REVIEW article
Environmental and health impacts of air pollution: a review.

- 1 Delphis S.A., Kifisia, Greece
- 2 Laboratory of Hygiene and Environmental Protection, Faculty of Medicine, Democritus University of Thrace, Alexandroupolis, Greece
- 3 Centre Hospitalier Universitaire Vaudois (CHUV), Service de Médicine Interne, Lausanne, Switzerland
- 4 School of Social and Political Sciences, University of Glasgow, Glasgow, United Kingdom
One of our era's greatest scourges is air pollution, on account not only of its impact on climate change but also its impact on public and individual health due to increasing morbidity and mortality. There are many pollutants that are major factors in disease in humans. Among them, Particulate Matter (PM), particles of variable but very small diameter, penetrate the respiratory system via inhalation, causing respiratory and cardiovascular diseases, reproductive and central nervous system dysfunctions, and cancer. Despite the fact that ozone in the stratosphere plays a protective role against ultraviolet irradiation, it is harmful when in high concentration at ground level, also affecting the respiratory and cardiovascular system. Furthermore, nitrogen oxide, sulfur dioxide, Volatile Organic Compounds (VOCs), dioxins, and polycyclic aromatic hydrocarbons (PAHs) are all considered air pollutants that are harmful to humans. Carbon monoxide can even provoke direct poisoning when breathed in at high levels. Heavy metals such as lead, when absorbed into the human body, can lead to direct poisoning or chronic intoxication, depending on exposure. Diseases occurring from the aforementioned substances include principally respiratory problems such as Chronic Obstructive Pulmonary Disease (COPD), asthma, bronchiolitis, and also lung cancer, cardiovascular events, central nervous system dysfunctions, and cutaneous diseases. Last but not least, climate change resulting from environmental pollution affects the geographical distribution of many infectious diseases, as do natural disasters. The only way to tackle this problem is through public awareness coupled with a multidisciplinary approach by scientific experts; national and international organizations must address the emergence of this threat and propose sustainable solutions.

Approach to the Problem
The interactions between humans and their physical surroundings have been extensively studied, as multiple human activities influence the environment. The environment is a coupling of the biotic (living organisms and microorganisms) and the abiotic (hydrosphere, lithosphere, and atmosphere).
Pollution is defined as the introduction into the environment of substances harmful to humans and other living organisms. Pollutants are harmful solids, liquids, or gases produced in higher than usual concentrations that reduce the quality of our environment.
Human activities have an adverse effect on the environment by polluting the water we drink, the air we breathe, and the soil in which plants grow. Although the industrial revolution was a great success in terms of technology, society, and the provision of multiple services, it also introduced the production of huge quantities of pollutants emitted into the air that are harmful to human health. Without any doubt, the global environmental pollution is considered an international public health issue with multiple facets. Social, economic, and legislative concerns and lifestyle habits are related to this major problem. Clearly, urbanization and industrialization are reaching unprecedented and upsetting proportions worldwide in our era. Anthropogenic air pollution is one of the biggest public health hazards worldwide, given that it accounts for about 9 million deaths per year ( 1 ).
Without a doubt, all of the aforementioned are closely associated with climate change, and in the event of danger, the consequences can be severe for mankind ( 2 ). Climate changes and the effects of global planetary warming seriously affect multiple ecosystems, causing problems such as food safety issues, ice and iceberg melting, animal extinction, and damage to plants ( 3 , 4 ).
Air pollution has various health effects. The health of susceptible and sensitive individuals can be impacted even on low air pollution days. Short-term exposure to air pollutants is closely related to COPD (Chronic Obstructive Pulmonary Disease), cough, shortness of breath, wheezing, asthma, respiratory disease, and high rates of hospitalization (a measurement of morbidity).
The long-term effects associated with air pollution are chronic asthma, pulmonary insufficiency, cardiovascular diseases, and cardiovascular mortality. According to a Swedish cohort study, diabetes seems to be induced after long-term air pollution exposure ( 5 ). Moreover, air pollution seems to have various malign health effects in early human life, such as respiratory, cardiovascular, mental, and perinatal disorders ( 3 ), leading to infant mortality or chronic disease in adult age ( 6 ).
National reports have mentioned the increased risk of morbidity and mortality ( 1 ). These studies were conducted in many places around the world and show a correlation between daily ranges of particulate matter (PM) concentration and daily mortality. Climate shifts and global planetary warming ( 3 ) could aggravate the situation. Besides, increased hospitalization (an index of morbidity) has been registered among the elderly and susceptible individuals for specific reasons. Fine and ultrafine particulate matter seems to be associated with more serious illnesses ( 6 ), as it can invade the deepest parts of the airways and more easily reach the bloodstream.
Air pollution mainly affects those living in large urban areas, where road emissions contribute the most to the degradation of air quality. There is also a danger of industrial accidents, where the spread of a toxic fog can be fatal to the populations of the surrounding areas. The dispersion of pollutants is determined by many parameters, most notably atmospheric stability and wind ( 6 ).
In developing countries ( 7 ), the problem is more serious due to overpopulation and uncontrolled urbanization along with the development of industrialization. This leads to poor air quality, especially in countries with social disparities and a lack of information on sustainable management of the environment. The use of fuels such as wood fuel or solid fuel for domestic needs due to low incomes exposes people to bad-quality, polluted air at home. It is of note that three billion people around the world are using the above sources of energy for their daily heating and cooking needs ( 8 ). In developing countries, the women of the household seem to carry the highest risk for disease development due to their longer duration exposure to the indoor air pollution ( 8 , 9 ). Due to its fast industrial development and overpopulation, China is one of the Asian countries confronting serious air pollution problems ( 10 , 11 ). The lung cancer mortality observed in China is associated with fine particles ( 12 ). As stated already, long-term exposure is associated with deleterious effects on the cardiovascular system ( 3 , 5 ). However, it is interesting to note that cardiovascular diseases have mostly been observed in developed and high-income countries rather than in the developing low-income countries exposed highly to air pollution ( 13 ). Extreme air pollution is recorded in India, where the air quality reaches hazardous levels. New Delhi is one of the more polluted cities in India. Flights in and out of New Delhi International Airport are often canceled due to the reduced visibility associated with air pollution. Pollution is occurring both in urban and rural areas in India due to the fast industrialization, urbanization, and rise in use of motorcycle transportation. Nevertheless, biomass combustion associated with heating and cooking needs and practices is a major source of household air pollution in India and in Nepal ( 14 , 15 ). There is spatial heterogeneity in India, as areas with diverse climatological conditions and population and education levels generate different indoor air qualities, with higher PM 2.5 observed in North Indian states (557–601 μg/m 3 ) compared to the Southern States (183–214 μg/m 3 ) ( 16 , 17 ). The cold climate of the North Indian areas may be the main reason for this, as longer periods at home and more heating are necessary compared to in the tropical climate of Southern India. Household air pollution in India is associated with major health effects, especially in women and young children, who stay indoors for longer periods. Chronic obstructive respiratory disease (CORD) and lung cancer are mostly observed in women, while acute lower respiratory disease is seen in young children under 5 years of age ( 18 ).
Accumulation of air pollution, especially sulfur dioxide and smoke, reaching 1,500 mg/m3, resulted in an increase in the number of deaths (4,000 deaths) in December 1952 in London and in 1963 in New York City (400 deaths) ( 19 ). An association of pollution with mortality was reported on the basis of monitoring of outdoor pollution in six US metropolitan cities ( 20 ). In every case, it seems that mortality was closely related to the levels of fine, inhalable, and sulfate particles more than with the levels of total particulate pollution, aerosol acidity, sulfur dioxide, or nitrogen dioxide ( 20 ).
Furthermore, extremely high levels of pollution are reported in Mexico City and Rio de Janeiro, followed by Milan, Ankara, Melbourne, Tokyo, and Moscow ( 19 ).
Based on the magnitude of the public health impact, it is certain that different kinds of interventions should be taken into account. Success and effectiveness in controlling air pollution, specifically at the local level, have been reported. Adequate technological means are applied considering the source and the nature of the emission as well as its impact on health and the environment. The importance of point sources and non-point sources of air pollution control is reported by Schwela and Köth-Jahr ( 21 ). Without a doubt, a detailed emission inventory must record all sources in a given area. Beyond considering the above sources and their nature, topography and meteorology should also be considered, as stated previously. Assessment of the control policies and methods is often extrapolated from the local to the regional and then to the global scale. Air pollution may be dispersed and transported from one region to another area located far away. Air pollution management means the reduction to acceptable levels or possible elimination of air pollutants whose presence in the air affects our health or the environmental ecosystem. Private and governmental entities and authorities implement actions to ensure the air quality ( 22 ). Air quality standards and guidelines were adopted for the different pollutants by the WHO and EPA as a tool for the management of air quality ( 1 , 23 ). These standards have to be compared to the emissions inventory standards by causal analysis and dispersion modeling in order to reveal the problematic areas ( 24 ). Inventories are generally based on a combination of direct measurements and emissions modeling ( 24 ).
As an example, we state here the control measures at the source through the use of catalytic converters in cars. These are devices that turn the pollutants and toxic gases produced from combustion engines into less-toxic pollutants by catalysis through redox reactions ( 25 ). In Greece, the use of private cars was restricted by tracking their license plates in order to reduce traffic congestion during rush hour ( 25 ).
Concerning industrial emissions, collectors and closed systems can keep the air pollution to the minimal standards imposed by legislation ( 26 ).
Current strategies to improve air quality require an estimation of the economic value of the benefits gained from proposed programs. These proposed programs by public authorities, and directives are issued with guidelines to be respected.
In Europe, air quality limit values AQLVs (Air Quality Limit Values) are issued for setting off planning claims ( 27 ). In the USA, the NAAQS (National Ambient Air Quality Standards) establish the national air quality limit values ( 27 ). While both standards and directives are based on different mechanisms, significant success has been achieved in the reduction of overall emissions and associated health and environmental effects ( 27 ). The European Directive identifies geographical areas of risk exposure as monitoring/assessment zones to record the emission sources and levels of air pollution ( 27 ), whereas the USA establishes global geographical air quality criteria according to the severity of their air quality problem and records all sources of the pollutants and their precursors ( 27 ).
In this vein, funds have been financing, directly or indirectly, projects related to air quality along with the technical infrastructure to maintain good air quality. These plans focus on an inventory of databases from air quality environmental planning awareness campaigns. Moreover, pollution measures of air emissions may be taken for vehicles, machines, and industries in urban areas.
Technological innovation can only be successful if it is able to meet the needs of society. In this sense, technology must reflect the decision-making practices and procedures of those involved in risk assessment and evaluation and act as a facilitator in providing information and assessments to enable decision makers to make the best decisions possible. Summarizing the aforementioned in order to design an effective air quality control strategy, several aspects must be considered: environmental factors and ambient air quality conditions, engineering factors and air pollutant characteristics, and finally, economic operating costs for technological improvement and administrative and legal costs. Considering the economic factor, competitiveness through neoliberal concepts is offering a solution to environmental problems ( 22 ).
The development of environmental governance, along with technological progress, has initiated the deployment of a dialogue. Environmental politics has created objections and points of opposition between different political parties, scientists, media, and governmental and non-governmental organizations ( 22 ). Radical environmental activism actions and movements have been created ( 22 ). The rise of the new information and communication technologies (ICTs) are many times examined as to whether and in which way they have influenced means of communication and social movements such as activism ( 28 ). Since the 1990s, the term “digital activism” has been used increasingly and in many different disciplines ( 29 ). Nowadays, multiple digital technologies can be used to produce a digital activism outcome on environmental issues. More specifically, devices with online capabilities such as computers or mobile phones are being used as a way to pursue change in political and social affairs ( 30 ).
In the present paper, we focus on the sources of environmental pollution in relation to public health and propose some solutions and interventions that may be of interest to environmental legislators and decision makers.
Sources of Exposure
It is known that the majority of environmental pollutants are emitted through large-scale human activities such as the use of industrial machinery, power-producing stations, combustion engines, and cars. Because these activities are performed at such a large scale, they are by far the major contributors to air pollution, with cars estimated to be responsible for approximately 80% of today's pollution ( 31 ). Some other human activities are also influencing our environment to a lesser extent, such as field cultivation techniques, gas stations, fuel tanks heaters, and cleaning procedures ( 32 ), as well as several natural sources, such as volcanic and soil eruptions and forest fires.
The classification of air pollutants is based mainly on the sources producing pollution. Therefore, it is worth mentioning the four main sources, following the classification system: Major sources, Area sources, Mobile sources, and Natural sources.
Major sources include the emission of pollutants from power stations, refineries, and petrochemicals, the chemical and fertilizer industries, metallurgical and other industrial plants, and, finally, municipal incineration.
Indoor area sources include domestic cleaning activities, dry cleaners, printing shops, and petrol stations.
Mobile sources include automobiles, cars, railways, airways, and other types of vehicles.
Finally, natural sources include, as stated previously, physical disasters ( 33 ) such as forest fire, volcanic erosion, dust storms, and agricultural burning.
However, many classification systems have been proposed. Another type of classification is a grouping according to the recipient of the pollution, as follows:
Air pollution is determined as the presence of pollutants in the air in large quantities for long periods. Air pollutants are dispersed particles, hydrocarbons, CO, CO 2 , NO, NO 2 , SO 3 , etc.
Water pollution is organic and inorganic charge and biological charge ( 10 ) at high levels that affect the water quality ( 34 , 35 ).
Soil pollution occurs through the release of chemicals or the disposal of wastes, such as heavy metals, hydrocarbons, and pesticides.
Air pollution can influence the quality of soil and water bodies by polluting precipitation, falling into water and soil environments ( 34 , 36 ). Notably, the chemistry of the soil can be amended due to acid precipitation by affecting plants, cultures, and water quality ( 37 ). Moreover, movement of heavy metals is favored by soil acidity, and metals are so then moving into the watery environment. It is known that heavy metals such as aluminum are noxious to wildlife and fishes. Soil quality seems to be of importance, as soils with low calcium carbonate levels are at increased jeopardy from acid rain. Over and above rain, snow and particulate matter drip into watery ' bodies ( 36 , 38 ).
Lastly, pollution is classified following type of origin:
Radioactive and nuclear pollution , releasing radioactive and nuclear pollutants into water, air, and soil during nuclear explosions and accidents, from nuclear weapons, and through handling or disposal of radioactive sewage.
Radioactive materials can contaminate surface water bodies and, being noxious to the environment, plants, animals, and humans. It is known that several radioactive substances such as radium and uranium concentrate in the bones and can cause cancers ( 38 , 39 ).
Noise pollution is produced by machines, vehicles, traffic noises, and musical installations that are harmful to our hearing.
The World Health Organization introduced the term DALYs. The DALYs for a disease or health condition is defined as the sum of the Years of Life Lost (YLL) due to premature mortality in the population and the Years Lost due to Disability (YLD) for people living with the health condition or its consequences ( 39 ). In Europe, air pollution is the main cause of disability-adjusted life years lost (DALYs), followed by noise pollution. The potential relationships of noise and air pollution with health have been studied ( 40 ). The study found that DALYs related to noise were more important than those related to air pollution, as the effects of environmental noise on cardiovascular disease were independent of air pollution ( 40 ). Environmental noise should be counted as an independent public health risk ( 40 ).
Environmental pollution occurs when changes in the physical, chemical, or biological constituents of the environment (air masses, temperature, climate, etc.) are produced.
Pollutants harm our environment either by increasing levels above normal or by introducing harmful toxic substances. Primary pollutants are directly produced from the above sources, and secondary pollutants are emitted as by-products of the primary ones. Pollutants can be biodegradable or non-biodegradable and of natural origin or anthropogenic, as stated previously. Moreover, their origin can be a unique source (point-source) or dispersed sources.
Pollutants have differences in physical and chemical properties, explaining the discrepancy in their capacity for producing toxic effects. As an example, we state here that aerosol compounds ( 41 – 43 ) have a greater toxicity than gaseous compounds due to their tiny size (solid or liquid) in the atmosphere; they have a greater penetration capacity. Gaseous compounds are eliminated more easily by our respiratory system ( 41 ). These particles are able to damage lungs and can even enter the bloodstream ( 41 ), leading to the premature deaths of millions of people yearly. Moreover, the aerosol acidity ([H+]) seems to considerably enhance the production of secondary organic aerosols (SOA), but this last aspect is not supported by other scientific teams ( 38 ).
Climate and Pollution
Air pollution and climate change are closely related. Climate is the other side of the same coin that reduces the quality of our Earth ( 44 ). Pollutants such as black carbon, methane, tropospheric ozone, and aerosols affect the amount of incoming sunlight. As a result, the temperature of the Earth is increasing, resulting in the melting of ice, icebergs, and glaciers.
In this vein, climatic changes will affect the incidence and prevalence of both residual and imported infections in Europe. Climate and weather affect the duration, timing, and intensity of outbreaks strongly and change the map of infectious diseases in the globe ( 45 ). Mosquito-transmitted parasitic or viral diseases are extremely climate-sensitive, as warming firstly shortens the pathogen incubation period and secondly shifts the geographic map of the vector. Similarly, water-warming following climate changes leads to a high incidence of waterborne infections. Recently, in Europe, eradicated diseases seem to be emerging due to the migration of population, for example, cholera, poliomyelitis, tick-borne encephalitis, and malaria ( 46 ).
The spread of epidemics is associated with natural climate disasters and storms, which seem to occur more frequently nowadays ( 47 ). Malnutrition and disequilibration of the immune system are also associated with the emerging infections affecting public health ( 48 ).
The Chikungunya virus “took the airplane” from the Indian Ocean to Europe, as outbreaks of the disease were registered in Italy ( 49 ) as well as autochthonous cases in France ( 50 ).
An increase in cryptosporidiosis in the United Kingdom and in the Czech Republic seems to have occurred following flooding ( 36 , 51 ).
As stated previously, aerosols compounds are tiny in size and considerably affect the climate. They are able to dissipate sunlight (the albedo phenomenon) by dispersing a quarter of the sun's rays back to space and have cooled the global temperature over the last 30 years ( 52 ).
Air Pollutants
The World Health Organization (WHO) reports on six major air pollutants, namely particle pollution, ground-level ozone, carbon monoxide, sulfur oxides, nitrogen oxides, and lead. Air pollution can have a disastrous effect on all components of the environment, including groundwater, soil, and air. Additionally, it poses a serious threat to living organisms. In this vein, our interest is mainly to focus on these pollutants, as they are related to more extensive and severe problems in human health and environmental impact. Acid rain, global warming, the greenhouse effect, and climate changes have an important ecological impact on air pollution ( 53 ).
Particulate Matter (PM) and Health
Studies have shown a relationship between particulate matter (PM) and adverse health effects, focusing on either short-term (acute) or long-term (chronic) PM exposure.
Particulate matter (PM) is usually formed in the atmosphere as a result of chemical reactions between the different pollutants. The penetration of particles is closely dependent on their size ( 53 ). Particulate Matter (PM) was defined as a term for particles by the United States Environmental Protection Agency ( 54 ). Particulate matter (PM) pollution includes particles with diameters of 10 micrometers (μm) or smaller, called PM 10 , and extremely fine particles with diameters that are generally 2.5 micrometers (μm) and smaller.
Particulate matter contains tiny liquid or solid droplets that can be inhaled and cause serious health effects ( 55 ). Particles <10 μm in diameter (PM 10 ) after inhalation can invade the lungs and even reach the bloodstream. Fine particles, PM 2.5 , pose a greater risk to health ( 6 , 56 ) ( Table 1 ).
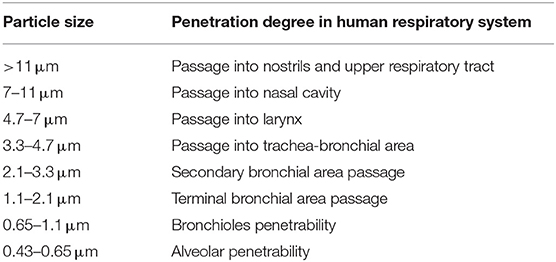
Table 1 . Penetrability according to particle size.
Multiple epidemiological studies have been performed on the health effects of PM. A positive relation was shown between both short-term and long-term exposures of PM 2.5 and acute nasopharyngitis ( 56 ). In addition, long-term exposure to PM for years was found to be related to cardiovascular diseases and infant mortality.
Those studies depend on PM 2.5 monitors and are restricted in terms of study area or city area due to a lack of spatially resolved daily PM 2.5 concentration data and, in this way, are not representative of the entire population. Following a recent epidemiological study by the Department of Environmental Health at Harvard School of Public Health (Boston, MA) ( 57 ), it was reported that, as PM 2.5 concentrations vary spatially, an exposure error (Berkson error) seems to be produced, and the relative magnitudes of the short- and long-term effects are not yet completely elucidated. The team developed a PM 2.5 exposure model based on remote sensing data for assessing short- and long-term human exposures ( 57 ). This model permits spatial resolution in short-term effects plus the assessment of long-term effects in the whole population.
Moreover, respiratory diseases and affection of the immune system are registered as long-term chronic effects ( 58 ). It is worth noting that people with asthma, pneumonia, diabetes, and respiratory and cardiovascular diseases are especially susceptible and vulnerable to the effects of PM. PM 2.5 , followed by PM 10 , are strongly associated with diverse respiratory system diseases ( 59 ), as their size permits them to pierce interior spaces ( 60 ). The particles produce toxic effects according to their chemical and physical properties. The components of PM 10 and PM 2.5 can be organic (polycyclic aromatic hydrocarbons, dioxins, benzene, 1-3 butadiene) or inorganic (carbon, chlorides, nitrates, sulfates, metals) in nature ( 55 ).
Particulate Matter (PM) is divided into four main categories according to type and size ( 61 ) ( Table 2 ).
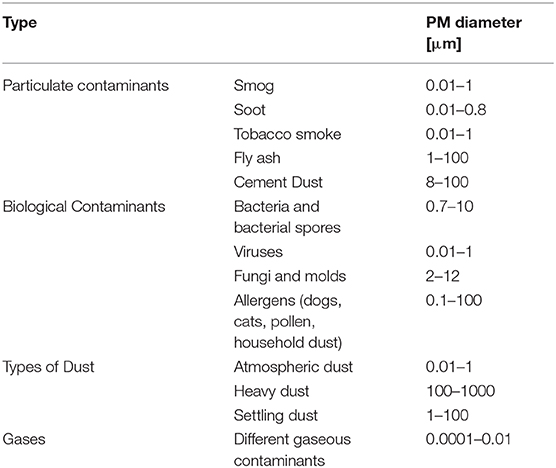
Table 2 . Types and sizes of particulate Matter (PM).
Gas contaminants include PM in aerial masses.
Particulate contaminants include contaminants such as smog, soot, tobacco smoke, oil smoke, fly ash, and cement dust.
Biological Contaminants are microorganisms (bacteria, viruses, fungi, mold, and bacterial spores), cat allergens, house dust and allergens, and pollen.
Types of Dust include suspended atmospheric dust, settling dust, and heavy dust.
Finally, another fact is that the half-lives of PM 10 and PM 2.5 particles in the atmosphere is extended due to their tiny dimensions; this permits their long-lasting suspension in the atmosphere and even their transfer and spread to distant destinations where people and the environment may be exposed to the same magnitude of pollution ( 53 ). They are able to change the nutrient balance in watery ecosystems, damage forests and crops, and acidify water bodies.
As stated, PM 2.5 , due to their tiny size, are causing more serious health effects. These aforementioned fine particles are the main cause of the “haze” formation in different metropolitan areas ( 12 , 13 , 61 ).
Ozone Impact in the Atmosphere
Ozone (O 3 ) is a gas formed from oxygen under high voltage electric discharge ( 62 ). It is a strong oxidant, 52% stronger than chlorine. It arises in the stratosphere, but it could also arise following chain reactions of photochemical smog in the troposphere ( 63 ).
Ozone can travel to distant areas from its initial source, moving with air masses ( 64 ). It is surprising that ozone levels over cities are low in contrast to the increased amounts occuring in urban areas, which could become harmful for cultures, forests, and vegetation ( 65 ) as it is reducing carbon assimilation ( 66 ). Ozone reduces growth and yield ( 47 , 48 ) and affects the plant microflora due to its antimicrobial capacity ( 67 , 68 ). In this regard, ozone acts upon other natural ecosystems, with microflora ( 69 , 70 ) and animal species changing their species composition ( 71 ). Ozone increases DNA damage in epidermal keratinocytes and leads to impaired cellular function ( 72 ).
Ground-level ozone (GLO) is generated through a chemical reaction between oxides of nitrogen and VOCs emitted from natural sources and/or following anthropogenic activities.
Ozone uptake usually occurs by inhalation. Ozone affects the upper layers of the skin and the tear ducts ( 73 ). A study of short-term exposure of mice to high levels of ozone showed malondialdehyde formation in the upper skin (epidermis) but also depletion in vitamins C and E. It is likely that ozone levels are not interfering with the skin barrier function and integrity to predispose to skin disease ( 74 ).
Due to the low water-solubility of ozone, inhaled ozone has the capacity to penetrate deeply into the lungs ( 75 ).
Toxic effects induced by ozone are registered in urban areas all over the world, causing biochemical, morphologic, functional, and immunological disorders ( 76 ).
The European project (APHEA2) focuses on the acute effects of ambient ozone concentrations on mortality ( 77 ). Daily ozone concentrations compared to the daily number of deaths were reported from different European cities for a 3-year period. During the warm period of the year, an observed increase in ozone concentration was associated with an increase in the daily number of deaths (0.33%), in the number of respiratory deaths (1.13%), and in the number of cardiovascular deaths (0.45%). No effect was observed during wintertime.
Carbon Monoxide (CO)
Carbon monoxide is produced by fossil fuel when combustion is incomplete. The symptoms of poisoning due to inhaling carbon monoxide include headache, dizziness, weakness, nausea, vomiting, and, finally, loss of consciousness.
The affinity of carbon monoxide to hemoglobin is much greater than that of oxygen. In this vein, serious poisoning may occur in people exposed to high levels of carbon monoxide for a long period of time. Due to the loss of oxygen as a result of the competitive binding of carbon monoxide, hypoxia, ischemia, and cardiovascular disease are observed.
Carbon monoxide affects the greenhouses gases that are tightly connected to global warming and climate. This should lead to an increase in soil and water temperatures, and extreme weather conditions or storms may occur ( 68 ).
However, in laboratory and field experiments, it has been seen to produce increased plant growth ( 78 ).
Nitrogen Oxide (NO 2 )
Nitrogen oxide is a traffic-related pollutant, as it is emitted from automobile motor engines ( 79 , 80 ). It is an irritant of the respiratory system as it penetrates deep in the lung, inducing respiratory diseases, coughing, wheezing, dyspnea, bronchospasm, and even pulmonary edema when inhaled at high levels. It seems that concentrations over 0.2 ppm produce these adverse effects in humans, while concentrations higher than 2.0 ppm affect T-lymphocytes, particularly the CD8+ cells and NK cells that produce our immune response ( 81 ).It is reported that long-term exposure to high levels of nitrogen dioxide can be responsible for chronic lung disease. Long-term exposure to NO 2 can impair the sense of smell ( 81 ).
However, systems other than respiratory ones can be involved, as symptoms such as eye, throat, and nose irritation have been registered ( 81 ).
High levels of nitrogen dioxide are deleterious to crops and vegetation, as they have been observed to reduce crop yield and plant growth efficiency. Moreover, NO 2 can reduce visibility and discolor fabrics ( 81 ).
Sulfur Dioxide (SO 2 )
Sulfur dioxide is a harmful gas that is emitted mainly from fossil fuel consumption or industrial activities. The annual standard for SO 2 is 0.03 ppm ( 82 ). It affects human, animal, and plant life. Susceptible people as those with lung disease, old people, and children, who present a higher risk of damage. The major health problems associated with sulfur dioxide emissions in industrialized areas are respiratory irritation, bronchitis, mucus production, and bronchospasm, as it is a sensory irritant and penetrates deep into the lung converted into bisulfite and interacting with sensory receptors, causing bronchoconstriction. Moreover, skin redness, damage to the eyes (lacrimation and corneal opacity) and mucous membranes, and worsening of pre-existing cardiovascular disease have been observed ( 81 ).
Environmental adverse effects, such as acidification of soil and acid rain, seem to be associated with sulfur dioxide emissions ( 83 ).
Lead is a heavy metal used in different industrial plants and emitted from some petrol motor engines, batteries, radiators, waste incinerators, and waste waters ( 84 ).
Moreover, major sources of lead pollution in the air are metals, ore, and piston-engine aircraft. Lead poisoning is a threat to public health due to its deleterious effects upon humans, animals, and the environment, especially in the developing countries.
Exposure to lead can occur through inhalation, ingestion, and dermal absorption. Trans- placental transport of lead was also reported, as lead passes through the placenta unencumbered ( 85 ). The younger the fetus is, the more harmful the toxic effects. Lead toxicity affects the fetal nervous system; edema or swelling of the brain is observed ( 86 ). Lead, when inhaled, accumulates in the blood, soft tissue, liver, lung, bones, and cardiovascular, nervous, and reproductive systems. Moreover, loss of concentration and memory, as well as muscle and joint pain, were observed in adults ( 85 , 86 ).
Children and newborns ( 87 ) are extremely susceptible even to minimal doses of lead, as it is a neurotoxicant and causes learning disabilities, impairment of memory, hyperactivity, and even mental retardation.
Elevated amounts of lead in the environment are harmful to plants and crop growth. Neurological effects are observed in vertebrates and animals in association with high lead levels ( 88 ).
Polycyclic Aromatic Hydrocarbons(PAHs)
The distribution of PAHs is ubiquitous in the environment, as the atmosphere is the most important means of their dispersal. They are found in coal and in tar sediments. Moreover, they are generated through incomplete combustion of organic matter as in the cases of forest fires, incineration, and engines ( 89 ). PAH compounds, such as benzopyrene, acenaphthylene, anthracene, and fluoranthene are recognized as toxic, mutagenic, and carcinogenic substances. They are an important risk factor for lung cancer ( 89 ).
Volatile Organic Compounds(VOCs)
Volatile organic compounds (VOCs), such as toluene, benzene, ethylbenzene, and xylene ( 90 ), have been found to be associated with cancer in humans ( 91 ). The use of new products and materials has actually resulted in increased concentrations of VOCs. VOCs pollute indoor air ( 90 ) and may have adverse effects on human health ( 91 ). Short-term and long-term adverse effects on human health are observed. VOCs are responsible for indoor air smells. Short-term exposure is found to cause irritation of eyes, nose, throat, and mucosal membranes, while those of long duration exposure include toxic reactions ( 92 ). Predictable assessment of the toxic effects of complex VOC mixtures is difficult to estimate, as these pollutants can have synergic, antagonistic, or indifferent effects ( 91 , 93 ).
Dioxins originate from industrial processes but also come from natural processes, such as forest fires and volcanic eruptions. They accumulate in foods such as meat and dairy products, fish and shellfish, and especially in the fatty tissue of animals ( 94 ).
Short-period exhibition to high dioxin concentrations may result in dark spots and lesions on the skin ( 94 ). Long-term exposure to dioxins can cause developmental problems, impairment of the immune, endocrine and nervous systems, reproductive infertility, and cancer ( 94 ).
Without any doubt, fossil fuel consumption is responsible for a sizeable part of air contamination. This contamination may be anthropogenic, as in agricultural and industrial processes or transportation, while contamination from natural sources is also possible. Interestingly, it is of note that the air quality standards established through the European Air Quality Directive are somewhat looser than the WHO guidelines, which are stricter ( 95 ).
Effect of Air Pollution on Health
The most common air pollutants are ground-level ozone and Particulates Matter (PM). Air pollution is distinguished into two main types:
Outdoor pollution is the ambient air pollution.
Indoor pollution is the pollution generated by household combustion of fuels.
People exposed to high concentrations of air pollutants experience disease symptoms and states of greater and lesser seriousness. These effects are grouped into short- and long-term effects affecting health.
Susceptible populations that need to be aware of health protection measures include old people, children, and people with diabetes and predisposing heart or lung disease, especially asthma.
As extensively stated previously, according to a recent epidemiological study from Harvard School of Public Health, the relative magnitudes of the short- and long-term effects have not been completely clarified ( 57 ) due to the different epidemiological methodologies and to the exposure errors. New models are proposed for assessing short- and long-term human exposure data more successfully ( 57 ). Thus, in the present section, we report the more common short- and long-term health effects but also general concerns for both types of effects, as these effects are often dependent on environmental conditions, dose, and individual susceptibility.
Short-term effects are temporary and range from simple discomfort, such as irritation of the eyes, nose, skin, throat, wheezing, coughing and chest tightness, and breathing difficulties, to more serious states, such as asthma, pneumonia, bronchitis, and lung and heart problems. Short-term exposure to air pollution can also cause headaches, nausea, and dizziness.
These problems can be aggravated by extended long-term exposure to the pollutants, which is harmful to the neurological, reproductive, and respiratory systems and causes cancer and even, rarely, deaths.
The long-term effects are chronic, lasting for years or the whole life and can even lead to death. Furthermore, the toxicity of several air pollutants may also induce a variety of cancers in the long term ( 96 ).
As stated already, respiratory disorders are closely associated with the inhalation of air pollutants. These pollutants will invade through the airways and will accumulate at the cells. Damage to target cells should be related to the pollutant component involved and its source and dose. Health effects are also closely dependent on country, area, season, and time. An extended exposure duration to the pollutant should incline to long-term health effects in relation also to the above factors.
Particulate Matter (PMs), dust, benzene, and O 3 cause serious damage to the respiratory system ( 97 ). Moreover, there is a supplementary risk in case of existing respiratory disease such as asthma ( 98 ). Long-term effects are more frequent in people with a predisposing disease state. When the trachea is contaminated by pollutants, voice alterations may be remarked after acute exposure. Chronic obstructive pulmonary disease (COPD) may be induced following air pollution, increasing morbidity and mortality ( 99 ). Long-term effects from traffic, industrial air pollution, and combustion of fuels are the major factors for COPD risk ( 99 ).
Multiple cardiovascular effects have been observed after exposure to air pollutants ( 100 ). Changes occurred in blood cells after long-term exposure may affect cardiac functionality. Coronary arteriosclerosis was reported following long-term exposure to traffic emissions ( 101 ), while short-term exposure is related to hypertension, stroke, myocardial infracts, and heart insufficiency. Ventricle hypertrophy is reported to occur in humans after long-time exposure to nitrogen oxide (NO 2 ) ( 102 , 103 ).
Neurological effects have been observed in adults and children after extended-term exposure to air pollutants.
Psychological complications, autism, retinopathy, fetal growth, and low birth weight seem to be related to long-term air pollution ( 83 ). The etiologic agent of the neurodegenerative diseases (Alzheimer's and Parkinson's) is not yet known, although it is believed that extended exposure to air pollution seems to be a factor. Specifically, pesticides and metals are cited as etiological factors, together with diet. The mechanisms in the development of neurodegenerative disease include oxidative stress, protein aggregation, inflammation, and mitochondrial impairment in neurons ( 104 ) ( Figure 1 ).
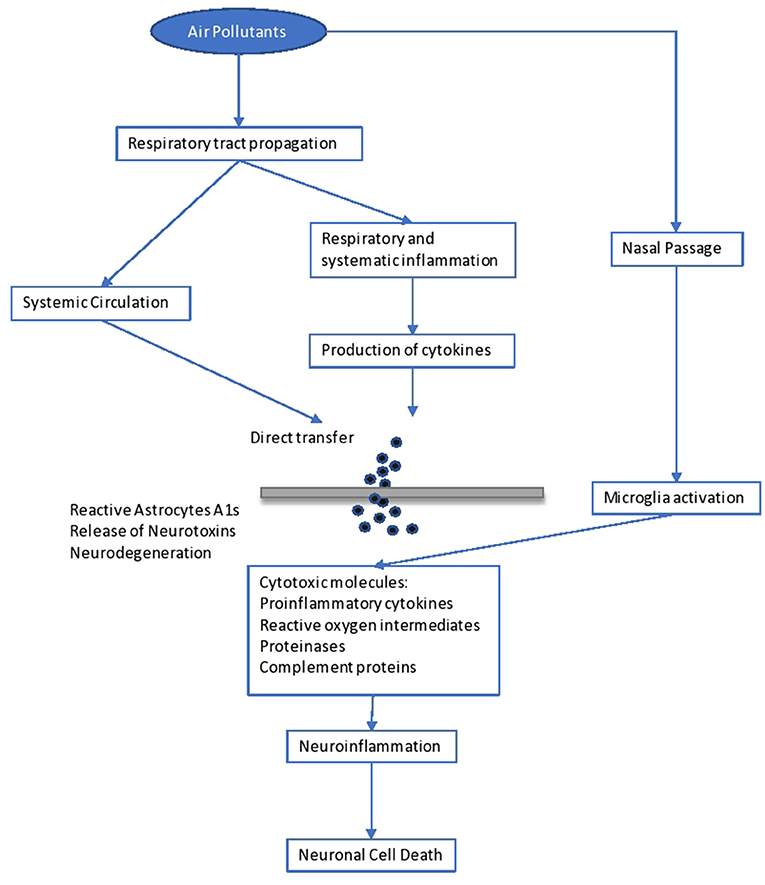
Figure 1 . Impact of air pollutants on the brain.
Brain inflammation was observed in dogs living in a highly polluted area in Mexico for a long period ( 105 ). In human adults, markers of systemic inflammation (IL-6 and fibrinogen) were found to be increased as an immediate response to PNC on the IL-6 level, possibly leading to the production of acute-phase proteins ( 106 ). The progression of atherosclerosis and oxidative stress seem to be the mechanisms involved in the neurological disturbances caused by long-term air pollution. Inflammation comes secondary to the oxidative stress and seems to be involved in the impairment of developmental maturation, affecting multiple organs ( 105 , 107 ). Similarly, other factors seem to be involved in the developmental maturation, which define the vulnerability to long-term air pollution. These include birthweight, maternal smoking, genetic background and socioeconomic environment, as well as education level.
However, diet, starting from breast-feeding, is another determinant factor. Diet is the main source of antioxidants, which play a key role in our protection against air pollutants ( 108 ). Antioxidants are free radical scavengers and limit the interaction of free radicals in the brain ( 108 ). Similarly, genetic background may result in a differential susceptibility toward the oxidative stress pathway ( 60 ). For example, antioxidant supplementation with vitamins C and E appears to modulate the effect of ozone in asthmatic children homozygous for the GSTM1 null allele ( 61 ). Inflammatory cytokines released in the periphery (e.g., respiratory epithelia) upregulate the innate immune Toll-like receptor 2. Such activation and the subsequent events leading to neurodegeneration have recently been observed in lung lavage in mice exposed to ambient Los Angeles (CA, USA) particulate matter ( 61 ). In children, neurodevelopmental morbidities were observed after lead exposure. These children developed aggressive and delinquent behavior, reduced intelligence, learning difficulties, and hyperactivity ( 109 ). No level of lead exposure seems to be “safe,” and the scientific community has asked the Centers for Disease Control and Prevention (CDC) to reduce the current screening guideline of 10 μg/dl ( 109 ).
It is important to state that impact on the immune system, causing dysfunction and neuroinflammation ( 104 ), is related to poor air quality. Yet, increases in serum levels of immunoglobulins (IgA, IgM) and the complement component C3 are observed ( 106 ). Another issue is that antigen presentation is affected by air pollutants, as there is an upregulation of costimulatory molecules such as CD80 and CD86 on macrophages ( 110 ).
As is known, skin is our shield against ultraviolet radiation (UVR) and other pollutants, as it is the most exterior layer of our body. Traffic-related pollutants, such as PAHs, VOCs, oxides, and PM, may cause pigmented spots on our skin ( 111 ). On the one hand, as already stated, when pollutants penetrate through the skin or are inhaled, damage to the organs is observed, as some of these pollutants are mutagenic and carcinogenic, and, specifically, they affect the liver and lung. On the other hand, air pollutants (and those in the troposphere) reduce the adverse effects of ultraviolet radiation UVR in polluted urban areas ( 111 ). Air pollutants absorbed by the human skin may contribute to skin aging, psoriasis, acne, urticaria, eczema, and atopic dermatitis ( 111 ), usually caused by exposure to oxides and photochemical smoke ( 111 ). Exposure to PM and cigarette smoking act as skin-aging agents, causing spots, dyschromia, and wrinkles. Lastly, pollutants have been associated with skin cancer ( 111 ).
Higher morbidity is reported to fetuses and children when exposed to the above dangers. Impairment in fetal growth, low birth weight, and autism have been reported ( 112 ).
Another exterior organ that may be affected is the eye. Contamination usually comes from suspended pollutants and may result in asymptomatic eye outcomes, irritation ( 112 ), retinopathy, or dry eye syndrome ( 113 , 114 ).
Environmental Impact of Air Pollution
Air pollution is harming not only human health but also the environment ( 115 ) in which we live. The most important environmental effects are as follows.
Acid rain is wet (rain, fog, snow) or dry (particulates and gas) precipitation containing toxic amounts of nitric and sulfuric acids. They are able to acidify the water and soil environments, damage trees and plantations, and even damage buildings and outdoor sculptures, constructions, and statues.
Haze is produced when fine particles are dispersed in the air and reduce the transparency of the atmosphere. It is caused by gas emissions in the air coming from industrial facilities, power plants, automobiles, and trucks.
Ozone , as discussed previously, occurs both at ground level and in the upper level (stratosphere) of the Earth's atmosphere. Stratospheric ozone is protecting us from the Sun's harmful ultraviolet (UV) rays. In contrast, ground-level ozone is harmful to human health and is a pollutant. Unfortunately, stratospheric ozone is gradually damaged by ozone-depleting substances (i.e., chemicals, pesticides, and aerosols). If this protecting stratospheric ozone layer is thinned, then UV radiation can reach our Earth, with harmful effects for human life (skin cancer) ( 116 ) and crops ( 117 ). In plants, ozone penetrates through the stomata, inducing them to close, which blocks CO 2 transfer and induces a reduction in photosynthesis ( 118 ).
Global climate change is an important issue that concerns mankind. As is known, the “greenhouse effect” keeps the Earth's temperature stable. Unhappily, anthropogenic activities have destroyed this protecting temperature effect by producing large amounts of greenhouse gases, and global warming is mounting, with harmful effects on human health, animals, forests, wildlife, agriculture, and the water environment. A report states that global warming is adding to the health risks of poor people ( 119 ).
People living in poorly constructed buildings in warm-climate countries are at high risk for heat-related health problems as temperatures mount ( 119 ).
Wildlife is burdened by toxic pollutants coming from the air, soil, or the water ecosystem and, in this way, animals can develop health problems when exposed to high levels of pollutants. Reproductive failure and birth effects have been reported.
Eutrophication is occurring when elevated concentrations of nutrients (especially nitrogen) stimulate the blooming of aquatic algae, which can cause a disequilibration in the diversity of fish and their deaths.
Without a doubt, there is a critical concentration of pollution that an ecosystem can tolerate without being destroyed, which is associated with the ecosystem's capacity to neutralize acidity. The Canada Acid Rain Program established this load at 20 kg/ha/yr ( 120 ).
Hence, air pollution has deleterious effects on both soil and water ( 121 ). Concerning PM as an air pollutant, its impact on crop yield and food productivity has been reported. Its impact on watery bodies is associated with the survival of living organisms and fishes and their productivity potential ( 121 ).
An impairment in photosynthetic rhythm and metabolism is observed in plants exposed to the effects of ozone ( 121 ).
Sulfur and nitrogen oxides are involved in the formation of acid rain and are harmful to plants and marine organisms.
Last but not least, as mentioned above, the toxicity associated with lead and other metals is the main threat to our ecosystems (air, water, and soil) and living creatures ( 121 ).
In 2018, during the first WHO Global Conference on Air Pollution and Health, the WHO's General Director, Dr. Tedros Adhanom Ghebreyesus, called air pollution a “silent public health emergency” and “the new tobacco” ( 122 ).
Undoubtedly, children are particularly vulnerable to air pollution, especially during their development. Air pollution has adverse effects on our lives in many different respects.
Diseases associated with air pollution have not only an important economic impact but also a societal impact due to absences from productive work and school.
Despite the difficulty of eradicating the problem of anthropogenic environmental pollution, a successful solution could be envisaged as a tight collaboration of authorities, bodies, and doctors to regularize the situation. Governments should spread sufficient information and educate people and should involve professionals in these issues so as to control the emergence of the problem successfully.
Technologies to reduce air pollution at the source must be established and should be used in all industries and power plants. The Kyoto Protocol of 1997 set as a major target the reduction of GHG emissions to below 5% by 2012 ( 123 ). This was followed by the Copenhagen summit, 2009 ( 124 ), and then the Durban summit of 2011 ( 125 ), where it was decided to keep to the same line of action. The Kyoto protocol and the subsequent ones were ratified by many countries. Among the pioneers who adopted this important protocol for the world's environmental and climate “health” was China ( 3 ). As is known, China is a fast-developing economy and its GDP (Gross Domestic Product) is expected to be very high by 2050, which is defined as the year of dissolution of the protocol for the decrease in gas emissions.
A more recent international agreement of crucial importance for climate change is the Paris Agreement of 2015, issued by the UNFCCC (United Nations Climate Change Committee). This latest agreement was ratified by a plethora of UN (United Nations) countries as well as the countries of the European Union ( 126 ). In this vein, parties should promote actions and measures to enhance numerous aspects around the subject. Boosting education, training, public awareness, and public participation are some of the relevant actions for maximizing the opportunities to achieve the targets and goals on the crucial matter of climate change and environmental pollution ( 126 ). Without any doubt, technological improvements makes our world easier and it seems difficult to reduce the harmful impact caused by gas emissions, we could limit its use by seeking reliable approaches.
Synopsizing, a global prevention policy should be designed in order to combat anthropogenic air pollution as a complement to the correct handling of the adverse health effects associated with air pollution. Sustainable development practices should be applied, together with information coming from research in order to handle the problem effectively.
At this point, international cooperation in terms of research, development, administration policy, monitoring, and politics is vital for effective pollution control. Legislation concerning air pollution must be aligned and updated, and policy makers should propose the design of a powerful tool of environmental and health protection. As a result, the main proposal of this essay is that we should focus on fostering local structures to promote experience and practice and extrapolate these to the international level through developing effective policies for sustainable management of ecosystems.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
IM is employed by the company Delphis S.A.
The remaining authors declare that the present review paper was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. WHO. Air Pollution . WHO. Available online at: http://www.who.int/airpollution/en/ (accessed October 5, 2019).
Google Scholar
2. Moores FC. Climate change and air pollution: exploring the synergies and potential for mitigation in industrializing countries. Sustainability . (2009) 1:43–54. doi: 10.3390/su1010043
CrossRef Full Text | Google Scholar
3. USGCRP (2009). Global Climate Change Impacts in the United States. In: Karl TR, Melillo JM, Peterson TC, editors. Climate Change Impacts by Sectors: Ecosystems . New York, NY: United States Global Change Research Program. Cambridge University Press.
4. Marlon JR, Bloodhart B, Ballew MT, Rolfe-Redding J, Roser-Renouf C, Leiserowitz A, et al. (2019). How hope and doubt affect climate change mobilization. Front. Commun. 4:20. doi: 10.3389/fcomm.2019.00020
5. Eze IC, Schaffner E, Fischer E, Schikowski T, Adam M, Imboden M, et al. Long- term air pollution exposure and diabetes in a population-based Swiss cohort. Environ Int . (2014) 70:95–105. doi: 10.1016/j.envint.2014.05.014
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Kelishadi R, Poursafa P. Air pollution and non-respiratory health hazards for children. Arch Med Sci . (2010) 6:483–95. doi: 10.5114/aoms.2010.14458
7. Manucci PM, Franchini M. Health effects of ambient air pollution in developing countries. Int J Environ Res Public Health . (2017) 14:1048. doi: 10.3390/ijerph14091048
8. Burden of Disease from Ambient and Household Air Pollution . Available online: http://who.int/phe/health_topics/outdoorair/databases/en/ (accessed August 15, 2017).
9. Hashim D, Boffetta P. Occupational and environmental exposures and cancers in developing countries. Ann Glob Health . (2014) 80:393–411. doi: 10.1016/j.aogh.2014.10.002
10. Guo Y, Zeng H, Zheng R, Li S, Pereira G, Liu Q, et al. The burden of lung cancer mortality attributable to fine particles in China. Total Environ Sci . (2017) 579:1460–6. doi: 10.1016/j.scitotenv.2016.11.147
11. Hou Q, An XQ, Wang Y, Guo JP. An evaluation of resident exposure to respirable particulate matter and health economic loss in Beijing during Beijing 2008 Olympic Games. Sci Total Environ . (2010) 408:4026–32. doi: 10.1016/j.scitotenv.2009.12.030
12. Kan H, Chen R, Tong S. Ambient air pollution, climate change, and population health in China. Environ Int . (2012) 42:10–9. doi: 10.1016/j.envint.2011.03.003
13. Burroughs Peña MS, Rollins A. Environmental exposures and cardiovascular disease: a challenge for health and development in low- and middle-income countries. Cardiol Clin . (2017) 35:71–86. doi: 10.1016/j.ccl.2016.09.001
14. Kankaria A, Nongkynrih B, Gupta S. Indoor air pollution in india: implications on health and its control. Indian J Comm Med . 39:203–7. doi: 10.4103/0970-0218.143019
15. Parajuli I, Lee H, Shrestha KR. Indoor air quality and ventilation assessment of rural mountainous households of Nepal. Int J Sust Built Env . (2016) 5:301–11. doi: 10.1016/j.ijsbe.2016.08.003
16. Saud T, Gautam R, Mandal TK, Gadi R, Singh DP, Sharma SK. Emission estimates of organic and elemental carbon from household biomass fuel used over the Indo-Gangetic Plain (IGP), India. Atmos Environ . (2012) 61:212–20. doi: 10.1016/j.atmosenv.2012.07.030
17. Singh DP, Gadi R, Mandal TK, Saud T, Saxena M, Sharma SK. Emissions estimates of PAH from biomass fuels used in rural sector of Indo-Gangetic Plains of India. Atmos Environ . (2013) 68:120–6. doi: 10.1016/j.atmosenv.2012.11.042
18. Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M BN. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ . (2008) 86:390–4. doi: 10.2471/BLT.07.044529
19. Kassomenos P, Kelessis A, Petrakakis M, Zoumakis N, Christides T, Paschalidou AK. Air Quality assessment in a heavily-polluted urban Mediterranean environment through Air Quality indices. Ecol Indic . (2012) 18:259–68. doi: 10.1016/j.ecolind.2011.11.021
20. Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med . (1993) 329:1753–9. doi: 10.1056/NEJM199312093292401
21. Schwela DH, Köth-Jahr I. Leitfaden für die Aufstellung von Luftreinhalteplänen [Guidelines for the Implementation of Clean Air Implementation Plans]. Landesumweltamt des Landes Nordrhein Westfalen. State Environmental Service of the State of North Rhine-Westphalia (1994).
22. Newlands M. Environmental Activism, Environmental Politics, and Representation: The Framing of the British Environmental Activist Movement . Ph.D. thesis. University of East London, United Kingdom (2015).
23. NEPIS (National Service Center for Environmental Publications) US EPA (Environmental Protection Agency) (2017). Available online at: https://www.epa.gov/clean-air-act-overview/air-pollution-current-and-future-challenges (accessed August 15, 2017).
24. NRC (National Research Council). Available online at: https://www.nap.edu/read/10728/chapter/1,2014 (accessed September 17, 2019).
25. Bull A. Traffic Congestion: The Problem and How to Deal With It . Santiago: Nationes Unidas, Cepal (2003).
26. Spiegel J, Maystre LY. Environmental Pollution Control, Part VII - The Environment, Chapter 55, Encyclopedia of Occupational Health and Safety . Available online at: http://www.ilocis.org/documents/chpt55e.htm (accessed September 17, 2019).
27. European Community Reports. Assessment of the Effectiveness of European Air Quality Policies and Measures: Case Study 2; Comparison of the EU and US Air Quality Standards and Planning Requirements. (2004). Available online at: https://ec.europa.eu/environment/archives/cafe/activities/pdf/case_study2.pdf (accessed September 22, 2019).
28. Gibson R, Ward S. Parties in the digital age; a review. J Represent Democracy . (2009) 45:87–100. doi: 10.1080/00344890802710888
29. Kaun A, Uldam J. Digital activism: after the hype. New Media Soc. (2017) 20:2099–106. doi: 10.1177/14614448177319
30. Sivitanides M, Shah V. The era of digital activism. In: 2011 Conference for Information Systems Applied Research(CONISAR) Proceedings Wilmington North Carolina, USA . Available online at: https://www.arifyildirim.com/ilt510/marcos.sivitanides.vivek.shah.pdf (accessed September 22, 2019).
31. Möller L, Schuetzle D, Autrup H. Future research needs associated with the assessment of potential human health risks from exposure to toxic ambient air pollutants. Environ Health Perspect . (1994) 102(Suppl. 4):193–210. doi: 10.1289/ehp.94102s4193
32. Jacobson MZ, Jacobson PMZ. Atmospheric Pollution: History, Science, and Regulation. Cambridge University Press (2002). p. 206. doi: 10.1256/wea.243.02
33. Stover RH. Flooding of soil for disease control. In: Mulder D, editor. Chapter 3. Developments in Agricultural and Managed Forest Ecology . Elsevier (1979). p. 19–28. Available online at: http://www.sciencedirect.com/science/article/pii/B9780444416926500094 doi: 10.1016/B978-0-444-41692-6.50009-4 (accessed July 1, 2019).
34. Maipa V, Alamanos Y, Bezirtzoglou E. Seasonal fluctuation of bacterial indicators in coastal waters. Microb Ecol Health Dis . (2001) 13:143–6. doi: 10.1080/089106001750462687
35. Bezirtzoglou E, Dimitriou D, Panagiou A. Occurrence of Clostridium perfringens in river water by using a new procedure. Anaerobe . (1996) 2:169–73. doi: 10.1006/anae.1996.0022
36. Kjellstrom T, Lodh M, McMichael T, Ranmuthugala G, Shrestha R, Kingsland S. Air and Water Pollution: Burden and Strategies for Control. DCP, Chapter 43. 817–32 p. Available online at: https://www.dcp-3.org/sites/default/files/dcp2/DCP43.pdf (accessed September 17, 2017).
37. Pathak RK, Wang T, Ho KF, Lee SC. Characteristics of summertime PM2.5 organic and elemental carbon in four major Chinese cities: implications of high acidity for water- soluble organic carbon (WSOC). Atmos Environ . (2011) 45:318–25. doi: 10.1016/j.atmosenv.2010.10.021
38. Bonavigo L, Zucchetti M, Mankolli H. Water radioactive pollution and related environmental aspects. J Int Env Appl Sci . (2009) 4:357–63
39. World Health Organization (WHO). Preventing Disease Through Healthy Environments: Towards an Estimate of the Environmental Burden of Disease . 1106 p. Available online at: https://www.who.int/quantifying_ehimpacts/publications/preventingdisease.pdf (accessed September 22, 2019).
40. Stansfeld SA. Noise effects on health in the context of air pollution exposure. Int J Environ Res Public Health . (2015) 12:12735–60. doi: 10.3390/ijerph121012735
41. Ethical Unicorn. Everything You Need To Know About Aerosols & Air Pollution. (2019). Available online at: https://ethicalunicorn.com/2019/04/29/everything-you-need-to-know-about-aerosols-air-pollution/ (accessed October 4, 2019).
42. Colbeck I, Lazaridis M. Aerosols and environmental pollution. Sci Nat . (2009) 97:117–31. doi: 10.1007/s00114-009-0594-x
43. Incecik S, Gertler A, Kassomenos P. Aerosols and air quality. Sci Total Env . (2014) 355, 488–9. doi: 10.1016/j.scitotenv.2014.04.012
44. D'Amato G, Pawankar R, Vitale C, Maurizia L. Climate change and air pollution: effects on respiratory allergy. Allergy Asthma Immunol Res . (2016) 8:391–5. doi: 10.4168/aair.2016.8.5.391
45. Bezirtzoglou C, Dekas K, Charvalos E. Climate changes, environment and infection: facts, scenarios and growing awareness from the public health community within Europe. Anaerobe . (2011) 17:337–40. doi: 10.1016/j.anaerobe.2011.05.016
46. Castelli F, Sulis G. Migration and infectious diseases. Clin Microbiol Infect . (2017) 23:283–9. doi: 10.1016/j.cmi.2017.03.012
47. Watson JT, Gayer M, Connolly MA. Epidemics after natural disasters. Emerg Infect Dis . (2007) 13:1–5. doi: 10.3201/eid1301.060779
48. Fenn B. Malnutrition in Humanitarian Emergencies . Available online at: https://www.who.int/diseasecontrol_emergencies/publications/idhe_2009_london_malnutrition_fenn.pdf . (accessed August 15, 2017).
49. Lindh E, Argentini C, Remoli ME, Fortuna C, Faggioni G, Benedetti E, et al. The Italian 2017 outbreak Chikungunya virus belongs to an emerging Aedes albopictus –adapted virus cluster introduced from the Indian subcontinent. Open Forum Infect Dis. (2019) 6:ofy321. doi: 10.1093/ofid/ofy321
50. Calba C, Guerbois-Galla M, Franke F, Jeannin C, Auzet-Caillaud M, Grard G, Pigaglio L, Decoppet A, et al. Preliminary report of an autochthonous chikungunya outbreak in France, July to September 2017. Eur Surveill . (2017) 22:17-00647. doi: 10.2807/1560-7917.ES.2017.22.39.17-00647
51. Menne B, Murray V. Floods in the WHO European Region: Health Effects and Their Prevention . Copenhagen: WHO; Weltgesundheits organisation, Regionalbüro für Europa (2013). Available online at: http://www.euro.who.int/data/assets/pdf_file/0020/189020/e96853.pdf (accessed 15 August 2017).
52. Schneider SH. The greenhouse effect: science and policy. Science . (1989) 243:771–81. doi: 10.1126/science.243.4892.771
53. Wilson WE, Suh HH. Fine particles and coarse particles: concentration relationships relevant to epidemiologic studies. J Air Waste Manag Assoc . (1997) 47:1238–49. doi: 10.1080/10473289.1997.10464074
54. US EPA (US Environmental Protection Agency) (2018). Available online at: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics (accessed September 22, 2018).
55. Cheung K, Daher N, Kam W, Shafer MM, Ning Z, Schauer JJ, et al. Spatial and temporal variation of chemical composition and mass closure of ambient coarse particulate matter (PM10–2.5) in the Los Angeles area. Atmos Environ . (2011) 45:2651–62. doi: 10.1016/j.atmosenv.2011.02.066
56. Zhang L, Yang Y, Li Y, Qian ZM, Xiao W, Wang X, et al. Short-term and long-term effects of PM2.5 on acute nasopharyngitis in 10 communities of Guangdong, China. Sci Total Env. (2019) 688:136–42. doi: 10.1016/j.scitotenv.2019.05.470.
57. Kloog I, Ridgway B, Koutrakis P, Coull BA, Schwartz JD. Long- and short-term exposure to PM2.5 and mortality using novel exposure models, Epidemiology . (2013) 24:555–61. doi: 10.1097/EDE.0b013e318294beaa
58. New Hampshire Department of Environmental Services. Current and Forecasted Air Quality in New Hampshire . Environmental Fact Sheet (2019). Available online at: https://www.des.nh.gov/organization/commissioner/pip/factsheets/ard/documents/ard-16.pdf (accessed September 22, 2019).
59. Kappos AD, Bruckmann P, Eikmann T, Englert N, Heinrich U, Höppe P, et al. Health effects of particles in ambient air. Int J Hyg Environ Health . (2004) 207:399–407. doi: 10.1078/1438-4639-00306
60. Boschi N (Ed.). Defining an educational framework for indoor air sciences education. In: Education and Training in Indoor Air Sciences . Luxembourg: Springer Science & Business Media (2012). 245 p.
61. Heal MR, Kumar P, Harrison RM. Particles, air quality, policy and health. Chem Soc Rev . (2012) 41:6606–30. doi: 10.1039/c2cs35076a
62. Bezirtzoglou E, Alexopoulos A. Ozone history and ecosystems: a goliath from impacts to advance industrial benefits and interests, to environmental and therapeutical strategies. In: Ozone Depletion, Chemistry and Impacts. (2009). p. 135–45.
63. Villányi V, Turk B, Franc B, Csintalan Z. Ozone Pollution and its Bioindication. In: Villányi V, editor. Air Pollution . London: Intech Open (2010). doi: 10.5772/10047
64. Massachusetts Department of Public Health. Massachusetts State Health Assessment . Boston, MA (2017). Available online at: https://www.mass.gov/files/documents/2017/11/03/2017%20MA%20SHA%20final%20compressed.pdf (accessed October 30, 2017).
65. Lorenzini G, Saitanis C. Ozone: A Novel Plant “Pathogen.” In: Sanitá di Toppi L, Pawlik-Skowrońska B, editors. Abiotic Stresses in Plant Springer Link (2003). p. 205–29. doi: 10.1007/978-94-017-0255-3_8
66. Fares S, Vargas R, Detto M, Goldstein AH, Karlik J, Paoletti E, et al. Tropospheric ozone reduces carbon assimilation in trees: estimates from analysis of continuous flux measurements. Glob Change Biol . (2013) 19:2427–43. doi: 10.1111/gcb.12222
67. Harmens H, Mills G, Hayes F, Jones L, Norris D, Fuhrer J. Air Pollution and Vegetation . ICP Vegetation Annual Report 2006/2007. (2012)
68. Emberson LD, Pleijel H, Ainsworth EA, den Berg M, Ren W, Osborne S, et al. Ozone effects on crops and consideration in crop models. Eur J Agron . (2018) 100:19–34. doi: 10.1016/j.eja.2018.06.002
69. Alexopoulos A, Plessas S, Ceciu S, Lazar V, Mantzourani I, Voidarou C, et al. Evaluation of ozone efficacy on the reduction of microbial population of fresh cut lettuce ( Lactuca sativa ) and green bell pepper ( Capsicum annuum ). Food Control . (2013) 30:491–6. doi: 10.1016/j.foodcont.2012.09.018
70. Alexopoulos A, Plessas S, Kourkoutas Y, Stefanis C, Vavias S, Voidarou C, et al. Experimental effect of ozone upon the microbial flora of commercially produced dairy fermented products. Int J Food Microbiol . (2017) 246:5–11. doi: 10.1016/j.ijfoodmicro.2017.01.018
71. Maggio A, Fagnano M. Ozone damages to mediterranean crops: physiological responses. Ital J Agron . (2008) 13–20. doi: 10.4081/ija.2008.13
72. McCarthy JT, Pelle E, Dong K, Brahmbhatt K, Yarosh D, Pernodet N. Effects of ozone in normal human epidermal keratinocytes. Exp Dermatol . (2013) 22:360–1. doi: 10.1111/exd.12125
73. WHO. Health Risks of Ozone From Long-Range Transboundary Air Pollution . Available online at: http://www.euro.who.int/data/assets/pdf_file/0005/78647/E91843.pdf (accessed August 15, 2019).
74. Thiele JJ, Traber MG, Tsang K, Cross CE, Packer L. In vivo exposure to ozone depletes vitamins C and E and induces lipid peroxidation in epidermal layers of murine skin. Free Radic Biol Med. (1997) 23:365–91. doi: 10.1016/S0891-5849(96)00617-X
75. Hatch GE, Slade R, Harris LP, McDonnell WF, Devlin RB, Koren HS, et al. Ozone dose and effect in humans and rats. A comparison using oxygen- 18 labeling and bronchoalveolar lavage. Am J Respir Crit Care Med . (1994) 150:676–83. doi: 10.1164/ajrccm.150.3.8087337
76. Lippmann M. Health effects of ozone. A critical review. JAPCA . (1989) 39:672–95. doi: 10.1080/08940630.1989.10466554
77. Gryparis A, Forsberg B, Katsouyanni K, Analitis A, Touloumi G, Schwartz J, et al. Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. Am J Respir Crit Care Med . (2004) 170:1080–7. doi: 10.1164/rccm.200403-333OC
78. Soon W, Baliunas SL, Robinson AB, Robinson ZW. Environmental effects of increased atmospheric carbon dioxide. Climate Res . (1999) 13:149–64 doi: 10.1260/0958305991499694
79. Richmont-Bryant J, Owen RC, Graham S, Snyder M, McDow S, Oakes M, et al. Estimation of on-road NO2 concentrations, NO2/NOX ratios, and related roadway gradients from near-road monitoring data. Air Qual Atm Health . (2017) 10:611–25. doi: 10.1007/s11869-016-0455-7
80. Hesterberg TW, Bunn WB, McClellan RO, Hamade AK, Long CM, Valberg PA. Critical review of the human data on short-term nitrogen dioxide (NO 2 ) exposures: evidence for NO2 no-effect levels. Crit Rev Toxicol . (2009) 39:743–81. doi: 10.3109/10408440903294945
81. Chen T-M, Gokhale J, Shofer S, Kuschner WG. Outdoor air pollution: nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am J Med Sci . (2007) 333:249–56. doi: 10.1097/MAJ.0b013e31803b900f
82. US EPA. Table of Historical SO 2 NAAQS, Sulfur US EPA . Available online at: https://www3.epa.gov/ttn/naaqs/standards/so2/s_so2_history.html (accessed October 5, 2019).
83. WHO Regional Office of Europe (2000). Available online at: https://euro.who.int/_data/assets/pdf_file/0020/123086/AQG2ndEd_7_4Sulfuroxide.pdf
84. Pruss-Ustun A, Fewrell L, Landrigan PJ, Ayuso-Mateos JL. Lead exposure. Comparative Quantification of Health Risks . World Health Organization. p. 1495–1542. Available online at: https://www.who.int/publications/cra/chapters/volume2/1495-1542.pdf?ua=1
PubMed Abstract | Google Scholar
85. Goyer RA. Transplacental transport of lead. Environ Health Perspect . (1990) 89:101–5. doi: 10.1289/ehp.9089101
86. National Institute of Environmental Health Sciences (NIH). Lead and Your Health . (2013). 1–4 p. Available online at: https://www.niehs.nih.gov/health/materials/lead_and_your_health_508.pdf (accessed September 17, 2019).
87. Farhat A, Mohammadzadeh A, Balali-Mood M, Aghajanpoor-Pasha M, Ravanshad Y. Correlation of blood lead level in mothers and exclusively breastfed infants: a study on infants aged less than six months. Asia Pac J Med Toxicol . (2013) 2:150–2.
88. Assi MA, Hezmee MNM, Haron AW, Sabri MYM, Rajion MA. The detrimental effects of lead on human and animal health. Vet World . (2016) 9:660–71. doi: 10.14202/vetworld.2016.660-671
89. Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt J Pet . (2016) 25:107–23. doi: 10.1016/j.ejpe.2015.03.011
90. Kumar A, Singh BP, Punia M, Singh D, Kumar K, Jain VK. Assessment of indoor air concentrations of VOCs and their associated health risks in the library of Jawaharlal Nehru University, New Delhi. Environ Sci Pollut Res Int . (2014) 21:2240–8. doi: 10.1007/s11356-013-2150-7
91. Molhave L, Clausen G, Berglund B, Ceaurriz J, Kettrup A, Lindvall T, et al. Total Volatile Organic Compounds (TVOC) in Indoor Air Quality Investigations. Indoor Air . 7:225–240. doi: 10.1111/j.1600-0668.1997.00002.x
92. Gibb T. Indoor Air Quality May be Hazardous to Your Health . MSU Extension. Available online at: https://www.canr.msu.edu/news/indoor_air_quality_may_be_hazardous_to_your_health (accessed October 5, 2019).
93. Ebersviller S, Lichtveld K, Sexton KG, Zavala J, Lin Y-H, Jaspers I, et al. Gaseous VOCs rapidly modify particulate matter and its biological effects – Part 1: simple VOCs and model PM. Atmos Chem Phys Discuss . (2012) 12:5065–105. doi: 10.5194/acpd-12-5065-2012
94. WHO (World Health Organization). Dioxins and Their Effects on Human Health. Available online at: https://www.who.int/news-room/fact-sheets/detail/dioxins-and-their-effects-on-human-health (accessed October 5, 2019).
95. EEA (European Environmental Agency). Air Quality Standards to the European Union and WHO . Available online at: https://www.eea.europa.eu/themes/data-and-maps/figures/air-quality-standards-under-the
96. Nakano T, Otsuki T. [Environmental air pollutants and the risk of cancer]. (Japanese). Gan To Kagaku Ryoho . (2013) 40:1441–5.
97. Kurt OK, Zhang J, Pinkerton KE. Pulmonary health effects of air pollution. Curr Opin Pulm Med . (2016) 22:138–43. doi: 10.1097/MCP.0000000000000248
98. Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet . (2014) 383:1581–92. doi: 10.1016/S0140-6736(14)60617-6
99. Jiang X-Q, Mei X-D, Feng D. Air pollution and chronic airway diseases: what should people know and do? J Thorac Dis . (2016) 8:E31–40.
100. Bourdrel T, Bind M-A, Béjot Y, Morel O, Argacha J-F. Cardiovascular effects of air pollution. Arch Cardiovasc Dis . (2017) 110:634–42. doi: 10.1016/j.acvd.2017.05.003
101. Hoffmann B, Moebus S, Möhlenkamp S, Stang A, Lehmann N, Dragano N, et al. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation . (2007) 116:489–496. doi: 10.1161/CIRCULATIONAHA.107.693622
102. Katholi RE, Couri DM. Left ventricular hypertrophy: major risk factor in patients with hypertension: update and practical clinical applications. Int J Hypertens . (2011) 2011:495349. doi: 10.4061/2011/495349
103. Leary PJ, Kaufman JD, Barr RG, Bluemke DA, Curl CL, Hough CL, et al. Traffic- related air pollution and the right ventricle. the multi-ethnic study of atherosclerosis. Am J Respir Crit Care Med . (2014) 189:1093–100. doi: 10.1164/rccm.201312-2298OC
104. Genc S, Zadeoglulari Z, Fuss SH, Genc K. The adverse effects of air pollution on the nervous system. J Toxicol . (2012) 2012:782462. doi: 10.1155/2012/782462
105. Calderon-Garciduenas L, Azzarelli B, Acuna H, et al. Air pollution and brain damage. Toxicol Pathol. (2002) 30:373–89. doi: 10.1080/01926230252929954
106. Rückerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect . (2007) 115:1072–80. doi: 10.1289/ehp.10021
107. Peters A, Veronesi B, Calderón-Garcidueñas L, Gehr P, Chen LC, Geiser M, et al. Translocation and potential neurological effects of fine and ultrafine particles a critical update. Part Fibre Toxicol . (2006) 3:13–8. doi: 10.1186/1743-8977-3-13
108. Kelly FJ. Dietary antioxidants and environmental stress. Proc Nutr Soc . (2004) 63:579–85. doi: 10.1079/PNS2004388
109. Bellinger DC. Very low lead exposures and children's neurodevelopment. Curr Opin Pediatr . (2008) 20:172–7. doi: 10.1097/MOP.0b013e3282f4f97b
110. Balbo P, Silvestri M, Rossi GA, Crimi E, Burastero SE. Differential role of CD80 and CD86 on alveolar macrophages in the presentation of allergen to T lymphocytes in asthma. Clin Exp Allergy J Br Soc Allergy Clin Immunol . (2001) 31:625–36. doi: 10.1046/j.1365-2222.2001.01068.x
111. Drakaki E, Dessinioti C, Antoniou C. Air pollution and the skin. Front Environ Sci Eng China . (2014) 15:2–8. doi: 10.3389/fenvs.2014.00011
112. Weisskopf MG, Kioumourtzoglou M-A, Roberts AL. Air pollution and autism spectrum disorders: causal or confounded? Curr Environ Health Rep . (2015) 2:430–9. doi: 10.1007/s40572-015-0073-9
113. Mo Z, Fu Q, Lyu D, Zhang L, Qin Z, Tang Q, et al. Impacts of air pollution on dry eye disease among residents in Hangzhou, China: a case-crossover study. Environ Pollut . (2019) 246:183–9. doi: 10.1016/j.envpol.2018.11.109
114. Klopfer J. Effects of environmental air pollution on the eye. J Am Optom Assoc . (1989) 60:773–8.
115. Ashfaq A, Sharma P. Environmental effects of air pollution and application of engineered methods to combat the problem. J Indust Pollut Control . (2012) 29.
116. Madronich S, de Gruijl F. Skin cancer and UV radiation. Nature . (1993) 366:23–9. doi: 10.1038/366023a0
117. Teramura A. Effects of UV-B radiation on the growth and yield of crop plants. Physiol Plant . (2006) 58:415–27. doi: 10.1111/j.1399-3054.1983.tb04203.x
118. Singh E, Tiwari S, Agrawal M. Effects of elevated ozone on photosynthesis and stomatal conductance of two soybean varieties: a case study to assess impacts of one component of predicted global climate change. Plant Biol Stuttg Ger . (2009) 11(Suppl. 1):101–8. doi: 10.1111/j.1438-8677.2009.00263.x
119. Manderson L. How global Warming is Adding to the Health Risks of Poor People . The Conversation. University of the Witwatersrand. Available online at: http://theconversation.com/how-global-warming-is-adding-to-the-health-risks-of-poor-people-109520 (accessed October 5, 2019).
120. Ministers of Energy and Environment. Federal/Provincial/Territorial Ministers of Energy and Environment (Canada), editor. The Canada-Wide Acid Rain Strategy for Post-2000 . Halifax: The Ministers (1999). 11 p.
121. Zuhara S, Isaifan R. The impact of criteria air pollutants on soil and water: a review. (2018) 278–84. doi: 10.30799/jespr.133.18040205
122. WHO. First WHO Global Conference on Air Pollution and Health. (2018). Available online at: https://www.who.int/airpollution/events/conference/en/ (accessed October 6, 2019).
123. What is the Kyoto Protocol? UNFCCC . Available online at: https://unfccc.int/kyoto__protocol (accessed October 6, 2019).
124. CopenhagenClimate Change Conference (UNFCCC) . Available online at: https://unfccc.int/process-and-meetings/conferences/past-conferences/copenhagen-climate-change-conference-december-2009/copenhagen-climate-change-conference-december-2009 (accessed October 6, 2019).
125. Durban Climate Change Conference,. UNFCCC (2011). Available online at: https://unfccc.int/process-and-meetings/conferences/past-conferences/copenhagen-climate-change-conference-december-2009/copenhagen-climate-change-conference-december-2009 (accessed October 6, 2019).
126. Paris Climate Change Agreement,. (2016). Available online at: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement
Keywords: air pollution, environment, health, public health, gas emission, policy
Citation: Manisalidis I, Stavropoulou E, Stavropoulos A and Bezirtzoglou E (2020) Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 8:14. doi: 10.3389/fpubh.2020.00014
Received: 17 October 2019; Accepted: 17 January 2020; Published: 20 February 2020.
Reviewed by:
Copyright © 2020 Manisalidis, Stavropoulou, Stavropoulos and Bezirtzoglou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ioannis Manisalidis, giannismanisal@gmail.com ; Elisavet Stavropoulou, elisabeth.stavropoulou@gmail.com
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Accessibility Links
- Skip to content
- Skip to search IOPscience
- Skip to Journals list
- Accessibility help
- Accessibility Help
Click here to close this panel.

