

How to Become a Clinical Project Manager

Becoming a clinical project manager (CPM) is a fulfilling career path that offers the opportunity to lead and manage clinical trials, ensuring that research is conducted smoothly and in accordance with regulatory standards. Clinical project managers are vital in the healthcare industry, as they help bring new medical innovations to the market. If you are considering this career, here’s a detailed guide on the steps you need to take, the qualifications required, and the skills you will need to succeed in this role.
Step 1: Obtain a Relevant Bachelor's Degree
The first step toward becoming a clinical project manager is earning a bachelor’s degree in a relevant field. While there isn’t a specific degree titled “Clinical Project Management,” degrees in life sciences, health sciences, biology, chemistry, or pharmacology are common paths. A background in these areas provides the foundational knowledge necessary for understanding clinical trials and the drug development process.
Popular Undergraduate Degrees for Clinical Project Managers:
Biology: Offers a deep understanding of human biology and laboratory practices.
Chemistry: Focuses on drug formulation and chemical processes.
Nursing: Provides insight into patient care and clinical settings.
Public Health: Covers broader health issues and epidemiology, which are relevant to clinical trials.
Additionally, some students pursue degrees in business administration or project management to strengthen their understanding of managing complex projects.
Step 2: Gain Experience in Clinical Research
A crucial step in becoming a clinical project manager is gaining experience in the clinical research field. Many start their careers in entry-level roles such as clinical research coordinators (CRCs), clinical trial assistants (CTAs), or clinical research associates (CRAs). These positions allow you to learn the ins and outs of clinical trials, from patient recruitment to data collection and regulatory compliance.
Common Entry-Level Roles:
Clinical Research Coordinator (CRC): Coordinates the daily operations of clinical trials.
Clinical Trial Assistant (CTA): Provides administrative support to project teams.
Clinical Research Associate (CRA): Monitors trial sites to ensure compliance with protocols and regulations.
Working in these roles helps you understand the regulatory framework and operational challenges that clinical project managers will eventually oversee. Experience in these positions also offers valuable insights into patient management, protocol adherence, and communication with study stakeholders.
For those looking to jumpstart their career, enrolling in a specialized course like the Clinical Research Coordinator training or the Clinical Trials Assistant Training course can give you the necessary skills and certification to enter the field.
Step 3: Earn Certifications and Advanced Training
While experience in clinical research is important, certifications are increasingly sought after by employers looking to hire clinical project managers. Earning certifications in clinical research management demonstrates your commitment to professional development and validates your expertise.
Some of the most recognized certifications include:
Project Management Professional (PMP): Offered by the Project Management Institute (PMI), this certification covers general project management practices applicable to all industries, including clinical trials.
Certified Clinical Research Professional (CCRP): Provided by the Society of Clinical Research Associates (SOCRA), this certification is specifically focused on clinical research professionals.
Certified Clinical Project Manager (CCPM): This is a specialized certification that focuses on the unique responsibilities and challenges of managing clinical trials.
To gain a deeper understanding of the regulatory and operational aspects of clinical trials, consider enrolling in advanced courses such as the Advanced Clinical Research Project Manager Certification or CRA training, which will equip you with the necessary skills to excel in this role.
Step 4: Develop Essential Skills for Clinical Project Management
Successful clinical project managers possess a unique combination of scientific knowledge, leadership abilities, and organizational skills. Here are some of the key skills required:
Leadership and Team Management:
CPMs oversee multidisciplinary teams, including scientists, clinicians, and regulatory experts. Strong leadership skills are needed to motivate, coordinate, and manage these diverse teams.
Communication:
CPMs must be excellent communicators, as they often serve as a liaison between research teams, sponsors, and regulatory authorities. Effective communication ensures that all stakeholders are informed and aligned on the trial’s progress.
Budgeting and Financial Management:
Managing the financial aspects of clinical trials, including budgeting and resource allocation, is a critical responsibility. CPMs must ensure that the project stays within budget without compromising the quality of the research.
Regulatory Knowledge:
Clinical trials are governed by strict regulations, including the International Council for Harmonisation (ICH) guidelines and Good Clinical Practice (GCP) standards. CPMs must stay up-to-date with these regulations to ensure compliance throughout the trial.
Problem-Solving and Risk Management:
Clinical trials often encounter unforeseen challenges, such as patient recruitment delays or data inconsistencies. CPMs must be skilled in identifying potential risks early on and developing strategies to mitigate them.
Data Analysis and Interpretation:
