Doing a Meta-Analysis: A Practical, Step-by-Step Guide
Saul McLeod, PhD
Editor-in-Chief for Simply Psychology
BSc (Hons) Psychology, MRes, PhD, University of Manchester
Saul McLeod, PhD., is a qualified psychology teacher with over 18 years of experience in further and higher education. He has been published in peer-reviewed journals, including the Journal of Clinical Psychology.
Learn about our Editorial Process
Olivia Guy-Evans, MSc
Associate Editor for Simply Psychology
BSc (Hons) Psychology, MSc Psychology of Education
Olivia Guy-Evans is a writer and associate editor for Simply Psychology. She has previously worked in healthcare and educational sectors.
On This Page:

What is a Meta-Analysis?
Meta-analysis is a statistical procedure used to combine and synthesize findings from multiple independent studies to estimate the average effect size for a particular research question.
Meta-analysis goes beyond traditional narrative reviews by using statistical methods to integrate the results of several studies, leading to a more objective appraisal of the evidence.
This method addresses limitations like small sample sizes in individual studies, providing a more precise estimate of a treatment effect or relationship strength.
Meta-analyses are particularly valuable when individual study results are inconclusive or contradictory, as seen in the example of vitamin D supplementation and the prevention of fractures.
For instance, a meta-analysis published in JAMA in 2017 by Zhao et al. examined 81 randomized controlled trials involving 53,537 participants.
The results of this meta-analysis suggested that vitamin D supplementation was not associated with a lower risk of fractures among community-dwelling adults. This finding contradicted some earlier beliefs and individual study results that had suggested a protective effect.
What’s the difference between a meta-analysis, systematic review, and literature review?
Literature reviews can be conducted without defined procedures for gathering information. Systematic reviews use strict protocols to minimize bias when gathering and evaluating studies, making them more transparent and reproducible.
While a systematic review thoroughly maps out a field of research, it cannot provide unbiased information on the magnitude of an effect. Meta-analysis statistically combines effect sizes of similar studies, going a step further than a systematic review by weighting each study by its precision.
What is Effect Size?
Statistical significance is a poor metric in meta-analysis because it only indicates whether an effect is likely to have occurred by chance. It does not provide information about the magnitude or practical importance of the effect.
While a statistically significant result may indicate an effect different from zero, this effect might be too small to hold practical value. Effect size, on the other hand, offers a standardized measure of the magnitude of the effect, allowing for a more meaningful interpretation of the findings
Meta-analysis goes beyond simply synthesizing effect sizes; it uses these statistics to provide a weighted average effect size from studies addressing similar research questions. The larger the effect size the stronger the relationship between two variables.
If effect sizes are consistent, the analysis demonstrates that the findings are robust across the included studies. When there is variation in effect sizes, researchers should focus on understanding the reasons for this dispersion rather than just reporting a summary effect.
Meta-regression is one method for exploring this variation by examining the relationship between effect sizes and study characteristics.
T here are three primary families of effect sizes used in most meta-analyses:
- Mean difference effect sizes : Used to show the magnitude of the difference between means of groups or conditions, commonly used when comparing a treatment and control group.
- Correlation effect sizes : Represent the degree of association between two continuous measures, indicating the strength and direction of their relationship.
- Odds ratio effect sizes : Used with binary outcomes to compare the odds of an event occurring between two groups, like whether a patient recovers from an illness or not.
The most appropriate effect size family is determined by the nature of the research question and dependent variable. All common effect sizes are able to be transformed from one version to another.
Real-Life Example
Brewin, C. R., Andrews, B., & Valentine, J. D. (2000). Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. Journal of Consulting and Clinical Psychology , 68 (5), 748.
This meta-analysis of 77 articles examined risk factors for posttraumatic stress disorder (PTSD) in trauma-exposed adults, with sample sizes ranging from 1,149 to over 11,000. Several factors consistently predicted PTSD with small effect sizes (r = 0.10 to 0.19), including female gender, lower education, lower intelligence, previous trauma, childhood adversity, and psychiatric history. Factors occurring during or after trauma showed somewhat stronger effects (r = 0.23 to 0.40), including trauma severity, lack of social support, and additional life stress. Most risk factors did not predict PTSD uniformly across populations and study types, with only psychiatric history, childhood abuse, and family psychiatric history showing homogeneous effects. Notable differences emerged between military and civilian samples, and methodological factors influenced some risk factor effects. The authors concluded that identifying a universal set of pretrauma predictors is premature and called for more research to understand how vulnerability to PTSD varies across populations and contexts.
How to Conduct a Meta-Analysis
Researchers should develop a comprehensive research protocol that outlines the objectives and hypotheses of their meta-analysis.
This document should provide specific details about every stage of the research process, including the methodology for identifying, selecting, and analyzing relevant studies.
For example, the protocol should specify search strategies for relevant studies, including whether the search will encompass unpublished works.
The protocol should be created before beginning the research process to ensure transparency and reproducibility.
Research Protocol
- To estimate the overall effect of growth mindset interventions on the academic achievement of students in primary and secondary school.
- To investigate if the effect of growth mindset interventions on academic achievement differs for students of different ages (e.g., elementary school students vs. high school students).
- To examine if the duration of the growth mindset intervention impacts its effectiveness.
- Growth mindset interventions will have a small, but statistically significant, positive effect on student academic achievement.
- Growth mindset interventions will be more effective for younger students than for older students.
- Longer growth mindset interventions will be more effective than shorter interventions.
Eligibility Criteria
- Published studies in English-language journals.
- Studies must include a quantitative measure of academic achievement (e.g., GPA, course grades, exam scores, or standardized test scores).
- Studies must involve a growth mindset intervention as the primary focus (including control vs treatment group comparison).
- Studies that combine growth mindset training with other interventions (e.g., study skills training, other types of psychological interventions) should be excluded.
Search Strategy
The researchers will search the following databases:
Keywords Combined with Boolean Operators:
- (“growth mindset” OR “implicit theories of intelligence” OR “mindset theory”) AND (“intervention” OR “training” OR “program”) ” OR “educational outcomes”) * OR “pupil ” OR “learner*”)**
Additional Search Strategies:
- Citation Chaining: Examining the reference lists of included studies can uncover additional relevant articles.
- Contacting Experts: Reaching out to researchers in the field of growth mindset can reveal unpublished studies or ongoing research.
Coding of Studies
The researchers will code each study for the following information:
- Sample size
- Age of participants
- Duration of intervention
- Type of academic outcome measured
- Study design (e.g., randomized controlled trial, quasi-experiment)
Statistical Analysis
- The researchers will calculate an effect size (e.g., standardized mean difference) for each study.
- The researchers will use a random-effects model to account for variation in effect sizes across studies.
- The researchers will use meta-regression to test the hypotheses about moderators of the effect of growth mindset interventions.
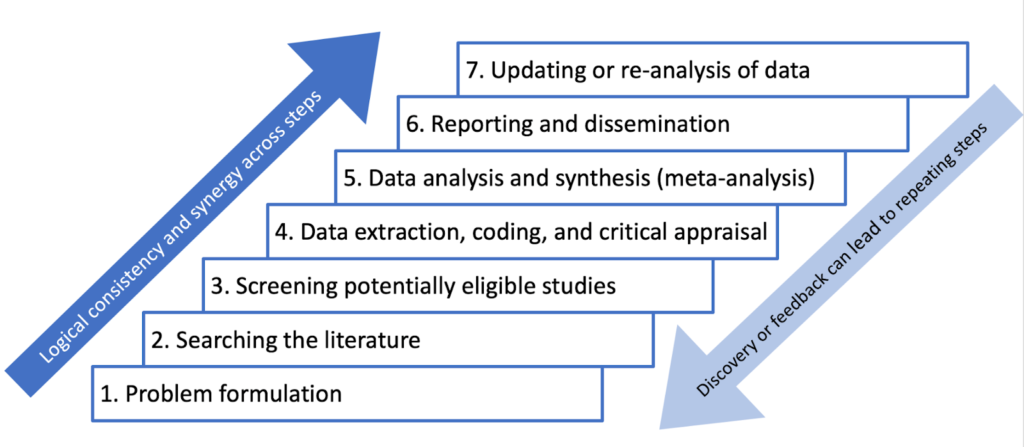
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) is a reporting guideline designed to improve the transparency and completeness of systematic review reporting.
PRISMA was created to tackle the issue of inadequate reporting often found in systematic reviews
- Checklist : PRISMA features a 27-item checklist covering all aspects of a meta-analysis, from the rationale and objectives to the synthesis of findings and discussion of limitations. Each checklist item is accompanied by detailed reporting recommendations in an Explanation and Elaboration document .
- Flow Diagram : PRISMA also includes a flow diagram to visually represent the study selection process, offering a clear, standardized way to illustrate how researchers arrived at the final set of included studies
Step 1: Defining a Research Question
A well-defined research question is a fundamental starting point for any research synthesis. The research question should guide decisions about which studies to include in the meta-analysis, and which statistical model is most appropriate.
For example:
- How do dysfunctional attitudes and negative automatic thinking directly and indirectly impact depression?
- Do growth mindset interventions generally improve students’ academic achievement?
- What is the association between child-parent attachment and prosociality in children?
- What is the relation of various risk factors to Post Traumatic Stress Disorder (PTSD)?
Step 2: Search Strategy
Present the full search strategies for all databases, registers and websites, including any filters and limits used. PRISMA 2020 Checklist
A search strategy is a comprehensive and reproducible plan for identifying all relevant research studies that address a specific research question.
This systematic approach to searching helps minimize bias.
It’s important to be transparent about the search strategy and document all decisions for auditability. The goal is to identify all potentially relevant studies for consideration.
PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) provide appropriate guidance for reporting quantitative literature searches.
Information Sources
The primary goal is to find all published and unpublished studies that meet the predefined criteria of the research question. This includes considering various sources beyond typical databases
Information sources for a meta-analysis can include a wide range of resources like scholarly databases, unpublished literature, conference papers, books, and even expert consultations.
Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. PRISMA 2020 Checklist
An exhaustive, systematic search strategy is developed with the assistance of an expert librarian.
- Databases: Searches should include seven key databases: CINAHL, Medline, APA PsycArticles, Psychology and Behavioral Sciences Collection, APA PsycInfo, SocINDEX with Full Text, and Web of Science: Core Collections.
- Grey Literature : In addition to databases, forensic or ‘expansive’ searches can be conducted. This includes: grey literature database searches (e.g. OpenGrey , WorldCat , Ethos ), conference proceedings, unpublished reports, theses , clinical trial databases , searches by names of authors of relevant publications. Independent research bodies may also be good sources of material, e.g. Centre for Research in Ethnic Relations , Joseph Rowntree Foundation , Carers UK .
- Citation Searching : Reference lists often lead to highly cited and influential papers in the field, providing valuable context and background information for the review.
- Contacting Experts: Reaching out to researchers or experts in the field can provide access to unpublished data or ongoing research not yet publicly available.
It is important to note that this may not be an exhaustive list of all potential databases.
Search String Construction
It is recommended to consult topic experts on the review team and advisory board in order to create as complete a list of search terms as possible for each concept.
To retrieve the most relevant results, a search string is used. This string is made up of:
- Keywords: Search terms should be relevant to the research questions, key variables, participants, and research design. Searches should include indexed terms, titles, and abstracts. Additionally, each database has specific indexed terms, so a targeted search strategy must be created for each database.
- Synonyms: These are words or phrases with similar meanings to the keywords, as authors may use different terms to describe the same concepts. Including synonyms helps cover variations in terminology and increases the chances of finding all relevant studies. For example, a drug intervention may be referred to by its generic name or by one of its several proprietary names.
- Truncation symbols : These broaden the search by capturing variations of a keyword. They function by locating every word that begins with a specific root. For example, if a user was researching interventions for smoking, they might use a truncation symbol to search for “smok*” to retrieve records with the words “smoke,” “smoker,” “smoking,” or “smokes.” This can save time and effort by eliminating the need to input every variation of a word into a database.
- Boolean operators: The use of Boolean operators (AND/OR/NEAR/NOT) helps to combine these terms effectively, ensuring that the search strategy is both sensitive and specific. For instance, using “AND” narrows the search to include only results containing both terms, while “OR” expands it to include results containing either term.
When conducting these searches, it is important to combine browsing of texts (publications) with periods of more focused systematic searching. This iterative process allows the search to evolve as the review progresses.
It is important to note that this information may not be entirely comprehensive and up-to-date.
Studies were identified by searching PubMed, PsycINFO, and the Cochrane Library. We conducted searches for studies published between the first available year and April 1, 2009, using the search term mindfulness combined with the terms meditation, program, therapy, or intervention and anxi , depress , mood, or stress. Additionally, an extensive manual review was conducted of reference lists of relevant studies and review articles extracted from the database searches. Articles determined to be related to the topic of mindfulness were selected for further examination.
Specify the inclusion and exclusion criteria for the review. PRISMA 2020 Checklist
Before beginning the literature search, researchers should establish clear eligibility criteria for study inclusion
To maintain transparency and minimize bias, eligibility criteria for study inclusion should be established a priori. Ideally, researchers should aim to include only high-quality randomized controlled trials that adhere to the intention-to-treat principle.
The selection of studies should not be arbitrary, and the rationale behind inclusion and exclusion criteria should be clearly articulated in the research protocol.
When specifying the inclusion and exclusion criteria, consider the following aspects:
- Intervention Characteristics: Researchers might decide that, in order to be included in the review, an intervention must have specific characteristics. They might require the intervention to last for a certain length of time, or they might determine that only interventions with a specific theoretical basis are appropriate for their review.
- Population Characteristics: A meta-analysis might focus on the effects of an intervention for a specific population. For instance, researchers might choose to focus on studies that included only nurses or physicians.
- Outcome Measures: Researchers might choose to include only studies that used outcome measures that met a specific standard.
- Age of Participants: If a meta-analysis is examining the effects of a treatment or intervention for children, the authors of the review will likely choose to exclude any studies that did not include children in the target age range.
- Diagnostic Status of Participants: Researchers conducting a meta-analysis of treatments for anxiety will likely exclude any studies where the participants were not diagnosed with an anxiety disorder.
- Study Design: Researchers might determine that only studies that used a particular research design, such as a randomized controlled trial, will be included in the review.
- Control Group: In a meta-analysis of an intervention, researchers might choose to include only studies that included certain types of control groups, such as a waiting list control or another type of intervention.
- Publication status : Decide whether only published studies will be included or if unpublished works, such as dissertations or conference proceedings, will also be considered.
Studies were selected if (a) they included a mindfulness-based intervention, (b) they included a clinical sample (i.e., participants had a diagnosable psychological or physical/medical disorder), (c) they included adult samples (18 – 65 years of age), (d) the mindfulness program was not coupled with treatment using acceptance and commitment therapy or dialectical behavior therapy, (e) they included a measure of anxiety and/or mood symptoms at both pre and postintervention, and (f) they provided sufficient data to perform effect size analyses (i.e., means and standard deviations, t or F values, change scores, frequencies, or probability levels). Studies were excluded if the sample overlapped either partially or completely with the sample of another study meeting inclusion criteria for the meta-analysis. In these cases, we selected for inclusion the study with the larger sample size or more complete data for measures of anxiety and depression symptoms. For studies that provided insufficient data but were otherwise appropriate for the analyses, authors were contacted for supplementary data.
Iterative Process
The iterative nature of developing a search strategy stems from the need to refine and adapt the search process based on the information encountered at each stage.
A single attempt rarely yields the perfect final strategy. Instead, it is an evolving process involving a series of test searches, analysis of results, and discussions among the review team.
Here’s how the iterative process unfolds:
- Initial Strategy Formulation: Based on the research question, the team develops a preliminary search strategy, including identifying relevant keywords, synonyms, databases, and search limits.
- Test Searches and Refinement: The initial search strategy is then tested on chosen databases. The results are reviewed for relevance, and the search strategy is refined accordingly. This might involve adding or modifying keywords, adjusting Boolean operators, or reconsidering the databases used.
- Discussions and Iteration: The search results and proposed refinements are discussed within the review team. The team collaboratively decides on the best modifications to improve the search’s comprehensiveness and relevance.
- Repeating the Cycle: This cycle of test searches, analysis, discussions, and refinements is repeated until the team is satisfied with the strategy’s ability to capture all relevant studies while minimizing irrelevant results.
By constantly refining the search strategy based on the results and feedback, researchers can be more confident that they have identified all relevant studies.
This iterative process ensures that the applied search strategy is sensitive enough to capture all relevant studies while maintaining a manageable scope.
Throughout this process, meticulous documentation of the search strategy, including any modifications, is crucial for transparency and future replication of the meta-analysis.
Step 3: Search the Literature
Conduct a systematic search of the literature using clearly defined search terms and databases.
Applying the search strategy involves entering the constructed search strings into the respective databases’ search interfaces. These search strings, crafted using Boolean operators, truncation symbols, wildcards, and database-specific syntax, aim to retrieve all potentially relevant studies addressing the research question.
The researcher, during this stage, interacts with the database’s features to refine the search and manage the retrieved results.
This might involve employing search filters provided by the database to focus on specific study designs, publication types, or other relevant parameters.
Applying the search strategy is not merely a mechanical process of inputting terms; it demands a thorough understanding of database functionalities and a discerning eye to adjust the search based on the nature of retrieved results.
Step 4: Screening & Selecting Research Articles
Once the literature search is complete, the next step is to screen and select the studies that will be included in the meta-analysis.
This involves carefully reviewing each study to determine its relevance to the research question and its methodological quality.
The goal is to identify studies that are both relevant to the research question and of sufficient quality to contribute to a meaningful synthesis.
Studies meeting the eligibility criteria are usually saved into electronic databases, such as Endnote or Mendeley , and include title, authors, date and publication journal along with an abstract (if available).
Selection Process
Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. PRISMA 2020 Checklist
The selection process in a meta-analysis involves multiple reviewers to ensure rigor and reliability.
Two reviewers should independently screen titles and abstracts, removing duplicates and irrelevant studies based on predefined inclusion and exclusion criteria.
- Initial screening of titles and abstracts: After applying a strategy to search the literature,, the next step involves screening the titles and abstracts of the identified articles against the predefined inclusion and exclusion criteria. During this initial screening, reviewers aim to identify potentially relevant studies while excluding those clearly outside the scope of the review. It is crucial to prioritize over-inclusion at this stage, meaning that reviewers should err on the side of keeping studies even if there is uncertainty about their relevance. This cautious approach helps minimize the risk of inadvertently excluding potentially valuable studies.
- Retrieving and assessing full texts: For studies which a definitive decision cannot be made based on the title and abstract alone, reviewers need to obtain the full text of the articles for a comprehensive assessment against the predefined inclusion and exclusion criteria. This stage involves meticulously reviewing the full text of each potentially relevant study to determine its eligibility definitively.
- Resolution of Disagreements : In cases of disagreement between reviewers regarding a study’s eligibility, a predefined strategy involving consensus-building discussions or arbitration by a third reviewer should be in place to reach a final decision. This collaborative approach ensures a fair and impartial selection process, further strengthening the review’s reliability.
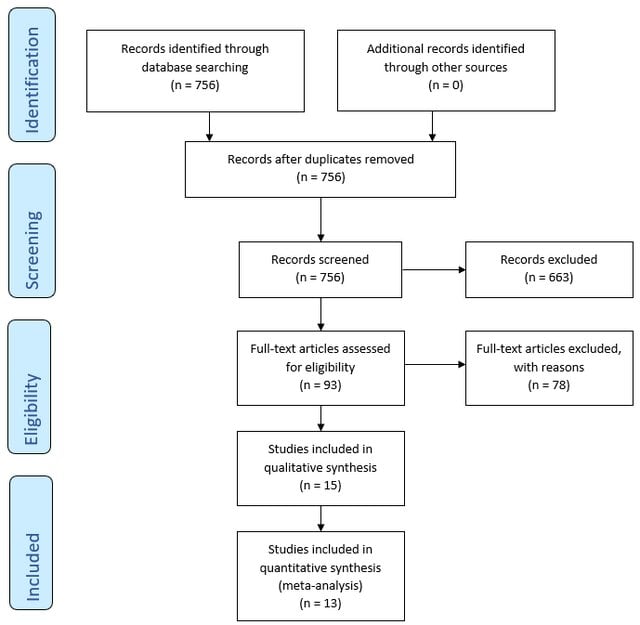
PRISMA Flowchart
The PRISMA flowchart is a visual representation of the study selection process within a systematic review.
The flowchart illustrates the step-by-step process of screening, filtering, and selecting studies based on predefined inclusion and exclusion criteria.
The flowchart visually depicts the following stages:
- Identification: The initial number of titles and abstracts identified through database searches.
- Screening: The screening process, based on titles and abstracts.
- Eligibility: Full-text copies of the remaining records are retrieved and assessed for eligibility.
- Inclusion: Applying the predefined inclusion criteria resulted in the inclusion of publications that met all the criteria for the review.
- Exclusion: The flowchart details the reasons for excluding the remaining records.
This systematic and transparent approach, as visualized in the PRISMA flowchart, ensures a robust and unbiased selection process, enhancing the reliability of the systematic review’s findings.
The flowchart serves as a visual record of the decisions made during the study selection process, allowing readers to assess the rigor and comprehensiveness of the review.
- How to fill a PRISMA flow diagram
Step 5: Evaluating the Quality of Studies
Data collection process.
Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. PRISMA 2020 Checklist
Data extraction focuses on information relevant to the research question, such as risk or recovery factors related to a particular phenomenon.
Extract data relevant to the research question, such as effect sizes, sample sizes, means, standard deviations, and other statistical measures.
It can be useful to focus on the authors’ interpretations of findings rather than individual participant quotes, as the latter lacks the full context of the original data.
The coding of studies in a meta-analysis involves carefully and systematically extracting data from each included study in a standardized and reliable manner. This step is essential for ensuring the accuracy and validity of the meta-analysis’s findings.
This information is then used to calculate effect sizes, examine potential moderators, and draw overall conclusions.
Coding procedures typically involve creating a standardized record form or coding protocol. This form guides the extraction of data from each study in a consistent and organized manner. Two independent observers can help to ensure accuracy and minimize errors during data extraction.
Beyond basic information like authors and publication year, code crucial study characteristics relevant to the research question.
For example, if the meta-analysis focuses on the effects of a specific therapy, relevant characteristics to code might include:
- Study characteristics : Publicatrion year, authors, country of origin, publication status ( Published : Peer-reviewed journal articles and book chapters Unpublished : Government reports, websites, theses/dissertations, conference presentations, unpublished manuscripts).
- Intervention : Type (e.g., CBT), duration of treatment, frequency (e.g., weekly sessions), delivery method (e.g., individual, group, online), intention-to-treat analysis (Yes/No)
- Outcome measures : Primary vs. secondary outcomes, time points of measurement (e.g., post-treatment, follow-up).
- Moderators : Participant characteristics that might moderate the effect size. (e.g., age, gender, diagnosis, socioeconomic status, education level, comorbidities).