Purpose-led Publishing is a coalition of three not-for-profit publishers in the field of physical sciences: AIP Publishing, the American Physical Society and IOP Publishing.
Together, as publishers that will always put purpose above profit, we have defined a set of industry standards that underpin high-quality, ethical scholarly communications.
We are proudly declaring that science is our only shareholder.
Extreme weather impacts of climate change: an attribution perspective
Ben Clarke 5,1 , Friederike Otto 2 , Rupert Stuart-Smith 1,3 and Luke Harrington 4
Published 28 June 2022 • © 2022 The Author(s). Published by IOP Publishing Ltd Environmental Research: Climate , Volume 1 , Number 1 Climate Variability and Change: Causes, Consequences and Solutions Citation Ben Clarke et al 2022 Environ. Res.: Climate 1 012001 DOI 10.1088/2752-5295/ac6e7d
Article metrics
65621 Total downloads
Share this article
Author e-mails.
Author affiliations
1 Environmental Change Institute, University of Oxford, Oxford OX1 3QY, United Kingdom
2 Grantham Institute, Imperial College London, London SW7 2AZ, United Kingdom
3 Oxford Sustainable Law Programme, University of Oxford, Oxford OX1 3QY, United Kingdom
4 New Zealand Climate Change Research Institute, Victoria University of Wellington, Wellington 6012, New Zealand
Author notes
5 Author to whom any correspondence should be addressed.
Ben Clarke https://orcid.org/0000-0002-9498-6266
Friederike Otto https://orcid.org/0000-0001-8166-5917
Luke Harrington https://orcid.org/0000-0002-1699-6119
- Received 14 January 2022
- Accepted 11 May 2022
- Published 28 June 2022
Peer review information
Method : Single-anonymous Revisions: 1 Screened for originality? Yes
Buy this article in print
Extreme event attribution aims to elucidate the link between global climate change, extreme weather events, and the harms experienced on the ground by people, property, and nature. It therefore allows the disentangling of different drivers of extreme weather from human-induced climate change and hence provides valuable information to adapt to climate change and to assess loss and damage. However, providing such assessments systematically is currently out of reach. This is due to limitations in attribution science, including the capacity for studying different types of events, as well as the geographical heterogeneity of both climate and impact data availability. Here, we review current knowledge of the influences of climate change on five different extreme weather hazards (extreme temperatures, heavy rainfall, drought, wildfire, tropical cyclones), the impacts of recent extreme weather events of each type, and thus the degree to which various impacts are attributable to climate change. For instance, heat extremes have increased in likelihood and intensity worldwide due to climate change, with tens of thousands of deaths directly attributable. This is likely a significant underestimate due to the limited availability of impact information in lower- and middle-income countries. Meanwhile, tropical cyclone rainfall and storm surge height have increased for individual events and across all basins. In the North Atlantic basin, climate change amplified the rainfall of events that, combined, caused half a trillion USD in damages. At the same time, severe droughts in many parts of the world are not attributable to climate change. To advance our understanding of present-day extreme weather impacts due to climate change developments on several levels are required. These include improving the recording of extreme weather impacts around the world, improving the coverage of attribution studies across different events and regions, and using attribution studies to explore the contributions of both climate and non-climate drivers of impacts.
Export citation and abstract BibTeX RIS
Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license . Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Every year, climate change manifests through more intense extreme weather events, including heatwaves, droughts and heavy rainfall. This leads to impacts upon people, property and nature that would not have occurred in the absence of these increases in events' likelihood and intensity. Unlike some other impacts of climate change, extreme weather events are manifesting on immediate timescales and changes in extremes are poorly described by the climatological means studied in many projections. Unfortunately, there is currently no standardised way of, or effort towards, documenting climate change related harms systematically. As a result, there is no systematic basis to quantify the major contribution of human influence on extreme weather to the costs of climate change. This contributes to the challenge that measures taken to mitigate and adapt to current (of ∼1.20 °C at the time of writing (Masson-Delmotte et al 2021 )) and future levels of global warming are not based on what could be the best available evidence.
Extreme event attribution is the method through which the role of climate change in an individual event can be assessed and quantified (Allen 2003 , Philip et al 2020 , van Oldenborgh et al 2021 ). Over the past two decades, more than 350 studies have quantified the role of climate change in over 400 extreme events (Carbon Brief 2021 ). This growing body of evidence is complementary to other analysis, such as work documenting observed and modelled trends in extremes due to climate change, and projections of future risk. The evidence from attribution studies adds value by highlighting the role of climate as a risk driver in experienced events, which in turn is useful for building future resilience (Raju et al 2022 ), and it enables the attribution of impacts, which is useful for cost-benefit analysis of mitigation and is a potential avenue for the exploration of drivers of loss and damage from climate-related extremes (James et al 2019 ). There is currently very little discussion of the role of science in determining loss and damage from anthropogenic climate change.
However, the number of events that have been studied using attribution methods is just a small fraction of all impactful extreme weather events that occurred over the same period. It is almost impossible to document this comprehensively due to data, time and resource constraints (Harrington and Otto 2020 , van Oldenborgh et al 2021 ). Furthermore, these studies overwhelmingly focus on events that occurred in the global north (Otto et al 2020a ). This pattern is mirrored in the unequal recording of impacts from extreme events, although nations in the global south are experiencing the most rapid changes in risk (Byers et al 2018 ) and often have high levels of underlying exposure and vulnerability to climate-related events.
The Sixth Assessment Report (AR6) of the Intergovernmental Panel on Climate Change (IPCC) provides a synthesis of attributable changes in extremes in regions across the world (Masson-Delmotte et al 2021 ). This forms a core part of the evidence base in this literature review. However, in order to link regional assessments of attributable risk to impacts that have occurred, it is necessary to compile attribution statements for individual events. In doing so this review builds on the IPCC assessment, linking broad changes in hazards to the ways in which these manifest and interact with exposure and vulnerability, and result in tangible impacts, with a bottom-up methodology, in which we develop insight into human influence on assessed extreme weather event categories based on literature on individual events. Further, the distinction between regional assessment and individual events is particularly important in adding evidence for more complex events, such as droughts, and at smaller spatial scales than the IPCC's regional aggregations, at which other phenomena may affect the influence of climate change.
Overall, the review brings together the best evidence we have on changes in extreme weather hazards and the impacts of past events, covering five key hazards: heatwaves, rainfall-based flooding, droughts, wildfires, and tropical cyclones. It is important to note that several of these hazards fall along different parts of the causative chain that stretches from anthropogenic climate change to impacts; each was selected as an appropriate intersection between attribution science and impact-relevance. As such, for each hazard, we assess the degree to which past impacts can be attributed to anthropogenic climate change, and the limiting factors associated with this. In doing so, we also build a picture of where evidence is most lacking, and therefore most urgent, for both attribution science and the documenting of impacts.
Each hazard section is laid out as follows. First, we describe attributed changes in extremes on global and regional scales, then how individual event attribution fits within this. Second, we discuss why it matters, describing the causative chain that results in key impacts arising due to such hazards. Finally, we discuss the 'attributable impacts' for each hazard. The intent of this subsection is not to suggest that the impacts of an event with any degree of anthropogenic influence, no matter the scale, are all attributable to climate change. Instead, these sections contain individual attribution statements alongside the impacts of those events, in the context of their connections as described previously. This should be interpreted as a snapshot, constrained by the body of existing attribution statements and to date, of the types and magnitude of impacts that have manifested to some degree by anthropogenic climate change. We posit that this is currently the most we can know based on existing evidence. And, in the absence of science to accurately attribute impacts using an end-to-end system for every event (and understanding that a fractional attributable risk (FAR)-based attribution of impacts is most applicable to classes of event (Perkins-Kirkpatrick et al 2022 )) this is nonetheless more useful than neglecting to combine such information at all. Tables 1 and 2 summarise this information on a global scale.
Table 1. Direct physical health impacts of different types of disaster between 2000 and 2020, as recorded by EMDAT, and the attributable influence of climate change on each hazard (EMDAT 2019 ).
| Observed direct impacts | Attributable influence of climate change on hazard severity/likelihood (confidence level) | |||
|---|---|---|---|---|
| Hazard | Deaths | Injured | Total affected | |
| Heatwaves | 157 000 | 193 000 | 320 000 | Increase (high) |
| Cold waves and severe winter conditions | 14 900 | 1.86 million | 96.1 million | Decrease (high) |
| Floods | 111 000 | 304 000 | 1.66 billion | Increase (medium) |
| Droughts | 21 300 | N/A | 1.44 billion | Increase (medium) |
| Wildfires | 1570 | 7260 | 3.38 million | Increase (medium) |
| Storms | 201 000 | 337 000 | 773 million | Rainfall increase (high) Other impacts no change (low) |
Table 2. Direct damages of different types of disaster between 2000 and 2020, as recorded by EMDAT, and the attributable influence of climate change on each hazard (EMDAT 2019 ). Note that these values are likely to be substantial underestimates of the true magnitude of damages.
| Observed direct impacts | Attributable influence of climate change on hazard severity/likelihood (confidence level) | ||
|---|---|---|---|
| Hazard | Insured damages (USD) | Total damages (USD) | |
| Heatwaves | 10 000 | 13.4 bn | Increase (high) |
| Cold waves and severe winter conditions | 4.63 bn | 31.3 bn | Decrease (high) |
| Floods | 74.1 bn | 610 bn | Increase (medium) |
| Droughts | 21 bn | 119 bn | Increase (medium) |
| Wildfires | 51.3 bn | 94.3 bn | Increase (medium) |
| Storms | 499 bn | 1.30 trillion | Rainfall Increase (high) Other impacts no change (low) |
This is not a review of risk, because that is impossible without also discussing vulnerability and exposure to changing hazards. However, it provides a platform on which to begin such a discussion. Further, it is a basis for placing a price tag on the diverse impacts of climate change, with implications for mitigation and adaptation considerations at all levels of decision-making. It concludes with several key areas in which further work will improve upon quantification of climate-related impacts, and the applications of this to address the inequity at the heart of the climate crisis (Pelling and Garschagen 2019 , Stone et al 2021 , Raju et al 2022 ).
2. Climate change and extreme weather impacts
2.1.1. changes in extremes.
The most dramatic changes in extreme weather induced by climate change are in the rate and intensity of heat and cold extremes. The rate and intensity of cold extremes are declining while heat extremes are increasing, with dire consequences for communities around the world. By 2015, the chance of the most extreme daily maximum temperatures (above the 99.9th percentile) averaged over land had increased fivefold (Fischer and Knutti 2015 ). Globally, as a direct result of climate change, previously very rare heat is now just unusual (Donat et al 2016 , King 2017 , Dunn et al 2020 , Seong et al 2021 ), while, in some cases, events now considered 'extreme' reach temperatures that were formerly all but impossible (Rahmstorf and Coumou 2011 , Imada et al 2019 , Sippel et al 2020 , Robinson et al 2021 ). The 2021 IPCC report is unequivocal in stating that average and extreme heat are increasing on every continent and that this is due to human-caused climate change (Masson-Delmotte et al 2021 ):
- A heatwave that would once have had a chance of 1 in 10 to occur in any given year in the pre-industrial climate will now occur 2.8 (1.8–3.2) times more frequently and be 1.2 °C hotter. At 2 °C of global warming, it will occur 5.6 (3.8–6.0) times more frequently and be 2.6 °C hotter.
- A heatwave that would have had a 1 in 50 chance to occur in any given year in the pre-industrial climate will now occur 4.8 (2.3–6.4) times more frequently and be 1.2 °C hotter. At 2 °C of global warming, it will occur 13.9 (6.9–16.6) times more frequently and be 2.7 °C hotter.
For even rarer events and on local scales the changes are even more dramatic. The increasing regularity of formerly rare events is particularly consequential: societies tend not to prepare for events that were historically so unlikely that they have never occurred (Woo 2016 ). Societies are especially vulnerable to the exceptionally extreme events that are now possible in a changing climate (Ciavarella et al 2021 ). In addition to this global picture, regional trends in heat extremes are attributed to climate change in Asia (Dong et al 2018 , Yin et al 2019 , Chen and Sun 2021 ), Africa (Stott et al 2011 ), Australia (Alexander and Arblaster 2017 ), Europe (Christidis and Stott 2016 ) and South America (Rusticucci and Zazulie 2021 ). A synthesis of current regional changes due to human influence are shown in figure 1 . We reproduce this figure and others from Working Group 1 (WG1) contribution to the IPCC AR6 (Masson-Delmotte et al 2021 ) to give context for our discussion of where attribution can add value to these global and regional pictures.

