An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List


Current challenges in metastasis research and future innovation for clinical translation
Amelia l. parker.
1 Matrix and Metastasis Lab, Kinghorn Cancer Centre, Garvin Institute of Medical Research, Darlinghurst, NSW 2010 Australia
2 St Vincent’s Clinical School, UNSW Sydney, Sydney, 2052 Australia
Madeleine Benguigui
3 Cell Biology and Cancer Science, Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, 31096 Haifa, Israel
Jaime Fornetti
4 Department of Oncological Sciences, Huntsman Cancer Institute, University of Utah, Salt Lake, UT USA
Erica Goddard
5 Public Health Sciences Division/Translational Research Program, Fred Hutchinson Cancer Research Center, Seattle, WA USA
Serena Lucotti
6 Children’s Cancer and Blood Foundation Laboratories, Departments of Pediatrics, and Cell and Developmental Biology, Drukier Institute for Children’s Health, Meyer Cancer Center, Weill Cornell Medicine, NY New York, USA
Jacob Insua-Rodríguez
7 Department of Physiology and Biophysics, Department of Biological Chemistry, Chao Family Comprehensive Cancer Centre, University of California, Irvine, CA USA
Adrian P. Wiegmans
8 Cancer and Ageing Research Program, Centre for Genomics and Personalised Health, Queensland University of Technology (QUT), Translational Research Institute, Woolloongabba, QLD 4121 Australia
Early Career Leadership Council of the Metastasis Research Society
Associated data.
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
While immense strides have been made in understanding tumor biology and in developing effective treatments that have substantially improved the prognosis of cancer patients, metastasis remains the major cause of cancer-related death. Improvements in the detection and treatment of primary tumors are contributing to a growing, detailed understanding of the dynamics of metastatic progression. Yet challenges remain in detecting metastatic dissemination prior to the establishment of overt metastases and in predicting which patients are at the highest risk of developing metastatic disease. Further improvements in understanding the mechanisms governing metastasis have great potential to inform the adaptation of existing therapies and the development of novel approaches to more effectively control metastatic disease. This article presents a forward-looking perspective on the challenges that remain in the treatment of metastasis, and the exciting emerging approaches that promise to transform the treatment of metastasis in cancer patients.
Introduction
Advances in understanding the key features of primary tumors have relied on innovation across specialized fields, culminating in improved clinical staging and patient survival rates. These advances have also enhanced our understanding of secondary tumor formation, or metastasis, that remains the major cause of cancer-related deaths. Metastasis is defined as the process in which cancer spreads from the primary tumor and establishes at anatomically distinct sites. While tremendous technology-driven advances in our understanding of the metastatic process are revealing promising targetable mechanisms, improving the outcomes of patients with metastatic disease remains a significant challenge. In this perspectives article we identify opportunities for emerging fields of investigation that have the potential to fundamentally revolutionize not only our understanding of the metastatic process, but also the way in which metastasis is treated.
Despite advances in cancer detection and treatment, residual disseminated disease remains present but undetected in a considerable proportion of patients whose primary tumor has been successfully treated. This residual disease can be present as micrometastases, defined as multicellular secondary tumor cell clusters, or as disseminated single tumor cells (DTCs) that are currently too small to detect in clinical diagnostic scans and persist as potential sources of subsequent metastatic relapse [ 1 ]. The latency period between initial diagnosis and metastatic recurrence varies between months and years and a number of models have been proposed to explain these dynamics. One predominant model is that disseminated cells undergo a period of cellular dormancy prior to awakening and giving rise to overt metastases [ 2 , 3 ]. Alternative models propose that the growth rate of disseminated cells remains relatively constant and instead it is the balance between proliferation and cell death in the disseminated cells that constrains the emergence of overt metastases until proliferation rates dominate [ 4 ]. The maturation models suggests that disseminated tumour cells must first acquire further genomic alterations that enable their overt growth at secondary sites, and this maturation process results in delayed formation of detectable secondary tumors following dissemination [ 4 ]. It is likely that latency periods and the evolution of metastatic disease results from mixtures of these models, and that the contribution of each model to patient outcome differs between tumour types, secondary sites and is influenced by multiple host factors. Highly variable latency periods present a challenge in monitoring patients for metastatic emergence. Furthermore, current diagnostic approaches lack the sensitivity to detect this minimal residual disease, and as a result, the temporal dynamics of tumor cell dissemination and the overall burden of disseminated disease remains unclear for most cancer types. Yet, while the presence of substantial micrometastatic disease is suggestive of a high risk of relapse, not all patients will develop overt metastases from these disseminated cells. Monitoring and modelling tumor latency dynamics, in particular dormancy and reawakening, using patient avatars is crucial for understanding relapse mechanisms and predicting patient populations at risk.
The processes that govern the dynamics of tumor cell dissemination from the primary site, seeding at secondary sites, and ultimately their outgrowth into overt metastases are emerging as complex, dynamic and spatially compartmentalized interactions between cancer cells and the local tissue microenvironment [ 5 – 7 ]. The additional impacts of host and environmental factors adds further complexity to the myriad regulators of tumor progression, confounding our ability to accurately predict the trajectory of each patient’s cancer and the most effective treatment. This intra- and inter-individual complexity heralds an era of precision medicine that exploits our understanding of these factors to tailor therapies that specifically target metastatic disease.
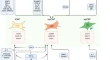
To achieve a precision medicine framework that improves the outcome of patients with metastatic disease, we must understand the collective influence of cancer cell intrinsic, tumor microenvironmental, host, and environmental factors on tumor behavior before translating this knowledge into targeted therapies. Growing evidence indicates that despite the unambivalent utility of chemotherapy in successfully treating the primary tumor and improving patient outcomes, it has been demonstrated that some chemotherapies and dosing regimes can accelerate metastatic progression in some in vivo models in a context-specific manner [ 8 , 9 ] and further study is required to determine if similar effects are seen in patients. Understanding how primary tumor treatments affect metastatic dissemination provides an opportunity to more effectively implement existing treatments to also inhibit metastasis, while also suggesting that novel therapeutic approaches may be required to specifically target metastasis. Precision medicine approaches that take into account the dynamics of metastatic latency, outgrowth and response to primary tumor therapies for those metastases that progress will revolutionize the clinical management and outcomes for cancer patients. As depicted in Fig. 1 , major challenges to realizing improved outcomes in metastatic disease can be summarized as:

Current challenges in the management of metastatic disease and areas of research addressing these challenges. Created with BioRender.com
- Detecting and quantifying the metastatic burden throughout treatment.
- Predicting which patients will develop overt metastatic disease.
- Understanding the mechanisms governing tumor metastasis.
- Developing more effective metastasis-targeted treatments.
Emerging advances overcoming these challenges have the potential to transform the clinical management of metastatic disease across cancer types (Fig. 2 ).