- Study design : Design (RCT quasi-experiment, etc.), blinding, control group used (e.g., waitlist control, treatment as usual), study setting (clinical, community, online/remote, inpatient vs. outpatient), pre-registration (yes/no), allocation method (simple randomization, block randomization, etc.).
- Sample : Recruitment method (snowball, random, etc.), sample size (total and groups), sample location (treatment & control group), attrition rate, overlap with sample(s) from another study?
- Adherence to reporting guidelines : e.g., CONSORT, STROBE, PRISMA
- Funding source : Government, industry, non-profit, etc.
- Effect Size : Comprehensive meta-analysis program is used to compute d and/or r. Include up to 3 digits after the decimal point for effect size information and internal consistency information. Also record the page number and table number from which the information is coded. This information helps when checking reliability and accuracy to ensure we are coding from the same information.
Before applying the coding protocol to all studies, it’s crucial to pilot test it on a small subset of studies. This helps identify any ambiguities, inconsistencies, or areas for improvement in the coding protocol before full-scale coding begins.
It’s common to encounter missing data in primary research articles. Develop a clear strategy for handling missing data, which might involve contacting study authors, using imputation methods, or performing sensitivity analyses to assess the impact of missing data on the overall results.
Quality Appraisal Tools
Researchers use standardized tools to assess the quality and risk of bias in the quantitative studies included in the meta-analysis. Some commonly used tools include:
- Recommended by the Cochrane Collaboration for assessing randomized controlled trials (RCTs).
- Evaluates potential biases in selection, performance, detection, attrition, and reporting.
- Used for assessing the quality of non-randomized studies, including case-control and cohort studies.
- Evaluates selection, comparability, and outcome assessment.
- Assesses risk of bias in non-randomized studies of interventions.
- Evaluates confounding, selection bias, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported results.
- Specifically designed for diagnostic accuracy studies.
- Assesses risk of bias and applicability concerns in patient selection, index test, reference standard, and flow and timing.
By using these tools, researchers can ensure that the studies included in their meta-analysis are of high methodological quality and contribute reliable quantitative data to the overall analysis.
Step 6: Choice of Effect Size
The choice of effect size metric is typically determined by the research question and the nature of the dependent variable.
- Odds Ratio (OR) : For instance, if researchers are working in medical and health sciences where binary outcomes are common (e.g., yes/no, failed/success), effect sizes like relative risk and odds ratio are often used.
- Mean Difference : Studies focusing on experimental or between-group comparisons often employ mean differences. The raw mean difference, or unstandardized mean difference, is suitable when the scale of measurement is inherently meaningful and comparable across studies.
- Standardized Mean Difference (SMD) : If studies use different scales or measures, the standardized mean difference (e.g., Cohen’s d) is more appropriate. When analyzing observational studies, the correlation coefficient is commonly chosen as the effect size.
- Pearson correlation coefficient (r) : A statistical measure frequently employed in meta-analysis to examine the strength of the relationship between two continuous variables.
Conversion of efect sizes to a common measure
May be necessary to convert reported findings to the chosen primary effect size. The goal is to harmonize different effect size measures to a common metric for meaningful comparison and analysis.
This conversion allows researchers to include studies that report findings using various effect size metrics. For instance, r can be approximately converted to d, and vice versa, using specific equations. Similarly, r can be derived from an odds ratio using another formula.
Many equations relevant to converting effect sizes can be found in Rosenthal (1991).
Step 7: Assessing Heterogeneity
Heterogeneity refers to the variation in effect sizes across studies after accounting for within-study sampling errors.
Heterogeneity refers to how much the results (effect sizes) vary between different studies, where no variation would mean all studies showed the same improvement (no heterogeneity), while greater variation indicates more heterogeneity.
Assessing heterogeneity matters because it helps us understand if the study intervention works consistently across different contexts and guides how we combine and interpret the results of multiple studies.
While little heterogeneity allows us to be more confident in our overall conclusion, significant heterogeneity necessitates further investigation into its underlying causes.
How to assess heterogeneity
- Homogeneity Test : Meta-analyses typically include a homogeneity test to determine if the effect sizes are estimating the same population parameter. The test statistic, denoted as Q, is a weighted sum of squares that follows a chi-square distribution. A significant Q statistic suggests that the effect sizes are heterogeneous.
- I2 Statistic : The I2 statistic is a relative measure of heterogeneity that represents the ratio of between-study variance (τ2) to the total variance (between-study variance plus within-study variance). Higher I2 values indicate greater heterogeneity.
- Prediction Interval : Examining the width of a prediction interval can provide insights into the degree of heterogeneity. A wide prediction interval suggests substantial heterogeneity in the population effect size.
Step 8: Choosing the Meta-Analytic Model
Meta-analysts address heterogeneity by choosing between fixed-effects and random-effects analytical models.
Use a random-effects model if heterogeneity is high. Use a fixed-effect model if heterogeneity is low, or if all studies are functionally identical and you are not seeking to generalize to a range of scenarios.
Although a statistical test for homogeneity can help assess the variability in effect sizes across studies, it shouldn’t dictate the choice between fixed and random effects models.
The decision of which model to use is ultimately a conceptual one, driven by the researcher’s understanding of the research field and the goals of the meta-analysis.
If the number of studies is limited, a fixed-effects analysis is more appropriate, while more studies are required for a stable estimate of the between-study variance in a random-effects model.
It is important to note that using a random-effects model is generally a more conservative approach.
Fixed-effects models
- Assumes all studies are measuring the exact same thing
- Gives much more weight to larger studies
- Use when studies are very similar
Fixed-effects models assume that there is one true effect size underlying all studies. The goal is to estimate this common effect size with the greatest precision, which is achieved by minimizing the within-study (sampling).
Consequently, studies are weighted by the inverse of their variance.
This means that larger studies, which generally have smaller variances, are assigned greater weight in the analysis because they provide more precise estimates of the common effect size
- Simplicity: The fixed-effect model is straightforward to implement and interpret, making it computationally simpler.
- Precision: When the assumption of a common effect size is met, fixed-effect models provide more precise estimates with narrower confidence intervals compared to random-effects models.
- Suitable for Conditional Inferences: Fixed-effect models are appropriate when the goal is to make inferences specifically about the studies included in the meta-analysis, without generalizing to a broader population.
- Restrictive Assumptions: The fixed-effect model assumes all studies estimate the same population parameter, which is often unrealistic, particularly with studies drawn from diverse methodologies or populations.
- Limited Generalizability: Findings from fixed-effect models are conditional on the included studies, limiting their generalizability to other contexts or populations.
- Sensitivity to Heterogeneity: Fixed-effect models are sensitive to the presence of heterogeneity among studies, and may produce misleading results if substantial heterogeneity exists.
Random-effects models
- Assumes studies might be measuring slightly different things
- Gives more balanced weight to both large and small studies
- Use when studies might vary in methods or populations
Random-effects models assume that the true effect size can vary across studies. The goal here is to estimate the mean of these varying effect sizes, considering both within-study variance and between-study variance (heterogeneity).
This approach acknowledges that each study might estimate a slightly different effect size due to factors beyond sampling error, such as variations in study populations, interventions, or designs.
This balanced weighting prevents large studies from disproportionately influencing the overall effect size estimate, leading to a more representative average effect size that reflects the distribution of effects across a range of studies.
- Realistic Assumptions: Random-effects models acknowledge the presence of between-study variability by assuming true effects are randomly distributed, making it more suitable for real-world research scenarios.
- Generalizability: Random-effects models allow for broader inferences to be made about a population of studies, enhancing the generalizability of findings.
- Accommodation of Heterogeneity: Random-effects models explicitly model heterogeneity, providing a more accurate representation of the overall effect when studies have varying effect sizes.
- Complexity: Random-effects models are computationally more complex, requiring the estimation of additional parameters, such as between-study variance.
- Reduced Precision: Confidence intervals tend to be wider compared to fixed-effect models, particularly when between-study heterogeneity is substantial.
- Requirement for Sufficient Studies: Accurate estimation of between-study variance necessitates a sufficient number of studies, making random-effects models less reliable with smaller meta-analyses.
Step 9: Perform the Meta-Analysis
This step involves statistically combining effect sizes from chosen studies. Meta-analysis uses the weighted mean of effect sizes, typically giving larger weights to more precise studies (often those with larger sample sizes).
The main function of meta-analysis is to estimate effects in a population by combining the effect sizes from multiple articles.
It uses a weighted mean of the effect sizes, typically giving larger weights to more precise studies, often those with larger sample sizes.
This weighting scheme makes statistical sense because an effect size with good sampling accuracy (i.e., likely to be an accurate reflection of reality) is weighted highly.
On the other hand, effect sizes from studies with lower sampling accuracy are given less weight in the calculations.
the process:
- Calculate weights for each study
- Multiply each study’s effect by its weight
- Add up all these weighted effects
- Divide by the sum of all weights
Estimating effect size using fixed effects
The fixed-effects model in meta-analysis operates under the assumption that all included studies are estimating the same true effect size.
This model focuses solely on within-study variance when determining the weight of each study.
The weight is calculated as the inverse of the within-study variance, which typically results in larger studies receiving substantially more weight in the analysis.
This approach is based on the idea that larger studies provide more precise estimates of the true effect.
The weighted mean effect size (M) is calculated by summing the products of each study’s effect size (ESi) and its corresponding weight (wi) and dividing that sum by the total sum of the weights:
1. Calculate weights (wi) for each study:
The weight is often the inverse of the variance of the effect size. This means studies with larger sample sizes and less variability will have greater weight, as they provide more precise estimates of the effect size
This weighting scheme reflects the assumption in a fixed-effect model that all studies are estimating the same true effect size, and any observed differences in effect sizes are solely due to sampling error. Therefore, studies with less sampling error (i.e., smaller variances) are considered more reliable and are given more weight in the analysis.
Here’s the formula for calculating the weight in a fixed-effect meta-analysis:
Wi = 1 / VYi 1
- Wi represents the weight assigned to study i.
- VYi is the within-study variance for study i.
Practical steps:
- The weight for each study is calculated as: Weight = 1 / (within-study variance)
- For example: Let’s say a study reports a within-study variance of 0.04. The weight for this study would be: 1 / 0.04 = 25
- Calculate the weight for every study included in your meta-analysis using this method.
- These weights will be used in subsequent calculations, such as computing the weighted mean effect size.
- Note : In a fixed-effects model, we do not calculate or use τ² (tau squared), which represents between-study variance. This is only used in random-effects models.
2. Multiply each study’s effect by its weight:
After calculating the weight for each study, multiply the effect size by its corresponding weight. This step is crucial because it ensures that studies with more precise effect size estimates contribute proportionally more to the overall weighted mean effect size
- For each study, multiply its effect size by the weight we just calculated.
3. Add up all these weighted effects:
Sum up all the products from step 2.
4. Divide by the sum of all weights:
- Add up all the weights we calculated in step 1.
- Divide the sum from step 3 by this total weight.
Implications of the fixed-effects model
- Larger studies (with smaller within-study variance) receive substantially more weight.
- This model assumes that differences between study results are due only to sampling error.
- It’s most appropriate when studies are very similar in methods and sample characteristics.
Estimating effect size using random effects
Random effects meta-analysis is slightly more complicated because multiple sources of differences potentially affecting effect sizes must be accounted for.
The main difference in the random effects model is the inclusion of τ² (tau squared) in the weight calculation. This accounts for between-study heterogeneity, recognizing that studies might be measuring slightly different effects.
This process results in an overall effect size that takes into account both within-study and between-study variability, making it more appropriate when studies differ in methods or populations.
The model estimates the variance of the true effect sizes (τ²). This requires a reasonable number of studies, so random effects estimation might not be feasible with very few studies.
Estimation is typically done using statistical software, with restricted maximum likelihood (REML) being a common method.
1. Calculate weights for each study:
In a random-effects meta-analysis, the weight assigned to each study (W*i) is calculated as the inverse of that study’s variance, similar to a fixed-effect model. However, the variance in a random-effects model considers both the within-study variance (VYi) and the between-studies variance (T^2).
The inclusion of T^2 in the denominator of the weight formula reflects the random-effects model’s assumption that the true effect size can vary across studies.
This means that in addition to sampling error, there is another source of variability that needs to be accounted for when weighting the studies. The between-studies variance, T^2, represents this additional source of variability.
Here’s the formula for calculating the weight in a random-effects meta-analysis:
W*i = 1 / (VYi + T^2)
- W*i represents the weight assigned to study i.
- T^2 is the estimated between-studies variance.
First, we need to calculate something called τ² (tau squared). This represents the between-study variance.
The estimation of T^2 can be done using different methods, one common approach being the method of moments (DerSimonian and Laird method).
The formula for T^2 using the method of moments is: T^2 = (Q – df) / C
- Q is the homogeneity statistic.
- df is the degrees of freedom (number of studies -1).
- C is a constant calculated based on the study weights
- The weight for each study is then calculated as: Weight = 1 / (within-study variance + τ²). This is different from the fixed effects model because we’re adding τ² to account for between-study variability.
Add up all the weights we calculated in step 1. Divide the sum from step 3 by this total weight
Implications of the random-effects model
- Weights are more balanced between large and small studies compared to the fixed-effects model.
- It’s most appropriate when studies vary in methods, sample characteristics, or other factors that might influence the true effect size.
- The random-effects model typically produces wider confidence intervals, reflecting the additional uncertainty from between-study variability.
- Results are more generalizable to a broader population of studies beyond those included in the meta-analysis.
- This model is often more realistic for social and behavioral sciences, where true effects may vary across different contexts or populations.
Step 10: Sensitivity Analysis
Assess the robustness of your findings by repeating the analysis using different statistical methods, models (fixed-effects and random-effects), or inclusion criteria. This helps determine how sensitive your results are to the choices made during the process.
Sensitivity analysis strengthens a meta-analysis by revealing how robust the findings are to the various decisions and assumptions made during the process. It helps to determine if the conclusions drawn from the meta-analysis hold up when different methods, criteria, or data subsets are used.
This is especially important since opinions may differ on the best approach to conducting a meta-analysis, making the exploration of these variations crucial.
Here are some key ways sensitivity analysis contributes to a more robust meta-analysis:
- Assessing Impact of Different Statistical Methods : A sensitivity analysis can involve calculating the overall effect using different statistical methods, such as fixed and random effects models. This comparison helps determine if the chosen statistical model significantly influences the overall results. For instance, in the meta-analysis of β-blockers after myocardial infarction, both fixed and random effects models yielded almost identical overall estimates. This suggests that the meta-analysis findings are resilient to the statistical method employed.
- Evaluating the Influence of Trial Quality and Size : By analyzing the data with and without trials of questionable quality or varying sizes, researchers can assess the impact of these factors on the overall findings.
- Examining the Effect of Trials Stopped Early : Including trials that were stopped early due to interim analysis results can introduce bias. Sensitivity analysis helps determine if the inclusion or exclusion of such trials noticeably changes the overall effect. In the example of the β-blocker meta-analysis, excluding trials stopped early had a negligible impact on the overall estimate.
- Addressing Publication Bias : It’s essential to assess and account for publication bias, which occurs when studies with statistically significant results are more likely to be published than those with null or nonsignificant findings. This can be accomplished by employing techniques like funnel plots, statistical tests (e.g., Begg and Mazumdar’s rank correlation test, Egger’s test), and sensitivity analyses.
By systematically varying different aspects of the meta-analysis, researchers can assess the robustness of their findings and address potential concerns about the validity of their conclusions.
This process ensures a more reliable and trustworthy synthesis of the research evidence.
Common Mistakes
When conducting a meta-analysis, several common pitfalls can arise, potentially undermining the validity and reliability of the findings. Sources caution against these mistakes and offer guidance on conducting methodologically sound meta-analyses.
- Insufficient Number of Studies: If there are too few primary studies available, a meta-analysis might not be appropriate. While a meta-analysis can technically be conducted with only two studies, the research community might not view findings based on a limited number of studies as reliable evidence. A small number of studies could suggest that the research field is not mature enough for meaningful synthesis.
- Inappropriate Combination of Studies : Meta-analyses should not simply combine studies indiscriminately. Avoid the “apples and oranges” problem, where studies with different research objectives, designs, measures, or samples are inappropriately combined. Such practices can obscure important differences between studies and lead to misleading conclusions.
- Misinterpreting Heterogeneity : One common mistake is using the Q statistic or p-value from a test of heterogeneity as the sole indicator of heterogeneity. While these statistics can signal heterogeneity, they do not quantify the extent of variation in effect sizes.
- Over-Reliance on Published Studies : This dependence on published literature introduces the risk of publication bias, where studies with statistically significant or favorable results are more likely to be published. Failure to acknowledge and address publication bias can lead to overestimating the true effect size.
- Neglecting Study Quality : Including studies with poor methodological quality can bias the results of a meta-analysis leading to unreliable and inaccurate effect size estimates. The decision of which studies to include should be based on predefined eligibility criteria to ensure the quality and relevance of the synthesis.
- Fixation on Statistical Significance : Placing excessive emphasis on the statistical significance of an overall effect while neglecting its practical significance is a critical mistake in meta-analysis, as is the case in primary studies. Considers both statistical and clinical or substantive significance.
- Misinterpreting Significance Testing in Subgroup Analyses : When comparing effect sizes across subgroups, merely observing that an effect is statistically significant in one subgroup but not another is insufficient. Conduct formal tests of statistical significance for the difference in effects between subgroups or to calculate the difference in effects with confidence intervals.
- Ignoring Dependence : Neglecting dependence among effect sizes, particularly when multiple effect sizes are extracted from the same study, is a mistake. This oversight can inflate Type I error rates and lead to inaccurate estimations of average effect sizes and standard errors.
- Inadequate Reporting : Failing to transparently and comprehensively report the meta-analysis process is a crucial mistake. A meta-analysis should include a detailed written protocol outlining the research question, search strategy, inclusion criteria, and analytical methods.
Reading List
- Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., & Van Ijzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study . Psychological bulletin , 133 (1), 1.
- Borenstein, M., Hedges, L. V., Higgins, J. P., & Rothstein, H. R. (2021). Introduction to meta-analysis . John Wiley & Sons.
- Crits-Christoph, P. (1992). A Meta-analysis . American Journal of Psychiatry , 149 , 151-158.
- Duval, S. J., & Tweedie, R. L. (2000). A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. Journal of the American Statistical Association, 95 (449), 89–98.
- Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test . BMJ, 315 (7109), 629–634.
- Egger, M., Smith, G. D., & Phillips, A. N. (1997). Meta-analysis: principles and procedures . Bmj , 315 (7121), 1533-1537.
- Field, A. P., & Gillett, R. (2010). How to do a meta‐analysis . British Journal of Mathematical and Statistical Psychology , 63 (3), 665-694.
- Hedges, L. V., & Pigott, T. D. (2004). The power of statistical tests for moderators in meta-analysis . Psychological methods , 9 (4), 426.
- Hedges, L. V., & Olkin, I. (2014). Statistical methods for meta-analysis . Academic press.
- Hofmann, S. G., Sawyer, A. T., Witt, A. A., & Oh, D. (2010). The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review . Journal of consulting and clinical psychology , 78 (2), 169.
- Littell, J. H., Corcoran, J., & Pillai, V. (2008). Systematic reviews and meta-analysis . Oxford University Press.
- Lyubomirsky, S., King, L., & Diener, E. (2005). The benefits of frequent positive affect: Does happiness lead to success? . Psychological bulletin , 131 (6), 803.
- Macnamara, B. N., & Burgoyne, A. P. (2022). Do growth mindset interventions impact students’ academic achievement? A systematic review and meta-analysis with recommendations for best practices. Psychological Bulletin .
- Polanin, J. R., & Pigott, T. D. (2015). The use of meta‐analytic statistical significance testing . Research Synthesis Methods , 6 (1), 63-73.
- Rodgers, M. A., & Pustejovsky, J. E. (2021). Evaluating meta-analytic methods to detect selective reporting in the presence of dependent effect sizes . Psychological methods , 26 (2), 141.
- Rosenthal, R. (1991). Meta-analysis: a review. Psychosomatic medicine , 53 (3), 247-271.
- Tipton, E., Pustejovsky, J. E., & Ahmadi, H. (2019). A history of meta‐regression: Technical, conceptual, and practical developments between 1974 and 2018 . Research synthesis methods , 10 (2), 161-179.
- Zhao, J. G., Zeng, X. T., Wang, J., & Liu, L. (2017). Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: a systematic review and meta-analysis. Jama , 318 (24), 2466-2482.
- How it works
"Christmas Offer"
Terms & conditions.
As the Christmas season is upon us, we find ourselves reflecting on the past year and those who we have helped to shape their future. It’s been quite a year for us all! The end of the year brings no greater joy than the opportunity to express to you Christmas greetings and good wishes.
At this special time of year, Research Prospect brings joyful discount of 10% on all its services. May your Christmas and New Year be filled with joy.
We are looking back with appreciation for your loyalty and looking forward to moving into the New Year together.
"Claim this offer"
In unfamiliar and hard times, we have stuck by you. This Christmas, Research Prospect brings you all the joy with exciting discount of 10% on all its services.
Offer valid till 5-1-2024
We love being your partner in success. We know you have been working hard lately, take a break this holiday season to spend time with your loved ones while we make sure you succeed in your academics
Discount code: RP0996Y

