameters were assessed by calculating Spearman’s correlation coefficients, and differences between groups were assessed with Mann-Whitney, Kruskall-Wallis or ordinary one-way or 2-way ANOVA tests, depending on sample distribution and the number of groups analyzed. To elaborate, nonparametric tests like Mann-Whitney and Kruskall-Wallis were employed when the data violated assumptions of normality, while ANOVA tests were applied when the data met parametric assumptions.
Unsupervised RNA-seq analysis reveals an antiviral gene expression signature of circulating neutrophils in COVID-19 that is strongly influenced by maturity
Different neutrophil subsets have gained a lot of attention as modulators of COVID-19 pathogenesis. We recently found increased frequencies of one neutrophil subset, referred to as low-density granulocytes (LDGs, isolated from the PBMC fraction), during COVID-19 [ 7 ]. In the current study we sought to understand in more detail the transcriptomic profile of different neutrophil subsets, including LDGs and their “normal” density counterpart, the circulating polymorphonuclear cells (PMNs) [ 31 ], which typically consists of mainly mature neutrophils. Neutrophils isolated from different cohorts comprised three PMN groups (severe COVID-19, mild COVID-19, and healthy controls), and one LDG group. Initial deconvolution of the RNA sequencing (RNA-seq) data allowed us to gain a comprehensive understanding of the cellular composition within PMN and LDG fractions and verified that most cells present in the samples were neutrophils ( S1A Fig ). This analysis also demonstrated that cells in the LDG fraction were predominantly immature neutrophils, meanwhile PMNs were composed of mainly mature neutrophils.
The samples with predominant neutrophil cell populations were selected for subsequent gene expression analysis (neutrophils ≥ 65%). The high variance in gene expression between PMNs and LDGs was confirmed by principal component analysis (PCA) ( Fig 1A ), which revealed that the gene expression patterns of COVID-19 LDGs differed from those of all PMNs regardless of the patients’ disease state. Functional enrichment analyses through gene overrepresentation (ORA) and gene-set enrichment analyses (GSEA) ( Fig 1B ) compared PMNs with LDGs from severe COVID-19 patients. The most statistically significant result was an overrepresentation of the NOD-like receptor signaling pathway in PMNs in contrast with LDGs, highlighting that the different neutrophil fractions have a distinct inflammatory profile. This was supported by GSEA, where the most obvious increases in fold changes were the enrichment of the interferon signaling pathways. Another relevant difference was the cell cycle and DNA replication pathways, identified by both ORA and GSEA, which supported our previous findings suggesting LDGs to be predominantly immature cells [ 7 ]. Furthermore, a heatmap of selected type I IFN (IFN-I) related genes confirmed a robust IFN-I gene signature in severe COVID-19 PMNs, while LDGs from severe COVID-19 distinctively lacked this signature ( Fig 1C ). Unsupervised clustering analysis, namely Iterative Clustering and Guide Gene Selection (ICGS) using the AltAnalyze software, supported these findings by identifying the top 118 differentially expressed (DE) genes, including several IFN-related genes ( S1B Fig ). Similarly to the selected samples included in Fig 1 , this analysis classified the samples into two major clusters: a first one containing all isolated LDG samples, and a second one comprising all isolated PMN samples. The former cluster consisted of neutrophil antimicrobial and granule marker genes (e.g. DEFA3 , DEFA4 , SERPINB10 , CTSG ), while in the latter cluster the most significantly upregulated genes in the PMNs from severe COVID-19 subgroup were mainly interferon inducible (e.g. IFI44L , IFI6 , GBP3 , IRF7 ). These differences were supported by a detailed gene analysis ( S2A Fig ).
The analysis was reduced to include only the samples with the highest purity (cell fraction over 0.65 of neutrophils), as identified by CIBERSORTx. ( A ) Principal component analysis (PCA) of the RNA-seq samples (n = 7 PMNs from HC, n = 10 PMNs from severe COVID-19, n = 8 PMNs from mild COVID-19, and n = 6 LDGs from severe COVID-19). ( B ) Ridgeline diagrams depicting the top 20 enriched signal pathways from the genes differentially expressed by PMNs versus LDGs during severe COVID-19: overrepresentation analysis (ORA) using KEGG database and gene-set enrichment analysis (GSEA) according to Reactome database. Both enrichment analyses were made using ExpressAnalyst and are sorted by P-value, obtained from Welch’s t-test. ( C ) Heatmap of differentially expressed IFN-related genes in COVID-19 PMNs and LDGs as compared to HC PMNs. RNA sequencing was performed on purified PMNs from healthy controls, mild COVID-19 and severe COVID-19, as well as LDGs from severe COVID-19. The heatmap was clustered by complete linkage and ordered by Spearman’s rank. FC = fold change .
https://doi.org/10.1371/journal.ppat.1012368.g001
Increased expression of inflammasome related genes in severe COVID-19 PMNs
In addition to the strong IFN-I signature, PMNs of severe COVID-19 upregulated several genes involved in inflammatory processes, such as the formation of inflammasomes. We further analyzed the differential expression of selected inflammasome related genes across all samples using RNA-seq ( Fig 2A ). PMNs of severe COVID-19 displayed higher levels of inflammasome genes such as NLRP3 and caspases 1, 4 and 5. LDGs did not display similar upregulation of inflammasome genes, with the notable exception IL-18 and NLRC4, which were not upregulated by PMNs. These findings prompted us to look more closely into PMN fractions between different disease states. Pathway analyses identified the inflammasome related NOD-like and RIG-like receptor signaling pathways among the most significantly overrepresented pathways, differentially expressed in severe COVID-19 PMNs versus HC PMNs (Figs 2B and S2B and S2C ) or mild COVID-19 PMNs (Figs 2C and S2D and S2E ). However, mild COVID-19 PMNs did not significantly differ from HC PMNs in their inflammatory profile ( S2F Fig ). The increased expression of selected IFN-I ( OAS1 , OAS2 , and IFIT1 ) and inflammasome related genes ( CASP1 , CASP5 , NLRC5 and NAIP ) between COVID-19 and HC PMNs was confirmed by RT-qPCR. However, some inflammasome related genes ( IL-1β , NLRP3 and NLRC4 ) were seemingly downregulated, although not statistically significant ( S3 Fig ).
( A ) Heatmap depicting selected differentially expressed inflammasome related genes from RNA sequencing performed in PMNs from HC, mild and severe COVID-19, as well as severe COVID-19 LDGs. Only the samples with the highest purity, determined by a cell fraction over 0.65 of neutrophils (identified by CIBERSORTx) are included. The heatmap was clustered by complete linkage and ordered by Spearman’s rank. ( B-C ) Ridgeline diagrams of overrepresentation analyses (ORA) according to KEGG database, depicting the top 10 enriched signaling pathways in PMNs during severe COVID-19 compared to ( B ) healthy controls and ( C ) mild COVID-19. ( D ) UMAP analysis of the COVID-19 Immune Atlas, which integrates 5 public COVID-19 PBMC single-cell transcriptomics datasets, created using CELLxGENE. (Top) UMAP showing the clustering of CD16+ cells (mature, FCGR3B expressing cells) and CD66b+ cells (immature, CEACAM8 expressing cells). Each dot represents a single cell colored according to the expression level of a selected gene. The color scale ranges from green (low expression) to purple (high expression). (Bottom) Pie chart summarizing the percentage of mature (black) and immature (blue) cells in the data. ( E ) The fraction of mature and immature neutrophils cells expressing inflammasome related genes identified in ( D ) are shown in bar graphs. For each gene, the proportion of expressing cells is shown in light blue, while the proportion of negative or not-expressing cells is shown in gray. Zoomed-in bar graph depicts the proportion of mature and immature cells expressing each gene.
https://doi.org/10.1371/journal.ppat.1012368.g002
Single cell sequencing data from the COVID-19 immune atlas, which integrates data from 5 independent studies analyzing COVID-19 PBMCs, confirmed our transcriptomic results ( Fig 2D ), from which a detailed gene by gene analysis of the most relevant inflammasome related genes is shown (Figs 2E and S4 ). Briefly, PYCARD gene coding for the ASC protein was expressed similarly in mature and immature neutrophils ( S4 Fig ), suggesting that both cell types may have ASC-dependent inflammasome forming capacity. However, most of the inflammasome gene expressions differed significantly and in the same manner as in our transcriptomic analysis.