Emerging advances in metastasis research. Created with BioRender.com
Dynamically defining metastatic burden during treatment
Current approaches: improving pathological assessments.
In order to improve the management of metastatic disease, it is imperative to obtain an accurate picture of when metastatic dissemination occurs, and how this process defines the risk profile for individuals both at diagnosis and throughout treatment. While great gains have been made in mapping the dynamics of tumor progression in many cancer types, much work remains to create a comprehensive temporal map of metastatic progression for each tumor type. To date, advancements in accurately assessing disseminated tumor burden in patients have been hampered by the limited sensitivity of radiological diagnostic scans for micrometastatic disease. Lymph node assessment, including sentinel node mapping with pathological assessment and ultrastaging (e.g. cytokeratin staining) as a surrogate for metastatic dissemination, has instead been used as a mainstay of metastatic disease staging in surgically resected cancers [ 10 ]. However, the recent integration of deep learning image analysis algorithms, which use multiple tissue features to define the presence of cancer cells, into clinically-established pipelines are improving the sensitivity and reproducibility of pathological metastasis staging [ 11 , 12 ]. In particular, Convolutional Neural Networks (CNN) have been the most widely studied artificial intelligence (AI) architecture for segmenting pathological images to classify tumour-associated regions of interest [ 13 , 14 ]. While the large amount of data required to train CNN architectures remains a significant hurdle in developing robust algorithms, these network architectures have been used to develop the most advanced AI algorithms to improve pathological staging, such as those developed from the CAncer MEtastases in LYmph Nodes Challenge (CAMELYON) [ 15 ]. The best performing algorithms to come out of this challenge were able to diagnose positive lymph node metastases with improved accuracy and efficiently compared with a panel of 11 pathologists [ 16 ]. This has led to the next phase of challenges that test AI capability to identify metastatic tissue in histopathologic scans demonstrating a pooled sensitivity of 82%, specificity of 84% and AUC of 0.90 for identification of tumor metastasis based on a summary of 2620 studies [ 16 ]. As the morphological and biochemical characteristics of tissue sites primed for metastatic colonisation become more clearly defined, we propose that the capacity of AI to detect overt metastasis will soon transfer to the ability to detect these pre-metastatic niches with equivalent accuracy. Such improvements in current clinical diagnostic pipelines will enable the early detection of metastatic dissemination to accurately prioritize patients for the most effective treatment.
Despite these improvements, metastatic lymph node assessment, by its nature, only captures cells that are within the lymphatic system and fails to detect cancer cells that have already disseminated to distant organs. Therefore, there is a clear need to improve the sensitivity and specificity of current diagnostic scans and develop label-free technologies that together can provide a whole-body picture of micrometastatic burden to define prognosis. In this regard, cross-platform imaging technologies that extend existing radiographic and radiological imaging technology to detect micrometastatic sites are showing promise [ 17 ].
The next phase in developing AI and imaging technology is in the detection of sites primed for metastatic colonization, thereby enabling the earliest assessment of metastatic risk and the opportunity to prevent the establishment of micrometastatic disease. These sites, known as pre-metastatic niches, are regions within distant organs that, under the influence of primary tumor-derived systemic factors, are primed to support the establishment and persistence of metastatic disease [ 18 ]. These pre-metastatic niches have now been characterized in the lungs [ 19 – 21 ], liver [ 22 ], lymph nodes [ 23 ], bone marrow [ 24 ] and brain [ 19 ] in both pre-clinical models and cancer patients. While the specific features of these niches appear to be tumor- and organ-dependent, vascular leakiness, increased inflammation (e.g. TLR4 activation), alteration of the extracellular matrix (ECM), recruitment of immunosuppressive cells (including macrophages, bone marrow derived cells (BMDCs) and regulatory T cells) as well as the activation and metabolic reprogramming of resident stromal cells are all common features of the pre-metastatic niche [ 26 ]. Together, these changes shape the pre-metastatic niche to be more receptive of tumor cell settlement by enhancing nutrient availability, vessel permeability, inflammation and cancer cell migration, survival and adhesion to ECM components at these distant sites. Altered textural features in radiological scans of axillary lymph nodes and metastatic sites that reflect increased matrix deposition or tissue density for example [ 17 , 22 ], are showing promise in the detection of these pre-metastatic niches [ 25 ], as are emerging imaging agents targeted against specific features of the pre-metastatic niche, such as overexpression of the α4β1 integrin receptor [ 27 ] or the presence of specific fibronectin isoforms in the ECM [ 17 ]. For example, radiomic analysis of the liver parenchyma on presurgical CT scans has been shown to predict the future development of hepatic metastases in colon cancer patients following primary tumor resection [ 25 ].
Further progress developing these emerging technologies will facilitate the dynamic monitoring of metastatic dissemination, allowing for rapid adaptation of therapies to maximize clinical responses (Table 1 ).
Observational clinical trials to improve metastasis detection and understand risk associations
Monitoring metastatic spread during treatment response
Liquid biopsy biomarker detection is a promising complementary approach to image-based detection of metastatic disease. Liquid biopsies are derived from plasma, serum or urine, and their minimally invasive nature makes them amenable to longitudinal tracking of metastatic progression in response to therapy. Detecting circulating tumor cells (CTCs) or their DNA (circulating tumor DNA, ctDNA) in plasma and serum are currently regarded as the most direct methods for assessing disseminated tumor burden using liquid biopsies. The number of CTCs or amount of ctDNA detected after therapy are robust readouts for treatment efficacy and with their superior sensitivity, enable the detection of relapse many months earlier than current radiological imaging procedures allow [ 28 ].
Despite the promise of monitoring CTCs as a direct measure of tumor cell dissemination [ 29 ], clinical implementation of CTC biomarkers as decision-making tools has been hampered by the scarcity of CTCs in blood specimens, a lack of standardized cell isolation approaches and inadequate sensitivity. CELLSEARCH TM , currently the only FDA-approved molecular pathology assay to detect CTCs, has overcome sensitivity limitations by implementing an EpCAM-positivity CTC enrichment step followed by an imaging-based tumor cell detection using cytokeratin and nuclear staining [ 30 , 31 ]. Although EpCAM+ enrichment and cytokeratin staining is a standard approach in a research setting, growing evidence indicates that EpCAM enrichment likely captures a subset of epithelial tumor cells and may not capture those tumor cells that have undergone a mesenchymal transition during metastatic dissemination [ 31 ], thereby limiting the assay’s applicability to specific epithelial cancers. Such approaches to standardize the isolation and enrichment of CTCs will provide insight into the dynamics of tumor cell dissemination as well as the opportunity to identify pro-metastatic features of cancer cells that can be therapeutically targeted [ 32 , 33 ].
Comparatively, ctDNA biomarker development has leveraged the sequencing revolution to demonstrate superior sensitivity and specificity compared with CTC analysis [ 29 ]. CtDNA markers that show considerable promise for clinical translation are mapped to mutated DNA regions corresponding to prevalent cancer drivers and are therefore cancer-type specific. These include mutant adenomatous polyposis coli ( APC ), epidermal growth factor receptor ( EGFR ) and Kirsten rat sarcoma virus ( KRAS ) DNA in the plasma of colorectal cancer patients as indicators of response to conventional and EGFR-targeted therapies, respectively, which are being developed for clinical use [ 34 , 35 ]. More recently, digital drop PCR pre-amplification and fluorescent probes have been developed into a promising standardized assay that detects ctDNA derived from mutant histone H3-genes in pediatric diffuse midline glioma patients, a tumor type that is generally not surgically accessible [ 36 ]. While these highly specific single-gene approaches are valuable for monitoring targeted therapy response, a panel of multiple ctDNA gene targets will be required to capture the burden of DTCs derived from diverse tumor types and highly clonal, genetically heterogeneous tumors. Multi-gene profiling of ctDNA in plasma samples, such as that implemented by MSK-ACCESS (MSK-Analysis of Circulating Cell-free DNA to Evaluate Somatic Status), overcomes the cancer- and subclone-specific nature of these targeted approaches to provide a potential multi-cancer or clonal diagnostic to guide clinical decision making [ 37 ]. The application of existing technology to different cell types in liquid biopsies also has the potential to improve ctDNA biomarker performance in detecting disseminated cancer cells derived from heterogeneous tumors. For example, comparative deep sequencing of ctDNA and matched healthy hematopoietic cell DNA from patients with highly clonal lung tumors was more accurate in predicting patient prognosis compared to ctDNA analysis alone [ 38 ]. In addition, combining ctDNA biomarkers with existing imaging modalities, such as specialized positron emission tomography - computed tomography (PET–CT), can improve the sensitivity and specificity of micrometastasis detection [ 38 ], suggesting that a multifaceted approach may achieve clinical benefit in the near term.
While ctDNA analysis is a promising technology for indirectly detecting DTCs, it cannot indicate their discrete anatomical location, thereby limiting its application as a stand-alone diagnostic tool. Fragmentomics and epigenetic profiling of ctDNA are emerging fields of investigation that have the potential to overcome these limitations by enabling identification of the ctDNA organ of origin, and therefore the primary tumor location [ 39 , 40 ]. Fragmentomics analysis is founded on the principles that DNA fragmentation occurs in a tissue-specific manner due to the influence of nucleosomal organization, chromatin structure, gene expression, and nuclease content of the tissue of origin, resulting in characteristic organ-specific signatures of ctDNA fragment size, nucleotide motifs at the fragment ends, and the genomic locations of the fragmentation endpoints [ 40 ]. Similarly, the detection of aberrant epigenetic methylation patterns, which are more prevalent and penetrant than genetic mutations in ctDNA, provides a more sensitive detection of ctDNA than mutational analysis alone, and also indicates tissue of origin [ 39 ]. However, methylome analysis is not yet capable of indicating the location of metastatic sites. With over 20 currently active clinical trials evaluating ctDNA methylation in the diagnosis and monitoring of various cancers (as of April 2021), the development of these ctDNA detection approaches holds immense promise not only in monitoring metastatic dissemination but also in cancer diagnosis.
The monitoring of circulating protein markers and extracellular vesicles derived from cancer cells are also being developed as markers of tumor burden and as surrogates for metastatic disease in tumors that have been surgically resected, for example, CA19-9 in pancreatic cancer [ 41 ]. However, circulating levels of primary tumor markers alone are not always predictive of metastatic prognosis, such as biochemical recurrence in prostate cancer as indicated by PSA levels [ 42 ]. By specifically detecting secreted factors and extracellular vesicles derived from micrometastatic sites or those that play a critical role in priming distant pre-metastatic niches [ 20 , 22 , 24 , 43 – 45 ] it may be possible to monitor for metastatic propensity and likely secondary sites at earlier stages of recurence. For example, integrin signatures of tumor-derived exosomes orchestrate tumor cell organotropism [ 46 ] and, together with other exosomal protein and miRNA cargos, educate resident cells such as Kupffer cells, BMDCs, fibroblasts and endothelial cells to promote metastasis [ 19 , 22 , 44 – 46 ]. Importantly, high levels of exosomal proteins and miRNAs involved in pre-malignant niche establishment correlate with poor prognosis and higher risk of metastatic disease [ 19 , 22 , 44 – 46 ]. Therefore, the use of metastatis-specific protein- and RNA-based biomarkers may leverage existing diagnostic technology to enable monitoring of metastatic burden throughout treatment.
Translating disseminated tumor cell burden into clinical risk measures
While the DTC burden reflects the potential for metastatic disease to develop, the establishment of overt metastasis from CTCs and micrometastases is a highly inefficient process [ 47 ]. For example, in breast cancer, less than half of the patients with detectable micrometastases in the bone marrow develop distant recurrence within 10 years [ 48 ]. Therefore, the volume of disseminated disease when present as small cell clusters or single cells does not always directly correlate with the incidence of overt metastasis. Seminal studies have begun to dissect the key intrinsic tumor cell features that confer the capacity to form overt metastases, and have shown that these features are present in a specific subset of tumor cells [ 49 ]. This highlights a need to understand the cell intrinsic and extrinsic factors that act in concert with the disseminated disease burden to define a patient’s metastatic propensity.
Different secondary sites have different propensities for the development of overt recurrence [ 50 ]. For example, lymph nodes, liver, lung, and bone are common metastatic sites across a multitude of primary cancer types, yet overt metastases in skeletal muscle are relatively rare [ 51 ]. While the mechanisms underlying these patterns are not yet well defined, it is known that the site at which metastasis develops can impact survival outcomes; therefore, the discovery of biomarkers indicative of the secondary seeding site(s) will be critical to evaluate the risk of site-specific recurrence [ 52 – 54 ]. Information from rapid autopsies will be fundamental to our understanding of these processes and in identifying site-specific biomarkers. Furthermore, the role of broader host and environmental factors in promoting metastasis, such as surgical removal of the primary tumor, age- and biomechanics-induced bone remodeling, as well as systemic stress hormones, are still emerging [ 55 – 60 ]. To robustly capture the long-term metastatic risk on this complex background, clinical studies will need to follow large cohorts over a sufficiently long period of time. This long-term data can then be used to understand the effects of these myriad factors on metastatic risk and will underpin the implementation of biomarkers in future precision medicine approaches.
Understanding and modelling the dynamics of metastatic dissemination
Research autopsies: an abundant resource to study human metastasis.
Successfully dissecting and targeting the mechanisms that drive metastasis will depend on (1) a foundational understanding of metastasis mechanisms in patients, as well as (2) our ability to model metastasis in the laboratory. DTCs are difficult to detect, and secondary tumors at distant sites are often not surgically resected. Therefore, viable human tissue for researching metastasis mechanisms is scarce. Furthermore, as described above, assessing DTC/micrometastatic burden in patients and identifying features distinguishing indolent from aggressive DTCs has remained challenging. Research autopsies, also termed ‘warm’ or ‘rapid’ autopsies, of deceased cancer patients (1–6 h post-mortem) reveal the burden of disseminated disease at the end stages of disease and represent a valuable source of viable metastatic tissue [ 61 ]. The high integrity of tissues derived from research autopsies enables broad multi-omics analysis at the bulk and single cell resolutions to study cancer cells within the metastatic microenvironment. Importantly, research autopsies enable the establishment of patient-derived xenografts, cell lines and organoids for mechanistic studies [ 61 ]. Increased establishment of research autopsy protocols will require the multidisciplinary involvement of clinicians and researchers, together with the generosity of cancer patients and their families, in an effort that will continue to provide invaluable insight into metastatic burden and its drivers.
Technological advances revealing tumor heterogeneity and spatial compartmentalization of the metastatic microenvironment
Emerging advances point to cellular heterogeneity and the microenvironment of primary tumors, pre-metastatic niches and metastatic sites as having a profound influence on the propensity and dynamics of metastatic dissemination. Interactions between cancer, stromal and immune cells as well as with the extracellular matrix spatiotemporally regulate metastasis [ 62 , 63 ]. In this regard, the advent of single cell genomics and spatial profiling technologies are revolutionizing our understanding of cellular heterogeneity, cellular interactions and microenvironmental characteristics that support and promote metastasis.
Tumor heterogeneity and the evolution of highly metastatic subclones during the progression of heterogeneous tumors is thought to significantly contribute to metastatic propensity. Bulk analysis approaches have revealed specific mutational profiles and cellular states associated with polyclonal and monoclonal metastasis mechanisms in multiple cancer types [ 64 – 67 ]. More recently, single cell genomics approaches have been revolutionary in further revealing cellular subtypes within the broader heterogeneous tumor community that drive metastatic dissemination. For example, single cell RNA sequencing (scRNA-seq) of primary and metastatic patient-derived xenografts (PDXs) of mammary and lung tumors revealed an enrichment of stem-like/progenitor cell states in cancer cells within metastases as compared to primary tumors [ 68 , 69 ]. Due to its single-cell resolution, single cell genomics will be particularly relevant in identifying mutationally- and transcriptionally-driven mechanisms of therapy resistance operating in subclonal cells that then outgrow treatment-sensitive subclones to drive continued tumor progression. For instance, single cell genomics was applied to chemo-refractory triple negative breast tumors, which revealed that resistant genotypes exist prior to neoadjuvant chemotherapy, and transcriptional programs that emerge in resistant cancer cell populations are induced by the treatment [ 70 ]. Using single-cell genomics in therapy-naïve primary lesions and paired, pan-resistant, anachronous secondary tumors in patient samples seems a challenging endeavor, but will significantly expand our understanding on therapy evasion mechanisms in metastasis.
Insights derived from early laser capture microdissection profiling of the tumor microenvironment have been accelerated by the application of multiplexed imaging (e.g. CODEX [ 71 ]), spatial transcriptomics (including the commercially available 10x Genomics Visium platform and emerging technologies slide-SEQ, non-destructive FISSEQ) and spatial proteomics analysis (e.g. imaging mass spectrometry) [ 72 ], together with the layering of these technologies on the more mature bulk and single cell genomics approaches. For example, multiplex imaging coupled with next generation sequencing has revealed that the close proximity of highly proliferative cancer cells with specific lymphocyte subtypes regulate immunological surveillance as metastases develop [ 73 ]. More detailed spatial characterization is now afforded by multiplexed ion beam imaging (MIBI)[ 74 , 75 ], which harbors greater spectral depth than traditional fluorescence-based multispectral imaging, to enable the spatial identification of approximately 40 proteins within the microenvironment. This targeted technology has been integrated with spatial transcriptomics and single cell RNAseq in primary cutaneous squamous carcinoma to reveal the importance of spatially regulated cellular crosstalk nodes in tumorigenesis [ 76 ], demonstrating the potential of this approach in revealing key spatial relationships within tumors that could provide invaluable insight when specifically applied to metastatic disease.
Mass spectrometry imaging further extends the capacity of MIBI by mapping hundreds to thousands of metabolomic and proteomic analytes across tissue sections. Importantly, this technology identifies metabolic and proteomic features that cannot be obtained using the nucleotide-based spatial mapping technologies described above [ 77 ]. While metabolic and proteomic mass spectrometry imaging detection has been established for some time, recent improvements in sample preparation methods that more effectively preserve native tissue structure are unlocking the immense potential of this technology to reveal novel functional nodes of cell-cell and cell-matrix interactions governing metastatic processes [ 78 ]. This approach is particularly powerful in the burgeoning era of immunotherapy, where the interaction of cancer and immune cells is increasingly recognized to regulate metastasis. Recently, focused approaches have revealed a role for myeloid cells in activating dormant DTC proliferation through spatially compartmentalized laminin proteolysis [ 57 , 58 ] and lipid metabolism [ 56 ]. Further spatial profiling of these environments using the wide lens conferred by mass spectrometry imaging may identify additional local nodes of cell-cell interactions that together regulate anti-tumor surveillance in a nuanced, microenvironment-specific manner. These advances in spatial technologies will be critical to provide fundamental answers to (1) how the immune milieu influences metastatic burden and (2) how these processes can be harnessed to control metastasis using existing and novel immunotherapy approaches. Insights into both metabolic and ECM remodeling within and surrounding tumor cells gained from mass spectrometry imaging also have the potential to reveal actionable stromal co-targeting approaches to improve treatment sensitivity [ 7 , 79 ]. Overall, orthogonal spatial technologies have great potential to reveal novel mechanisms driving metastatic dissemination, which will underpin patient risk predictions and the development of metastasis-specific therapies.
Modelling metastasis to predict risk
The identification of tumor features that support metastasis and predict metastatic risk in patients will facilitate improvements in vitro [ 80 , 81 ] and in vivo models [ 82 , 83 ] that recapitulate the dynamics of metastatic dissemination, colonization and outgrowth. Patient-derived xenografts and three-dimensional organoid cultures derived during treatment and from research autopsies more accurately reflect clinical treatment responses, and are currently being implemented as patient avatars in precision medicine programs to identify the most effective treatment for individual patients [ 84 , 85 ]. As patient avatars, these models can be subjected to extensive drug screening in the laboratory to identify the most effective anti-tumor therapies that are likely to achieve a complete clinical response, thereby informing clinical practice to accelerate treatment. Improvements in animal models, including transplantable syngeneic mouse and human cancer lines, mouse xenografts, and genetically engineered- and humanized- mouse models are also being developed to mimic the behavior of human metastatic disease [ 86 , 87 ]. While models of primary tumor behavior have received considerable attention and have significantly contributed to developments in cancer treatment, investment in developing metastasis-specific preclinical models should be prioritized for their capacity to reveal metastatic mechanisms that can be readily exploited therapeutically to improve patient survival. These technological developments are anticipated to most profoundly impact poor prognosis cancers and disadvantaged populations where stage IV diagnoses are more prevalent.
Data gathered from preclinical and clinical studies of metastasis dynamics are underpinning the implementation of mathematical modelling and artificial intelligence to build computational predictors of metastatic burden and therapy response [ 88 ]. These mathematical models also confer the opportunity to infer the actual stage of progression for a patient’s tumor at diagnosis, to predict their likely burden of disseminated disease and to assess their risk of developing overt metastases [ 89 ]. Importantly, mathematical models have validated the non-linear relationship between primary tumor size and survival, indicating that metastatic propensity is not simply a function of tumor size but that tumor intrinsic, extrinsic and host factors all contribute to metastatic progression [ 90 ]. Furthermore, clinically-informed mathematical models can define when metastases develop to enable earlier metastasis detection. For example, models of brain metastases from primary lung tumors have identified that DTCs in the brain remain dormant for approximately 5 months before their outgrowth, and that a further 12-19 months of secondary tumor growth occurs before metastasis is clinically diagnosed [ 91 ], thereby indicating that there is a significant window of opportunity to inhibit the outgrowth of disseminated cancer cells to the brain as well as improve the detection of early brain metastases. This temporal understanding, coupled with the predictive power of these models [ 89 ], provides the foundation to improve protocols for the early detection of metastatic disease and reveals opportunities to treat these patients earlier than current protocols allow. Mathematical models that are able to integrate the myriad host-, tissue- and niche-specific factors in metastatic dissemination and outgrowth will be key to understanding, as well as predicting, how these microenvironmental factors interact with intrinsic features of a patient’s primary tumor to drive the organotypic nature of metastasis [ 92 ]. Fueled by increased clinical data collection, these modelling approaches represent a step towards the clinical implementation of mathematical modelling as a predictive tool in precision oncology.
Improving the treatment of metastatic disease
There are fundamental challenges in treating metastatic disease once it has been diagnosed. Tumor heterogeneity enables the persistence and expansion of treatment -refractory subclones at not only primary but also secondary sites. In addition, most current treatments are targeted to proliferative cell states, and therefore are less effective against quiescent metastatic disease prior to outgrowth. Disseminated disease, particularly when dormant, is commonly resistant to current standard-of-care therapies that target the primary tumor [ 93 ]. This enables residual disease to persist and re-emerge even after successful treatment of the primary tumor. Therefore, overcoming therapy resistance is paramount to prolonging the survival of metastatic patients.
Therapies that specifically target metastatic disease can be distinguished into two main categories: (1) treatments aimed at eradicating metastatic disease [ 29 ] and (2) treatments aimed at maintaining metastatic disease in a chronically dormant state [ 94 ].
Treatments aimed at eradicating metastatic disease
Metastatic tumors have higher levels of resistance to systemic conventional chemotherapies compared with primary tumors and this presents a major hurdle to eradicating metastatic disease [ 95 ]. Resistance mechanisms to conventional chemotherapies can be due to chemotherapy-induced acquisition of de novo genomic alterations [ 96 ], phenotypic adaptation to cytotoxic stress [ 97 ] or result from the expansion of pre-existing clones [ 98 ]. These resistance traits can arise during both the latent and overt stages of metastases [ 93 ].
Given that conventional chemotherapies indiscriminately target rapidly dividing cells, it was assumed that DTCs in a quiescent or dormant state would be relatively insensitive to cytotoxic therapies. Therefore, one approach to sensitizing dormant cells to conventional chemotherapies is to drive them into a highly proliferative state. Approaches to stimulate the awakening of dormant cells, including G-CSF and acute IFN-alpha treatments [ 99 , 100 ] or by driving epigenetic remodeling to transient drug-sensitive states (e.g. HDAC inhibition) [ 101 ], have improved the efficacy of cell cycle- and DNA damage response-dependent chemotherapeutic agents. However, these approaches carry a substantial risk of driving indiscriminate and uncontrollable metastatic outgrowth.
Recent data indicate that dormant cells can be targeted without the need to awaken them, thereby mitigating the significant risks associated with inducing them into a proliferative state. Rather than the quiescent state of dormant tumor cells giving rise to chemoresistance, these recent studies have identified cell-cell and cell-matrix interactions within the dormancy niche that protect DTCs from cytotoxic chemotherapies, independently of their proliferative state [ 9 , 102 ]. Such studies are beginning to reveal potential strategies for targeting these interactions to re-sensitize metastatic tumors to current standard-of-care agents without inducing uncontrolled metastatic outgrowth. Some of these approaches have the potential for near-immediate translation into clinical protocols. For example, additional rounds of docetaxel chemotherapy following standard-of-care fluorouracil, epirubicin, and cyclophosphamide (FEC) treatment eliminated dormant tumor cells in the bone marrow of some breast cancer patients [ 103 ], highlighting how additional rounds of currently available chemotherapies may be used to effectively prevent metastasis. Conversely, emerging evidence points to the ability of standard-of-care chemotherapy to potentiate metastatic dissemination and drive the awakening of dormant DTCs in specific in vivo models [ 8 ]. This contrasts with evidence that neoadjuvant chemotherapy has been shown to be as efficacious as adjuvant chemotherapy in terms of relapse-free survival in some cancers, and therefore a more thorough understanding of the effects of therapy on metastatic dynamics in patients should inform treatment strategies in the future. Finally, identification of survival mechanisms exercised by DTCs has revealed potential therapeutic targets including autophagy [ 104 ], Srk and Mek1/2 in combination [ 105 ], and integrin-signaling [ 62 , 102 ]. Overall, a more comprehensive understanding of these mechanisms is likely to reveal novel, specific targets of metastatic disease for further clinical development and provides hope that dormant DTCs can be eradicated without risking their uncontrolled proliferation.
Novel therapeutic strategies targeting metastasis are also emerging as our understanding of cancer cell-niche interactions develops. In bone metastases, identification of Jagged1 as a mediator of crosstalk between cancer cells and the bone microenvironment has led to the development of a new humanized anti-Jagged1 antibody that sensitizes bone metastases to chemotherapy [ 106 ]. Similarly, targeting integrin-mediated interactions in the perivascular niche of the bone microenvironment also prevents metastatic outgrowth in preclinical models [ 102 ]. Significant improvements in targeting metastatic disease will also rely on further development of nanoparticles and other chemotherapy carriers that can be targeted directly to specific secondary sites and pre-metastatic niches [ 17 ], thereby enabling combination therapies to simultaneously target multiple metastases. Defining how these niche-targeted treatments operate at different secondary sites and interact with each other will be critical in defining effective precision therapies.
Treatments enforcing dormancy
Given the challenges in eliminating dormant DTCs, and the low penetrance by which dormant DTCs manifest as overt metastases, developing therapies that maintain DTCs in a dormant state is regarded as a promising therapeutic approach. Estrogen receptor (ER) antagonists such as tamoxifen, commonly used to treat ER+ breast cancer, are an example of anti-metastatic therapies administered in an adjuvant setting, although it remains unclear whether this treatment maintains dormancy or enables the elimination of dormant cells. Studies of tamoxifen treatment for 10 years found an additional reduction in metastatic recurrence rates when compared to the standard-of-care 5-year treatment regimen [ 107 ], highlighting the potential for this approach to achieve long term remission. Similarly, RANKL inhibitors, such as denosumab, have demonstrated utility in reducing bone resorption-driven activation of dormant DTCs in bone metastases of prostate cancer [ 108 ].
More recent proposed approaches to enforce metastatic dormancy include small molecules and monoclonal antibody therapies that drive DTCs into a dormant state. Induction of DTC dormancy has been achieved through CDK4/6 inhibition (e.g. Palbociclib) [ 109 ] or by inhibiting outside-in pro-proliferative signaling [ 95 , 110 ]. Immunotherapy that harnesses immunological surveillance to control DTCs also has great potential to both eradicate and control metastatic disease since neutrophils and myeloid subtypes regulate microenvironmental cues that control dormancy [ 55 – 57 ]. For these reasons, immunotherapy is likely to become an essential component of metastasis management. Gaining a better understanding of the relationship between tissue-specific immune populations and DTCs, and how these interactions govern dormancy and outgrowth, will be essential to developing effective immunotherapies that control metastatic burden.
While treatments that eradicate or maintain cancer cell dormancy promise a future where metastatic latency can be effectively treated, therapies specifically targeting dormant metastatic disease are not yet clinically available. Fortunately, several clinical trials are ongoing with the explicit purpose of targeting dormancy and/or using DTCs or micrometastatic burden (for example, via detection of DTCs in bone marrow aspirates) as a readout of efficacy (Table 2 ). Nevertheless, substantial challenges remain in designing and completing clinical trials for metastasis-specific therapies. Regulatory frameworks around the world currently limit the feasibility of clinical trials with metastatic relapse at endpoints because the long time period required of such studies often exceeds that of intellectual property protection that underpins the financial support of the trial. Embarking on such a large and long trial is considered feasible when supported by substantial efficacy in the acute setting, and this hinders the development of cytostatic metastatic therapies that may not have substantial short term effects [ 97 ]. Furthermore, there must be a willingness from regulatory bodies to accept ctDNA and other biomarkers as clinical trial endpoints of metastasis, rather than the traditional RECIST-based progression-free survival readouts that encompass only overt metastases. The growing inclusion of disseminated disease burden as secondary endpoints in clinical trials will serve to (1) identify potential therapies for targeting dormancy after initial standard-of-care therapy has been completed and, (2) introduce the use of DTCs as a readout for therapeutic efficacy. Taken together, including disseminated disease burden in clinical trials is fundamental to developing more effective treatments against metastatic disease.
Interventional clinical trials targeting metastatic disease
Concluding remarks
Metastatic disease remains the major cause of cancer death, yet in most cancer types we are only beginning to understand the processes that govern the dissemination of cancer cells from the primary tumor, their seeding to distant sites and their eventual outgrowth into overt metastases. It is a significant challenge to dissect the numerous host, environmental and microenvironmental factors that intersect with intrinsic features of cancer cells to spatiotemporally regulate metastatic progression. However, our ability to accurately detect DTCs and garner an accurate measure of metastatic burden in patients throughout treatment will underpin efforts to treat metastatic disease more effectively (Challenge 1). On this front, substantial strides have been made in improving the sensitivity of metastatic detection through machine-learning fueled pathological assessment and the use of liquid biopsy biomarkers to collectively detect CTCs in patients. Standardization of these approaches, together with increased access to research autopsies, will illuminate a hitherto opaque understanding of metastatic dynamics and will predict which patients are likely to develop metastatic recurrence (Challenge 2). This clinical data will continue to inform accurate in vitro and in vivo modelling of metastasis, to dissect the complex spatiotemporal interplay of cell intrinsic, microenvironmental and host factors (Challenge 3). Patient avatars developed from these models most accurately represent the patient’s treatment response and could become central to precision medicine frameworks that identify the most effective treatment for each individual. With further standardization, these tools may also prove fundamental to drug development pipelines for metastasis-targeted therapies. Dose-intensity modulation of existing therapies or novel therapies that eradicate or suppress metastases are being developed as biological mechanisms governing these processes are revealed (Challenge 4). Ultimately, translating these treatments to the clinic will require large, long-term clinical trials capable of supporting the complexity of individualized precision medicine protocols and with disseminated tumor burden, at both the micro- and macro-metastatic scales, as primary endpoints. Continued progress in overcoming these major challenges will require the collective interdisciplinary effort of researchers, clinicians, patients and funding agencies to transform metastatic cancer into a highly manageable and ultimately curable disease in all patients across all cancer types.
Open Access funding enabled and organized by CAUL and its Member Institutions.
Data Availability
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Amelia L. Parker and Early Career Leadership Council of the Metastasis Research Society have contributed eqully to this study.
Metastasis Research Network (MetNet)