Meta-Analysis – Definition, Purpose And How To Conduct It
Published by Owen Ingram at April 26th, 2023 , Revised On September 23, 2024
The number of studies being published in biomedical and clinical literature is increasing day by day. The massive abundance of research studies in these areas makes it rather difficult to synthesise all data and accumulate knowledge from various studies. All of this delays certain clinical decisions and conclusions to be made.
To determine the validity of a hypothesis , it is necessary to look into multiple studies rather than just one. For this purpose, systematic reviews or narrative reviews have been used to synthesise data from multiple studies, which often leads to an objective approach as different people can have different opinions. Meta-analysis, on the other hand, provides an objective and quantitative approach to combining evidence from various studies.
What Is Meta-Analysis?
The meaning of meta-analysis is a statistical method of combining results from numerous studies on a certain research question. The term was first used in 1976 and can be used to determine if the effect reported in the literature is real or not.
To conduct a quality meta-analysis, you need to identify an area in which the effect of treatment is uncertain. It is also recommended that you collect as many studies similar to the effect as possible so that you can compare them and get a better picture of it. This assists the researcher in understanding how big or small the effect is, and how different the results are from other studies.
Purpose Of Meta-Analysis In Research
The purpose of meta-analysis is more than just combining results from studies to give a statistical assessment. It also helps to point out:
- Any potential reasons for variations and differences in results, also known as heterogeneity in meta-analysis. Some popular reasons for this might be differences in sample size, or differences in analysis methods in research.
- The real estimate of the effect size that is reported in literature than any individual study. Combining multiple studies reduces research bias and the chances of random errors.
Meta-Analysis In Applied And Basic Research
Basic research involves seeking knowledge and gathering data on any subject, whereas applied research is more experimental and uses methods to solve real-life problems. Meta-analyses are used in both types of research that is applied and basic research.
- Pharmaceutical companies use meta-analyses to gain approval for new drugs, such as antibiotics for bacterial infections. Even regulatory authorities use this research method to gain approval for different processes. Hence, meta-analysis is used in medicine, crime, education and psychology for applied research.
- In terms of basic research, it is used in various fields such as sociology, finance, economics, marketing and social psychology. An example of meta-analysis in basic research is studying the effect of caffeine on cognitive performance.
Strengths and Limitations Of Meta-Analysis
There are many key benefits of meta-analysis in research studies, as it is a powerful tool. Here are some strengths of it:
- A meta-analysis takes place after a systematic review, which means the end product will be reliable and accurate.
- It has great statistical power, as it combines multiple studies rather than one individual study, which might suffer from a lack of sufficient data.
- This analysis can confirm existing research or refute it. Either way, it gives a confirmatory data analysis.
- It is regarded highly in the scientific community, as it provides an objective and solid analysis of evidence.
Challenges Associated With Meta-Analysis
Meta-analysis also has certain challenges that result in limitations while carrying out this statistical quantitative approach. Some challenges faced by meta-analyses are:
- It is not always possible to predict the outcome of a large-scale study. This is because meta-analysis mostly rely on small-scale studies which do not represent the broad population.
- A decent meta-analysis can not make up for flawed or bad research designs. Thus, it can not control the potential for bias to arise in studies. Therefore, it is urged to only include research with sound methodologies known as “best evidence synthesis”.
Hire an Expert Writer
Orders completed by our expert writers are
- Formally drafted in an academic style
- Free Amendments and 100% Plagiarism Free – or your money back!
- 100% Confidential and Timely Delivery!
- Free anti-plagiarism report
- Appreciated by thousands of clients. Check client reviews
How To Conduct A Meta-Analysis
Before conducting a meta-analysis and defining the research scope, it is necessary to evaluate the number of publications that have grown over the years. It can be quite hard to scan and skim through a large number of studies and literature reviews, which is why it is necessary to define the research question with care, including only relevant aspects. Here are steps on how to perform a meta-analysis:
- Formulate a research question that showcases the effects or interventions to be studied. This is mostly a binary question, such as “Does drug X improve outcome Y” in clinical studies.
- Conduct a systematic review that analyses and synthesises all data related to the one research question.
- Gather all data such as sample sizes and research methods used to indicate data variability. All decide which dependent variables are allowed.
- The selection of criteria is also a crucial step as it is necessary to understand whether published or unpublished studies are to be included or not. Based on the research question, it is important to choose studies that are quality-based and relevant.
- Choosing the right meta-analytic methods and meta-analysis software to be used in meta-analysis is another significant step. Some methods used are traditional univariate meta-analysis, meta-regression and meta-analytic structural equation modelling methods.
- While evaluating the data, it is necessary to use a meta-analysis forest plot, which is the graphical representation of the results of a meta-analysis studies. Its visual representation helps understand the heterogeneity among studies and helps compare the overall effect sizes of an intervention.
- The final step of literature meta-analysis is to report the results. They should be comprehensive and precise for the reader’s understanding.
Meta-Analysis Vs Systematic Review
A systematic review is a comprehensive analysis of existing research, whereas a meta-analysis is a statistical analysis or combination of results from two separate studies. Here’s how the two differ from each other:
Frequently Asked Questions
Where does meta-analysis fit in the research process.
It plays a key role in planning new studies and identifying answers to research questions. It is also widely sought for publications. Lastly, it is also used for grant applications that are used to justify the need for a new study.
Which fields use meta-analysis?
Common fields where meta-analyses are used are medicine, psychology, sociology, education, and health. It may also be used in finance, marketing and economics.
Is meta-analysis qualitative or quantitative?
Meta-analysis is a quantitative method that uses statistical methods to synthesise and collect data from various studies to estimate the size of the effect of a particular intervention or treatment.
You May Also Like
Quantitative research is associated with measurable numerical data. Qualitative research is where a researcher collects evidence to seek answers to a question.
In correlational research, a researcher measures the relationship between two or more variables or sets of scores without having control over the variables.
A case study is a detailed analysis of a situation concerning organizations, industries, and markets. The case study generally aims at identifying the weak areas.
As Featured On

USEFUL LINKS
LEARNING RESOURCES

COMPANY DETAILS

Splash Sol LLC
- How It Works

Meta-analysis
Reviewed by Psychology Today Staff
Meta-analysis is an objective examination of published data from many studies of the same research topic identified through a literature search. Through the use of rigorous statistical methods, it can reveal patterns hidden in individual studies and can yield conclusions that have a high degree of reliability. It is a method of analysis that is especially useful for gaining an understanding of complex phenomena when independent studies have produced conflicting findings.
Meta-analysis provides much of the underpinning for evidence-based medicine. It is particularly helpful in identifying risk factors for a disorder, diagnostic criteria, and the effects of treatments on specific populations of people, as well as quantifying the size of the effects. Meta-analysis is well-suited to understanding the complexities of human behavior.
- How Does It Differ From Other Studies?
- When Is It Used?
- What Are Some Important Things Revealed by Meta-analysis?

There are well-established scientific criteria for selecting studies for meta-analysis. Usually, meta-analysis is conducted on the gold standard of scientific research—randomized, controlled, double-blind trials. In addition, published guidelines not only describe standards for the inclusion of studies to be analyzed but also rank the quality of different types of studies. For example, cohort studies are likely to provide more reliable information than case reports.
Through statistical methods applied to the original data collected in the included studies, meta-analysis can account for and overcome many differences in the way the studies were conducted, such as the populations studied, how interventions were administered, and what outcomes were assessed and how. Meta-analyses, and the questions they are attempting to answer, are typically specified and registered with a scientific organization, and, with the protocols and methods openly described and reviewed independently by outside investigators, the research process is highly transparent.

Meta-analysis is often used to validate observed phenomena, determine the conditions under which effects occur, and get enough clarity in clinical decision-making to indicate a course of therapeutic action when individual studies have produced disparate findings. In reviewing the aggregate results of well-controlled studies meeting criteria for inclusion, meta-analysis can also reveal which research questions, test conditions, and research methods yield the most reliable results, not only providing findings of immediate clinical utility but furthering science.
The technique can be used to answer social and behavioral questions large and small. For example, to clarify whether or not having more options makes it harder for people to settle on any one item, a meta-analysis of over 53 conflicting studies on the phenomenon was conducted. The meta-analysis revealed that choice overload exists—but only under certain conditions. You will have difficulty selecting a TV show to watch from the massive array of possibilities, for example, if the shows differ from each other in multiple ways or if you don’t have any strong preferences when you finally get to sit down in front of the TV.

A meta-analysis conducted in 2000, for example, answered the question of whether physically attractive people have “better” personalities . Among other traits, they prove to be more extroverted and have more social skills than others. Another meta-analysis, in 2014, showed strong ties between physical attractiveness as rated by others and having good mental and physical health. The effects on such personality factors as extraversion are too small to reliably show up in individual studies but real enough to be detected in the aggregate number of study participants. Together, the studies validate hypotheses put forth by evolutionary psychologists that physical attractiveness is important in mate selection because it is a reliable cue of health and, likely, fertility.

The stress generation hypothesis views the symptoms of psychopathology as a unique source of stress that may contribute to the development and maintenance of disordered states.

Mistakenly categorizing one type of thing as another can lead to erroneous—and sometimes paranormal—beliefs.

How many common teaching techniques have actually been tested? Of those that have been tested, what were the results? The answers may surprise you. They surprised this educator!

The replication crisis, publication bias, and careerism undermine scientific rigor and ethical responsibility in counseling to provide effective and safe care for clients.

The human brain has two halves. A new study highlights when differences between them start.

When considered across a lifetime, no within-person association exists between religiosity and psychological well-being.

What are the prevalence rates of psychosis for displaced refugees?

A recent review provides compelling evidence that arts engagement significantly reduces cognitive decline and enhances the quality of life among healthy older adults.

Personal Perspective: Mental healthcare AI is evolving beyond administrative roles. By automating routine tasks, therapists can spend sessions focusing on human interactions.

Investing in building a positive classroom climate holds benefits for students and teachers alike.
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

It’s increasingly common for someone to be diagnosed with a condition such as ADHD or autism as an adult. A diagnosis often brings relief, but it can also come with as many questions as answers.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
A Guide to Conducting a Meta-Analysis
Affiliations.
- 1 Department of Psychology, Faculty of Arts and Social Sciences, National University of Singapore, Block AS4, Level 2, 9 Arts Link, Singapore, 117570, Singapore. [email protected].
- 2 Department of Psychology, Faculty of Arts and Social Sciences, National University of Singapore, Block AS4, Level 2, 9 Arts Link, Singapore, 117570, Singapore.
- PMID: 27209412
- DOI: 10.1007/s11065-016-9319-z
Meta-analysis is widely accepted as the preferred method to synthesize research findings in various disciplines. This paper provides an introduction to when and how to conduct a meta-analysis. Several practical questions, such as advantages of meta-analysis over conventional narrative review and the number of studies required for a meta-analysis, are addressed. Common meta-analytic models are then introduced. An artificial dataset is used to illustrate how a meta-analysis is conducted in several software packages. The paper concludes with some common pitfalls of meta-analysis and their solutions. The primary goal of this paper is to provide a summary background to readers who would like to conduct their first meta-analytic study.
Keywords: Literature review; Meta-analysis; Moderator analysis; Systematic review.
Publication types
- Research Support, Non-U.S. Gov't
- Data Interpretation, Statistical
- Meta-Analysis as Topic*
- Publication Bias
- Review Literature as Topic
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Practical Guide to Meta-analysis
- 1 Stanford-Surgery Policy Improvement, Research and Education (S-SPIRE) Center, Palo Alto, California
- 2 Department of Biostatistics, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill
- 3 Department of Surgery, University of Michigan, Ann Arbor
- Editorial Maximizing the Impact of Surgical Health Services Research Amir A. Ghaferi, MD, MS; Adil H. Haider, MD, MPH; Melina R. Kibbe, MD JAMA Surgery
- Guide to Statistics and Methods Practical Guide to Qualitative Analysis Margaret L. Schwarze, MD, MPP; Amy H. Kaji, MD, PhD; Amir A. Ghaferi, MD, MS JAMA Surgery
- Guide to Statistics and Methods Practical Guide to Mixed Methods Lesly A. Dossett, MD, MPH; Amy H. Kaji, MD, PhD; Justin B. Dimick, MD, MPH JAMA Surgery
- Guide to Statistics and Methods Practical Guide to Cost-effectiveness Analysis Benjamin S. Brooke, MD, PhD; Amy H. Kaji, MD, PhD; Kamal M. F. Itani, MD JAMA Surgery
- Guide to Statistics and Methods Practical Guide to Comparative Effectiveness Research Using Observational Data Ryan P. Merkow, MD, MS; Todd A. Schwartz, DrPH; Avery B. Nathens, MD, MPH, PhD JAMA Surgery
- Guide to Statistics and Methods Practical Guide to Health Policy Evaluation Using Observational Data John W. Scott, MD, MPH; Todd A. Schwartz, DrPH; Justin B. Dimick, MD, MPH JAMA Surgery
- Guide to Statistics and Methods Practical Guide to Survey Research Karen Brasel, MD, MPH; Adil Haider, MD, MPH; Jason Haukoos, MD, MSc JAMA Surgery
- Guide to Statistics and Methods Practical Guide to Assessment of Patient-Reported Outcomes Giana H. Davidson, MD, MPH; Jason S. Haukoos, MD, MSc; Liane S. Feldman, MD JAMA Surgery
- Guide to Statistics and Methods Practical Guide to Implementation Science Heather B. Neuman, MD, MS; Amy H. Kaji, MD, PhD; Elliott R. Haut, MD, PhD JAMA Surgery
- Guide to Statistics and Methods Practical Guide to Decision Analysis Dorry L. Segev, MD, PhD; Jason S. Haukoos, MD, MSc; Timothy M. Pawlik, MD, MPH, PhD JAMA Surgery
Meta-analysis is a systematic approach of synthesizing, combining, and analyzing data from multiple studies (randomized clinical trials 1 or observational studies 2 ) into a single effect estimate to answer a research question. Meta-analysis is especially useful if there is debate around the research question in the literature published to date or the individual published studies are underpowered. Vital to a high-quality meta-analysis is a comprehensive literature search, prespecified hypothesis and aims, reporting of study quality, consideration of heterogeneity and examination of bias. In the hierarchy of evidence, meta-analysis appears above observational studies and randomized clinical trials because it rigorously collates evidence across a larger body of literature; however, meta-analysis is largely dependent on the quality of the primary data.
- Editorial Maximizing the Impact of Surgical Health Services Research JAMA Surgery
Read More About
Arya S , Schwartz TA , Ghaferi AA. Practical Guide to Meta-analysis. JAMA Surg. 2020;155(5):430–431. doi:10.1001/jamasurg.2019.4523
Manage citations:
© 2024
Surgery in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Digital Health
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Sexual Health
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Study Design 101: Meta-Analysis
- Case Report
- Case Control Study
- Cohort Study
- Randomized Controlled Trial
- Practice Guideline
- Systematic Review
Meta-Analysis
- Helpful Formulas
- Finding Specific Study Types
A subset of systematic reviews; a method for systematically combining pertinent qualitative and quantitative study data from several selected studies to develop a single conclusion that has greater statistical power. This conclusion is statistically stronger than the analysis of any single study, due to increased numbers of subjects, greater diversity among subjects, or accumulated effects and results.
Meta-analysis would be used for the following purposes:
- To establish statistical significance with studies that have conflicting results
- To develop a more correct estimate of effect magnitude
- To provide a more complex analysis of harms, safety data, and benefits
- To examine subgroups with individual numbers that are not statistically significant
If the individual studies utilized randomized controlled trials (RCT), combining several selected RCT results would be the highest-level of evidence on the evidence hierarchy, followed by systematic reviews, which analyze all available studies on a topic.
- Greater statistical power
- Confirmatory data analysis
- Greater ability to extrapolate to general population affected
- Considered an evidence-based resource
Disadvantages
- Difficult and time consuming to identify appropriate studies
- Not all studies provide adequate data for inclusion and analysis
- Requires advanced statistical techniques
- Heterogeneity of study populations
Design pitfalls to look out for
The studies pooled for review should be similar in type (i.e. all randomized controlled trials).
Are the studies being reviewed all the same type of study or are they a mixture of different types?
The analysis should include published and unpublished results to avoid publication bias.
Does the meta-analysis include any appropriate relevant studies that may have had negative outcomes?
Fictitious Example
Do individuals who wear sunscreen have fewer cases of melanoma than those who do not wear sunscreen? A MEDLINE search was conducted using the terms melanoma, sunscreening agents, and zinc oxide, resulting in 8 randomized controlled studies, each with between 100 and 120 subjects. All of the studies showed a positive effect between wearing sunscreen and reducing the likelihood of melanoma. The subjects from all eight studies (total: 860 subjects) were pooled and statistically analyzed to determine the effect of the relationship between wearing sunscreen and melanoma. This meta-analysis showed a 50% reduction in melanoma diagnosis among sunscreen-wearers.
Real-life Examples
Goyal, A., Elminawy, M., Kerezoudis, P., Lu, V., Yolcu, Y., Alvi, M., & Bydon, M. (2019). Impact of obesity on outcomes following lumbar spine surgery: A systematic review and meta-analysis. Clinical Neurology and Neurosurgery, 177 , 27-36. https://doi.org/10.1016/j.clineuro.2018.12.012
This meta-analysis was interested in determining whether obesity affects the outcome of spinal surgery. Some previous studies have shown higher perioperative morbidity in patients with obesity while other studies have not shown this effect. This study looked at surgical outcomes including "blood loss, operative time, length of stay, complication and reoperation rates and functional outcomes" between patients with and without obesity. A meta-analysis of 32 studies (23,415 patients) was conducted. There were no significant differences for patients undergoing minimally invasive surgery, but patients with obesity who had open surgery had experienced higher blood loss and longer operative times (not clinically meaningful) as well as higher complication and reoperation rates. Further research is needed to explore this issue in patients with morbid obesity.
Nakamura, A., van Der Waerden, J., Melchior, M., Bolze, C., El-Khoury, F., & Pryor, L. (2019). Physical activity during pregnancy and postpartum depression: Systematic review and meta-analysis. Journal of Affective Disorders, 246 , 29-41. https://doi.org/10.1016/j.jad.2018.12.009
This meta-analysis explored whether physical activity during pregnancy prevents postpartum depression. Seventeen studies were included (93,676 women) and analysis showed a "significant reduction in postpartum depression scores in women who were physically active during their pregnancies when compared with inactive women." Possible limitations or moderators of this effect include intensity and frequency of physical activity, type of physical activity, and timepoint in pregnancy (e.g. trimester).
Related Terms
A document often written by a panel that provides a comprehensive review of all relevant studies on a particular clinical or health-related topic/question.
Publication Bias
A phenomenon in which studies with positive results have a better chance of being published, are published earlier, and are published in journals with higher impact factors. Therefore, conclusions based exclusively on published studies can be misleading.
Now test yourself!
1. A Meta-Analysis pools together the sample populations from different studies, such as Randomized Controlled Trials, into one statistical analysis and treats them as one large sample population with one conclusion.
a) True b) False
2. One potential design pitfall of Meta-Analyses that is important to pay attention to is:
a) Whether it is evidence-based. b) If the authors combined studies with conflicting results. c) If the authors appropriately combined studies so they did not compare apples and oranges. d) If the authors used only quantitative data.
Evidence Pyramid - Navigation
- Meta- Analysis
- Case Reports
- << Previous: Systematic Review
- Next: Helpful Formulas >>