Figure 1. Synthesis of assessment of observed change in hot extremes and confidence in human contribution to the observed changes in the AR6 land-regions, excluding Antarctica. The evidence is mostly drawn from changes in metrics based on daily maximum temperatures; regional studies using other indices (heatwave duration, frequency and intensity) are used in addition. From figure SPM.3 of the WG1 contribution to the IPCC AR6. See Masson-Delmotte et al ( 2021 ) for more details and Otto et al ( 2021 ) for the dataset.
Download figure:
2.1.2. Why it matters
The impact of increased temperatures on mortality is widely established in the epidemiological literature. As climate change intensifies heatwaves around the world, the risk of heat-related deaths increases unless exposed populations' vulnerability is reduced. The fraction of the burden of heat-related mortality that is due to climate change is large and growing, with 37% of heat-related deaths attributed to climate change worldwide (Vicedo-Cabrera et al 2021 ), equivalent to tens of thousands of deaths per year. Increases in the number of hot days, and intensity of heatwaves results in a range of heat-related illnesses. Such illnesses include cardiovascular and respiratory complications, renal failure, electrolyte imbalance, and harm to foetal health (Moyce et al 2016 , Ebi et al 2018 , Parsons et al 2021 ). Increasing temperatures and heatwaves have also increased the prevalence and range of temperature-sensitive pathogens, such as Vibrio , which can cause cholera and gastroenteritis (Ebi et al 2017 ).
Increases in the occurrence of heat extremes result in substantial increases in mortality, and this effect is particularly pronounced the higher the temperatures. Climate change increases the likelihood of reaching very high temperatures, at which point the human body may no longer be able to cool itself. The theoretical limit for human survival is a 'wet bulb' temperature of 35 °C, at which point even the healthiest human in shade and with water would die from severe heat stroke in a matter of hours (Raymond et al 2020 ). Both mortality and morbidity rise significantly at far lower temperatures than this upper limit, affecting the elderly, very young and those with pre-existing medical conditions, such as respiratory and cardiovascular illness (Michelozzi et al 2009 , Sugg et al 2016 , Watts et al 2018 , Green et al 2019 ). Heatwaves are also strongly associated with rises in harmful pollutants such as ozone, particulate matter, sulphur dioxide, carbon monoxide and nitrogen dioxide, which further contribute to respiratory health impacts (Garcia-Herrera et al 2010 , Analitis et al 2014 , Li et al 2017 , Kalisa et al 2018 ).
A direct consequence of the health impacts of heat is a loss of labour productivity during hot periods, as workers must slow down, take more breaks, and hydrate to remain safe (Sahu et al 2013 , Kjellstrom et al 2016 , Spector et al 2019 , Borg et al 2021 ). In the US alone, labour productivity losses due to extreme heat cost around USD 2 bn annually (Zhang and Shindell 2021 ), while across the world total losses amount to USD 280–311 bn (Borg et al 2021 ). These are concentrated in lower- and middle-income countries, in tropical and subtropical areas, and in heavy manual labour and outdoor industries such as agriculture and construction (Kjellstrom et al 2016 , Borg et al 2021 , Parsons et al 2021 ). Across the world, the labour capacity of rural populations during summer months fell by 5.3% between 2000 and 2016 due to rising heat—in tropical regions capacity fell by up to 30% (Watts et al 2018 ). While some adaptation policies have already been put into place, such as moving work hours earlier in the day, these measures may come with unintended consequences, and decrease in effectiveness as warming continues (Masuda et al 2019 , Parsons et al 2021 ).
While there have been very few observations of wet bulb temperatures over the critical 35 °C threshold, the occurrence of dangerous humid heat extremes, exceeding wet bulb temperatures of at least 27 °C, has more than doubled since 1979 (Raymond et al 2020 ). By another measure, 40% of the total land surface has already entered an 'unusual' climate in the warmest months—that is, temperatures exceed a signal-to-noise ratio of 1 (Frame et al 2017 , Hawkins et al 2020 ). Increases in severe heat hazards cause a disproportionate increase in the associated health risks because a disproportionately large fraction of the global population lives in hotter regions (Watts et al 2018 ). On top of that, between 2000 and 2016, the number of vulnerable people (over 65 years) exposed to extreme heat increased by 125 million, reaching 175 million in 2015 (Watts et al 2018 ).
2.1.3. Attributable impacts
Climate change amplifies the temperature of most heat extremes (Otto et al 2016 ). Attribution research has found that the most extreme heatwaves have become substantially more likely, or even only possible at all (Imada et al 2019 ), due to climate change. This includes, but is not limited to, events in Europe in 2003 (Stott et al 2004 , Christidis et al 2015 ) and 2018 (Leach et al 2020 ), Russia 2010 (Rahmstorf and Coumou 2011 , Otto et al 2012 ), the US (Shiogama et al 2014 ), China (Sparrow et al 2018 , Zhou et al 2019 ), Argentina (Hannart et al 2015 ), North Africa (Mitchell 2016 ), Australasia (Hope et al 2016 ), South and Southeast Asia (Azhar et al 2014 , Wehner et al 2016 , Mazdiyasni et al 2017 , Imada et al 2018 , Christidis et al 2018b ), and across the world (Peterson et al 2012 , 2015 , Herring et al 2014 , 2015 , 2016 , 2018 , 2019 , 2020 ). In some cases, events were effectively impossible in the absence of climate change (Herring et al 2019 , 2020 , 2021 , Imada et al 2019 , Fischer et al 2021 , World Weather Attribution 2021a ), including the emerging possibility of simultaneous heat extremes across regions and continents (Kornhuber et al 2019 , Vogel et al 2019 ).
Between the years 2000 and 2020, the disaster database EMDAT recorded approximately 157 000 deaths from heatwaves across the planet (table 2 , EMDAT 2019 ), although it is acknowledged that this is likely to be a substantial underestimate due to reporting limitations and because deaths due to heat occur outside of officially-declared events (Otto et al 2020a , see below). Around 125 000 of these deaths occurred during just two events, the European heatwave of 2003 and Russian heatwave of 2010, which resulted in 70 000 and 55 000 deaths, respectively. Both of these events were made substantially more likely by climate change, as noted above (Stott et al 2004 , Rahmstorf and Coumou 2011 , Otto et al 2012 ). In the case of the 2003 heatwave, this was made at least twice as likely to occur, due to climate change, and has since become 10 times more likely again (Christidis et al 2015 ). The Russian heatwave, meanwhile, was found to have been made 5 times more likely to occur by the climate change observed since 1960 (Rahmstorf and Coumou 2011 ). In the UK, estimates using simple hazard-based FAR methodology link around 1,500 excess deaths from three heatwaves directly to climate change (Clarke et al 2021 ). And another study on the 2003 heatwave used an end-to-end approach, combining meteorological attribution with the effect of temperatures on mortality, to directly attribute deaths in Greater London and Central Paris; 64 additional Londoners (∼20% of the total) and 506 Parisians (∼70% of the total) died due to the influence of climate change (Mitchell et al 2016 ).
2.1.3.1. CASE STUDY: Western Russia, 2010
From early July until mid-August 2010, an intense high-pressure system over Eastern Europe and Western Russia caused temperatures to soar above 30 °C throughout the region, breaking 40 °C in many major cities. An event of this magnitude was made approximately 3–5 times more likely by climate change (Rahmstorf and Coumou 2011 , Otto et al 2012 ).
This extreme heat led to widespread drought conditions that decimated 25% of the entire annual crop yield and also amplified wildfires across more than 10 million hectares of dried-out forests, steppe and peat regions (Barriopedro et al 2011 , Bondur 2011 ) . This compounded an already growing fire hazard in rural Western Russia, resulting from changes in land use and sustainable management, among other factors (Goldammer 2010 ) . The destruction of grain crops and subsequent export ban led to rising food prices domestically and abroad. Pakistan was Russia's fourth largest customer and experienced a 16% rise in the price of wheat, linked with a 1.6% rise in poverty (Welton 2010 ) . The destruction of thousands of properties left over 3500 people homeless. Harmful gases and aerosols from the fires became trapped in the stagnant high-pressure system, resulting in poor air quality in many major cities. This exacerbated the already-unprecedented public health crisis, particularly affecting those with severe asthma and heart problems. In the city of Moscow alone, around 5000 more deaths were recorded than for the same period in the previous year, and across the whole country this was closer to 55 000 from a combination of heat and poor air quality (Barriopedro et al 2011 ).
2.1.4. Underestimation of impacts
These Europe-focused results are far from a complete tally of climate change-amplified heatwave impacts. This is largely due to data limitations. Both assessments of health associated with extreme heat (Green et al 2019 ) and weather observations, crucial for assessing the link to climate change (Otto et al 2020a ), are concentrated within higher income countries. EMDAT lists 147 instances of impactful heat events from individual countries for the period 2000–2020, only an improbably low 58 of which are from all of Asia, Africa, South and Central America and the Caribbean combined (EMDAT 2019 ). Of the 157 000 total deaths recorded, only 10 000—or 6.3%—were recorded in these regions, which together constitute almost 85% of the world's population, over 60% of the land mass, and many of the hottest and most humid climates. These data are subject to recording biases that limit the number of heatwaves registered to EMDAT to those that are officially declared by national meteorological services, many of which do not currently have formal heatwave definitions, and recorded locally as exceeding one of several impact thresholds; thus, they are limited by the capacity of governments or NGOs to attribute heat-related impacts. Further, this dataset focuses only on heatwaves, periods of relatively extreme temperatures, whereas many heat-related deaths in fact occur outside of heatwaves, when temperatures are also increased by climate change, but are not captured within these data (Gasparrini et al 2015 ).
In the two most impactful European heatwaves recorded, the maximum recorded wet-bulb temperature peaked at 28 °C; temperatures frequently exceed this in other regions of the world such as south Asia (Raymond et al 2020 ), with many more lethal heat events likely already occurring than are reported (Mora et al 2017 ).
In addition to the attributable trends in exposure to extreme heat, we can elicit evidence from the few attribution studies that exist. For instance, in 2015 in the Indian city of Hyderabad, heat extremes over a 5 day period were made more than 30 times more likely by climate change (Wehner et al 2016 ). Including this event, three devastating heatwaves in India in 2010, 2013 and 2015 resulted in the deaths of at least 5000 people (Azhar et al 2014 , Mazdiyasni et al 2017 ). Meanwhile in neighbouring Pakistan, also in 2015, the city of Karachi experienced an extreme heat event which by the same measure would have been effectively impossible without climate change (Wehner et al 2016 ).
The impacts from heatwaves in hotter climates may be somewhat mitigated by factors such as the natural acclimatisation of populations—which might also be considered as longer-term impacts upon behaviour, culture and architecture—and age demographics (Gasparrini et al 2015 , Green et al 2019 ). However, these mitigating factors are more than likely offset by greater population density, higher frequency of more intense extremes, and greater vulnerability in many of the regions with few reported impacts (Harrington and Otto 2018 , Rohat et al 2019 ). We are therefore confident that the reported deaths from heatwaves and extreme heat, and thus those linked to climate change, are a vast underestimate.
2.1.5. Is increased heatwave-related mortality offset by a reduction in cold extremes?
Cold extremes are decreasing in frequency and intensity across most of the world and at continental and subcontinental scales (Christidis and Stott 2016 , King 2017 , Dunn et al 2020 , Hu et al 2020 ). In the Arctic, the rise in heat extremes (Sui et al 2017 , Dobricic et al 2020 ) and decrease in cold extremes (Sui et al 2017 ) is especially pronounced, in line with its rapid warming (Box et al 2019 ). Specific cold spells of recent years have displayed this decreased probability due to climate change, including in the UK (Christidis and Stott 2020 ), US (Bellprat et al 2016 ), Europe (Peterson et al 2012 , Christiansen et al 2018 ) and China (Sun et al 2018 ).
On average, mortality and morbidity rates are higher in winter than summer months in temperate regions (Conlon et al 2011 , Ebi and Mills 2013 , Gronlund et al 2018 ), with a more complex relationship in locations across the tropics and subtropics (McMichael et al 2008 , Singh et al 2019 ). The direct effect of cold on health remains obscured by the wide array of seasonal factors at play (Staddon et al 2014 , Kinney et al 2015 , Hajat 2017 ), including cardiovascular disease which is only weakly linked to cold temperatures (Ebi and Mills 2013 ). For the effect of extremes specifically, there are two key factors to consider. First, temperature-mortality relationships are generally far steeper for extreme heat than extreme cold (Gronlund et al 2018 ). Second, the most severe winter cold spells contribute little to overall winter mortality, and even in some temperate regions there is evidence that climate change will not decrease winter mortality (Staddon et al 2014 ). Thus the reduction in frequency and intensity of cold extremes has likely not affected overall changes in mortality substantially, nor offset those from hot extremes (Ebi and Mills 2013 ) and the impact of increasing heat-related mortality are assessed to far exceed any reductions in cold-related mortality as a result of climate change (Gasparrini et al 2017 , Huber et al 2017 , Vicedo-Cabrera et al 2018 ).
2.2. Rainfall and flooding
2.2.1. changes in high rainfall extremes.
By 2015, climate change increased the likelihood of daily precipitation extremes exceeding the 99.9th percentile of pre-industrial events by 18%, averaged over all land regions (Fischer and Knutti 2015 ). A warmer atmosphere holds more moisture at a given pressure: the Clausius–Clapeyron relation states that the increase in moisture held at a given pressure is 6%–7% per 1 °C. Extra water in the atmosphere combines with changes in weather patterns to affect rainfall extremes in a given region (O'Gorman 2015 , Pfahl et al 2017 ). In contrast to heat, these changes vary greatly across regions and seasons. For example, extreme rainfall is increasing in Northern Europe in winter but decreasing in the Southern part of the continent in summer.
Nonetheless, since the 1950s, heavy rainfall has become more frequent and intense across most parts of the world, which is now known to be mainly due to human climate change (Fischer and Knutti 2015 ). It has not strongly decreased in likelihood anywhere. A synthesis of current regional changes due to human influence are shown in figure 2 . Globally, the IPCC reports that, in a given location, what would once have been a one-in-10 year rainfall event currently occurs 1.3 (1.2–1.4) times every 10 years and is 6.7% wetter. At 2 °C of global warming, this will be 1.7 (1.6–2.0) times per 10 years and 14% wetter (Masson-Delmotte et al 2021 ). Sub-daily extreme rainfall events are intensifying at a rate at or exceeding Clausius–Clapeyron scaling (Fowler et al 2021 ), while regional attribution shows that deluges generally are becoming more frequent and intense especially across North America, Asia and Europe (Chen and Sun 2017a , 2021 , Paik et al 2020 , Dong et al 2021 , Sun et al 2021 ), though this may also be true in other regions with a lower availability of observational data.

Figure 2. Synthesis of assessment of observed change in heavy precipitation and confidence in human contribution to the observed changes in the AR6 land-regions, excluding Antarctica. The evidence is mostly drawn from changes in indices based on one-day or five-day precipitation amounts using global and regional studies. From Figure SPM.3 of the WG1 contribution to the IPCC AR6. See Masson-Delmotte et al ( 2021 ) for more details and Otto et al ( 2021 ) for the dataset.
2.2.2. Link with flooding
Flooding is a major source of the impacts that extreme rainfall has upon human societies. In general, changes in the risk of flooding due to heavy precipitation also depend on changes in other factors including the susceptibility of areas to flooding, land use change and river management (Ji et al 2020 ), as well as other climate-related factors such as soil moisture, storm extent and snowmelt (Sharma et al 2018 , Wasko and Nathan 2019 ). As a result, there is high regional and sub-regional variation in trends in streamflow (Do et al 2017 , Gudmundsson et al 2019 ), but many of the observed changes can only be explained by human influence on the climate (Gudmundsson et al 2021 ). Evidence from attribution-science literature shows that growing numbers of floods have been made more intense by the effect of climate change on precipitation (Cho et al 2016 , Pall et al 2017 , van der Wiel et al 2017 , Philip et al 2018a , Teufel et al 2019 ).
2.2.3. Why it matters
Flooding damages property and infrastructure, as evidenced by disaster data for the years 2000–2020 in which floods globally caused USD 610 bn in damage (table 2 ). It also places people in direct danger of injury and death. The flood events recorded in the EMDAT database led to 111 000 deaths and affected 1.66 bn people over the period 2000–2020 (table 1 , EMDAT 2019 ). Indeed, flooding is the disaster that is recorded as affecting the greatest number of people—though this may be due to a bias arising from the variable ease of recording impacts for different disasters; heatwaves are more pervasive but ill-defined than flood extent, for example. One further study that considered only 'large floods' found that 255–290 million people were directly affected by flooding between 2000 and 2018, the population in areas affected by inundation grew by 58–86 million between 2000 and 2015, and the number of people affected by flooding continues to increase due to population increases and climate change (Tellman et al 2021 ).
Floods impact both physical and mental health. Physical impacts result directly from dangerous water flows and inundation, as well as 'cascading impacts', in which the destruction of infrastructure limits access to services and utilities including clean water and sanitation, resulting in ill health (Ramana Dhara et al 2013 ). In turn, this enhances the spread of and vulnerability to water-borne disease, including leptospirosis, cholera and other diarrhoeal diseases such as giardiasis, salmonellosis, and cryptosporidiosis (Marcheggiani et al 2010 , Ramana Dhara et al 2013 ). This occurrence of such outbreaks following floods is well documented. This evidence includes an inventory of 87 extreme events between 1910 and 2010 (Cann et al 2013 ), known associations between flood events and gastrointestinal illness in the US (Patz et al 2008 , Uejio et al 2014 ) and India (Bush et al 2014 ), and has been observed in the aftermath of floods in Pakistan (Baqir et al 2012 ), Mozambique (Devi 2019 ), China (Zhang et al 2019 ), Ecuador (Carlton et al 2014 ), the Solomon Islands (Jones et al 2016 ) and many others (Fredrick et al 2015 , Levy et al 2016 ). This is especially impactful in areas of pre-existing high vulnerability, such as those without access to improved sanitation and water sources, on top of other poverty- and conflict-related factors such as access to healthcare, education and early warning systems (Cann et al 2013 , Davies et al 2015 ).
In addition, vector-borne diseases such as malaria, dengue and West Nile Fever may spread further following flooding, as more widespread stagnant water bodies provide breeding grounds for mosquitoes (Ramana Dhara et al 2013 , Hinz et al 2019 ). Finally, many diseases are also enhanced by the effect of warmth and high humidity, because this increases the longevity of many pathogens and mosquitoes (Moors et al 2013 , Levy et al 2016 , Hinz et al 2019 ). The combination of climate change impacts on precipitation, temperature, and yet other factors that amplify the resulting impacts, such as societal capacity to deal with health impacts, create compound risks.
Other compound risks are also associated with flooding. For example, low-lying coastal areas are increasingly affected by high sea levels, due to storm surges and sea-level rise, which combine with heavy rainfall to amplify flood damages (Moftakhari et al 2017 , Bevacqua et al 2019 , Marsooli and Lin 2020 ). Similarly, tropical cyclones result in damage to infrastructure including power lines, water supplies and roads, increasing vulnerability to high temperatures as air conditioning is disabled, and access to clean water and healthcare are restricted (Lin 2019 ; Matthews et al 2019 , Mejia Manrique et al 2021 , Yu et al 2020 ).
The mental health impacts of disasters are also becoming documented and understood more widely, with an emerging literature (Hayes et al 2018 , Watts et al 2018 , Cianconi et al 2020 ), especially on the impacts of floods (Tunstall et al 2006 , Stanke et al 2012 , Alderman et al 2013 , Azuma et al 2014 , Fernandez et al 2015 , Burton et al 2016 , Waite et al 2017 ). These impacts include post-traumatic stress disorder (PTSD), anxiety, depression and suicidal thoughts, among other conditions (Dodgen et al 2016 ) and persist long after the disaster itself. First responders are severely impacted by the mental health effects of working in the aftermath of disasters, with local first responders most heavily affected (Osofsky et al 2011 , Rusiecki et al 2014 ). These effects are more likely to occur in those with pre-existing mental health conditions (Dodgen et al 2016 , Hayes et al 2018 ). Quantitative attribution of mental health impacts to climate change remains challenging. This is due to the diverse nature of such impacts, and because attribution studies typically consider one aspect of the causal chain (climate-meteorological event or meteorological event-mental health impacts), not both (Hayes et al 2018 ). However, a few cases exist in which mental health impacts are attributed to an event and the event itself is attributed to climate change. For example, the 2013/14 UK floods were made more likely by climate change (Huntingford et al 2014 , Christidis and Stott 2015 , van Oldenborgh et al 2015 , Schaller et al 2016 , Kay et al 2018 ) and caused increased psychological morbidity among those both flooded and disrupted (Waite et al 2017 ).
2.2.4. Attributable impacts
Annual monsoons are a critical source of rainfall for at least 60% of the world's population in areas including south and east Asia, Australia, and east and west Africa (Li et al 2016 ). The south Asian monsoon is of particular societal importance, providing 80% of the water to the subcontinent, which contains nearly a fifth of the world's population and is heavily reliant upon agriculture (Katzenberger et al 2021 ). In the 20th Century, a decline in the East Asian summer monsoon rains was observed, with the most intense rains becoming shorter but more intense, including flooding and droughts (Burke and Stott 2017 ). Since 2000, the strength of south Asian monsoon rains has increased, with the most pronounced increases occurring in the most intense events (Katzenberger et al 2021 ). This pattern covers all monsoon regions, to varying degrees, and crucially an associated increase in both drought and flooding (Burke and Stott 2017 , Wang et al 2021 ). In response to future warming, and if aerosol emissions are reduced, significant and substantial increases in monsoon rains are expected, resulting in growing flash flooding risks (Masson-Delmotte et al 2021 ), especially in East Asia (Samset et al 2018 ). However, as increased precipitation is expected to occur over fewer days of more intense rainfall, worsening of droughts also becomes more likely (Burke and Stott 2017 ).
According to EMDAT, around 49 000 deaths due to flooding occurred in south Asia from 2000 to 2020, almost half of recorded global flood mortality (EMDAT 2019 ). The region has also suffered damages of around USD 104 bn, only around USD 4 bn of which is recorded as insured damages. Many of the deadliest and most destructive floods in this subset occurred during the monsoon season, including in 2000 (India and Bangladesh), 2007 (across south Asia), 2010 (Pakistan), 2017 (Bangladesh), and 2005, 2008, 2013, 2019 and 2020 (India). However, even outside of the monsoon season, rainfall extremes have been amplified by climate change (Rimi et al 2018 ).
Outside of south Asia, the most impactful flood events in terms of both mortality and numbers of people affected by flooding also occurred primarily in low- and middle-income countries in Africa, including Sudan, Ethiopia, and Nigeria; South America, including Peru, Colombia and Brazil; and the Caribbean, including Haiti and the Dominican Republic. While few attribution assessments on specific events are available in these regions, there is nonetheless evidence of links between these types of events and climate change as described above. Further, trends in increased flooding have been identified in regions including parts of Brazil (Bartiko et al 2019 ) and Ethiopia (Mamo et al 2019 ), which combine with other factors to pose greater danger to people. For example, the Metropolitan Region of São Paulo has simultaneously undergone rapid urban expansion and an increase in the number of extremely heavy precipitation days. Such events were exceedingly rare in the 1950s, but by the 2010s occurred 2–5 times per year. This has placed people at a rapidly rising risk of flash flooding.
Not including tropical cyclones, extreme rainfall events with detected anthropogenic influence have occurred in Europe (Pall et al 2011 , Schaller et al 2016 , van Oldenborgh et al 2016 , Otto et al 2018a ), the Mediterranean (Vautard et al 2015 ), US (Herring et al 2014 , Eden et al 2016 , van der Wiel et al 2017 ), parts of South America (De Abreu et al 2018 , Christidis et al 2018a ), New Zealand (Rosier et al 2015 ), southeast Asia (Yun et al 2020 ), Japan (Imada et al 2020 , Kawase et al 2020 ) and China (Burke et al 2016 , Sun and Miao 2018 , Zhou et al 2018 , Yuan et al 2018b ). Collectively, these events represent financial losses and destruction of property of more than USD 60 bn.
In certain areas, attribution studies on rainfall have directly estimated the fraction of damages incurred due to climate change. For example, in the UK between 2000 and 2020 approximately USD 9 bn in flood damages have been attributed to climate change (Clarke et al 2021 ). In New Zealand, USD 140 million in insured damages attributable to climate change occurred over 2007–2017, although this is likely a significant underestimate of overall costs (Frame et al 2020a ). These studies provide broad estimates using a simple hazard-based FAR methodology. End-to-end attribution using hydrological models and taking account of exposure and vulnerability remains challenging (Schaller et al 2016 ), though research using modelling chains (Kay et al 2018 ) and a storyline approach (Schaller et al 2020 ) is ongoing. While changing weather patterns can be complex in a given area, the general trend is increasingly extreme rainfall resulting in destructive flooding over a large portion of the world's surface.
2.3. Drought
2.3.1. changes in extremes.
Droughts are complex but extremely impactful events that affect billions of people worldwide (table 1 ). There are many different types of drought with varying impacts. The main categories include meteorological, agricultural and hydrological drought. All are connected, and each simply refers to an anomalous moisture deficit in part of the hydrological system relative to some baseline, be it in precipitation, soil moisture, or groundwater reservoirs, respectively (Cook et al 2018 ). The fingerprint of climate change on increasing drought has been observed in several drought-prone regions of the world, including California, the Pacific Northwest, parts of China, western North America, and the Mediterranean (Gudmundsson and Seneviratne 2016 , Chen and Sun 2017b , Cook et al 2018 ), as well as globally (Marvel et al 2019 ). With the exception of the Mediterranean, which is already receiving markedly less precipitation, this is largely due to amplified temperatures driving evaporation and melting snowpack, reducing the meltwater contribution to river flows (Cook et al 2018 ). Other smaller Mediterranean-like regions such as central Chile, the far southwest tip of southern Africa and southwest Australia have also dried due to climate change, and are now more prone to drought (Seager et al 2019 ). A synthesis of current regional changes due to human influence are shown in figure 3 .

Figure 3. Synthesis of assessment of observed change in agricultural and ecological drought and confidence in human contribution to the observed changes in the AR6 land-regions, excluding Antarctica. These are assessed based on observed and simulated changes in total column soil moisture, complemented by evidence on changes in surface soil moisture, water balance (precipitation minus evapotranspiration) and indices driven by precipitation and atmospheric evaporative demand. From Figure SPM.3 of the WG1 contribution to the IPCC AR6. See Masson-Delmotte et al ( 2021 ) for more details and Otto et al ( 2021 ) for the dataset.
'Flash droughts' are a type of soil moisture, or agricultural, drought that occurs extremely rapidly, with little warning (Yuan et al 2019 ) and can have severe consequences for agricultural productivity. In recent years, there has been a notable rise in such events in the US, China and South Africa (Cook et al 2018 ). Meanwhile, some of the most catastrophic droughts in the world continue to occur in East Africa (Gebremeskel et al 2019 ). No single drought there has been linked directly to climate change, partly due to a relatively short observational record, high uncertainties and high natural variability, especially for precipitation (Uhe et al 2018 , Philip et al 2018b , Kew et al 2021 ). There is limited evidence that anthropogenic warming of Western Pacific sea surface temperatures may contribute to more frequent drought (Funk 2012 , Funk et al 2019 ). More generally, the drying of the major rainy season in the region, the 'long rains' (Lyon and Dewitt 2012 ), is likely connected to climate change (Tierney et al 2015 , Hoell et al 2017 ).
2.3.2. Why it matters
In 2019, there were approximately 690 million undernourished people. Food insecurity is linked to conflict, alongside climate-related shocks such as drought (FAO 2020 ). The least food secure regions of the world are the most vulnerable to drought, and thus any increase in drought severity due to climate change. In Brazil, an ongoing drought since 2019 has led to water scarcity, severe crop losses including corn and coffee, and amplified fire activity in the Amazon (Marengo et al 2021 ). In south Asia, the changing patterns of monsoon rainfall as well as rising temperatures and other types of extreme weather have already caused a decline in food security (Bandara and Cai 2014 ). In East Africa, the major drought in 1984/85 led to a famine that caused the deaths of around 450 000 people. More recently, a drought in 2008–10 affected 13 million people, another in 2010–11 affected 12 million and caused the deaths of 250 000 people in Somalia alone. Since 2005, droughts have increased in frequency in East Africa and caused substantial livestock death, disruption of livelihoods and rising food prices (Nicholson 2017 , Gebremeskel et al 2019 ). In turn, this has contributed to internal migration and further socio-economic instabilities in the region (Gebremeskel et al 2019 ). From South Asia across the middle east and most of Africa, hunger is a growing challenge that drought is exacerbating. More broadly, extension of drought across water-scarce regions is exceptionally costly through its impact on ecosystems, agriculture and wider society (Cook et al 2018 ).
2.3.3. Attributable impacts
The fingerprint of climate change has manifested very clearly on several recent droughts. California provides an exemplary case. From 2011 to 2017, it suffered an extended drought, possibly the worst in a thousand years (Osaka and Bellamy 2020 ). Even as this event unfolded, scientists demonstrated that various contributing factors were attributable to climate change, including reduced snowpack (Mote et al 2016 , Berg and Hall 2017 ) and warm dry years (Diffenbaugh et al 2015 , Williams et al 2015 ). This drought was then alleviated by incredibly intense seasonal rainfall that led to destructive flooding, with damages of at least USD 1 bn (The Weather Channel 2017 ), in a compound event that has been linked to climate change (Simon Wang et al 2017 ). Similar compound droughts and floods have occurred in the UK (Parry et al 2013 ) and East Africa (Gebremeskel et al 2019 ). Not only that, new research shows that the California drought was a smaller part of a larger mega-drought stretching from 2000 to 2018, which itself was pushed from a moderate event to the worst in 1200 years by climate change (Williams et al 2020 ). From 2014 to 2016, economic losses in the agriculture industry amounted to at least USD 5.5 bn, and the loss of 42 000 jobs (Howitt et al 2014 , 2015 , Medellín-Azuara et al 2016 ). Furthermore, during the first three years of the drought, hundreds of millions of trees perished due to water stress, wildfires and proliferating bark beetles; in parts of the Sierra Nevada almost half of all trees died (Fettig et al 2019 ).
There are several other cases of drought across the world that have been shown to have been intensified by climate change. This includes South Africa 2015–17 (Yuan et al 2018a , Otto et al 2018b ), Europe 2016–17 (García-Herrera et al 2019 ), Indonesia 2015 (King et al 2016 ), New Zealand (Harrington et al 2016 ) and Canada (Szeto et al 2016 ).
The impacts of these droughts vary greatly in severity and form, being acutely related to exposure and vulnerability in the affected region. In Canada, drought conditions led to forest fires that created a serious public health risk. In New Zealand, economic costs of the 2013 drought totalled at least USD 1.3 bn. In Europe, drought costs an average of €6.8 bn per year (García-Herrera et al 2019 ). Against this backdrop, the extreme 2016–17 event caused loss of many types of crops, including cereals, olives, tomatoes, wine grapes, and almonds, with losses of at least €2 bn in Italy alone (García-Herrera et al 2019 ). Episodic drought is becoming more common in Brazil, and though the number of fatalities has fallen drastically, the number of people affected is still increasing; since 1990, hundreds of droughts affected over a billion people (Sena et al 2014 ). In South Africa, economic losses totalled USD 400 million, cost tens of thousands of jobs and months of extreme water restrictions for citizens in late 2017 (Stanford University 2020 ). Cape Town also narrowly avoided 'day zero', when there would have been no water remaining in city pipes. Attribution research has demonstrated that climate change amplified all of these impacts.
2.3.3.1. CASE STUDY: Indonesia, 2015
In the dry season of July–October 2015, Indonesia experienced a combination of severe heat and extremely low precipitation that created drought conditions. This was partly due to the occurrence of a strong El Niño, which is linked to high temperatures and strongly linked to lower-than-normal precipitation rates in the dry season in Indonesia. In addition, the resulting land surface temperatures were also amplified significantly by anthropogenic warming ( King et al 2016 ).
The impacts of this drought were myriad and severe. Farmland drought affected over 111 000 hectares of crops ( DMCDD 2015 ), which led to widespread loss of income, rises in food prices ( Webb and Wadhwa 2016 ) and poverty ( Reuters 2015 ). It triggered the worst fire season since 1997, resulting in air pollution that detrimentally affected the health of millions and caused in the deaths of over 100 300 people across Indonesia, Malaysia and Singapore ( Huijnen et al 2016 , Koplitz et al 2016 ). The impact on vegetation more widely disrupted local wildlife, causing thousands of long-tailed monkeys to attack and steal from villages in search of food ( Rohmah 2015 ).
2.4. Wildfire
2.4.1. changes in extremes.
Wildfire risk is inextricably tied to dry and hot conditions, and is greatest during periods of 'Fire weather', classified using various metrics as some combination of high temperature, low humidity, lack of rain, fuel availability and high wind speed (Van Wagner 1987 , Dowdy et al 2009 ). The risk of wildfire has already substantially increased in many regions, including the western US, Alaska and Canada (Jarraud and Steiner 2012 , Dennison et al 2014 , Balch et al 2018 , Goss et al 2020 ), the Mediterranean (Abatzoglou et al 2019 , Barbero et al 2020 , Touma et al 2021 ), Amazonia (Alencar et al 2011 , 2015 , Abatzoglou et al 2019 , Touma et al 2021 ), southeast Asia (Touma et al 2021 ) and Australia (Dowdy 2018 , Dowdy and Pepler 2018 , Harris and Lucas 2019 ). A synthesis of current regional changes due to human influence are shown in figure 4 .