Metastasis Research Network (MetNet)
Understanding the spectrum of complex metastatic processes is important to the development of a comprehensive and cohesive understanding of cancer metastasis. The Metastasis Research Network (MetNet) supports several U54 Specialized Centers ( RFA-CA-20-029 ). These collaborative, multidisciplinary projects focus on several themes of the metastatic process and utilize integrative systems-level approaches.
The MetNet will advance the understanding of metastasis as a non-linear, dynamic, and emergent process and promote advances in mechanistic understanding of early dissemination, cellular and physical microenvironment crosstalk, dormancy, and mechanisms of responses by metastatic cells to therapies. The network integrates multiple perspectives and expertise to advance metastasis research, including scientific experts in different fields, physicians, and research advocates.
MetNet Funding Opportunity
PAR-22-234 : MetNet Research Projects (U01 Clinical Trial Not Allowed)
MetNet Research Advocates
MetNet brings together scientific experts in different disease and research areas to find answers to why and how metastasis occurs in cancer and discover solutions to prevent, control and ultimately eradicate it. Patient advocates bring a unique perspective to each MetNet center, working as valued partners with the researchers. They incorporate the patient experience into basic and translational research, having experienced cancer or supported someone with cancer.
The advocates have three primary roles:
- Engage in a scientific discussion with the investigators to communicate patient priorities, preferences, and concerns.
- Mentor researchers to broaden their understanding of the disease, help them communicate their work in a way that is accessible for those outside the scientific community, and gain perspective on the impact they have on patients’ lives.
- Serve as a liaison to their communities by translating the progress of scientific research from MetNet for a general audience.
The research advocates are each a member of one of the five MetNet academic institutions’ centers, and they also work together on common advocacy goals across the centers.
MetNet News and Publications
Congratulations to the poster and flash talk award winners at the 2023 MetNet Investigators Meeting!
Poster Presentation Awards
Thank you to the MetNet Advocacy Working Group for reviewing the meeting posters and presenting the awards.
- Elena Cambria, Ph.D. (Massachusetts Institute of Technology): Influence of primary tumor stiffness on metastatic progression via “cell mechanical memory”
- Aparajita Khan, Ph.D. (Stanford University): Distinct genetic alterations driving aggressive brain metastasis in colorectal cancer
- Andrey Rubanov (NYU Langone Health): Chromatin remodeling by CHD7 regulates melanoma metastasis and anti-tumor immunity
Flash Talk Presentation Award
- Kat Liu (Stanford University): Tumor-intrinsic and 3D-specific type I interferon production and signaling in MCF7 spheroids
Upcoming and Past Meetings
October 11-12, 2023 - MetNet Investigators Meeting
December 2, 2021 - MetNet Kickoff Meeting
September 24, 2021 - MetNet Center Introductions
MetNet Social Media
MetNet research and funding opportunities are shared on Twitter: @NCIsysbio
Contacts for MetNet
For additional information about the MetNet, please contact Dr. Joanna Watson , Dr. Christine Nadeau , or Dr. Brunilde Gril .
Funded Projects

Cancer and Metastasis Reviews
- Focuses on the molecular and cellular biology of cancer metastasis and tumor progression.
- Presents expert contributions on a single theme or topic in each issue.
- Erica Golemis

Latest issue
Volume 43, Issue 1
Defining and Understanding Cancer Cell Plasticity in Therapy Resistant
Latest articles
The effect of glp-1r agonists on the medical triad of obesity, diabetes, and cancer.
- Shahad Sabaawi Ibrahim
- Raghad Sabaawi Ibrahim
- Dietrich Büsselberg

Multiomics approach towards characterization of tumor cell plasticity and its significance in precision and personalized medicine
- Mohammad Azhar Aziz

Healthcare disparities, screening, and molecular testing in the changing landscape of non–small cell lung cancer in the United States: a review
- Razelle Kurzrock
- Aadel A. Chaudhuri
- Ignacio I. Wistuba

The roles of PD-L1 in the various stages of tumor metastasis
- Weiqin Jiang

Cancer-associated fibroblasts: a versatile mediator in tumor progression, metastasis, and targeted therapy
- Tianchen Guo
Journal updates
Introducing new editorial team, trending articles from the past 12 months.
Find out which Cancer and Metastasis Reviews articles are attracting the most attention on the social web.
Altmetric Report

Journal information
- CAB Abstracts
- Chemical Abstracts Service (CAS)
- Google Scholar
- INIS Atomindex
- Japanese Science and Technology Agency (JST)
- Norwegian Register for Scientific Journals and Series
- OCLC WorldCat Discovery Service
- Pathway Studio
- Science Citation Index Expanded (SCIE)
- TD Net Discovery Service
- UGC-CARE List (India)
Rights and permissions
Editorial policies
© Springer Science+Business Media, LLC, part of Springer Nature
- Find a journal
- Publish with us
- Track your research
When you choose to publish with PLOS, your research makes an impact. Make your work accessible to all, without restrictions, and accelerate scientific discovery with options like preprints and published peer review that make your work more Open.
- PLOS Biology
- PLOS Climate
- PLOS Complex Systems
- PLOS Computational Biology
- PLOS Digital Health
- PLOS Genetics
- PLOS Global Public Health
- PLOS Medicine
- PLOS Mental Health
- PLOS Neglected Tropical Diseases
- PLOS Pathogens
- PLOS Sustainability and Transformation
- PLOS Collections
- Collections Home
- About Collections
- Browse Collections
- Calls for Papers
CALL FOR PAPERS
Cancer Metastasis Call for Papers
Submit to PLOS ONE, the #1 publisher of cancer metastasis research. Due to COVID-19 restrictions, PLOS ONE is extending the deadline for submissions to the Cancer Metastasis collection. This unique opportunity will allow authors to continue to submit their research to this Call for Papers, including post collection launch, until December 14, 2020.
Submit to PLOS ONE
Cancer metastasis is responsible for 90% of all cancer deaths. The metastatic cascade is a multistep and sequential process involving cancer cell invasion, intravasation, circulation and colonization. However, our knowledge of the molecular and biochemical mechanisms that govern this process are still poorly understood. Furthermore, there is an urgent need to develop therapeutic interventions that target each part of the cascade.
In this Call for Papers, we are inviting submissions that report on the biochemical and cell biological basis of metastasis, including but not limited to cell adhesion, cell migration, cytoskeletal dynamics, cell polarity, tumour heterogeneity, tumour dormancy and the tumour microenvironment. In addition, we welcome submissions that report on disease progression and outcomes, potential biomarkers for metastatic cancer as well as the development of anti-metastatic therapies, including drug development, drug delivery and personalized medicine. In this collection, we aim to highlight the diversity of research that is being conducted in the cancer metastasis field, including studies that focus on basic and translational research.
- Public health and epidemiology
- Gender and sexuality studies
- Medicine and health care
- Mental health and psychiatry
- Health care - Health policy
- Health care - Health services research