Activation of neutrophil inflammasome related pathways during respiratory distress is not specific to COVID-19
We also reanalyzed the RNA-seq data generated by LaSalle et al . [ 8 ], focusing on neutrophil transcriptomics in patients with COVID-19 versus non-COVID-19 patients, and healthy controls. The non-COVID-19 patients exhibited acute respiratory distress and clinical suspicion for COVID-19. However, they tested negative for SARS-CoV-2 by PCR, unlike those classified as COVID-19 patients. Our analysis included IFN-α response, IL-1β production, TLR signaling, NLRP3 inflammasome, and pyroptosis pathways, using the Gene Ontology (GO) database; the NLR signaling pathway using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database; and inflammasome pathway using the REACTOME database ( Fig 3 ). These pathways were significantly enriched in COVID-19 patients, supporting our findings. Importantly, the genes from the above-mentioned pathways were also induced in non-COVID-19 patients, suggesting that these pathways represent a general neutrophil response to inflammatory stimuli rather than a COVID-19 specific response.
Bar graphs represent the activation levels of selected pathways and processes as identified by neutrophil transcriptomics. The analysis includes interpheron alpha (IFN-α) responses, interleukin (IL)-1β production, Toll-like receptor (TLR) signaling, NLRP3 inflammasome, and pyroptosis, as determined through the Gene Ontology (GO) database. The NOD-like receptor signaling pathway was investigated using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, and the inflammasome pathway was explored via the REACTOME database (more information in S1 Table ). The graphs compare the activation levels of these pathways in healthy controls (HC), non-COVID patients with similar symptoms (COVID-19 negative), and COVID-19 positive individuals. Statistical significance is denoted as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. P values were calculated with Kruskall-Wallis test.
https://doi.org/10.1371/journal.ppat.1012368.g003
Inflammasomes are activated in severe COVID-19 PMNs, but not directly by SARS-CoV-2
Given the strong upregulation of many inflammasome related genes during severe COVID-19, we assessed whether PMNs exhibit active inflammasome formation in vivo . To evaluate spontaneous inflammasome mediated cytokine secretion, fresh PMNs isolated from severe COVID-19 patients and HC were cultured ex vivo for 24 hours. We measured the levels of IL-1β and IL-18 in the supernatant and found that IL-1β secretion was significantly increased in the supernatant of severe COVID-19 PMNs compared to HC PMNs ( Fig 4A ), whereas the IL-18 levels did not differ significantly ( Fig 4B ). Additionally, since SARS-CoV-2 viral particles were previously implicated to induce inflammasome formation in macrophages [ 17 ], the IL-1β and IL-18 levels after HC PMNs exposure to SARS-CoV-2 were also assessed but no significant effects in the secretion of these cytokines were observed ( Fig 4C and 4D ).
PMNs were freshly isolated from blood and cultured at 2 million cells/ml ( A ) IL-1β and ( B ) IL-18 levels in 24 h cell culture supernatants from COVID-19 (n = 11 for IL-1β and 9 for IL-18) and HC PMNs (n = 6 for both). ( C ) IL-1β and ( D ) IL-18 levels in 24 h cell culture supernatant from PMNs exposed or non-exposed to purified SARS-CoV-2 viral particles (10 virus particles / PMN) (n = 3). ( E ) Caspase1 activity in PMNs following a 2 h stimulation with nigericin or purified SARS-CoV-2 viral particles (10 virus particles / PMN). For HC PMNs, n = 9 for mock and nigericin and n = 6 for SARS-CoV-2 exposure. For COVID-19 PMNs, n = 12 for mock and nigericin and n = 9 for SARS-CoV-2 exposure. *p < 0.05 and **p < 0.01. Data presented as mean ± SD. Tukey’s multiple comparisons test for mixed-effect analysis was applied for ( E ), meanwhile P values for ( A-D ) were calculated with the Mann-Whitney U-test.
https://doi.org/10.1371/journal.ppat.1012368.g004
The spontaneous secretion of IL-1β by COVID-19 PMNs suggests that these cells are actively producing and releasing IL-1β through inflammasome formation which is dependent on caspase1 activity [ 32 ]. We assessed caspase1 activity in response to the second signal required for inflammasome activation, induced by nigericin, and observed increased caspase1 activity in severe COVID-19 PMNs compared to HC PMNs ( Fig 4E ). These findings suggest that severe COVID-19 PMNs have an increased capacity for inflammasome activation, potentially due to an existing priming signal during acute disease in vivo . However, no significant difference in caspase1 activity between non-exposed and virus-exposed PMNs were observed ( Fig 4F ), indicating that caspase1 activation in COVID-19 PMNs is not directly triggered by the virus.
Type I IFNs prime PMNs for inflammasome activation
Since PMNs from COVID-19 patients concomitantly display a strong IFN-I signature ( Fig 1B and 1C ) and an increased propensity for inflammasome activation, we hypothesized that IFN-I could act as the priming signal for PMN inflammasomes during COVID-19. Isolated HC PMNs were stimulated ex vivo with exogenous IFN-I and the well-described inflammasome priming (1 st signal) and activator (2 nd signal) agents LPS and nigericin, respectively [ 33 , 34 ]. After stimulation, both priming signals induced pro-IL-1β (31 kDa) in the cell lysates, followed by the release of active IL-1β (17 kDa) into the supernatant in response to nigericin ( Fig 5A ), confirming the ability of IFN-I to prime PMNs for inflammasome activation.
Isolated HC or COVID-19 PMNs were non-stimulated or stimulated 4h with IFN-I (combination of 2.7*10 4 IU/ml IFN-α and IFN-β) or 20 ng/ml LPS (1 st signal), followed by 4h with 2.5 μM nigericin or purified SARS-CoV-2 (10,1 virus/PMNs) (2 nd signal). Then, ( A ) western blot of pro-IL-1β (31 kD) and active IL-1β (17 kD) was performed from HC PMNs supernatant and cell lysates, ( B ) IL-1β (n = 5 HC PMN and 9 COVID-19 PMN) and ( C ) MPO (n = 5 HC PMN and 9 COVID-19 PMN) were measured from supernatants by ELISA. ( D-E ) Effect of inflammasome inhibitor MCC950 (2 μg/ml, added simultaneously with nigericin) on IL-1β secretion in ( D ) HC and ( E ) severe COVID-19 PMN supernatant (n = 3). ( F ) LDH and ( G ) IL-8 in HC and severe COVID-19 PMN supernatants (n = 3). ( H-K ) RT-qPCR of selected mRNAs in IFN-I or LPS-primed HC and COVID-19 PMNs (n = 6–8 HC PMN and 7–10 COVID-19 PMN). ( L-M ) HC PMNs were stimulated with high dose IFN-I (2.7*10 5 IU/ml), normal dose IFN-I (2.7*10 4 IU/ml) and 20 ng/ml LPS. After 4 hr stimulation caspase1 activity was measured using median fluorescence intensity (MFI) of FAM-FLICA by flow cytometry ( L , n = 5, representative histogram of one donor shown) and after 24 hr stimulation IL-1β release was measured by ELISA ( M, n = 5 ) . P values calculated with Kruskall-Wallis test for the comparison between treatments by group (HC or COVID-19 PMNs), and Mann-Whitney test for the comparison between HC and COVID-19 PMNs by individual treatment for ( B-G ), and Two-way ANOVA Tukey’s multiple comparisons test for ( B, H-K ). The treatments in L-M were compared to mock by one-way ANOVA for repeated measures, corrected for multiple comparisons with the two-stage step-up method of Benjamini, Krieger and Yekutieli. *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001. Data presented as mean ± SD.
https://doi.org/10.1371/journal.ppat.1012368.g005
To assess inflammasome formation in circulating neutrophils during COVID-19, PMNs from HC and COVID-19 patients underwent similar stimulation assays as above, followed by IL-1β measurement from supernatants by ELISA. In addition, to further assess the role of SARS-CoV-2 virus particles in neutrophil inflammasome activation, HC PMNs were cultured in the presence of purified viruses (10 infectious units/PMN). HC PMNs responded to both LPS and IFN-I by increasing their IL-1β secretion, which was exponentiated after exposure to nigericin ( Fig 5A and 5B ), confirming the ability of IFN-Is to prime for inflammasome assembly in PMNs, albeit less efficiently than LPS. Furthermore, as expected, the ability of IFN-I to prime for nigericin-mediated inflammasome activation was dependent on the IFN-I receptor IFNAR1 for both IFN-α and IFN-β ( S5A Fig ).