While not directly responsible for analyzing trial data, CPMs must have a thorough understanding of how clinical data is collected and interpreted to ensure that study results are accurate and reliable.
Step 5: Gain Experience in Project Management
In addition to clinical research experience, it is essential to gain hands-on experience in managing projects. This can be done by taking on leadership roles within clinical trials or managing smaller projects under the guidance of an experienced clinical project manager.
Many clinical project managers start in roles such as associate project managers, where they learn the skills needed to oversee timelines, budgets, and team coordination. This experience prepares them for the more complex responsibilities of leading entire clinical trials.
Step 6: Pursue a Master’s Degree (Optional but Beneficial)
While a bachelor’s degree is sufficient for entry-level roles, a master’s degree in clinical research, project management, or business administration can significantly enhance your prospects of becoming a clinical project manager. Graduate programs provide advanced knowledge in research methodologies, project management strategies, and regulatory affairs, equipping you for higher-level positions.
Some institutions offer specialized master's degrees in clinical research management or healthcare project management. These programs are designed to provide the skills needed to oversee clinical trials effectively and prepare for leadership roles in the field.
Step 7: Network and Stay Current with Industry Trends
The healthcare and clinical research industries are constantly evolving, with new regulations, technologies, and methodologies emerging regularly. Staying informed about these changes is crucial for clinical project managers. Attending industry conferences, webinars, and continuing education courses can help you stay updated on the latest trends.
Networking is equally important. Building relationships with other professionals in the field can provide opportunities for career advancement, mentorship, and collaborative projects. Consider joining professional organizations such as the Association of Clinical Research Professionals (ACRP) or the Society of Clinical Research Associates (SOCRA) to stay connected with the latest developments in the industry.
Career Outlook and Opportunities for Clinical Project Managers
The demand for clinical project managers is growing due to the increasing number of clinical trials worldwide, particularly in areas like oncology, cardiology, and neurology. According to the U.S. Bureau of Labor Statistics, employment in clinical research is expected to grow faster than the average for all occupations over the next decade.
Clinical project managers can work for:
Contract Research Organizations (CROs): These organizations are often contracted by pharmaceutical companies to manage clinical trials.
Pharmaceutical and Biotech Companies: Clinical project managers may work directly for drug development companies to manage in-house trials.
Academic and Research Institutions: Universities and research centers also conduct clinical trials and require CPMs to manage them.
Final Thoughts
Becoming a clinical project manager requires a blend of education, experience, and specialized skills. While it is a challenging role, it is also incredibly rewarding, offering the chance to contribute to cutting-edge medical research that can improve patient outcomes worldwide.
If you are interested in advancing your career in clinical project management, consider exploring advanced certifications and training programs, such as the Advanced Clinical Research Project Manager Certification, to give yourself a competitive edge.
By following these steps, you can build a successful career in clinical project management and play a crucial role in the future of healthcare innovation.
Reference Links:
U.S. National Library of Medicine – Clinical Research Careers
Harvard T.H. Chan School of Public Health – Master's in Clinical Research
Project Management Institute (PMI) – Project Management Professional (PMP) Certification
Society of Clinical Research Associates (SOCRA) – Certification Programs
Relevant Course Links:
Clinical Research Coordinator
Advanced Clinical Research Project Manager Certification
CRA (Clinical Research Associate)
ICH-GCP (Good Clinical Practice)
Acronym of a Title in Clinical Research: Understanding the Key Terminology
Clinical trials new protocol training checklist for study nurses.
- Certified ScrumMaster
- PMI-ACP Exam Prep
- Leading SAFe® 6.0 Certification
- SAFe Scrum Master
- Certified Scrum Product Owner (CSPO)
- SAFe for Teams
- Agile Scrum Foundation
- AgilePM Foundation and Practitioner Certification
- Agile Scrum Master (ASM)
- Kanban Training
- Scrum Fundamentals
PMP Certification
Project Management Fundamentals
CAPM Exam Prep
- Change Management Foundation and Practitioner Certification
- PRINCE2 Foundation & Practitioner Certification (7th Edition)
- PRINCE2 Agile Foundation & Practitioner Certification
- Business Analysis Foundation and Practitioner Certification
- Microsoft Project Training
- JIRA Certification Training
- Lean Project Management
- ITIL 4 Foundation
- VeriSM™ Foundation
- SIAM Foundation
- SIAM Professional
- 7 QC Tools Training
- Minitab Essentials
- Lean Six Sigma Yellow Belt
- Six Sigma Awareness
- Lean Six Sigma Green Belt
- Design for Six Sigma
- Lean Six Sigma Black Belt
- Lean Fundamentals
- Value Stream Mapping
- Quality by Design
- Quality Function Deployment
- BPM and Six Sigma
- RCA through Six Sigma
- DevOps Foundation
- DevOps Master
- DevOps Professional
- Continuous Delivery Architecture
- COBIT 5 Certification
- Corporate Group Training
- 1-to-1 Training
- Join as a Trainer