Joe Ramos University of Hawaii, USA Webpage

Shengyu Yang Penn State University, USA Webpage

Helen Fillmore University of Portsmouth, United Kingdom Webpage

Tobias Zech University of Liverpool, United Kingdom Webpage
PLOS ONE is the #1 publisher of cancer metastasis papers with over 30,000 papers published.
Days to first decision on average
Citations of PLOS ONE cancer metastasis papers
Monthly site visitors
Disciplines represented by authors from 200 countries
Years of publishing interdisciplinary cancer metastasis
Interdisciplinary cancer metastasis papers published
PLOS ONE publishes primary research across all areas of science, including interdisciplinary research. Research articles are selected for scientific rigor rather than perceived impact.
Email questions to [email protected]
PLOS ONE A Distinct Macrophage Population Mediates Metastatic Breast Cancer Cell Extravasation, Establishment and Growth August 10, 2009 / Binzhi Qian, Yan Deng, Jae Hong Im, Ruth J. Muschel, Yiyu Zou, Jiufeng Li, Richard A. Lang, Jeffrey W. Pollard
Submit your paper to PLOS ONE to be considered for this Collection.
Mention the Cancer Metastasis Call for Papers in your cover letter. The Collection will publish Summer 2020.
- Subscribe to receive updates about this Collection
- Explore more cancer metastasis research
- History, Facts & Figures
- YSM Dean & Deputy Deans
- YSM Administration
- Department Chairs
- YSM Executive Group
- YSM Board of Permanent Officers
- FAC Documents
- Current FAC Members
- Appointments & Promotions Committees
- Ad Hoc Committees and Working Groups
- Chair Searches
- Leadership Searches
- Organization Charts
- Faculty Demographic Data
- Professionalism Reporting Data
- 2022 Diversity Engagement Survey
- State of the School Archive
- Faculty Climate Survey: YSM Results
- Strategic Planning
- Mission Statement & Process
- Beyond Sterling Hall
- COVID-19 Series Workshops
- Previous Workshops
- Departments & Centers
- Find People
- Biomedical Data Science
- Health Equity
- Inflammation
- Neuroscience
- Global Health
- Diabetes and Metabolism
- Policies & Procedures
- Media Relations
- A to Z YSM Lab Websites
- A-Z Faculty List
- A-Z Staff List
- A to Z Abbreviations
- Dept. Diversity Vice Chairs & Champions
- Dean’s Advisory Council on Lesbian, Gay, Bisexual, Transgender, Queer and Intersex Affairs Website
- Minority Organization for Retention and Expansion Website
- Office for Women in Medicine and Science
- Committee on the Status of Women in Medicine Website
- Director of Scientist Diversity and Inclusion
- Diversity Supplements
- Frequently Asked Questions
- Recruitment
- By Department & Program
- News & Events
- Executive Committee
- Aperture: Women in Medicine
- Self-Reflection
- Portraits of Strength
- Mindful: Mental Health Through Art
- Event Photo Galleries
- Additional Support
- MD-PhD Program
- PA Online Program
- Joint MD Programs
- How to Apply
- Advanced Health Sciences Research
- Clinical Informatics & Data Science
- Clinical Investigation
- Medical Education
- Visiting Student Programs
- Special Programs & Student Opportunities
- Residency & Fellowship Programs
- Center for Med Ed
- Organizational Chart
- Leadership & Staff
- Committee Procedural Info (Login Required)
- Faculty Affairs Department Teams
- Recent Appointments & Promotions
- Academic Clinician Track
- Clinician Educator-Scholar Track
- Clinican-Scientist Track
- Investigator Track
- Traditional Track
- Research Ranks
- Instructor/Lecturer
- Social Work Ranks
- Voluntary Ranks
- Adjunct Ranks
- Other Appt Types
- Appointments
- Reappointments
- Transfer of Track
- Term Extensions
- Timeline for A&P Processes
- Interfolio Faculty Search
- Interfolio A&P Processes
- Yale CV Part 1 (CV1)
- Yale CV Part 2 (CV2)
- Samples of Scholarship
- Teaching Evaluations
- Letters of Evaluation
- Dept A&P Narrative
- A&P Voting
- Faculty Affairs Staff Pages
- OAPD Faculty Workshops
- Leadership & Development Seminars
- List of Faculty Mentors
- Incoming Faculty Orientation
- Faculty Onboarding
- Past YSM Award Recipients
- Past PA Award Recipients
- Past YM Award Recipients
- International Award Recipients
- Nominations Calendar
- OAPD Newsletter
- Fostering a Shared Vision of Professionalism
- Academic Integrity
- Addressing Professionalism Concerns
- Consultation Support for Chairs & Section Chiefs
- Policies & Codes of Conduct
- First Fridays
- Fund for Physician-Scientist Mentorship
- Grant Library
- Grant Writing Course
- Mock Study Section
- Research Paper Writing
- Establishing a Thriving Research Program
- Funding Opportunities
- Join Our Voluntary Faculty
- Child Mental Health: Fostering Wellness in Children
- Faculty Resources
- Research by Keyword
- Research by Department
- Research by Global Location
- Translational Research
- Research Cores & Services
- Program for the Promotion of Interdisciplinary Team Science (POINTS)
- CEnR Steering Committee
- Experiential Learning Subcommittee
- Goals & Objectives
- Issues List
- Print Magazine PDFs
- Print Newsletter PDFs
- YSM Events Newsletter
- Social Media
- Patient Care
INFORMATION FOR
- Residents & Fellows
- Researchers
Promising New Treatment for Patients with HR+ HER-2 Negative Metastatic Breast Cancer
Metastatic breast cancer image.
Breast cancer that has spread to other parts of the body ( metastatic ) is particularly hard to treat. New research from Yale Cancer Center reveals first-of-its-kind data from a phase I study in patients with hormone receptor positive HER2-negative metastatic breast cancer. The results, which assess the safety and efficacy of a treatment known as PF-07248144, offer new hope for treating this aggressive type of breast cancer.
Yale researchers found that PF-07248144, both as a standalone therapy and in combination with the hormone therapyfulvestrant (an estrogen receptor antagonist agent), was well-tolerated and effective at treating patients with hormone receptor-positive, HER2-negative metastatic breast cancer. PF-07248144 targets and blocks the proteins KAT6A and KAT6B, which can help cancer grow and spread when they are not working properly, or are dysregulated.
Senior author of the study, Patricia LoRusso, DO , Associate Director for Experimental Therapeutics at Yale Cancer Center, will present the findings at the American Society of Clinical Oncology (ASCO) Annual Meeting on June 1.
“We're now realizing how important epigenetics (field of study focused on modification of gene expression rather than the alteration of the underlying DNA) is in terms of understanding the biology of cancer and targeting the disease for treatment interventions,” said LoRusso.
For nearly three years, 107 patients were enrolled in the phase I trial and received at least one dose of PF-07248144. 29 patients received PF-07248144 at 5 dose levels, 35 patients received PF-07248144 at 5 mg daily, and 43 patients received PF-07248144 at 5 mg daily in combination with 500 mg fulvestrant. According to LoRusso, “the goal of the study was to overcome the patient’s ESR1 mutation and enhance, or resume response in combination with hormonal therapy (fulvestrant).”
In a review of the data, the combination of PF-07248144 with fulvestrant showed an objective response rate (ORR) of 30.2% and a median duration of response (DOR) of nine months. For patients who were treated with only PF-07248144, the ORR was lower at 11%, however the median DOR was slightly better at 12 months. Most side effects related to the treatment were manageable. The most common were taste alteration, a decrease in white blood cell count, and anemia.
The phase I trial is still ongoing, however the preliminary findings are encouraging and support further research of PF-07248144 in larger trials.
“Although this was not a randomized trial, the degree and duration of response of the combination of PF-07248144 with fulvestrant appear at least preliminarily in this trial to be better than historical data with fulvestrant alone,” said LoRusso.
Toru Mokuhara from National Cancer Hospital East in Kashiwa, Japan, was the study’s first author. Other authors include Yeon Hee Park, David Sommerhalder, Kan Yonemori, Erika Hamilton, Sung-Bae Kim, Jee Hyun Kim, Hiroji Iwata, Toshinari Yamashita, Rachel M. Layman, Timothy Clay, Yee Soo Chae, Catherine Oakman, Fengting Yan,Gun Min Kim,Seock-Ah Im, Geoffrey J. Lindeman, Hope S. Rugo, Marlon Liyanage, Michelle Saul, Christophe Le Corre, Athanasia Skoura, Li Liu, and Meng Li.
Featured in this article
- Patricia LoRusso, DO Amy and Joseph Perella Professor of Medicine (Medical Oncology); Chief, Experimental Therapeutics; Associate Cancer Center Director, Experimental Therapeutics
Cancer Biology
Focus areas.
The mission of the Department of Cancer Biology is to identify and understand the causes of cancer, to develop innovative approaches to reduce cancer incidence, to create and test novel and more effective therapies, and to translate these findings into clinical care for the benefit of patients.
Research in our department is highly collaborative and is potentiated through close interactions with other basic science departments and translated through clinical collaborations.
Our faculty members are aligned into eight research focus areas that address cancer development, progression and treatment.
Cancer disparities
While cancer affects everyone, certain groups have higher rates of cancer cases, deaths and health complications. These differences can be associated with genetics, sex, racial and ethnic populations, socioeconomic status, or specific geographic areas. Studying the factors that lead to cancer disparities leads to more-effective prevention and treatment approaches for the affected populations.
- Read more about the cancer disparities focus area .
Cancer stem cells
The stem cell theory of cancer proposes that among the many different types of cells within a cancer, there exists a subpopulation of cells called cancer stem cells that multiply indefinitely, are resistant to chemotherapy, and are thought to be responsible for relapse after therapy. Cancer stem cells also give rise to highly metastatic cells that spread to other organs and tissues within the body. A deeper understanding of cancer stem cells is leading to better implementation of existing anti-cancer therapies and identification of new approaches that target the cancer stem cells specifically.
- Read more about the cancer stem cells focus area .
Cancer systems biology
Cancer is a complex disease with many molecular, genetic and cellular causes. Considering these causes as a system of interactions can lead to a better understanding of the processes involved in the development of cancer and response to therapies. Cancer systems biology integrates advanced experimental models, insights from genome sequencing and other large-scale data projects, and computational models to create unified models of cancer behavior.
- Read more about the cancer systems biology focus area .
Oncogenic gene dysregulation and carcinogenesis
Cancer initiates from genetic alterations, including mutations, deletions and copy number gains, that function to activate cancer-promoting pathways or to block processes that normally inhibit cancer development. Through better understanding of pro- and anti-cancer signaling processes and how they become dysregulated in carcinogenesis and tumor progression, our team can improve biological tools for clinicians and devise molecularly targeted therapies to intervene.
- Read more about the oncogenic gene dysregulation and carcinogenesis focus area .
Precision cancer medicine and translational therapeutics
Cancers develop and respond to therapies differently from one patient to the next. A better understanding of the specific processes driving cancer growth and spread in an individual patient allows for tailored therapeutic strategies that are more effective with minimal side effects. Profiling the mutations and abnormalities that drive a tumor, in combination with development of experimental models that assess the specific responses of cancer cells to therapeutics that target those abnormalities, has dramatically improved outcomes in many cancer types.
- Read more about the precision cancer medicine and translational therapeutics focus area .
Tumor immunology and immunotherapy
Therapeutic strategies that stimulate the immune system to target cancer cells can lead to long-lasting tumor regression and minimize relapse. Integrated efforts of laboratory researchers and clinicians are leading to improved knowledge of how the immune system interacts with cancer cells and how immune processes can be intentionally manipulated for therapeutic effect.
- Read more about the tumor immunology and immunotherapy focus area .
Tumor invasion and metastasis
A fundamental property of malignant tumor cells is the ability to invade surrounding tissues and to metastasize to other organs. These abilities underlie the majority of cancer-associated deaths. While invasion and metastasis are often thought of as the final stages of tumor development, recent studies have shown that tumor spread can occur even at early stages of tumor development, sometimes even before the primary tumor has been identified. Development of therapies targeting invasion and metastasis has the promise to significantly reduce cancer mortality.
- Read more about the tumor invasion and metastasis focus area .
Tumor microenvironment
Tumors require complex interactions with surrounding blood vessels, immune cells, supportive tissue structures and cell types that are distinct to the tumor site in order to grow, become invasive and metastasize. Tumors influence their microenvironment by releasing soluble signals that lead to degradation and remodeling of the tissue structures that constrain their growth. Targeting the interactions of tumors with the microenvironment is an important and developing area of study.
- Read more about the tumor microenvironment focus area .
More about research at Mayo Clinic
- Research Faculty
- Laboratories
- Core Facilities
- Centers & Programs
- Departments & Divisions
- Clinical Trials
- Institutional Review Board
- Postdoctoral Fellowships
- Training Grant Programs
- Publications
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Policy & Insight
- Charity News
- Science & Technology
Stopping the spread: A revolution in how we think about metastasis

18 October 2022
When a cancer spreads from a primary tumour, the place where it first started to grow, to another area of the body, this is referred to as metastasis .
To spread, some cells from a primary tumour need to break away and travel to another place in the body via the bloodstream. These cells then form another tumour, called a secondary tumour, in another organ.
For example, breast cancer cells may travel to the lungs and form a secondary tumour there.
Metastasis is associated with very poor prognosis, and despite being the main cause of death in people with cancer, it has remained incredibly difficult to prevent and treat. This is largely because we haven’t been able to identify key drivers of this process that could act as therapeutic targets. Until now.
Ground-breaking research from scientists at our Cambridge Institute, recently published in Nature Genetics , has identified a protein in our cells, called NALCN, as a key regulator of metastasis.
What’s more, their findings show us that metastasis isn’t a process unique to cancer cells. Instead, cancer cells exploit a process that healthy tissues use to shed cells and move around the body, revolutionising the way we think about metastasis.
These findings are among the most important to have come out of my lab for three decades. Not only have we identified one of the elusive drivers of metastasis, but we have also turned a commonly held understanding of this on its head, showing how cancer hijacks this process in healthy cells for its own gains. Professor Richard Gilbertson
Finding the right channel
In their previous research, the team had identified a gene that was ‘turned off’ when a normal stem cell became a malignant stem cell in stomach cancers.
That gene makes the protein NALCN, which is a channel in the membrane of our cells that lets tiny molecules of salt pass in and out of them. The mutations caused the channel to be blocked off so it can’t perform its usual function.
This was unexpected, as the normal function of NALCN seemed to have no bearing otherwise on cancer development or progression.
So, in their most recent study they tested the effect of switching off this gene in mice with gastrointestinal cancers.
What they found was that losing the function of this gene had no effect on the primary tumour, but it caused the cancer to become extremely metastatic.
To investigate this phenomenon further, they switched the gene off in mice without cancer. And that’s where things got more interesting.
In these mice that had no NALCN, they found that healthy cells were metastasising around the body at a level similar to that of tumour cells in the mice with cancer.
Crucially, when these circulating cells reached another organ, they formed normal tissue in that organ. For example, if cells that had shed from the stomach had moved to the kidney, they turned into normal kidney cells.
“Traditionally, we think about our organs a bit like houses in a street, and those houses never share bricks with each other throughout life,” says Professor Richard Gilbertson, senior group leader at our Cambridge Institute.
“What this opens the possibility for is that organs can actually share their cells with each other.
“In fact, we see this at low rates going on all the time, even when we don’t delete this channel, suggesting that organs actually are much more fluid than we used to think.”
It may be that the body is therefore using this process as a repair mechanism. If some of the cells in one organ are damaged, cells from other organs can be mobilised to replace them.
This is the first time it’s been shown that metastasis is in fact a normal process, and it’s not only cancer cells that can spread around the body, a belief that’s been held for decades.
Solving the mysteries
In addition to elucidating the mechanism of metastasis, these findings might help to explain other current mysteries surrounding metastasis. For example, secondary tumours can appear in a person that had their primary tumour removed many years earlier or, in very rare cases, never had a primary tumour.
In these cases, it may be that healthy cells acquired some cancer-causing mutations but did not develop into cancer at their primary location. These ‘normal’ cells then shed from their original site, and move to other organs, where they formed normal tissue.
These cells then go on to become cancerous in the future after accumulating more mutations, creating metastases even though a primary tumour has been removed, or never formed in the tissue they originally came from.
And on the flipside, it provides an explanation as to why, for many years, dormant circulating tumour cells (CTCs) have been observed in people with cancer that have not gone on to form secondary tumours, which raised the question of why some CTCs form tumours and others don’t.
It’s because these circulating cells aren’t actually tumour cells, they’re cells from healthy tissues, we just didn’t know they could circulate before now.
“We’ve been tied to the concept that this has to be abnormal, therefore, they have to be cancer cells,” says Gilbertson. “But now we know that isn’t the case. They’re not tumour cells, they’re actually part of this normal process.”
Opening the possibilities
Having identified the driver of metastasis, we now have a potential therapeutic target for preventing it, which has huge implications for cancer survival.
The team are therefore looking into ways to restore the function of NALCN in cancer cells to prevent metastasis from occurring.
This might be tricky, as drugs that target this type of channel usually aim to block them, rather than hold them open. However, it has been achieved before in drugs used to treat other conditions like cystic fibrosis, and the team are investigating whether there are existing drugs that could be repurposed to prevent metastasis.
But that isn’t the only implication of this research.
“We’ve got lots of different avenues to explore,” says Dr Eric Rahrmann, lead researcher on the study and senior research associate at our Cambridge Institute.
“Can we use the mutations in this gene as a diagnostic marker? Like an early detection approach. Can we make predictions on whether people will have metastatic disease?
“We’re also looking at regenerative therapy medicines. If we enhance dissemination of cells from one organ to go into another organ to repair it, can we use this more as a truly reparative mechanism?”
This discovery has the potential to truly change the research landscape, both in the field of cancer research and beyond.
“If you could stop metastasis,” Gilbertson concludes. “Or significantly suppress it, you’re getting towards managing cancer for the long term, and that’s the Holy Grail.”
This is such an amazing discovery and really great news. I do so enjoy reading about the fantasic work being done by CRUK and associated research groups.
I have been reading your observations which clearly show great strides in your work which show great advancement in your research.
Keep up the good work so eventually you will develop enough experience gained from trials with mice to start trials in humans .
I am thrilled beyond words with this progress albeit nearly 8 years too late for my beloved husband
I hope and pray that this study can be fast tracked. If meds for Covid came so fast I truly hope this can too. Think of the lives that could be saved. As I’m living with MBC and also living with HOPE.
They need to realize time is critical. My young daughter has mtnbc and will likely never make it as “oversight” agencies spend years and years dragging things out. They will never convince me this is necessary for something absolutely lethal…
As someone living with MBC I am hopeful and thankful to all who are working to eradicate this horrible disease. A lot of these discoveries may not come to fruition in time for me with human trials but I am thankful that they may save so many lives.
Excellent news and as a stage IV breast cancer patient some inspirational news. Let’s have the drug available sooner than later please.
Amazing news about metastatic cancer Hopefully one day it can be treated and controlled, if not cured
An extremely exciting decision development!
This is fantastic research, congratulations to the team. Any chance we can get it fast tracked so that we are not waiting at least 10 years before it can save lives, either through repurposed drugs or novel drugs. We need urgent action now to save life and end the terrible suffering currently taking place with this disease.
Highlighted content
Sarah harding's legacy: finding women who may have higher breast cancer risks, general election 2024: what does this mean for the tobacco and vapes bill, skin cancer cases reach all-time high, an animal's guide to staying safe in the sun, hpv vaccine slashes cervical cancer rates across society, one to one with penny: volunteering special 1 - that cancer conversation podcast, the ‘mystery’ culprit causing kidney cancer worldwide, proteins in blood could give cancer warning seven years earlier, related topics.
- Cancer Research UK-funded research
- Cancer spread (metastasis)
- Stomach cancer
- Bowel (colorectal) cancer
- CRUK Cambridge Institute
- Cancer in the news
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Gene variants foretell the biology of future breast cancers in Stanford Medicine study
In a finding that vastly expands the understanding of tumor evolution, researchers discover genetic biomarkers that can predict the breast cancer subtype a patient is likely to develop.
May 30, 2024 - By Krista Conger