Interestingly, COVID-19 PMNs produced less IL-1β than HC PMNs upon exogenous inflammasome activation primed by either LPS or IFN-I, while SARS-CoV-2 particles did not have any effect on PMN inflammasome activation ( Fig 5B ). As with 24 h cultures ( Fig 4B ), we did not detect any significant changes in IL-18 secretion in either HC or COVID-19 PMNs ( S5B Fig ). However, the release of myeloperoxidase (MPO), used as a marker of degranulation and/or NETosis, in response to nigericin was similar between COVID-19 PMNs and HC PMNs ( Fig 5C ), and therefore the observed diminished IL-1β release by COVID-19 PMNs is not due to general cellular inertia but may be specific to the ex vivo induced inflammasome pathway. Furthermore, additional stimulation assays in the presence of the NLRP3 inhibitor MCC950 ( Fig 5D and 5E ) and caspase1 inhibitor YVAD ( S5C Fig ) confirmed that induced IL-1β secretion is dependent on canonical NLRP3 inflammasome activation. Unlike IL-1β ( S5D Fig ), increased IL-18 secretion was not detectable even after 24 h stimulation ( S5E Fig ). Furthermore, the observed residual IL-18 was not affected by inflammasome inhibitors, suggesting its secretion to be unrelated to inflammasome activity in PMNs.
We further assessed the specificity of inflammasome activation by measuring LDH and IL-8 levels in the supernatants from the same cells and under the same experimental conditions as shown in Fig 5D and 5E . The measurements of the former were done to assess inflammasome mediated cell death by pyroptosis in response to nigericin, while the latter was assessed to demonstrate the responsiveness of PMNs to an inflammasome unrelated inflammatory cascade. As with IL-1β secretion, COVID-19 PMNs were less responsive than HC PMNs to nigericin- and LPS-mediated LDH ( Fig 5F ) and IL-8 ( Fig 5G ) release, respectively. This suggests that COVID-19 PMNs are generally poorly responsive to inflammatory stimuli.
To examine this reduced responsiveness to external inflammatory priming, we evaluated the inflammasome related gene expression following ex vivo stimulation with IFN-I or LPS (Figs 5H–5K and S5F–S5I ). OAS1 gene, an interferon stimulated gene (ISG), showed significant upregulation by IFN-I in COVID-19 PMNs as compared to HC PMNs ( Fig 5H ), while the inflammasome related genes IL-1β ( Fig 5I ), CASP1 ( Fig 5J ) and NLRC5 ( Fig 5K ) were more efficiently induced in HC PMNs than COVID-19 PMNs. This suggests that the inflammasome defect in COVID-19 PMNs is at the transcriptional level when using IFN-I as the priming factor, while high OAS1 gene expression indicates transcriptional defect is restricted to individual genes.
Next, we investigated whether IFN-I could also activate caspase1 directly without the 2 nd signal to boost inflammasome activation. In addition to treating HC PMNs with IFN-I and LPS as in previous experiments, we also included another group with a higher dose of IFN-I (ten-fold) and assessed caspase1 activity after 4 hours with FAM-FLICA, a fluorescent caspase1 reactive dye ( Fig 5L ) as well as IL-1β release after 24 hours by ELISA ( Fig 5M ). Results showed that similar to LPS, high-dose IFN-I induced significant caspase1 activity, which was not observed with the normal IFN-I concentration. Despite this, the normal IFN-I concentration still resulted in increased IL-1β levels in the supernatant. Taken together, these findings suggest that the 2 nd signal is not essential for inflammasome activation by IFN-I and that IL-1β release is a more sensitive method of detecting inflammasome activity as compared to caspase1 activity in our assay setup. Thus, these results can also explain our previous observation of increased spontaneous release of IL-1β by COVID-19 PMNs even though significant caspase1 activity is only detected after nigericin-mediated boosting in vitro ( Fig 4A and 4E ).
Association between ex vivo inflammasome activation and disease severity
Our analysis of the association between ex vivo inflammasome activation (caspase1 activity and IL-1β release) and clinical markers of disease severity, including neutrophil responses, revealed intriguing links. Calprotectin is a marker of neutrophil activation or death [ 35 ] but also potentially activates the inflammasome [ 36 ]. A significant positive correlation between calprotectin plasma levels and PMN caspase1 activity ( Fig 6A and 6B ) underscores this latter possibility and highlights the interplay between inflammation and inflammasome activation in PMNs of COVID-19 patients. Furthermore, the negative association of PMN IL-1β levels (after ex vivo stimulation with IFN and nigericin) with disease severity (WHO ordinal scale, Fig 6A ) and patient neutrophil counts ( Fig 6A and 6C ) supports the exhaustion hypothesis, wherein PMNs from severe COVID-19 patients may be less responsive to stimuli due to prior in vivo activation. While these findings provide intriguing insights into the complex interplay between calprotectin release, caspase1 activity, and inflammasome activation in COVID-19, additional research is required to further elucidate these connections.
( A ) Spearman’s correlation matrix depicting the relationships among clinical parameters and results of ex vivo experimentation. For the WHO ordinal scale, the baseline parameters were used. ( B-C ) Linear regression analysis demonstrating the associations between: ( B ) Positive association between PMN Caspase1 activity, measured after ex vivo nigericin stimulation, and the levels of Calprotectin in the matched patient’s peripheral blood; ( C ) Negative association between e x vivo stimulated PMN IL-1β levels (IFN+Nig) and the blood neutrophil count in matched patients at the time of sampling (n = 12). LOS = length of stay . WHO = World Health Organization . Min = minimum . Casp1 = caspase1 . LPS or IFN + nig = lipopolysaccharide or type I interferon + nigericin ex vivo stimulation .
https://doi.org/10.1371/journal.ppat.1012368.g006
LDGs differ from PMNs in their ability to release IL-18 upon inflammasome activation
Transcriptomic analysis presented above revealed a distinct lack of IFN-I responsive and inflammasome related gene expression in LDGs as compared to PMNs of severe COVID-19 patients. This suggested that inflammasomes are not similarly regulated in LDGs as compared to PMNs during COVID-19. To assess the inflammasome forming capacity of LDGs, we conducted ex vivo stimulation assays using LDGs isolated from COVID-19 patients, similar to the approach used for PMNs described earlier. Like PMNs, IL-1β secretion by LDGs was elevated in the presence of a priming signal (IFN-I or LPS), which exponentially increased when the inflammasome activation signaling molecule nigericin was added ( Fig 7A ). Contrary to PMNs and in line with the transcriptomics data, an increased IL-18 secretion was detected ( Fig 7B ). Additionally, the secretion of both ILs by LDGs was inhibited in the presence of inflammasome specific inhibitors MCC950 and YVAD ( Fig 7A and 7B ).
These findings suggested that IFN-I can prime for inflammasome activation also in LDGs. Furthermore, the ability to release IL-18 upon neutrophil inflammasome activation varies based on cellular maturation state. To explore this further, we conducted in vitro stimulation studies using differentiated HL-60 cells, an immature neutrophil-like model [ 37 ]. Similar to LDGs from COVID-19 patients, HL-60 displayed comparable IL-18 secretion pattern upon LPS or IFN-I stimulation and nigericin-induced activation. Notably, their IL-1β release was only detected with LPS priming ( Fig 7C and 7D ). Furthermore, transcriptomic analysis revealed an upregulation of inflammasome related genes upon differentiation ( Fig 7E ). Overall, these findings suggest that neutrophils may lose the ability to secrete IL-18 in response to inflammasome activity during maturation, and increased release of neutrophil-derived IL-18 occurs primarily in disease states associated with extensive granulopoiesis and increased immature granulocyte counts in the blood, like COVID-19 [ 38 ].