- Last Updated: Sep 25, 2023 10:59 AM
- URL: https://guides.himmelfarb.gwu.edu/studydesign101

- Himmelfarb Intranet
- Privacy Notice
- Terms of Use
- GW is committed to digital accessibility. If you experience a barrier that affects your ability to access content on this page, let us know via the Accessibility Feedback Form .
- Himmelfarb Health Sciences Library
- 2300 Eye St., NW, Washington, DC 20037
- Phone: (202) 994-2962
- [email protected]
- https://himmelfarb.gwu.edu
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 08 March 2018
Meta-analysis and the science of research synthesis
- Jessica Gurevitch 1 ,
- Julia Koricheva 2 ,
- Shinichi Nakagawa 3 , 4 &
- Gavin Stewart 5
Nature volume 555 , pages 175–182 ( 2018 ) Cite this article
58k Accesses
955 Citations
735 Altmetric
Metrics details
- Biodiversity
- Outcomes research
Meta-analysis is the quantitative, scientific synthesis of research results. Since the term and modern approaches to research synthesis were first introduced in the 1970s, meta-analysis has had a revolutionary effect in many scientific fields, helping to establish evidence-based practice and to resolve seemingly contradictory research outcomes. At the same time, its implementation has engendered criticism and controversy, in some cases general and others specific to particular disciplines. Here we take the opportunity provided by the recent fortieth anniversary of meta-analysis to reflect on the accomplishments, limitations, recent advances and directions for future developments in the field of research synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Eight problems with literature reviews and how to fix them

The past, present and future of Registered Reports
Reporting guidelines for precision medicine research of clinical relevance: the BePRECISE checklist
Jennions, M. D ., Lortie, C. J. & Koricheva, J. in The Handbook of Meta-analysis in Ecology and Evolution (eds Koricheva, J . et al.) Ch. 23 , 364–380 (Princeton Univ. Press, 2013)
Article Google Scholar
Roberts, P. D ., Stewart, G. B. & Pullin, A. S. Are review articles a reliable source of evidence to support conservation and environmental management? A comparison with medicine. Biol. Conserv. 132 , 409–423 (2006)
Bastian, H ., Glasziou, P . & Chalmers, I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 7 , e1000326 (2010)
Article PubMed PubMed Central Google Scholar
Borman, G. D. & Grigg, J. A. in The Handbook of Research Synthesis and Meta-analysis 2nd edn (eds Cooper, H. M . et al.) 497–519 (Russell Sage Foundation, 2009)
Ioannidis, J. P. A. The mass production of redundant, misleading, and conflicted systematic reviews and meta-analyses. Milbank Q. 94 , 485–514 (2016)
Koricheva, J . & Gurevitch, J. Uses and misuses of meta-analysis in plant ecology. J. Ecol. 102 , 828–844 (2014)
Littell, J. H . & Shlonsky, A. Making sense of meta-analysis: a critique of “effectiveness of long-term psychodynamic psychotherapy”. Clin. Soc. Work J. 39 , 340–346 (2011)
Morrissey, M. B. Meta-analysis of magnitudes, differences and variation in evolutionary parameters. J. Evol. Biol. 29 , 1882–1904 (2016)
Article CAS PubMed Google Scholar
Whittaker, R. J. Meta-analyses and mega-mistakes: calling time on meta-analysis of the species richness-productivity relationship. Ecology 91 , 2522–2533 (2010)
Article PubMed Google Scholar
Begley, C. G . & Ellis, L. M. Drug development: Raise standards for preclinical cancer research. Nature 483 , 531–533 (2012); clarification 485 , 41 (2012)
Article CAS ADS PubMed Google Scholar
Hillebrand, H . & Cardinale, B. J. A critique for meta-analyses and the productivity-diversity relationship. Ecology 91 , 2545–2549 (2010)
Moher, D . et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6 , e1000097 (2009). This paper provides a consensus regarding the reporting requirements for medical meta-analysis and has been highly influential in ensuring good reporting practice and standardizing language in evidence-based medicine, with further guidance for protocols, individual patient data meta-analyses and animal studies.
Moher, D . et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 4 , 1 (2015)
Nakagawa, S . & Santos, E. S. A. Methodological issues and advances in biological meta-analysis. Evol. Ecol. 26 , 1253–1274 (2012)
Nakagawa, S ., Noble, D. W. A ., Senior, A. M. & Lagisz, M. Meta-evaluation of meta-analysis: ten appraisal questions for biologists. BMC Biol. 15 , 18 (2017)
Hedges, L. & Olkin, I. Statistical Methods for Meta-analysis (Academic Press, 1985)
Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36 , 1–48 (2010)
Anzures-Cabrera, J . & Higgins, J. P. T. Graphical displays for meta-analysis: an overview with suggestions for practice. Res. Synth. Methods 1 , 66–80 (2010)
Egger, M ., Davey Smith, G ., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 315 , 629–634 (1997)
Article CAS Google Scholar
Duval, S . & Tweedie, R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56 , 455–463 (2000)
Article CAS MATH PubMed Google Scholar
Leimu, R . & Koricheva, J. Cumulative meta-analysis: a new tool for detection of temporal trends and publication bias in ecology. Proc. R. Soc. Lond. B 271 , 1961–1966 (2004)
Higgins, J. P. T . & Green, S. (eds) Cochrane Handbook for Systematic Reviews of Interventions : Version 5.1.0 (Wiley, 2011). This large collaborative work provides definitive guidance for the production of systematic reviews in medicine and is of broad interest for methods development outside the medical field.
Lau, J ., Rothstein, H. R . & Stewart, G. B. in The Handbook of Meta-analysis in Ecology and Evolution (eds Koricheva, J . et al.) Ch. 25 , 407–419 (Princeton Univ. Press, 2013)
Lortie, C. J ., Stewart, G ., Rothstein, H. & Lau, J. How to critically read ecological meta-analyses. Res. Synth. Methods 6 , 124–133 (2015)
Murad, M. H . & Montori, V. M. Synthesizing evidence: shifting the focus from individual studies to the body of evidence. J. Am. Med. Assoc. 309 , 2217–2218 (2013)
Rasmussen, S. A ., Chu, S. Y ., Kim, S. Y ., Schmid, C. H . & Lau, J. Maternal obesity and risk of neural tube defects: a meta-analysis. Am. J. Obstet. Gynecol. 198 , 611–619 (2008)
Littell, J. H ., Campbell, M ., Green, S . & Toews, B. Multisystemic therapy for social, emotional, and behavioral problems in youth aged 10–17. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004797.pub4 (2005)
Schmidt, F. L. What do data really mean? Research findings, meta-analysis, and cumulative knowledge in psychology. Am. Psychol. 47 , 1173–1181 (1992)
Button, K. S . et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14 , 365–376 (2013); erratum 14 , 451 (2013)
Parker, T. H . et al. Transparency in ecology and evolution: real problems, real solutions. Trends Ecol. Evol. 31 , 711–719 (2016)
Stewart, G. Meta-analysis in applied ecology. Biol. Lett. 6 , 78–81 (2010)
Sutherland, W. J ., Pullin, A. S ., Dolman, P. M . & Knight, T. M. The need for evidence-based conservation. Trends Ecol. Evol. 19 , 305–308 (2004)
Lowry, E . et al. Biological invasions: a field synopsis, systematic review, and database of the literature. Ecol. Evol. 3 , 182–196 (2013)
Article PubMed Central Google Scholar
Parmesan, C . & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421 , 37–42 (2003)
Jennions, M. D ., Lortie, C. J . & Koricheva, J. in The Handbook of Meta-analysis in Ecology and Evolution (eds Koricheva, J . et al.) Ch. 24 , 381–403 (Princeton Univ. Press, 2013)
Balvanera, P . et al. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9 , 1146–1156 (2006)
Cardinale, B. J . et al. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443 , 989–992 (2006)
Rey Benayas, J. M ., Newton, A. C ., Diaz, A. & Bullock, J. M. Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science 325 , 1121–1124 (2009)
Article ADS PubMed CAS Google Scholar
Leimu, R ., Mutikainen, P. I. A ., Koricheva, J. & Fischer, M. How general are positive relationships between plant population size, fitness and genetic variation? J. Ecol. 94 , 942–952 (2006)
Hillebrand, H. On the generality of the latitudinal diversity gradient. Am. Nat. 163 , 192–211 (2004)
Gurevitch, J. in The Handbook of Meta-analysis in Ecology and Evolution (eds Koricheva, J . et al.) Ch. 19 , 313–320 (Princeton Univ. Press, 2013)
Rustad, L . et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126 , 543–562 (2001)
Adams, D. C. Phylogenetic meta-analysis. Evolution 62 , 567–572 (2008)
Hadfield, J. D . & Nakagawa, S. General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi-trait models for continuous and categorical characters. J. Evol. Biol. 23 , 494–508 (2010)
Lajeunesse, M. J. Meta-analysis and the comparative phylogenetic method. Am. Nat. 174 , 369–381 (2009)
Rosenberg, M. S ., Adams, D. C . & Gurevitch, J. MetaWin: Statistical Software for Meta-Analysis with Resampling Tests Version 1 (Sinauer Associates, 1997)
Wallace, B. C . et al. OpenMEE: intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol. Evol. 8 , 941–947 (2016)
Gurevitch, J ., Morrison, J. A . & Hedges, L. V. The interaction between competition and predation: a meta-analysis of field experiments. Am. Nat. 155 , 435–453 (2000)
Adams, D. C ., Gurevitch, J . & Rosenberg, M. S. Resampling tests for meta-analysis of ecological data. Ecology 78 , 1277–1283 (1997)
Gurevitch, J . & Hedges, L. V. Statistical issues in ecological meta-analyses. Ecology 80 , 1142–1149 (1999)
Schmid, C. H . & Mengersen, K. in The Handbook of Meta-analysis in Ecology and Evolution (eds Koricheva, J . et al.) Ch. 11 , 145–173 (Princeton Univ. Press, 2013)
Eysenck, H. J. Exercise in mega-silliness. Am. Psychol. 33 , 517 (1978)
Simberloff, D. Rejoinder to: Don’t calculate effect sizes; study ecological effects. Ecol. Lett. 9 , 921–922 (2006)
Cadotte, M. W ., Mehrkens, L. R . & Menge, D. N. L. Gauging the impact of meta-analysis on ecology. Evol. Ecol. 26 , 1153–1167 (2012)
Koricheva, J ., Jennions, M. D. & Lau, J. in The Handbook of Meta-analysis in Ecology and Evolution (eds Koricheva, J . et al.) Ch. 15 , 237–254 (Princeton Univ. Press, 2013)
Lau, J ., Ioannidis, J. P. A ., Terrin, N ., Schmid, C. H . & Olkin, I. The case of the misleading funnel plot. Br. Med. J. 333 , 597–600 (2006)
Vetter, D ., Rucker, G. & Storch, I. Meta-analysis: a need for well-defined usage in ecology and conservation biology. Ecosphere 4 , 1–24 (2013)
Mengersen, K ., Jennions, M. D. & Schmid, C. H. in The Handbook of Meta-analysis in Ecology and Evolution (eds Koricheva, J. et al.) Ch. 16 , 255–283 (Princeton Univ. Press, 2013)
Patsopoulos, N. A ., Analatos, A. A. & Ioannidis, J. P. A. Relative citation impact of various study designs in the health sciences. J. Am. Med. Assoc. 293 , 2362–2366 (2005)
Kueffer, C . et al. Fame, glory and neglect in meta-analyses. Trends Ecol. Evol. 26 , 493–494 (2011)
Cohnstaedt, L. W. & Poland, J. Review Articles: The black-market of scientific currency. Ann. Entomol. Soc. Am. 110 , 90 (2017)
Longo, D. L. & Drazen, J. M. Data sharing. N. Engl. J. Med. 374 , 276–277 (2016)
Gauch, H. G. Scientific Method in Practice (Cambridge Univ. Press, 2003)
Science Staff. Dealing with data: introduction. Challenges and opportunities. Science 331 , 692–693 (2011)
Nosek, B. A . et al. Promoting an open research culture. Science 348 , 1422–1425 (2015)
Article CAS ADS PubMed PubMed Central Google Scholar
Stewart, L. A . et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. J. Am. Med. Assoc. 313 , 1657–1665 (2015)
Saldanha, I. J . et al. Evaluating Data Abstraction Assistant, a novel software application for data abstraction during systematic reviews: protocol for a randomized controlled trial. Syst. Rev. 5 , 196 (2016)
Tipton, E. & Pustejovsky, J. E. Small-sample adjustments for tests of moderators and model fit using robust variance estimation in meta-regression. J. Educ. Behav. Stat. 40 , 604–634 (2015)
Mengersen, K ., MacNeil, M. A . & Caley, M. J. The potential for meta-analysis to support decision analysis in ecology. Res. Synth. Methods 6 , 111–121 (2015)
Ashby, D. Bayesian statistics in medicine: a 25 year review. Stat. Med. 25 , 3589–3631 (2006)
Article MathSciNet PubMed Google Scholar
Senior, A. M . et al. Heterogeneity in ecological and evolutionary meta-analyses: its magnitude and implications. Ecology 97 , 3293–3299 (2016)
McAuley, L ., Pham, B ., Tugwell, P . & Moher, D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 356 , 1228–1231 (2000)
Koricheva, J ., Gurevitch, J . & Mengersen, K. (eds) The Handbook of Meta-Analysis in Ecology and Evolution (Princeton Univ. Press, 2013) This book provides the first comprehensive guide to undertaking meta-analyses in ecology and evolution and is also relevant to other fields where heterogeneity is expected, incorporating explicit consideration of the different approaches used in different domains.
Lumley, T. Network meta-analysis for indirect treatment comparisons. Stat. Med. 21 , 2313–2324 (2002)
Zarin, W . et al. Characteristics and knowledge synthesis approach for 456 network meta-analyses: a scoping review. BMC Med. 15 , 3 (2017)
Elliott, J. H . et al. Living systematic reviews: an emerging opportunity to narrow the evidence-practice gap. PLoS Med. 11 , e1001603 (2014)
Vandvik, P. O ., Brignardello-Petersen, R . & Guyatt, G. H. Living cumulative network meta-analysis to reduce waste in research: a paradigmatic shift for systematic reviews? BMC Med. 14 , 59 (2016)
Jarvinen, A. A meta-analytic study of the effects of female age on laying date and clutch size in the Great Tit Parus major and the Pied Flycatcher Ficedula hypoleuca . Ibis 133 , 62–67 (1991)
Arnqvist, G. & Wooster, D. Meta-analysis: synthesizing research findings in ecology and evolution. Trends Ecol. Evol. 10 , 236–240 (1995)
Hedges, L. V ., Gurevitch, J . & Curtis, P. S. The meta-analysis of response ratios in experimental ecology. Ecology 80 , 1150–1156 (1999)
Gurevitch, J ., Curtis, P. S. & Jones, M. H. Meta-analysis in ecology. Adv. Ecol. Res 32 , 199–247 (2001)
Lajeunesse, M. J. phyloMeta: a program for phylogenetic comparative analyses with meta-analysis. Bioinformatics 27 , 2603–2604 (2011)
CAS PubMed Google Scholar
Pearson, K. Report on certain enteric fever inoculation statistics. Br. Med. J. 2 , 1243–1246 (1904)
Fisher, R. A. Statistical Methods for Research Workers (Oliver and Boyd, 1925)
Yates, F. & Cochran, W. G. The analysis of groups of experiments. J. Agric. Sci. 28 , 556–580 (1938)
Cochran, W. G. The combination of estimates from different experiments. Biometrics 10 , 101–129 (1954)
Smith, M. L . & Glass, G. V. Meta-analysis of psychotherapy outcome studies. Am. Psychol. 32 , 752–760 (1977)
Glass, G. V. Meta-analysis at middle age: a personal history. Res. Synth. Methods 6 , 221–231 (2015)
Cooper, H. M ., Hedges, L. V . & Valentine, J. C. (eds) The Handbook of Research Synthesis and Meta-analysis 2nd edn (Russell Sage Foundation, 2009). This book is an important compilation that builds on the ground-breaking first edition to set the standard for best practice in meta-analysis, primarily in the social sciences but with applications to medicine and other fields.
Rosenthal, R. Meta-analytic Procedures for Social Research (Sage, 1991)
Hunter, J. E ., Schmidt, F. L. & Jackson, G. B. Meta-analysis: Cumulating Research Findings Across Studies (Sage, 1982)
Gurevitch, J ., Morrow, L. L ., Wallace, A . & Walsh, J. S. A meta-analysis of competition in field experiments. Am. Nat. 140 , 539–572 (1992). This influential early ecological meta-analysis reports multiple experimental outcomes on a longstanding and controversial topic that introduced a wide range of ecologists to research synthesis methods.
O’Rourke, K. An historical perspective on meta-analysis: dealing quantitatively with varying study results. J. R. Soc. Med. 100 , 579–582 (2007)
Shadish, W. R . & Lecy, J. D. The meta-analytic big bang. Res. Synth. Methods 6 , 246–264 (2015)
Glass, G. V. Primary, secondary, and meta-analysis of research. Educ. Res. 5 , 3–8 (1976)
DerSimonian, R . & Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 7 , 177–188 (1986)
Lipsey, M. W . & Wilson, D. B. The efficacy of psychological, educational, and behavioral treatment. Confirmation from meta-analysis. Am. Psychol. 48 , 1181–1209 (1993)
Chalmers, I. & Altman, D. G. Systematic Reviews (BMJ Publishing Group, 1995)
Moher, D . et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 354 , 1896–1900 (1999)
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 , 1539–1558 (2002)
Download references