Stanford Medicine researchers found that inherited gene sequences can predict what type of breast cancer a patient is likely to develop, along with how aggressive that cancer may be. Emily Moskal
A Stanford Medicine study of thousands of breast cancers has found that the gene sequences we inherit at conception are powerful predictors of the breast cancer type we might develop decades later and how deadly it might be.
The study challenges the dogma that most cancers arise as the result of random mutations that accumulate during our lifetimes. Instead, it points to the active involvement of gene sequences we inherit from our parents — what’s known as your germline genome — in determining whether cells bearing potential cancer-causing mutations are recognized and eliminated by the immune system or skitter under the radar to become nascent cancers.
“Apart from a few highly penetrant genes that confer significant cancer risk, the role of hereditary factors remains poorly understood, and most malignancies are assumed to result from random errors during cell division or bad luck,” said Christina Curtis , PhD, the RZ Cao Professor of Medicine and a professor of genetics and of biomedical data science. “This would imply that tumor initiation is random, but that is not what we observe. Rather, we find that the path to tumor development is constrained by hereditary factors and immunity. This new result unearths a new class of biomarkers to forecast tumor progression and an entirely new way of understanding breast cancer origins.”
Curtis is the senior author of the study, which will be published May 31 in Science . Postdoctoral scholar Kathleen Houlahan , PhD, is the lead author of the research.
“Back in 2015, we had posited that some tumors are ‘born to be bad’ — meaning that their malignant and even metastatic potential is determined early in the disease course,” Curtis said. “We and others have since corroborated this finding across multiple tumors, but these findings cast a whole new light on just how early this happens.”
A new take on cancer’s origin
The study, which gives a nuanced and powerful new understanding of the interplay between newly arisen cancer cells and the immune system, is likely to help researchers and clinicians better predict and combat breast tumors.
Currently, only a few high-profile cancer-associated mutations in genes are regularly used to predict cancers, but these account for a small minority of cases. Those include BRCA1 and BRCA2, which occur in about one of every 500 women and confer an increased risk of breast or ovarian cancer, and rarer mutations in a gene called TP53 that causes a disease called Li Fraumeni syndrome, which predisposes to childhood and adult-onset tumors.

Christina Curtis
The findings suggest there are tens or hundreds of additional gene variants — identifiable in healthy people — that through interactions with the immune system pull the strings that determine why some people remain cancer-free throughout their lives.
“Our findings not only explain which subtype of breast cancer an individual is likely to develop,” Houlahan said, “but they also hint at how aggressive and prone to metastasizing that subtype will be. Beyond that, we speculate that these inherited variants may influence a person’s risk of developing breast cancer. However, future studies will be needed to examine this.”
The genes we inherit from our parents are known as our germline genome. They’re mirrors of our parents’ genetic makeup, and they can vary among people in small ways that give some of us blue eyes, brown hair or type O blood. Some inherited genes include mutations that confer increased cancer risk from the get-go, such as BRCA1, BRCA2 and TP53.
In contrast, most cancer-associated genes are part of what’s known as our somatic genome. As we live our lives, our cells divide and die in the tens of millions. Each time the DNA in a cell is copied, mistakes happen and mutations can accumulate. DNA in tumors is often compared with the germline genomes in blood or normal tissues in an individual to pinpoint which changes likely led to the cell’s cancerous transformation.
Classifying breast cancers
In 2012, Curtis began a deep dive — assisted by machine learning — into the types of somatic mutations that occur in thousands of breast cancers. She was eventually able to categorize the disease into 11 subtypes with varying prognoses and risk of recurrence, finding that four of the 11 groups were significantly more likely to recur even 10 or 20 years after diagnosis — critical information for clinicians making treatment decisions and discussing long-term prognoses with their patients.
Prior studies had shown that people with inherited BRCA1 mutations tend to develop a subtype of breast cancer known as triple negative breast cancer. This correlation implies some behind-the-scenes shenanigans by the germline genome that affects what subtype of breast cancer someone might develop.
“We wanted to understand how inherited DNA might sculpt how a tumor evolves,” Houlahan said. To do so, they took a close look at the immune system.
It’s a quirk of biology that even healthy cells routinely decorate their outer membranes with small chunks of the proteins they have bobbing in their cytoplasm — an outward display that reflects their inner style.

Kathleen Houlahan
The foundations for this display are what’s known as HLA proteins, and they are highly variable among individuals. Like fashion police, immune cells called T cells prowl the body looking for any suspicious or overly flashy bling (called epitopes) that might signal something is amiss inside the cell. A cell infected with a virus will display bits of viral proteins; a sick or cancerous cell will adorn itself with abnormal proteins. These faux pas trigger the T cells to destroy the offenders.
Houlahan and Curtis decided to focus on oncogenes, normal genes that, when mutated, can free a cell from regulatory pathways meant to keep it on the straight and narrow. Often, these mutations take the form of multiple copies of the normal gene, arranged nose to tail along the DNA — the result of a kind of genomic stutter called amplification. Amplifications in specific oncogenes drive different cancer pathways and were used to differentiate one breast cancer subtype from another in Curtis’ original studies.

The importance of bling
The researchers wondered whether highly recognizable epitopes would be more likely to attract T cells’ attention than other, more modest displays (think golf-ball-sized, dangly turquoise earrings versus a simple silver stud). If so, a cell that had inherited a flashy version of an oncogene might be less able to pull off its amplification without alerting the immune system than a cell with a more modest version of the same gene. (One pair of overly gaudy turquoise earrings can be excused; five pairs might cause a patrolling fashionista T cell to switch from tutting to terminating.)
The researchers studied nearly 6,000 breast tumors spanning various stages of disease to learn whether the subtype of each tumor correlated with the patients’ germline oncogene sequences. They found that people who had inherited an oncogene with a high germline epitope burden (read: lots of bling) — and an HLA type that can display that epitope prominently — were significantly less likely to develop breast cancer subtypes in which that oncogene is amplified.
There was a surprise, though. The researchers found that cancers with a large germline epitope burden that manage to escape the roving immune cells early in their development tended to be more aggressive and have a poorer prognosis than their more subdued peers.
“At the early, pre-invasive stage, a high germline epitope burden is protective against cancer,” Houlahan said. “But once it’s been forced to wrestle with the immune system and come up with mechanisms to overcome it, tumors with high germline epitope burden are more aggressive and prone to metastasis. The pattern flips during tumor progression.”
“Basically, there is a tug of war between tumor and immune cells,” Curtis said. “In the preinvasive setting, the nascent tumor may initially be more susceptible to immune surveillance and destruction. Indeed, many tumors are likely eliminated in this manner and go unnoticed. However, the immune system does not always win. Some tumor cells may not be eliminated and those that persist develop ways to evade immune recognition and destruction. Our findings shed light on this opaque process and may inform the optimal timing of therapeutic intervention, as well as how to make an immunologically cold tumor become hot, rendering it more sensitive to therapy.”
The researchers envision a future when the germline genome is used to further stratify the 11 breast cancer subtypes identified by Curtis to guide treatment decisions and improve prognoses and monitoring for recurrence. The study’s findings may also give additional clues in the hunt for personalized cancer immunotherapies and may enable clinicians to one day predict a healthy person’s risk of developing an invasive breast cancer from a simple blood sample.
“We started with a bold hypothesis,” Curtis said. “The field had not thought about tumor origins and evolution in this way. We’re examining other cancers through this new lens of hereditary and acquired factors and tumor-immune co-evolution.”
The study was funded by the National Institutes of Health (grants DP1-CA238296 and U54CA261719), the Canadian Institutes of Health Research and the Chan Zuckerberg Biohub.

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

- Frontiers in Oncology
- Cancer Metabolism
- Research Topics
Metastatic cancer: Cell behavior and the pre-metastatic niche
Total Downloads
Total Views and Downloads
About this Research Topic
The behavior of metastatic cancer cells is dependent on the type of cancer and the specific characteristics of the cells. In general, these cells are more aggressive and invasive than the primary tumor cells, and they have the ability to evade the immune system and resist chemotherapy and radiation therapy. ...
Keywords : Metastatic cascade, organ tropisms and colonization, primary tumor factors, extracellular vesicles, cancer cell dormancy, cancer treatment
Important Note : All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
Topic Editors
Topic coordinators, recent articles, submission deadlines, participating journals.
Manuscripts can be submitted to this Research Topic via the following journals:
total views
- Demographics
No records found
total views article views downloads topic views
Top countries
Top referring sites, about frontiers research topics.
With their unique mixes of varied contributions from Original Research to Review Articles, Research Topics unify the most influential researchers, the latest key findings and historical advances in a hot research area! Find out more on how to host your own Frontiers Research Topic or contribute to one as an author.
Scientists create tailored drug for aggressive breast cancer
Scientists have used breast cancer cells' weakness against themselves by linking a tumour-selective antibody with a cell-killing drug to destroy hard-to-treat tumours.
The research, published today in Clinical Cancer Research by a team from King's College London and funded by Breast Cancer Now, marks a new method in cancer treatment.
The discovery is particular to triple negative breast cancer, which makes up 15% of all diagnosed breast cancer. This type of breast cancer is typically aggressive, resistant to chemotherapy, has a lower survival rate and is more common in women under 40.
Usual treatment involves surgery, chemotherapy and radiotherapy, however this type of cancer can evade the drugs and return to spread again.
The scientists conducted data analysis using over 6000 breast cancer samples to investigate the properties of breast cancer cells that are associated with aggressive and chemotherapy-resistant cancers.
They studied the cancer's biology, what is expressed in the tumour and the cell surface, and the cell's insides to understand how the cancer cells escape from cancer drugs. They established the presence of the cancer cell surface marker EGFR along with oncogenic molecules cyclin-dependent kinases (CDK), which are responsible for cell division and proliferation.
They used this knowledge against the cancer cells to link cetuximab, a tumour-selective antibody that targets the EGFR protein expressed in this type of cancer, with a CDK-blocking drug to create a tailored drug for breast cancer. Because the antibody drug conjugate specifically targets the cancer cell, it may be possible to administer a lower inhibitor dose than usual which means it's less toxic for the patient.
Lead author Professor Sophia Karagiannis, from King's College London, said: "We were on the hunt for cancer's vulnerabilities and now we've found out how we can guide our therapies to one of these. We combined these two drugs to create a tailored antibody drug conjugate for patients with this aggressive cancer. The antibody guides the toxic drug directly to the cancer cell which offers the possibility for a lower dose and less adverse side effects to be experienced.
"More work needs to be done before this therapy can reach the clinic, but we expect that this can offer new treatment options for cancers with unfavourable prognosis. Beyond this antibody drug conjugate, we hope that our concept will lead the way for new antibody drug conjugates of this type to be tailored to patient groups likely to benefit."
Lead research scientist Dr Anthony Cheung from King's College London said: ''Triple negative breast cancer represents a molecularly and clinically diverse disease. By exploiting EGFR overexpression and dysregulated cell cycle molecules in selected patient groups, the antibody drug conjugate, but not the antibody alone, could stop the cancer cell from dividing and engender cytotoxic functions specifically against the cancer cells.''
Dr Simon Vincent, director of services, support and influencing at Breast Cancer Now, which funded this research, said: "Each year, around 8,000 women in the UK are diagnosed with triple negative breast cancer, which is typically more aggressive than other breast cancers and more likely to return or spread following treatment.
"This exciting research has not only improved our understanding of the properties of aggressive breast cancer cells that are resistant to chemotherapy but has also brought us closer to developing a targeted therapy that destroys these cancer cells while minimising side effects for patients.
"While further research is needed before this treatment can be used in people, this is an exciting step forward in developing targeted therapies for triple negative breast cancer, and we look forward to seeing how these findings could lead to new and effective ways of tackling this devastating disease."
- Breast Cancer
- Lung Cancer
- Colon Cancer
- Brain Tumor
- Ovarian Cancer
- Monoclonal antibody therapy
- Chemotherapy
- Mammography
- Breast cancer
- Esophageal cancer
Story Source:
Materials provided by King's College London . Note: Content may be edited for style and length.
Journal Reference :
- Anthony Cheung, Alicia M. Chenoweth, Annelie Johansson, Roman Laddach, Naomi Guppy, Jennifer Trendell, Benjamina Esapa, Antranik Mavousian, Blanca Navarro-Llinas, Syed Haider, Pablo Romero-Clavijo, Ricarda M. Hoffmann, Paolo Andriollo, Khondaker Miraz Rahman, Paul Jackson, Sophia Tsoka, Sheeba Irshad, Ioannis Roxanis, Anita Grigoriadis, David E. Thurston, Christopher J. Lord, Andrew N.J. Tutt, Sophia N. Karagiannis. Anti-EGFR antibody-drug conjugate carrying an inhibitor targeting CDK restricts triple-negative breast cancer growth . Clinical Cancer Research , 2024; DOI: 10.1158/1078-0432.CCR-23-3110
Cite This Page :
Explore More
- Extinct Saber-Toothed Cat On Texas Coast
- Some Black Holes Survive in Globular Clusters
- People Altering Decomposition in Waterways
- Historic Iceberg Surges
- Food Groups Based On Level of Processing
- Computer Vision, Machine Learning Aid Driving
- Most Distant Known Galaxy
- A New Dinosaur from Zimbabwe
- The Case of the Missing Black Holes
- Menstrual Periods Arriving Earlier
Trending Topics
Strange & offbeat.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 31, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency
Pfizer drug extends life for people with rare form of lung cancer

A Pfizer lung cancer drug has been shown to greatly reduce tumor progression and improve survival outcomes for people in the advanced stages of a rare form of the disease, according to trial results published Friday.
Lorlatinib, which is already approved and available under the brand name Lorbrena in the United States, was tested in a clinical trial of hundreds of people with anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC).
Roughly half received lorlatinib while the rest received crizotinib, an earlier generation drug.
After five years of follow-up, more than half of patients treated with lorlatinib did not see their cancer progress.
"We're talking about patients with advanced metastatic disease—so this is actually a truly unprecedented finding," Pfizer's thoracic oncology strategy lead Despina Thomaidou told AFP.
Sixty percent of patients receiving lorlatinib, an oral one a day tablet, were alive without disease progression after five years compared to 8 percent on crizotinib.
"There is an 81 percent reduction in the risk of progression or death," added Thomaidou.
Lung cancer is the leading cause of cancer deaths globally.
NSCLC accounts for more than 80 percent of lung cancers, with ALK-positive tumors responsible for roughly five percent of NSCLC cases, translating to around 72,000 new cases each year worldwide.
ALK-positive NSCLC mostly affects younger patients and is not strongly linked to smoking. It is also very aggressive—25-40 percent of people with ALK-positive NSCLC develop brain metastases within the first two years.
Lorlatinib penetrates the blood-brain barrier better than prior generation medicines, said Thomaidou, and works to inhibit tumor mutations that drive resistance.
Patients on the lorlatinib arm had a 94 percent risk reduction in the progression of brain metastases compared to crizotinib.
Side effects of lorlatinib included swellings, weight gain and mental health problems such as depression.
"The progression-free survival is outstanding—we have not seen anything close to this," said oncologist David Spigel of Sarah Cannon Research Institute in Nashville, who was not involved in the study.
One critique he had was that lorlatinib was compared to crizotinib, which was "an outstanding drug in its time," but has since fallen out of use in the United States.
The results were published at the annual meeting of the American Society of Clinical Oncology and in the Journal of Clinical Oncology .
Explore further
Feedback to editors

Neuroscience research suggests ketones can enhance cognitive function and protect brain networks
51 minutes ago
New research finds antidepressants may help deliver other drugs into the brain

Mediterranean diet tied to one-fifth lower risk of death in women

Scientists find new method to enhance efficacy of bispecific antibodies for solid tumors

Cardiomyocytes study discovers new way to regenerate damaged heart cells
Study: The route into the cell influences the outcome of SARS-CoV-2 infection
Prenatal testing offers a window for finding a mother's cancer risk
2 hours ago

Study shows most doctors endorsing drugs on X are paid to do so

Mobile app effective in smoking cessation, triples chance of success

Infants hear significantly more speech than music at home, study finds
Related stories.