( A-D ) Isolated COVID-19 LDGs or HL-60 cells (differentiated for 5 days with 1% DMSO) were non-stimulated or stimulated 4h with IFN-I or LPS (1 st signal), followed by 4h with nigericin (2 nd signal) in the presence or absence of inflammasome inhibitors MCC950 or YVAD as previously. Secretion of ( A, C ) IL-1β and ( B, D ) IL-18 were measured from the supernatants by ELISA (n = 2 for LDGs and 3–5 for HL-60). *p < 0.05 and **p < 0.01. P values calculated with Kruskal-Wallis test. Data presented as mean ± SD. ( E ) Volcano plot of differentiated vs undifferentiated HL-60 cells gene expression from GSE93996, with inflammasome related genes marked in blue. Only significant DE genes are shown (adjusted p value < 0.05).
https://doi.org/10.1371/journal.ppat.1012368.g007
Neutrophils are recruited to the lungs in SARS-CoV-2 infected mice
Hamsters and human ACE2 expressing mice infected with SARS-CoV-2 develop pulmonary inflammation including neutrophil recruitment [ 39 – 41 ]. To further assess the role of neutrophils in COVID-19, we utilized a recently developed SARS-CoV-2 mouse model [ 18 ]. This model employs the MaVie strain, serially passaged in mouse lungs and causing pneumonia like human COVID-19 in wild-type BALB-C mice [ 18 ]. Infected mice started losing weight by day 2 post-infection, with some mice reaching the clinical endpoint of 20% weight loss by day 4 ( S6A Fig , includes animals from 4 independent infection experiments, details of animal usage in S3 Table ). The first experiments were performed to study infection kinetics, histopathology and Ly-6G+ neutrophil accumulation in lungs.
Viral loads, as assessed by RT-qPCR and titration of infectious virus, were significantly higher at 2 dpi than at 4 dpi ( Fig 8A and 8B ), and viral antigen expression, widespread at 2 dpi in bronchioles and alveoli, matched this pattern (Figs 8C and S6D ). The extensive viral replication at 2 dpi was associated with degeneration of infected epithelial cells, most prominent in the respiratory epithelium, accompanied by neutrophil (Ly6G+) infiltration ( S6D Fig ). An increase in neutrophil numbers in the lungs of infected mice was observed as represented by increased Ly6G+ neutrophil/lymphocyte ratio and total Ly6G+ cell counts as assessed by flow cytometry of lung single cell suspension ( Fig 8D and 8E ; the gating strategy for Ly6G+ neutrophils is shown in S6B Fig ). A significant increase in the number of neutrophils in the lungs of infected mice compared to PBS-inoculated mice was also confirmed in situ , by morphometrical quantification of lungs sections stained for Ly6G (Figs 8F and S6D ). Detailed information on the histological and immunohistochemical features is provided in S3 Table .
Female BALB/c mice were intranasally inoculated with 5*10 5 TCID50 SARS-CoV-2 MaVie strain or PBS as control and euthanized at 2 dpi or 4 dpi. ( A ) RNA was isolated from lungs and subjected to RT-qPCR targeting viral subE and GAPDH as housekeeping gene. The relative expression of subE was measured using the comparative Ct method as compared to mock-infected control (in which subE was undetectable but set to 40 Ct) **** p < 0.0001. P values calculated with Welch’s t-test. ( B ) Infectious virus was calculated from supernatants of lung single cell suspensions of infected mice as fluorescence focus forming units (FFU) in Vero E6 cells. * p < 0.05. P values calculated with Mann-Whitney test. ( C ) Quantification based on morphometric analysis that determines the area of immunolabelling for SARS-CoV-2 nucleoprotein in relation to total tissue area. ( D ) Quantification of Ly6G neutrophil/lymphocyte ratio in lung single cell suspensions by flow cytometry. ( E ) Quantification of total Ly6G neutrophil counts in lung single cell suspensions by flow cytometry. ( F ) Quantification of Ly6G based on morphometric analysis that determines the area of immunolabelling for Ly6G in relation to total tissue area in mock-infected controls. ( G ) Quantification of median fluorescence intensity (MFI) of CD11b expression in ly6G neutrophils by flow cytometry. Representative histograms of CD11b expression in Ly6G+ neutrophils is shown. P values for C-G were calculated with ordinary one-way ANOVA using Tukey’s multiple comparison correction. Black line represents the mean. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. ( H ) Fluorescent nuclear staining of representative magnetic-bead isolated Ly6G neutrophils by Hoechst33342. Panels A, C and F are representative of two independent experiments.
https://doi.org/10.1371/journal.ppat.1012368.g008
Furthermore, flow cytometry revealed diminished surface expression of the maturation and activation marker CD11b in Ly6G+ neutrophils of infected mice both at 2 and 4 dpi as compared to PBS controls ( Fig 8G ). However, morphologically, neutrophils from infected mice were similar to those in the PBS control mice in displaying equally multilobed nuclei (DNA staining of isolated Ly6G+ neutrophils shown in Fig 8H ; a representative flow cytometry histogram showing the purity of Ly6G+ neutrophils after isolation is provided in S6C Fig ). This suggests that the reduced expression of CD11b on the surface of neutrophils from infected mice was not the consequence of an accumulation of CD11b-negative immature neutrophils but rather an activation-related phenomenon, such as shedding or internalization.
Neutrophils from SARS-CoV-2 infected mice display IFN-I dependent caspase1 activation
Next, we investigated whether neutrophils of SARS-CoV-2 infected mice show increased caspase1 activation. We utilized the fluorescent FAM-FLICA caspase1 reactive probe in conjunction with Ly6G+ neutrophil staining to detect caspase1 activity in neutrophils by flow cytometry. Indeed, we observed significantly an increased median fluorescent intensity of FAM-FLICA in Ly6G+ neutrophils at 4 dpi, but not at 2 dpi, compared to PBS-inoculated controls ( Fig 9A ). This finding suggests increased neutrophil caspase1 activity at latter stages of the infection, concomitant with decreased viral loads.
Female BALB/c mice were intranasally inoculated with 5* 10 5 TCID50 SARS-CoV-2 MaVie strain or PBS as control. ( A ) Lungs were harvested at 2 and 4 dpi and single cell suspensions stained with FAM-FLICA and Ly6G antibody followed by flow cytometric analysis. FAM-FLICA median fluorescence intensity (MFI) was recorded in Ly6G+ neutrophils (n = 4, representative histogram image shown). * p < 0.05, ** p < 0.01. P values calculated with one-way ANOVA using Tukey’s multiple comparisons. ( B-E ) Ly-6G+ neutrophils isolated from lung single cell suspensions based on positive selection with magnetic beads. ( B - D ) RNA was isolated and subjected to transcriptomic analysis by RNA-seq. ( B ) Principal component analysis (PCA) of the PBS-inoculated control and SARS-CoV-2 infected mice lung neutrophil RNA-seq samples. ( C ) Heatmap of the top differentially expressed genes (DEGs). ( D ) Volcano plots of DEGs between neutrophils isolated from SARS-CoV-2 infected mice versus uninfected PBS-inoculated mice. Blue points represent significant terms (adjusted p-value < 0.05), while smaller gray points represent non-significant terms. Relevant inflammasome and interferon related genes are shown with larger and darker blue points. ( E ) Caspase1 activity in isolated mice neutrophils following a 2 h stimulation with nigericin was assessed by a bioluminescence method (Caspase-Glo 1 Inflammasome Assay). ( F-I ) Mice were intraperitoneally inoculated with 250 μg anti-IFNAR or IgG1 isotype control directly after infection with SARS-CoV-2 and lung neutrophils isolated at 2 dpi (including also intranasally PBS-inoculated control mice without intraperitoneal injection). ( F ) Caspase1 activity was assessed following a 2 h stimulation with nigericin by bioluminescence method. ( G-I ) RNA was isolated from isolated neutrophils and fold change mRNA expressions of ( G ) Oasl2, ( H ) Caspase1 (Casp1) and ( I ) IL-1β (Il1b) was assessed by RT-qPCR in isotype control and anti-IFNAR treated infected mice as compared to mock-infected control mice. *p < 0.05, **p < 0.01 and ***p < 0.001. P values for A, E and F panels were calculated with ordinary one-way ANOVA using Tukey’s multiple comparisons correction, while Welch’s t-test was used for panels G-I. Data presented as mean ± SD.
https://doi.org/10.1371/journal.ppat.1012368.g009
The potential role of IFN-I in mediating caspase1 activity in neutrophils of infected mice was initially assessed by isolating neutrophils from the lungs of infected mice at 2 and 4 dpi, as well as from non-infected mice, for transcriptomic analysis by RNA-seq. PCA showed differences between neutrophils from infected and non-infected mice, with slight variation between the 2 and 4 dpi time points ( Fig 9B ). These differences were reflected in many DEGs, including several IFN-I responsive and inflammasome related genes, which showed strong upregulation at 2 dpi with slightly lower but still significantly elevated levels at 4 dpi, compared to non-infected mice (highlighted in the DEG heatmap; Fig 9C ). The volcano plot ( Fig 9D ) provided a comprehensive view of the DEG pattern between neutrophils from SARS-CoV-2 infected and mock-infected mice. In addition to confirming the upregulation of IFN-I responsive and inflammasome related genes observed in the heatmap, the plot revealed a broader transcriptional response to viral infection with several additional DEG.