Figure 4. Synthesis of observed, attributed and projected changes in fire weather, from the Interactive Atlas of WG1 of the AR6 of the IPCC (Gutiérrez et al 2021 , Iturbide et al 2021 , Masson-Delmotte et al 2021 ).
Recent blazes across the world have proved to be violent manifestations of this. For instance, in British Columbia in 2017 and 2021 severe hot and dry summers led to unprecedented forest fires. In 2017, the burned area was made 7–11 times larger by climate change and, equivalently, the event was made 2–4 times more likely (Kirchmeier‐Young et al 2019 ). Similar results were found in an analysis of fire risk in Western Canada, where fires as large as those that burned almost 600 000 ha near Fort McMurray, Alberta, in 2016, were found to have become 1.5–6 times more likely to occur as a result of climate change (Kirchmeier-Young et al 2017 ). In Sweden in 2018, extensive forest fires were made 10% more likely by climate change (Krikken et al 2019 ). And using the same method, the record-breaking Australian bushfire season of 2019/20 was made at least 30% more likely by climate change (van Oldenborgh et al 2020 ). From 1984 to 2015, over 4 million ha of burned area in the western US is directly attributed to climate change (Abatzoglou and Williams 2016 ). And in southern China, extreme wildfires of 2019 were made over seven times more likely by climate change (Du et al 2021 ).
2.4.2. Why it matters
Wildfires can cause direct mortality, although the total number of direct deaths are typically lower than for other extreme events (table 1 ). However, wildfire smoke consists of fine particulate matter (known as PM 2.5 and PM 10 ) that reaches deep into the lungs when inhaled, can reach the bloodstream, and is likely more toxic than ambient particulates of the same scale (Aguilera et al 2021 ). The hazardous air pollutants that constitute the smoke aggravate existing respiratory health issues, trigger new conditions and may also have links to cardiovascular health impacts (Reid et al 2016 , Matz et al 2020 , Chen et al 2021 ), as well as adverse effects on pregnancy outcomes (Abdo et al 2019 ). In Canada, short term effects of wildfire smoke include 54–240 premature deaths and USD 0.41–1.8 bn annually, while long-term chronic issues are responsible for 570–2500 premature deaths and costs of USD 4.3–19 bn annually (Matz et al 2020 ). A similar study for the US from 2008 to 2012 showed that short-term effects cost thousands of lives and additional hospital admissions for respiratory and cardiovascular illness annually, while long-term exposure cost tens of thousands of lives annually—the economic costs of these health burdens was estimated as USD 11–20 bn (2010$) per year for short-term, and USD 76–130 bn per year for long-term effects (Fann et al 2018 ). Finally, across the world total attributable deaths to landscape fire smoke are in the hundreds of thousands (262 000 in La Niña years, compared with 532 000 during El Niño), with the worst affected areas being sub-Saharan Africa and southeast Asia (Johnston et al 2012 ).
2.4.3. Attributable impacts
Severe impacts have also been recorded for attributed weather and fire events. For instance, during the anthropogenically amplified European heatwave of 2003, the central and Algarve regions of Portugal experienced the worst mega-fires in history (Tedim et al 2013 ). The resultant smoke dispersed across Europe, increasing the concentrations of PM 2.5 by 20%–200% in many places (Hodzic et al 2007 ), where several hundred deaths were linked to air pollution in the UK and Netherlands alone (Solberg et al 2008 ). Fires across Indonesia in 2015 led to over 100 000 excess deaths. Similarly, in Russia in 2010 smoke from burning forests and peatlands became trapped over population centres, exacerbating the public health crisis and causing up to 2000 excess deaths in Moscow alone (Shaposhnikov et al 2014 ). The Black Saturday bushfires in Victoria, Australia in 2009 were made more likely by climate change (Black 2016 ), and resulted in PTSD in a significant minority of the most affected groups (Bryant et al 2014 ). Finally, the 2016 Alberta wildfires displaced over 80 000 people and caused over CAD 3.5 bn in insured losses. As noted above, these fires were made substantially more likely due to climate change. Across Canada, wildfires burn 2.1 million ha per year, approximately the area of Wales (Kirchmeier-Young et al 2017 ).
2.4.3.1. CASE STUDY: Australia, 2019/20
In the summer of 2019/20, New South Wales experienced the worst fire season on record, since dubbed the 'Black Summer fires'. In the 2019 fire year, the burned area totalled almost three times that in any of the previous 32 years (Canadell et al 2021 ) . This event was made at least 30% more likely by climate change (van Oldenborgh et al 2020 ) , which adds further evidence that dangerous fire weather in southeast Australia has emerged outside of the range of historical experience (Abram et al 2021 ) . Not only that, the sheer scale of the fires went beyond anything either simulated in CMIP6 models or widely discussed even within the large uncertainties associated with wildfire hazards;this led to calls for urgent improvement of both risk modelling and uncertainty interpretation for accurately informing society of such unprecedented risks going forwards (Sanderson and Fisher 2020 ) . This case study thus illustrates a broader point about the urgent need to provide risk guidance acknowledging both known and unknown unknowns, especially in a changing climate (Sanderson and Fisher 2020 , Clarke et al 2021 ).
These fires burned a record 19 million hectares of forest and woodland (Khan 2021 ) , resulting in the direct destruction of 5900 buildings and tens of thousands of livestock being killed. An estimated 3 bn mammals, reptiles, birds and frogs were killed or displaced, making it 'one of the worst wildlife disasters in modern history.' (WWF Australia 2020 ) , with fears of possible extinctions of endangered species (Filkov et al 2020 , Ward et al 2020 ).
Across the region, levels of PM 2.5 exceeded the WHO guideline levels fourfold (Yu et al 2020 ) . Smoke from the fires was responsible for '417 excess deaths, 1124 hospitalisations for cardiovascular problems and 2027 for respiratory problems, and 1305 presentations to emergency departments with asthma' ( Borchers Arriagada et al 2020 , Filkov et al 2020 ) . The costs associated with this totalled AUD 1.95 bn, approximately 10 times the annual health burden due to fire smoke (Johnston et al 2021 ).
2.5. Tropical cyclones
2.5.1. changes in extremes.
Trends indicate no significant change in the frequency of tropical cyclones globally, but a greater fraction of those that do occur are the most intense Saffir-Simpson category 4 and 5 superstorms (Walsh et al 2019 , Kossin et al 2020 ), which usually dominate the societal impacts (Christensen et al 2013 ). Tropical cyclones are also shifting poleward in most regions, affecting the areas impacted (Kossin et al 2016 ). Further, a slowing in tropical cyclone movement has been observed (Kossin 2018 , Yamaguchi and Maeda 2020 ), accompanied by deposition of higher rainfall intensities (Patricola and Wehner 2018 ), affecting the severity of impacts.
There is substantial variability between basins. Increasing trends in the number of storms are most significant in the central Pacific, Arabia Sea and North Atlantic, and decreases are observed in the Bay of Bengal, the southern Indian Ocean and western North Pacific. This spatial distribution change is too large to be explained by natural variability alone and is linked to climate change (Murakami et al 2020 ). In the North Atlantic, an observed increase in intensification rate is likely too large for natural variability (Bhatia et al 2019 ), likewise for the significant slowing of translation speed over the US (Kossin 2018 ), while the observed increase in overall activity is significant yet not attributable to climate change (Ting et al 2015 ). In the Bay of Bengal, despite the decreasing numbers, there is a clear increasing trend in the fraction of high intensity storms and overall cyclone energy (Balaji et al 2018 ). Changes in overall activity are less certain in the west Pacific due to high variability, but northward shift in storm tracks since the 1980s is significant (Kossin et al 2016 , Lee et al 2020 ), as is a slowdown of translation speed (Yamaguchi and Maeda 2020 ).
There have also been several notable events amplified by climate change in recent years, including Hurricanes Irma, Maria, Katrina, Harvey, Florence, Sandy, Typhoons Haiyan and Morakot, and others. Additionally, notable recent seasons of high cyclone activity could not be explained without anthropogenic influence, including in the Arabian sea in 2015 (Murakami et al 2017 ), in the western North Pacific in 2015 (Zhang et al 2016 , Yang et al 2018 , Yamada et al 2019 ), and in the North Atlantic in 2017 (Murakami et al 2018 ).
2.5.2. Why it matters
Tropical cyclones often cause flooding, including due to storm surges affecting coastal areas. In addition, storms generate high winds that fell trees, and destroy property and power lines, thus creating further disruption. For instance, in the wake of Hurricane Irma in 2017, services on Puerto Rico were hindered by blackouts after a partial collapse of the power system (Zorrilla 2017 ). When Hurricane Maria struck just two weeks later it caused devastation exacerbated by this additional vulnerability. Further, it extended the spatial and temporal aspects of disruption to services and the power grid across the island and for months into the future (Kishore et al 2018 , Kwasinski et al 2019 ). The subsequent reliance on generators led to worsening air quality in San Juan (Subramanian et al 2018 ). The extreme rainfall also triggered over 40 000 landslides across the island, wiping out other power lines, roads and other structures (Bessette-Kirton et al 2019 ). The storm's passage also severely damaged vegetation across the island, which took months to fully recover (Hu and Smith 2018 ). There were also more long-term impacts. For example, in 2017 in Puerto Rico, in the context of an already-struggling economy, the severity of the 2017 hurricane season may have led between 129 000 and 477 000 Puerto Ricans to migrate away from the island (Acosta et al 2020 ).
2.5.3. Attributable impacts
Rainfall from Hurricanes Katrina, Maria and Irma was amplified by climate change (Patricola and Wehner 2018 ). In Puerto Rico, Maria and Irma resulted in widespread anxiety-mood disorders (Ferré et al 2019 , Galea et al 2007 , Scaramutti et al 2019 , Whaley 2009 ), as in Hurricane Katrina (Galea et al 2007 , Whaley 2009 ), especially prevalent among the most marginalised groups (Rhodes et al 2010 ) and the young (Orengo-Aguayo et al 2019 ). Additionally, at least 1000, and potentially as many as 4645, people died (Kishore et al 2018 , Santos-Burgoa et al 2018 ). We note an illustrative pair of case studies is provided by the relative impacts of Hurricane Maria in Puerto Rico and Hurricane Irma in Cuba; both islands experienced devastating landfall which caused mass destruction of property and infrastructure that affected the entire population. However, in Cuba the loss of life was far lower and the recovery was much swifter, which in turn reduced longer-term impacts. This is due to an array of factors, including engaging the public in disaster preparation and incorporating science into risk planning (Zakrison et al 2020 ), which are crucial to consider in any direct end-to-end attribution of impacts.
Other high-mortality tropical cyclones include Typhoon Haiyan (Lagmay et al 2015 ) and Cyclone Idai (Devi 2019 ), which are estimated to have led to over 7000 and 1300 deaths in southeast Asia and across south-eastern Africa, respectively. Typhoon Haiyan was shown to have been strengthened by climate change, increasing the height of the resulting storm surge by 20% (Takayabu et al 2015 ). During Cyclone Idai, flooding destroyed over 800 000 hectares of croplands belonging to half a million households (Club of Mozambique, 2019 ). In the Philippines, Haiyan severely impacted the livelihoods of 3.4 million coconut farmers and thus disrupted a major component of the nation's agriculture industry (Seriño et al 2021 ). The deadliest cyclone in the global record in the 21st Century, representing nearly 70% of all recorded mortality for storms in the period, was Cyclone Nargis, which struck Myanmar in 2008 and caused over 138 000 fatalities (Fritz et al 2009 ). This cyclone formed due to anomalously warm waters in the Bay of Bengal (Lin et al 2009 ), where such storms are becoming less frequent but more intense due to climate change (Balaji et al 2018 ).
In early 2022 in Malawi, Mozambique and Madagascar, tropical cyclones Ana and Batsirai triggered widespread flooding after causing extreme rainfall in the midst of a heavy rainy season. This led to a range of impacts, including destruction of thousands of homes and classrooms, as well as water supply systems, crops, roads, bridges, healthcare facilities and churches, and overall affecting hundreds of thousands of people. Rainfall from each was amplified by climate change, and the impacts were further compounded by high exposure and ongoing vulnerabilities in the region, including from recent flooding, conflict in northern Mozambique, and severe food insecurity in Madagascar (Otto et al 2022 ).
The extreme rainfall from Hurricanes Katrina, Irma, Maria, Harvey, Dorian, and Florence and Typhoon Morakot were each individually amplified by climate change (Van Oldenborgh et al 2017 , Patricola and Wehner 2018 , Wang et al 2018 , 2019 , Reed et al 2020 , 2021 , Frame et al 2020b ). Furthermore, analysis of specific drivers of Hurricane Harvey showed that such an event was linked with anomalously high ocean temperatures (both in the Gulf of Mexico and globally), therefore suggesting direct causality to global warming (Trenberth et al 2018 ). Together, the six storms listed above caused almost half a trillion dollars in damage to property and infrastructure.
In the North Atlantic basin alone, it is likely that other hurricanes constituting damages in excess of USD 200 bn follow a similar pattern (EMDAT 2019 ). Furthermore, while Hurricane Sandy was not significantly intensified by climate change (Lackmann 2015 ), the probability of storm surges as high have more than tripled due to sea level rise (Lin et al 2016 ). The added effect of climate change on this storm surge resulted in an extra USD 8 bn in damage and affected a further 71 000 people (Strauss et al 2021 ). It is reasonable to conclude that both storm surge and rainfall totals from all tropical cyclones are being amplified by climate change, while other aspects of such events vary between basins.
3. Discussion and conclusions
Developments in climate change attribution, improved understanding of the myriad impacts of extreme weather, and documenting its harms, have meant that an increasingly diverse and societally-relevant range of impacts can be assessed for their connection to anthropogenic climate change. This includes those occurring on local and regional scales beyond those assessed in the recent IPCC AR6.
However, both the impacts of climate change and the current degree of understanding of these vary across hazards and regions. In order to adapt to changing risks effectively, and to optimise mitigation of further warming, it is crucial that understanding continues to develop and does so equitably. This review and other work (Otto et al 2020b , Clarke et al 2021 ) provides a starting point for more systematic documenting of the costs (monetary and non-monetary) of human-caused climate change today and the losses and damages caused. In order to build on this, there are three areas in which scientific developments will add great value.
First, recording the impacts of extreme weather far more systematically around the world. A lack of data on past impacts of extreme events is a major barrier to mitigating future damages, simply because there is no direct evidence upon which to base the necessary measures. In particular, the impacts of extreme heat are chronically under-recorded in the global south. As explored by Harrington and Otto ( 2020 ) for heat in sub-Saharan Africa, this is likely due to institutional differences in the way impacts are recorded (government agencies vs NGOs), and would benefit from a collaborative effort to create databases for documenting mortality, morbidity and impacts on transport and power infrastructure, especially in cities. Furthermore, developing official heatwave definitions for nations currently lacking them would improve the chance that they are recorded in international disaster databases such as EMDAT.
Second, improve the coverage of attribution for more regions around the world, for a more diverse range of hazards, and with a focus on event definitions that are most pertinent to the impacts upon people. Attribution allows us to identify whether and to what degree climate change influenced a given event, as well as a trajectory of change over time. This is just part of the full picture of risk, as explored below. However, it remains useful to link between lived impacts and global climate change for the purposes of communication, adaptation and mitigation. It is therefore important everywhere.
Attribution studies on individual events are currently lacking for a number of regions and hazards. This includes key flood events highlighted earlier in this review as some of the most impactful, in South Asia; in Africa, including Sudan, Ethiopia, and Nigeria; in South America, including Peru, Colombia and Brazil; and the Caribbean, including Haiti and the Dominican Republic. Attribution of extreme heat is limited across South America and Africa largely due to a lack of research capacity across the world. For tropical cyclones, while rainfall and storm surge heights are increasingly well understood, especially in the North Atlantic, changes in intensity are not. Further, basins with some of the most devastating storms of the past, such as Cyclone Nargis in the Bay of Bengal and Cyclone Idai in the southwestern Indian Ocean, remain understudied. Finally, for wildfires, the IPCC AR6 documents regional observed changes. However, these are large spatial averages that may dampen the signal of individual attribution studies if they only apply to smaller subregions. Further, additional attribution work would add value in vulnerable and drought-prone regions such as Amazonia, the Mediterranean and Southern Africa.
Going forwards, the coverage of attribution studies can be improved in several ways: operationalising attribution and recording impact information as part of national weather services; incorporating local experts into any attribution analysis; building capacity for local experts to conduct such analyses in the future; improving understanding of compound event attribution, especially for drought; utilising the existing body of attribution literature to make statements without requiring new analysis in regions that are already well understood; creating a standardised language for impacts and risk.
The third and final area for future work involves a broader consideration of risk, rather than simply hazards and impacts. The context of a disaster, in the form of the exposure and vulnerability of affected individuals, infrastructure, agricultural systems and property, is crucial to a more complete understanding, whether this is included in quantitative analysis (e.g. Otto et al 2015 , Ebi et al 2017 ), or provides a way to frame a study and define an event that is suitable for a particular use (Stone et al 2021 ). For instance, East Africa is frequently subjected to droughts with devastating humanitarian consequences. Despite substantial research into these events, no significant connection to climate change is detectable. In part, this is due to a dearth of observational data. However, the reality in the region is that high levels of vulnerability due to poverty and socio-cultural factors, and very high regional exposure are already significant drivers of disasters.
One consequence of this is that even a relatively small climate change signal would lead to vastly-amplified impacts in the region—this framing tells part of the story. Most importantly, however, it also shows that to reduce risks it is more pertinent to tackle these non-climate drivers head-on, rather than blaming the external forces of 'nature' or 'climate' (Raju et al 2022 ).
Therefore, in the illustrative case of East Africa, expending additional resources purely to identify a climate-related signal from the noise is relatively unimportant, and headlines about climate change driving drought in the region are actively unhelpful. Instead, in order to mitigate impacts, the focus of research and resources ought to be the development of reliable seasonal forecasting, the effective distribution of this information, and other measures to reduce vulnerability (Coughlan de Perez et al 2019 , Gebremeskel et al 2019 ). In this situation, attribution provides a crucial source of information if it is framed in such a way to identify all key drivers of impacts, not simply answering the climate question or considering meteorological extremes (Stone et al 2021 , Raju et al 2022 ).
A recent study on famine in Madagascar exemplifies this approach (World Weather Attribution 2021b ). Analysis found that low precipitation totals, which contributed to the crisis, were not significantly changed in likelihood by human-caused climate change. Instead, the main drivers of the famine were food insecurity due to poverty, compounded by outbreaks of pests and COVID-19 restrictions. This information has great utility for risk reduction, which promises significant near-term co-benefits yet may be overlooked in analysis focused solely on climate change. Building on this, the study also notes an emerging future connection to climate change, which will likely only amplify such droughts significantly at global warming levels of greater than 2 °C. This could be important information for mitigation going forwards.
This work is in line with other analyses in which climate change is found to be the single most important driver of an event. For example, in Siberia in 2020, the extreme heat would have been all but impossible without human-caused climate change (World Weather Attribution 2021c ). In essence, to address climate change appropriately, it is important to understand all of the key drivers of an event that may be hidden behind the headlines—neither ignoring climate change nor focusing solely upon it.
Acknowledgments
This work was supported by a NERC Doctoral Training Partnership Grant NE/L002612/1 and the European Union's Horizon 2020 research and innovation program under Grant Agreement No. 101003469. R F S-S acknowledges support from the Natural Environment Research Council Grant NE/S007474/1, Climate Analytics and the Oxford Martin Programme on the Post-Carbon Transition. L J H acknowledges funding from the New Zealand MBIE Endeavour Fund Whakahura programme (Grant ID: RTVU1906). This work was also supported by the Austrian non-profit organisation AllRise (Reg. No. 1958321055). These funding bodies had no direct involvement in the conduct of the research or production of the article.
Data availability statement
The data that support the findings of this study are available upon reasonable request from the authors.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.

International Journal of Environmental Research
- Hamidreza Jafari
Societies and partnerships
- University of Tehran
Latest articles
Analysing complete street design principles using space syntax methodology in a case of haft-e-tir square, tehran.
- Azadeh Mohajer Milani

Photocatalytic Degradation of Methyl Red and Methyl Blue Dyes with Doped-Titanium Dioxide Nanoparticles in the Presence of Peroxymonosulfate Oxidant
Seasonal Distribution, Characterization, Indexing and Risk Assessment of Micro- and Nanoplastics in Coastal Sediments: a Case Study from Istanbul
- Ceyhun Akarsu
- Vildan Zülal Sönmez
- Nüket Sivri

Hydration and Pb Stabilization Mechanisms of Fly Ash–Slag-Based Mine Backfilling Binders
- Chutong Zhao
- Xiaona Wang
- Qunhui Wang

Pollution Characteristics and Risk Assessment of Heavy Metals in Dujiangyan Irrigation District, China
- Hongling Yin
Journal updates
Previous editors, journal information.
- Astrophysics Data System (ADS)
- CAB Abstracts
- Chemical Abstracts Service (CAS)
- Engineering Village – GEOBASE
- Google Scholar
- INIS Atomindex
- Japanese Science and Technology Agency (JST)
- OCLC WorldCat Discovery Service
- Science Citation Index Expanded (SCIE)
- Semantic Scholar
- TD Net Discovery Service
- UGC-CARE List (India)
- Zoological Record
Rights and permissions
Editorial policies
© University of Tehran
- Find a journal
- Publish with us
- Track your research
- Introduction
- Conclusions
- Article Information
Participants continued to live in their home environment without any prescribed diet or physical activity during the 28 consecutive days of the study. Error bars are SEs of the mean. The vertical dashed line separates the two 2-week sleep periods.
A-D, Data are in ascending order of change in sleep duration for the control group and sleep extension group. E, Data were from 74 participants. All available data were used. The line represents the line of best fit from the linear regression model. One participant in the control group and 3 participants in the sleep extension group had missing data in change in sleep duration (ie, missing mean data in at least 1 of 2 study periods). One participant in the control group and 4 participants in the sleep extension group had missing data in change in energy intake. Overall, 1 participant in the control group and 5 participants in the sleep extension group had missing data in either change in sleep duration or change in energy intake.
Trial Protocol
eMethods. Participants, Inclusion and Exclusion Criteria
eReferences
eTable 1. Effect of Treatment on Actigraphy-Based Time in Bed and Sleep Duration on All Days, Workdays and Free Days
eTable 2. Effect of Treatment on Actigraphy-Based Outcomes
eTable 3. Baseline Characteristics of Participants With Complete vs Incomplete Data
eTable 4. Self-Reported Outcomes by Visual Analog Scales
Data Sharing Statement
- Good Sleep, Better Life—Enhancing Health and Safety With Optimal Sleep JAMA Internal Medicine Invited Commentary April 1, 2022 Mark R. Rosekind, PhD; Rafael Pelayo, MD; Debra A. Babcock, MD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
Tasali E , Wroblewski K , Kahn E , Kilkus J , Schoeller DA. Effect of Sleep Extension on Objectively Assessed Energy Intake Among Adults With Overweight in Real-life Settings : A Randomized Clinical Trial . JAMA Intern Med. 2022;182(4):365–374. doi:10.1001/jamainternmed.2021.8098
Manage citations:
© 2024
- Permissions
Effect of Sleep Extension on Objectively Assessed Energy Intake Among Adults With Overweight in Real-life Settings : A Randomized Clinical Trial
- 1 Department of Medicine, The University of Chicago, Chicago, Illinois
- 2 Department of Public Health Sciences, The University of Chicago, Chicago, Illinois
- 3 Biotechnology Center, Department of Nutritional Sciences, University of Wisconsin–Madison, Madison
- Invited Commentary Good Sleep, Better Life—Enhancing Health and Safety With Optimal Sleep Mark R. Rosekind, PhD; Rafael Pelayo, MD; Debra A. Babcock, MD JAMA Internal Medicine
Question What is the effect of sleep extension on objectively assessed energy intake in adults with overweight in their usual home environment?
Findings In this randomized clinical trial of 80 adults with overweight and habitual sleep less than 6.5 hours per night, those randomized to a 2-week sleep extension intervention significantly reduced their daily energy intake by approximately 270 kcal compared with the control group. Total energy expenditure did not significantly differ between the sleep extension and control groups, resulting in a negative energy balance with sleep extension.
Meaning The findings suggest that improving and maintaining adequate sleep duration could reduce weight and be a viable intervention for obesity prevention and weight loss programs.
Importance Short sleep duration has been recognized as a risk factor for obesity. Whether extending sleep duration may mitigate this risk remains unknown.
Objective To determine the effects of a sleep extension intervention on objectively assessed energy intake, energy expenditure, and body weight in real-life settings among adults with overweight who habitually curtailed their sleep duration.
Design, Setting, and Participants This single-center, randomized clinical trial was conducted from November 1, 2014, to October 30, 2020. Participants were adults aged 21 to 40 years with a body mass index (calculated as weight in kilograms divided by height in meters squared) between 25.0 and 29.9 and had habitual sleep duration of less than 6.5 hours per night. Data were analyzed according to the intention-to-treat principle.
Interventions After a 2-week habitual sleep period at baseline, participants were randomized to either an individualized sleep hygiene counseling session that was intended to extend their bedtime to 8.5 hours (sleep extension group) or to continue their habitual sleep (control group). All participants were instructed to continue daily routine activities at home without any prescribed diet or physical activity.
Main Outcomes and Measures The primary outcome was change in energy intake from baseline, which was objectively assessed as the sum of total energy expenditure and change in body energy stores. Total energy expenditure was measured by the doubly labeled water method. Change in body energy stores was computed using regression of daily home weights and body composition changes from dual-energy x-ray absorptiometry. Sleep duration was monitored by actigraphy. Changes from baseline were compared between the 2 groups using intention-to-treat analysis.
Results Data from 80 randomized participants (mean [SD] age, 29.8 [5.1] years; 41 men [51.3%]) were analyzed. Sleep duration was increased by approximately 1.2 hours per night (95% CI, 1.0 to 1.4 hours; P < .001) in the sleep extension group vs the control group. The sleep extension group had a significant decrease in energy intake compared with the control group (−270 kcal/d; 95% CI, −393 to −147 kcal/d; P < .001). The change in sleep duration was inversely correlated with the change in energy intake ( r = −0.41; 95% CI, −0.59 to −0.20; P < .001). No significant treatment effect in total energy expenditure was found, resulting in weight reduction in the sleep extension group vs the control group.
Conclusions and Relevance This trial found that sleep extension reduced energy intake and resulted in a negative energy balance in real-life settings among adults with overweight who habitually curtailed their sleep duration. Improving and maintaining healthy sleep duration over longer periods could be part of obesity prevention and weight loss programs.
Trial Registration ClinicalTrials.gov Identifier: NCT02253368
Obesity is a major public health concern. 1 The obesity epidemic appears to coincide with a pattern of sleeping less that has been observed in society over the past several decades. For example, one-third of the US population reported not getting the recommended 7 to 9 hours of sleep per night. 2 - 4 Substantial evidence suggests that sleeping less than 7 hours per night on a regular basis is associated with adverse health consequences. 5 Particularly, insufficient sleep duration has been increasingly recognized as an important risk factor for obesity. 6 , 7 Prospective epidemiologic studies suggest that short sleep duration is an important risk factor for weight gain. 8 - 10 However, it remains unknown whether extending sleep duration can be an effective strategy for preventing or reversing obesity. Although sleep hygiene education is encouraged by obesity experts, 11 most health professionals and patients do not implement obtaining adequate sleep duration as part of the strategies to combat the obesity epidemic. 12
At the population level, the association between energy flux and body weight implicates that increased energy intake is the main factor in higher body weights in modern society. 13 According to dynamic prediction models, a sustained increase in energy intake of even 100 kcal/d would result in a weight gain of about 4.5 kg over 3 years. 14 , 15 Factors that underlie the observed persistent increase in energy intake and mean weight gain at the population level need to be better understood. One such factor is insufficient sleep duration. Short-term experimental laboratory studies have found that sleep restriction in healthy individuals is associated with an increased mean energy intake of about 250 to 350 kcal/d with minimal to no change in energy expenditure. 16 - 19 However, these laboratory studies do not represent real life. The magnitude of sleep restriction was extreme in most cases, and energy intake was ascertained from a single or a few meals. In a real-life setting in which participants continue their normal daily activities, multiple interacting factors (eg, social interactions and free-living physical activity) can influence energy intake or expenditure and weight.
To date, it remains unknown whether and to what extent an intervention that is intended to increase sleep duration in a real-life setting affects energy balance and body weight. We conducted a randomized clinical trial (RCT) to determine the effects of a sleep extension intervention on objectively assessed energy intake, energy expenditure, and body weight in real-life settings among adults with overweight who habitually curtailed their sleep duration.
This single-center, parallel-group RCT was conducted from November 1, 2014, to October 30, 2020. The protocol was approved by The University of Chicago Institutional Review Board, and participants provided written informed consent. The study protocol is available in Supplement 1 . We followed the Consolidated Standards of Reporting Trials ( CONSORT ) reporting guideline.
Adult men and women aged 21 to 40 years with a body mass index (calculated as weight in kilograms divided by height in meters squared) between 25.0 and 29.9 and a mean habitual sleep duration of less than 6.5 hours per night were eligible. Individuals were required to have stable self-reported sleep habits for the past 6 months. They were recruited from the community and completed an initial online survey followed by a face-to-face interview. Race and ethnicity data were self-reported at this time and included the following race and ethnicity categories: Asian, Black or African American, Hispanic, and White. Those who met the inclusion criteria underwent laboratory screening (polysomnography, oral glucose tolerance test, and blood tests) to determine eligibility. Habitual sleep duration was confirmed by a 1-week screening wrist actigraphy at home. Those who had obstructive sleep apnea confirmed by laboratory polysomnography (apnea-hypopnea index >5), insomnia or history of any other sleep disorder, or night shift and rotating shift work (current or in the past 2 years) were excluded. Detailed eligibility criteria are provided in the eMethods in Supplement 2 .
After a 2-week habitual sleep period at baseline, participants were randomized to either 2-week sleep extension (sleep extension group) or 2-week continued habitual sleep (control group) ( Figure 1 ). Participants continued their daily routine activities at home without any prescribed diet or physical activity.
To blind participants to the sleep extension intervention, we described the study in the recruitment materials as follows: “we will collect information about sleep habits and metabolism.” The sleep extension group was blinded to randomization until after the 2-week baseline assessments, and the control group was blinded until the end of the 4-week study. This approach allowed us to capture habitual sleep-wake patterns without influencing participants' usual behavior or creating selection bias with only participants interested in improving sleep habits. After study completion, all participants were provided with information about the health benefits of optimal sleep duration. Block randomization, stratified by sex, was performed using computer-generated random numbers. Before the trial, randomization assignments were prepared by a biostatistician (K.W.) using opaque, sealed, and numbered envelopes and were given to the research coordinator (E.K.).
Sleep-wake patterns were continuously monitored at home by wrist actigraphy throughout the 4-week study. Participants were asked to wear an accelerometer (motion)-based monitor (Actiwatch Spectrum Plus; Philips) and to press a built-in event marker button when they went to bed to sleep each night and when they got out of bed each morning. Sleep was automatically scored (Actiware, version 6.0.9; Philips) using validated algorithms as the sum of all epochs that were scored as sleep during the total time spent in bed. 20 , 21
During the 2-week baseline, all participants were instructed to continue their habitual sleep patterns at home. On the morning of day 15, participants met with study investigators (E.T. and E.K.) in the research center. Those who were randomized to the sleep extension group received individualized sleep hygiene counseling through a structured interview (E.T.) (eMethods in Supplement 2 ). 22 At the end of the interview, participants were provided with individualized recommendations to follow at home for 2 weeks, with the aim of extending their bedtime duration to 8.5 hours. On day 22, participants returned for a brief follow-up visit. Actigraphy data from the first intervention week were reviewed, and further sleep counseling was provided as needed.
To minimize any imbalance in contact with the investigators between the 2 groups, we asked participants in the control group to meet with the study investigators on days 15 and 22. Actigraphy data of these participants were downloaded, but the participants did not receive any specific sleep recommendations and were instructed to continue their daily routine and habitual sleep behaviors until the end of the study.
For each 2-week period, the energy intake was calculated from the sum of total energy expenditure and change in body energy stores using the principle of energy balance. 14 , 23 , 24 Total energy expenditure was measured by the doubly labeled water method. 25 - 29 For each 2-week period, the change in body energy stores was computed from the regression (slope, grams per day) of daily home weights and change in body composition (ie, fat mass and fat-free mass) using dual-energy x-ray absorptiometry. Participants were provided a cellular-enabled weight scale (BodyTrace; BodyTrace Inc) and instructed to take their nude weights twice every morning after awakening before eating or drinking. Weight values were hidden from the participants to minimize potential influence on behavior. Changes in body composition were converted to changes in energy stores using 9.5 kcal/g as the energy coefficient of fat mass and 1.0 kcal/g as the energy coefficient of fat-free mass. 30 Resting metabolic rate was measured by indirect calorimetry for 30 minutes after fasting and for 4 hours after eating a standardized breakfast. Thermic effect of the meal was calculated, which was previously described elsewhere. 31 Activity energy expenditure was calculated by subtracting the resting metabolic rate and thermic effect of the meal from the total energy expenditure. 31 , 32 Additional details are provided in the eMethods in Supplement 2 .
The primary outcome was change in energy intake from baseline. A total final sample size of 80 participants (40 per group) was originally planned and provided 80% power to detect a true difference in energy intake between groups of 207 kcal/d using a 2-sided α = .05 significance threshold (trial protocol in Supplement 1 ). An intention-to-treat analysis was conducted in Stata, version 16 (StataCorp LLC) using 2-tailed tests with statistical significance set at P < .05. Categorical data are presented as counts and percentages. Continuous data are presented as means and SDs. Linear mixed-effects models were fit to determine the treatment differences between the groups. 33 Models included the randomization group, 2-week baseline period (period 1) vs 2-week intervention (period 2) and their interaction, and random effects for each participant. The treatment effect (95% CI) was estimated by the treatment group and period interaction, which is equivalent to testing the difference in change from baseline (period 2 minus period 1) in the sleep extension group vs the control group. To confirm the robustness of primary findings, we fit additional models using the analysis of covariance approach with the period 2 value as the dependent variable, treatment group as the independent variable, and period 1 value as covariates.
In secondary analyses, mixed models that adjusted for sex or menstrual cycle were also fit; these covariates were chosen because of the known influence of menstrual cycle on short-term changes in weight. A Pearson correlation coefficient was calculated to assess the relationships between the changes from baseline in sleep duration and the changes from baseline in energy intake. No adjustments were made to P values or CIs for multiple comparisons. Baseline characteristics of participants with complete data were compared with those of participants with incomplete data using unpaired, 2-tailed t tests and Fisher exact tests. No imputation for missing values was performed.
Of the 210 adults who provided consent and were assessed for eligibility, 81 were randomized (41 to the control group and 40 to the sleep extension group) initially ( Figure 1 ). One participant in the control group revealed adhering to a weight loss regimen and thus did not meet the study inclusion criteria and was deemed ineligible after randomization. 34 The 80 participants had a mean (SD) age of 29.8 (5.1) years and consisted of 41 men (51.3%) and 39 women (48.7%). Baseline characteristics of participants were similar between randomization groups ( Table 1 ). None of the participants were using any antihypertensive or lipid-lowering agents or any prescription medication that can affect sleep or metabolism.
Figure 2 illustrates the mean nightly sleep duration by actigraphy in each group throughout the 4-week study. Participants in the sleep extension group had a significant increase from baseline in mean sleep duration by actigraphy compared with those in the control group (1.2 hours; 95% CI, 1.0-1.4 hours; P < .001). The findings were similar with regard to change in sleep duration when only participants' workdays (1.3 hours; 95% CI, 1.0-1.5 hours; P < .001) or free days (1.1 hours; 95% CI, 0.7-1.5 hours; P < .001) were considered (eTable 1 in Supplement 2 ). No difference was found in change in sleep efficiency (percentage of time spent asleep during time in bed) between the 2 groups (–0.6 hours; 95% CI, –2.1 to 1.0 hours; P = .48), confirming the success of the intervention (eTable 2 in Supplement 2 ).
Energy intake was statistically significantly decreased in the sleep extension group compared with the control group (−270.4 kcal/d; 95% CI, −393.4 to −147.4 kcal/d; P < .001). Figure 3 A through D illustrates the changes from baseline in energy intake and the changes from baseline in sleep duration in individual participants. There was a significant increase in energy intake from baseline in the control group (114.9 kcal/d; 95% CI, 29.6 to 200.2 kcal/d) and a significant decrease in energy intake from baseline in the sleep extension group (−155.5 kcal/d; 95% CI, −244.1 to −66.9 kcal/d) ( Table 2 ). Considering all participants, the change in sleep duration was inversely correlated with the change in energy intake ( r = −0.41; 95% CI, −0.59 to −0.20; P < .001) ( Figure 3 E). Each 1-hour increase in sleep duration was associated with a decrease in energy intake of approximately 162 kcal/d (−162.3 kcal/d; 95% CI, −246.8 to −77.7 kcal/d; P < .001).
No statistically significant treatment effect was found in total energy expenditure or other measures of energy expenditure ( Table 2 ). Participants in the sleep extension group had a statistically significant reduction in weight compared with those in the control group (−0.87 kg; 95% CI, −1.39 to −0.35 kg; P = .001). There was weight gain from baseline in the control group (0.39 kg; 95% CI, 0.02 to 0.76 kg) and weight reduction from baseline in the sleep extension group (−0.48 kg; 95% CI, −0.85 to −0.11 kg) ( Table 2 ).
The findings on energy intake, energy expenditure, and weight were similar after adjustment for the effects of sex or menstrual cycle. No statistically significant differences in baseline characteristics were found between the 75 participants (93.8%) who had complete data on energy intake (primary outcome) vs participants with missing data on energy intake. The proportion of participants with complete data on energy intake was not significantly different between the sleep extension and control groups (90.0% vs 97.5%; P = .36). When all reported outcomes were considered, no significant differences (except for depressive symptoms) in baseline characteristics were found between participants with complete data and participants with incomplete or missing data (eTable 3 in Supplement 2 ). The proportion of participants with complete data on all reported outcomes was similar between the sleep extension and control groups (82.5% vs 85.0%; P > .99).
In this RCT of adults with overweight who habitually curtailed their sleep duration, sleep extension reduced energy intake and resulted in a negative energy balance (ie, energy intake that is less than energy expenditure) in real-life settings. To our knowledge, this study provides the first evidence of the beneficial effects of extending sleep to a healthy duration on objectively assessed energy intake and body weight in participants who continued to live in their home environment. Modest lifestyle changes in energy intake or expenditure are increasingly promoted as viable interventions to reverse obesity.
According to the Hall dynamic prediction model, a decrease in energy intake of approximately 270 kcal/d, which we observed after short-term sleep extension, would predict an approximately 12-kg weight loss over 3 years if the effects were sustained over a long term. 14 , 15 However, this study cannot infer how long healthy sleep habits may be sustained. Nevertheless, these modeling predictions on weight change suggest that continued adequate sleep duration and beneficial effect on energy intake could translate into clinically meaningful weight loss and help reverse or prevent obesity. Thus, the findings of this study may have important public health implications for weight management and policy recommendations.
The findings of decreased energy intake, negative energy balance, and weight reduction resulting from sleep extension are in agreement with the findings of short-term laboratory sleep-restriction studies showing increased energy intake and weight gain 17 as well as the findings of prospective epidemiologic studies linking sleep restriction to obesity risk. 8 A recent meta-analysis of randomized controlled laboratory studies found that short-term sleep restriction over 1 to 14 days of duration in healthy individuals was associated with increases of mean energy intake by approximately 253 kcal/d, as assessed during a single meal. 17 Another meta-analysis of prospective cohort studies found that the risk of obesity increased by 9% for each 1-hour decrease in sleep duration. 8 We did not observe a statistically significant change in total energy expenditure by doubly labeled water method or mean daytime activity counts by actigraphy (eTable 2 in Supplement 2 ). Although some laboratory sleep-restriction studies reported an increase in total energy expenditure of approximately 92 to 111 kcal/d, using a whole-room calorimeter, 35 , 36 other studies observed no change. 16 , 37 We found a modest reduction in weight after sleep extension, and the composition of weight change was primarily in fat-free mass, which is consistent with the short-term changes in body composition. 38 , 39 If sleep is extended over longer periods, weight loss in the form of fat mass would likely increase over time. A few observations suggest that sleeping 7 to 8 hours per night is associated with greater success in weight loss interventions. 40 - 43
In this RCT, we found an overall increase in objective sleep duration of approximately 1.2 hours in participants who habitually slept less than 6.5 hours per night. The change in sleep duration from baseline varied between participants and from night to night in the real-life setting. Overall, the sleep extension group compared with the control group had significantly higher subjective scores in obtaining sufficient sleep, with more daytime energy and alertness and better mood (eTable 4 in Supplement 2 ). Similar to a previous study of sleep extension, 22 the present RCT used an individualized counseling approach. Another study used bedtime extension in habitual short sleepers in real-life conditions but obtained variable benefits on sleep, likely because of a lack of an individualized approach or appropriate blinding. 44 None of these previous studies objectively measured energy intake.
Future similarly rigorous intervention studies of longer duration and using objective assessments of energy balance under real-life conditions are warranted to elucidate the underlying mechanisms and to investigate whether sleep extension could be an effective, scalable strategy for reversing obesity in diverse populations. Along with a healthy diet and regular physical activity, healthy sleep habits should be integrated into public messages to help reduce the risk of obesity and related comorbidities.
This study has several strengths. The major strengths are the randomized design and the objective tracking of energy intake and sleep in real-life settings. Most epidemiologic studies linking short sleep duration to body weight relied on self-reported dietary intake. 45 We did not collect self-reported dietary data because this method is subject to bias and has been shown to be inaccurate compared with the doubly labeled water method. 46 , 47 Most experimental studies that measured energy intake used a single meal under unnatural laboratory conditions. We used a validated method to objectively track energy intake by the doubly labeled water method and change in energy stores. 23 , 48 , 49 In this trial, we objectively quantified energy intake after sleep extension while individuals continued their daily routine in their usual environment. Participant blinding and use of actigraphy allowed us to capture true habitual sleep patterns at baseline. 22 , 50 In addition, we excluded insomnia and sleep apnea.
This study also has several limitations. We enrolled adults with overweight and used selective eligibility criteria, which may limit generalizability to more diverse populations. The increase in energy intake and weight from baseline that we observed in the control group may have contributed to the significant treatment effects. However, in RCTs, performing a between-group comparison, rather than separate tests against baseline within the groups, is strongly recommended. 51 The study did not provide information on how long healthy sleep habits could be maintained over longer periods. 44 We did not systematically assess the factors that may have influenced sleep behavior, but limiting the use of electronic devices appeared to be a key intervention among the participants (eTable 4 in Supplement 2 ). The doubly labeled water method has a precision of 5%, which may translate into some degree of uncertainty in the energy intake calculations. Although whole-room calorimeters can measure energy expenditure with a higher precision of approximately 1% to 2%, they do not represent real-life measurement and are not feasible over longer periods. We did not assess the underlying biological mechanisms of food frequency and the circadian timing of food intake. Multiple interrelated factors could contribute to the finding of decreased energy intake after sleep extension. 6 , 52 Evidence from laboratory sleep restriction studies suggests that increased hunger, alterations in appetite-regulating hormones, and changes in brain regions related to reward-seeking behavior are potential mechanisms that promote overeating after sleep restriction. 6 , 45
This RCT found that short-term sleep extension reduced objectively measured energy intake and resulted in a negative energy balance in real-life settings in adults with overweight who habitually curtailed their sleep duration. The findings highlighted the importance of improving and maintaining adequate sleep duration as a public health target for obesity prevention and increasing awareness about the benefits of adequate sleep duration for healthy weight maintenance.
Accepted for Publication: November 14, 2021.
Published Online: February 7, 2022. doi:10.1001/jamainternmed.2021.8098
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2022 Tasali E et al. JAMA Internal Medicine .
Corresponding Author: Esra Tasali, MD, Department of Medicine, The University of Chicago, 5841 S Maryland Ave, Chicago, IL 60637 ( [email protected] ).
Author Contributions: Author Dr Tasali and Ms Wroblewski had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Tasali, Schoeller.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Tasali, Schoeller.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Tasali, Wroblewski.
Obtained funding: Tasali.
Administrative, technical, or material support: Tasali, Kahn, Kilkus, Schoeller.
Supervision: Tasali.
Other - research coordination duties: Kahn.
Conflict of Interest Disclosures: None reported.
Funding/Support: This study was funded by grants R01DK100426, CTSA-UL1 TR0002389, and UL1TR002389 from the National Institutes of Health and by the Diabetes Research and Training Center at The University of Chicago.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data Sharing Statement : See Supplement 3 .
Additional Contributions: Timothy Shriver, MS, University of Wisconsin–Madison, assisted with doubly labeled water measurements. Maureen Costello, MS, The University of Chicago, assisted with dual-energy x-ray absorptiometry scans. Becky Tucker, BA, Harry Whitmore, RPSGT, and Kristin Hoddy, PhD, RD, The University of Chicago, assisted with data collection. We thank the nurses, dieticians, and technicians at the Clinical Research Center at The University of Chicago for their expert assistance in data collection. We also thank the staff of the Sleep Research Center at The University of Chicago for their support. These individuals received no additional compensation, outside of their usual salary, for their contributions. We thank the volunteers for participating in this study.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Microbiol
Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review
Vinay mohan pathak.
1 Department of Microbiology, University of Delhi, New Delhi, India
Vijay K. Verma
Balwant singh rawat.
2 Department of Pharmaceutical Sciences, Gurukul Kangri Deemed to be University, Haridwar, India
Baljinder Kaur
3 Indian Institute of Technology Bombay, Mumbai, Maharashtra, India
Neelesh Babu
4 Department of Microbiology, Baba Farid Institute of Technology, Sudhowala, India
Akansha Sharma
5 Allergy and Immunology Section, CSIR-IGIB, New Delhi, India
Seeta Dewali
6 Laboratory of Alternative Protocols in Zoology and Biotechnology Research Laboratory, Department of Zoology, Kumaun University, Nainital, India
Monika Yadav
7 Cancer Biology Laboratory, School of Life Sciences, Jawaharlal Nehru University, New Delhi, India
Reshma Kumari
8 Department of Botany & Microbiology, Gurukul Kangri Deemed to be University, Haridwar, India
Sevaram Singh
9 Multidisciplinary Clinical Translational Research, Translational Health Science and Technology Institute, NCR Biotech Science Cluster, Faridabad, India
10 Jawaharlal Nehru University, New Delhi, India
Asutosh Mohapatra
11 Food Process Engineering, National Institute of Food Technology, Entrepreneurship and Management, Thanjavur, India
Varsha Pandey
12 Department of Bioscience and Biotechnology, Banasthali Vidyapith, Newai Tonk, India
Nitika Rana
13 Department of Environmental Science, Dr. Yashwant Singh Parmar University of Horticulture and Forestry, Solan, India
Jose Maria Cunill
14 Biotechnology Engineering, Universidad Politécnica Metropolitana de Puebla, Mexico, Mexico
Pesticides are either natural or chemically synthesized compounds that are used to control a variety of pests. These chemical compounds are used in a variety of sectors like food, forestry, agriculture and aquaculture. Pesticides shows their toxicity into the living systems. The World Health Organization (WHO) categorizes them based on their detrimental effects, emphasizing the relevance of public health. The usage can be minimized to a least level by using them sparingly with a complete grasp of their categorization, which is beneficial to both human health and the environment. In this review, we have discussed pesticides with respect to their global scenarios, such as worldwide distribution and environmental impacts. Major literature focused on potential uses of pesticides, classification according to their properties and toxicity and their adverse effect on natural system (soil and aquatic), water, plants (growth, metabolism, genotypic and phenotypic changes and impact on plants defense system), human health (genetic alteration, cancer, allergies, and asthma), and preserve food products. We have also described eco-friendly management strategies for pesticides as a green solution, including bacterial degradation, myco-remediation, phytoremediation, and microalgae-based bioremediation. The microbes, using catabolic enzymes for degradation of pesticides and clean-up from the environment. This review shows the importance of finding potent microbes, novel genes, and biotechnological applications for pesticide waste management to create a sustainable environment.
Introduction
Pesticides are chemical compounds that are used to eliminate insects, rodents, fungi, and weeds. They include insecticides, herbicides, nematicides, fungicides, molluscicides, rodenticides, plant growth regulators, and other compounds ( Zhan et al., 2020 ; Bhatt et al., 2021a ; Zhang et al., 2021 ). It is generally used to prevent illnesses spread by vectors, including crop protection, food preservation, and significant roles in commercial as well as food based industrial practices, i.e., aquaculture, agriculture, food processing, and storage ( Mieldazys et al., 2015 ; Sharma et al., 2019 ). Any living bodies, either animals or plants, which are harmful for human or animals are known as pests. Pesticides are substances that are used to either kill or prevent the growth of pests.
According to the United States Code of Federal Regulations (CFR), a pesticide is any component or mixture of compounds intended for use as a plant regulator, defoliant, or desiccant ( United States Environmental Protection Agency, 2004 ). Pesticides are defined by the Food and Agriculture Organization (FAO) of the United Nations as substance or mixture of substances attended for controlling, preventing, destroying any pest, animal, or human disease causing vectors, undesirable plants, or animal species affecting food production, managing, selling, storage, and transportation ( World Health Organization, 2015 ). Since ancient times, a variety of chemical compounds have been used to control pests. Sulfur compounds are well known example of such insect and mite control pesticides ( Gyawali, 2018 ). Pyrethrum, a plant ( Chrysanthemum cinerariaefolium ) derived pesticide, has been used for over 2000 years ( Unsworth, 2010 ). Salty water and chemical compounds (organics as well as inorganic) were widely used to control pests’ populations until the introduction of dichloro diphenyl trichloroethane (DDT) by Paul Herman Muller in 1939 as a potent pesticide ( Abubakar et al., 2020 ). However, use of DDT is helpful to increasing the food productivity and shelf-life of food products. Thus, the global demand for DDT increased day by day, which opened the door to synthesizing new chemical substances that act as pesticides. DDT was replaced by organophosphates (OPs) and carbamates (CMs) in the United States in 1975 ( Barnhoorn et al., 2009 ).
The global pesticide consumption in 2019 was approximately 4.19 million metric tons, where China was by far the largest pesticide-consuming country (1.76 million metric tons), followed by the United States (408 thousand tons), Brazil (377 thousand tons), and Argentina (204 thousand tons) ( Fernández, 2021 ). In southeast Asia, WHO reported an annual increase in pesticide usage with 20% of developing countries as pesticide-consumers, including Cambodia, Laos, and Vietnam ( Schreinemachers and Tipraqsa, 2012 ; Schreinemachers et al., 2015 ). India belongs to one of the major pesticide producing countries in Asia, having 90 thousand tons annual production of organochlorine pesticides including benzene hexachloride and DDT ( Khan et al., 2010 ; Pozo et al., 2011 ). Between 2010 and 2014, the average cost/benefit ratio was 0.645 g of total pesticides per kilogram of crop yield, with an average yearly consumption of 2.784 kg ha –1 . Japan (18.94 kg ha –1 ) had the greatest average pesticide usage from 2010 to 2014, followed by China (10.45 kg ha –1 ), Mexico (7.87 kg ha –1 ), Brazil (6.16 kg ha –1 ), Germany (5.12 kg ha –1 ), France (4.85 kg ha –1 ), United Kingdom (4.03 kg ha –1 ), United Status (3.88 kg ha –1 ), and India (0.26 kg ha –1 ) ( Zhang, 2018 ).
Herbicides account for 47.5% of pesticide contributions, followed by insecticides 29.5%, fungicides 17.5%, and other types of insecticides 5.5%, as shown in Figure 1 ( Gill and Garg, 2014 ; Zhang, 2018 ; Sharma et al., 2019 ). Pesticides are classified based on a variety of variables. The most often used criteria for pesticide classification are the mode of entry, chemical makeup, and the target it kills. On the other hand, the WHO and Globally Harmonized System (GHS) classified pesticides based on their toxicity or harmful effects, prioritizing public health.