Early treatment with lorlatinib improves survival in some lung cancer patients
Nov 18, 2020
Patient-reported outcomes from the randomized phase III CROWN study of first-line Lorlatinib versus in ALK+ NSCLC
Jan 31, 2021
Treatment of advanced non-small cell lung cancer with driver mutations
Mar 27, 2024
Alecensa approved as first and only anaplastic lymphoma kinase inhibitor for non-small cell lung cancer
Apr 23, 2024
Alectinib halts lung cancer growth more than a year longer than crizotinib
Jun 5, 2017

Targeted lung cancer treatment gets initial 'no' for NHS in England
Feb 5, 2020
Recommended for you

Team finds new potential causes of rare and lethal bone cancer
4 hours ago

Gene variants foretell the biology of future breast cancers, study finds
23 hours ago

Study: Access to targeted lung cancer drug is cost-prohibitive globally
20 hours ago

Study confirms effectiveness of 'watch-and-wait' approach to prostate cancer
21 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Featured Topics
Featured series.
A series of random questions answered by Harvard experts.
Explore the Gazette
Read the latest.
New Alzheimer’s study suggests genetic cause of specific form of disease

Had a bad experience meditating? You’re not alone.
Rethinking life-support decisions

Harvard-led study IDs statin that may block pathway to some cancers
Cholesterol-lowering drug suppresses chronic inflammation that creates dangerous cascade
Tracy Hampton
MGH Communications
Statins, commonly used cholesterol-lowering drugs, may block a pathway that leads to the development of cancer from chronic inflammation, according to a new study led by investigators from Harvard-affiliated Mass General Cancer Center .
The team’s experiments showed that environmental toxins, such as those caused by exposure to allergens and chemical irritants, create a cascade effect that stimulates inflammation in the skin and pancreas that, when chronic, can result in cancer. Their findings suggest that using statins to suppress this pathway may have a protective effect.
The findings are published in Nature Communications.
In mice, pitavastatin suppressed environmentally induced inflammation in the skin and the pancreas and prevented the development of inflammation-related pancreatic cancers.
“Chronic inflammation is a major cause of cancer worldwide,” said senior author Shawn Demehri, a principal investigator at the Center for Cancer Immunology and Cutaneous Biology Research Center of Massachusetts General Hospital and an associate professor of dermatology at Harvard Medical School and the Bob and Rita Davis Family MGH Research Scholar 2023-2028 .
“We investigated the mechanism by which environmental toxins drive the initiation of cancer-prone chronic inflammation in the skin and pancreas. Furthermore, we examined safe and effective therapies to block this pathway in order to suppress chronic inflammation and its cancer aftermath,” Demehri said.
The study relied on cell lines, animal models, human tissue samples, and epidemiological data. The group’s cell-based experiments demonstrated that environmental toxins (such as exposure to allergens and chemical irritants) activate two connected signaling pathways called the TLR3/4 and TBK1-IRF3 pathways. This activation leads to the production of the interleukin-33 (IL-33) protein, which stimulates inflammation in the skin and pancreas that can contribute to the development of cancer.
When they screened a library of U.S. Food and Drug Administration-approved drugs, the researchers found that the statin pitavastatin effectively suppresses IL-33 expression by blocking the activation of the TBK1-IRF3 signaling pathway. In mice, pitavastatin suppressed environmentally induced inflammation in the skin and pancreas and prevented development of inflammation-related pancreatic cancers.
In human pancreas tissue samples, IL-33 was overexpressed in samples from patients with chronic pancreatitis (inflammation) and pancreatic cancer compared with normal pancreatic tissue. Also, in analyses of electronic health records data on more than 200 million people across North America and Europe, use of pitavastatin was linked to a significantly reduced risk of chronic pancreatitis and pancreatic cancer.
The findings demonstrate that blocking IL-33 production with pitavastatin may be a safe and effective preventive strategy to suppress chronic inflammation and the subsequent development of certain cancers.
“Next, we aim to further examine the impact of statins in preventing cancer development in chronic inflammation in liver and gastrointestinal tract and to identify other novel, therapeutic approaches to suppress cancer-prone chronic inflammation” said Demehri.
Research support was provided by the Burroughs Wellcome Fund, the LEO Foundation, the Sidney Kimmel Foundation, and the National Institutes of Health.
Share this article
You might like.
Findings eventually could pave way to earlier diagnosis, treatment, and affect search for new therapies

Altered states of consciousness through yoga, mindfulness more common than thought and mostly beneficial, study finds — though clinicians ill-equipped to help those who struggle

Of the survivors within one study group, more than 40% recovered at least some independence.
Six receive honorary degrees
Harvard recognizes educator, conductor, theoretical physicist, advocate for elderly, writer, and Nobel laureate
When should Harvard speak out?
Institutional Voice Working Group provides a roadmap in new report
Day to remember
One journey behind them, grads pause to reflect before starting the next
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 28 May 2024
Mechanisms of metastatic colorectal cancer
- Adrià Cañellas-Socias ORCID: orcid.org/0000-0002-8373-7803 1 , 2 nAff4 ,
- Elena Sancho 1 , 2 &
- Eduard Batlle ORCID: orcid.org/0000-0003-2422-0326 1 , 2 , 3
Nature Reviews Gastroenterology & Hepatology ( 2024 ) Cite this article
52 Altmetric
Metrics details
- Colon cancer
Despite extensive research and improvements in understanding colorectal cancer (CRC), its metastatic form continues to pose a substantial challenge, primarily owing to limited therapeutic options and a poor prognosis. This Review addresses the emerging focus on metastatic CRC (mCRC), which has historically been under-studied compared with primary CRC despite its lethality. We delve into two crucial aspects: the molecular and cellular determinants facilitating CRC metastasis and the principles guiding the evolution of metastatic disease. Initially, we examine the genetic alterations integral to CRC metastasis, connecting them to clinically marked characteristics of advanced CRC. Subsequently, we scrutinize the role of cellular heterogeneity and plasticity in metastatic spread and therapy resistance. Finally, we explore how the tumour microenvironment influences metastatic disease, emphasizing the effect of stromal gene programmes and the immune context. The ongoing research in these fields holds immense importance, as its future implications are projected to revolutionize the treatment of patients with mCRC, hopefully offering a promising outlook for their survival.
Despite metastatic disease being the main cause of death in colorectal cancer (CRC), its molecular basis remains poorly characterized, which hinders the development of therapeutic strategies.
Data support the view that most driver events necessary for metastatic dissemination are acquired early during cancer evolution; however, mutations in oncogenes and tumour suppressor genes influence metastatic disease progression.
Tumour cell plasticity is pervasive and necessary for metastatic progression; tumour cells co-opt different cell states during metastatic dissemination, relapse, outgrowth and therapy resistance.
Metastases show either an immune-excluded or an immune-desert phenotype, implying a major role of the immune system in shaping metastatic CRC; different metastases from the same patient might exhibit distinct immune environments.
A holistic view of immune evasion mechanisms in metastases is lacking, and emerging evidence suggests that metastases of different organs co-opt distinct tumour microenvironments and respond differently to immune therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

Molecular principles of metastasis: a hallmark of cancer revisited

Genetic and microenvironmental intra-tumor heterogeneity impacts colorectal cancer evolution and metastatic development