Acknowledgements
We dedicate this Review to the memory of Ingram Olkin and William Shadish, founding members of the Society for Research Synthesis Methodology who made tremendous contributions to the development of meta-analysis and research synthesis and to the supervision of generations of students. We thank L. Lagisz for help in preparing the figures. We are grateful to the Center for Open Science and the Laura and John Arnold Foundation for hosting and funding a workshop, which was the origination of this article. S.N. is supported by Australian Research Council Future Fellowship (FT130100268). J.G. acknowledges funding from the US National Science Foundation (ABI 1262402).
Author information
Authors and affiliations.
Department of Ecology and Evolution, Stony Brook University, Stony Brook, 11794-5245, New York, USA
Jessica Gurevitch
School of Biological Sciences, Royal Holloway University of London, Egham, TW20 0EX, Surrey, UK
Julia Koricheva
Evolution and Ecology Research Centre and School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, 2052, New South Wales, Australia
Shinichi Nakagawa
Diabetes and Metabolism Division, Garvan Institute of Medical Research, 384 Victoria Street, Darlinghurst, Sydney, 2010, New South Wales, Australia
School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK
Gavin Stewart
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed equally in designing the study and writing the manuscript, and so are listed alphabetically.
Corresponding authors
Correspondence to Jessica Gurevitch , Julia Koricheva , Shinichi Nakagawa or Gavin Stewart .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks D. Altman, M. Lajeunesse, D. Moher and G. Romero for their contribution to the peer review of this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
PowerPoint slides
Powerpoint slide for fig. 1, rights and permissions.
Reprints and permissions
About this article
Cite this article.
Gurevitch, J., Koricheva, J., Nakagawa, S. et al. Meta-analysis and the science of research synthesis. Nature 555 , 175–182 (2018). https://doi.org/10.1038/nature25753
Download citation
Received : 04 March 2017
Accepted : 12 January 2018
Published : 08 March 2018
Issue Date : 08 March 2018
DOI : https://doi.org/10.1038/nature25753
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Accelerating evidence synthesis for safety assessment through clinicaltrials.gov platform: a feasibility study.
BMC Medical Research Methodology (2024)
Investigate the relationship between the retraction reasons and the quality of methodology in non-Cochrane retracted systematic reviews: a systematic review
- Azita Shahraki-Mohammadi
- Leila Keikha
- Razieh Zahedi
Systematic Reviews (2024)
A meta-analysis on global change drivers and the risk of infectious disease
- Michael B. Mahon
- Alexandra Sack
- Jason R. Rohr
Nature (2024)
Systematic review of the uncertainty of coral reef futures under climate change
- Shannon G. Klein
- Cassandra Roch
- Carlos M. Duarte
Nature Communications (2024)
A population-scale analysis of 36 gut microbiome studies reveals universal species signatures for common diseases
- Qiulong Yan
npj Biofilms and Microbiomes (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Systematic Reviews and Meta-analysis: Understanding the Best Evidence in Primary Healthcare
S gopalakrishnan, p ganeshkumar.
- Author information
- Copyright and License information
Address for correspondence: Dr. P Ganeshkumar, Department of Community Medicine, SRM Medical College, Hospital and Research Centre, Kattankulathur, Tamil Nadu, India. E-mail: [email protected]
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Healthcare decisions for individual patients and for public health policies should be informed by the best available research evidence. The practice of evidence-based medicine is the integration of individual clinical expertise with the best available external clinical evidence from systematic research and patient's values and expectations. Primary care physicians need evidence for both clinical practice and for public health decision making. The evidence comes from good reviews which is a state-of-the-art synthesis of current evidence on a given research question. Given the explosion of medical literature, and the fact that time is always scarce, review articles play a vital role in decision making in evidence-based medical practice. Given that most clinicians and public health professionals do not have the time to track down all the original articles, critically read them, and obtain the evidence they need for their questions, systematic reviews and clinical practice guidelines may be their best source of evidence. Systematic reviews aim to identify, evaluate, and summarize the findings of all relevant individual studies over a health-related issue, thereby making the available evidence more accessible to decision makers. The objective of this article is to introduce the primary care physicians about the concept of systematic reviews and meta-analysis, outlining why they are important, describing their methods and terminologies used, and thereby helping them with the skills to recognize and understand a reliable review which will be helpful for their day-to-day clinical practice and research activities.
Keywords: Evidence-based medicine, meta-analysis, primary care, systematic review
Introduction
Evidence-based healthcare is the integration of best research evidence with clinical expertise and patient values. Green denotes, “Using evidence from reliable research, to inform healthcare decisions, has the potential to ensure best practice and reduce variations in healthcare delivery.” However, incorporating research into practice is time consuming, and so we need methods of facilitating easy access to evidence for busy clinicians.[ 1 ] Ganeshkumar et al . mentioned that nearly half of the private practitioners in India were consulting more than 4 h per day in a locality,[ 2 ] which explains the difficulty of them in spending time in searching evidence during consultation. Ideally, clinical decision making ought to be based on the latest evidence available. However, to keep abreast with the continuously increasing number of publications in health research, a primary healthcare professional would need to read an insurmountable number of articles every day, covered in more than 13 million references and over 4800 biomedical and health journals in Medline alone. With the view to address this challenge, the systematic review method was developed. Systematic reviews aim to inform and facilitate this process through research synthesis of multiple studies, enabling increased and efficient access to evidence.[ 1 , 3 , 4 ]
Systematic reviews and meta-analyses have become increasingly important in healthcare settings. Clinicians read them to keep up-to-date with their field and they are often used as a starting point for developing clinical practice guidelines. Granting agencies may require a systematic review to ensure there is justification for further research and some healthcare journals are moving in this direction.[ 5 ]
This article is intended to provide an easy guide to understand the concept of systematic reviews and meta-analysis, which has been prepared with the aim of capacity building for general practitioners and other primary healthcare professionals in research methodology and day-to-day clinical practice.
The purpose of this article is to introduce readers to:
The two approaches of evaluating all the available evidence on an issue i.e., systematic reviews and meta-analysis,
Discuss the steps in doing a systematic review,
Introduce the terms used in systematic reviews and meta-analysis,
Interpret results of a meta-analysis, and
The advantages and disadvantages of systematic review and meta-analysis.
Application
What is the effect of antiviral treatment in dengue fever? Most often a primary care physician needs to know convincing answers to questions like this in a primary care setting.
To find out the solutions or answers to a clinical question like this, one has to refer textbooks, ask a colleague, or search electronic database for reports of clinical trials. Doctors need reliable information on such problems and on the effectiveness of large number of therapeutic interventions, but the information sources are too many, i.e., nearly 20,000 journals publishing 2 million articles per year with unclear or confusing results. Because no study, regardless of its type, should be interpreted in isolation, a systematic review is generally the best form of evidence.[ 6 ] So, the preferred method is a good summary of research reports, i.e., systematic reviews and meta-analysis, which will give evidence-based answers to clinical situations.
There are two fundamental categories of research: Primary research and secondary research. Primary research is collecting data directly from patients or population, while secondary research is the analysis of data already collected through primary research. A review is an article that summarizes a number of primary studies and may draw conclusions on the topic of interest which can be traditional (unsystematic) or systematic.
Terminologies
Systematic review.
A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize on a specific issue. It synthesizes the results of multiple primary studies related to each other by using strategies that reduce biases and random errors.[ 7 ] To this end, systematic reviews may or may not include a statistical synthesis called meta-analysis, depending on whether the studies are similar enough so that combining their results is meaningful.[ 8 ] Systematic reviews are often called overviews.
The evidence-based practitioner, David Sackett, defines the following terminologies.[ 3 ]
Review: The general term for all attempts to synthesize the results and conclusions of two or more publications on a given topic.
Overview: When a review strives to comprehensively identify and track down all the literature on a given topic (also called “systematic literature review”).
Meta-analysis: A specific statistical strategy for assembling the results of several studies into a single estimate.
Systematic reviews adhere to a strict scientific design based on explicit, pre-specified, and reproducible methods. Because of this, when carried out well, they provide reliable estimates about the effects of interventions so that conclusions are defensible. Systematic reviews can also demonstrate where knowledge is lacking. This can then be used to guide future research. Systematic reviews are usually carried out in the areas of clinical tests (diagnostic, screening, and prognostic), public health interventions, adverse (harm) effects, economic (cost) evaluations, and how and why interventions work.[ 9 ]
Cochrane reviews
Cochrane reviews are systematic reviews undertaken by members of the Cochrane Collaboration which is an international not-for-profit organization that aims to help people to make well-informed decisions about healthcare by preparing, maintaining, and promoting the accessibility of systematic reviews of the effects of healthcare interventions.
Cochrane Primary Health Care Field is a systematic review of primary healthcare research on prevention, treatment, rehabilitation, and diagnostic test accuracy. The overall aim and mission of the Primary Health Care Field is to promote the quality, quantity, dissemination, accessibility, applicability, and impact of Cochrane systematic reviews relevant to people who work in primary care and to ensure proper representation in the interests of primary care clinicians and consumers in Cochrane reviews and review groups, and in other entities. This field would serve to coordinate and promote the mission of the Cochrane Collaboration within the primary healthcare disciplines, as well as ensuring that primary care perspectives are adequately represented within the Collaboration.[ 10 ]
Meta-analysis
A meta-analysis is the combination of data from several independent primary studies that address the same question to produce a single estimate like the effect of treatment or risk factor. It is the statistical analysis of a large collection of analysis and results from individual studies for the purpose of integrating the findings.[ 11 ] The term meta-analysis has been used to denote the full range of quantitative methods for research reviews.[ 12 ] Meta-analyses are studies of studies.[ 13 ] Meta-analysis provides a logical framework to a research review where similar measures from comparable studies are listed systematically and the available effect measures are combined wherever possible.[ 14 ]
The fundamental rationale of meta-analysis is that it reduces the quantity of data by summarizing data from multiple resources and helps to plan research as well as to frame guidelines. It also helps to make efficient use of existing data, ensuring generalizability, helping to check consistency of relationships, explaining data inconsistency, and quantifies the data. It helps to improve the precision in estimating the risk by using explicit methods.
Therefore, “systematic review” will refer to the entire process of collecting, reviewing, and presenting all available evidence, while the term “meta-analysis” will refer to the statistical technique involved in extracting and combining data to produce a summary result.[ 15 ]
Steps in doing systematic reviews/meta-analysis
Following are the six fundamental essential steps while doing systematic review and meta-analysis.[ 16 ]
Define the question
This is the most important part of systematic reviews/meta-analysis. The research question for the systematic reviews may be related to a major public health problem or a controversial clinical situation which requires acceptable intervention as a possible solution to the present healthcare need of the community. This step is most important since the remaining steps will be based on this.
Reviewing the literature
This can be done by going through scientific resources such as electronic database, controlled clinical trials registers, other biomedical databases, non-English literatures, “gray literatures” (thesis, internal reports, non–peer-reviewed journals, pharmaceutical industry files), references listed in primary sources, raw data from published trials and other unpublished sources known to experts in the field. Among the available electronic scientific database, the popular ones are PUBMED, MEDLINE, and EMBASE.
Sift the studies to select relevant ones
To select the relevant studies from the searches, we need to sift through the studies thus identified. The first sift is pre-screening, i.e., to decide which studies to retrieve in full, and the second sift is selection which is to look again at these studies and decide which are to be included in the review. The next step is selecting the eligible studies based on similar study designs, year of publication, language, choice among multiple articles, sample size or follow-up issues, similarity of exposure, and or treatment and completeness of information.
It is necessary to ensure that the sifting includes all relevant studies like the unpublished studies (desk drawer problem), studies which came with negative conclusions or were published in non-English journals, and studies with small sample size.
Assess the quality of studies
The steps undertaken in evaluating the study quality are early definition of study quality and criteria, setting up a good scoring system, developing a standard form for assessment, calculating quality for each study, and finally using this for sensitivity analysis.
For example, the quality of a randomized controlled trial can be assessed by finding out the answers to the following questions:
Was the assignment to the treatment groups really random?
Was the treatment allocation concealed?
Were the groups similar at baseline in terms of prognostic factors?
Were the eligibility criteria specified?
Were the assessors, the care provider, and the patient blinded?
Were the point estimates and measure of variability presented for the primary outcome measure?
Did the analyses include intention-to-treat analysis?
Calculate the outcome measures of each study and combine them
We need a standard measure of outcome which can be applied to each study on the basis of its effect size. Based on their type of outcome, following are the measures of outcome: Studies with binary outcomes (cured/not cured) have odds ratio, risk ratio; studies with continuous outcomes (blood pressure) have means, difference in means, standardized difference in means (effect sizes); and survival or time-to-event data have hazard ratios.
Combining studies
Homogeneity of different studies can be estimated at a glance from a forest plot (explained below). For example, if the lower confidence interval of every trial is below the upper of all the others, i.e., the lines all overlap to some extent, then the trials are homogeneous. If some lines do not overlap at all, these trials may be said to be heterogeneous.
The definitive test for assessing the heterogeneity of studies is a variant of Chi-square test (Mantel–Haenszel test). The final step is calculating the common estimate and its confidence interval with the original data or with the summary statistics from all the studies. The best estimate of treatment effect can be derived from the weighted summary statistics of all studies which will be based on weighting to sample size, standard errors, and other summary statistics. Log scale is used to combine the data to estimate the weighting.
Interpret results: Graph
The results of a meta-analysis are usually presented as a graph called forest plot because the typical forest plots appear as forest of lines. It provides a simple visual presentation of individual studies that went into the meta-analysis at a glance. It shows the variation between the studies and an estimate of the overall result of all the studies together.
Forest plot
Meta-analysis graphs can principally be divided into six columns [ Figure 1 ]. Individual study results are displayed in rows. The first column (“study”) lists the individual study IDs included in the meta-analysis; usually the first author and year are displayed. The second column relates to the intervention groups and the third column to the control groups. The fourth column visually displays the study results. The line in the middle is called “the line of no effect.” The weight (in %) in the fifth column indicates the weighting or influence of the study on the overall results of the meta-analysis of all included studies. The higher the percentage weight, the bigger the box, the more influence the study has on the overall results. The sixth column gives the numerical results for each study (e.g., odds ratio or relative risk and 95% confidence interval), which are identical to the graphical display in the fourth column. The diamond in the last row of the graph illustrates the overall result of the meta-analysis.[ 4 ]
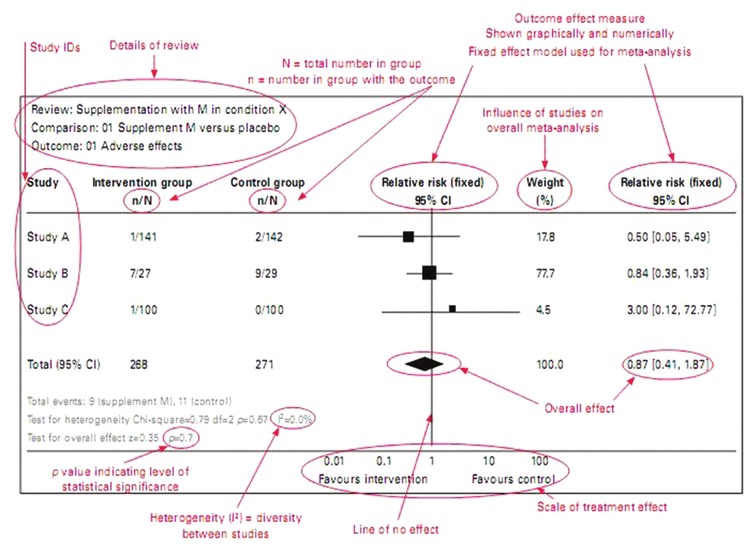
Interpretation of meta-analysis[ 4 ]
Thus, the horizontal lines represent individual studies. Length of line is the confidence interval (usually 95%), squares on the line represent effect size (risk ratio) for the study, with area of the square being the study size (proportional to weight given) and position as point estimate (relative risk) of the study.[ 7 ]
For example, the forest plot of the effectiveness of dexamethasone compared with placebo in preventing the recurrence of acute severe migraine headache in adults is shown in Figure 2 .[ 17 ]
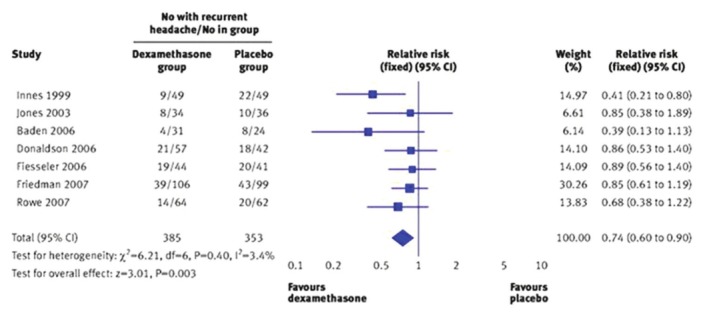
Forest plot of the effectiveness of dexamethasone compared with placebo in preventing the recurrence of acute severe migraine headache in adults[ 17 ]
The overall effect is shown as diamond where the position toward the center represents pooled point estimate, the width represents estimated 95% confidence interval for all studies, and the black plain line vertically in the middle of plot is the “line of no effect” (e.g., relative risk = 1).
Therefore, when examining the results of a systematic reviews/meta-analysis, the following questions should be kept in mind:
Were apples combined with oranges?
- Heterogeneity among studies may make any pooled estimate meaningless.
Were all of the apples rotten?
- The quality of a meta-analysis cannot be any better than the quality of the studies it is summarizing.
Were some apples left on the tree?
- An incomplete search of the literature can bias the findings of a meta-analysis.
Did the pile of apples amount to more than just a hill of beans?
- Make sure that the meta-analysis quantifies the size of the effect in units that you can understand.
Subgroup analysis and sensitivity analysis
Subgroup analysis looks at the results of different subgroups of trials, e.g., by considering trials on adults and children separately. This should be planned at the protocol stage itself which is based on good scientific reasoning and is to be kept to a minimum.
Sensitivity analysis is used to determine how results of a systematic review/meta-analysis change by fiddling with data, for example, what is the implication if the exclusion criteria or excluded unpublished studies or weightings are assigned differently. Thus, after the analysis, if changing makes little or no difference to the overall results, the reviewer's conclusions are robust. If the key findings disappear, then the conclusions need to be expressed more cautiously.
Advantages of Systematic Reviews
Systematic reviews have specific advantages because of using explicit methods which limit bias, draw reliable and accurate conclusions, easily deliver required information to healthcare providers, researchers, and policymakers, help to reduce the time delay in the research discoveries to implementation, improve the generalizability and consistency of results, generation of new hypotheses about subgroups of the study population, and overall they increase precision of the results.[ 18 ]
Limitations in Systematic Reviews/Meta-analysis
As with all research, the value of a systematic review depends on what was done, what was found, and the clarity of reporting. As with other publications, the reporting quality of systematic reviews varies, limiting readers’ ability to assess the strengths and weaknesses of those reviews.[ 5 ]
Even though systematic review and meta-analysis are considered the best evidence for getting a definitive answer to a research question, there are certain inherent flaws associated with it, such as the location and selection of studies, heterogeneity, loss of information on important outcomes, inappropriate subgroup analyses, conflict with new experimental data, and duplication of publication.
Publication Bias
Publication bias results in it being easier to find studies with a “positive” result.[ 19 ] This occurs particularly due to inappropriate sifting of the studies where there is always a tendency towards the studies with positive (significant) outcomes. This effect occurs more commonly in systematic reviews/meta-analysis which need to be eliminated.
The quality of reporting of systematic reviews is still not optimal. In a recent review of 300 systematic reviews, few authors reported assessing possible publication bias even though there is overwhelming evidence both for its existence and its impact on the results of systematic reviews. Even when the possibility of publication bias is assessed, there is no guarantee that systematic reviewers have assessed or interpreted it appropriately.[ 20 ]
To overcome certain limitations mentioned above, the Cochrane reviews are currently reported in a format where at the end of every review, findings are summarized in the author's point of view and also give an overall picture of the outcome by means of plain language summary. This is found to be much helpful to understand the existing evidence about the topic more easily by the reader.
A systematic review is an overview of primary studies which contains an explicit statement of objectives, materials, and methods, and has been conducted according to explicit and reproducible methodology. A meta-analysis is a mathematical synthesis of the results of two or more primary studies that addressed the same hypothesis in the same way. Although meta-analysis can increase the precision of a result, it is important to ensure that the methods used for the reviews were valid and reliable.
High-quality systematic reviews and meta-analyses take great care to find all relevant studies, critically assess each study, synthesize the findings from individual studies in an unbiased manner, and present balanced important summary of findings with due consideration of any flaws in the evidence. Systematic review and meta-analysis is a way of summarizing research evidence, which is generally the best form of evidence, and hence positioned at the top of the hierarchy of evidence.
Systematic reviews can be very useful decision-making tools for primary care/family physicians. They objectively summarize large amounts of information, identifying gaps in medical research, and identifying beneficial or harmful interventions which will be useful for clinicians, researchers, and even for public and policymakers.
Source of Support: Nil
Conflict of Interest: None declared.
- 1. Green S. Systematic reviews and meta-analysis. Singapore Med J. 2005;46:270–3. [ PubMed ] [ Google Scholar ]
- 2. Ganeshkumar P, Arun Kumar S, Rajoura OP. Evaluation of computer usage in healthcare among private practitioners of NCT Delhi. Stud Health Technol Inform. 2011;169:960–4. [ PubMed ] [ Google Scholar ]
- 3. Sackett D, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: What it is and what it isn't. BMJ. 1996;312:71–2. doi: 10.1136/bmj.312.7023.71. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Ried K. Interpreting and understanding meta-analysis graphs--a practical guide. Aust Fam Physician. 2006;35:635–8. [ PubMed ] [ Google Scholar ]
- 5. Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Glasziou P, Vanderbroucke J, Chalmers I. Assessing the quality of research. BMJ. 2004;328:39–41. doi: 10.1136/bmj.328.7430.39. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Cook DJ, Mulrow CD, Haynes RB. Systematic reviews: Synthesis of best evidence for clinical decisions. Ann Intern Med. 1997;126:376–80. doi: 10.7326/0003-4819-126-5-199703010-00006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Clarke M. The cochrane collaboration and systematic reviews. Br J Surg. 2007;94:391–2. doi: 10.1002/bjs.5812. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Published by CRD, University of York; 2009. Jan, Systematic Reviews: CRD's guidance for undertaking reviews in health care. Centre for Reviews and Dissemination, University of York, 2008. [ Google Scholar ]
- 10. Cochrane Primary Health Care Field, The Cochrane Library, The Cochrane Collaboration, 23rd Mar. 2012. [Last accessed on 2012 Apr 30]. Available from: http://www.cochraneprimarycare.org/welcome .
- 11. Glass GV. Primary, secondary, and meta-analysis of research. Educ Res. 1976;5:3–8. [ Google Scholar ]
- 12. Goldman L, Feinstein AR. Anticoagulants and myocardial infarction. The problems of pooling, drowning, and floating. Ann Intern Med. 1979;90:92–4. doi: 10.7326/0003-4819-90-1-92. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Kassirer JP. Clinical trials and meta-analysis. What do they do for us? N Engl J Med. 1992;327:273–4. doi: 10.1056/NEJM199207233270411. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Kavale KA, Glass GV. Meta-analysis and the integration of research in special education. J Learn Disabil. 1981;14:531–8. doi: 10.1177/002221948101400909. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. An introduction to meta-analysis, Cochrane Collaboration open learning material for reviewers, Version 1.1, November. 2002. [Last accessed on 2013 Jan 15]. Available from: http://www.cochrane-net.org/openlearning/html/mod3-2.htm .
- 16. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. The Cochrane Collaboration. 2009. [Last accessed on 2009 Sep]. Available from: http://www.cochrane-handbook.org .
- 17. Sedgwick P. Meta-analyses I. [Last accessed on 2013 Jan 15];BMJ. 2011 342:d45. Available from: http://www.bmj.com/content/342/bmj.d45 . [ Google Scholar ]
- 18. Greenhalgh T. Papers that summarise other papers (systematic reviews and meta-analyses) BMJ. 1997;315:672–5. doi: 10.1136/bmj.315.7109.672. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Publication Bias, Cochrane Collaboration open learning material for reviewers, Version 1.1, November. 2002. [Last accessed on 2013 Jan 15]. Available from: http://www.cochrane-net.org/openlearning/html/mod15-2.htm .
- 20. Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. [Last accessed on 2012 Apr 30];PLoS Med. 2007 4:e78. doi: 10.1371/journal.pmed.0040078. Available from: http://www.plosmedicine.org/article/info%3Adoi% 2F10.1371%2Fjournal.pmed.0040078 . [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (990.1 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