Percentage distribution of pesticides ( Nicolopoulou-Stamati et al., 2016 ; Alengebawy et al., 2021 ).
The main advantages of pesticides are the expected immediate gains after application, e.g., eliminating caterpillars, which has the primary benefit of raising cabbage yields and quality. The three major outcomes result in 26 key advantages, ranging from the preservation of recreational grass to the saving of human lives. Secondary benefits are those that arise as a result of the primary advantages but are less obvious or immediate. They might be subtle, less visible at first glance, or long-term in character. As a result, proving cause and effect for secondary benefits is more difficult, although they can still be strong pesticide reasons. Increased productivity of cabbage leads to an increase in economic wealth, which helps to improve children’s health and education systems. Secondary benefits have been identified, including healthier individuals and permanently cultivated land that conserves biodiversity. This accomplishment was aided by the use of high-yield seed types, advanced irrigation technologies, and agricultural herbicides ( Bureau, 1993 ). Similarly, most nations’ productivity and output have increased significantly, such as wheat yields in the United Kingdom and maize yields in the United States. A multitude of factors have been blamed for increased productivity, including better cultivars, machinery use, and fertilizer usage. Pests, insects, diseases, and weeds can substantially reduce the production of harvestable crops; as a result, pesticides have played a crucial role in food production and processing. Warren (1998) also highlighted the huge increase in food production in the United States over the 20th century. Pesticides are used to increase agricultural output and food preservation while ignoring their associated risks. Overuse, exposure, and harmful consequences can all be mitigated by applying it judiciously and utilizing different pesticide categories ( World Health Organization, 2009 ). Many detrimental effects have been seen as a result of widespread pesticide usage, and effective waste management strategies are necessary to address pesticide issues.
Pesticide biodegradation is a new way of environmentally acceptable pesticide pollution control for a long-term environmental benefit. Microorganisms play a significant role in the breakdown of pesticides and have been recognized for their influence and many uses in human welfare. Several recent studies have demonstrated the potential of microorganisms, isolated from sewage or soil to degrade pesticides. These microbes include several bacterial and fungal strains, actinomycetes, algae, etc. ( Kafilzadeh et al., 2015 ). The process of pesticide biodegradation, which involves bacteria and enzymes, is described in detail in the biodegradation portion of this review. The entire process of pesticide synthesis or formulation, manufacturing or mass industrial production, detrimental effects on the environment and human health, and biodegradation of pesticides has been shown in Figure 2 . To date, there is scant information about the detailed classifications, toxicity, and remediation of pesticides in the environment. Therefore, this review article exploring the new dimensions for removal of pesticides from the environment. This review discusses the impact on living systems, bioremediation approaches, and complete residual removal of pesticides from the environment.

Thematic diagram of the synthesis, production, uses, effects, and eco-friendly management of pesticides.
Classification of pesticides
The pesticides show the toxicity in the living systems on the basis of their chemical formulations and quantity in an instance. Pesticides are a broad category of products that include antiseptics, disinfectants, anti-bacterial, fungicides, algicides, rodenticides, and herbicides ( Garcia et al., 2012 ). Pesticides are classified into two major categories based on their physical and chemical properties. Pesticide classification by nature of pesticide (synthetic and natural) and acting on pest type is illustrated in Figure 3 . Organic chemicals made up the majority of synthetic pesticides, which were grouped into the following four groups: Organophosphates, organochlorines, carbamates, and pyrethroids. Some widely used pesticides and their structures are shown in Table 1 . Naturally occurring pesticides, also known as biopesticides, are formed by living creatures such as plants, bacteria, and fungus ( Abubakar et al., 2020 ; Bhatt et al., 2020a , 2021b ).

Classification of pesticides ( Jayaraj et al., 2016 ; Hassaan and El Nemr, 2020 ; Malhotra et al., 2021 ; Souto et al., 2021 ; Parra-Arroyo et al., 2022 ).
Generally used pesticides and their chemical structures.
| Name | Structure | Name | Structure |
| DDT (Dichlorodiphenyltrichloroethane) | Lindane | ||
| DDD (Dichlorodiphenyldichloroethane) | HCH | ||
| DDE (Dichlorodiphenyldichloroethylene) | Chlordecone | ||
| Dieldrin | Toxaphene | ||
| Aldrin | Mirex | ||
| Endrin | Endosulfan | ||
| Heptachlor | Chlordane | ||
Classification of pesticides on the basis of toxicity
The amount of pesticides used (dose) and exposure period (time) are the two most important factors for pesticide toxicity that define the acute and chronic toxicity of pesticides. Acute toxicity refers to a pesticide’s toxicity to animals, plants, and humans following a definite short-term exposure of pesticide. A pesticide with a high acute toxicity is fatal, even if only a tiny quantity is absorbed into body. The World Health Organization (WHO) recognizes only acute toxicity for pesticide categorization and based on lethal dosage (LD50) divided into two types, i.e., acute cutaneous (dermal) toxicity (e.g., extremely: less than 50-mg/kg body weight of rat; highly: 50-200-mg/kg body weight of rat; moderately: 200-2,000-mg/kg body weight of rat, etc.) and acute oral toxicity (e.g., extremely: less than 5-mg/kg body weight of rat; highly: 5-50-mg/kg body weight of rat; moderately: 50-2,000-mg/kg body weight of rat, etc.) are shown in Table 2 ( World Health Organization, 2009 ).
Pesticides classification according to WHO guidelines ( World Health Organization, 2009 ).
| Class | LD of rat | Hazardous level | |
| Dermal | Oral | ||
| Ia | Less than 50 mg/kg body weight | Less than 5 mg/kg body weight | Extremely |
| Ib | 50–200 mg/kg body weight | 5–50 mg/kg body weight | Highly |
| II | 200–2000 mg/kg body weight | 50–2000 mg/kg body weight | Moderately |
| III | Above 2000 | Above 2000 | Slightly |
| U | 5000 or above | 5000 or above | Unlikely to present acute hazard |
The deadly impact of pesticide exposure that persists over time is known as chronic toxicity. Chronic toxicity of pesticides is a worry for the general population and those who work with pesticides directly because of possible exposure to pesticides. Pesticides are now classified into “WHO Hazard classifications” according to the widely used “WHO Recommended Categorization of Pesticides by Hazard.” Following a change in 2009, such a classification was merged with the “Globally Harmonized System (GHS) Acute Toxicity Hazard Category” shown in Table 3 ( Mieldazys et al., 2015 ). Pesticides are also classified based on pest type, mode of action, and disease management strategies as shown in Table 4 . Another type of classification is based on its mode of entry, which is divided into the following five categories: (1) Systemic pesticides (absorbed by animals or plants and transferred to other locations, such as in plants, entering into untreated tissues of roots, stems, or leaves via multidirectional movement through the vascular system), (2) non-systemic or contact pesticides (they require physical contact with the pest for their action), (3) stomach toxicants (it enters the digestive tract and is absorbed inside the insect’s body; such toxicants are effective for vector control and are used for mosquito or black fly management by malathion application), (4) fumigants (these pesticides are used as poisonous gases or vapor that enter the pest respiratory system via spiracles and kill it), and (5) repellents (it is used to keep pests away from treated objects) ( Yadav and Devi, 2017 ).
Pesticides classification according to the Globally Harmonized System GHS ( World Health Organization, 2009 ).
| Category | Classification criteria | |||
| LD of rat dermal | Hazardous description | LD of rat oral | Hazardous description | |
| 1 | Less than 50 mg/kg body weight | Lethal if come in skin contact | Less than 5 mg/kg body weight | Lethal if consumed |
| 2 | 50–200 mg/kg body weight | Lethal if come in skin contact | 5–50 mg/kg body weight | Lethal if consumed |
| 3 | 200–1000 mg/kg body weight | Toxic if come in skin contact | 50–300 mg/kg body weight | Toxic if consumed |
| 4 | 1000–2000 mg/kg body weight | Harmful if come in skin contact | 300–2000 mg/kg body weight | Harmful if consumed |
| 5 | 2000–5000 mg/kg body weight | Possibly harmful if come in skin contact | 2000–5000 mg/kg body weight | Possibly harmful if consumed |
Pesticides classification according to pest type, functions, and management strategies.
| Type of pesticide | Type of pests | Functions | Pests and disease management | References |
| Aldicarb | Nematicides | Inhibit nematodes (plants parasites) | Damage tissue oxidative stress, and also binds and inhibits acetylcholinesterase (AChE) (controlling acetylcholine neurotransmitter) | ; |
| Insecticides | Inhibit insects and other arthropods also | |||
| Atrazine | Herbicides | Destroy weeds and other plants, photosystem-II (PSII)–inhibiting | Use to control grasses and broadleaf weeds in sorghum, corn, and sugar cane crops | ; |
| Avitrol | Avicides | Chemicals that lethal to small seed-eating birds | Used for population management of certain birds (crows, gulls, cowbirds, blackbirds, starlings, grackles, pigeons, sparrows, red-winged blackbirds) | ; ; |
| Azoxystrobin | Fungicides | Kill fungi (blights, rusts, molds, and mildews), azoxystrobin act fungal mitochondrion, binds to cytochrome bc complex and inhibit electron transport thorough oxidative phosphorylation. | Uses to kill Oomycetes, Ascomycetes, Deuteromycetes, BasidiomycetesAnd it controls disease like apple scab rusts, rice blast, powdery and downey mildew. | ; |
| Benzoxazin | Ovicides | Prevention of mites and insects egg growth | In pest managmeent | ; |
| Bifenazate | Acaricides | Control spiders and mites that feed on plants and animals by altering their growth and development. Target site of Bifenazate is mitochondrial, particularly the Q site of that encoded for cytochrome b | Bifenazate uses as an acaricide on strawberry, flowering plants, and nursery ornamentals | ; ; |
| Boric acid | Desiccants | Act on plants by drying their tissues | Use to bed bug control | ; |
| Copper complexes | Bactericides | Prevent bacteria with greater doses, copper works as a broad-spectrum biocide by interfering with nucleic acids, disrupting enzyme active sites, interfering with the energy transport system, cell membranes integrity disrupted | Copper complexes are used to prevent infection of seedlings from plant pathogens by seed treatment | ; |
| Copper sulfate | Algaecides | Control or kill growth of algae | Alter the algal growth and photosynthesis | ; |
| Dichlorobenzene | Moth balls | Inhibit molds and moth larvae and prevent cloths damage | Commonly used to control moths, molds, and mildew | ; |
| Fipronil | Termiticides | Fipronil inhibits termites by acting as a GABA antagonist and leads to excessive CNS excitation and causes death | Used in seed coatings and granular soil treatments to control unwanted arthropods in many kinds of food, horticultural, and turf plants | ; ; |
| Methiocarb | Repellents | Repel pest vertebrates and invertebrates by its taste or smell | Use as a seedling bird repellant and also effective against frit fly larvae. | ; |
| Methoprene | Larvicides | Prevents larvae growth | Uses as mosquito larvicide, also effective against horn flies, mushroom flies in compost, dipteran pests of livestocks, nuisance flies, highly selectivity for insects and no acute toxicity is expected in humans | ; ; |
| Metaldehyde | Molluscicides | Prevent mollusk’s (snail’s) usually disturbing growth of plants or crops | Use in vegetables and gardens, to kill slugs, snails, other garden pests | ; |
| Rotenone | Piscicides | Toxic and act on fishes | Uses in fisheries and fish management strategies (where unbalanced population of fish) | ; |
| Scytovirin | Virucides | Acts against viruses | Control of viral infections and diseases | |
| Tebuthiuron | Silvicides | Specific to woody vegetation and act on it | Uses to manage the undesirable plants or unwanted forest species and apply to eliminate trees and brush or “entire forest” | |
| Trifluromethyl nitrophenol (TFM) | Lampricides | Target larvae of lampreys by uncoupling mitochondrial oxidative phosphorylation and ATP production reduces which ultimately leads to death | TFM used to control invasive sea lamprey ( ) | ; |
Migration and behavior of pesticides in the ecosystem
When pesticides are administered to a specific area or plant by a farmer, they have the potential to migrate and degrade into the environment and using indigenous microbial strains and physicochemical factors. They show a variety of effects on non-targeted plants as well as kingdom animalia after entering into the ecosystem ( Tudi et al., 2021 ). Pesticides are degraded in our ecosystem by a variety of physical and microbiological processes, including light, temperature, moisture, oxygen, and microorganisms. Pesticides degrade into new chemical entities called metabolites, which can be hazardous or non-toxic depending on their chemical composition ( Liu et al., 2015 ; Marie et al., 2017 ). Pesticides and their metabolites are transported from a targeted to a non-targeted area via adsorption, leaching, volatilization, or surface runoff ( Tudi et al., 2021 ). Because there is an attraction between soil particles and pesticides in sorption systems (attraction influenced by soil organic matter and soil texture), pesticides linger in the soil for a long period of time and have a harmful effect on the soil and ecosystem ( Qin et al., 2014 ).
Impact of physical and chemical factors on the transformation of pesticides in soil and water
Physical and chemical properties such as molecular weight, ionizability, lipophilicity, polarizability, and volatility of pesticides decide their behavior and biological activity in soil ( Bailey and White, 1970 ; Pignatello and Xing, 1995 ; Gevao et al., 2000 ; Beulke et al., 2004 ). In general, pesticide fate in a soil ecosystem depends on the abiotic transformation related to its physicochemical properties and also on biological transformation related to the presence of live organisms ( Różański, 1992 ). The physical properties make them resistant, reducing losses while chemical structures determine the persistence of pesticides in soil or the environment. These physical and chemical properties of chemical compounds are linked to their movement in soil and aquatic systems and robustness under adverse conditions ( Pereira et al., 2016 ).
Some crucial processes, including adsorption, degradation, and movement, control the behavior and fate of pesticides in soil. Depending on how the pesticide moves, these processes are further classified into leaching, transmission, runoff, microbial and plant absorption. Pesticide transformations in the soil system may vary. Adsorption processes are based on physical forces such as van der Waals or chemical nature, such as electronic interactions ( Gevao et al., 2000 ). Degradation of the pesticides leads to formation of free and bound residues with some altered molecular structures, which are difficult to extract ( Roberts, 1984 ; Gevao et al., 2000 ). Through diffusion and volatilization, pesticides can dissipate into the atmosphere and wind or runoff leading to subsequent contamination of water bodies. The physical and chemical properties of soil and pesticides, along with other environmental conditions, are mainly responsible for their adsorption by target and non-target organisms, a phenomenon known as bioaccumulation. Chemical and physical characteristics have an impact on leaching, and vertical downward shifting from soil systems. Through the leaching process, pesticides can reach up to groundwater level, making water vulnerable to pollution. Leaching of pesticide into the groundwater in sufficient quantities can pose a hazardous risk to animal and human health. The soil with a sandy nature and low organic content acted as an unstable holding system and weakly absorbed or persistent compounds were most likely to leach-out easily. The chemical, physical, and biological factors of soil with pesticides applied for agriculture practices may influence the leaching process ( Steffens et al., 2013 ). The various agriculture practices are responsible for pesticides translocation in soil or water and the period of their persistence in that environment can be short or longer for weeks, months, or even years due to a number of factors, which include climate change, texture of soil, pH, temperature, moisture, and the content of mineral and organic compounds ( Bailey and White, 1970 ; Gevao et al., 2000 ; Gupta and Gajbhiye, 2002 ). Additionally, the leaching and seepage of chemical compounds depends on their mobility as well as persistence, which increases the risk of water pollution ( Pereira et al., 2016 ).
Pesticide impact on the natural system
Pesticides safeguard around a third of all agricultural goods globally, yet their extensive usage has negative consequences for ecosystems ( Zhang et al., 2011 ). Pesticides harm and accumulate in more other places than crops due to poor management/mishandling, or a lack of information (misuse and overuse). Label instructions on how to use and safety recommendations such as donning rubber gloves and protecting eyeglasses from exposure are not effectively followed by users (EPA Common cause of pesticide incidents) ( Qu et al., 2019 ). Pesticides have a wide range of effects on non-targeted creatures, resulting in environmental issues ( Rosell et al., 2008 ). In the case of air pollution by persistence organic pesticide (POP), is caused by ground and spray. Pesticides that are semi-volatile in nature adsorbed on aerosol particles. The half-lives of these particles are few days to more than a month, it depends on gas-phase reactivity. POP (which are present in the air) undergo a transformation from their native form to a highly toxic form via oxidation and photochemical reactions. The migration of these pesticides (POP) depends on the low solubility in water, climate-weather, temperature and humidity ( Woodrow et al., 2018 ). Current use pesticides (CUPs) are more biodegradable in nature as well as less toxic and persistent as compared to previously used organochlorine pesticides ( Chen et al., 2020 ).
Pesticide impact on the soil system
Pesticides are generally used to protect the crop, but there are several ways in which they can also contaminate the soil. Some of the common reasons include inappropriate use, a lack of information on how to use them in terms of amount, a high amount of runoff into water bodies, and pesticides that are adsorbed, desorb, and broken down during their passage through soil, and these phenomena are dependent on pesticide properties such as persistence, bio-accumulation, and toxicity. Because of this process, the soils become secondary sources of the pollutants with respect to air soil exchange ( Pokhrel et al., 2018 ). According to the report, in European countries, the distribution of 76 pesticide residues was evaluated in 317 agricultural top soil samples, either they contained one pesticide or more than one ( Silva et al., 2019 ).
The bioavailability of pesticides in the food web, pesticide uptake, toxic kinetics, dispersion, metabolism, and excretion all have an impact on species. Pesticides are used excessively and arbitrarily on various crop species, causing harm to beneficial biota such as microorganisms, honey bees, predators, birds, plants, and small animals ( Alengebawy et al., 2021 ).
Pesticide impact on the aquatic system
Persistence organic pesticide and CUP pesticides enter into the water bodies through a variety of mechanisms, including atmospheric precipitation, chemical or pesticide manufacturing industries releasing unprocessed chemical waste into running water sources (rivers) and other water bodies, where these pesticides travel for miles and contaminate aquatic or water bodies, negatively impacting aquatic ecosystems ( Socorro et al., 2016 ). These pesticides accumulate and transmit from lower to higher trophic levels in aquatic systems, affecting aquatic flora and fauna directly, from which these pesticides have an impact on human health through intake or other means ( Woodrow et al., 2018 ). Chen et al. (2020) studied the aquatic system of shanghai, China and reported a high concentration of CUP (napropamide, atrazine, and chlorpyriphos).
Effect of pesticide on water eco system
Water is one of the essential elements for all forms of life on earth. About 71% of the water is covered by the earth’s surface. Groundwater constitutes about 30% of the world’s freshwater resources ( Marsala et al., 2020 ). Groundwater quality is under threat due to fast population growth, urbanization, industrialization agricultural pesticides, and population stress ( Jayaraj et al., 2016 ; Wagh et al., 2020 ). Pesticides may get into groundwater as a result of agricultural runoff from the field or even direct application. The presence of pesticides in water sources is a cause for worry. Pesticides are a type of hazardous chemical that poses a health risk to humans. In many places in the world, groundwater is the most significant source of drinking water. Pesticide pollution is generated from poorly managed agricultural operations and contaminates the surface and ground water. It reduces the quality of drinking water available ( Khatri and Tyagi, 2015 ).
Among the pesticides, organochlorine pesticides (OCPs) have been widely used across the world to control agricultural pests and vector borne diseases (malaria and dengue). Organochlorine pesticides are non-volatile compounds. The problem with using them is that they linger for a long time in natural systems. The use of these substances in an indiscriminate manner has the potential to affect the environment, drinking water systems, and human health. The OCPs’ exposure over time can result in cancer, birth deformities, neurological impairment, reproductive problems, and immune system disease ( Agbeve et al., 2014 ; Fosu-Mensah et al., 2016 ).
The entry of pesticides into both ground and surface water should be protected. Surface runoff and leaching carry pesticides into water bodies. These pesticides are taken up by plants in the soil, reduced into different chemical forms, and then leached into groundwater. High rainfall increases the risk of pesticides contaminating water. Pesticides that enter groundwater impair the quality of the water, making it unsafe for human consumption as well as for flora and animals. Eliminating pesticides from groundwater is a challenging process. Pesticides in drinking water have negative consequences for both individuals and the ecosystems. According to WHO, around 1 million people are poisoned acutely because of pesticide contact ( Hassaan and El Nemr, 2020 ). To improve production, pesticides will always be a part of human existence and the environment. For pest management, an Integrated Pest Management (IPM) method should be used, which is meant to cause the least amount of environmental disruption by pesticides.
Effects of pesticides on aquatic animals
Pesticide exposure does not just harm target creatures; it also affects a variety of non-target organisms, with fish being the most notable one. Acute exposure to several pesticides resulted in the mortality of fish in certain cases, whereas lower exposure to the same chemicals resulted in deadly alterations. In many species of fish exposed to various pesticides, changes in hematological parameters such as red blood cells, white blood cells, or plasma and serum level modifications lead to histological abnormalities affecting the liver, kidneys, gills, muscles, brain, and gut ( Tahir et al., 2021 ). Furthermore, genotoxicity has been documented in numerous cases caused by several pesticides. Fish are the lowest rung of the aquatic food chain; thus, they mirror the state of water quality and contamination. Submissive phenomena allow them to collect and store compounds such as heavy metals and pesticides, allowing contaminants in their environment to be recognized. Fish ingest a higher amount of pesticide-infected algae, phytoplankton, and other aquatic plants, causing toxic toxins to progressively accumulate in the tissues and organs of the fish. A small number of these compounds can be regulated by metabolism, while the rest bio-accumulate in the organs and organ systems of fish. Different pollutants are absorbed by the fish’s gills, skin, and alimentary canal, which then disseminate into various organs and tissues, altering physiological and natural phenomena ( Banaee et al., 2011 ). Because the gills are completely exposed to water, they are the most polluted organs. Toxicants enter the body through the gills, increasing oxygen demand. As a result, monitoring any hazardous stress in the aquatic environment is an important metric ( Panigrahi et al., 2014 ).
The following components of a global bicycle should be addressed when determining the principal pathways of pesticide exposure to aquatic systems and biota: (1) The water column, which is frequently the first to be exposed to pesticides, (2) Algae, mosses, vascular hydrophytes, leaf litter, and branches are examples of organic substrates, (3) Inorganic substrates ranging from fine silt to coarse sand particles ( Murthy et al., 2013 ). Pesticide levels in interstitial water and sediments are often lower than in the water column, and lithic biotopes are typically less polluted than the standing waters. Pesticides have toxic effects on aquatic creatures, including fish, at sub-lethal and deadly dosages ( Khafaga et al., 2020 ).
Hematological causes by pesticide in fish
Fish hematological research has grown in importance as a reliable and sensitive index for assessing biological and pathological changes caused by natural or anthropogenic factors such as microbial infection or levels of contamination in aquatic sources. As a result, hematological parameters are regarded as a crucial tool for determining the body’s functioning condition in response to various stresses ( Ali and Rani, 2009 ). Pesticides changed the hematological parameters of fish in a relatively short time ( Rezania et al., 2018 ). As a result, the hematologic index may be used to efficiently monitor the health and reaction of fish and aquatic creatures to various toxicants, displaying the ecological position of the environment and a typical way to determine the contaminant’s sub-lethal effects ( Pimpao et al., 2007 ). According to Rios et al. (2002) , the blood parameters of fish were altered by several genetical and environmental factors. Pesticides affect a variety of fish characteristics, with a focus on blood parameters.
Pesticide-induced behavioral changes in fish
In several fish species, including Tor putitora and Cyprinus carpio , pesticides can cause schooling behavior, mucus formation via skin’s goblet cells (sliminess), motionlessness, transformations in migration activities, tumbling toward base, jumping, non-responsiveness with hyperexcitability, irregular activities, greater opercular rate (respiration increases), and modifications in body color. Furthermore, they have the ability to change and disturb aquatic vertebrate swimming behavior, such as that of fish and amphibians, as well as impair their growth rates ( Stehle and Schulz, 2015 ). Pyrethroid exposure, decreased the function of the dopamine active transporter, resulting in unpredictable behavior ( Wang et al., 2020 ).
Malformations and reproductive disorders caused by pesticides in fish
Pesticides may cause reproductive issues in brown trout ( Salmo trutta ) and in Atlantic salmon ( Salmo salar ) ( Jaensson et al., 2007 ). In addition, additional studies discovered a range of developmental abnormalities in fish exposed to the herbicide ( Dawar et al., 2016 ). Pyrethroids have been found in various studies to be harmful to fish reproductive and early embryonic stages. Pyrethroids such as bifenthrin and permethrin can cause egg proteins (choriogenin and vitellogenin) to be delayed in juvenile fish ( Brander et al., 2012 ). Deltamethrin [second-generation (type II) pyrethroid neurotoxin insecticide] at concentrations of 20 and 40 g/L was shown to be damaging to the development of the swim bladder in zebrafish embryos reported by Wu et al. (2020) .
Common effects of pesticides on fish
Pesticides have been shown to have effects on the activity of acetylcholinesterase (AChE), causing an impact on the neurological system and triggering numerous neurotoxic effects (neurotoxicity) in fish ( Sharbidre et al., 2011 ). Fish species such as Rhamdia quelen , C. carpio , Colisa fasciatus, Oreochromis mossambicus , and Labeo rohita are affected by pesticide exposure and have also shown the alteration in AChE activity ( Joseph and Raj, 2011 ). In addition, cypermethrin (CYP) caused neurotoxicity and apoptosis in the Catla catla brain ( Jindal and Sharma, 2019 ). Pesticides also harm fish’s endocrine systems ( Brodeur et al., 2013 ). When used in large numbers, these chemical compounds may induce molecular toxicity in fish such as Cirrhinus mrigala , Carassius auratus (goldfish), and L. rohita ( Ullah et al., 2014 ). According to histopathological examinations, they have a negative effect on the endocrine systems of Oncorhynchus mykiss and L. rohita ( Dey and Saha, 2014 ). Pesticides also cause oxidative stress in T. putitora , Lepomis macrochirus , Hoplias malabaricus , Oreochromis niloticus , Clarias gariepinus , and L. rohita by affecting antioxidant defense enzyme activities and reducing the lipid peroxidation marker malondialdehyde, glutathione- S -transferase, glutathione reductase, and glutathione level ( Muthukumaravel et al., 2013 ).
Effect of chemical pesticides on plants
Nowadays, chemical pesticides are widely used by farmers on agricultural land to control weeds, insects, bacteria, fungus, mollusks, rodents, etc. To combat their needs, an increasing population is demanding more foods. Pesticides are used for better crop production ( Tomer, 2013 ). The pesticide defends crops in agricultural land and also minimizes the risk of damage during post-harvest storage. It is very effective and successful in controlling a number of diseases in plants as well as humans, such as malaria and typhoid, but on the other hand, it decreases the soil quality of agricultural land, which is the reason that their negative effects are kept in mind. In 1960, most of the technologically advanced countries banned or restricted the use of pesticides. Ideally, a synthetic or chemical pesticide must be toxic or lethal to the targeted or non-target species. Because of extensive use of pesticides, the pests and insects are going to develop resistance to transformed pesticides like DDT and escape from it.
Effect of pesticides on vegetables and fruits
The use of pesticides provides a protective layer against pod infection by other pod-feeding insect pests, but damaged pods may not yield seeds or be of poor quality and unfit for use ( Mugo, 1998 ). The usage of chitosan at an early developmental stage boosted plant growth and development and produced higher seed output in rice and soybeans ( Chibu et al., 2002 ). Similar work has been done by Boonlertnirun et al. (2005) in rice and Rehim et al. (2009) in maize and bean.
Pesticides impact on plant growth and metabolism
Although all pesticides are designed to eliminate or prevent certain plant or animal species, it is a great deal to know about the increasing biological as well as physiological effects of these chemicals on their target organisms. Simultaneously, there are many advantages and potential risks to the use of agrochemicals. Chemically treated seeds are often exposed to substantially greater chemical concentrations than the mature plants during cultivation, so these benefits are countered by the danger of phytotoxicity. Herbicides suppress or control plant weeds by a variety of mechanisms with biological processes such as photosynthesis activity, mitosis cell division, function of enzymes, root and leaf development, DNA and protein synthesis, cell membrane destruction, or encouraging uncontrolled growth. The use of pesticides involves a variety of enzymatic and non-enzymatic alterations in biochemical and physiological antioxidants that can have an initial effect on plant growth from germination and ultimately affect the production of plant yield, e.g., vegetables, fruits, and seeds ( Choudhury, 2019 ; Yengkokpam and Mazumder, 2020 ).
Effect of pesticides on plant growth and development
Plant (crop) growth and development do not proceed normally and lead to growth due to the life cycle of the crop, which increases seed size, dry matter accumulation, food storage material in leaves, stems, fruits and roots ( Jan et al., 2012 ). Despite the fact that plant development is influenced by a variety of environmental, genetic, exogenous, and endogenous variables, as well as hormonal situations. Plant development, on the other hand, is an essential phase in determining their producing capability. Brecke and Duke (1980) introduced glyphosate to reduce leaf dry matter accumulation in Phaseolus vulgaris L. Basantani et al. (2011) observed an overall decrease in germination rate, dry weight, and root length of Vigna radiata after treatment with glyphosate (10 mm). Mishra et al. found that spraying high quantities of pesticides (dimethoate) shortens root and shoot length. Due to increasing levels, dimethoate concentrations in the root are higher than in the shoot ( Mishra et al., 2008 ). Murthy et al. (2005) conducted similar research on Glycin max L.
Effect of pesticides on plant physiology
In the field of pesticide studies, the plant growth is hampered by pesticide accumulation in plants and causes a variety of metabolic disorders, such as chlortoluron affected the plant photosynthetic electron transport chain mechanism ( Fuerst and Norman, 1991 ; Sharples et al., 1997 ), and Barry et al. (1990) was observed that the PS II reaction center was disrupted. During the photosynthetic pathway, uracil-type herbicides prevent the hill reaction and photosystem II. Reduction of total chlorophyll as well as chlorophyll a, b, and carotenoid content is increased with the increasing application of fungicide doses to plant leaves ( Tort and Turkyilmaz, 2003 ). Sharma et al. (2018a) stated that employment of herbicide causes noxious effects on plants like necrosis, stunting, burns, chlorosis and twisting of leaves. However, Donald (2004) has observed in his experiment that excessive application of pesticides can cause a major reduction in structural vegetation of diversity. Most scientists have been recorded that use of pesticides adversely affects the plant growth and development ( Sharma et al., 2015 , 2016a , b , c ; Shahzad et al., 2018 ).
Effect of pesticides on plant defense systems
The use of pesticides causes oxidative stress due to the formation of reactive oxygen species (ROS), which can finally lead to growth deficiency and reduced efficiency of photosynthesis in plants. Plants improve the toxicity because of pesticides by increasing the activity of their antioxidative defense system, which includes non-enzymatic antioxidants and antioxidative enzymes ( Xia et al., 2009 ; Sharma et al., 2015 , 2017a , b , 2018b ). Plant proteins, chlorophyll pigments, and photosynthetic efficacy are all reduced by oxidative stress ( Xia et al., 2006 ).
Effect of pesticides on human health
The human body gets exposure to pesticides either directly or indirectly. By using pesticides on crops, humans come in direct contact with them and they affect the skin, eyes, mouth, and respiratory tract, and cause acute reactions such as headache, irritation, vomiting, sneezing, and rashes on the skin. The severity of these pesticides on humans depends upon exposure time and concentration. Generally, pesticides are released from the body in the form of excretion (urinary, biliary, and secretory gland). The consumption of such vegetables and fruits that are grown in pesticide contaminated soil and water used for long-term, accumulation increase the concentration of toxins inside the body organs and causes chronic diseases such as neurotoxicity, cancer, necrosis, asthma, reproductive disorder, cardiac disease, diabetes, etc. ( Kalyabina et al., 2021 ). The quaternary nitrogen compounds such as paraquat are associated with neurodegenerative diseases like Parkinson’s, but their molecular mechanism are still not well known ( Franco et al., 2010 ). Similarly, pesticide group of carbamates inhibits the acetylcholinesterase (AChE) activity and is used as a biomarker of neurotoxicity ( Gupta et al., 2016 ). The cancer problem is caused by the various pesticides, but breast cancer is the most common in all cancer types and is associated with organophosphorus (malathion and parathion) that affect cellular growth and proliferation ( Calaf, 2021 ). Similarly, autoinhibitory M2 muscarinic receptors on parasympathetic neurons that innervate airway smooth muscle are implicated in the case of asthma by organophosphorus ( Calaf, 2021 ). It also reduces fertility and creates genital tract anomalies in both males and females by affecting the action of endocrine hormones, their release timing, and imitating these hormones. According to several studies, organophosphorus reduces paraoxonase activity and increases the risk of coronary artery disease ( Kabir et al., 2015 ). In several African nations, hunger and undernutrition are the most serious concerns.
Role of pesticides in genetic damage
The DNA is an important biomolecule present in living organisms that carries hereditary information and controls the biological synthesis of proteins and enzymes. It acts as the key molecular target of drugs and environmental chemicals such as pesticides. Pesticides interact with DNA and cause conformational changes that could induce gene mutations and lead to adverse health consequences such as carcinogenesis. The acute effects of such chemically synthesized compounds on human health are generally tested and reported before the market launch of these pesticides ( Van der Plaat et al., 2018 ). However, the long-term effect of chronic exposure to pesticides has become a major concern in the last decade.
Pesticide exposure is of the following three types. (1) Direct occupational: Farmworkers who mix and spray the pesticides in agriculture fields; (2) Direct non-occupational: Rural-resident people who live near agriculture fields; (3) Indirect exposure: People who stay far from agriculture areas but get exposed to pesticides through agriculture products, the food chain and contaminated water. Occupational exposure is the most dangerous one as it is linked to a broad range of immediate effects or diseases such as lung disease and airway obstruction. A study conducted in the Dutch population reflects a significant association between the airway obstruction in farmworkers and the corresponding genomic methylation of 31 CpGs ( Van der Plaat et al., 2018 ). Alteration in the genomic methylation pattern affects the expression and repression of genes.
Paredes-Céspedes et al. (2019) reported a notable increase of %5mC in the CpG sites of the WRAP53α gene, “antisense” gene of the p53, in mestizo urban fumigation sprayers who generally use organophosphate insecticides and pyrethroids. Such genetic modifications could act as carcinogenic agents. Differentially methylated CpGs have been found to be unique to the active ingredients of marketed pesticides such as mesotrione, dicamba, acetochlor, picloram atrazine, malathion, glyphosate, and metolachlor ( Hoang et al., 2021 ). Occupational and non-occupational pesticide exposure, as well as chronic and high pesticide exposure in human beings, lead to altered genomic methylation. Various pesticides, including DDT, vinclozolin, methoxychlor, chlorpyrifos methyl, and organochlorine, have been reported to increase or decrease the epigenetic methylation pattern in human beings ( Mahna et al., 2021 ).
The possible genetic damage initiated by occupational pesticide exposure is much greater than that caused by smoking and alcohol consumption ( Nascimento et al., 2022 ). This points to the commonly unacceptable fact that pesticide exposure is much more dangerous than quitting smoking. The random effect of DNA damage in the pesticide-exposed group is roughly 4.63 times more than in the control-exposed group, according to a meta-analytical evaluation addressing probable DNA damage arising from pesticide exposure to farmers ( Nascimento et al., 2022 ). A total of 42 studies were included in the study, with a total number of individuals 2,885 and 2,543 in the exposed and control groups, respectively. In contrast to previous studies, this study found that DNA damage induced by pesticides was not affected by the usage of personal protective equipment, pesticide type, or an individual’s age and gender.
Non-farm employees who reside near agricultural grounds are exposed to pesticides through passive exposure and are thus at risk of pesticide-induced genetic destruction. Non-occupational exposure to pesticides generally corresponds to a high blood concentration of pesticides and increased DNA damage. The pesticides, being oxidizing in nature induces DNA damage via oxidative stress ( Doǧanlar et al., 2018 ). The literature represents that aged people, females, and children are more vulnerable to non-occupational pesticide exposure. Increased micronuclei (MN) numbers, oxidative damage, and strand breaks in DNA were seen in the peripheral blood lymphocytes of toddlers living in pesticide-sprayed areas ( Kapka-Skrzypczak et al., 2019 ).
Non-occupational exposure to pyrethroids, a key pesticide used in agricultural and commercial locations, occurs primarily via residues through contaminated air and diet. The presence of pyrethroids metabolites in the human urine, including CDCCA [ cis -3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid], DBCA ( cis -2,2-dibromovinyl-2,2-dimethylcyclopropane-carboxylic acid), TDCCA [ trans -3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid], and 3PBA (3-phenoxybenzoic acid) provides an indication of non-occupational pesticide exposure. The studies to estimate the effect of non-occupational pesticide exposure on human sperm are generally conducted on men recruited from infertility clinics with normal sperm concentrations. The presence of pyrethroid metabolites in human urine is linked to sperm DNA damage increasing and the quality of semen reduces ( Meeker et al., 2008 ).
A positive association were examined with the medium DNA fragmentation index (M DFI) percentage and CDCCA 450th as well as the percentile of 3PBA 450th and high DNA fragmentation index (H DFI) ( Jurewicz et al., 2015 ). Non-occupational exposure to pyrethroids also increases the risk of sex chromosome disomy in sperm nuclei. Radwan et al. (2015) reported disomy in sperm chromosome YY (3PBA), XY (3PBA, TDCCA), 18 (3PBA, CDCCA), 21 (3PBA), and total disomy (3PBA). Those with higher levels of TDCCA and CDCCA have a consistent increased risk of XY, YY, XX, and disomy in the total sex chromosome (7–30%). Males with higher levels of 3PBA displayed an increased risk of YY disomy (28%), a decreased rate of XY disomy (16%), a decreased total disomy (7%), and an increased chromosome 18 disomy ( Young et al., 2013 ).
In reality, human beings and animals are exposed to multiple pesticides and herbicides simultaneously, which may act independently or interdependently. The pesticides organophosphates (OP) and pyrethroids (PYR) act in synergism to increase the risk of germ cell abnormalities ( Figueroa et al., 2019 ). Earlier, Salazar-Arredondo et al. (2008) also reported the chromatin as well as DNA damage in human spermatozoa caused by in vitro exposure to a mixture of various organophosphorus pesticides including CPO (chlorpyrifos-oxon), CPF (chlorpyrifos), DZO (diazoxon) or DZN (diazinon), and MePO (methyl-paraoxon).
The pesticides cause DNA damage by interacting with the DNA backbone in either of three ways (1) Intercalation, (2) Grove binding, and (3) Methylation. Extensive studies have been reported in the literature that show the type of interaction between DNA and pesticides ( Table 5 ). The genetic damage caused by pesticides is generally studied in animal models such as mice or rats. Dinitroaniline herbicide, pendimethalin (PND), causes significant DNA damage in the liver and kidney cells of treated rats. This damage is shown to disturb the oxidative balance and activate apoptosis genes ( Ahmad et al., 2018 ).
Mode of interaction of various pesticides with DNA.
| Pesticide | Pesticide group | Mode of interaction | References |
| Chloridazon or Pyrazon | Organochlorine herbicide | Intercalation via GC region | |
| Fenitrothion | Organophosphorus insecticide | Partially intercalation via NO and the C Form conformation | |
| Permethrin, deltamethrin | Synthetic pyrethroid insecticides | Groove binding and partial intercalation | |
| Methyl Thiophanate | Fungicide | Non-intercalative groove binding via AT region | |
| Propyzamide | Herbicide | Intercalation via AT region | |
| Edifenphos | Organophosphate pesticide | Electrostatic binding minor groove binding via AT region | |
| Tau-fluvalinate, flumethrin | Synthetic pyrethroid pesticide | Hydrogen bonding and Van der Waals forces, minor groove binding via AT region | |
| Dinitramine | Herbicide | Hydrophobic interactions, major groove binding | |
| Resmethrin | Synthetic pyrethroid insecticides | Hydrogen bonds and Van der Waals forces, groove binding via GC region | |
| Pendimethalin | Herbicide | Intercalation via GC region | |
| Organophosphates | Pesticide | DNA methylation | |
| Organophosphate, pyrethroids | Fumigation insecticide | DNA methylation | |
| Endosulfan | Pesticide | DNA hypomethylation | |
| Glyphosate | Pesticide | DNA hypermethylation | |
| Diazinon | Pesticide | DNA hypermethylation | |
| Fonofos, parathion, terbufos | Pesticide | DNA hypermethylation |
Pesticides’ role in cancer
Several epidemiological and molecular research highlighted a close association between persistent pesticides exposure and increased risk of diseases such as neurodegenerative disorders, endocrine disruptors, respiratory complications, reproductive disorders, and birth defects ( García et al., 2017 ; Larsen et al., 2017 ; Addissie et al., 2020 ; Bast et al., 2021 ; Bhadauriya et al., 2021 ; Witczak et al., 2021 ; Gea et al., 2022 ; Iteire et al., 2022 ). In addition, the carcinogenic, teratogenic, and mutagenic nature of these compounds are also believed to be a contributing source of cancer development in the human population.
It has been observed that a person with a direct exposure to pesticides is highly susceptible to several human malignancies such as cancer including head, neck, breast, thyroid, brain, colorectal, pancreatic, lung, leukemia, prostate, non-Hodgkin lymphoma and ovarian cancer ( Obiri et al., 2013 ; Pardo et al., 2020 ; Leonel et al., 2021 ; Lerro et al., 2021 ). Several pathways have been discovered to date; however, the major molecular mechanism that is likely to cause pesticide-induced carcinogenesis involves oxidative stress, genetic and epigenetic changes, and endocrine disruptions ( Figure 4 ). For instance, excessive production of ROSs as a result of pesticide exposure can disrupt the cellular equilibrium between pro and anti-oxidant molecules and induce oxidative stress to induce macromolecule damage, leading to dysregulation of several fundamental processes and subsequently stimulating cancer initiation, growth, progression, metastasis, and chemotherapeutic resistance ( Pardo et al., 2020 ; Leonel et al., 2021 ; Lerro et al., 2021 ).