- Best Project Management Blogs
What Does a Clinical Project Manger Do? Roles & Responsibilities

Clinical Project Managers (CPM) play a crucial role in advancing the progress of a clinical trial. The significance of CPMs lies in their ability to navigate complex clinical trials, ensuring precision, compliance, and efficiency.
As stewards of the entire research lifecycle, from planning to execution, Clinical Project Managers wield strategic thinking, leadership insight, and a deep understanding of regulatory landscapes.
In a world where the pursuit of groundbreaking therapies intensifies, the demand for skilled managers is reaching new heights, with organizations recognizing their pivotal role in trial success.
This blog explores the pivotal responsibilities of clinical project managers and sheds light on why their expertise is becoming increasingly coveted, underscoring the crucial role they play in shaping the future of healthcare.
Table of Contents:
What is a Clinical Project Manager?
What does a clinical project manager do, skills required to become a clinical project manager.
- Essential Certifications or Degrees Required to become a Clinical Project Manager
How to Become a Clinical Project Manager?
Salary and job outlook for a clinical project manager.
A Clinical Project Manager (CPM) is an experienced expert in clinical research and healthcare management who is responsible for managing and coordinating the different aspects of clinical trials.
This multifaceted role encompasses strategic planning, execution, and monitoring of clinical research projects to ensure they adhere to regulatory standards, timelines, and budgets.
Clinical Project Manager acts as a connecting point between research teams, sponsors, regulatory authorities, and other stakeholders, facilitating effective communication and collaboration.
Clinical Project Manager responsibilities include protocol development, risk management , team leadership, and navigating the complexities of regulatory compliance. By leveraging their expertise in project management, scientific understanding, and regulatory knowledge, they contribute significantly to successful clinical trials, ultimately advancing medical knowledge and bringing novel treatments to needy patients.
A Clinical Project Manager (CPM) is a pivotal figure in clinical trials, overseeing the intricate processes that lead to the successful execution of healthcare research. Their role encompasses many responsibilities, blending scientific expertise with project management skills to ensure the seamless progression of clinical trials.
Other key roles and responsibilities of a Clinical Project Manager:
- Strategic Planning: Develop comprehensive plans for the initiation, execution, and completion of clinical trials, aligning them with project goals and timelines
- Protocol Development: Contribute to the creation and refinement of study protocols, outlining the methodology, objectives, and criteria for participant selection
- Site Selection: Identify and evaluate suitable clinical trial sites, considering factors such as patient demographics, facilities, and regulatory compliance
- Regulatory Compliance: Navigate and ensure adherence to the complex web of regulatory requirements, obtaining necessary approvals and permissions for the clinical trial
- Budget Oversight: Manage the financial aspects of the clinical trial, ensuring adherence to the allocated budget and making informed decisions to optimize resource utilization
- Data Integrity: Oversee data collection and management processes, emphasizing the importance of data accuracy, completeness, and compliance with regulatory standards
- Problem Resolution: Address challenges and obstacles that may arise during the trial, making decisions that safeguard patient safety and ensure the integrity of the study
- Quality Assurance: Maintain a focus on the overall quality of the clinical trial, implementing measures to uphold ethical standards, patient welfare, and the reliability of research outcomes
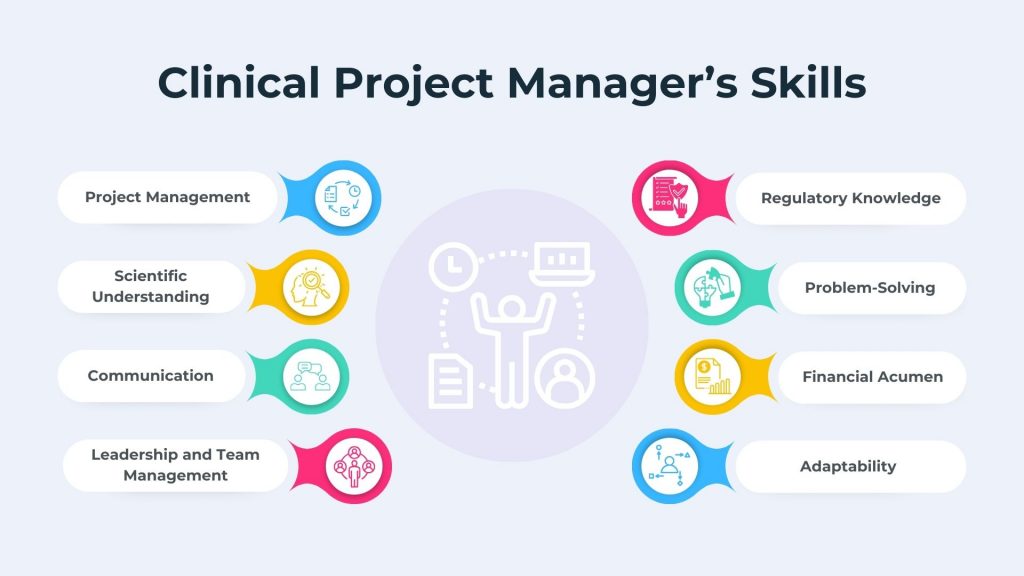
Becoming a successful clinical project manager requires a diverse set of skills that combines scientific knowledge, project management proficiency, and effective communication. Below are some of the key skills that a clinical project manager is required to excel in the role:
1. Project Management Skills
- Planning and Organization: Ability to develop and execute comprehensive project plans, ensuring all aspects of the clinical trial are well-coordinated
- Time Management: Efficiently allocate resources, manage timelines, and prioritize tasks to meet project milestones
- Risk Management: Identify potential risks and proactively implement strategies to mitigate them, ensuring smooth project progression
2. Scientific Understanding Skills
- Clinical Research Knowledge: Familiarity with the principles and processes of clinical research, including study design, protocols, and ethical considerations
- Medical Terminology: Ability to understand and interpret medical and scientific terminology crucial for effective communication with research teams and stakeholders
3. Communication Skills
- Interpersonal Communication: Build strong professional relationships with diverse stakeholders , including research teams, sponsors, regulatory authorities, and site personnel
- Presentation Skills: Effectively convey complex information clearly and concisely, verbally and in written form
4. Leadership and Team Management Skills
- Team Building: Foster collaboration and cohesion within cross-functional teams, inspiring motivation and commitment to project goals
- Decision-Making: Make informed decisions promptly, especially in high-pressure situations, to address challenges and keep the project on track
5. Regulatory Knowledge and Skills
- Regulatory Compliance: Stay updated on and ensure adherence to relevant regulations and guidelines governing clinical trials in different regions
- Ethical Considerations: Understand and navigate the ethical considerations in clinical research, prioritizing patient safety and welfare
6. Problem-Solving Skills
- Critical Thinking: Analyze complex situations, identify root causes of issues, and develop effective solutions to keep the project moving forward
7. Financial Acumen
- Budget Management: Proficiency in managing project budgets, optimizing resource allocation, and ensuring financial accountability throughout the trial
8. Adaptability Skills
- Flexibility: Navigate unforeseen challenges and changes in project scope with adaptability, adjusting strategies and plans as needed
- Learning Agility: Stay abreast of advancements in clinical research, project management methodologies, and regulatory requirements
Essential Certifications or Degrees Required to Become a Clinical Project Manager
Becoming a Clinical Project Manager requires a combination of education, relevant degrees, and professional certifications. The specific requirements may vary based on the employer, industry sector, and the clinical trials complexity.
Here are some essential certifications and degrees that can enhance the qualifications of individuals aspiring to become Clinical Project Managers:
1. Educational Background
- Bachelor’s Degree: A bachelor’s degree in an appropriate field such as life sciences, healthcare, nursing, pharmacy, or a related discipline is frequently the minimum educational requirement
- Advanced Degrees: While not always mandatory, having a master’s degree (e.g., Master of Public Health, Master of Science in Clinical Research) or a Ph.D. can be advantageous, especially for more senior or specialized roles
2. Project Management Professional (PMP) Certification
The PMP certification is offered by the Project Management Institute (PMI), is widely recognized, and demonstrates proficiency in project management principles. It is valuable for Clinical Project Managers as they oversee complex clinical trials.
Achieve global recognition with the PMP certification from Invensis Learning. Benefit from expert trainers, flexible learning options, and success guarantees to propel your career to new heights. Enroll now to access exclusive discounts and become a certified leader in project management.
3. Certified Clinical Research Professional (CCRP) Certification
The Certified Clinical Research Professional (CCRP) certification is a professional designation offered by the Society of Clinical Research Associates (SoCRA). It is a worldwide recognized credential that demonstrates an individual’s skills and understanding of the principles and practices of clinical research.