Molecular characterization of colorectal cancer related peritoneal metastatic disease
Dekker, E., Tanis, P. J., Vleugels, J. L. A., Kasi, P. M. & Wallace, M. B. Colorectal cancer. Lancet 394 , 1467–1480 (2019).
Article PubMed Google Scholar
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A. & Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 73 , 233–254 (2023).
Douillard, J.-Y. et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 369 , 1023–1034 (2013).
Article CAS PubMed Google Scholar
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350 , 2335–2342 (2004).
Cervantes, A. et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34 , 10–32 (2022).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366 , 2455–2465 (2012).
Article CAS PubMed PubMed Central Google Scholar
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366 , 2443–2454 (2012).
Fearon, E. R. & Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 61 , 759–767 (1990).
Lannagan, T. R., Jackstadt, R., Leedham, S. J. & Sansom, O. J. Advances in colon cancer research: in vitro and animal models. Curr. Opin. Genet. Dev. 66 , 50–56 (2021).
Batlle, E. & Clevers, H. Cancer stem cells revisited. Nat. Med. 23 , 1124–1134 (2017).
Tilg, H., Adolph, T. E., Gerner, R. R. & Moschen, A. R. The intestinal microbiota in colorectal cancer. Cancer Cell 33 , 954–964 (2018).
Kuipers, E. J. et al. Colorectal cancer. Nat. Rev. Dis. Prim. 1 , 15065 (2015).
Li, J., Ma, X., Chakravarti, D., Shalapour, S. & DePinho, R. A. Genetic and biological hallmarks of colorectal cancer. Genes. Dev. 38 , 787–820 (2021).
Article Google Scholar
Rahbari, N. N. et al. Time of metastasis and outcome in colorectal cancer. Ann. Surg. 269 , 494–502 (2019).
Chen, K., Collins, G., Wang, H. & Toh, J. W. T. Pathological features and prognostication in colorectal cancer. Curr. Oncol. 28 , 5356–5383 (2021).
Article PubMed PubMed Central Google Scholar
Gustavsson, B. et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin. Colorectal Cancer 14 , 1–10 (2015).
Kopetz, S. et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J. Clin. Oncol. 27 , 3677–3683 (2009).
Pastorino, U. et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J. Thorac. Cardiovasc. Surg. 113 , 37–49 (1997).
Lipsyc, M. & Yaeger, R. Impact of somatic mutations on patterns of metastasis in colorectal cancer. J. Gastrointest. Oncol. 6 , 645–649 (2015).
PubMed PubMed Central Google Scholar
Yaeger, R. et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 33 , 125–136.e3 (2018).
Moertel, C. G. et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 322 , 352–358 (1990).
Varghese, A. Chemotherapy for stage II colon cancer. Clin. Colon. Rectal Surg. 28 , 256–261 (2015).
André, T. et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J. Clin. Oncol. 33 , 4176–4187 (2015).
Tie, J. et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N. Engl. J. Med. 386 , 2261–2272 (2022).
Kotani, D. et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 29 , 127–134 (2023).
Birkbak, N. J. & McGranahan, N. Cancer genome evolutionary trajectories in metastasis. Cancer Cell 37 , 8–19 (2020).
Priestley, P. et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 575 , 210–216 (2019).
Robinson, D. R. et al. Integrative clinical genomics of metastatic cancer. Nature 548 , 297–303 (2017).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23 , 703–713 (2017).
Martínez-Jiménez, F. et al. Pan-cancer whole-genome comparison of primary and metastatic solid tumours. Nature 618 , 333–341 (2023).
Nguyen, B. et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell 185 , 563–575.e11 (2022).
Sottoriva, A. et al. A Big Bang model of human colorectal tumor growth. Nat. Genet. 47 , 209–216 (2015).
Holch, J. W., Ricard, I., Stintzing, S., Modest, D. P. & Heinemann, V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur. J. Cancer 70 , 87–98 (2017).
Sartore-Bianchi, A. et al. Central nervous system as possible site of relapse in ERBB2 -positive metastatic colorectal cancer. JAMA Oncol. 6 , 927 (2020).
Strickler, J. H., Yoshino, T., Graham, R. P., Siena, S. & Bekaii-Saab, T. Diagnosis and treatment of ERBB2-positive metastatic colorectal cancer. JAMA Oncol. 8 , 760 (2022).
Schell, M. J. et al. A multigene mutation classification of 468 colorectal cancers reveals a prognostic role for APC. Nat. Commun. 7 , 1–12 (2016).
Elez, E. et al. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAF V600E metastatic colorectal cancer. Nat. Med. 28 , 2162–2170 (2022).
Parsons, M. J., Tammela, T. & Dow, L. E. WNT as a driver and dependency in cancer. Cancer Discov. 11 , 2413–2429 (2021).
Muzny, D. M. et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature 487 , 330–337 (2012).
Article CAS Google Scholar
Fujii, M. et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18 , 827–838 (2016).
Fumagalli, A. et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc. Natl Acad. Sci. USA 114 , E2357–E2364 (2017).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459 , 262–265 (2009).
Jung, P. et al. Isolation and in vitro expansion of human colonic stem cells. Nat. Med. 17 , 1225–1227 (2011).
Van De Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161 , 933–945 (2015).
Matano, M. et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat. Med. 21 , 256–262 (2015).
O’Rourke, K. P. et al. Transplantation of engineered organoids enables rapid generation of metastatic mouse models of colorectal cancer. Nat. Biotechnol. 35 , 577–582 (2017).
Tauriello, D. V. F. et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554 , 538–543 (2018).
Roper, J. et al. In vivo genome editing and organoid transplantation models of colorectal cancer and metastasis. Nat. Biotechnol. 35 , 569–576 (2017).
Hu, Z., Li, Z., Ma, Z. & Curtis, C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat. Genet. 52 , 701–708 (2020).
Roerink, S. F. et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 556 , 457–462 (2018).
Hu, Z. et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 51 , 1113–1122 (2019).
Naxerova, K. et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science 357 , 55–60 (2017).
Reiter, J. G. et al. Lymph node metastases develop through a wider evolutionary bottleneck than distant metastases. Nat. Genet. 52 , 692–700 (2020).
Ryser, M. D., Min, B. H., Siegmund, K. D. & Shibata, D. Spatial mutation patterns as markers of early colorectal tumor cell mobility. Proc. Natl Acad. Sci. USA 115 , 5774–5779 (2018).
Bretthauer, M. et al. Effect of colonoscopy screening on risks of colorectal cancer and related death. N. Engl. J. Med. 387 , 1547–1556 (2022).
Dang, H. X. et al. The clonal evolution of metastatic colorectal cancer. Sci. Adv. 6 , eaay9691 (2020).
Kok, S. Y. et al. Malignant subclone drives metastasis of genetically and phenotypically heterogenous cell clusters through fibrotic niche generation. Nat. Commun. 12 , 863 (2021).
Cheung, K. J. & Ewald, A. J. A collective route to metastasis: seeding by tumor cell clusters. Science 352 , 167–169 (2016).
Comen, E., Norton, L. & Massagué, J. Clinical implications of cancer self-seeding. Nat. Rev. Clin. Oncol. 8 , 369–377 (2011).
Campbell, K. et al. Collective cell migration and metastases induced by an epithelial-to-mesenchymal transition in Drosophila intestinal tumors. Nat. Commun. 10 , 2311 (2019).
Cañellas-Socias, A. et al. Metastatic recurrence in colorectal cancer arises from residual EMP1 + cells. Nature 611 , 603–613 (2022).
Massagué, J. & Ganesh, K. Metastasis-initiating cells and ecosystems. Cancer Discov. 11 , 971–994 (2021).
André, T. et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 27 , 3109–3116 (2009).
Nors, J., Iversen, L. H., Erichsen, R., Gotschalck, K. A. & Andersen, C. L. Incidence of recurrence and time to recurrence in stage I to III colorectal cancer. JAMA Oncol. 10 , 54–62 (2024).
Engstrand, J., Strömberg, C., Nilsson, H., Freedman, J. & Jonas, E. Synchronous and metachronous liver metastases in patients with colorectal cancer – towards a clinically relevant definition. World J. Surg. Oncol. 17 , 228 (2019).
Mizuno, T. et al. SMAD4 gene mutation predicts poor prognosis in patients undergoing resection for colorectal liver metastases. Eur. J. Surg. Oncol. 44 , 684–692 (2018).
Álvarez-Varela, A. et al. Mex3a marks drug-tolerant persister colorectal cancer cells that mediate relapse after chemotherapy. Nat. Cancer 3 , 1052–1070 (2022).
Gerstberger, S., Jiang, Q. & Ganesh, K. Metastasis. Cell 186 , 1564–1579 (2023).
Smith, H. A. & Kang, Y. Determinants of organotropic metastasis. Annu. Rev. Cancer Biol. 1 , 403–423 (2017).
Font-Clos, F., Zapperi, S. & La Porta, C. A. M. Blood flow contributions to cancer metastasis. iScience 23 , 101073 (2020).
Denève, E. et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 59 , 1384–1392 (2013).
Tsilimigras, D. I. et al. Liver metastases. Nat. Rev. Dis. Prim. 7 , 27 (2021).
Riihimaki, M., Hemminki, A., Sundquist, J. & Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 6 , 29765 (2016).
Wei, Q. et al. Multiregion whole-exome sequencing of matched primary and metastatic tumors revealed genomic heterogeneity and suggested polyclonal seeding in colorectal cancer metastasis. Ann. Oncol. 28 , 2135–2141 (2017).
Gassmann, P., Hemping-Bovenkerk, A., Mees, S. T. & Haier, J. Metastatic tumor cell arrest in the liver-lumen occlusion and specific adhesion are not exclusive. Int. J. Colorectal Dis. 24 , 851–858 (2009).
Haier, J., Korb, T., Hotz, B., Spiegel, H.-U. & Senninger, N. An intravital model to monitor steps of metastatic tumor cell adhesion within the hepatic microcirculation. J. Gastrointest. Surg. 7 , 507–515 (2003).
Brodt, P. et al. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int. J. Cancer 71 , 612–619 (1997).
Khatib, A.-M., Fallavollita, L., Wancewicz, E. V., Monia, B. P. & Brodt, P. Inhibition of hepatic endothelial E-selectin expression by C-raf antisense oligonucleotides blocks colorectal carcinoma liver metastasis. Cancer Res. 62 , 5393–5398 (2002).
CAS PubMed Google Scholar
Nakamori, S. et al. Involvement of carbohydrate antigen sialyl Lewis x in colorectal cancer metastasis. Dis. Colon. Rectum 40 , 420–431 (1997).
Timmers, M. et al. Interactions between rat colon carcinoma cells and Kupffer cells during the onset of hepatic metastasis. Int. J. Cancer 112 , 793–802 (2004).
Matsumura, H. et al. Kupffer cells decrease metastasis of colon cancer cells to the liver in the early stage. Int. J. Oncol. 45 , 2303–2310 (2014).
Urosevic, J. et al. Colon cancer cells colonize the lung from established liver metastases through p38 MAPK signalling and PTHLH. Nat. Cell Biol. 16 , 685–694 (2014).
Sonoshita, M. et al. Promotion of colorectal cancer invasion and metastasis through activation of NOTCH–DAB1–ABL–RHOGEF protein TRIO. Cancer Discov. 5 , 198–211 (2015).
Sonoshita, M. et al. Suppression of colon cancer metastasis by Aes through inhibition of Notch signaling. Cancer Cell 19 , 125–137 (2011).
Giannou, A. D. et al. Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity 56 , 125–142.e12 (2023).
Franko, J. et al. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J. Clin. Oncol. 30 , 263–267 (2012).
Franko, J. et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 17 , 1709–1719 (2016).
Bootsma, S., Bijlsma, M. F. & Vermeulen, L. The molecular biology of peritoneal metastatic disease. EMBO Mol. Med. 15 , e15914 (2023).
Enblad, M., Graf, W. & Birgisson, H. Risk factors for appendiceal and colorectal peritoneal metastases. Eur. J. Surg. Oncol. 44 , 997–1005 (2018).
Demuytere, J., Ernst, S., van Ovost, J., Cosyns, S. & Ceelen, W. The tumor immune microenvironment in peritoneal carcinomatosis. Int. Rev. Cell Mol. Biol. 371 , 63–95 (2022).
Lenos, K. J. et al. Molecular characterization of colorectal cancer related peritoneal metastatic disease. Nat. Commun. 13 , 4443 (2022).
Laoukili, J. et al. Peritoneal metastases from colorectal cancer belong to Consensus Molecular Subtype 4 and are sensitised to oxaliplatin by inhibiting reducing capacity. Br. J. Cancer 126 , 1824–1833 (2022).
Househam, J. et al. Phenotypic plasticity and genetic control in colorectal cancer evolution. Nature 611 , 744–753 (2022).
Joanito, I. et al. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat. Genet. 54 , 963–975 (2022).
Vermeulen, L. et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12 , 468–476 (2010).
Lenos, K. J. et al. Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nat. Cell Biol. 20 , 1193–1202 (2018).
Medema, J. P. & Vermeulen, L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 474 , 318–326 (2011).
De Sousa e Melo, F. et al. A distinct role for Lgr5 + stem cells in primary and metastatic colon cancer. Nature 543 , 676–680 (2017).
Fumagalli, A. et al. Plasticity of Lgr5-negative cancer cells drives metastasis in colorectal cancer. Cell Stem Cell 26 , 569–578.e7 (2020).
Heinz, M. C. et al. Liver colonization by colorectal cancer metastases requires YAP-controlled plasticity at the micrometastatic stage. Cancer Res. 82 , 1953–1968 (2022).
Ganesh, K. et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer 1 , 28–45 (2020).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449 , 1003–1007 (2007).
Ayyaz, A. et al. Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569 , 121–125 (2019).
Barry, E. R. et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493 , 106–110 (2013).
Gregorieff, A., Liu, Y., Inanlou, M. R., Khomchuk, Y. & Wrana, J. L. Yap-dependent reprogramming of Lgr5 + stem cells drives intestinal regeneration and cancer. Nature 526 , 715–718 (2015).
Yui, S. et al. YAP/TAZ-dependent reprogramming of colonic epithelium links ECM remodeling to tissue regeneration. Cell Stem Cell 22 , 35–49.e7 (2018).
Ohara, T. E., Colonna, M. & Stappenbeck, T. S. Adaptive differentiation promotes intestinal villus recovery. Dev. Cell 57 , 166–179.e6 (2022).
Nusse, Y. M. et al. Parasitic helminths induce fetal-like reversion in the intestinal stem cell niche. Nature 559 , 109–113 (2018).
Pikkupeura, L. M. et al. Transcriptional and epigenomic profiling identifies YAP signaling as a key regulator of intestinal epithelium maturation. Sci. Adv. 9 , eadf9460 (2023).
Guiu, J. et al. Tracing the origin of adult intestinal stem cells. Nature 570 , 107–111 (2019).
Mustata, R. C. et al. Identification of Lgr5-independent spheroid-generating progenitors of the mouse fetal intestinal epithelium. Cell Rep. 5 , 421–432 (2013).
Ohta, Y. et al. Cell-matrix interface regulates dormancy in human colon cancer stem cells. Nature 608 , 784–794 (2022).
Solé, L. et al. P53 wild-type colorectal cancer cells that express a fetal gene signature are associated with metastasis and poor prognosis. Nat. Commun. 13 , 2866 (2022).
Lupo, B. et al. Colorectal cancer residual disease at maximal response to EGFR blockade displays a druggable Paneth cell-like phenotype. Sci. Transl. Med. 12 , eaax8313 (2020).
Leach, J. D. G. et al. Oncogenic BRAF, unrestrained by TGFβ-receptor signalling, drives right-sided colonic tumorigenesis. Nat. Commun. 12 , 3464 (2021).
Martinez-Ordoñez, A. et al. Hyaluronan driven by epithelial aPKC deficiency remodels the microenvironment and creates a vulnerability in mesenchymal colorectal cancer. Cancer Cell 41 , 252–271.e9 (2023).
Vasquez, E. G. et al. Dynamic and adaptive cancer stem cell population admixture in colorectal neoplasia. Cell Stem Cell 29 , 1213–1228.e8 (2022).
Ramos Zapatero, M. et al. Trellis tree-based analysis reveals stromal regulation of patient-derived organoid drug responses. Cell 186 , 5606–5619.e24 (2023).
Moorman, A. R. et al. Progressive plasticity during colorectal cancer metastasis. Preprint at bioRxiv https://doi.org/10.1101/2023.08.18.553925 (2023).
Qin, X. et al. An oncogenic phenoscape of colonic stem cell polarization. Cell 186 , 5554–5568.e18 (2023).
Azzolin, L. et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell 158 , 157–170 (2014).
Cheung, P. et al. Regenerative reprogramming of the intestinal stem cell state via Hippo signaling suppresses metastatic colorectal cancer. Cell Stem Cell 27 , 590–604.e9 (2020).
Latacz, E. et al. Histopathological growth patterns of liver metastasis: updated consensus guidelines for pattern scoring, perspectives and recent mechanistic insights. Br. J. Cancer 127 , 988–1013 (2022).
Frentzas, S. et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat. Med. 22 , 1294–1302 (2016).
Nierop, P. M. H. et al. Preoperative systemic chemotherapy alters the histopathological growth patterns of colorectal liver metastases. J. Pathol. Clin. Res. 8 , 48–64 (2022).
Rubbia-Brandt, L. et al. Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann. Oncol. 18 , 299–304 (2007).
Messaoudi, N. et al. Prognostic implications of adaptive immune features in MMR-proficient colorectal liver metastases classified by histopathological growth patterns. Br. J. Cancer 126 , 1329–1338 (2022).
Stremitzer, S. et al. Immune phenotype and histopathological growth pattern in patients with colorectal liver metastases. Br. J. Cancer 122 , 1518–1524 (2020).
Liang, J. Y. et al. Histopathological growth patterns correlate with the immunoscore in colorectal cancer liver metastasis patients after hepatectomy. Cancer Immunol. Immunother. 69 , 2623–2634 (2020).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541 , 321–330 (2017).
Liu, Y. et al. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell 40 , 424–437.e5 (2022).
Che, L. H. et al. A single-cell atlas of liver metastases of colorectal cancer reveals reprogramming of the tumor microenvironment in response to preoperative chemotherapy. Cell Discov. 7 , 80 (2021).
Wang, F. et al. Single-cell and spatial transcriptome analysis reveals the cellular heterogeneity of liver metastatic colorectal cancer. Sci. Adv. 9 , eadf5464 (2023).
Friedman, E. et al. High levels of transforming growth factor beta 1 correlate with disease progression in human colon cancer. Cancer Epidemiol. Biomark. Prev. 4 , 549–554 (1995).
CAS Google Scholar
Calon, A. et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22 , 571–584 (2012).
Calon, A. et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 47 , 320–329 (2015).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16 , 582–598 (2016).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554 , 544–548 (2018).
Chakravarthy, A., Khan, L., Bensler, N. P., Bose, P. & De Carvalho, D. D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9 , 4692 (2018).
Tauriello, D. V. F., Sancho, E. & Batlle, E. Overcoming TGFβ-mediated immune evasion in cancer. Nat. Rev. Cancer 22 , 25–44 (2022).
Krishnamurty, A. T. et al. LRRC15 + myofibroblasts dictate the stromal setpoint to suppress tumour immunity. Nature 611 , 148–154 (2022).
Nicolas-Boluda, A. & Donnadieu, E. Obstacles to T cell migration in the tumor microenvironment. Comp. Immunol. Microbiol. Infect. Dis. 63 , 22–30 (2019).
Wolf, K., Müller, R., Borgmann, S., Bröcker, E.-B. & Friedl, P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood 102 , 3262–3269 (2003).
Kaur, A. et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 9 , 64–81 (2019).
Salmon, H. et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122 , 899–910 (2012).
Sadjadi, Z., Zhao, R., Hoth, M., Qu, B. & Rieger, H. Migration of cytotoxic T lymphocytes in 3D collagen matrices. Biophys. J. 119 , 2141–2152 (2020).
Shen, Y. et al. Reduction of liver metastasis stiffness improves response to bevacizumab in metastatic colorectal cancer. Cancer Cell 37 , 800–817.e7 (2020).
Friedl, P. & Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10 , 445–457 (2009).
Linares, J. et al. Long-term platinum-based drug accumulation in cancer-associated fibroblasts promotes colorectal cancer progression and resistance to therapy. Nat. Commun. 14 , 746 (2023).
Li, H. et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 49 , 708–718 (2017).
Lee, H. O. et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat. Genet. 52 , 594–603 (2020).
Kobayashi, H. et al. The origin and contribution of cancer-associated fibroblasts in colorectal carcinogenesis. Gastroenterology 162 , 890–906 (2022).
Heichler, C. et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut 69 , 1269–1282 (2020).
Koncina, E. et al. IL1R1 + cancer-associated fibroblasts drive tumor development and immunosuppression in colorectal cancer. Nat. Commun. 14 , 4251 (2023).
Kobayashi, H. et al. The balance of stromal BMP signaling mediated by GREM1 and ISLR drives colorectal carcinogenesis. Gastroenterology 160 , 1224–1239.e30 (2021).
Khaliq, A. M. et al. Refining colorectal cancer classification and clinical stratification through a single-cell atlas. Genome Biol. 23 , 113 (2022).
Nicolas, A. M. et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell 40 , 168–184.e13 (2022).
Bhattacharjee, S. et al. Tumor restriction by type I collagen opposes tumor-promoting effects of cancer-associated fibroblasts. J. Clin. Invest. 131 , e146987 (2021).
Hu, X. et al. Prediction of hepatic metastasis and relapse in colorectal cancers based on concordance analyses with liver fibrosis scores. Clin. Transl. Med. 9 , e13 (2020).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348 , 69–74 (2015).