Gerstein Science Information Centre
Knowledge syntheses: systematic & scoping reviews, and other review types.
- Before you start
- Getting Started
- Different Types of Knowledge Syntheses
- Assemble a Team
- Develop your Protocol
- Eligibility Criteria
- Screening for articles
- Data Extraction
- Critical appraisal
- What are Systematic Reviews?
When is conducting a meta-analysis appropriate?
Elements of a meta-analysis, methods and guidance.
- What are Scoping Reviews?
- What are Rapid Reviews?
- What are Realist Reviews?
- What are Mapping Reviews?
- What are Integrative Reviews?
- What are Umbrella Reviews?
- Standards and Guidelines
- Supplementary Resources for All Review Types
- Resources for Qualitative Synthesis
- Resources for Quantitative Synthesis
- Resources for Mixed Methods Synthesis
- Bibliography
- More Questions?
- Common Mistakes in Systematic Reviews, scoping reviews, and other review types
A meta-analysis is defined by Haidlich (2010) as "a quantitative, formal, epidemiological study design used to systematically assess previous research studies to derive conclusions about that body of research. Outcomes from a meta-analysis may include a more precise estimate of the effect of treatment or risk factor for disease, or other outcomes , than any individual study contributing to the pooled analysis" (p. 29).
According to Grant & Booth (2009) , a meta-analysis is defined as a "technique that statistically combines the results of quantitative studies to provide a more precise effect of the results" (p. 94).
When to Use It: According to the Cochrane Handbook , "an important step in a systematic review is the thoughtful consideration of whether it is appropriate to combine the numerical results of all, or perhaps some, of the studies. Such a meta-analysis yields an overall statistic (together with its confidence interval) that summarizes the effectiveness of an experimental intervention compared with a comparator intervention" (section 10.2).
Conducting meta-analyses can have the following benefits, according to Deeks et al. (2019, section 10.2) :
To improve precision. Many studies are too small to provide convincing evidence about intervention effects in isolation. Estimation is usually improved when it is based on more information.
To answer questions not posed by the individual studies. Primary studies often involve a specific type of participant and explicitly defined interventions. A selection of studies in which these characteristics differ can allow investigation of the consistency of effect across a wider range of populations and interventions. It may also, if relevant, allow reasons for differences in effect estimates to be investigated.
To settle controversies arising from apparently conflicting studies or to generate new hypotheses. Statistical synthesis of findings allows the degree of conflict to be formally assessed, and reasons for different results to be explored and quantified.
The following characteristics, strengths, and challenges of conducting meta-analyses in systematic reviews are derived from Grant & Booth (2009) , Haidlich (2010) and Deeks et al. (2019) .
Characteristics:
A meta-analysis can only be conducted after the completion of a systematic review, as the meta-analysis statistically summarizes the findings from the studies synthesized in a particular systematic review. A meta-analysis cannot exist without a pre-existing systematic review. Grant & Booth (2009) state that "although many systematic reviews present their results without statistically combining data [in a meta-analysis], a good systematic review is essential to a meta-analysis of the literature" (p. 98).
Conducting a meta-analysis requires all studies that will be statistically summarized to be similar - i.e. the population, intervention, and comparison. Grant & Booth (2009) state that "most importantly, it requires that the same measure or outcome be measured in the same way at the same time intervals" (p. 98).
The end product is a quantitative review that is often complex in nature
Consolidates individual studies that on their own do not have much practical impact, having a solid package of evidence benefits decision-makers who often can't afford to read many individual studies.
Challenges:
It can be challenging to ensure that studies used in a meta-analysis are similar enough, which is a crucial component
Meta-analyses can perhaps be misleading due to biases such as those concerning specific study designs, reporting, and biases within studies
The following resource provides further support on conducting a meta-analysis.
METHODS & GUIDANCE
- Cochrane Training: Chapter 10: Analysing data and undertaking meta-analyses
Provides a comprehensive overview on meta-analyses.
REPORTING GUIDELINE
- PRISMA 2020 Checklist
PRISMA (2020) is a 27-item checklist that replaces the PRISMA (2009) statement , which ensures proper and transparent reporting for each element in a systematic review and meta-analysis. "It is an expanded checklist that details reporting recommendations for each item, the PRISMA 2020 abstract checklist, and the revised flow diagrams for original and updated reviews."
SUPPLEMENTARY RESOURCES
Check out the supplementary resources page for additional information on meta-analyses.
- << Previous: What are Systematic Reviews?
- Next: What are Scoping Reviews? >>
- Last Updated: Aug 30, 2024 1:34 PM
- URL: https://guides.library.utoronto.ca/systematicreviews
Library links
- Gerstein Home
- U of T Libraries Home
- Renew items and pay fines
- Library hours
- Contact Gerstein
- University of Toronto Libraries
- UT Mississauga Library
- UT Scarborough Library
- Information Commons
- All libraries
© University of Toronto . All rights reserved.
Connect with us
Library Research Guides - University of Wisconsin Ebling Library
Uw-madison libraries research guides.
- Course Guides
- Subject Guides
- University of Wisconsin-Madison
- Research Guides
- Nursing Resources
- Meta-Analysis & Meta-Synthesis
Nursing Resources : Meta-Analysis & Meta-Synthesis
- Definitions of
- Professional Organizations
- Nursing Informatics
- Nursing Related Apps
- EBP Resources
- PICO-Clinical Question
- Types of PICO Question (D, T, P, E)
- Secondary & Guidelines
- Bedside--Point of Care
- Pre-processed Evidence
- Measurement Tools, Surveys, Scales
- Types of Studies
- Table of Evidence
- Qualitative vs Quantitative
- Types of Research within Qualitative and Quantitative
- Independent Variable VS Dependent Variable
- Sampling Methods and Statistics
- Cohort vs Case studies
- Review vs Systematic Review vs ETC...
- Scoping Reviews
- Systematic Reviews
- Standard, Guideline, Protocol, Policy
- Additional Guidelines Sources
- Systematic Reviews & Scoping Reviews
- Peer Reviewed Articles
- Conducting a Literature Review
- Writing a Research Paper or Poster
- Annotated Bibliographies
- Levels of Evidence (I-VII)
- Reliability
- Validity Threats
- Threats to Validity of Research Designs
- Nursing Theory
- Nursing Models
- PRISMA, RevMan, & GRADEPro
- ORCiD & NIH Submission System
- Understanding Predatory Journals
- Nursing Scope & Standards of Practice, 4th Ed
- Distance Ed & Scholarships
- Assess A Quantitative Study?
- Assess A Qualitative Study?
- Find Health Statistics?
- Choose A Citation Manager?
- Find Instruments, Measurements, and Tools
- Write a CV for a DNP or PhD?
- Find information about graduate programs?
- Learn more about Predatory Journals
- Get writing help?
- Choose a Citation Manager?
- Other questions you may have
- Search the Databases?
- Get Grad School information?
A Meta-Anayslsis
A meta-analysis goes beyond critique and integration and conducts secondary statistical analyses on the outcomes of similar studies. It is a systematic review that uses quantitative methods to synthesize and summarize the results.
An advantage of a meta-analysis is the ability to be completely objective in evaluating research findings. Not all topics, however, have sufficient research evidence to allow a meta-analysis to be conducted. In that case, an integrative review is an appropriate strategy.
A meta-analysis may be part of a systematic review.
Meta-synthesis
What is a meta-synthesis?
First of all, what is a meta-synthesis? According to Screiber et al. (1997, p.314), a meta-synthesis “is bringing together and breaking down of findings, examining them, discovering essential features and, in some way , combining phenomena into a transformed whole” In basic terms, a meta-synthesis is the ‘bringing together’ of Qualitative data to form a new interpretation of the research field.
Meta-synthesis Vs. Meta-analysis: Whats the difference?
Unlike a meta-analysis which is used to aggregate findings to establish ‘truths’, for example, if an intervention has a true effect on a variable, a meta-synthesis can lead to new interpretations of research. This can result in new theories being developed.
In summary, a meta-analysis is a way of testing a hypothesis whereas a meta-synthesis is a way of developing a new theory.
Three main types of Meta-synthesis
1) Theory Building – This form of meta-synthesis brings together findings on a theoretical level to build a tentative theory.
2) Theory Explication – This form of meta-synthesis is a way of reconceptualising the original phenomenon.
3) Descriptive – This form of meta-synthesis provides a broad description of the research phenomenon.
These forms of meta-synthesis are not discrete, they are complimentary. The aim of Meta-synthesis usually overlap as you will see in the example later on.
Why use a meta-synthesis?
Qualitative data is useful for providing a snapshot at one person’s interpretation of an event or phenomenon. By bringing together many different interpretations you are strengthening the evidence for an interpretation by discovering common themes and differences & building new interpretations of the topic of interest.
http://abbarker.wordpress.com/2013/04/25/meta-synthesis/
- << Previous: Systematic Reviews
- Next: Research & Literature Review Help >>
- Last Updated: Oct 23, 2024 10:19 AM
- URL: https://researchguides.library.wisc.edu/nursing
Cochrane UK is now closed
Due to changes in NIHR funding in evidence synthesis, Cochrane UK is now closed. We are so grateful to all our contributors and supporters.
If you would like to get involved with Cochrane, please visit the Join Cochrane pages
To learn more about Cochrane, visit Cochrane's homepage
If you would like to propose a topic for a Cochrane Review, please see our instructions for authors
If you have any queries, please contact Cochrane Support
Cochrane UK
Meta-analysis: what, why, and how.