Having established a robust IFN-I transcriptional signature in neutrophils from SARS-CoV-2 infected mice we wanted to assess whether they possess increased propensity for nigericin-induced caspase1 activation, similar to human PMNs after exogenous priming by IFN-I. Neutrophils from infected mice, harvested at 2 and 4 dpi, displayed increased caspase1 activity upon nigericin stimulation, compared to neutrophils from non-infected mice (as displayed by both bioluminescence and FAM-FLICA fluorescence assays; Figs 9E and S7A , respectively). To directly assess the role of IFN-I nigericin-induced caspase1 activity, we inoculated mice with an IFN-I blocking anti-IFNAR monoclonal antibody or an isotype control antibody post-infection. Remarkably, neutrophils from anti-IFNAR treated mice showed diminished nigericin-induced caspase1 activity (Figs 9F and S7A ). Furthermore, as expected due to their typical IFN-responsiveness, oasl2, caspase1 and IL-1β gene expressions were lower in anti-IFNAR treated than in isotype treated mice ( Fig 9G–9I ). Taken together, the results indicate that IFN-I is responsible for the increased caspase1 activity in neutrophils of infected mice.
Blocking IFN-I signaling did not significantly alter virus replication, virus-induced pathological changes, neutrophil CD11b expression or neutrophil caspase1 activity without exogenous stimuli ( S7B–S7D , S7H and S7I Fig and S3 Table ). Neutrophil counts indicated a significant increase in neutrophil accumulation in the lungs of anti-IFNAR treated mice; however, this was not confirmed by neutrophil/lymphocyte ratio or morphometry (S7E-S7G Fig). Interestingly, regardless of treatment, some neutrophils in infected mice displayed degeneration and NETosis, evidenced by histone H3cit staining ( Fig 7J ; S3 Table ).
Neutrophils, the largest cell population of the host immune system, are rapidly recruited to sites of infection and play an important role in orchestrating an early immune response [ 42 , 43 ]. The relevance of neutrophils in viral infections became increasingly apparent during the COVID-19 pandemic, as they have been shown to be key mediators of the observed pathological processes [ 44 ].
This study sheds light on the potential involvement of the inflammasome pathway in COVID-19, particularly by demonstrating its activation in mature neutrophils during SARS-CoV-2 infection. Our investigation of the inflammatory profile of neutrophils as the dominant population of peripheral blood polymorphonuclear cells (PMNs) revealed an increased ability of neutrophils from severe COVID-19 patients for inflammasome assembly as evidenced by their transcriptional profile, spontaneous release of IL-1β, and elevated caspase1 activity. These findings are consistent with previous reports indicating activation of the NLRP3 inflammasome and ASC specks in circulating neutrophils during acute COVID-19 [ 14 , 16 ]. Furthermore, despite showing increased caspase1 activity, neutrophils from COVID-19 patients exhibited diminished soluble IL-1β production upon exogenous activation of the NLRP3 inflammasome pathway compared to healthy controls, which suggests that this pathway is “exhausted” due to prior activation during the disease. Mechanistically, our findings show that IFN-I, elevated in COVID-19 patients [ 45 , 46 ], can prime inflammasome formation in neutrophils. Transcriptomic analyses revealed that circulating neutrophils during severe COVID-19 show increased expression of IFN-responsive genes, suggesting inflammasome priming by IFN-I also in vivo during COVID-19 [ 47 ]. Furthermore, the study found that immature neutrophils, which are prevalent in low-density granulocyte fraction (LDGs), exhibit unique inflammasome gene expression and outcomes compared to mature neutrophils (PMNs). Distinctively from PMNs, LDGs do not display the IFN-I signature or upregulation of major inflammasome related genes, which indicates their lower responsiveness to IFN-I during COVID-19. However, since we were able to show that LDGs can form inflammasomes when stimulated by IFN-I ex vivo , their lower responsiveness is probably due to LDGs not being similarly exposed to IFN-I as compared to PMNs during COVID-19.
SARS-CoV-2 infected mice also showed increased neutrophil caspase1 activity, reversible by an IFN-I receptor (IFNAR) blocking antibody. Transcriptional analysis revealed a robust IFN-I signature and elevated expression of inflammasome genes encoding for caspase1 and IL-1β in neutrophils of infected mice, which were also inhibited by blocking IFNAR signaling, suggesting that IFN-I may also prime for inflammasome activation in mice. Notably, the anti-IFNAR treatment did not affect neutrophil recruitment or NETosis, which is consistent with another COVID-19 model using transgenic human ACE2, where IFNAR knockout inhibited recruitment of monocytes and lymphocytes, but not neutrophils, to infected lungs [ 48 ].
Inflammasomes were first studied in macrophages, revealing many molecular mechanisms regulating inflammasome assembly [ 49 ]. Macrophage inflammasome activation has emerged as a major factor also in COVID-19 [ 17 ]. Interestingly, macrophage inflammasome activation was recognized to be IFN-I mediated in an experimental rhesus macaque COVID-19 model [ 50 ]. However, due to the abundance of neutrophils compared with cells of monocyte/macrophage lineage [ 51 , 52 ], the significance of neutrophil inflammasomes in COVID-19 is likely underestimated. Our results highlight inflammasomes as an additional important inflammatory mechanism in neutrophils [ 14 ], complementing their role in phagocytosis, reactive oxygen species generation, degranulation, and NETosis [ 31 ].
SARS-CoV-2 can directly activate inflammasomes in cells of the monocyte/macrophage lineage [ 17 ]. Our study investigated whether SARS-CoV-2 can provide the first or second signal for inflammasome activation in neutrophils. However, we found no evidence of direct virus-induced inflammasome activation in neutrophils. The difference between macrophages and neutrophils in their susceptibility to SARS-CoV-2 could depend on many factors. Both cell types express ACE2, the receptor for SARS-CoV-2, but may differ in ACE2 expression levels [ 53 ]. Furthermore, the intracellular environment of macrophages is better suited for viral replication [ 54 ], while neutrophils focus on phagocytosis and antimicrobial responses [ 31 , 55 ]. Additionally, pathogen opsonization can trigger inflammasomes in macrophages [ 56 ] but is not a primary function of neutrophils. Therefore, our findings suggest neutrophil inflammasome activation in response to SARS-CoV-2 likely results from interactions with infected and/or dying cells in the lungs, rather than direct virus activation. To note, whether SARS-CoV-2 can induce neutrophil inflammasomes through immune complex-mediated mechanisms, as seen in monocytes/macrophages [ 17 ] remains to be determined.
In this study, we demonstrated IFN-I as the first signal for NLRP3 inflammasome activation in neutrophils. While prior research has explored IFN-inflammasome crosstalk [ 57 ], priming capacity of IFN-I remained unclear. While IFN-I promotes inflammasomes in epithelial cells [ 58 ] it can also dampen IL-1β in macrophages [ 59 ]. Plausibly, initial IFN-I exposure may upregulate inflammasome genes, whereas prolonged activity could hinder IFN-I signaling via “negative feedback” loop, in line with our findings of inflammasome exhaustion in circulating neutrophils of severe COVID-19 patients. It should be noted that several SARS-CoV-2 encoded proteins have been shown to inhibit IFN-I signaling [ 60 ]. However, no evidence suggests that neutrophils can be infected by SARS-CoV-2 and therefore it seems unlikely that such direct virus mediated effects could play a role in the observed neutrophil unresponsiveness to IFN-I.