4. Project Management Fundamentals (PMF) Certification
The Project Management Fundamentals (PMF) Certification is an entry-level credential offered by the Association for Project Management (APM) that validates an individual’s understanding of the fundamental principles and practices of project management. It is designed for those new to the field or wanting to formalize their project management knowledge.
5. Certified Clinical Project Manager (CCPM) Certification
The Certified Clinical Project Manager (CCPM) certification is a professional designation offered by various organizations that demonstrates an individual’s expertise in managing clinical trials and research projects. It validates their ability to effectively plan, execute, monitor, and evaluate clinical research studies, ensuring adherence to regulatory and ethical guidelines.
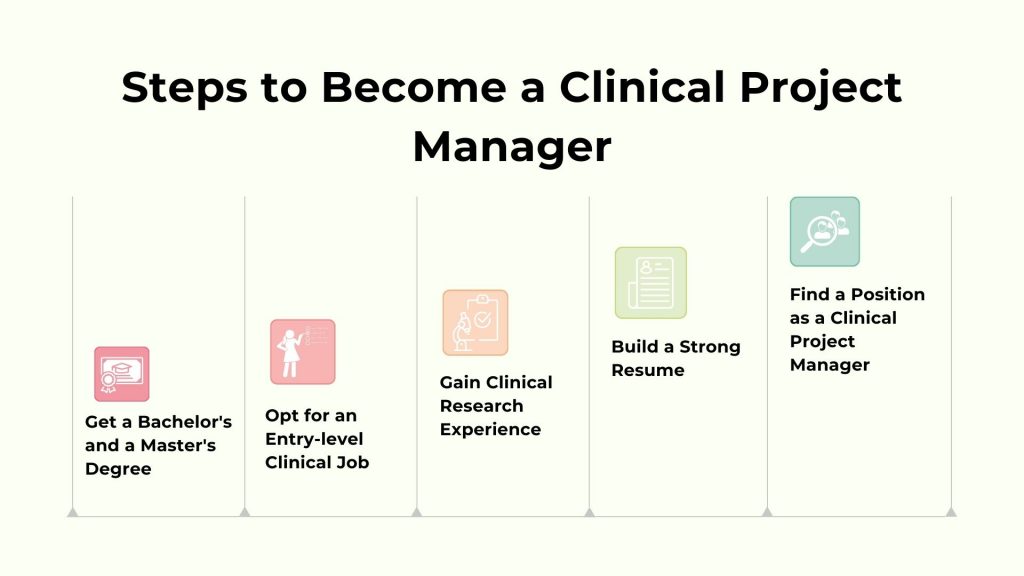
Becoming a Clinical Project Manager involves a strategic combination of education, experience, and professional development. Here’s a step-by-step guide on how to embark on a career as a Clinical Project Manager:
1. Get a Bachelor’s and a Master’s Degree
Embark on your journey by earning a bachelor’s degree in a relevant field, such as life sciences or healthcare. This foundational step equips you with essential knowledge for a career in clinical research.
To enhance your qualifications further, pursue a master’s degree, opting for specialized programs like a Master’s in Public Health (MPH) or a Master’s in Clinical Research.
2. Opt for an Entry-level Clinical Job
Kickstart your career with an entry-level position in clinical research, such as a Clinical Research Assistant or Coordinator. These roles expose you to the day-to-day operations of clinical trials, providing valuable insights into research protocols, data management, and regulatory compliance.
3. Gain Clinical Research Experience
Actively seek hands-on experience in clinical research, engaging in tasks like patient recruitment and study coordination. Develop a strong understanding of Good Clinical Practice (GCP) guidelines and ethical considerations. This practical experience lays the groundwork for a well-rounded skill set and prepares you for more advanced roles.
4. Build a Strong Resume
Create an effective resume that highlights your educational background, relevant coursework, and practical experience. Emphasize key skills such as attention to detail, data management, and knowledge of regulatory standards. Include certifications, like GCP, to underscore your commitment to maintaining high-quality standards in clinical research.
5. Find a Position as a Clinical Project Manager
Progress in your career by applying for roles with increasing responsibilities, focusing on project management within clinical trials. Leverage your educational background, practical experience, and certifications to showcase readiness for a Clinical Project Manager role. Highlight your ability to lead teams, manage timelines, and strictly adhere to regulatory standards.
Before switching any career, individuals should know two main things: one is salary growth and the other one is job opportunities. The salary and job outlook for a Clinical Project Manager (CPM) can vary based on factors such as experience, education, location, and the specific industry within healthcare or clinical research. It’s essential to note that salary trends and job outlook may evolve over time.
Salary of a Clinical Project Manager