Major molecular mechanisms associated with pesticides-induced carcinogenesis.
In a study by Želježić et al. (2018) , herbicide terbuthylazine exposure was reported to form reactive terbuthylazine metabolites, which induce DNA cross-links in both in vitro and in vivo systems. Thakur et al. (2018) reported that oxidative DNA damage induced by two extensively used organophosphate pesticides, monocrotophos and chlorpyrifos, modulate the AP endonuclease 1-dependent base excision repair pathway to promote the proliferation of lung cancer. Similar toxic effects were also observed for widely used insecticide, neonicotinoid (dinotefuran, nitenpyram, and acetamiprid) exposure, which resulted in disturbance of amino acid metabolism, accumulation of lipids, and enhance oxidative stress in ICR mice via decreasing glutathione (GSH) level and increasing superoxide dismutase (SOD) level ( Yan et al., 2020 ). Polymorphism in oxidative stress-related genes (catalase, glutathione peroxidase, glutathione- S -transferases, manganese superoxide dismutase, and paraoxonase) may not be directly linked to cancer; instead, they make people more vulnerable to pesticide-induced oxidative stress ( Kaur et al., 2018 ; Moradi et al., 2018 ; Costa et al., 2019 ; Mbah Ntepe et al., 2020 ).
Endocrine disruptions are caused by agents/EDCs (endocrine disrupting chemicals) that affect the natural function of the endocrine (hormone) systems of a body by disrupting the synthesis, release, binding, specific activity or abolition of normal hormone, which are responsible for the growth, development, fertility, and homeostasis maintenance of a cell. Pesticides are well known for disrupting endocrine function via mimicking or delaying the release of natural hormones, thus, being accountable for decreased fertility, neurological or behavioral dysfunctions, thyroid gland abnormalities, immunosuppression, and carcinogenesis ( Kori et al., 2018 ; Pizzorno, 2018 ; Requena et al., 2019 ; Brandt et al., 2020 ; Montes-Grajales and Olivero-Verbel, 2020 ). Most of the pesticides work as agonists to activate numerous hormone receptors for instance androgen receptors, estrogen receptors, pregnane X receptors, nuclear hormone receptors, and aryl hydrocarbon receptors ( Eve et al., 2020 ; Lacouture et al., 2022 ). Low dose of phenolic EDCs upregulated aromatase signaling and thus regulated aromatase-induced 17β-estradiol biosynthesis to support breast cancer cells proliferation ( Williams and Darbre, 2019 ). Furthermore, thiacloprid and imidacloprid exposure stimulates CYP19 promoter activity, which increases estrogen biosynthesis in vitro in a similar manner to hormone-dependent breast cancer ( Caron-Beaudoin et al., 2018 ). Recently, antagonistic effects of pesticides have also come into focus. For example, cypermethrin showed an inhibitory effect on the dihydrotestosterone activated interaction of the androgen receptor with its coactivators ARA70 and ARA55 ( Zhen et al., 2020 ). Zhang et al. (2018) discovered a novel mechanism of endocrine disruption, where 16 pesticides showed anti-mineralocorticoid activity, among which 14 interfere with nuclear translocation of the mineralocorticoid receptor to promote hepatocellular carcinoma. Another novel pathway involves fungicides (Prochloraz, vinclozolin, and M2) competing with the androgen receptor, ZIP9, to block pro-apoptotic signaling in prostate cancer cells ( Thomas and Dong, 2019 ). In another study, glyphosate was reported to inhibit aromatase signaling in a non-competitive manner while imidacloprid and thiacloprid inhibited estrogen receptor activity in MELN cells ( Zhang C. et al., 2020 ). Overall, we observed that pesticides can alter the cellular metabolism in multiple ways to induce cancer risk. It was also observed that a person with direct or occupational exposure along with inherent genetic susceptibilities is more prone to disease.
Pesticide exposure causes allergies and asthma
The salubrious nature of pesticides makes them ideal candidates for modern agriculture techniques and enhanced crop-production. However, extensive usage of pesticides leads to serious health conditions due to their bio-magnification and persistent nature ( Sharma et al., 2019 ). The vapors of pesticides can invade water, soil, air and finally enter the food chain, thereby threatening to human health ( Sharma et al., 2017c ). It has been found that food contaminated with pesticide residues leads to a higher level of toxicity compared to drinking or inhaling contaminated water or air ( Margni et al., 2002 ). Pesticides can mimic or antagonizes natural hormones, thus disbalancing hormonal homeostasis, reducing immunity, causing cancer and other reproduction-related problems ( Yadav et al., 2015 ).
Studies have reported that acute or chronic exposure to such pesticides leads to airway diseases such as allergic rhinitis or asthma. The population at high-risk of developing health issues due to pesticide exposure includes mainly farm workers, pest control workers, or workers from agricultural industry, and the other environmentally exposed individuals residing near farms or agriculture fields or the individuals exposed to household pesticides ( Ernst, 2002 ; Ndlovu et al., 2011 ).
More evidence of exposure to pesticides has been reported among farmers and their families along with insecticide producers or applicators across the globe, such as the United States, Canada, France, and Australia, with increased asthmatic conditions ( Baldi et al., 2014 ). Such exposures may lead to decreased FEV 1 (forced expiratory volume in 1 s) of forced breath with exacerbation of asthma and also induction of autonomic function and altered immune response ( Osteen and Fernandez-Cornejo, 2013 ; Henneberger et al., 2014 ). In relation to the use of domestic pesticides, exposure to insecticides has a particularly important role in the induction and worsening the asthma and asthma-like syndrome ( Osteen and Fernandez-Cornejo, 2013 ). In countries such as the United States, where asthma morbidity is high due to cockroach sensitization, insecticides are used to control exposure, which in turn increases pesticide exposure, and asthma morbidity ( Garthwaite et al., 2012 ).
Another study on farm operators showed a significant association between current asthma and lifetime allergic rhinitis by the use of carbaryl and 2,4-dichlorophenoxyacetic acid. Approximately 40% of 2.1 million farm operators had lifetime allergic rhinitis in 30% farmers and 5.1% has current asthma ( Patel et al., 2018 ). Some synthetic insecticides, such as pyrethroid, used to control mosquitoes are known to cause asthma attacks, while permethrin and Sumithrin are key contributors to headaches, tremors, convulsions, asthmatic attacks, and can be lethal in more serious conditions ( EPA et al., 2009 ; Amaral, 2014 ). Not much is known about specific pesticides responsible for allergic/asthmatic exposure. Studies from Canada, Spain, India, or South Africa demonstrated that pesticides belonging to class organophosphates and carbamates are particularly involved in causing asthmatic conditions ( Hernández, 2015 ). These studies mainly performed lung function assays such as spirometry, and lung volumes/capacity, but none has involved primary inhalation challenge testing.
Effect of pesticides on asthma
Pesticide use and asthma incidences were reported in the common people as reported by some of the studies performed in the United States population. The US urban population was found to be chemically intolerant to at least three commonly used chemicals such as paints, pesticides, perfumes, or car exhaust. Subjects reported asthmatic and respiratory symptoms such as shortness of breath with wheezing and chest tightness ( Baldwin et al., 1997 ; Amaral, 2014 ). A cross-sectional study of US National Health and Nutrition showed an association of residential pesticides with respiratory problems in children, mostly used in the kitchen or dining area ( Xu et al., 2012 ). The incidence of such residential exposures has increased in the United States from 1.1 to 4.4 per million ( Amaral, 2014 ; Hudson et al., 2014 ). Indoor air pollution, caused by pesticide spraying or the use of over-the-counter insecticides, has exacerbated symptoms such as irritation, lower respiratory pain, wheezing, dyspnea, and dry cough. In a randomized investigation of 25 asthmatic participants exposed to modest amounts of aerosols, asthmatic symptoms worsened when compared to a control group (given water). Asthmatic patients had a more than 15% decrease in FEV1 and severe bronchial responsiveness, with symptoms affecting the chest, nose, and eyes ( Salome et al., 2000 ).
Previously, it has been reported that allergic asthma was relatively more common in children than in adults. The risk of environmental exposure to pesticides was higher for school children, especially those living near farms or rural areas ( Matthews, 2005 ; De Barros Rodrigues et al., 2022 ). Children with acute symptoms have been reported due to pesticide drift near their schools, or they might be at even higher risk because of accidental contact while playing on agriculture farms with empty containers of contaminating materials ( Buralli et al., 2020 ). In a longitudinal study, children living in agricultural communities had higher amounts of the dialkylphosphate (DAP) metabolite in their urine. The DAP metabolites are general to organophosphorus pesticides and are responsible for the temporal pattern of children’s pesticide exposure upon pesticide spraying in an agricultural region ( Koch et al., 2002 ). Other factors for children’s hospitalization related to pesticide exposure are their increased respiratory rate, comparatively larger surface area of skin, and elevated metabolic rate ( Sharma et al., 2019 ).
A few studies investigated the role of allergic asthma as well as other respiratory symptoms due to pesticide exposure among women. The studies were mainly focused on male workers, associated directly or non-directly with agricultural fields, but it was evident that women are also increasingly affected and at high-risk due to pesticide exposure ( Ndlovu et al., 2011 ). In a study, Hoppin et al. (2008) evaluated pesticide and occupational exposures as risk factors for farm women. Out of 25,000 women with atopic and non-atopic asthma, who grew up on farms and used pesticides, were more likely to develop atopic asthma than the non-users. In an infant’s environmental health birth cohort study of 266 mothers in Costa Rica, by performing a survey, they investigated the outcomes of respiratory and allergic conditions in mothers upon exposure to pesticides and other environmental metabolites. The study found significant association of high asthma score and urinary levels of thiabendazole metabolite in women living near waste burning farms and women living in agriculture farms reported eczema and itch rash ( Garry, 2004 ; Alhanti et al., 2021 ). Another study linked pesticide exposure to changes in the serum metabolome after eating fruits and vegetables (FVs). The study analyzed 171 women under infertility treatment and showed significant associations of metabolic pathways upon the eating of either high or low-to-moderate pesticide residue FVs. Different biological pathways were associated with the intake of high or low pesticide residues, including metabolism (energy, cellular receptor, enzyme, lipid, and vitamin) and intracellular signaling ( Hood et al., 2022 ). There is a need to perform more such unique studies about associations between environmental and occupational pesticide exposures and respiratory and allergic diseases. Such an insightful study related to dietary intake of pesticides might provide information on potential mechanisms associated with human diseases.
A link between food allergies and pesticides
Food allergy affects up to 10% of the world population, with more severity in infants as compared to adults. It has been referred to as the “second wave” of the allergy epidemic, following asthma ( Loh and Tang, 2018 ). In parallel, the use of pesticides such as organophosphates has been increased in agriculture and industries. This increased use of organic agents might prolong the allergic manifestations in atopic individuals by potential mechanisms such as epigenetic control of allergen expression, modifying proteins to make them even more allergenic; or increased polyamine production in stressed condition ( Falak et al., 2012 ; Loh and Tang, 2018 ).
People who are exposed to chemicals either through chlorinated water or come into contact with foods that contain them or breathe polluted air are more likely to develop food allergies. Chemicals like dichlorophenols can alter the microbiota of the human body and in turn influence the body’s immune system to trigger such reactions. In contrary to hygiene hypothesis, dichlorophenols can kill microbes and clear the environment such that young children become prone to developing allergy risks. In an international survey of the United States (NHANES) in the period 2005–2006, 2,200 children aged 6 were checked for dichlorophenol levels in their urine along with allergies to peanuts, eggs, milk, and shrimp. It was found that children with high levels of urine dichlorophenol were 80% more likely to develop allergies ( Jerschow et al., 2012 ).
An ample number of studies have been performed related to pesticide exposure and asthma, but a lot more meticulous studies need to be accomplished. The previous data was generated accordingly self-reported or doctor-diagnosed asthma, which needs to be refurbished with bronchial responsiveness measurements and lung function. To strengthen the data, a detailed molecular and genetic phenotyping must be explored to study the effect of pesticides in different types of asthmatic conditions ( Jerschow et al., 2012 ; Loh and Tang, 2018 ). Studies on different active and organic ingredients or new formulations along with potent agents might provide important insights, such as between asthma and exposure to pesticides. The recent cohort studies identified certain biomarkers directly linked to pesticide exposure and asthma, thus new biomarkers for the different and generally used pesticides can be considered. More robust measurement of pesticide exposure depending upon the biomarkers should be the focus of the future comprehensive studies. Their metabolic rate, bioactivity, life time, and threshold levels must be recorded to understand the pathophysiology of the underlying asthmatic or atopic conditions. Finally, more longitudinal studies offering a large sample size over a longer period of time can be a big step toward understanding the biological pathways at the gene level that can directly link pesticide exposure to disease development.
Pesticide effects on preserved food
Pesticides play a global role in the protection, preservation, comfort of food, fiber, and human health ( Winteringham, 1971 ). However, the excessive and uncontrolled use and misuse of pesticides, as well as their long-run transportation and volatility, cause widespread environmental damage or contamination. Moreover, the occurrence of many highly toxic, non-patented, and eco-resistant chemicals creates severe health concerns that causes global impact simultaneously ( Ecobichon, 2001 ). In India, the value addition and processing of ready-to-eat (RTE) or ready-to-serve (RTS) packaged products impact a lot on monitoring the levels of pesticide residues during the final consumption. However, during the processing of raw agricultural commodities (RAC), the levels of pesticides are mostly governed by the concentration level and physico–chemical characteristics of the product to be processed ( Muralidhara et al., 2022 ). Researchers reported that pre- or post-processing steps are capable enough of reducing the load of pesticides in the final product. However, in certain specific cases, processing aids in the accumulation of pesticide residues (e.g., extraction of oil from oil seeds) ( Kaushik et al., 2009 ; Muralidhara et al., 2022 ). Therefore, a maximum residual limit (MRL) of pesticides needs to be established in the case of food products attaining paramount exposure to pesticides during their pre-harvesting phase ( Scholz et al., 2017 ).
Processing factor (Pf) – During the processing of foods, there is a chance that the whole mass of pesticide residues can be assimilated into processed products. Therefore, the effect of pesticide residues on food products can be expressed by a term “processing factor” and can be calculated as follows.
The processing factor is an integral tool to generate data for global regulatory authorities monitoring the residual limits and also helps in assessing the risks by estimating the refined dietary exposure of pesticides in a processed food commodity before consumption ( OECD, 2008 ).
Effect of pesticide residue on processing operations
Processing operations play a significant role in maintaining or lowering the pesticide limit in the final value-added processed products aiding enhanced shelf-life and better product quality; however, certain processing steps impact negatively by enriching the level pesticide residues in the final product by developing toxic metabolites or second- and third-generation derivatives. Post-harvesting operations such as washing, peeling, chopping, etc. help in reducing the pesticides on the surface of fruit and vegetable commodities ( Yigit and Velioglu, 2020 ). The heat treatments such as pasteurization, sterilization, blanching, frying, boiling, cooking, etc. help in the reduction of pesticides by chemical reactions due to oxidation and hydrolysis of chemical compounds. Also, low moisture content, pH, and time–temperature combination during cooking also modulate the residual pesticide limit in the final product. Similarly, unit operations such as drying and grinding of samples, canning of food products, etc. abundantly reduce the residual limits by evaporating water and altering the physico–chemical nature of pesticides ( Kaushik et al., 2009 ). However, the unit processing operations such as cereal grain processing, fruit processing, oil extraction, grape, egg drying, and so on have a high risk of increased levels of residual pesticides and are affected by a variety of factors such as the physico–chemical behavior of pesticide molecules, produced metabolites during the chemical process, their photostability, lipophilicity, thermal stability, and polarity ( Scholz et al., 2017 ).
Determination of pesticide residues in food matrix
The determination of the residual pesticide limit in RTE/RTS foods involves a complex phenomenon and requires some special criteria. The extractability of a pesticide residue depends on the biochemical nature and behavior of food. The complexity of a matrix behavior is often increased by the processing operations involved, which impacts the performance method by decreasing precision as well as accuracy. Therefore, usage of matrix-matched calibrations and selective clean-up practices are necessary to avoid such issues ( Law et al., 2019 ). The worldwide harmonization of maximum residual limits (MRLs) for pesticide residues in raw agricultural commodities has attained a high recognition. Similarly, in India, food technologists and central agency such as Food Safety and Standard Authority of India (FSSAI) are now emphasizing too much toward a sustainable growth in the processed food sector for making and consumption of value-added items with safe or lower residual limits of pesticides ( Muralidhara et al., 2022 ).
Eco-friendly management of pesticides as bioremediation
Physical and chemical cleaning of pesticides release more toxic compounds, and both are harmful as well as costly. To maintain a sustainable environment with a healthy and productive ecosystem, eco-friendly approach as bioremediation methods is available to remove harmful contaminants ( Desisa et al., 2022 ). Since plants, algae, fungi, bacteria, and their interactions are used to remove toxins via bioremediation, which serves as a cost-effective and environmentally benign method. Pesticide remediation today includes a variety of environment friendly techniques, such as phytoremediation, microalgae bioremediation, myco-remediation, and bacterial pesticide degradation ( Singh et al., 2020 ).
Phytoremediation is an economical, solar-powered method that involves the removal or reduction of harmful chemicals from damaged sites using effective plant species. Kochia sp., Triticum spp., Ricinus communis and Ceratophyllum demersum are well-known plant species that have played a significant role in the removal of atrazine, lindane, chlorpyrifos, and endrin, respectively. The absorption of pesticides by plants results in the conversion of hazardous pesticides into less toxic compounds, which helps to remove toxic pollutants from polluted sites. Plants use various mechanisms to remove pollutants, including pollutant transpiration (phytovolatilization), clean-up through the rhizosphere microbiome (rhizo-degradation), enzymatic degradation (phytodegradation), and pesticide accumulation in different plant parts (phytoextraction). Such plants also improve the landscapes, reduce soil erosion, and prevent pollutant seepage. In addition, phytoremediation serves as an economic, safe, and green approach for chemical waste treatment ( Subashini et al., 2007 ; Gill and Garg, 2014 ; Mishra et al., 2015 ; Rissato et al., 2015 ; Kuppusamy et al., 2016 ; Main et al., 2017 ; Mir et al., 2017 ; Koranteng et al., 2018 ; Perez-Lucas et al., 2018 ; Singh et al., 2020 ).
Microalgae are also known as effective biosorbents of heavy metals and pesticides and can remove them from contaminated areas. Chlamydomonas reinhardtii , Chlamydomonas mexicana , and Dunaliella sp. have been reported for the removal of prometryne, atrazine, and mirex pesticides, respectively. Such photoautotrophic organisms exist in different forms in nature and are involved in the conversion of radiant energy (light energy to chemical energy). The use of microalgae results in the production of oxygen, which preserves the environment’s balance. Oxygen generated from microalgae also helps the bacteria during the biodegradation process. Microalgae have been found to use chemical pollutants as an energy alternate and to accelerate the biodegradation process. It can be used to achieve a variety of objectives, including nutrient recovery from wastewater, biomass formation, removal of contaminants (bioaccumulation and biosorption), and being able to grow under stress conditions. In which, bioaccumulation is an energy-dependent active process involving living organisms that metabolize pollutants. Whereas biosorption is an energy-independent process that involves both dead and living organisms for the removal of contaminant form polluted environments. The use of such technology in a two-way manner, such as pesticide accumulation as well as conversion of toxic into less toxic compounds. The degradation is influenced by the introduction of potent microalgae, optimum conditions, and the chemical composition of pesticides. In addition, there are some major factors that alter the degradation process of pesticides, such as molecular weight, functional group, concentration, and water solubility. Under stress conditions, these microalgae act mixotropically and derive their energy from light and organic carbon, which gives them an advantage over bacteria and fungi during biodegradation ( Velasquez and Dussan, 2009 ; Chojnacka, 2010 ; Mata et al., 2010 ; John et al., 2011 ; Subashchandrabose et al., 2011 ; Monteiro et al., 2012 ; Rath, 2012 ; Chekroun et al., 2014 ; Kabra et al., 2014 ; Torres et al., 2017 ; Singh et al., 2020 ).
Myco-remediation is another type of biological approach to pesticide waste management, where fungi can use such pollutants as a carbon source and convert them into less toxic compounds, thus cleaning them from the water and soil system. Fungi are ideal among microorganisms due to their structural morphology, which contains hyphae, that allows the transfer of small chemical molecules by microscopic pores easily. The mycelium networks have a multi-functional role, in addition to accelerating pesticide degradation, they also improve the plant’s nutrient and water availability. Ligninolytic fungi are known to secrete a variety of extracellular enzymes that aid in the transformation of recalcitrant chemical compounds. While saprotrophic fungi excrete the most enzymes, followed by other fungi (soft rot, white rot, and brown rot). White-rot fungi ( P. Pleurotus ostreatus , Trametes hirsutus , and Cyathus bulleri ) are widely known for pesticide biodegradation due to their extracellular enzyme complex (e.g., laccase, manganese peroxidase, and lignin peroxidase) acting non-specifically. The consortium of potent fungal species was found to be suitable for chlorpyrifos and DDT biodegradation. The phyla Zygomycota, Ascomycota, and Basidiomycota are reported for biodegradation via attacking on functional groups (dehydrogenation, demethylation, hydroxylation, etc.). This process is also influenced by other factors such as optimal temperature, pH, moisture, nutrient, and water availability, all of which play a significant role in pesticide degradation. Nowadays, many developing countries cannot afford biopesticides or cannot avoid the use of chemical pesticides, so they need to use myco-remediation or other bioremediation approaches to control pesticide pollution in a parallel manner ( Tortella et al., 2005 ; Huang et al., 2008 ; Sagar and Singh, 2011 ; Adenipekun and Lawal, 2012 ; Chen et al., 2012 ; Wu et al., 2015 ; Maqbool et al., 2016 ; Janusz et al., 2017 ; Singh et al., 2020 ).
Bacteria have been widely reported to degrade and remove pesticides as compared to other remedial approaches. Pseudomonas , Azotobacter , Flavobacterium , and Arthrobacter are the major bacterial genus involved in the removal of pesticides from polluted environments. The discovery of pollutant-degrading bacteria aided by advances in genetic engineering methods. These microbes use the pesticide for nutrients, generate H 2 O and CO 2 , and overcome the environmental risk associated with pesticides. In the soil system, such pesticides accumulate and act as electron donors and carbon sources for soil microorganisms. The environmental conditions, pesticide exposure time, and concentration, bacterial type, and growth factors (such as temperature, pH, moisture, nutrient, and water availability) all are important for efficient biodegradation. However, the presence of sulfate and chloride act as anion and bind strongly to microbes that blocks the microbial action on pesticides. The chemical structure is the first target of microbial degradation and converted into inorganic components that are further utilized by the microorganism. Advanced approaches such as bioaugmentation, bio-stimulation and natural attenuation are employed to increase the pesticide biodegradability, which includes potent bacteria, nutrient addition, and the introduction of native species to the contaminated site respectively. Alcaligenes, Flavobacterium, Acinetobacter are reported as endosulfan degrading bacteria. Similarly, Stenotrophomonas sp. also known for almost 100% removal of diazinon from the contaminated site. The bacterial system is well studied as compared to other bioremediation technologies. The diverse bacterial groups and their corresponding enzymes responsible for degradation are explained in the “Biodegradation of Pesticide Pollutants” section ( Gavrilescu, 2005 ; Singh and Walker, 2006 ; Arias-Estevez et al., 2008 ; Huang et al., 2008 ; Singh et al., 2011 , 2020 ; Laura et al., 2013 ; Rani and Dhania, 2014 ; Adams et al., 2015 ; Deng et al., 2015 ).
Biodegradation of pesticide pollutants
Biodegradation of pesticides is mainly mediated by using microbial systems. Microbes are able to produce a specific group of enzymes that are able to catalyze the pesticides from contaminated sites. The pure culture and mixed cultures of the bacteria and fungi were found to be effective in the removal of pesticide residues from the water and soil environment. Microbial consortium was found with superior degradation abilities ( Bhatt et al., 2021c ). Singh et al. (1999) found that microbes have developed a number of metabolic routes to breakdown or detoxify a variety of environmental contaminants, including pesticides. Conde-Avila et al. (2021) reported bacteria from the genera Streptomyces , Flavimonas , Burkholderia , Micrococcus , Sphingomonas , Brevibacterium , Flavobacterium , Pseudomonas , Agrobacterium , Arthrobacter , Enterobacter , and Bacillus are associated with pesticide biodegradation. There is a diverse group of bacteria and fungi that are capable of degrading pesticides. The different phyla include Bacteroidetes, Basidiomycota, Chlorophyta, Cyanobacteria, Actinomycetota, Firmicutes, and Proteobacteria. The bacteria that fall under Actinobacteria have a tremendous capability to degrade several classes of chemical pesticides as most of the strains have high GC content and are actively used for the recycling of complex polymers. Streptomyces , Nocardioides , Arthrobacter , Rhodococcus , Micrococcus , and Microbacterium are members of the Actinomycetota phylum and can metabolize a variety of chemical compounds such as organochlorides, organophosphates, carbamates, triazinones, and others ( Kim et al., 2017 ). Similarly, Firmicutes are also play a critical role in pesticide biodegradation. Among them, several strains possess endospores that are resistant to any adverse condition and are reported as extremophiles. There are a number of firmicutes that are capable of degrading pesticides, including Paenibacillus polymyxa, Bacillus licheniformis , Bacillus thuringiensis , Bacillus pumilus , Bacillus subtilis , and Bacillus cereus ( Patil et al., 1970 ). Moreover, among the proteobacteria, α-, β-, and γ-proteobacteria have also been reported for their pesticide degradation activity.
Among the α-proteobacteria strains that have been reported are Sphingomonas , Rhizobium , Methylobacterium , Azospirillum , Pseudaminobacter , Bosea , Mesorhizobium , Shinella , and Ochrobactrum . Moreover, Ralstonia , Alcaligenes , Burkholderia , Achromobacter , and Cupriavidus are the reported bacterial strains among β-proteobacteria. Furthermore, reported bacterial strains among γ-proteobacteria are Yersinia , Pseudomonas , Klebsiella , Acinetobacter , Serratia , and Xanthomonas ( Bhatt et al., 2020b ; Kumar et al., 2021 ). Microbes and their enzymes associated with biodegradation of different types of pesticide are shown in Tables 6 , ,7 7 .
Pesticides degrading microorganisms.
| Type of pesticide | Example | Microorganism | References |
| Organophosphorus | Chlorpyrifos | spp., spp., spp., spp., | ; |
| Organophosphorus | Parathion | spp., spp., spp., spp. | ; |
| Organophosphorus | Methyl parathion | spp., spp., spp., , | ; ; |
| Glyphosate | spp., spp., spp., spp., , | ; | |
| Organophosphorus | Coumaphos | spp., | ; |
| Organophosphorus | Monocrotophos | spp., spp., | |
| Organophosphorus | Fenitrothion | spp., spp. | ; |
| Organophosphorus | Fenthion | spp. | |
| Organophosphorus | Diazinon | spp., spp., spp. | ; |
| Organophosphorus | DDT | ||
| Organochlorine | Aldrin | ||
| Organochlorine | Dieldrin | ||
| Organochlorine | Endosulfan | sp., | ; |
| Organochlorine | Alpha endosulfan | ; | |
| Organochlorine | Beta endosulfan | ||
| Organochlorine | Dichlorodiphenyl-trichloroethane | sp., sp., sp., , | ; ; |
| Organochlorine | Lindane | sp. P27, sp. NITDBR1, sp. A5, sp. M7, , | ; ; |
| Triazone | Atrazine | spp. spp., spp. | |
| Carbamate | Carbafuron | spp., spp., spp. | |
| EPTC | spp., spp. | ||
| Carbafuron | |||
| Avermectin | Emamectin Benzoate | ||
| Neonicotinoid | Thiamethoxam |
Bacterial enzymes, responsible for the degradation of pesticides ( Ortiz-Hernández et al., 2013 ).
| Pesticide | Enzyme | Bacteria |
| Gylphosate | Oxidoreductase (Gox) | spp., spp. |
| Endosulfan, aldrin, malathion, DDT, endosulfate | Monooxygenases (Esd) | spp., spp. |
| Hexachlorobenzene, Pentachlorobenzene | P450 | |
| Trifluralin | Dioxygenases (TOD) | |
| Hexachlorocyclohexane | Haloalkane Dehalogenases (Lin B) | spp. |
| Chloro-S-trazina | AtzA | spp. |
| Chloro-S-trazina | TrzN | spp. |
| Hexachlorocyclohexane (Gamma isomer) | Lin A | spp. |
| 2,4-dichlorophenoxyacetic acid | TfdA | |
| Pyridyl-oxyacetic acid | TfdA | |
| Pyridyl-oxyacetic acid | DMO | |
| Phosphotriester | Phosphotriesterases (OPH/OpdA) | spp. |
The basic stages of pesticide conversion were characterized by Kumar et al., 1996 as follows: (1) Mineralization: Carbon dioxide or methane as an end-product of complete degradation; (2) Detoxification: Conversion of toxic to non-toxic compounds; (3) Co-metabolism: Microbes involved in the metabolism process of compounds without benefiting themselves from these compounds; (4) Activation: Activation of compounds. During the beginning of 1064, hydrolases and oxygenases came in knowledge and Singh et al. also reported involvement of these enzymes in pesticide biodegradation ( Bollag et al., 1968 ; Tiedje et al., 1969 ; Singh et al., 1999 ). Under both denitrifying and aerobic conditions, hydrolytic dehalogenation (the substitution of a halogen group by a hydroxyl) can occur, but only methanogenic and sulfonic circumstances result in reductive dehalogenation, which involves the substitution of a halogen group by a hydrogen group. Furthermore, biotransformation events such as polymerization and methylation may occur, resulting in more hazardous or recalcitrant compounds. Different methods for converting hazardous pesticides were used, depending on their chemical constituents and the microbes that were used for bioconversion ( Singh et al., 1999 ). Factors such as the microbial culture, cultivation technique, size of inoculum, growth under elevated pesticide percentage, adaptation, rhizosphere interactions, and response against the environmental factors can all affect the pesticide degradation process ( Conde-Avila et al., 2021 ). Research has concentrated on the practice of microbial cellular immobilization (CI) technology in several materials and supports the long-lasting survival of microbes. Now, research has shifted to the use of microbial cells as CI, which protects and allows them to be reused. Such a strategy enhances the possibilities of techniques lasting and succeeding in a pesticide-contaminated environment for a long period and has been found suitable for pesticide biodegradation ( Colla et al., 2014 ; Pradeep and Subbaiah, 2016 ; Fernández-López et al., 2017 ; Conde-Avila et al., 2021 ).
The CI technology has served as an environmentally approachable processes for waste management practices. The use of CI of degrading microbes in the elimination and or degradation of pollutants, the CI system has developed as an eco-friendly alternative approach. There are certain disadvantages to CI technology, such as microbial interactions with the immobilization material and its impact on microbial survivability ( Conde-Avila et al., 2021 ). When CI is utilized instead of free cells, the percentage of clearance and efficiency increases for pesticides including chlorpyrifos, atrazine, difenoconazole cypermethrin, carbaryl, endosulfan, and carbofuran. The benefits of utilizing CI are independently supported by the immobilization method or substance employed ( Bhadbhade et al., 2002 ; Pattanasupong et al., 2004 ; Adinarayana et al., 2005 ; López-Pérez et al., 2006 ; Fuentes et al., 2013 ; Zucca and Sanjust, 2014 ; Abigail and Das, 2015 ; Chen et al., 2015 ; Tallur et al., 2015 ; Bhatt et al., 2016 ; Fernández-López et al., 2017 ). Because pesticides come in such a wide variety of chemical groups, the factors that influence their presence, transit, and mobility are complicated and difficult to anticipate. Extrinsic and intrinsic variables govern adsorption-desorption, biodegradation, volatilization, photodegradation and breakdown phenomena, which mediate pesticide occurrence, and migration ( Conde-Avila et al., 2021 ). Soils with high organic matter reduce pesticide availability through adsorption to a larger percentage than sandy soils ( Yanez-Ocampo et al., 2016 ).
Bioremediation procedures frequently include organic wastes and/or specialist strains with catabolic capabilities against contaminants to assist the breakdown of more persistent pesticides or to reduce their influence on microorganisms. Using genetically engineered strains to breakdown pesticides might be an effective method. Pesticide-exposed native species can develop the capacity to degrade toxic chemicals. Such technology was created to clean up pesticide-related pollutants ( Barreiros et al., 2012 ; Nikel et al., 2014 ; Castillo et al., 2016 ; Bhatt et al., 2019a , b , c , 2020c , 2021d ).
In comparison to pure cultures, the introduction of consortia or pesticide primed materials has been found to improve pesticide breakdown and mineralization capability in BPSs (bio-purification systems) ( Sniegowski and Springael, 2015 ). Furthermore, Biobed bioremediation systems can be an ideal microcosm for developing specialized microorganisms capable of enhancing pesticide residue metabolization from wastewaters ( Dunon et al., 2013 ). However, the bioaugmentation strategy for various pesticide biodegradations in wastewaters at high concentrations, as occurs in real-world scenarios, is still little known ( Sniegowski and Springael, 2015 ).
Pesticide use has expanded extensively in the recent years, resulting in the environmental damage, particularly water and soil contamination. Pesticides come in a variety of forms, but organophosphates, organochlorine, carbamate, and pyrethroids are the most abundantly uses pesticides and have human and environmental concerns. Refined knowledge of various properties related to the physical and chemical background of pesticides are necessary to determine the impact and behavior of pesticide transformation in that environment. Such pesticides need proper management strategies for converting them to non-toxic compounds before releasing them into the environment. They are the most persistent and generally resistant to degradation under natural conditions. The scientific community has been working hard to come up with creative approaches to pesticide pollution reduction. Environmentally friendly management strategies include several bioremediation approaches and servers to solve pesticide problems or develop alternative green solutions. Bioremediation strategies such as phytoremediation, microalgae bioremediation, myco-remediation, and microbial degradation are also cost-effective and environmentally benign methods. Nowadays, microbial degradation methods are used extensively. Microorganisms and their enzymes play a key role in the breakdown of chemical compounds and synthetic pesticides. Although these methods are environmentally friendly, they have certain limitation such as metabolic routes followed by microbes are highly influenced by external factors. As a result, further study is needed in specific areas before this approach can be declared successful. Enzymatic degradation appears to be a viable method. It is becoming increasingly vital to do significant research to find enzymes capable of degrading synthetic pesticides. Microbial degradation occurs at a considerably slower rate and is not always as efficient or straightforward to carry out as traditional bioremediation technologies. It needed to find more potent microbes, novel genes, and bioremediation approaches for proper waste management of pesticide pollutants. Genetically engineered microorganisms and biotechnology also play a significant role in this area. The above discussion illustrates the utilization of pesticide degrading microorganisms in a constructive way to manage the pesticide pollutants in an eco-friendly manner. Hence, the further studies on the screening of effective microbial strains and enzymes are essential to reduce pesticide risks for the environment and human health.
Author contributions
VMP: conception and design of study and revising the manuscript critically for important intellectual content, approval of the version of the manuscript to be published. VMP and VV: analysis and/or interpretation of data. VMP, VV, BR, BK, NB, AS, SD, MY, RK, SS, AM, VP, NR, and JC: acquisition of data. VMP, BR, and BK: drafting the manuscript. All authors approved the version of the manuscript to be published.
VP would like to acknowledge the Faculty of Inter-Disciplinary and Applied Sciences, University of Delhi, South Campus, New Delhi, India for providing financial assistance as the V. N. Bakshi Post-Doctoral Fellowship (UDSC/FIAS/VNB-PDF2019).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- Abigail M. E. A., Das N. (2015). Removal of atrazine from the aqueous environment using immobilized Pichia kudriavzevii Atz-EN-01 by two different methods. Int. Biodeterior. Biodegrad. 104 53–58. 10.1016/j.ibiod.2015.05.014 [ CrossRef ] [ Google Scholar ]
- Abubakar Y., Tijjani H., Egbuna C., Adetunji C. O., Kala S., Kryeziu T. L., et al. (2020). “ Pesticides, history, and classification ,” in Natural Remedies for Pest, Disease and Weed Control , eds Egbuna C., Sawicka B. (Amsterdam: Academic Press; ), 29–42. [ Google Scholar ]
- Adams G. O., Fufeyin P. T., Okoro S. E., Ehinomen I. (2015). Bioremediation, biostimulation and bio-augmention: a review. Int. J. Environ. Bioremed. Biodegrad. 3 28–39. 10.12691/ijebb-3-1-5 [ CrossRef ] [ Google Scholar ]
- Addissie Y. A., Kruszka P., Troia A., Wong Z. C., Everson J. L., Kozel B. A., et al. (2020). Prenatal exposure to pesticides and risk for holoprosencephaly: a case-control study. Environ. Health 19 1–13. 10.1186/s12940-020-00611-z [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Adenipekun C. O., Lawal R. (2012). Uses of mushrooms in bioremediation: a review. Biotechnol. Mol. Biol. Rev. 7 62–68. 10.5897/BMBR12.006 [ CrossRef ] [ Google Scholar ]
- Adinarayana K., Jyothi B., Ellaiah P. (2005). Production of alkaline protease with immobilized cells of Bacillus subtilis PE-11 in various matrices by entrapment technique. AAPS Phar. Sci. Tech . 6 E391–E397. 10.1208/pt060348 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Agbeve S., Osei-Fosu P., Carboo D. (2014). Levels of organochlorine pesticide residues in Mondia whitei , a medicinal plant used in traditional medicine for erectile dysfunction in Ghana. Int. J. Adv. Agric. Res. 1 9–16. [ Google Scholar ]
- Ahmad A., Ahmad M. (2018). Deciphering the mechanism of interaction of edifenphos with calf thymus DNA. Spectro. Act. Part A: Mol. Biomol. Spectro. 188 244–251. 10.1016/j.saa.2017.07.014 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahmad I., Ahmad A., Ahmad M. (2016). Binding properties of pendimethalin herbicide to DNA: multispectroscopic and molecular docking approaches. Phys. Chem. Chem. Phys. 18 6476–6485. 10.1039/c5cp07351k [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahmad M., Zafeer M. F., Javed M., Ahmad M. (2018). Pendimethalin-induced oxidative stress, DNA damage and activation of anti-inflammatory and apoptotic markers in male rats. Sci. Rep . 8 1–9. 10.1038/s41598-018-35484-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahmadi F., Ghanbari K. (2014). Proposed model for binding of permethrin and deltamethrin insecticides with ct-DNA, a structural comparative study. Ecotoxicol. Environ. Saf . 106 136–145. 10.1016/j.ecoenv.2014.02.018 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahmadi F., Jafari B., Rahimi-Nasrabadi M., Ghasemi S., Ghanbari K. (2013). Proposed model for in vitro interaction between fenitrothion and DNA, by using competitive fluorescence, 31P NMR, 1H NMR, FT-IR, CD and molecular modeling. Toxicol Int. Vit. 27 641–650. 10.1016/j.tiv.2012.11.004 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ahmadi F., Jamali N., Jahangard-Yekta S., Jafari B., Nouri S., Najafi F., et al. (2011). The experimental and theoretical QM/MM study of interaction of chloridazon herbicide with ds-DNA. Spectroch. Act. Part A 79 1004–1012. 10.1016/j.saa.2011.04.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Aislabie J., Lloyd-Jones G. (1995). A review of bacterial-degradation of pesticides. Soil Res. 33 925–942. [ Google Scholar ]
- Alengebawy A., Abdelkhalek S. T., Qureshi S. R., Wang M.-Q. (2021). Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxicology 9 : 42 . 10.3390/toxics9030042 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Alhanti B., De Joode B. V. W., Martinez M. S., Mora A. M., Gamboa L. C., Reich B., et al. (2021). Environmental exposures contribute to respiratory and allergic symptoms among women living in the banana growing regions of Costa Rica. Occup. Environ. Med. 79 469–476. 10.1136/oemed-2021-107611 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ali H. A. J., Rani V. J. (2009). Effect of phosalone on haematological indices in the tilapia, Oreochromis mossambicus . Tur. J. Vet. Anim. Sci . 33 407–411. [ Google Scholar ]
- Amaral A. F. (2014). Pesticides and asthma: Challenges for epidemiology . Front. Public Health 2 : 6 . 10.3389/fpubh.2014.00006 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Arias-Estevez M., Lopez-Periago E., Martinez-Carballo E., Simal-Gandara J., Mejuto J. C., Garcia-Rio L. (2008). The mobility and degradation of pesticides in soils and the pollution of groundwater resources . Agric. Ecosyst. Environ. 123 , 247–260. 10.1016/j.agee.2007.07.011 [ CrossRef ] [ Google Scholar ]
- Authority E. F. S. A., Alvarez F., Arena M., Auteri D., Borroto J., Brancato A., et al. (2021). Updated peer review of the pesticide risk assessment of the active substance bifenazate. EFSA J. 19 : e06818 . 10.2903/j.efsa.2021.6818 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bailey G. W., White J. L. (1970). “ Factors influencing the adsorption, desorption, and movement of pesticides in soil ,” in Single Pesticide Volume: The Triazine Herbicides , eds Gunther F. A., Gunther J. D. (New York, NY: Springer; ), 29–92. 10.1007/978-1-4615-8464-3_4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baldi I., Robert C., Piantoni F., Tual S., Bouvier G., Lebailly P., et al. (2014). Agricultural exposure and asthma risk in the AGRICAN French cohort. Int. J. Hygi. Environ. Health 217 435–442. 10.1016/j.ijheh.2013.08.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Baldwin C., Bell I., O’rourke M., Lebowitz M. (1997). The association of respiratory problems in a community sample with self-reported chemical intolerance. Euro. J. Epidem. 13 547–552. 10.1023/a:1007341813396 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Banaee M., Mirvaghefi A., Amiri B. M., Rafiee G., Nematdost B. (2011). Hematological and histopathological effects of diazinon poisoning in common carp ( Cyprinus carpio ). J. Fish 64 Pe1–Pe12. [ Google Scholar ]
- Barnhoorn I. E., Bornman M., Van Rensburg C. J., Bouwman H. (2009). DDT residues in water, sediment, domestic and indigenous biota from a currently DDT-sprayed area. Chemo 77 1236–1241. 10.1016/j.chemosphere.2009.08.045 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Barreiros L., Peres J., Azevedo N. F., Manaia C. M., Nunes O. C. (2012). Environmental factors influencing molinate biodegradation by a two-member mixed culture in rice paddy field floodwater. Int. Biodeter. Biodegr. 72 52–58. [ Google Scholar ]
- Barry P., Young A. J., Britton G. (1990). Photodestruction of pigments in higher plants by herbicide action: I. The effect of DCMU (diuron) on isolated chloroplasts. J. Experiment. Bot. 41 123–129. [ Google Scholar ]
- Basantani M., Srivastava A., Sen S. (2011). Elevated antioxidant response and induction of tau-class glutathione S-transferase after glyphosate treatment in Vigna radiata (L.) Wilczek. Pest. Biochem. Phys . 99 111–117. 10.1016/j.pestbp.2010.11.007 [ CrossRef ] [ Google Scholar ]
- Bast A., Semen K. O., Drent M. (2021). Pulmonary toxicity associated with occupational and environmental exposure to pesticides and herbicides. Curr. Opin. Pulmo. Med. 27 278–283. 10.1097/MCP.0000000000000777 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Beasley V. R. (2020). Direct and Indirect Effects of Environmental Contaminants on Amphibians. Amsterdam: Elsevier. 10.1016/b978-0-12-409548-9.11274-6 [ CrossRef ] [ Google Scholar ]
- Beulke S., Brown C. D., Fryer C. J., Van Beinum W. (2004). Influence of kinetic sorption and diffusion on pesticide movement through aggregated soils. Chemo 57 481–490. 10.1016/j.chemosphere.2004.06.026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhadauriya P., Parihar R., Ganesh S. (2021). Pesticides DEET, fipronil and maneb induce stress granule assembly and translation arrest in neuronal cells. Biochem. Biophys. Rep. 28 : 101110 . 10.1016/j.bbrep.2021.101110 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhadbhade B., Sarnaik S., Kanekar P. (2002). Biomineralization of an organophosphorus pesticide, Monocrotophos, by soil bacteria. J. Appl. Microbiol. 93 224–234. 10.1046/j.1365-2672.2002.01680.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Bhatt K., Huang Y., Lin Z., Chen S. (2020a). Esterase is a powerful tool for the biodegradation of pyrethroid insecticides. Chemo 244 : 125507 . 10.1016/j.chemosphere.2019.125507 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Huang Y., Rene E. R., Kumar A. J., Chen S. (2020b). Mechanism of allethrin biodegradation by a newly isolated Sphingomonas trueperi strain CW3 from wastewater sludge. Bioresour. Technol. 305 : 123074 . 10.1016/j.biortech.2020.123074 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Huang Y., Zhang W., Sharma A., Chen S. (2020c). Enhanced cypermethrin degradation kinetics and metabolic pathway in Bacillus thuringiensis strain SG4. Microorganisms 8 : 223 . 10.3390/microorganisms8020223 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Gangola S., Chaudhary P., Khati P., Kumar G., Sharma A., et al. (2019a). Pesticide induced up-regulation of esterase and aldehyde dehydrogenase in indigenous Bacillus spp. Bioremed. J . 23 42–52. 10.1080/10889868.2019.1569586 [ CrossRef ] [ Google Scholar ]
- Bhatt P., Huang Y., Zhan H., Chen S. (2019b). Insight into microbial applications for the biodegradation of pyrethroid insecticides. Front. Microbiol. 10 : 1778 . 10.3389/fmicb.2019.01778 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Pal K., Bhandari G., Barh A. (2019c). Modelling of the methyl halide biodegradation in bacteria and its effect on environmental systems. Pest. Biochem. Phys. 158 88–100. 10.1016/j.pestbp.2019.04.015 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Joshi T., Bhatt K., Zhang W., Huang Y., Chen S. (2021a). Binding interaction of glyphosate with glyphosate oxidoreductase and C–P lyase: Molecular docking and molecular dynamics simulation studies. J. Hazar. Mat . 409 : 124927 . 10.1016/j.jhazmat.2020.124927 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Zhou X., Huang Y., Zhang W., Chen S. (2021b). Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J. Hazar. Mat. 411 : 125026 . 10.1016/j.jhazmat.2020.125026 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Bhatt K., Sharma A., Zhang W., Mishra S., Chen S. (2021c). Biotechnological basis of microbial consortia for the removal of pesticides from the environment. Crit. Rev. Biotechnol. 41 317–338. 10.1080/07388551.2020.1853032 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Gangola S., Bhandari G., Zhang W., Maithani D., Mishra S., et al. (2021d). New insights into the degradation of synthetic pollutants in contaminated environments. Chemo 268 : 128827 . 10.1016/j.chemosphere.2020.128827 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bhatt P., Sharma A., Gangola S., Khati P., Kumar G., Srivastava A. (2016). Novel pathway of cypermethrin biodegradation in a Bacillus sp. strain SG2 isolated from cypermethrin-contaminated agriculture field. 3 Bio 6 1–11. 10.1007/s13205-016-0372-3 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Birceanu O., Wilkie M. P. (2018). Post-exposure effects of the piscicide 3-trifluoromethyl-4-nitrophenol (TFM) on the stress response and liver metabolic capacity in rainbow trout ( Oncorhynchus mykiss ). PLoS One 13 : e0200782 . 10.1371/journal.pone.0200782 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Blatchford P. A., Scott C., French N., Rehm B. H. (2012). Immobilization of organophosphohydrolase OpdA from Agrobacterium radiobacter by overproduction at the surface of polyester inclusions inside engineered Escherichia coli . Biotechnol. Bioeng. 109 1101–1108. 10.1002/bit.24402 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bollag J., Helling C., Alexander M. (1968). 2, 4-D metabolism. Enzymic hydroxylation of chlorinated phenols. J. Agri. Food Chem. 16 826–828. 10.1021/jf60159a037 [ CrossRef ] [ Google Scholar ]
- Boonlertnirun S., Boonlertnirun K., Sooksathan I. (2005). “ Effect of chitosan application on growth and yield of rice ( Oryza sativa ) var. Suphunburi 1 ,” in Proceedings of 43rd Kasetsart University Annual Conference , (Thailand: ). [ Google Scholar ]
- Bose S., Kumar P. S., Vo D.-V. N., Rajamohan N., Saravanan R. (2021). Microbial degradation of recalcitrant pesticides: a review. Environ. Chem. Lett. 19 3209–3228. 10.1007/s10311-021-01236-5 [ CrossRef ] [ Google Scholar ]
- Brander S. M., He G., Smalling K. L., Denison M. S., Cherr G. N. (2012). The in vivo estrogenic and in vitro anti-estrogenic activity of permethrin and bifenthrin. Environ. Toxicol. Chem. 31 2848–2855. 10.1002/etc.2019 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brandt A., Hohnheiser B., Sgolastra F., Bosch J., Meixner M. D., Büchler R. (2020). Immunosuppression response to the neonicotinoid insecticide thiacloprid in females and males of the red mason bee Osmia bicornis L. Scientific Reports 10 1–10. 10.1038/s41598-020-61445-w [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brecke B. J., Duke W. B. (1980). Effect of glyphosate on intact bean plants ( Phaseolus vulgaris L.) and isolated cells. Plant Physiol. 66 656–659. 10.1104/pp.66.4.656 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Brodeur J. C., Sassone A., Hermida G. N., Codugnello N. (2013). Environmentally-relevant concentrations of atrazine induce non-monotonic acceleration of developmental rate and increased size at metamorphosis in Rhinella arenarum tadpoles. Ecotoxicol. Environ. Saf. 92 10–17. 10.1016/j.ecoenv.2013.01.019 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Buralli R. J., Dultra A. F., Ribeiro H. (2020). Respiratory and allergic effects in children exposed to Pesticides—A systematic review. Int. J. Environ Res. Public Health 17 : 2740 . 10.3390/ijerph17082740 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bureau L. (1993). Indian Labour Statistics. India: Government of India. [ Google Scholar ]
- Calaf G. M. (2021). Role of organophosphorous pesticides and acetylcholine in breast carcinogenesis. Semin. Can. Biol. 76 206–217. 10.1016/j.semcancer.2021.03.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Cannon R. D., Ruha A. M. (2013). Insecticides, Herbicides, and Rodenticides. Emergency Medicine: Clinical Essentials , 2nd Edn, Vol. 146 . Philadelphia, PA: Elsevier Saunders, 1246–1256. [ Google Scholar ]
- Caron-Beaudoin É, Viau R., Sanderson J. T. (2018). Effects of neonicotinoid pesticides on promoter-specific aromatase (CYP19) expression in Hs578t breast cancer cells and the role of the VEGF pathway. Environ. Health Perspect. 126 : 047014 . 10.1289/EHP2698 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Castillo J. M., Beguet J., Martin-Laurent F., Romero E. (2016). Multidisciplinary assessment of pesticide mitigation in soil amended with vermicomposted agroindustrial wastes. J. Haz. Mat. 304 379–387. 10.1016/j.jhazmat.2015.10.056 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chekroun K. B., Sánchez E., Baghour M. (2014). The role of algae in bioremediation of organic pol- lutants. Int. Res. J. Public. Environ. Health 1 19–32. [ Google Scholar ]
- Chen C., Wu T.-W., Wang H.-L., Wu S.-H., Tien C.-J. (2015). The ability of immobilized bacterial consortia and strains from river biofilms to degrade the carbamate pesticide methomyl. Int. J. Environ. Sci. Technol. 12 2857–2866. 10.1007/s13762-014-0675-z [ CrossRef ] [ Google Scholar ]
- Chen C., Zou W., Cui G., Tian J., Wang Y., Ma L. (2020). Ecological risk assessment of current-use pesticides in an aquatic system of Shanghai, China. Chemo 257 : 127222 . 10.1016/j.chemosphere.2020.127222 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chen S., Liu C., Peng C., Liu H., Hu M., Zhong G. (2012). Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PLoS One 7 : 47205 . 10.1371/journal.pone.0047205 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chibu H., Shibayama H., Arima S. (2002). Effects of chitosan application on the shoot growth of rice and soybean. Jpn. J. Crop Sci. 71 206–211. 10.1626/jcs.71.206 [ CrossRef ] [ Google Scholar ]
- Chojnacka K. (2010). Biosorption and bioaccumulation–the prospects for practical applications. Environ. Int. 36 299–307. 10.1016/j.envint.2009.12.001 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Choudhury P. P. (2019). “ Transformation of Herbicides in the Environment ,” in Herbicide Residue Research in India. Environmental Chemistry for a Sustainable World , eds Sondhia S., Choudhury P., Sharma A. (Singapore: Springer; ), 415–442. [ Google Scholar ]
- Colla T. S., Andreazza R., Bücker F., de Souza M. M., Tramontini L., Prado G. R., et al. (2014). Bioremediation assessment of diesel–biodiesel-contaminated soil using an alternative bioaugmentation strategy. Environ. Sci. Pol. Res. 21 2592–2602. 10.1007/s11356-013-2139-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Conde-Avila V., Ortega-Martínez L. D., Loera O., El Kassis E. G., Dávila J. G., Valenzuela C. M., et al. (2021). Pesticides degradation by immobilised microorganisms. Int. J. Environ. Anal. Chem. 101 2975–3005. 10.1080/03067319.2020.1715375 [ CrossRef ] [ Google Scholar ]
- Costa C., Miozzi E., Teodoro M., Fenga C. (2019). Influence of genetic polymorphism on pesticide-induced oxidative stress. Curr. Opin. Toxicol. 13 1–7. [ Google Scholar ]
- Daneshmehr M.-A., Ahmadi F., Ahmadi B., Shakiba E. (2016). Deciphering the binding mode of dinitramine herbicide to ct-DNA, a thermodynamic discussion. Food Agric. Immun. 27 23–39. 10.1080/09540105.2015.1055555 [ CrossRef ] [ Google Scholar ]
- Dawar F. U., Zuberi A., Azizullah A., Khattak M. N. K. (2016). Effects of cypermethrin on survival, morphological and biochemical aspects of rohu (Labeo rohita) during early development. Chemo 144 697–705. 10.1016/j.chemosphere.2015.09.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- De Barros Rodrigues M., De Carvalho D. S., Chong-Silva D. C., de Pereira M. N. E. U., de Albuquerque G. S. C., Cieslak F., et al. (2022). Association between exposure to pesticides and allergic diseases in children and adolescents: a systematic review with meta-analysis. J. Peditr. [Online ahead of print]. 10.1016/j.jped.2021.10.007 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Deng S., Chen Y., Wang D., Shi T., Wu X., Ma X. (2015). Rapid biodegradation of organophos- phorus pesticides by Stenotrophomonas sp. G1. J. Hazard. Mater. 297 17–24. 10.1016/j.jhazmat.2015.04.052 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Desisa B., Getahun A., Muleta D. (2022). “ Advances in biological treatment technologies for some emerging pesticides ,” in Pesticides Bioremediation , eds Siddiqui S., Meghvansi M. K., Chaudhary K. K. (Cham: Springer; ), 10.1007/978-3-030-97000-0_10 [ CrossRef ] [ Google Scholar ]