The dualistic nature of the IFN-I response in COVID-19 has been recognized previously. It seems that a strong initial IFN-I response to SARS-CoV-2 is more likely to result in asymptomatic or mild COVID-19 whereas a decreased initial IFN-I activity, due to e.g. genetic defects or increased levels of IFN-I autoantibodies, can lead to more severe COVID-19 [ 61 ]. This initial beneficial effect of IFN-I is probably due to its ability to limit viral replication at early stages of the infection. However, at later stages of the disease IFN-I can be detrimental by promoting inflammatory pathways instead of direct antiviral effects [ 62 ]. Thus, similarly to the IFN-I response in general, the role of neutrophil inflammasomes in development and severity of COVID-19 might be dualistic in nature with an initial protective effect while damaging when sustained for prolonged periods.
Our study demonstrated a strong association between PMN caspase1 activity and plasma levels of calprotectin, a marker of neutrophil activation. It is of interest to note that calprotectin can also promote inflammasome activity in neutrophils [ 63 , 64 ]. Therefore, in addition to IFN-I discussed in this study, it is possible that calprotectin also contributes to neutrophil inflammasome formation during COVID-19. Additionally, increased disease severity, as assessed by the WHO ordinal scale, was significantly linked to PMNs being less responsive to ex vivo IFN-induced inflammasome activation. Thus, these results suggest that neutrophil inflammasomes would play a role in disease severity, rather than being protective in COVID-19.
Our study also unveiled distinct gene profiles in LDGs and PMNs from severe COVID-19 patients. LDGs exhibited upregulation of genes related to DNA replication and cell cycle, indicating immaturity, and confirming our prior findings [ 7 ]. Conversely, PMNs displayed heightened NLR signaling, suggesting a robust response to pathogens. While our study compared PMNs and LDGs, and the COVID-19 Immune Atlas single cell analysis represented a broader classification of mature and immature neutrophils, the alignment of our results with the atlas provides further support for the distinct characteristics of these two neutrophil populations in severe COVID-19. Notably, IL-18 gene expression and secretion after ex vivo stimulation were higher in LDGs than PMNs. To note, PMN’s lack of IL-18 secretion is not due to lack of protein, as they constitutively express significant amounts intracellularly [ 65 ]. This indicates a similarity between LDGs and monocytes/macrophages in inflammasome mediated IL-18 processing, possibly lost during neutrophil maturation.
The present study has some limitations worth discussing. Firstly, the relatively small human sample size may limit the generalizability of the findings. While RNA-seq provided valuable insights into gene expression profiles of PMNs and LDGs, we did not perform functional validation of the identified pathways in this study. Regarding our experimental SARS-CoV-2 disease model, the high virus input might trigger robust immune responses that differ from typical human infections, and the short-lived virus replication in the applied model does not capture the effect of prolonged antigen exposure or the complex inflammatory milieu seen in human cases. Importantly, our results do not directly assess the role of neutrophil inflammasomes in COVID-19 pathogenesis in humans or in the animal disease model. Further studies are therefore needed to understand the relative contribution of neutrophil inflammasomes in COVID-19 disease progression, compared to the better described macrophage inflammasomes as well as to other inflammatory pathways engaged by neutrophils such as degranulation, reactive oxygen species production and NETosis. Furthermore, due to the ubiquitous expression of IFNAR, the observed inhibitory effects on neutrophil inflammasome activity by IFNAR blockade does not exclude the possibility that IFN-I could promote neutrophil inflammasome formation by indirect effects such as regulating the interplay between neutrophils and other immune cells or stimulating the release of pro-inflammatory cytokines by other cell types. In addition, investigating the effects of IFNAR blockade in other time points than the chosen 2 dpi might have been more valuable in revealing its effects on viral replication and neutrophil accumulation in the infected lungs. Finally, since the prominent role of neutrophils in the immune response to viral infections is widely recognized [ 42 , 43 ] and it would be valuable to compare these findings to neutrophil responses in other viral respiratory infections.
Taken together, our findings provide valuable insights into neutrophil involvement in COVID-19 and possibly other viral respiratory infections. However, further research is needed to fully grasp the role of neutrophil inflammasomes in COVID-19 pathogenesis. This increased understanding may facilitate the development of targeted treatment approaches for COVID-19. For example, pharmacologically targeting the inflammasome pathway in neutrophils with novel inhibiting molecules [ 66 ], may help mitigate the exaggerated inflammatory response observed in severe cases. The next steps involve validating the pathways and genes identified as potential therapeutic targets and assessing their COVID-19 specificity. Prospectively, these strategies could be extended to address upcoming respiratory virus pandemics, where neutrophils and inflammasomes provide major pathogenic contributions.
Supporting information
S1 fig. comparison of gene expression in granulocyte populations of covid-19 patients using rna-seq analysis..
( A ) Deconvoluted RNA-seq data. The cellular composition in isolated PMN and LDG fractions was estimated using CIBERSORTx through the identification of cell populations based on RNA-seq. The bar plots in the figure represent the cell composition of each RNA-seq sample, offering insights on sample purity. ( B ) Heatmap of the top 118 differentially expressed genes between PMNs from healthy controls, mild and severe COVID-19, as well as LDGs from severe disease, identified by unsupervised ICGS analysis based on correlation, using AltAnalyze software. IFN-related genes, identified by GENESHOT, are shown in bold.
https://doi.org/10.1371/journal.ppat.1012368.s001
S2 Fig. Enriched differentially expressed genes and pathways in severe COVID-19 PMNs and LDGs.
( A-B ) Volcano plots of DEGs between severe COVID-19 PMNs versus ( A ) severe COVID-19 LDGs and ( B ) HC PMNs. ( C-D ) Volcano plots of enriched gene sets in severe COVID-19 PMNs versus ( C ) HC PMNs and ( D ) severe COVID-19 LDGs, using KEGG database. Each point represents a single gene set, where the x-axis measures its odds ratio, while the y-axis shows its -log10(p-value). ( E-F ) Volcano plots of ( E ) severe COVID-19 PMNs versus mild COVID-19 PMNs and ( F ) mild COVID-19 PMNs vs HC PMN. For all panels, blue points represent significant terms (adjusted p-value < 0.05), while smaller gray points represent non-significant terms. DEG = differentially expressed genes .
https://doi.org/10.1371/journal.ppat.1012368.s002
S3 Fig. Differential expression of interferon and inflammasome related genes in PMNs during COVID-19.
RNA was extracted from isolated HC PMNs (n = 8–13) versus severe COVID-19 PMNs (n = 29–32) and subjected to comparative RT-qPCR using specific primers for OAS1, OAS2, IFIT1, IFI16, caspase1, caspase5, IL1B, NLRC4, NLRC5, NLRP3 and NAIP. *p < 0.05, **p < 0.01, ***p < 0.001 and **** p < 0.0001. P values calculated with Mann-Whitney U-test. Data presented as mean ± SD.
https://doi.org/10.1371/journal.ppat.1012368.s003
S4 Fig. Expression of inflammasome related genes in mature and immature neutrophils from COVID-19 PBMCs.
The fraction of mature and immature neutrophils cells expressing 17 inflammasome related genes identified in Fig 2D (shown in black and blue, respectively) are shown in a bar graph. For each gene, the proportion of expressing cells is shown in light blue, while the proportion of negative or not-expressing cells is shown in gray. Zoomed-in bar graph depicts the proportion of mature and immature cells expressing each gene.
https://doi.org/10.1371/journal.ppat.1012368.s004
S5 Fig. Ex vivo stimulation of isolated PMNs.