The salary prospects for a clinical project manager are generally quite positive. They play a crucial role in the healthcare industry, overseeing the planning, execution, and monitoring of clinical trials and research projects.
Their expertise in project management, clinical research methodology, and regulatory compliance ensures the successful completion of these studies, leading to the development of new drugs, treatments, and medical devices.
Experience is a significant factor in determining salary. The salary ranges for clinical project managers are as follows:
Clinical project managers have the potential to experience significant salary growth throughout their careers. With increasing experience, specialized skills, and advanced certifications, clinical project managers can advance into senior-level positions with higher earning potential.
Additionally, the demand for clinical project managers is expected to grow faster than average in the coming years, further contributing to positive salary prospects.
Job Outlook of a Clinical Project Manager
The job outlook for clinical project managers is exceptionally promising, driven by the increasing demand for clinical trials, the growing complexity of research projects, and the expanding healthcare needs of an aging population.
As per the US Bureau of Labor Statistics (BLS) , employment of medical and health services managers, which includes clinical project managers, will expand by 32% from 2020 to 2030, much faster than the average for all professions. This growth is related to the aging population and the increasing demand for healthcare services.
Here are some specific factors that contribute to the positive job outlook for clinical project managers:
- Increasing demand for clinical trials
- Growing complexity of clinical trials
- The aging population and rising healthcare needs
- Expansion of medical group practices
Clinical project managers can pursue diverse career paths and advance into senior-level positions with increasing responsibilities and higher compensation.
Some potential career trajectories include:
- Clinical Research Associate (CRA)
- Clinical Research Coordinator
- Clinical Research Manager
- Director of Clinical Research
- Clinical Research Program Manager
- Clinical Research Portfolio Manager
- Clinical Project Manager Specialist
- Clinical Project Manager Lead
- Global Clinical Project Manager
- Senior Clinical Project Manager
- Executive Clinical Project Manager
A clinical project manager plays a pivotal role in the healthcare industry, ensuring the successful execution of clinical trials and research projects. Their expertise in project management, clinical research methodology, and regulatory compliance is crucial for bringing new drugs, devices, and therapies to patients, improving healthcare outcomes, and advancing medical knowledge.
If you are passionate about healthcare, have strong organizational skills, and possess a keen eye for detail, a career as a clinical project manager could be a rewarding and fulfilling path. With the right education, experience, and certifications, you can significantly impact the future of healthcare by overseeing the development of life-saving treatments and technologies.
EXIN Business Analysis Foundation and Practitioner Training
PRINCE2 Foundation and Practitioner Certification Training
Change Management Foundation and Practiitioner Certification Training
RELATED ARTICLES MORE FROM AUTHOR