- Dey C., Saha S. (2014). A comparative study on the acute toxicity bioassay of dimethoate and lambda-cyhalothrin and effects on thyroid hormones of freshwater teleost fish Labeo rohita (Hamilton). Int. J. Environ. Res. 8 1085–1092. 10.22059/IJER.2014.802 [ CrossRef ] [ Google Scholar ]
- Doǧanlar Z. B., Doǧanlar O., Tozkir H., Gökalp F. D., Doǧan A., Yamaç F., et al. (2018). Nonoccupational exposure of agricultural area residents to pesticides: pesticide accumulation and evaluation of genotoxicity. Arch. Environ. Conta. Toxicol. 75 530–544. 10.1007/s00244-018-0545-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Donald P. F. (2004). Biodiversity impacts of some agricultural commodity production systems. Conserv. Biol. 18 17–37. 10.1111/j.1523-1739.2004.01803.x [ CrossRef ] [ Google Scholar ]
- Dong Y.-J., Bartlam M., Sun L., Zhou Y.-F., Zhang Z.-P., Zhang C.-G., et al. (2005). Crystal structure of methyl parathion hydrolase from Pseudomonas sp. WBC-3. J. Mol. Biol. 353 655–663. 10.1016/j.jmb.2005.08.057 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dunon V., Sniegowski K., Bers K., Lavigne R., Smalla K., Springael D. (2013). High prevalence of IncP-1 plasmids and IS 1071 insertion sequences in on-farm biopurification systems and other pesticide-polluted environments. FEMS Microbiol. Ecol. 86 415–431. 10.1111/1574-6941.12173 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eastmond D. A., Balakrishnan S. (2010). “ Genotoxicity of pesticides ,” in Hayes’ Handbook of Pesticide Toxicology ed. Krieger R. (Riverside, CA: University of California; ), 357–380. 10.1016/B978-0-12-374367-1.00011-2 [ CrossRef ] [ Google Scholar ]
- Ecobichon D. J. (2001). Pesticide use in developing countries. Toxicology 160 27–33. 10.1016/s0300-483x(00)00452-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- EPA, OPPTS, OPP, and SRRD. (2009). US Environmental Protection Agency Office of Pesticide Programs A Review of the Relationship between Pyrethrins Corrected Version. Washington, DC: United States Environmental Protection Agency [ Google Scholar ]
- Ernst P. (2002). Pesticide exposure and asthma. Am. J. Respir. Critic. Care Med. 165 563–564. 10.1164/ajrccm.165.5.2201001c [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Eve L., Fervers B., Le Romancer M., Etienne-Selloum N. (2020). Exposure to endocrine disrupting chemicals and risk of breast cancer. Int. J. Mol. Sci. 21 : 9139 . 10.3390/ijms21239139 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Falak R., Sankian M., Varasteh A. (2012). The possible role of organophosphorus pesticides in Augmentation of food allergenicity: a putative hypothesis. Res. J. Environ. Toxicol. 6 : 88 . 10.3923/rjet.2012.88.100 [ CrossRef ] [ Google Scholar ]
- Fernández L. (2021). Global pesticide use by country | Statista. Available online at: https://www.statista.com/statistics/1263069/global-pesticide-use-by-country/ (accessed May 31, 2022). [ Google Scholar ]
- Fernández-López M. G., Popoca-Ursino C., Sánchez-Salinas E., Tinoco-Valencia R., Folch-Mallol J. L., Dantán-González E., et al. (2017). Enhancing methyl parathion degradation by the immobilization of Burkholderia sp. isolated from agricultural soils. Microbiol. Open 6 : e00507 . 10.1002/mbo3.507 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Figueroa Z. I., Young H. A., Mumford S. L., Meeker J. D., Barr D. B., Gray G. M., et al. (2019). Pesticide interactions and risks of sperm chromosomal abnormalities. Int. J. Hygien. Environ. Health 222 1021–1029. 10.1016/j.ijheh.2019.07.001 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Finch S., Samuel A., Lane G. P. (2014). Lockhart and wiseman’s crop husbandry including grassland. Else 433–453. [ Google Scholar ]
- Fosu-Mensah B. Y., Okoffo E. D., Darko G., Gordon C. (2016). Assessment of organochlorine pesticide residues in soils and drinking water sources from cocoa farms in Ghana. Springer Plus . 5 1–13. 10.1186/s40064-016-2352-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Franco R., Li S., Rodriguez-Rocha H., Burns M., Panayiotidis M. I. (2010). Molecular mechanisms of pesticide-induced neurotoxicity: relevance to Parkinson’s disease. Chem. Biol. Interact. 188 289–300. 10.1016/j.cbi.2010.06.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fuentes M. S., Briceńo G. E., Saez J. M., Benimeli C. S., Diez M. C., Amoroso M. J. (2013). Enhanced removal of a pesticides mixture by single cultures and consortia of free and immobilized Streptomyces strains. Biomed. Res. Int. 2013 : 392573 . 10.1155/2013/392573 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fuerst E. P., Norman M. A. (1991). Interactions of herbicides with photosynthetic electron transport. Weed Sci. 39 458–464. 10.1017/S0043174500073227 [ CrossRef ] [ Google Scholar ]
- Garcia F. P., Ascencio S. C., Oyarzún J. C. G., Hernandez A. C., Alavarado P. V. (2012). Pesticides: classification, uses and toxicity. Measures of exposure and genotoxic risks. J. Res. Environ. Sci. Toxicol . 1 279–293. [ Google Scholar ]
- García J., Ventura M. I., Requena M., Hernández A. F., Parrón T., Alarcón R. (2017). Association of reproductive disorders and male congenital anomalies with environmental exposure to endocrine active pesticides. Reprod. Toxicol. 71 95–100. 10.1016/j.reprotox.2017.04.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garry V. (2004). Pesticides and children. Toxicol. Appl. Pharmacol. 198 152–163. 10.1016/j.taap.2003.11.027 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Garthwaite D., Barker I., Parrish G., Smith L., Hudson S., Pietravalle S. (2012). Pesticide Usage Survey Report 243: Outdoor Vegetable Crops in the United Kingdom 2011. England: Fera Science Ltd. [ Google Scholar ]
- Gavrilescu M. (2005). Fate of pesticides in the environment and its bioremediation. Eng. Life Sci. 5 497–526. 10.1002/elsc.200520098 [ CrossRef ] [ Google Scholar ]
- Gea M., Zhang C., Tota R., Gilardi G., Di Nardo G., Schilirò T. (2022). Assessment of five pesticides as endocrine-disrupting chemicals: effects on estrogen receptors and aromatase. Int. J. Environ. Res. Public Health 19 : 1959 . 10.3390/ijerph19041959 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gevao B., Semple K. T., Jones K. C. (2000). Bound pesticide residues in soils: a review. Environ. Pol 108 3–14. 10.1016/s0269-7491(99)00197-9 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gill H., Garg H. (2014). Pesticides: Environmental Impacts and Management Strategies, Pesticides-Toxic Aspects. London: IntechOpen, 10.5772/57399 [ CrossRef ] [ Google Scholar ]
- Gupta R. C. (2014). “ Rotenone ,” in Encyclopedia of Toxicology , 3rd Edn, ed. Philip W. (London: Elsevier; ), 185–187. [ Google Scholar ]
- Gupta R. C., Crissman J. W. (2013). “ Agricultural chemicals ,” in Haschek and Rousseaux’s Handbook of Toxicologic Pathology , eds Haschek W., Bolon B., Ochoa R., Rousseaux C., Wallig M. (London: Elsevier; ), 1349–1372. [ Google Scholar ]
- Gupta S., Gajbhiye V. T. (2002). Effect of concentration, moisture and soil type on the dissipation of flufenacet from soil. Chemo 47 901–906. 10.1016/S0045-6535(02)00017-6 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gupta V. K., Pathak A., Siddiqi N. J., Sharma B. (2016). Carbofuran modulating functions of acetylcholinesterase from rat brain in vitro. Adv. Biol. 2016 : 3760967 . 10.1155/2016/3760967 [ CrossRef ] [ Google Scholar ]
- Gyawali K. (2018). Pesticide uses and its effects on public health and environment. J. Health Promot. 6 28–36. 10.3126/jhp.v6i0.21801 [ CrossRef ] [ Google Scholar ]
- Hassaan M. A., El Nemr A. (2020). Pesticides pollution: classifications, human health impact, extraction and treatment techniques. Egypt. J. Aquat. Res. 46 207–220. 10.1016/j.ejar.2020.08.007 [ CrossRef ] [ Google Scholar ]
- Henneberger P. K., Liang X., London S. J., Umbach D. M., Sandler D. P., Hoppin J. A. (2014). Exacerbation of symptoms in agricultural pesticide applicators with asthma. Int. Arch. Occupat. Environ. Health 87 423–432. 10.1007/s00420-013-0881-x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hernández A. F. (2015). Pesticides and asthma | *Project S E.N.S.O.R , Vol. 26 . Cham: Springer. [ Google Scholar ]
- Hoang T. T., Qi C., Paul K. C., Lee M., White J. D., Richards M., et al. (2021). Epigenome-Wide DNA methylation and pesticide use in the agricultural lung health study. Environ. Health Perspect. 129 : 097008 . 10.1289/EHP8928 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hong Q., Zhang Z., Hong Y., Li S. (2007). A microcosm study on bioremediation of fenitrothion-contaminated soil using Burkholderia sp. FDS-1. Int. Biodeter. Biodegr. 59 55–61. 10.1016/j.ibiod.2006.07.013 [ CrossRef ] [ Google Scholar ]
- Hood R. B., Liang D., Chiu Y. H., Sandoval-Insausti H., Chavarro J. E., Jones D., et al. (2022). Pesticide residue intake from fruits and vegetables and alterations in the serum metabolome of women undergoing infertility treatment . Envir. Inter. 160 , 107061 . 10.1016/j.envint.2021.107061 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hoppin J. A., Umbach D. M., London S. J., Henneberger P. K., Kullman G. J., Alavanja M. C., et al. (2008). Pesticides and atopic and nonatopic asthma among farm women in the Agricultural Health Study . Am. J. Respir. Crit. Car. Med. 177 , 11–18. 10.1164/rccm.200706-821OC [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Huang D. L., Zeng G. M., Feng C. L., Hu S., Jiang X. Y., Tang L., et al. (2008). Degradation of lead-contaminated lignocellulosic waste by Phanerochaete chrysosporium and the reduction of lead toxicity. Environ. Sci. Technol. 42 4946–4951. 10.1021/es800072c [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Huch M., Stoll D. A., Kulling S. E., Soukup S. T. (2022). Metabolism of glyphosate by the human fecal microbiota. Toxicol. Lett. 358 1–5. 10.1016/j.toxlet.2021.12.013 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Hudson N. L., Kasner E. J., Beckman J., Mehler L., Schwartz A., Higgins S., et al. (2014). Characteristics and magnitude of acute pesticide-related illnesses and injuries associated with pyrethrin and pyrethroid exposures—11 states, 2000–2008. Am. J Indus. Med. 57 15–30. 10.1002/ajim.22216 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Iteire K., Sowole A., Ogunlade B. (2022). Exposure to pyrethroids induces behavioral impairments, neurofibrillary tangles and tau pathology in Alzheimer’s type neurodegeneration in adult Wistar rats. Drug Chem. Toxicol. 45 839–849. 10.1080/01480545.2020.1778020 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jaensson A., Scott A. P., Moore A., Kylin H., Olsén K. H. (2007). Effects of a pyrethroid pesticide on endocrine responses to female odours and reproductive behaviour in male parr of brown trout ( Salmo trutta L.). Aquat. Toxicol. 81 1–9. 10.1016/j.aquatox.2006.10.011 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jan S., Parween T., Siddiqi T. (2012). Effect of gamma radiation on morphological, biochemical, and physiological aspects of plants and plant products. Environ. Rev. 20 17–39. 10.1139/a11-021 [ CrossRef ] [ Google Scholar ]
- Janusz G., Pawlik A., Sulej J., Sìwiderska-Burek U., Jarosz-Wilkołazka A., Paszczyński A. (2017). Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 41 941–962. 10.1093/femsre/fux049 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jayaraj R., Megha P., Sreedev P. (2016). Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 9 :, 90–100. 10.1515/intox-2016-0012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jerschow E., McGinn A. P., De Vos G., Vernon N., Jariwala S., Hudes G., et al. (2012). Dichlorophenol-containing pesticides and allergies: results from the US National Health and Nutrition Examination Survey 2005-2006. Ann. Allergy Asthma Immunol. 109 420–425. 10.1016/j.anai.2012.09.005 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Jindal R., Sharma R. (2019). Neurotoxic responses in brain of Catla catla exposed to cypermethrin: a semiquantitative multibiomarker evaluation. Ecol. Indicat. 106 : 105485 . 10.1016/j.ecolind.2019.105485 [ CrossRef ] [ Google Scholar ]
- John R. P., Anisha G. S., Nampoothiri K. M., Pandey A. (2011). Micro and macroalgal biomass: a renewable source for bioethanol. Bioresour. Technol. 102 186–193. 10.1016/j.biortech.2010.06.139 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Joseph B., Raj S. J. (2011). Impact of pesticide toxicity on selected biomarkers in fishes. Int. J. Zool. Res. 7 212–222. 10.3923/ijzr.2011.212.222 [ CrossRef ] [ Google Scholar ]
- Jurewicz J., Radwan M., Wielgomas B., Sobala W., Piskunowicz M., Radwan P., et al. (2015). The effect of environmental exposure to pyrethroids and DNA damage in human sperm. Syst. Biol. Reprod. Med. 61 37–43. 10.3109/19396368.2014.981886 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kabir E. R., Rahman M. S., Rahman I. (2015). A review on endocrine disruptors and their possible impacts on human health. Environ. Toxicol. Pharmacol. 40 241–258. 10.1016/j.etap.2015.06.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kabra A. N., Ji M. K., Choi J., Kim J. R., Govindwar S. P., Jeon B. H. (2014). Toxicity of atrazine and its bioaccumulation and biodegradation in a green microalga, Chlamydomonas mexicana . Environ. Sci. Pol. 21 12270–12278. 10.1007/s11356-014-3157-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kafilzadeh F., Ebrahimnezhad M., Tahery Y. (2015). Isolation and identification of endosulfan-degrading bacteria and evaluation of their bioremediation in Kor River, Iran . Osong Public Health Res. Perspect. 6 , 39–46. 10.1016/j.phrp.2014.12.003 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kalyabina V. P., Esimbekova E. N., Kopylova K. V., Kratasyuk V. A. (2021). Pesticides: formulants, distribution pathways and effects on human health–a review. Toxicol. Rep. 8 1179–1192. 10.1016/j.toxrep.2021.06.004 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kapka-Skrzypczak L., Czajka M., Sawicki K., Matysiak-Kucharek M., Gabelova A., Sramkova M., et al. (2019). Assessment of DNA damage in Polish children environmentally exposed to pesticides. Mutat. Res. Gen. Toxicol. Environ. Muta. 843 52–56. 10.1016/j.mrgentox.2018.12.012 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kaur G., Dogra N., Singh S. (2018). Health risk assessment of occupationally pesticide-exposed population of cancer prone area of Punjab. Toxicol. Sci. 165 157–169. 10.1093/toxsci/kfy140 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kaur R., Goyal D. (2019). Toxicity and degradation of the insecticide monocrotophos. Environ. Chem. Lett. 17 1299–1324. 10.1007/s10311-019-00884-y [ CrossRef ] [ Google Scholar ]
- Kaushik G., Satya S., Naik S. (2009). Food processing a tool to pesticide residue dissipation–A review. Food Res. Int. 42 26–40. 10.1016/j.foodres.2008.09.009 [ CrossRef ] [ Google Scholar ]
- Khafaga A. F., Naiel M. A., Dawood M. A., Abdel-Latif H. M. (2020). Dietary Origanum vulgare essential oil attenuates cypermethrin-induced biochemical changes, oxidative stress, histopathological alterations, apoptosis, and reduces DNA damage in Common carp ( Cyprinus carpio ). Aquat. Toxicol . 228 : 105624 . 10.1016/j.aquatox.2020.105624 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Khan M. J., Zia M. S., Qasim M. (2010). Use of pesticides and their role in environmental pollution. World Acad. Sci. Eng. Technol . 72 122–128. [ Google Scholar ]
- Khatri N., Tyagi S. (2015). Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 8 23–39. 10.1080/21553769.2014.933716 [ CrossRef ] [ Google Scholar ]
- Kim H., Kim D.-U., Lee H., Yun J., Ka J.-O. (2017). Syntrophic biodegradation of propoxur by Pseudaminobacter sp. SP1a and Nocardioides sp. SP1b isolated from agricultural soil. Int. Biodeteri. Biodegra. 118 1–9. 10.1016/j.ibiod.2017.01.024 [ CrossRef ] [ Google Scholar ]
- Koch D., Lu C., Fisker-Andersen J., Jolley L., Fenske R. A. (2002). Temporal association of children’s pesticide exposure and agricultural spraying: report of a longitudinal biological monitoring study. Environ. Health Perspect. 110 829–833. 10.1289/ehp.02110829 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koranteng S. S., Darko D. A., Nukpezah D., Ameka G. K. (2018). Pesticides bioconcentration potential of aquatic plants in the Volta Lake. West. Afr. J. App. Ecol. 26 193–202. [ Google Scholar ]
- Kori R. K., Singh M. K., Jain A. K., Yadav R. S. (2018). Neurochemical and behavioral dysfunctions in pesticide exposed farm workers: a clinical outcome. Ind. J. Clini. Biochem. 33 372–381. 10.1007/s12291-018-0791-5 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kumar M., Yadav A. N., Saxena R., Paul D., Tomar R. S. (2021). Biodiversity of pesticides degrading microbial communities and their environmental impact. Biocata. Agric. Biotechnol. 31 : 101883 . 10.1016/j.bcab.2020.101883 [ CrossRef ] [ Google Scholar ]
- Kumar S., Mukerji K., Lai R. (1996). Molecular aspects of pesticide degradation by microorganisms. Crit. Rev. Microbiol. 22 1–26. 10.3109/10408419609106454 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kuppusamy S., Palanisami T., Megharaj M., Venkateswarlu K., Naidu R. (2016). In-situ remediation approaches for the management of contaminated sites: a comprehensive overview. Rev. Environ. Contam. Toxicol. 236 1–115. 10.1007/978-3-319-20013-2_1 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lacouture A., Lafront C., Peillex C., Pelletier M., Audet-Walsh E. (2022). Impacts of endocrine-disrupting chemicals on prostate function and cancer. Environ. Res. 204 : 112085 . 10.1016/j.envres.2021.112085 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lamichhane J. R., Osdaghi E., Behlau F., Köhl J., Jones J. B., Aubertot J.-N. (2018). Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sust. Dev. 38 1–18. 10.1007/s13593-018-0503-9 [ CrossRef ] [ Google Scholar ]
- Larsen A. E., Gaines S. D., Deschênes O. (2017). Agricultural pesticide use and adverse birth outcomes in the San Joaquin Valley of California. Nat. Commun. 8 1–9. 10.1038/s41467-017-00349-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Laura M., Snchez-Salinas E., Dantn Gonzlez E., Luisa M. (2013). “ Pesticide biodegradation: Mechanisms, genetics and strategies to enhance the process, ” in Biodegradation: Life of Science , ed. Chamy R. (London: IntechOpen; ). 10.5772/56098 [ CrossRef ] [ Google Scholar ]
- Law C., Green R., Suneetha Kadiyala B. S., Knai C., Brown K. A., Dangour A. D., et al. (2019). Purchase trends of processed foods and beverages in urban India. Glob. Food Secur. 23 191–204. 10.1016/j.gfs.2019.05.007 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Leonel A. C., Bonan R. F., Pinto M. B., Kowalski L. P., Perez D. E. (2021). The pesticides use and the risk for head and neck cancer: a review of case-control studies’. Med. Oral. Patol. Oral. Cir. Bucal. 26 e56–e63. 10.4317/medoral.23962 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lerro C. C., Freeman L. E. B., DellaValle C. T., Andreotti G., Hofmann J. N., Koutros S., et al. (2021). Pesticide exposure and incident thyroid cancer among male pesticide applicators in agricultural health study. Environ. Int. 146 : 106187 . 10.1016/j.envint.2020.106187 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu Y., Mo R., Tang F., Fu Y., Guo Y. (2015). Influence of different formulations on chlorpyrifos behavior and risk assessment in bamboo forest of China. Environ. Sci. Pol. Res. 22 20245–20254. 10.1007/s11356-015-5272-2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Loh W., Tang M. L. (2018). The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 15 : 2043 . 10.3390/ijerph15092043 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- López-Pérez G. C., Arias-Estévez M., López-Periago E., Soto-González B., Cancho-Grande B., Simal-Gándara J. (2006). Dynamics of pesticides in potato crops. J. Agric. Food Chem. 54 1797–1803. 10.1016/j.envadv.2021.100114 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lourthuraj A. A., Hatshan M. R., Hussein D. S. (2022). Biocatalytic degradation of organophosphate pesticide from the wastewater and hydrolytic enzyme properties of consortium isolated from the pesticide contaminated water. Environ. Res. 205 : 112553 . 10.1016/j.envres.2021.112553 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mahna D., Puri S., Sharma S. (2021). DNA methylation modifications: mediation to stipulate pesticide toxicity. Int. J. Environ. Sci. Technol. 18 531–544. 10.1007/s13762-020-02807-9 [ CrossRef ] [ Google Scholar ]
- Main A. R., Fehr J., Liber K., Headley J. V., Peru K. M., Morrissey C. A. (2017). Reduction of neonicotinoid insecticide residues in Prairie wetlands by common wetland plants. Sci. Total Environ. 579 1193–1202. 10.1016/j.scitotenv.2016.11.102 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Malhotra H., Kaur S., Phale P. S. (2021). Conserved metabolic and evolutionary themes in microbial degradation of carbamate pesticides. Front. Microbiol. 12 : 648868 10.3389/fmicb.2021.648868 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mali H., Shah C., Rudakiya D. M., Patel D. H., Trivedi U., Subramanian R. (2022). A novel organophosphate hydrolase from Arthrobacter sp. HM01: characterization and applications. Bioresour. Technol. 349 : 126870 . 10.1016/j.biortech.2022.126870 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maqbool Z., Hussain S., Imran M., Mahmood F., Shahzad T., Ahmed Z., et al. (2016). Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: a critical review. Environ. Sci. Pol. Res. 23 16904–16925. 10.1007/s11356-016-7003-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Margni M., Rossier D., Crettaz P., Jolliet O. (2002). Life cycle impact assessment of pesticides on human health and ecosystems. Agric. Ecosyst Environ. 93 379–392. 10.1016/S0167-8809(01)00336-X [ CrossRef ] [ Google Scholar ]
- Marie L., Sylvain P., Benoit G., Maurice M., Gwenaël I. (2017). Degradation and transport of the chiral herbicide s-metolachlor at the catchment scale: combining observation scales and analytical approaches. Environ. Sci. Technol. 51 13231–13240. 10.1021/acs.est.7b02297 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Marsala R. Z., Capri E., Russo E., Bisagni M., Colla R., Lucini L., et al. (2020). First evaluation of pesticides occurrence in groundwater of Tidone Valley, an area with intensive viticulture. Sci. Tot. Environ. 736 : 139730 . 10.1016/j.scitotenv.2020.139730 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mata T. M., Martins A. A., Caetano N. S. (2010). Microalgae for biodiesel production and other applications: a review. Renew. Sust. Energ. Rev. 14 217–232. 10.1016/j.rser.2009.07.020 [ CrossRef ] [ Google Scholar ]
- Matthews D. (2005). Asthma. Child. Pest. 25 1–10. [ Google Scholar ]
- Mbah Ntepe L. J., Habib R., Judith Laure N., Raza S., Nepovimova E., Kuca K., et al. (2020). Oxidative Stress and Analysis of Selected SNPs of ACHE (rs 2571598). BCHE (rs 3495), CAT (rs 7943316), SIRT1 (rs 10823108), GSTP1 (rs 1695), and Gene GSTM1, GSTT1 in Chronic Organophosphates Exposed Groups from Cameroon and Pakistan. Int. J. Mol. Sci. 21 : 6432 . 10.3390/ijms21176432 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Meeker J. D., Barr D. B., Hauser R. (2008). Human semen quality and sperm DNA damage in relation to urinary metabolites of pyrethroid insecticides. Hum. Reprod. 23 1932–1940. 10.1093/humrep/den242 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mieldazys A., Mieldazys R., Vilkevicius G., Stulginskis A. (2015). Agriculture-Use of Pesticides/Plant Protection Products. Bilbao: EU-OSHA. [ Google Scholar ]
- Mir Z. A., Bharose R., Lone A. H., Malik Z. A. (2017). Review on phytoremediation: an eco-friendly and green technology for removal of heavy metals. Crop. Res. 52 74–82. [ Google Scholar ]
- Mishra R. K., Mohammad N., Roychoudhury N. (2015). Soil pollution: causes, effects and control. Trop. For. Res. Instruct. 3 20–30. [ Google Scholar ]
- Mishra V., Srivastava G., Prasad S. M., Abraham G. (2008). Growth, photosynthetic pigments and photosynthetic activity during seedling stage of cowpea ( Vigna unguiculata ) in response to UV-B and dimethoate. Pest. Biochem. Phys. 92 30–37. [ Google Scholar ]
- Monteiro C. M., Castro P. M. L., Malcata F. W. (2012). Metal uptake by microalgae biotechnology progress: underlying mechanisms and practical applications. Biotechnol. Prog. 28 299–311. 10.1002/btpr.1504 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Monteiro J. P., Jurado A. S. (2014). “ Methoprene ,” in Encyclopedia of Toxicology , 3rd. Edn, ed. Wexler P. (), 246–249. [ Google Scholar ]
- Montes-Grajales D., Olivero-Verbel J. (2020). Structure-based identification of endocrine disrupting pesticides targeting breast cancer proteins. Toxicology 439 : 152459 . 10.1016/j.tox.2020.152459 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Moradi M.-T., Khazaei M., Khazaei M. (2018). The effect of catalase C262T gene polymorphism in susceptibility to ovarian cancer in Kermanshah province, Western Iran. J. Obstet. Gynaecol. 38 562–566. 10.1080/01443615.2017.1381672 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Mugo H. M. (1998). Studies of Insect Pests of Pigeonpea, Cajanus cajan (l) Millsp, During the Flowering and Post-Flowering Stages and their Impact on Seed Yield in Kenya. Kenya: University of Nairobi [ Google Scholar ]
- Muralidhara M., Mithyantha S., Rajendran T., Banerjee K. (2022). Regulatory landscape of risk assessment of pesticide residues in processed foods in India: a perspective. J. Food Sci. Technol. 1–11. 10.1007/s13197-022-05388-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Murthy G. P., Prasad G. M., Sudarshana M. (2005). Toxicity of different imbibition periods of dimethoate on germination, chlorophyll a/b and dry matter of Glycine max (L) Merrill. cv. KHSB-2, during early seedling growth. J. Phytol. Res. 18 199–201. [ Google Scholar ]
- Murthy K. S., Kiran B., Venkateshwarlu M. (2013). A review on toxicity of pesticides in Fish. Int. J. Open Sci. Res. 1 15–36. [ Google Scholar ]
- Muthukumaravel K., Sivakumar B., Kumarasamy P., Govindarajan M. (2013). Studies on the toxicity of pesticide monocrotophos on the biochemical constituents of the freshwater fish Labeo rohita . Int. J. Curr. Biochem. Biotechnol. 2 20–26. [ Google Scholar ]
- Nandhini A., Muthukumar H., Gummadi S. N. (2021). Chlorpyrifos in environment and foods: a critical review of detection methods and degradation pathways. Environ. Sci. Pro. Imp. 23 , 1255–1277. 10.1371/journal.pone.0038137 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nascimento F. D. A., Pedroso T. M. A., Ramos J. S. A., Parise M. R. (2022). Farmers exposed to pesticides have almost five times more DNA damage: a meta-analysis study. Environ. Sci. Pol. Res. 29 805–816. 10.1007/s11356-021-15573-z [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ndlovu V., Dalvie M. A., Jeebhay M. F. (2011). Pesticides and the airways-a review of the literature: allergies in the workplace. Curr. Allergy Clin. Immun. 24 212–217. [ Google Scholar ]
- Nicolopoulou-Stamati P., Maipas S., Kotampasi C., Stamatis P., Hens L. (2016). Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front. Public Health. 4 : 148 . 10.3389/fpubh.2016.00148 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Nikel P. I., Martínez-García E., De Lorenzo V. (2014). Biotechnological domestication of Pseudomonads using synthetic biology. Nat. Rev. Microbiol . 12 368–379. [ PubMed ] [ Google Scholar ]
- NIPHM (2018). Pesticide management division, syllabus, pesticide classification on use, chemical nature, formulation toxicity and action etc. Hyderabad: NIPHM, 1–17. [ Google Scholar ]
- OECD (2008). Magnitude of the pesticide residues in processed commodities, OECD guidelines for the testing of chemicals, section 5. OECD Test Guideline No. 508. Paris: OECD Publishing. [ Google Scholar ]
- Obiri S., Cobbina S. J., Armah F. A., Luginaah I. (2013). Assessment of cancer and noncancer health risks from exposure to PAHs in street dust in the Tamale Metropolis, Ghana. J. Environ. Sci. Health Part A 48 408–416. 10.1080/10934529.2013.728914 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ortiz-Hernández M. L., Sánchez-Salinas E., Dantán-González E., Castrejón-Godínez M. L. (2013). Pesticide biodegradation: mechanisms, genetics and strategies to enhance the process. Biodegr. Life Sci. 251–287. 10.5772/56098 [ CrossRef ] [ Google Scholar ]
- Osteen C. D., Fernandez-Cornejo J. (2013). Economic and policy issues of US agricultural pesticide use trends. Pest. Manag. Sci. 69 1001–1025. 10.1002/ps.3529 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Panigrahi A. K., Choudhury N., Tarafdar J. (2014). Pollutional impact of some selective agricultural pesticides on fish Cyprinus carpio . IMPACT 2 71–76. [ Google Scholar ]
- Parakhia M. V., Tomar R. S., Vadukia M. R., Malviya B. J., Rathod V. M., Thakkar J. R., et al. (2014). Draft genome sequence of the methyl parathion (pesticide) degrading bacterium Pseudomonas spp. MR3. Ind. J. Microbiol. 54 120–121. 10.1007/s12088-013-0433-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pardo L. A., Beane Freeman L. E., Lerro C. C., Andreotti G., Hofmann J. N., Parks C. G., et al. (2020). Pesticide exposure and risk of aggressive prostate cancer among private pesticide applicators. Environ. Health 19 1–12. 10.1186/s12940-020-00583-0 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Paredes-Céspedes D. M., Herrera-Moreno J. F., Bernal-Hernández Y. Y., Medina-Díaz I. M., Salazar A. M., Ostrosky-Wegman P., et al. (2019). Pesticide exposure modifies DNA methylation of coding region of WRAP53α, an antisense sequence of p53, in a Mexican population. Chem. Res. Toxicol. 32 1441–1448. 10.1021/acs.chemrestox.9b00153 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Parra-Arroyo L., González-González R. B., Castillo-Zacarías C., Martínez E. M. M., Sosa-Hernández J. E., Bilal M., et al. (2022). Highly hazardous pesticides and related pollutants: toxicological, regulatory, and analytical aspects. Sci. Total Environ. 807 : 151879 . 10.1016/j.scitotenv.2021.151879 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patel O., Syamlal G., Henneberger P. K., Alarcon W. A., Mazurek J. M. (2018). Pesticide use, allergic rhinitis, and asthma among US farm operators. J. Agromed. 23 327–335. 10.1080/1059924X.2018.1501451 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Patil K., Matsumura F., Boush G. (1970). Degradation of endrin, aldrin, and DDT by soil microorganisms. Appl. Microbiol. 19 879–881. 10.1128/am.19.5.879-881.1970 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pattanasupong A., Nagase H., Sugimoto E., Hori Y., Hirata K., Tani K., et al. (2004). Degradation of carbendazim and 2, 4-dichlorophenoxyacetic acid by immobilized consortium on loofa sponge. J. Biosci. Bioeng. 98 28–33. 10.1016/S1389-1723(04)70238-8 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Paul K. C., Chuang Y.-H., Cockburn M., Bronstein J. M., Horvath S., Ritz B. (2018). Organophosphate pesticide exposure and differential genome-wide DNA methylation. Sci. Total Environ. 645 1135–1143. 10.1016/j.scitotenv.2018.07.143 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pereira V. J., da Cunha J. P. A. R., de Morais T. P., Ribeiro-Oliveira J. P., de Morais J. B. (2016). Physical-chemical properties of pesticides: concepts, applications, and interactions with the environment. Biosci. J. 32 627–641. [ Google Scholar ]
- Perez-Lucas G., Vela N., El Aatik A., Navarro S. (2018). “ Environmental risk of groundwater pollution by pesticide leaching through the soil profile ,” in Pesticides, Anthropogenic Activities and the Health of our Environment , (London: IntechOpen; ), 10.5772/intechopen.82418 [ CrossRef ] [ Google Scholar ]
- Pignatello J. J., Xing B. (1995). Mechanisms of slow sorption of organic chemicals to natural particles. Environ. Sci. Technol. 30 1–11. 10.1021/es940683g [ CrossRef ] [ Google Scholar ]
- Pimpao C., Zampronio A., De Assis H. S. (2007). Effects of deltamethrin on hematological parameters and enzymatic activity in Ancistrus multispinis (Pisces, Teleostei). Pest. Biochem. Phys. 88 122–127. 10.1016/j.pestbp.2006.10.002 [ CrossRef ] [ Google Scholar ]
- Pizzorno J. (2018). Environmental toxins and infertility. Integr. Med. 17 : 8 . [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pokhrel B., Gong P., Wang X., Chen M., Wang C., Gao S. (2018). Distribution, sources, and air–soil exchange of OCPs, PCBs and PAHs in urban soils of Nepal. Chemo 200 532–541. 10.1016/j.chemosphere.2018.01.119 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pozo K., Harner T., Lee S. C., Sinha R. K., Sengupta B., Loewen M., et al. (2011). Assessing seasonal and spatial trends of persistent organic pollutants (POPs) in Indian agricultural regions using PUF disk passive air samplers. Environ. Pol. 159 646–653. 10.1016/j.envpol.2010.09.025 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Pradeep V., Subbaiah U. M. (2016). Use of Ca-alginate immobilized Pseudomonas aeruginosa for repeated batch and continuous degradation of Endosulfan. 3 Bio 6 1–13. 10.1007/s13205-016-0438-2 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Qin F., Gao Y. X., Guo B. Y., Xu P., Li J. Z., Wang H. L. (2014). Environmental behavior of benalaxyl and furalaxyl enantiomers in agricultural soils. J. Environ. Sci. Health Part B 49 738–746. 10.1080/03601234.2014.929482 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Qu C., Albanese S., Lima A., Hope D., Pond P., Fortelli A., et al. (2019). The occurrence of OCPs, PCBs, and PAHs in the soil, air, and bulk deposition of the Naples metropolitan area, southern Italy: implications for sources and environmental processes. Environ. Int. 124 89–97. 10.1016/j.envint.2018.12.031 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Radwan M., Jurewicz J., Wielgomas B., Piskunowicz M., Sobala W., Radwan P., et al. (2015). The association between environmental exposure to pyrethroids and sperm aneuploidy. Chemo 128 42–48. 10.1016/j.chemosphere.2014.12.077 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rahman M. A., Arefin A. S., Saha O., Rahaman M. M. (2018). Isolation and identification of pesticides degrading bacteria from farmland soil. Bang. J. Microbiol. 35 90–94. 10.3329/bjm.v35i2.42635 [ CrossRef ] [ Google Scholar ]
- Ramaseshadri P., Farkaš R., Palli S. R. (2012). Recent progress in juvenile hormone analogs (JHA) research. Adv. Ins. Phys. 43 353–436. [ Google Scholar ]
- Rani K., Dhania G. (2014). Bioremediation and biodegradation of pesticide from contaminated soil and water - a noval approach. Int. J. Curr. Microbiol. App. Sci. 3 23–33. [ Google Scholar ]
- Rath B. (2012). Microalgal bioremediation: current practices and perspectives. J. Biochem. Technol. 3 299–304. [ Google Scholar ]
- Rehim H., Hegazy E., El-Barbary A. (2009). Radiation modification of natural polysaccharides for applacation in agriculture. Poly. 50 1952–1957. [ Google Scholar ]
- Requena M., López-Villén A., Hernández A. F., Parrón T., Navarro Á, Alarcón R. (2019). Environmental exposure to pesticides and risk of thyroid diseases. Toxicol. Lett. 315 55–63. 10.1016/j.toxlet.2019.08.017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rezania S., Park J., Din M. F. M., Taib S. M., Talaiekhozani A., Yadav K. K., et al. (2018). Microplastics pollution in different aquatic environments and biota: a review of recent studies. Mar. Pol. Bull. 133 191–208. 10.1016/j.marpolbul.2018.05.022 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rigas F., Dritsa V., Marchant R., Papadopoulou K., Avramides E., Hatzianestis I. (2005). Biodegradation of lindane by Pleurotus ostreatus via central composite design. Environ. Int. 31 , 191–196. 10.1016/j.envint.2004.09.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rios F., Kalinin A., Rantin F. (2002). The effects of long-term food deprivation on respiration and haematology of the neotropical fish Hoplias malabaricus . J. Fish Biol. 61 85–95. 10.1111/j.1095-8649.2002.tb01738.x [ CrossRef ] [ Google Scholar ]
- Rissato S. R., Galhiane M. S., Fernandes J. R., Gerenutti M., Gomes H. M., Ribeiro R., et al. (2015). Evaluation of Ricinus communis L. for the phytoremediation of polluted soil with organochlorine pesticides. Biomed. Res. Int. 8 : 549863 . 10.1155/2015/549863 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Roberts T. R. (1984). Non-extractable pesticide residues in soils and plants. Pur. Appl. Chem. 56 945–956. 10.1351/pac198456070945 [ CrossRef ] [ Google Scholar ]
- Romero-Aguilar M., Tovar-Sánchez E., Sánchez-Salinas E., Mussali-Galante P., Sánchez-Meza J. C., Castrejón-Godínez M. L., et al. (2014). Penicillium sp. as an organism that degrades endosulfan and reduces its genotoxic effects. Springer Plus 3 1–11. 10.1186/2193-1801-3-536 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rosell G., Quero C., Coll J., Guerrero A. (2008). Biorational insecticides in pest management. J. Pest. Sci. 33 103–121. 10.1584/jpestics.R08-01 [ CrossRef ] [ Google Scholar ]
- Różański L. (1992). Przemiany pestycydów w organizmach żywych i środowisku. Warszawa: PWRiL. [ Google Scholar ]
- Russo F., Ceci A., Pinzari F., Siciliano A., Guida M., Malusà E., et al. (2019). Bioremediation of dichlorodiphenyltrichloroethane (DDT)-contaminated agricultural soils: potential of two autochthonous saprotrophic fungal strains. Appl. Environ. Microbiol. 85 e1720–e1719. 10.1128/AEM.01720-19 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sagar V., Singh D. P. (2011). Biodegradation of lindane pesticide by non white-rots soil fungus Fusarium sp. World J. Microbiol. Biotechnol. 27 1747–1754. 10.1007/s11274-010-0628-8 [ CrossRef ] [ Google Scholar ]
- Salazar-Arredondo E., De Jesús Solís-Heredia M., Rojas-García E., Hernández-Ochoa I., Quintanilla-Vega B. (2008). Sperm chromatin alteration and DNA damage by methyl-parathion, chlorpyrifos and diazinon and their oxon metabolites in human spermatozoa. Reproduct. Toxicol. 25 455–460. 10.1016/j.reprotox.2008.05.055 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Salome C., Marks G., Savides P., Xuan W., Woolcock A. (2000). The effect of insecticide aerosols on lung function, airway responsiveness and symptoms in asthmatic subjects. Eur. Respir. J. 16 38–43. 10.1034/j.1399-3003.2000.16a07.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saquib Q., Al-Khedhairy A. A., Alarifi S. A., Dutta S., Dasgupta S., Musarrat J. (2010). Methyl thiophanate as a DNA minor groove binder produces MT–Cu (II)–DNA ternary complex preferably with AT rich region for initiation of DNA damage. Int. J. Bio. Macromol. 47 68–75. 10.1016/j.ijbiomac.2010.03.017 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Saravanan A., Kumar P. S., Jeevanantham S., Harikumar P., Bhuvaneswari V., Indraganti S. (2022). Identification and sequencing of bacteria from crop field: Application of bacteria—agro-waste biosorbent for rapid pesticide removal. Environ. Technol. Innovat. 25 : 102116 . 10.1016/j.eti.2021.102116 [ CrossRef ] [ Google Scholar ]
- Scholz R., Herrmann M., Michalski B. (2017). Compilation of processing factors and evaluation of quality controlled data of food processing studies. J. Consum. Protect. Food Saf. 12 3–14. 10.1007/s00003-016-1043-3 [ CrossRef ] [ Google Scholar ]
- Schreinemachers P., Afari-Sefa V., Heng C. H., Dung P. T. M., Praneetvatakul S., Srinivasan R. (2015). Safe and sustainable crop protection in Southeast Asia: status, challenges and policy options. Environ. Sci. Pol. 54 357–366. 10.1016/J.ENVSCI.2015.07.017 [ CrossRef ] [ Google Scholar ]
- Schreinemachers P., Tipraqsa P. (2012). Agricultural pesticides and land use intensification in high, middle and low income countries. Food Pol. 37 616–626. 10.1016/j.foodpol.2012.06.003 [ CrossRef ] [ Google Scholar ]
- Shahzad B., Tanveer M., Che Z., Rehman A., Cheema S. A., Sharma A., et al. (2018). Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: a review. Ecotoxicol. Environ. Saf 147 935–944. 10.1016/j.ecoenv.2017.09.066 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharbidre A. A., Metkari V., Ka Patode P. (2011). Effect of diazinon on acetylcholinesterase activity and lipid. Res. J. Environ. Toxicol. 5 152–161. 10.3923/rject.2011.152.161 [ CrossRef ] [ Google Scholar ]
- Sharma A., Kumar V., Kanwar M., Thukral A., Bhardwaj R. (2017a). Ameliorating imidacloprid induced oxidative stress by 24-epibrassinolide in Brassica juncea L. Rus. J. Plant Phys. 64 509–517. 10.1134/S1021443717040124 [ CrossRef ] [ Google Scholar ]
- Sharma A., Thakur S., Kumar V., Kesavan A. K., Thukral A. K., Bhardwaj R. (2017b). 24-epibrassinolide stimulates imidacloprid detoxification by modulating the gene expression of Brassica juncea L. BMC Plant Biol. 17 : 56 . 10.1186/s12870-017-1003-9 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharma A., Kumar V., Bhardwaj R., Thukral A. K. (2017c). Seed pre-soaking with 24-epibrassinolide reduces the imidacloprid pesticide residues in green pods of Brassica juncea L. Toxicol. Environ. Chem. 99 95–103. 10.1080/02772248.2016.1146955 [ CrossRef ] [ Google Scholar ]
- Sharma A., Kumar V., Kumar R., Shahzad B., Thukral A. K., Bhardwaj R. (2018a). Brassinosteroid-mediated pesticide detoxification in plants: a mini-review. Cogn. Food Agric . 4 : 1436212 . 10.1080/23311932.2018.1436212 [ CrossRef ] [ Google Scholar ]
- Sharma A., Kumar V., Yuan H., Kanwar M. K., Bhardwaj R., Thukral A. K., et al. (2018b). Jasmonic acid seed treatment stimulates insecticide detoxification in Brassica juncea L. Front. Plant Sci. 9 : 1609 . 10.3389/fpls.2018.01609 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharma A., Kumar V., Shahzad B., Tanveer M., Sidhu G. P. S., Handa N., et al. (2019). Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 1 1–16. 10.1007/s42452-019-1485-1 [ CrossRef ] [ Google Scholar ]
- Sharma A., Kumar V., Singh R., Thukral A. K., Bhardwaj R. (2016a). Effect of seed pre-soaking with 24-epibrassinolide on growth and photosynthetic parameters of Brassica juncea L. in imidacloprid soil. Ecotoxicol. Environ. Saf. 133 195–201. 10.1016/j.ecoenv.2016.07.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharma A., Bhardwaj R., Kumar V., Thukral A. K. (2016b). GC-MS studies reveal stimulated pesticide detoxification by brassinolide application in Brassica juncea L. plants. Environ. Sci. Pol. Res. 23 14518–14525. 10.1007/s11356-016-6650-0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharma A., Thakur S., Kumar V., Kanwar M. K., Kesavan A. K., Thukral A. K., et al. (2016c). Pre-sowing seed treatment with 24-epibrassinolide ameliorates pesticide stress in Brassica juncea L. through the modulation of stress markers. Front. Plant Sci. 7 : 1569 . 10.3389/fpls.2016.01569 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Sharma I., Bhardwaj R., Pati P. K. (2015). Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J. Plant Growth Regul. 34 509–518. [ Google Scholar ]
- Sharples C. R., Hull M. R., Cobb A. H. (1997). Growth and photosynthetic characteristics of two biotypes of the weed black-grass ( Alopecurus myosuroides Huds.) resistant and susceptible to the herbicide chlorotoluron. Ann. Bot. 79 455–461. [ Google Scholar ]
- Siddique T., Okeke B. C., Arshad M., Frankenberger W. T. (2003). Enrichment and isolation of endosulfan-degrading microorganisms. J. Environ. Qual. 32 47–54. 10.2134/jeq2003.4700 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Silva V., Mol H. G., Zomer P., Tienstra M., Ritsema C. J., Geissen V. (2019). Pesticide residues in European agricultural soils–A hidden reality unfolded. Sci. Total. Environ. 653 1532–1545. 10.1016/j.scitotenv.2018.10.441 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Singh B. K., Kuhad R. C., Singh A., Lal R., Tripathi K. (1999). Biochemical and molecular basis of pesticide degradation by microorganisms. Crit. Rev. Biotechnol. 19 197–225. 10.1080/0738-859991229242 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Singh B. K., Walker A. (2006). Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 30 428–471. 10.1111/j.1574-6976.2006.00018.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Singh D. V., Ali R., Kulsum M., Bhat R. A. (2020). “ Ecofriendly approaches for remediation of pesticides in contaminated environs ,” in Bioremediation and Biotechnology , eds Bhat R., Hakeem K., Saud Al-Saud N. (Cham: Springer; ), 10.1007/978-3-030-46075-4_8 [ CrossRef ] [ Google Scholar ]
- Singh J. S., Pandey V. C., Singh D. P. (2011). Efficient soil microorganisms: a new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 140 339–353. 10.1016/j.agee.2011.01.017 [ CrossRef ] [ Google Scholar ]
- Sniegowski K., Springael D. (2015). Establishment of multiple pesticide biodegradation capacities from pesticide-primed materials in on-farm biopurification system microcosms treating complex pesticide-contaminated wastewater. Pest Manag. Sci. 71 986–995. 10.1002/ps.3876 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Socorro J., Durand A., Temime-Roussel B., Gligorovski S., Wortham H., Quivet E. (2016). The persistence of pesticides in atmospheric particulate phase: An emerging air quality issue. Sci. Rep. 6 1–7. 10.1038/srep33456 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Souto A. L., Sylvestre M., Tölke E. D., Tavares J. F., Barbosa-Filho J. M., Cebrián-Torrejón G. (2021). Plant-derived pesticides as an alternative to pest management and sustainable agricultural production: prospects, applications and challenges. Molecules 26 : 4835 . 10.3390/molecules26164835 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Steffens K., Larsbo M., Moeys J., Jarvis N., Lewan E. (2013). Predicting pesticide leaching under climate change: Importance of model structure and parameter uncertainty. Agri. Ecol. Environ. 172 24–34. 10.1016/j.agee.2013.03.018 [ CrossRef ] [ Google Scholar ]
- Stehle S., Schulz R. (2015). Agricultural insecticides threaten surface waters at the global scale. Proc. Natl. Acad. Sci. 112 5750–5755. 10.1073/pnas.1500232112 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Subashchandrabose S. R., Ramakrishnan B., Megharaj M., Venkateswarlu K., Naidu R. (2011). Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol. Adv. 29 896–907. 10.1016/j.biotechadv.2011.07.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Subashini H. D., Sekar S., Devi V. R. S., Rajam A., Malarvannan S. (2007). Biodegradation of pesticidal residue using traditional plants with medicinal properties and Trichoderma . Res. J. Environ. Toxicol. 1 124–130. 10.3923/rjet.2007.124.130 [ CrossRef ] [ Google Scholar ]
- Tahir R., Ghaffar A., Abbas G., Turabi T. H., Kausar S., Xiaoxia D., et al. (2021). Pesticide induced hematological, biochemical and genotoxic changes in fish: a review. Agrobiol. Rec . 3 41–57. [ Google Scholar ]
- Tallur P. N., Mulla S. I., Megadi V. B., Talwar M. P., Ninnekar H. Z. (2015). Biodegradation of cypermethrin by immobilized cells of Micrococcus sp. strain CPN 1. Braz. J. Microbiol. 46 667–672. 10.1590/S1517-838246320130557 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tao M., Zhang G., Pan J., Xiong C. (2016). Deciphering the groove binding modes of tau-fluvalinate and flumethrin with calf thymus DNA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 155 28–37. 10.1016/j.saa.2015.11.006 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tao M., Zhang G., Xiong C., Pan J. (2015). Characterization of the interaction between resmethrin and calf thymus DNA in vitro. New J. Chem. 39 3665–3674. [ Google Scholar ]
- Thakur S., Dhiman M., Mantha A. K. (2018). APE1 modulates cellular responses to organophosphate pesticide-induced oxidative damage in non-small cell lung carcinoma A549 cells. Mol. Cell Biochem. 441 201–216. 10.1007/s11010-017-3186-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Thomas P., Dong J. (2019). Novel mechanism of endocrine disruption by fungicides through binding to the membrane androgen receptor, ZIP9 (SLC39A9), and antagonizing rapid testosterone induction of the intrinsic apoptotic pathway. Steroids 149 : 108415 . 10.1016/j.steroids.2019.05.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tiedje J. M., Duxbury J., Alexander M., Dawson J. (1969). 2, 4-D metabolism: pathway of degradation of chlorocatechols by Arthrobacter sp. J. Agric. Food Chem. 17 1021–1026. 10.1021/jf60165a037 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tomer N. (2013). Determination of chlorinated pesticide in vegetables, cereals and pulses by gas chromatography in east national capital region, Delhi, India. Res. J. Agric. Sci. 1 27–28. [ Google Scholar ]
- Torres E. M., Hess D., McNeil B. T., Guy T., Quinn J. C. (2017). Impact of inorganic contaminants on microalgae productivity and bioremediation potential. Ecotoxicol. Environ. Saf. 139 367–376. 10.1016/j.ecoenv.2017.01.034 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tort N., Turkyilmaz B. (2003). Physiological effects of captan fungicide on pepper ( Capsicum annuum L.) plant. Pak. J. Bio. Sci. 6 2026–2029. 10.3923/pjbs.2003.2026.2029 [ CrossRef ] [ Google Scholar ]
- Tortella G. R., Diez M. C., Durán N. (2005). Fungal diversity and use in decomposition of environmental pollutants. Crit. Rev. Microbiol 31 197–212. 10.1080/10408410500304066 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tudi M., Daniel Ruan H., Wang L., Lyu J., Sadler R., Connell D., et al. (2021). Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 18 : 1112 . 10.3390/ijerph18031112 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Ullah S., Hasan Z., Zuberi A., Younus N., Rauf S. (2014). Comparative study on body composition of two chinese carps, common carp ( Cyprinus carpio ) and silver carp ( Hypophthalmichthys molitrix ). Glob. Vet. 13 867–876. 10.5829/idosi.gv.2014.13.05.86234 [ CrossRef ] [ Google Scholar ]
- United States. Environmental Protection Agency (2004). Overview of the Ecological Risk Assessment Process in the Office of Pesticide Programs, US Environmental Protection Agency: Endangered and Threatened Species Effects Determinations. Collingdale, PA: DIANE Pub. [ Google Scholar ]
- Unsworth J. (2010). History of pesticide use. International Union of pure and applied chemistry (IUPAC). North Carolina: IUPAC [ Google Scholar ]
- United States Environmental Protection Agency [US-EPA] (2022). Pesticide to Control Bed Bug. Available online at: https://www.epa.gov/bedbugs/pesticides-control-bed-bugs#desiccants (accessed April 4, 2022). [ Google Scholar ]
- Van der Plaat D. A., de Jong K., de Vries M., van Diemen C. C., Nedeljković I., Amin N., et al. (2018). Occupational exposure to pesticides is associated with differential DNA methylation . Occup. Environ. Med. 75 , 427–435. 10.1136/oemed-2017-104787 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Van Nieuwenhuyse P., Demaeght P., Dermauw W., Khalighi M., Stevens C., Vanholme B., et al. (2012). On the mode of action of bifenazate: new evidence for a mitochondrial target site. Pest. Biochem. Phys. 104 88–95. 10.1016/j.pestbp.2012.05.013 [ CrossRef ] [ Google Scholar ]
- Velasquez L., Dussan J. (2009). Biosorption and bioaccumulation of heavy metals on dead and living biomass of Bacillus sphaericus . J. Haz. Mater. 167 713–716. 10.1016/j.jhazmat.2009.01.044 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wagh V., Mukate S., Muley A., Kadam A., Panaskar D., Varade A. (2020). Study of groundwater contamination and drinking suitability in basaltic terrain of Maharashtra, India through PIG and multivariate statistical techniques. J. Water Sup. Res. Technol. Aquat. 69 398–414. 10.2166/aqua.2020.108 [ CrossRef ] [ Google Scholar ]
- Wang H., Meng Z., Liu F., Zhou L., Su M., Meng Y., et al. (2020). Characterization of boscalid-induced oxidative stress and neurodevelopmental toxicity in zebrafish embryos. Chem. 238 : 124753 . 10.1016/j.chemosphere.2019.124753 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Warren G. (1998). Spectacular increases in crop yields in the United States in the twentieth century. Weed Technol. 12 752–760. [ Google Scholar ]
- Williams G. P., Darbre P. D. (2019). Low-dose environmental endocrine disruptors, increase aromatase activity, estradiol biosynthesis and cell proliferation in human breast cells. Mol. Cell Endocrinol. 486 55–64. 10.1016/j.mce.2019.02.016 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Winteringham F. (1971). Some global aspects of pesticide residue problems. Isr. J. Entomol. 6 171–181. [ Google Scholar ]
- Witczak A., Pohoryło A., Abdel-Gawad H. (2021). Endocrine-disrupting organochlorine pesticides in human breast milk: changes during lactation. Nutrition. 13 : 229 . 10.3390/nu13010229 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Woodrow J. E., Gibson K. A., Seiber J. N. (2018). Pesticides and related toxicants in the atmosphere. Rev. Environ. Conta. Toxicol. 247 147–196. 10.1007/398_2018_19 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- World Health Organization (2009). Children’s Health and the Environment. WHO Training Package for the Health Sector-World Health Organization. Geneva: World Health Organization [ Google Scholar ]
- World Health Organization (2015). International Code of Conduct on Pesticide Management: Guidelines on Pesticide Legislation. Geneva: World Health Organization [ Google Scholar ]
- Wu S., Zhang X., Sun Y., Wu Z., Li T., Hu Y., et al. (2015). Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM–EDS. TEM–EDS, and XAFS. Environ. Sci. Technol. 49 14036–14047. 10.1021/acs.est.5b03659 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wu Y., Li W., Yuan M., Liu X. (2020). The synthetic pyrethroid deltamethrin impairs zebrafish ( Danio rerio ) swim bladder development. Sci. Total Environ. 701 : 134870 . 10.1016/j.scitotenv.2019.134870 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xia X. J., Huang Y. Y., Wang L., Huang L. F., Yu Y. L., Zhou Y. H., et al. (2006). Pesticides-induced depression of photosynthesis was alleviated by 24-epibrassinolide pretreatment in Cucumis sativus L. Pest. Biochem Phys. 86 42–48. 10.1016/j.pestbp.2006.01.005 [ CrossRef ] [ Google Scholar ]
- Xia X. J., Zhang Y., Wu J. X., Wang J. T., Zhou Y. H., Shi K., et al. (2009). Brassinosteroids promote metabolism of pesticides in cucumber. J. Agric. Food Chem. 57 8406–8413. 10.1021/jf901915a [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xiao P., Kondo R. (2020). Potency of Phlebia species of white rot fungi for the aerobic degradation, transformation and mineralization of lindane. J. Microbiol. 58 395–404. 10.1007/s12275-020-9492-x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Xu X., Nembhard W. N., Kan H., Becker A., Talbott E. O. (2012). Residential pesticide use is associated with children’s respiratory symptoms. J. Occup. Environ. Med. 54 1281–1287. 10.1097/JOM.0b013e31825cb6ae [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yadav I. C., Devi N. L. (2017). Pesticides classification and its impact on human and environment. Environ. Sci. Eng. 6 140–158. [ Google Scholar ]
- Yadav I. C., Devi N. L., Syed J. H., Cheng Z., Li J., Zhang G., et al. (2015). Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighboring countries: a comprehensive review of India. Sci. Total Environ. 511 123–137. 10.1016/j.scitotenv.2014.12.041 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yan S., Meng Z., Tian S., Teng M., Yan J., Jia M., et al. (2020). Neonicotinoid insecticides exposure cause amino acid metabolism disorders, lipid accumulation and oxidative stress in ICR mice. Chemo 246 : 125661 . 10.1016/j.chemosphere.2019.125661 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yanez-Ocampo G., Wong-Villarreal A., Del Aguila-Juarez P., Lugo-de la Fuente J., Vaca-Paulin R. (2016). Composting of soils polluted with pesticides: a microbial approach and methods for monitoring. JSM Environ. Sci. Ecol. 4 : 1032 . 10.1016/j.envint.2011.06.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Yengkokpam P., Mazumder P. B. (2020). Phytotoxicity of malathion (PM) and tatafen (PTF) towards Solanum melongena L. cv. Longai: a case study. Plant Phys. Rep. 25 149–156. 10.1007/s40502-019-00498-0 [ CrossRef ] [ Google Scholar ]
- Yigit N., Velioglu Y. S. (2020). Effects of processing and storage on pesticide residues in foods. Crit. Rev. Food Sci. Nutr. 60 3622–3641. 10.1080/10408398.2019.1702501 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Young H. A., Meeker J. D., Martenies S. E., Figueroa Z. I., Barr D. B., Perry M. J. (2013). Environmental exposure to pyrethroids and sperm sex chromosome disomy: a cross-sectional study. Environ. Health 12 1–9. 10.1186/1476-069X-12-111 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Želježić D., Žunec S., Bjeliš M., Benković V., Mladinić M., Lovaković Tariba B., et al. (2018). Effects of the chloro-s-triazine herbicide terbuthylazine on DNA integrity in human and mouse cells. Environ. Sci. Pol. Res. 25 19065–19081. 10.1007/s11356-018-2046-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhan H., Huang Y., Lin Z., Bhatt P., Chen S. (2020). New insights into the microbial degradation and catalytic mechanism of synthetic pyrethroids. Environ. Res. 182 : 109138 . 10.1016/j.envres.2020.109138 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang C., Schilirò T., Gea M., Bianchi S., Spinello A., Magistrato A., et al. (2020). Molecular basis for endocrine disruption by pesticides targeting aromatase and estrogen receptor. Int. J. Environ. Res. Public Health 17 : 5664 . 10.3390/ijerph17165664 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang W., Lin Z., Pang S., Bhatt P., Chen S. (2020). Insights into the biodegradation of lindane (γ-hexachlorocyclohexane) using a microbial system. Front. Microbiol . 11 : 522 . 10.3389/fmicb.2020.00522 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang J., Huang X., Liu H., Liu W., Liu J. (2018). Novel pathways of endocrine disruption through pesticides interference with human mineralocorticoid receptors. Toxicol. Sci. 162 53–63. 10.1093/toxsci/kfx244 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang W. (2018). Global pesticide use: profile, trend, cost/benefit and more. Proc. Int. Acad. Ecol. Environ. Sci. 8 : 1 . [ Google Scholar ]
- Zhang W., Li J., Zhang Y., Wu X., Zhou Z., Huang Y., et al. (2022). Characterization of a novel glyphosate-degrading bacterial species, chryseobacterium sp. Y16C, and evaluation of its effects on microbial communities in glyphosate-contaminated soil. J. Haz. Mat. 432 : 128689 . 10.1016/j.jhazmat.2022.128689 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang W., Pang S., Lin Z., Mishra S., Bhatt P., Chen S. (2021). Biotransformation of perfluoroalkyl acid precursors from various environmental systems: Advances and perspectives . Environ. Pollut. 272 : 115908 . 10.1016/j.envpol.2020.115908 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zhang W.-J., van der Werf W., Pang Y. (2011). A simulation model for vegetable-insect pest-insect nucleopolyhedrovirus epidemic system. J. Environ. Entomol. 33 283–301. [ Google Scholar ]
- Zhang Y., Pan J., Zhang G., Zhou X. (2015). Intercalation of herbicide propyzamide into DNA using acridine orange as a fluorescence probe. Sensors Actuators B Chem. 206 630–639. 10.1016/j.snb.2014.09.114 [ CrossRef ] [ Google Scholar ]
- Zhen D., Shen J. Y., Hong J. W., Zhang R., Zheng L., Qi W., et al. (2020). Inhibitory Effects of Cypermethrin on Interactions of the Androgen Receptor with Coactivators ARA70 and ARA55. Biomed. Environ. Sci. 33 158–164. 10.3967/bes2020.022 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zucca P., Sanjust E. (2014). Inorganic materials as supports for covalent enzyme immobilization: methods and mechanisms. Molecules 19 14139–14194. 10.3390/molecules190914139 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]