This is an excerpt from a blog originally published on Students 4 Best Evidence
What is a meta-analysis?
Meta-analysis is a statistical technique for combining data from multiple studies on a particular topic.
Meta-analyses play a fundamental role in evidence-based healthcare. Compared to other study designs (such as randomized controlled trials or cohort studies), the meta-analysis comes in at the top of the evidence-based medicine pyramid. This is a pyramid which enables us to weigh up the different levels of evidence available to us. As we go up the pyramid, each level of evidence is less subject to bias than the level below it. Therefore, meta-analyses can be seen as the pinnacle of evidence-based medicine (1).
Meta-analyses began to appear as a leading part of research in the late 70s. Since then, they have become a common way for synthesizing evidence and summarizing the results of individual studies (2).
Read the full article here
RTI uses cookies to offer you the best experience online. By clicking “accept” on this website, you opt in and you agree to the use of cookies. If you would like to know more about how RTI uses cookies and how to manage them please view our Privacy Policy here . You can “opt out” or change your mind by visiting: http://optout.aboutads.info/ . Click “accept” to agree.
Meta-analysis of day treatment and contingency management dismantling research: Birmingham Homeless Cocaine Studies (1990-2006)
Schumacher, J., Milby, J. , Wallace, D. , Meehan, D., Kertesz, S., Vuchinich, R., Dunning, J., & Usdan, S. (2007). Meta-analysis of day treatment and contingency management dismantling research: Birmingham Homeless Cocaine Studies (1990-2006) . Journal of Consulting and Clinical Psychology , 75 (5), 823 - 828.
- Share on Facebook
- Share on X.com
- Share on Linkedin
To contact an RTI author, request a report, or for additional information about publications by our experts, send us your request.
- [email protected]
- +1 919 541 8787
Recent Publications
Adult vaccination coverage in the united states, harm reduction-focused behavioral activation for people who inject drugs, the impact of violations of expected utility theory on choices in the face of multiple risks, estimating rates of change to interpret quantitative wastewater surveillance of disease trends.
Here’s how you know
- U.S. Department of Health and Human Services
- National Institutes of Health
Probiotics: Usefulness and Safety
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What are probiotics?
Probiotics are live microorganisms that are intended to have health benefits when consumed or applied to the body. They can be found in yogurt and other fermented foods, dietary supplements , and beauty products. Cases of severe or fatal infections have been reported in premature infants who were given probiotics, and the U.S. Food and Drug Administration (FDA) has warned health care providers about this risk.
Although people often think of bacteria and other microorganisms as harmful “germs,” many are actually helpful. Some bacteria help digest food, destroy disease-causing cells, or produce vitamins. Many of the microorganisms in probiotic products are the same as or similar to microorganisms that naturally live in our bodies.
What types of bacteria are in probiotics?
Probiotics may contain a variety of microorganisms. The most common are bacteria that belong to groups called Lactobacillus and Bifidobacterium . Other bacteria may also be used as probiotics, and so may yeasts such as Saccharomyces boulardii .
Different types of probiotics may have different effects. For example, if a specific kind of Lactobacillus helps prevent an illness, that doesn’t necessarily mean that another kind of Lactobacillus or any of the Bifidobacterium probiotics would do the same thing.
Are prebiotics the same as probiotics?
No, prebiotics aren’t the same as probiotics. Prebiotics are nondigestible food components that selectively stimulate the growth or activity of desirable microorganisms.
What are synbiotics?
Synbiotics are products that combine probiotics and prebiotics.
How popular are probiotics?
The 2012 National Health Interview Survey (NHIS) showed that about 4 million (1.6 percent) U.S. adults had used probiotics or prebiotics in the past 30 days. Among adults, probiotics or prebiotics were the third most commonly used dietary supplement other than vitamins and minerals. The use of probiotics by adults quadrupled between 2007 and 2012. The 2012 NHIS also showed that 300,000 children age 4 to 17 (0.5 percent) had used probiotics or prebiotics in the 30 days before the survey.
How might probiotics work?
Probiotics may have a variety of effects in the body, and different probiotics may act in different ways.
Probiotics might:
- Help your body maintain a healthy community of microorganisms or help your body’s community of microorganisms return to a healthy condition after being disturbed
- Produce substances that have desirable effects
- Influence your body’s immune response.
How are probiotics regulated in the United States?
Government regulation of probiotics in the United States is complex. Depending on a probiotic product’s intended use, the FDA might regulate it as a dietary supplement, a food ingredient, or a drug.
Many probiotics are sold as dietary supplements, which don’t require FDA approval before they are marketed. Dietary supplement labels may make claims about how the product affects the structure or function of the body without FDA approval, but they aren’t allowed to make health claims, such as saying the supplement lowers your risk of getting a disease, without the FDA’s consent.
If a probiotic is going to be marketed as a drug for treatment of a disease or disorder, it has to meet stricter requirements. It must be proven safe and effective for its intended use through clinical trials and be approved by the FDA before it can be sold.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Learning About the Microbiome
The community of microorganisms that lives on us and in us is called the “microbiome,” and it’s a hot topic for research. The Human Microbiome Project, supported by the National Institutes of Health (NIH) from 2007 to 2016, played a key role in this research by mapping the normal bacteria that live in and on the healthy human body. With this understanding of a normal microbiome as the basis, researchers around the world, including many supported by NIH, are now exploring the links between changes in the microbiome and various diseases. They’re also developing new therapeutic approaches designed to modify the microbiome to treat disease and support health.
The National Center for Complementary and Integrative Health (NCCIH) is among the many agencies funding research on the microbiome. Researchers supported by NCCIH are studying the interactions between components of food and microorganisms in the digestive tract. The focus is on the ways in which diet-microbiome interactions may lead to the production of substances with beneficial health effects.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} What has science shown about the effectiveness of probiotics for health conditions?
A great deal of research has been done on probiotics, but much remains to be learned about whether they’re helpful and safe for various health conditions.
Probiotics have shown promise for a variety of health purposes, including prevention of antibiotic-associated diarrhea (including diarrhea caused by Clostridium difficile ), prevention of necrotizing enterocolitis and sepsis in premature infants, treatment of infant colic , treatment of periodontal disease , and induction or maintenance of remission in ulcerative colitis .
However, in most instances, we still don’t know which probiotics are helpful and which are not. We also don’t know how much of the probiotic people would have to take or who would be most likely to benefit. Even for the conditions that have been studied the most, researchers are still working toward finding the answers to these questions.
The following sections summarize the research on probiotics for some of the conditions for which they’ve been studied.
Gastrointestinal Conditions
.header_greentext{color:greenimportant;font-size:24pximportant;font-weight:500important;}.header_bluetext{color:blueimportant;font-size:18pximportant;font-weight:500important;}.header_redtext{color:redimportant;font-size:28pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;font-size:28pximportant;font-weight:500important;}.header_purpletext{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.header_yellowtext{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.header_blacktext{color:blackimportant;font-size:22pximportant;font-weight:500important;}.header_whitetext{color:whiteimportant;font-size:22pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;}.green_header{color:greenimportant;font-size:24pximportant;font-weight:500important;}.blue_header{color:blueimportant;font-size:18pximportant;font-weight:500important;}.red_header{color:redimportant;font-size:28pximportant;font-weight:500important;}.purple_header{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.yellow_header{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.black_header{color:blackimportant;font-size:22pximportant;font-weight:500important;}.white_header{color:whiteimportant;font-size:22pximportant;font-weight:500important;} antibiotic-associated diarrhea.
- Probiotics have been studied for antibiotic-associated diarrhea in general, as well as for antibiotic-associated diarrhea caused by one specific bacterium, Clostridium difficile . This section discusses the research on antibiotic-associated diarrhea in general. C. difficile is discussed in a separate section below.
- A 2017 review of 17 studies (3,631 total participants) in people who were not hospitalized indicated that giving probiotics to patients along with antibiotics was associated with a decrease of about half in the likelihood of antibiotic-associated diarrhea. However, this conclusion was considered tentative because the quality of the studies was only moderate. Patients who were given probiotics had no more side effects than patients who didn’t receive them.
- Probiotics may be helpful for antibiotic-associated diarrhea in young and middle-aged people, but a benefit has not been demonstrated in elderly people, according to a 2016 review of 30 studies (7,260 participants), 5 of which focused on people age 65 or older. It’s uncertain whether probiotics actually don’t work in elderly people or whether no effect was seen because there were only a few studies of people in this age group.
- A review of 23 studies (with 3,938 participants) of probiotics to prevent antibiotic-associated diarrhea in children provided moderate quality evidence that probiotics had a protective effect. No serious side effects were observed in children who were otherwise healthy, except for the infection for which they were being treated.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Clostridium difficile Infection
- The bacterium Clostridium difficile can infect the colon (large intestine) of patients who have received antibiotics, causing diarrhea that can range from mild to severe. C. difficile infection is difficult to treat and sometimes comes back after treatment. It’s more common in people who take antibiotics long-term and in elderly people, and it can spread in hospitals and nursing homes. C. difficile infection affects about half a million people a year in the United States and causes about 15,000 deaths.
- A 2017 analysis of 31 studies (8,672 total patients) concluded that it is moderately certain that probiotics can reduce the risk of C. difficile diarrhea in adults and children who are receiving antibiotics. Most of these studies involved hospital patients. The analysis also concluded that the use of probiotics along with antibiotics appears to be safe, except for patients who are very weak or have poorly functioning immune systems.
- The types of probiotics that would be most useful in reducing the risk of C. difficile diarrhea, the length of time for which they should be taken, and the most appropriate doses are uncertain.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Constipation
- A 2014 review of 14 studies (1,182 participants) of probiotics for constipation in adults showed some evidence of benefit, especially for Bifidobacterium lactis .
- A 2017 evaluation of 9 studies (778 participants) of probiotics for constipation in elderly people indicated that probiotics produced a small but meaningful benefit. The type of bacteria most often tested was Bifidobacterium longum . The researchers who performed the evaluation suggested that probiotics might be helpful for chronic constipation in older people as an addition to the usual forms of treatment.
- A 2017 review looked at 7 studies of probiotics for constipation in children (515 participants). The studies were hard to compare because of differences in the groups of children studied, the types of probiotics used, and other factors. The reviewers did not find evidence that any of the probiotics tested in the children were helpful. A second 2017 review, which included 4 of the same studies and 2 others (498 total participants in the 6 studies examined), took a more optimistic view of the evidence, noting that overall, probiotics did increase stool frequency, and that the effect was more noticeable in Asian than European children.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Diarrhea Caused by Cancer Treatment
- Diarrhea is a common side effect of chemotherapy or radiotherapy for cancer. It’s been suggested that probiotics might help prevent or treat this type of diarrhea. However, a 2018 review of 12 studies (1,554 participants) found that the evidence for a beneficial effect of probiotics was inconclusive.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Diverticular Disease
- In diverticulosis, small pouches develop at weak spots in the wall of the colon (large intestine). In most cases, this does not cause any symptoms. If symptoms (such as bloating, constipation, diarrhea, or cramping) do occur, the condition is called diverticular disease. If any of the pouches become inflamed, the condition is called diverticulitis. Patients with diverticulitis can have severe abdominal pain and may develop serious complications.
- A 2016 review of 11 studies (764 participants) of probiotics for diverticular disease was unable to reach conclusions on whether the probiotics were helpful because of the poor quality of the studies.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Inflammatory Bowel Disease
- Inflammatory bowel disease is a term for a group of conditions that cause a portion of the digestive system to become inflamed; the most common types are ulcerative colitis and Crohn’s disease. Symptoms may include abdominal pain, diarrhea (which may be bloody), loss of appetite, weight loss, and fever. The symptoms can range from mild to severe, and they may come and go. Treatment includes medicines and in some cases, surgery.
- A 2014 review of 21 studies in patients with ulcerative colitis (1,700 participants) indicated that adding probiotics, prebiotics, or synbiotics to conventional treatment could be helpful in inducing or maintaining remission of the disease. The same review also looked at 14 studies (746 participants) of probiotics, prebiotics, or synbiotics for Crohn’s disease and did not find evidence that they were beneficial.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Irritable Bowel Syndrome
- A 2018 review of 53 studies (5,545 total participants) of probiotics for irritable bowel syndrome (IBS) concluded that probiotics may have beneficial effects on global IBS symptoms and abdominal pain, but it was not possible to draw definite conclusions about their effectiveness or to identify which species, strains, or combinations of probiotics are most likely to be helpful.
For more information, see the NCCIH fact sheet on irritable bowel syndrome .
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Traveler’s Diarrhea
- A 2018 review evaluated 11 studies (5,143 participants) of probiotics or prebiotics for prevention of traveler’s diarrhea and found evidence that they may be helpful. However, the review didn’t assess the quality of the studies and didn’t include data on side effects.
- A 2017 clinical practice guideline by the International Society of Travel Medicine stated that there’s insufficient evidence to recommend probiotics or prebiotics to prevent or treat traveler’s diarrhea. The guidelines acknowledged that there’s evidence suggesting a small benefit but pointed out that studies vary greatly in terms of factors such as the probiotic strains used, the causes of the diarrhea, and geographic locations. Also, some studies had weaknesses in their design.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Conditions in Infants
.header_greentext{color:greenimportant;font-size:24pximportant;font-weight:500important;}.header_bluetext{color:blueimportant;font-size:18pximportant;font-weight:500important;}.header_redtext{color:redimportant;font-size:28pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;font-size:28pximportant;font-weight:500important;}.header_purpletext{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.header_yellowtext{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.header_blacktext{color:blackimportant;font-size:22pximportant;font-weight:500important;}.header_whitetext{color:whiteimportant;font-size:22pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;}.green_header{color:greenimportant;font-size:24pximportant;font-weight:500important;}.blue_header{color:blueimportant;font-size:18pximportant;font-weight:500important;}.red_header{color:redimportant;font-size:28pximportant;font-weight:500important;}.purple_header{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.yellow_header{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.black_header{color:blackimportant;font-size:22pximportant;font-weight:500important;}.white_header{color:whiteimportant;font-size:22pximportant;font-weight:500important;} infant colic.
- Colic is excessive, unexplained crying in young infants. Babies with colic may cry for 3 hours a day or more, but they eat well and grow normally. The cause of colic is not well understood, but studies have shown differences in the microbial community in the digestive tract between infants who have colic and those who don’t, which suggests that microorganisms may be involved.
- A 2018 review of 7 studies (471 participants) of probiotics for colic, 5 of which involved the probiotic Lactobacillus reuteri DSM 17938, found that this probiotic was associated with successful treatment (defined as a reduction of more than half in daily crying time). However, the effect was mainly seen in exclusively breastfed infants.
- No harmful effects were seen in a review of 4 studies (345 participants) of L. reuteri DSM 17938 for colic or in a small NCCIH-funded study that included repeated physical examinations and blood tests in infants with colic who were given this probiotic, as well as parents’ reports of symptoms.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Necrotizing Enterocolitis
- Necrotizing enterocolitis is a serious, sometimes fatal disease that occurs in premature infants. It involves injury or damage to the intestinal tract, causing death of intestinal tissue. Its exact cause is unknown, but an abnormal reaction to food components and the microorganisms that live in a premature baby’s digestive tract may play a role.
- A 2017 review of 23 studies (7,325 infants) showed that probiotics helped to prevent necrotizing enterocolitis in very-low-birth-weight infants. However, the results of individual studies varied; not all showed a benefit. Probiotics that included both Lactobacillus and Bifidobacterium seemed to produce the best results, but it was not possible to identify the most beneficial strains within these large groups of bacteria.
- None of the infants in the studies described above developed harmful short-term side effects from the probiotics. However, the long-term effects of receiving probiotics at such a young age are uncertain. Outside of these studies, there have been instances when probiotics did have harmful effects in newborns. In 2023, the FDA warned health care providers that premature infants who are given probiotics are at risk of severe, potentially fatal infections caused by the microorganisms in the products.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Sepsis in Infants
- Sepsis is a serious illness in which the body has a harmful, overwhelming response to an infection. It can cause major organs and body systems to stop working properly and can be life threatening. The risk of sepsis is highest in infants, children, the elderly, and people with serious medical problems. One group particularly at risk for sepsis is premature infants.
- A review of 37 studies (9,416 participants) found that probiotics were helpful in reducing the risk of sepsis in premature infants.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Dental Disorders
.header_greentext{color:greenimportant;font-size:24pximportant;font-weight:500important;}.header_bluetext{color:blueimportant;font-size:18pximportant;font-weight:500important;}.header_redtext{color:redimportant;font-size:28pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;font-size:28pximportant;font-weight:500important;}.header_purpletext{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.header_yellowtext{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.header_blacktext{color:blackimportant;font-size:22pximportant;font-weight:500important;}.header_whitetext{color:whiteimportant;font-size:22pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;}.green_header{color:greenimportant;font-size:24pximportant;font-weight:500important;}.blue_header{color:blueimportant;font-size:18pximportant;font-weight:500important;}.red_header{color:redimportant;font-size:28pximportant;font-weight:500important;}.purple_header{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.yellow_header{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.black_header{color:blackimportant;font-size:22pximportant;font-weight:500important;}.white_header{color:whiteimportant;font-size:22pximportant;font-weight:500important;} dental caries (tooth decay).
- A small amount of research, all in infants and young children, has examined the possibility that probiotics might be helpful in preventing dental caries (also called cavities or tooth decay). A review of 7 studies (1,715 total participants) found that the use of probiotics was associated with fewer cavities in 4 of the 7 studies, but the quality of the evidence was low, and no definite conclusions about the effectiveness of probiotics could be reached.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Periodontal Diseases (Gum Disease)
- Periodontal diseases result from infections and inflammation of the gums and bone that surround and support the teeth. If the disease is severe, the gums can pull away from the teeth, bone can be lost, and teeth may loosen or fall out.
- A 2016 review of 12 studies (452 participants) that evaluated probiotics for periodontal disease found evidence that they could be a helpful addition to treatment by reducing disease-causing bacteria and improving clinical signs of the disease. However, effects may differ for different probiotics.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Conditions Related to Allergy
.header_greentext{color:greenimportant;font-size:24pximportant;font-weight:500important;}.header_bluetext{color:blueimportant;font-size:18pximportant;font-weight:500important;}.header_redtext{color:redimportant;font-size:28pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;font-size:28pximportant;font-weight:500important;}.header_purpletext{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.header_yellowtext{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.header_blacktext{color:blackimportant;font-size:22pximportant;font-weight:500important;}.header_whitetext{color:whiteimportant;font-size:22pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;}.green_header{color:greenimportant;font-size:24pximportant;font-weight:500important;}.blue_header{color:blueimportant;font-size:18pximportant;font-weight:500important;}.red_header{color:redimportant;font-size:28pximportant;font-weight:500important;}.purple_header{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.yellow_header{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.black_header{color:blackimportant;font-size:22pximportant;font-weight:500important;}.white_header{color:whiteimportant;font-size:22pximportant;font-weight:500important;} allergic rhinitis (hay fever).
- A review of 23 studies (1,919 participants) in which probiotics were tested for treating allergic rhinitis found some evidence that they may be helpful for improving symptoms and quality of life. However, because the studies tested different probiotics and measured different effects, no recommendations about the use of probiotics could be made. Few side effects of probiotics were reported in these studies.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Asthma
- A review of 11 studies (910 participants) of probiotics for asthma in children had inconclusive results.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Atopic Dermatitis
- Atopic dermatitis is an itchy chronic skin disorder that’s associated with allergies but not caused by them. It’s most common in infants and may start as early as age 2 to 6 months. Many people outgrow it by early adulthood. Atopic dermatitis is one of several types of eczema.
- A 2017 review of 13 studies (1,271 participants) of probiotics for the treatment of atopic dermatitis in infants and children did not find consistent evidence of a beneficial effect. A review of 9 studies (269 participants) in adults provided preliminary evidence that some strains of probiotics might be beneficial for symptoms of atopic dermatitis.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Prevention of Allergies
- It’s been suggested that changes in people’s lifestyles and environment may have led to reduced contact with microorganisms early in life, and that this decrease may have contributed to an increase in allergies. This is sometimes called the “hygiene hypothesis,” although factors unrelated to hygiene, such as smaller family size and the use of antibiotics, may also play a role. Studies have been done in which probiotics were given to pregnant women and/or young infants in the hope of preventing the development of allergies.
- A 2015 review of 17 studies (4,755 participants) that evaluated the use of probiotics during pregnancy or early infancy found that infants exposed to probiotics had a lower risk of developing atopic dermatitis, especially if they were exposed to a mixture of probiotics. However, probiotics did not have an effect on the risks of asthma, wheezing, or hay fever (allergic rhinitis).
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Other Conditions
.header_greentext{color:greenimportant;font-size:24pximportant;font-weight:500important;}.header_bluetext{color:blueimportant;font-size:18pximportant;font-weight:500important;}.header_redtext{color:redimportant;font-size:28pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;font-size:28pximportant;font-weight:500important;}.header_purpletext{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.header_yellowtext{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.header_blacktext{color:blackimportant;font-size:22pximportant;font-weight:500important;}.header_whitetext{color:whiteimportant;font-size:22pximportant;font-weight:500important;}.header_darkred{color:#803d2fimportant;}.green_header{color:greenimportant;font-size:24pximportant;font-weight:500important;}.blue_header{color:blueimportant;font-size:18pximportant;font-weight:500important;}.red_header{color:redimportant;font-size:28pximportant;font-weight:500important;}.purple_header{color:purpleimportant;font-size:31pximportant;font-weight:500important;}.yellow_header{color:yellowimportant;font-size:20pximportant;font-weight:500important;}.black_header{color:blackimportant;font-size:22pximportant;font-weight:500important;}.white_header{color:whiteimportant;font-size:22pximportant;font-weight:500important;} acne.
- Research has identified mechanisms by which probiotics, either taken orally or used topically (applied to the skin), might influence acne. However, there has been very little research in people on probiotics for acne, and the American Academy of Dermatology’s 2016 guidelines for managing acne state that the existing evidence isn’t strong enough to justify any recommendations about the use of probiotics.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Hepatic Encephalopathy
- When the liver is damaged and unable to remove toxic substances from the blood, the toxins can build up in the bloodstream and affect the nervous system. This may lead to impairments of brain function called hepatic encephalopathy.
- A 2017 review looked at 21 studies (1,420 participants) of probiotics for hepatic encephalopathy and concluded that they were generally of low quality. There was evidence that compared with a placebo (an inactive substance) or no treatment, probiotics probably had beneficial effects on hepatic encephalopathy, but it was uncertain whether probiotics were better than lactulose, a conventional treatment for liver disease.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Upper Respiratory Infections
- Probiotics have been tested for their effects against upper respiratory infections (a group that includes the common cold, middle ear infections, sinusitis, and various throat infections). A 2015 evaluation of 12 studies with 3,720 total participants indicated that people taking probiotics may have fewer and shorter upper respiratory infections. However, the quality of the evidence was low because some of the studies were poorly conducted.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Urinary Tract Infections
- A 2015 review of 9 studies (735 participants) of probiotics for the prevention of urinary tract infection did not find evidence of a beneficial effect.
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Can probiotics be harmful?
- Probiotics have an extensive history of apparently safe use, particularly in healthy people. However, few studies have looked at the safety of probiotics in detail, so there’s a lack of solid information on the frequency and severity of side effects.
- The risk of harmful effects from probiotics is greater in people with severe illnesses or compromised immune systems. When probiotics are being considered for high-risk individuals, such as premature infants or seriously ill hospital patients, the potential risks of probiotics should be carefully weighed against their benefits. Cases of severe or fatal infections have been reported in premature infants who were given probiotics, and the U.S. Food and Drug Administration (FDA) has warned health care providers about this risk.
- Possible harmful effects of probiotics include infections, production of harmful substances by the probiotic microorganisms, and transfer of antibiotic resistance genes from probiotic microorganisms to other microorganisms in the digestive tract.
- Some probiotic products have been reported to contain microorganisms other than those listed on the label. In some instances, these contaminants may pose serious health risks.
NCCIH-Funded Research
NCCIH sponsors a variety of research projects related to probiotics or the microbiome. In addition to the previously mentioned studies on diet-microbiome interactions in the digestive tract, recent topics include:
- The mechanisms by which probiotics may help to reduce postmenopausal bone loss
- Engineering probiotics to synthesize natural substances for microbiome-brain research
- The mechanisms by which certain probiotics may relieve chronic pelvic pain
- The effects of a specific Bifidobacterium strain on changes in short-chain fatty acid production in the gut that may play a role in antibiotic-associated diarrhea.
More To Consider
- Don’t use probiotics as a reason to postpone seeing your health care provider about any health problem.
- If you’re considering a probiotic dietary supplement, consult your health care provider first. This is especially important if you have health problems. Anyone with a serious underlying health condition should be monitored closely while taking probiotics.
- Take charge of your health—talk with your health care providers about any complementary health approaches you use. Together, you can make shared, well-informed decisions.
For More Information
Nccih clearinghouse.
The NCCIH Clearinghouse provides information on NCCIH and complementary and integrative health approaches, including publications and searches of Federal databases of scientific and medical literature. The Clearinghouse does not provide medical advice, treatment recommendations, or referrals to practitioners.
Toll-free in the U.S.: 1-888-644-6226
Telecommunications relay service (TRS): 7-1-1
Website: https://www.nccih.nih.gov
Email: [email protected] (link sends email)
Know the Science
NCCIH and the National Institutes of Health (NIH) provide tools to help you understand the basics and terminology of scientific research so you can make well-informed decisions about your health. Know the Science features a variety of materials, including interactive modules, quizzes, and videos, as well as links to informative content from Federal resources designed to help consumers make sense of health information.
Explaining How Research Works (NIH)
Know the Science: How To Make Sense of a Scientific Journal Article
Understanding Clinical Studies (NIH)
A service of the National Library of Medicine, PubMed® contains publication information and (in most cases) brief summaries of articles from scientific and medical journals. For guidance from NCCIH on using PubMed, see How To Find Information About Complementary Health Approaches on PubMed .
Website: https://pubmed.ncbi.nlm.nih.gov/
MedlinePlus
To provide resources that help answer health questions, MedlinePlus (a service of the National Library of Medicine) brings together authoritative information from the National Institutes of Health as well as other Government agencies and health-related organizations.
Website: https://www.medlineplus.gov
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Key References
- Bafeta A, Koh M, Riveros C, et al. Harms reporting in randomized controlled trials of interventions aimed at modifying microbiota: a systematic review. Annals of Internal Medicine . 2018;169(4):240-247.
- Blaabjerg S, Artzi DM, Aabenhus R. Probiotics for the prevention of antibiotic-associated diarrhea in outpatients—a systematic review and meta-analysis. Antibiotics . 2017;6(4).pii:E21.
- Butel M-J. Probiotics, gut microbiota and health. Médecine et Maladies Infectieuses . 2014;44(1):1-8.
- Cohen PA. Probiotic safety—no guarantees. JAMA Internal Medicine . 2018;178(12):1577-1578.
- Degnan FH. The US Food and Drug Administration and probiotics: regulatory categorization. Clinical Infectious Diseases. 2008;46(Suppl 2):S133–S136.
- Didari T, Solki S, Mozaffari S, et al. A systematic review of the safety of probiotics. Expert Opinion on Drug Safety . 2014;13(2):227–239.
- Dryl R, Szajewska H. Probiotics for management of infantile colic: a systematic review of randomized controlled trials. Archives of Medical Science. 2018;14(5):1137-1143.
- Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. International Journal of Environmental Research and Public Health. 2014;11(5):4745-4767.
- Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Alimentary Pharmacology & Therapeutics . 2018;48(10):1044-1060.
- Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile -associated diarrhea in adults and children. Cochrane Database of Systematic Reviews. 2017;(12):CD006095. Accessed at www.cochranelibrary.com on January 23, 2018.
- Guarner F, Khan AG, Garisch J, et al. World Gastroenterology Organisation Global Guidelines. Probiotics and Prebiotics. October 2011. Journal of Clinical Gastroenterology . 2012;46(6):468–481.
- Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA . 2012;307(18):1959–1969.
- Hempel S, Newberry S, Ruelaz A, et al. Safety of Probiotics to Reduce Risk and Prevent or Treat Disease. Evidence Report/Technology Assessment no. 200. Rockville, MD: Agency for Healthcare Research and Quality; 2011. AHRQ publication no. 11-E007.
- Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137(3):e20153684.
- Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes . 2010;1(3):164-185.
- Thomas JP, Raine T, Reddy S, et al. Probiotics for the prevention of necrotizing enterocolitis in very low-birth-weight infants: a meta-analysis and systematic review. Acta Paediatrica . 2017;106(11):1729-1741.
- U.S. Food and Drug Administration. Warning Regarding Use of Probiotics in Preterm Infants. Issued September 29, 2023. Accessed at https://www.fda.gov/media/172606 on October 2, 2023.
- Zuccotti G, Meneghin F, Aceti A, et al. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70(11):1356-13
.header_greentext{color:green!important;font-size:24px!important;font-weight:500!important;}.header_bluetext{color:blue!important;font-size:18px!important;font-weight:500!important;}.header_redtext{color:red!important;font-size:28px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;font-size:28px!important;font-weight:500!important;}.header_purpletext{color:purple!important;font-size:31px!important;font-weight:500!important;}.header_yellowtext{color:yellow!important;font-size:20px!important;font-weight:500!important;}.header_blacktext{color:black!important;font-size:22px!important;font-weight:500!important;}.header_whitetext{color:white!important;font-size:22px!important;font-weight:500!important;}.header_darkred{color:#803d2f!important;}.Green_Header{color:green!important;font-size:24px!important;font-weight:500!important;}.Blue_Header{color:blue!important;font-size:18px!important;font-weight:500!important;}.Red_Header{color:red!important;font-size:28px!important;font-weight:500!important;}.Purple_Header{color:purple!important;font-size:31px!important;font-weight:500!important;}.Yellow_Header{color:yellow!important;font-size:20px!important;font-weight:500!important;}.Black_Header{color:black!important;font-size:22px!important;font-weight:500!important;}.White_Header{color:white!important;font-size:22px!important;font-weight:500!important;} Additional References
- Bae J-M. Prophylactic efficacy of probiotics on travelers’ diarrhea: an adaptive meta-analysis of randomized controlled trials. Epidemiology and Health . 2018;40:e2018043.
- Black LI, Clarke TC, Barnes PM, Stussman BJ, Nahin RL. Use of complementary health approaches among children aged 4-17 years in the United States: National Health Interview Survey, 2007-2012. National health statistics reports; no 78. Hyattsville, MD: National Center for Health Statistics. 2015.
- Cao L, Wang L, Yang L, et al. Long-term effect of early-life supplementation with probiotics on preventing atopic dermatitis: a meta-analysis. Journal of Dermatological Treatment . 2015;26(6):537-540.
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. National health statistics reports; no 79. Hyattsville, MD: National Center for Health Statistics. 2015.
- Dalal R, McGee RG, Riordan SM, et al. Probiotics for people with hepatic encephalopathy. Cochrane Database of Systematic Reviews . 2017;(2):CD008716. Accessed at www.cochranelibrary.com on November 15, 2018.
- Dimidi E, Christodoulides S, Fragkos KC, et al. The effect of probiotics on functional constipation in adults: a systematic review and meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition . 2014;100(4):1075-1084.
- Doron S, Snydman DR. Risk and safety of probiotics. Clinical Infectious Diseases . 2015;60(Suppl 2):S129-S134.
- Fatheree NY, Liu Y, Taylor CM, et al. Lactobacillus reuteri for infants with colic: a double-blind, placebo-controlled, randomized clinical trial. Journal of Pediatrics . 2017;191:170-178.
- Ghouri YA, Richards DM, Rahimi EF, et al. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clinical and Experimental Gastroenterology . 2014;7:473-487.
- Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews. Gastroenterology & Hepatology . 2017;14(8):491-502.
- Goldenberg JZ, Lytvyn L, Steurich J, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database of Systematic Reviews . 2015;(12):CD004827. Accessed at www.cochranelibrary.com on November 2, 2018.
- Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews . 2015;(2):CD006895. Accessed at www.cochranelibrary.com on March 6, 2018.
- Hoffmann DE, Fraser CM, Palumbo FB, et al. Probiotics: finding the right regulatory balance. Science . 2013;342(6156):314-315.
- Huang R, Hu J. Positive effect of probiotics on constipation in children: a systematic review and meta-analysis of six randomized controlled trials. Frontiers in Cellular and Infection Microbiology. 2017;7:153.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Frontiers in Cellular and Infection Microbiology. 2017;7:392.
- Jafarnejad S, Shab-Bidar S, Speakman JR, et al. Probiotics reduce the risk of antibiotic-associated diarrhea in adults (18-64 years) but not the elderly (>65 years): a meta-analysis. Nutrition in Clinical Practice . 2016;31(4):502-513.
- Jørgensen MR, Castiblanco G, Twetman S, et al. Prevention of caries with probiotic bacteria during early childhood. Promising but inconsistent findings. American Journal of Dentistry . 2016;29(3):127-131.
- Kechagia M, Basoulis D, Konstantopoulou S, et al. Health benefits of probiotics: a review. ISRN Nutrition . 2013;2013:48165.
- Kelesidis T, Pothoulakis C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therapeutic Advances in Gastroenterology . 2012;5(2):111-125.
- Kober M-M, Bowe WP. The effect of probiotics on immune regulation, acne, and photoaging. International Journal of Women’s Dermatology . 2015;1(2):85-89.
- Lahner E, Bellisario C, Hassan C, et al. Probiotics in the treatment of diverticular disease. A systematic review. Journal of Gastrointestinal and Liver Diseases . 2016;25(1):79-86.
- Lin J, Zhang Y, He C, et al. Probiotics supplementation in children with asthma: a systematic review and meta-analysis. Journal of Paediatrics and Child Health. 2018;54(9):953-961.
- Marcason W. Probiotics: where do we stand? Journal of the Academy of Nutrition and Dietetics. 2013;113(10):1424.
- Martínez-Martínez MI, Calabuig-Tolsá R, Cauli O. The effect of probiotics as a treatment for constipation in elderly people: a systematic review. Archives of Gerontology and Geriatrics. 2017;71:142-149.
- Matsubara VH, Bandara HM, Ishikawa KH, et al. The role of probiotic bacteria in managing periodontal disease: a systematic review. Expert Review of Anti-infective Therapy . 2016;14(7):643-655.
- Notay M, Foolad N, Vaughn AR, et al. Probiotics, prebiotics, and synbiotics for the treatment and prevention of adult dermatological diseases. American Journal of Clinical Dermatology . 2017;18(6):721-732.
- Oelschlaeger TA. Mechanisms of probiotic actions – A review. International Journal of Medical Microbiology . 2010;300(1):57-62.
- Osborn DA, Sinn JKH. Prebiotics in infants for prevention of allergy. Cochrane Database of Systematic Reviews . 2013(3):CD006474. Accessed at www.cochranelibrary.com on March 6, 2018.
- Petschow B, Doré J, Hibberd P, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Annals of the New York Academy of Sciences . 2013;1306:1–17.
- Reid G. Probiotics: definition, scope and mechanisms of action. Best Practice & Research: Clinical Gastroenterology. 2016;30(1):17-25.
- Riddle MS, Connor BA, Beeching NJ, et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. Journal of Travel Medicine . 2017;24(Suppl 1):S63-S80.
- Sanders ME, Guarner F, Guerrant R, et al. An update on the use and investigation of probiotics in health and disease. Gut. 2013;62(5):787-796.
- Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database of Systematic Reviews . 2015;(12):CD008772. Accessed at www.cochranelibrary.com on November 9, 2018.
- Shanahan F. A commentary on the safety of probiotics. Gastroenterology Clinics of North America . 2012;41(4):869–876.
- Steele SR, McCormick J, Melton GB, et al. Practice parameters for the management of Clostridium difficile infection. Diseases of the Colon and Rectum . 2015;58(1):10-24.
- Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics . 2018;141(1):e20171811.
- Vallabhaneni S, Walker TA, Lockhart SR, et al. Fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. Morbidity and Mortality Weekly Report. 2015;64(6):155-156.
- Venugopalan V, Shriner KA, Wong-Beringer A. Regulatory oversight and safety of probiotic use. Emerging Infectious Diseases. 2010;16(11):1661-1665.
- Wei D, Heus P, van de Wetering FT, et al. Probiotics for the prevention or treatment of chemotherapy- or radiotherapy-related diarrhoea in people with cancer. Cochrane Database of Systematic Reviews. 2018;(8):CD008831. Accessed at www.cochranelibrary.com on November 9, 2018.
- Wojtyniak K, Szajewska H. Systematic review: probiotics for functional constipation in children. European Journal of Pediatrics . 2017;176(9):1155-1162.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. Journal of the American Academy of Dermatology . 2016;74(5):945-973.
- Zajac AE, Adams AS, Turner JH. A systematic review and meta-analysis of probiotics for the treatment of allergic rhinitis. International Forum of Allergy and Rhinology . 2015;5(6):524-532.
Acknowledgments
NCCIH thanks Yisong Wang, Ph.D., and David Shurtleff, Ph.D., for their review of the 2019 update of this publication.
This publication is not copyrighted and is in the public domain. Duplication is encouraged.
NCCIH has provided this material for your information. It is not intended to substitute for the medical expertise and advice of your health care provider(s). We encourage you to discuss any decisions about treatment or care with your health care provider. The mention of any product, service, or therapy is not an endorsement by NCCIH.
For Consumers
5 Things To Know About Probiotics
For Health Care Providers
Risk of Invasive Disease in Preterm Infants Given Probiotics Formulated To Contain Live Bacteria or Yeast
Irritable Bowel Syndrome and Complementary Health Approaches
Probiotics - Systematic Reviews/Reviews/Meta-analyses (PubMed®)
Probiotics - Randomized Controlled Trials (PubMed®)
Research Results
A Probiotic/Prebiotic Combination Reduces Behavioral Symptoms Associated With Stress
Related Fact Sheets
Irritable Bowel Syndrome: What You Need To Know