Project Management vs Data Analytics: Complete Overview

What is Quality Assurance (QA) in Project Management?

What is Sensitivity Analysis in Project Management?
Leave a reply.
Save my name, email, and website in this browser for the next time I comment.
- 14,448 Likes
- 444 Followers
- 107k Subscribers
- 2,170 Followers
Related Articles

Best Practices to Handle Risks in an Enterprise

25+ PMP Formulas to Ace Your PMP Exam in 2024

Top 10 Misconceptions about Agile Development
Six Sigma Belt Level Rankings & Roles

ITIL Service Transition – FAQs
Popular posts.

The Project Management Life Cycle Explained

What are the Roles and Responsibilities of a Quality Control Inspector

7 Cs of Effective Communication with Example

Top 10 Factors for Project Success

Quality Analyst Job Roles, Responsibilities, and Skills Explained!
Suggested posts.
- 7 Cs of Effective Communication with Examples
- Project Management Lifecycle
- Project Success Factors
- Quality Control Inspector Job Description
- Risk Management Examples
- QA Manager Job Description
- Quality Management Team Roles and Responsibilities
- Risk Management Tools & Techniques
- Quality Analyst Job Description
- What is Business Value
- Who are Project Stakeholders
- Importance of Project Management
- What is Project Management
- Project Management Skills
- Project Manager Job Description
- Agile Project Manager Interview Questions
- Risk and Compliance Manager Job Description
- Risk Management Process
- Project Scope Management
- Healthcare Project Manager Job Description
- Six Sigma Project Examples
- Risk Analysis Methods
- ITIL Service Lifecycle
- Risk Manager Job Description
POPULAR CATEGORIES
- Best Project Management Blogs 265
- Top Agile Blog Posts 158
- Top Blogs on Quality Management 127
- Latest IT Service Management Blogs 108
- Trending Articles on DevOps 65
- Popular Blogs on IT Security and Governance 55
- Top Blogs on Professional Development 33
- Top Infographics Collection 8

Hurry Up and Enroll for the earliest bantch of
Price2 foundation & Practitioner
Download E-book Blog
Thank You for submitting your enquiry. One of our training consultants will get in touch with you shortly.
50+ Training and Certification Programs - Upskill Today Learn more about our training programs.


IMAGES
VIDEO