COMMENTS
Air pollution can have a disastrous effect on all components of the environment, including groundwater, soil, and air. Additionally, it poses a serious threat to living organisms. In this vein, our interest is mainly to focus on these pollutants, as they are related to more extensive and severe problems in human health and environmental impact.
Thus deforestation cause a chain effects which adversely affect the natural environment. North Asian International Research Journal of Social Science & Humanities ISSN: 24 54 -9827 Vol. 3, Issue 8 ...
1. Introduction. Climate change is a pervasive and growing global threat to biodiversity and ecosystems (Díaz et al., 2019).Climate change affects individual species and the way they interact with other organisms and their habitats, which alters the structure and function of ecosystems and the goods and services that natural systems provide to society (Díaz et al., 2019).
Moreover, air pollution seems to have various malign health effects in early human life, such as respiratory, cardiovascular, mental, and perinatal disorders ( 3 ), leading to infant mortality or chronic disease in adult age ( 6 ). National reports have mentioned the increased risk of morbidity and mortality ( 1 ).
To better understand the problem, gathered the information in this report from various media outlets, research agencies, policy papers, newspapers, and other sources. ... (2020) Technology transfer, climate change mitigation, and environmental patent impact on sustainability and economic growth: a comparison of European countries. Technol ...
This paper introduces a thematic issue dedicated to the interaction between climate change and the biosphere. ... often in interaction with other factors. In particular, it addressed research frontiers such as the effects of changes in climate variability and extremes; interactions among multiple stressors; thresholds and the potential for ...
Table 1: Major environmental pollutants, their sources, and impact on human health Pollutants and health effects are less studied because of the need for more information on exposure to pollutants in less developed countries, where waste management could be better, there is higher poverty, and the application of new technologies is limited The same situation is observed in many developed ...
3. Environmental effects of COVID-19. The global disruption caused by the COVID-19 has brought about several effects on the environment and climate. Due to movement restriction and a significant slowdown of social and economic activities, air quality has improved in many cities with a reduction in water pollution in different parts of the world.
Plastic pollution is a persistent challenge worldwide with the first reports evidencing its impact on the living and nonliving components of the environment dating back more than half a century. The rising concerns regarding the immediate and long-term consequences of plastic matter entrainment into foods and water cannot be overemphasized in light of our pursuit of sustainability (in terms of ...
Environmental Research is a multi-disciplinary journal publishing high quality and novel information about anthropogenic issues of global relevance and applicability in a wide range of environmental disciplines, and demonstrating …. View full aims & scope. $3590. Article publishing charge. for open access.
Abstract. Purpose: This review aims to critically analyze and summarize the existing literature on urbanization's effects on environmental sustainability. It delves deep into the nexus between ...
Climate dictates the critical aspects of human environmental conditions. The frequency and intensity of extreme weather conditions due to human-induced climate change have alarmingly increased ...
Environmental Research: Climate, Volume 1, Number 1 Climate Variability and Change: Causes, Consequences and Solutions Citation Ben Clarke et al 2022 Environ. Res.: ... The impact on vegetation more widely disrupted local wildlife, causing thousands of long-tailed monkeys to attack and steal from villages in search of food (Rohmah 2015).
We cannot solve this controversy in this paper. Instead, our research objective is to assess the total effect (i.e., direct and indirect effects) from population growth on the environment in Europe. ... Liddle B. Population, affluence, and environmental impact across development: evidence from panel cointegration modeling. Environmental ...
Water Environment Research is a multidisciplinary water and wastewater research journal, publishing fundamental and applied research related to water quality. Abstract This paper presents the reviews of scientific papers published in 2018 issues on the effects of anthropogenic pollution on the aquatic organisms dwelling in freshwater ecosystem ...
Increasing demands on ecosystems, decreasing biodiversity, and climate change are among the most pressing environmental issues of our time. As changing weather conditions are leading to increased vector-borne diseases and heat- and flood-related deaths, it is entering collective consciousness: environmental issues are human health issues. In public health, the field addressing these issues is ...
The environmental impact (GHG emissions) of declared professional expenses (transportation, accommodation and meals) is estimated based on their nature and economic value. From the diagnosis we derive a set of recommendations and action plans to support the transition towards sustainable research practices.
Abstract. Humans have actually reduced yield from ecosystem services, owing to human-induced changes to components of the Earth's biodiversity and ecosystems along with economic development ...
The International Journal of Environmental Research (IJER) is an international and multidisciplinary platform for researchers across the globe to swiftly publish, share and discuss new findings and developments in environmental science, engineering, and management.IJER is an interdisciplinary journal concerned with all aspects of the environment. These include but are not limited to air, water ...
2. Research Methods. In recent years, researchers have increasingly used quantitative and qualitative research (mixed methods) techniques to expand the scope and improve the analytic power of their studies [29,30].Quantitative research method is a statistical and interpretive technique used to describe or explain the meaning and relationships of a phenomenon under investigation.
The isoflavonoid genistein (GEN) derived from soy has been reported to have neuroprotective and antioxidant effects. The research explored the pathways for in vivo and in vitro Pb-induced neurotoxicity to guard against Pb-induced toxicity of GEN. Cell viability was decreased and cell apoptosis was increased with the exposure of the Pb. In vitro ...
radation of plastic products, "white pollution" has become. more and more serious. The large amount of disposable. plastic products and the low recycling rate have caused. serious pollution to ...
Other - research coordination duties: Kahn. Conflict of Interest Disclosures: None reported. Funding/Support: This study was funded by grants R01DK100426, CTSA-UL1 TR0002389, and UL1TR002389 from the National Institutes of Health and by the Diabetes Research and Training Center at The University of Chicago.
Fish hematological research has grown in importance as a reliable and sensitive index for assessing biological and pathological changes caused by natural or anthropogenic factors such as microbial infection or levels of contamination in aquatic sources. ... Direct and Indirect Effects of Environmental Contaminants on Amphibians. Amsterdam ...