Dolcetti, R. et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am. J. Pathol. 154 , 1805–1813 (1999).
Popat, S., Hubner, R. & Houlston, R. S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 23 , 609–618 (2005).
Pelka, K. et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell 184 , 4734–4752.e20 (2021).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372 , 2509–2520 (2015).
Le, D. T. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357 , 409–413 (2017).
Overman, M. J. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18 , 1182–1191 (2017).
André, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383 , 2207–2218 (2020).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26 , 566–576 (2020).
Chalabi, M. et al. Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: the NICHE-2 study [abstract LBA7]. Ann. Oncol. 33 (Suppl. 7), S1389 (2022).
Cercek, A. et al. PD-1 blockade in mismatch repair–deficient, locally advanced rectal cancer. N. Engl. J. Med. 386 , 2363–2376 (2022).
Yarchoan, M., Hopkins, A. & Jaffee, E. M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 377 , 2500–2501 (2017).
Galon, J. J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313 , 1960–1964 (2006).
Pagès, F. et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391 , 2128–2139 (2018).
Pagès, F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 353 , 2654–2666 (2005).
Mlecnik, B. et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl. Med. 8 , 327ra26 (2016).
Berghoff, A. S. et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 5 , e1057388 (2016).
Mlecnik, B. et al. Integrative analyses of colorectal cancer show Immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 44 , 698–711 (2016).
Hurtado, C. G., Wan, F., Housseau, F. & Sears, C. L. Roles for interleukin 17 and adaptive immunity in pathogenesis of colorectal cancer. Gastroenterology 155 , 1706–1715 (2018).
Van den Eynde, M. et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell 34 , 1012–1026.e3 (2018).
Angelova, M. et al. Evolution of metastases in space and time under immune selection. Cell 175 , 751–765.e16 (2018).
Crisafulli, G. et al. Temozolomide treatment alters mismatch repair and boosts mutational burden in tumor and blood of colorectal cancer patients. Cancer Discov. 12 , 1656–1675 (2022).
Amodio, V. et al. Genetic and pharmacological modulation of DNA mismatch repair heterogeneous tumors promotes immune surveillance. Cancer Cell 41 , 196–209.e5 (2023).
Zhang, L. et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181 , 442–459.e29 (2020).
Revel, M., Sautès-Fridman, C., Fridman, W.-H. & Roumenina, L. T. C1q + macrophages: passengers or drivers of cancer progression. Trends Cancer 8 , 517–526 (2022).
Sathe, A. et al. Colorectal cancer metastases in the liver establish immunosuppressive spatial networking between tumor-associated SPP1 + macrophages and fibroblasts. Clin. Cancer Res. 29 , 244–260 (2023).
Qi, J. et al. Single-cell and spatial analysis reveal interaction of FAP + fibroblasts and SPP1 + macrophages in colorectal cancer. Nat. Commun. 13 , 1742 (2022).
Ozato, Y. et al. Spatial and single-cell transcriptomics decipher the cellular environment containing HLA-G + cancer cells and SPP1 + macrophages in colorectal cancer. Cell Rep. 42 , 111929 (2023).
Ries, C. H. et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25 , 846–859 (2014).
Zheng, W. et al. Tumor-associated neutrophils in colorectal cancer development, progression and immunotherapy. Cancers 14 , 4755 (2022).
Singhal, S. et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 30 , 120–135 (2016).
Jaillon, S. et al. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 20 , 485–503 (2020).
Ogawa, R. et al. Loss of Smad4 promotes colorectal cancer progression by recruiting tumor-associated neutrophils via the CXCL1/8–CXCR2 axis. Clin. Cancer Res. 25 , 2887–2899 (2019).
Yamamoto, T. et al. Loss of SMAD4 promotes lung metastasis of colorectal cancer by accumulation of CCR1 + tumor-associated neutrophils through CCL15-CCR1 axis. Clin. Cancer Res. 23 , 833–844 (2017).
Jackstadt, R. et al. Epithelial NOTCH signaling rewires the tumor microenvironment of colorectal cancer to drive poor-prognosis subtypes and metastasis. Cancer Cell 36 , 319–336.e7 (2019).
Lepsenyi, M. et al. CXCL2-CXCR2 axis mediates αV integrin-dependent peritoneal metastasis of colon cancer cells. Clin. Exp. Metastasis 38 , 401–410 (2021).
Liao, W. et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 35 , 559–572.e7 (2019).
Germann, M. et al. Neutrophils suppress tumor‐infiltrating T cells in colon cancer via matrix metalloproteinase‐mediated activation of TGFβ. EMBO Mol. Med. 12 , e10681 (2020).
Tian, S. et al. Tumour-associated neutrophils secrete AGR2 to promote colorectal cancer metastasis via its receptor CD98hc–xCT. Gut 71 , 2489–2501 (2022).
Itatani, Y. et al. Suppressing neutrophil-dependent angiogenesis abrogates resistance to anti-VEGF antibody in a genetic model of colorectal cancer. Proc. Natl Acad. Sci. USA 117 , 21598–21608 (2020).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361 , eaao4227 (2018).
Yahagi, M. et al. Smoking is a risk factor for pulmonary metastasis in colorectal cancer. Colorectal Dis. 19 , O322–O328 (2017).
Bertocchi, A. et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell 39 , 708–724.e11 (2021).
Peuker, K. et al. Epithelial calcineurin controls microbiota-dependent intestinal tumor development. Nat. Med. 22 , 506–515 (2016).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359 , 91–97 (2018).
Yachida, S. et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25 , 968–976 (2019).
Roelands, J. et al. An integrated tumor, immune and microbiome atlas of colon cancer. Nat. Med. 29 , 1273–1286 (2023).
Peuker, K. et al. Microbiota-dependent activation of the myeloid calcineurin-NFAT pathway inhibits B7H3- and B7H4-dependent anti-tumor immunity in colorectal cancer. Immunity 55 , 701–717.e7 (2022).
Sahin, I. H., Ciombor, K. K., Diaz, L. A., Yu, J. & Kim, R. Immunotherapy for microsatellite stable colorectal cancers: challenges and novel therapeutic avenues. Am. Soc. Clin. Oncol. Educ. Book. 42 , 242–253 (2022).
Fukuoka, S. et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J. Clin. Oncol. 38 , 2053–2061 (2020).
Fakih, M. et al. Immunotherapy response in microsatellite stable metastatic colorectal cancer is influenced by site of metastases. Eur. J. Cancer 196 , 113437 (2024).
Chen, E. X. et al. Liver metastases and immune checkpoint inhibitor efficacy in patients with refractory metastatic colorectal cancer: a secondary analysis of a randomized clinical trial. JAMA Netw. Open. 6 , e2346094 (2023).
Yu, J. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 27 , 152–164 (2021).
Lee, J. C. et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci. Immunol. 5 , eaba0759 (2020).
Bando, H., Ohtsu, A. & Yoshino, T. Therapeutic landscape and future direction of metastatic colorectal cancer. Nat. Rev. Gastroenterol. Hepatol. 20 , 306–322 (2023).
Lote, H., Starling, N., Pihlak, R. & Gerlinger, M. Advances in immunotherapy for MMR proficient colorectal cancer. Cancer Treat. Rev. 111 , 102480 (2022).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16 , 563–580 (2019).
Huang, A. C. et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 25 , 454–461 (2019).
Derksen, J. W. G. et al. The Prospective Dutch Colorectal Cancer (PLCRC) cohort: real-world data facilitating research and clinical care. Sci. Rep. 11 , 3923 (2021).
Meguid, R. A., Slidell, M. B., Wolfgang, C. L., Chang, D. C. & Ahuja, N. Is there a difference in survival between right- versus left-sided colon cancers? Ann. Surg. Oncol. 15 , 2388–2394 (2008).
Alese, O. B. et al. Predictive and prognostic effects of primary tumor size on colorectal cancer survival. Front. Oncol. 11 , 728076 (2021).
Liu, Y. et al. Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell 33 , 721–735.e8 (2018).
Seshagiri, S. et al. Recurrent R-spondin fusions in colon cancer. Nature 488 , 660–664 (2012).
Birgin, E. et al. Prognostic value of disease-free interval in colorectal cancer: is it time? Eur. J. Surg. Oncol. 48 , 2032–2038 (2022).
Väyrynen, V. et al. Incidence and management of patients with colorectal cancer and synchronous and metachronous colorectal metastases: a population-based study. BJS Open. 4 , 685–692 (2020).
Hugen, N., van de Velde, C. J. H., de Wilt, J. H. W. & Nagtegaal, I. D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 25 , 651–657 (2014).
De Sousa E Melo, F. et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat. Med. 19 , 614–618 (2013).
Marisa, L. et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLoS Med. 10 , e1001453 (2013).
Sadanandam, A. et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat. Med. 19 , 619–625 (2013).
Isella, C. et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 47 , 312–319 (2015).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21 , 1350–1356 (2015).
Piskol, R. et al. A clinically applicable gene-expression classifier reveals intrinsic and extrinsic contributions to consensus molecular subtypes in primary and metastatic colon cancer. Clin. Cancer Res. 25 , 4431–4442 (2019).
Eide, P. W. et al. Metastatic heterogeneity of the consensus molecular subtypes of colorectal cancer. NPJ Genom. Med. 6 , 59 (2021).
Moosavi, S. H. et al. De novo transcriptomic subtyping of colorectal cancer liver metastases in the context of tumor heterogeneity. Genome Med. 13 , 143 (2021).
Gengenbacher, N., Singhal, M. & Augustin, H. G. Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat. Rev. Cancer 17 , 751–765 (2017).
Jackstadt, R. & Sansom, O. J. Mouse models of intestinal cancer. J. Pathol. 238 , 141–151 (2016).
Amirkhah, R. et al. MmCMS: mouse models’ consensus molecular subtypes of colorectal cancer. Br. J. Cancer 128 , 1333–1343 (2023).
Fumagalli, A. et al. A surgical orthotopic organoid transplantation approach in mice to visualize and study colorectal cancer progression. Nat. Protoc. 13 , 235–247 (2018).
Nguyen, T. L. A., Vieira-Silva, S., Liston, A. & Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model. Mech. 8 , 1–16 (2015).
Download references
Acknowledgements
The authors acknowledge the support of all members of the Batlle Laboratory. E.B. receives support from ERC (ERC AdvG 884623), AGAUR-SGR1278, Spanish Ministry of Science PID2020-119917RB-I00, IMI-PERSIST-SEQ, and AECC (GEACC19006BAT).
Author information
Adrià Cañellas-Socias
Present address: Center for Cancer Cell Therapy, Stanford Cancer Institute, Stanford University, Stanford, CA, USA
Authors and Affiliations
Institute for Research in Biomedicine (IRB Barcelona), The Barcelona Institute of Science and Technology (BIST), Barcelona, Spain
Adrià Cañellas-Socias, Elena Sancho & Eduard Batlle
Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Barcelona, Spain
Institució Catalana de Recerca i Estudis Avançats (ICREA), Barcelona, Spain
Eduard Batlle
You can also search for this author in PubMed Google Scholar
Contributions
All authors researched data for the article. E.B. and A.C.-S. contributed substantially to conceptualization of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Correspondence to Adrià Cañellas-Socias or Eduard Batlle .
Ethics declarations
Competing interests.
E.B. is author in a patent related to TGFβ inhibitors. E.B. is author in a patent describing bispecific antibodies to target cancer stem cells. The laboratory of E.B. has received research funding from MERUS and INCYTE. E.B. has received honoraria for consulting from Genentech. The remaining authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Gastroenterology & Hepatology thanks Florian Greten, Jacco van Rheenen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
NCT03026140: https://clinicaltrials.gov/study/NCT03026140
NCT04165772: https://clinicaltrials.gov/study/NCT04165772
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Cañellas-Socias, A., Sancho, E. & Batlle, E. Mechanisms of metastatic colorectal cancer. Nat Rev Gastroenterol Hepatol (2024). https://doi.org/10.1038/s41575-024-00934-z
Download citation
Accepted : 17 April 2024
Published : 28 May 2024
DOI : https://doi.org/10.1038/s41575-024-00934-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
Cancer metastasis is responsible for the vast majority of cancer‐related deaths worldwide. In contrast to numerous discoveries that reveal the detailed mechanisms leading to the formation of the primary tumor, the biological underpinnings of the metastatic disease remain poorly understood. Cancer metastasis is a complex process in which ...
At a recent NCI conference, survivors and researchers came together to discuss how to better address the needs of those living with metastatic cancer. Engineered immune cells deliver anticancer signal, prevent cancer from spreading. Posted: March 24, 2021. In an NCI study, treating mice with engineered immune cells shrank tumors and prevented ...
The US cancer mortality rate declined by 29% from 1991 to 2017, with an average decline of 1.5% per year between 2013 and 2017. The steepest declines have been observed in metastatic melanoma (− ...
Metastasis is the spread of cancer to a part of the body distant from the original primary cancer. This occurs through the transfer of malignant or cancerous cells via lymph or blood. The new ...
Cancer metastasis, or the systemic spread and growth of tumor cells throughout the body, is the principal cause of cancer-related death 1. Despite 70 years of drug development for cancer, survival ...
Patients with metastatic cancer have considerably lower 5-year survival rates than those with localized cancer [1], [2].More than 90% of cancer-related deaths result from metastatic disease [3].Metastasis is a dynamic, multifaceted process during which normal cells transform into oncogenic cells that proliferate uncontrollably, evade the immune system, become resistant to programmed cell death ...
Advances in understanding the key features of primary tumors have relied on innovation across specialized fields, culminating in improved clinical staging and patient survival rates. These advances have also enhanced our understanding of secondary tumor formation, or metastasis, that remains the major cause of cancer-related deaths.
Basic research supported by the Tumor Metastasis branch seeks to define the intrinsic and extrinsic mechanisms that allow tumor cells to leave the primary tumor, invade surrounding tissue, travel through the circulation or lymph, and enter, survive and colonize distant organs. Metastasis research encompasses both chronological progression and ...
Cell Metastasis is a journal that publishes cutting-edge research on the molecular and cellular mechanisms of cancer metastasis. Learn from the latest findings and insights on topics such as chromatin modifications, neuronal fate, and RNA interference.
The Metastasis Research Network (MetNet) supports several U54 Specialized Centers ( RFA-CA-20-029 ). These collaborative, multidisciplinary projects focus on several themes of the metastatic process and utilize integrative systems-level approaches. The MetNet will advance the understanding of metastasis as a non-linear, dynamic, and emergent ...
Overview. Cancer and Metastasis Reviews publishes outstanding reviews on recent developments in the biology and treatment of malignant disease and highlights promising new directions. Focuses on the molecular and cellular biology of cancer metastasis and tumor progression. Presents expert contributions on a single theme or topic in each issue.
This research topic aims to provide valuable insights into innovative small molecules and effective therapeutic strategies targeting tumor metastasis and stemness for cancer treatment, with the goal of improving the survival rate of cancer patients. • Discovery of key factors affecting CSC differentiation and cancer metastasis.
The aim of the current Research Topic is to cover promising, recent, and novel research trends in the field of cell adhesion, metastasis, and the immune response. Areas to be covered in this Research Topic may include, but are not limited to: • The molecular basis by which CAMs modulate the metastatic progress.
Keywords: premetastatic niche, metastasis, exosomes, cfDNA, tumor microenvironment, ECM-remodeling . Important Note: All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements.Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of ...
Submit to PLOS ONE, the #1 publisher of cancer metastasis research. Due to COVID-19 restrictions, PLOS ONE is extending the deadline for submissions to the Cancer Metastasis collection. This unique opportunity will allow authors to continue to submit their research to this Call for Papers, including post collection launch, until December 14, 2020.
Breast cancer that has spread to other parts of the body is particularly hard to treat. New research from Yale Cancer Center reveals first-of-its-kind data from a phase I study in patients with hormone receptor positive HER2-negative metastatic breast cancer. The results, which assess the safety and efficacy of a treatment known as PF-07248144 ...
The mission of the Department of Cancer Biology is to identify and understand the causes of cancer, to develop innovative approaches to reduce cancer incidence, to create and test novel and more effective therapies, and to translate these findings into clinical care for the benefit of patients. Research in our department is highly collaborative ...
Explore some of the most intensively pursued research topics at Memorial Sloan Kettering. ... Our scientists pursue every aspect of cancer research—from exploring the biology of genes and cells, to developing immune-based treatments, uncovering the causes of metastasis, and more. Programs & Centers;
Metastasis is the hallmark of cancer that is responsible for the greatest number of cancer-related deaths. Yet, it remains poorly understood. The continuous evolution of cancer biology research ...
Instead, cancer cells exploit a process that healthy tissues use to shed cells and move around the body, revolutionising the way we think about metastasis. These findings are among the most important to have come out of my lab for three decades. Not only have we identified one of the elusive drivers of metastasis, but we have also turned a ...
The research, published in Radiology: Imaging Cancer, showed that the AI model was significantly better at identifying patients with axillary metastasis than MRI or ultrasound. In clinical practice, the AI model would have helped avoid 51% of benign (noncancerous) or unnecessary surgical sentinel node biopsies while correctly detecting 95% of ...
This new result unearths a new class of biomarkers to forecast tumor progression and an entirely new way of understanding breast cancer origins." Curtis is the senior author of the study, which will be published May 31 in Science. Postdoctoral scholar Kathleen Houlahan, PhD, is the lead author of the research.
This research could transform cancer diagnosis and treatment, enhancing personalized medicine by facilitating rapid and accurate testing of cancer cell behaviors from patient biopsies and ...
Metastatic multiple myeloma is a form of cancer of the bone marrow that has spread to other parts of the body. Common sites of metastasis include the skin, muscles, and lymph nodes.
Recent research has shown that cell behavior and pre-metastatic niche are active participants in the metastatic process and play a critical role in the success or failure of cancer cell colonization and therapy. This Research Topic aims to provide a forum to advance research on new mechanisms that would lead to novel strategies and cancer ...
Scientists have used breast cancer cells' weakness against themselves by linking a tumour-selective antibody with a cell-killing drug to destroy hard-to-treat tumours. The research, published ...
A Pfizer medicine has been shown to greatly reduce cancer progression and improve survival outcomes for people in the advanced stages of a form of lung cancer, results published Friday showed.
We are pleased to invite you to read the highly cited Special Issues in 2022 and 2023 related to cancer metastasis in Cancers (ISSN: 2072-6694). We hope this announcement will provide useful information for this field. The list of relevant Special Issues is provided below:
Statins, commonly used cholesterol-lowering drugs, may block a pathway that leads to the development of cancer from chronic inflammation, according to a new study led by investigators from Harvard-affiliated Mass General Cancer Center. The team's experiments showed that environmental toxins, such as those caused by exposure to allergens and chemical irritants, create a cascade effect that ...
Abstract. Despite extensive research and improvements in understanding colorectal cancer (CRC), its metastatic form continues to pose a substantial challenge, primarily owing to limited ...