( A ) HC PMNs (1 million/ml) were primed for 4 hr by low dose IFN-α, low dose IFN-β (both 2.7*10 3 IU/ml) or 20 ng/ml LPS followed by 2.5 μM nigericin activation for 4 hr. IL-1β release was measured by ELISA and the assays were performed in the presence of either α-IFNAR1 or mouse IgG as control (both 100 μg/ml) (n = 5). *p < 0.05 and **p < 0.01. P values calculated using two-way ANOVA with Šídák multiple comparison test. ( B ) IL-18 (n = 2–3 HC PMN and 3 COVID-19 PMN) was measured from supernatants by ELISA following LPS or IFN-I priming (4 h) and subsequent nigericin activation (4 h). ( C-E ) Effect of different inflammasome specific inhibitors in cytokine secretion. ( C ) Effect of inflammasome inhibitor MCC950 (2 μg/ml) and YVAD (20 μg/ml) on LPS or IFN-I primed (4 h) and nigericin activated (4 h) IL-1β secretion in the supernatant of healthy control PMNs (n = 8). ( D-E ) Effect of inflammasome inhibitor MCC950 (2 μg/ml, added simultaneously with nigericin) on LPS or IFN-I primed (4 h) and nigericin activated (20 h). *p < 0.05, **p < 0.01, ***p < 0.001, **** p < 0.0001. P values calculated with Kruskal-Wallis test. Data presented as mean ± SD. IFN = interferon type I , LPS = lipopolysaccharide , Nig = nigericin , YVAD = tetrapeptide caspase1 inhibitor Tyr-Val-Ala-Asp . ( F-I ) Gene expressions in HC and COVID-19 PMNs after LPS or IFN-I stimulation. A comparison of gene expression in isolated healthy control PMNs versus COVID-19 PMNs after ex vivo stimulation with LPS or IFN-I. Extracted RNA was subjected to comparative RT-qPCR using specific primers for NLRP3, NLRC4, NAIP and CASP5 (n = 4–8 for HC PMN and 6–9 for COVID-19 PMN). *p < 0.05. Two-way ANOVA with Tukey’s multiple comparison test was applied. Data were presented as mean ± SD.
https://doi.org/10.1371/journal.ppat.1012368.s005
S6 Fig. Animal weight dynamics, flow cytometry gating strategy and immunohistochemistry in SARS-CoV-2 infected mice.
Female BALB/c mice were intranasally inoculated with 5*10 5 TCID50 SARS-CoV-2 MaVie strain or PBS as control and euthanized at 2 dpi or 4 dpi. ( A ) Daily tracking of animal weight performed throughout the experiment (n = 12 for SARS-CoV-2 infected animals, n = 6 for PBS-inoculated animals). The weights of the mice euthanized at 2 dpi (n = 26) did not show significant differences and are not reported. ( B ) Gating strategy to analyze Ly6G+ neutrophils in mouse lung single cell suspensions. Side scatter area (SSC-A) versus forward scatter area (FSC-A) plot followed by side scatter area versus height (SSC-A vs SSC-H) plot were used for the identification of single cells. BV605 yellow live/dead dye was used to discriminate dead cells, from which CD3/CD19+ lymphocytes and Ly6G+ neutrophils were gated as shown. ( C ) Representative histogram showing the percentage of Ly6G+ cells after isolation from lung single cell suspension using Ly6G-binding magnetic beads. ( D ) Left column: immunohistochemistry for SARS-CoV-2 nucleoprotein; right column: immunohistochemistry for Ly6G (neutrophil marker), hematoxylin counterstain. Bars = 500 μm (large images) and 50 μm (insets). At 2 dpi (top), the arrow points at a bronchus with viral antigen expression in epithelial cells. A close–up of the bronchus (bottom; B: bronchial lumen) shows degenerated and slough off antigen positive epithelial cells. Adjacent alveoli exhibit viral antigen expression in typeI (arrowhead) and typeII (arrow) pneumocytes. The overview (top) shows neutrophils between the infected bronchial (arrow) epithelial cells, in parenchymal areas (arrowhead; right inset) and in capillaries (arrowheads). A close-up of the bronchus (bottom; B: bronchial lumen) highlights numerous neutrophils between degenerate (arrowheads) epithelial cells. At 4 dpi (middle), there are focal areas with antigen expression in alveolar epithelial cells and infiltrating macrophages. Neutrophils are present among the infiltrating cells (arrow) as individual cells (inset: arrows) or in aggregates (inset: arrowhead). The bottom shows the lung of a mock-infected control animal. There is no viral antigen expression. Staining for Ly6G depicts individual neutrophils in larger vessels (inset: arrow) or in capillaries (inset: arrowheads).
https://doi.org/10.1371/journal.ppat.1012368.s006
S7 Fig. Impact of α-IFNAR treatment in SARS-CoV-2 infected mice.
Female BALB/c mice were intranasally inoculated with 5* 10 5 TCID50 SARS-CoV-2 MaVie strain or PBS as control. Mice were intraperitoneally inoculated with 250 μg anti-IFNAR or IgG1 isotype control directly after infection with SARS-CoV-2 and lung neutrophils isolated at 2 dpi (including also intranasally PBS-inoculated control mice without intraperitoneal injection) ( A ) Quantification of caspase1 positive cells in nigericin-activated isolated Ly6G neutrophils stained by FAM-FLICA. Representative histogram is shown. * p < 0.05. P values calculated using one-way ANOVA with multiple comparison test (Holm-Šídák correction). ( B ) RNA was isolated from mouse lungs and subjected to RT-qPCR targeting the replication-intermediate subgenomic E gene and GAPDH as housekeeping gene. RNA levels were assessed based on cycle threshold Ct levels. The expression levels of the target gene SubE were measured and normalized to GAPDH levels using the comparative Ct method (ΔΔCt). The fold change values were calculated by the formula 2^(-ΔΔCt), representing the relative gene expression compared to the PBS mock-infected control (in which subE was undetectable but set to 40 Ct). No significant differences are seen between the two groups, assessed with Welch’s t-test. ( C ) Infectious virus was calculated from supernatants of lung single cell suspensions of infected mice as fluorescence focus forming units (FFU) in Vero E6 cells. ( D ) Quantification based on morphometric analysis that determines the area of immunolabelling for SARS-CoV-2 nucleoprotein in relation to total tissue area. ( E ) Quantification of Ly6G neutrophil/lymphocyte ratio in lung single cell suspensions by flow cytometry. ( F ) Quantification of Ly6G cell counts extrapolated per lung in single cell suspensions by flow cytometry ( G ) Quantification of Ly-6G based on morphometric analysis that determines the area of immunolabelling for Ly6G in relation to total tissue area in mock-infected controls. ( H ) Quantification of median fluorescence intensity (MFI) of CD11b expression in ly6G neutrophils by flow cytometry. ( I ) Quantification of FAM-FLICA MFI in Ly6G+ neutrophils by flow cytometry. Panels B, D and G are representative of two independent experiments. ( J ) Histological features, viral antigen expression and extent of neutrophil influx and evidence of neutrophil damage in the lung of SARS-CoV-2 infected BALB/C mice after isotype control and anti-IFNAR treatment at 2dpi. Left column: Control isotype treated mice; right column: anti-IFNAR treated mice. HE stain (top layer) and immunohistology, hematoxylin counterstain (all other images). Bars: 250 μm (overview images) and 25 μm (insets). In control isotype treated mice, the lung exhibits degeneration and loss of bronchial and bronchiolar epithelial cells (HE stain: arrowhead; right inset), with mild inflammatory infiltration. The parenchyma exhibits focal areas of increased cellularity, with typeII pneumocyte activation and occasional degenerate alveolar epithelial cells (arrows; left inset: degenerate cells (arrowhead) and infiltrating neutrophil (arrow)). Staining for SARS-CoV-2 NP confirms epithelial cell infection in bronchus (arrowhead; right inset) and alveoli (arrow; left inset). Right inset: Viral antigen expression is seen in intact and sloughed off, degenerate epithelial cells. Left inset: Viral antigen expression is seen in both typeI (small arrowhead) and typeII (small arrow) pneumocytes; there are also degenerate positive cells (large arrowhead). Neutrophils (Ly6G+) are located within focal parenchymal areas of increased cellularity (arrows; left inset: arrowheads) and present between degenerate bronchial epithelial cells (arrowhead; right inset: arrowhead). Staining for histone H3 shows neutrophil degeneration/NETosis in parenchymal areas (arrow; left inset: arrowheads) and associated with degenerate epithelial cells (arrowhead; right inset: positive reaction between sloughed off epithelial cells (arrow) and between the intact epithelial layer (arrowhead)). In anti-IFNAR treated animals, the lung exhibits degeneration and loss of bronchial and bronchiolar epithelial cells (arrowhead; right inset: arrows), with mild inflammatory infiltration and individual neutrophils between intact and sloughed off degenerate epithelial cells (right inset: arrowheads). The parenchyma exhibits focal areas of increased cellularity, with typeII pneumocyte activation and occasional degenerate alveolar epithelial cells (arrows; left inset: degenerate cells (arrow) and infiltrating neutrophils (arrowhead). Staining for SARS-CoV-2 NP shows epithelial cell infection in bronchioles (arrowhead; right inset) and alveoli (arrow; left inset). Right inset: Viral antigen expression is seen in intact and sloughed off, degenerate epithelial cells. Left inset: Viral antigen expression is seen in pneumocytes (arrow) and infiltrating macrophages (arrowheads). Neutrophils (Ly6G+) locate within focal parenchymal areas of increased cellularity (arrows; left inset) and are present between intact (inset: arrowhead) and degenerate epithelial cells (arrowhead; right inset: arrow). Staining for histone H3cit shows neutrophil degeneration/NETosis in parenchymal areas (arrow; left inset) and associated with degenerate epithelial cells (arrowhead; right inset: positive reaction between sloughed off epithelial cells (arrow) and between the intact epithelial layer (arrowhead)). Dpi = days post infection; NP = nucleoprotein .