IMAGES
VIDEO
COMMENTS
Meta-analysis is a method of synthesizing quantitative data from multiple studies on a common research question. It involves extracting effect sizes, variance measures, and statistical models from the studies and combining them to improve statistical power and resolve uncertainties or discrepancies.
A meta-analysis is a quantitative synthesis of primary studies that aims to accumulate knowledge and test theoretical assumptions in various fields. This article provides a workflow of the essential steps, methodological recommendations, and references for conducting a meta-analysis in management research.
Meta-analysis is a statistical procedure used to combine and synthesize findings from multiple independent studies to estimate the average effect size for a particular research question. Meta-analysis goes beyond traditional narrative reviews by using statistical methods to integrate the results of several studies, leading to a more objective ...
Meta-analysis is a statistical method of combining results from numerous studies on a certain research question. Learn how to conduct a meta-analysis, its strengths and limitations, and how it differs from a systematic review.
Meta-analysis is an objective examination of published data from many studies of the same research topic, using rigorous statistical methods to reveal patterns and conclusions. Learn how meta ...
Meta-analysis is a research process that systematically synthesises the findings of single studies to calculate an overall effect. Learn the five steps of meta-analysis, how to interpret the results and why it is important for evidence-based practice and research.
Meta-analysis is widely accepted as the preferred method to synthesize research findings in various disciplines. This paper provides an introduction to when and how to conduct a meta-analysis. Several practical questions, such as advantages of meta-analysis over conventional narrative review and the number of studies required for a meta ...
Meta-analysis is a central method for knowledge accumulation in many scien-tic elds (Aguinis et al. 2011c; Kepes et al. 2013). Similar to a narrative review, it serves as a synopsis of a research question or eld. However, going beyond a narra-tive summary of key ndings, a meta-analysis adds value in providing a quantitative
Meta-analysis is a systematic approach of synthesizing, combining, and analyzing data from multiple studies (randomized clinical trials 1 or observational studies 2) into a single effect estimate to answer a research question.Meta-analysis is especially useful if there is debate around the research question in the literature published to date or the individual published studies are underpowered.
But meta-analysis is based on a uniquely laborious data collection that often takes months of expert researcher time. So, meta-analysis can benefit from AI more than most research fields. We believe that in a few years new versions of GPT (or some equivalent) will be able to assist with data collection from primary studies.
1. A Meta-Analysis pools together the sample populations from different studies, such as Randomized Controlled Trials, into one statistical analysis and treats them as one large sample population with one conclusion. a) True b) False. 2. One potential design pitfall of Meta-Analyses that is important to pay attention to is:
A systematic review may or may not include a meta-analysis, which provides a statistical approach to quantitatively combine results of studies eligible for a systematic review topic [2,3,4,5 ...
Meta-analysis is a statistical approach to synthesize the results of multiple studies on the same or similar topics. It involves systematic identification, evaluation, and interpretation of evidence, and can be used to guide policy decisions and assess the effectiveness of interventions.
Meta-analysis is the quantitative, scientific synthesis of research results. Since the term and modern approaches to research synthesis were first introduced in the 1970s, meta-analysis has had a ...
Whether a SR, meta-analysis, meta-synthesis, or meta-review, all SRs are a form of evidence or research syntheses. Although many narratives on the history of systematic reviewing call attention to Karl Pearson's ( Simpson and Pearson, 1904 ) integration of correlations pertaining to the effectiveness of a typhoid vaccine as the original SR ...
A meta-analysis is a statistical method that combines results from multiple studies on a similar topic. It can help identify common trends or differences, but also faces challenges such as time, expertise, bias and quality of data.
Systematic review and meta-analysis is a way of summarizing research evidence, which is generally the best form of evidence, and hence positioned at the top of the hierarchy of evidence. Systematic reviews can be very useful decision-making tools for primary care/family physicians.
A meta-analysis is defined by Haidlich (2010) as "a quantitative, formal, epidemiological study design used to systematically assess previous research studies to derive conclusions about that body of research. Outcomes from a meta-analysis may include a more precise estimate of the effect of treatment or risk factor for disease, or other outcomes, than any individual study contributing to the ...
Meta-Analysis. What meta-research has taught us about research and changes to research practices. John P. A. Ioannidis, Corresponding Author. ... Meta-research has offered on all of these fronts empirical evidence that sometimes pertains even to large effects of extreme biases. Continued surveys of research practices and results may offer ...
What is meta-analysis? Meta-analysis is a research process used to systematic-ally synthesise or merge the findings of single, inde-pendent studies, using statistical methods to calculate an overall or 'absolute' effect.2 Meta-analysis does not simply pool data from smaller studies to achieve a larger
A meta-analysis goes beyond critique and integration and conducts secondary statistical analyses on the outcomes of similar studies. It is a systematic review that uses quantitative methods to synthesize and summarize the results. An advantage of a meta-analysis is the ability to be completely objective in evaluating research findings.
A meta-analysis was performed to aggregate the results from the individual studies and, thus, obtain greater statistical power. Metaanalysis is a research process used to systematically synthesize ...
Meta-analysis is a statistical technique for combining data from multiple studies on a particular topic. Learn more about the role, benefits, and methods of meta-analysis from Cochrane UK, a trusted source of evidence-based healthcare.
Meta-analysis of day treatment and contingency management dismantling research: Birmingham Homeless Cocaine Studies (1990-2006). Journal of Consulting and Clinical Psychology , 75 (5), 823 - 828.
Our meta-analysis finds that the powerful relationship between employee engagement -- measured by Gallup's Q 12 survey -- and performance can be generalized across countries, industries ...
Learn about probiotics, live microorganisms that may have health benefits when consumed or applied to the body. Find out how probiotics work, how they are regulated, and what science has shown about their effectiveness and safety for various conditions.
A meta description is an HTML element that summarizes a web page and appears in search engine results. Learn how to write effective meta descriptions, how to preview them, and how they may affect your SEO and CTR.
Colorectal cancer (CRC) is one of the most common types of cancer worldwide. In addition to known risk factors, oncoviruses have attracted exceptional attention from recent research. Numerous hypotheses on interactions between the Epstein-Barr virus (EBV) and Human papillomavirus (HPV) in CRC are still based on sparse prevalence data of these coinfections. The aim of this study was to ...