https://doi.org/10.1371/journal.ppat.1012368.s007
S1 Table. Information for Fig 3 , detailing the Gene Set Enrichment Analysis (GSEA) databases used for pathway analyses.
https://doi.org/10.1371/journal.ppat.1012368.s008
S2 Table. qPCR primer sequences: gene-specific forward and reverse primers.
https://doi.org/10.1371/journal.ppat.1012368.s009
S3 Table. Histological changes as well as SARS-CoV-2 nucleoprotein and RNA expression in female BALB/C mice infected with SARS-CoV-2.
https://doi.org/10.1371/journal.ppat.1012368.s010
S1 Source Data. Original scan of IL-1β and actin western blot. HC PMNs were non-stimulated or stimulated 4h with IFN-I (combination of 2.7*104 IU/ml IFN-α and IFN-β) or 20 ng/ml LPS (1st signal), followed by 4h with 2.5 μM nigericin (2nd signal).
Western blots from supernatants and cell lysates were performed first for actin and then for IL-1β on the same membrane as indicated. Information for Fig 5A .
https://doi.org/10.1371/journal.ppat.1012368.s011
Acknowledgments
RNA isolation, library preparations and RNA sequencing was performed at the Institute for Molecular Medicine Finland FIMM, Genomics unit supported by HiLIFE and Biocenter Finland. The authors also thank M. Utriainen for expert technical assistance.
- View Article
- PubMed/NCBI
- Google Scholar
- 25. CZ CELLxGENE Discover. Chan Zuckerberg Initiative. Available: https://cellxgene.cziscience.com/

IMAGES
COMMENTS
Research Summary. Definition: A research summary is a brief and concise overview of a research project or study that highlights its key findings, main points, and conclusions. It typically includes a description of the research problem, the research methods used, the results obtained, and the implications or significance of the findings.
A research summary is a brief yet concise version of the research paper for a targeted audience. Read more to find out about structure of a research summary, tips to write a good research summary, and common mistakes to write a research summary. ... Results. Create a list of evidence obtained from the various experiments with a primary analysis ...
Checklist: Research results 0 / 7. I have completed my data collection and analyzed the results. I have included all results that are relevant to my research questions. I have concisely and objectively reported each result, including relevant descriptive statistics and inferential statistics. I have stated whether each hypothesis was supported ...
Research Results. Research results refer to the findings and conclusions derived from a systematic investigation or study conducted to answer a specific question or hypothesis. These results are typically presented in a written report or paper and can include various forms of data such as numerical data, qualitative data, statistics, charts, graphs, and visual aids.
Reporting Research Results in APA Style | Tips & Examples. Published on December 21, 2020 by Pritha Bhandari.Revised on January 17, 2024. The results section of a quantitative research paper is where you summarize your data and report the findings of any relevant statistical analyses.. The APA manual provides rigorous guidelines for what to report in quantitative research papers in the fields ...
Table of contents. When to write a summary. Step 1: Read the text. Step 2: Break the text down into sections. Step 3: Identify the key points in each section. Step 4: Write the summary. Step 5: Check the summary against the article. Other interesting articles. Frequently asked questions about summarizing.
A research summary is a piece of writing that summarizes your research on a specific topic. Its primary goal is to offer the reader a detailed overview of the study with the key findings. A research summary generally contains the article's structure in which it is written. You must know the goal of your analysis before you launch a project.
For most research papers in the social and behavioral sciences, there are two possible ways of organizing the results. Both approaches are appropriate in how you report your findings, but use only one approach. Present a synopsis of the results followed by an explanation of key findings. This approach can be used to highlight important findings.
A summary must be coherent and cogent and should make sense as a stand-alone piece of writing. It is typically 5% to 10% of the length of the original paper; however, the length depends on the length and complexity of the article and the purpose of the summary. Accordingly, a summary can be several paragraphs or pages, a single paragraph, or ...
A research paper summary is a short overview of a research paper. Generally, a research paper summary is about 300-400 words long, though with longer papers, they're usually no more than 10 percent the length of the original paper. Research paper summaries play an important role in academia.
A summary of the research findings. Information about participant flow, recruitment, retention, and attrition. If some participants started the study and later left or failed to complete the study, then this should be described. ... Pay attention to how the authors present the results of their research. Get a second opinion. If possible, take ...
A research article usually has seven major sections: Title, Abstract, Introduction, Method, Results, Discussion, and References. The first thing you should do is to decide why you need to summarize the article. If the purpose of the summary is to take notes to later remind yourself about the article you may want to write a longer summary ...
Next, the results section needs to communicate the findings of your research in a systematic manner. The section needs to be organized such that the primary research question is addressed first, then the secondary research questions. If the research addresses multiple questions, the results section must individually connect with each of the ...
Following this overall summary, the relevant data in the tables is broken down into greater detail in text form in the Results section. "Results on the bio-accumulation of cadmium were found to be the highest (17.5 mg kgG1) in the bulb, when the concentration of cadmium in the solution was 1×10G2 M and lowest (0.11 mg kgG1) in the leaves when the concentration was 1×10G3 M."
Present the results of the paper, in logical order, using tables and graphs as necessary. Explain the results and show how they help to answer the research questions posed in the Introduction. Evidence does not explain itself; the results must be presented and then explained. Avoid: presenting results that are never discussed; presenting ...
The results section of a research paper tells the reader what you found, while the discussion section tells the reader what your findings mean. The results section should present the facts in an academic and unbiased manner, avoiding any attempt at analyzing or interpreting the data. Think of the results section as setting the stage for the ...
Build coherence along this section using goal statements and explicit reasoning (guide the reader through your reasoning, including sentences of this type: 'In order to…, we performed….'; 'In view of this result, we ….', etc.). In summary, the general steps for writing the Results section of a research article are:
On a related note is the discussion of how to present non-significant results. Given that published research largely reflects findings that are novel or possess a large effect size (Prager et al ...
Finally, summarizing the research findings is crucial in the results section, as it provides readers with a concise summary of the study's main results and conclusions. This step should be written in a clear and straightforward manner, highlighting the most important findings and explaining their significance.
A research summary is a short, concise summary of an academic research paper. It is often used to summarize the results of an experiment, summarize the major findings and conclusions, and provide a brief overview of the methods and procedures used in the study.
The results chapter in a dissertation or thesis (or any formal academic research piece) is where you objectively and neutrally present the findings of your qualitative analysis (or analyses if you used multiple qualitative analysis methods ). This chapter can sometimes be combined with the discussion chapter (where you interpret the data and ...
The researcher usually organizes the results of his/her results section by research question or hypothesis, stating the results for each one, using statistics to show how the research question or hypothesis was answered in the study. ... In the presentation, spend the least amount of time on the literature review (a very brief summary will ...
Key Points: This chapter provides guidance on interpreting the results of synthesis in order to communicate the conclusions of the review effectively. Methods are presented for computing, presenting and interpreting relative and absolute effects for dichotomous outcome data, including the number needed to treat (NNT).
The Youth Anxiety Measure for DSM-5 (YAM-5) is a self- and parent-report scale specifically developed to assess symptoms of major anxiety disorders (part 1 or YAM-5-I) and specific phobias/agoraphobia (part 2 or YAM-5-II) in children and adolescents in terms of the contemporary psychiatric classification system. Since its introduction, the measure has been increasingly used in research, making ...
Author summary COVID-19, caused by the SARS-CoV-2, ranges from mild "flu"-like symptoms to severe respiratory distress or even death. Neutrophils are important cells of our immune system which are strongly involved in inflammatory responses, including those occurring in COVID-19. However, despite extensive research, the precise contribution of neutrophils to the pathogenesis of COVID-19 ...