| What happens next is that a proton (a hydrogen ion) gets transferred from the bottom oxygen atom to one of the others. It gets picked off by one of the other substances in the mixture (for example, by attaching to a lone pair on an unreacted ethanol molecule), and then dumped back onto one of the oxygens more or less at random. The net effect is: Now a molecule of water is lost from the ion. The product ion has been drawn in a shape to reflect the product which we are finally getting quite close to! The structure for the latest ion is just like the one we discusssed at length back in step 1. The positive charge is actually delocalised all over that end of the ion, and there will also be contributions from structures where the charge is on the either of the oxygens: It is easier to follow what is happening if we keep going with the structure with the charge on the carbon. The hydrogen is removed from the oxygen by reaction with the hydrogensulphate ion which was formed way back in the first step. And there we are! The ester has been formed, and the sulphuric acid catalyst has been regenerated. © Jim Clark 2002 (modified 2004) We use cookies to ensure that we give you the best experience on our website. By continuing to browse this repository, you give consent for essential cookies to be used. You can read more about our Privacy and Cookie Policy . - Departments
- University Research
- About the University
 Synthesis of ethyl ethanoate from ethanol by heterogeneous catalytic dehydrogenation, hydrogenation and purificationColley, Stephen William (2002) Synthesis of ethyl ethanoate from ethanol by heterogeneous catalytic dehydrogenation, hydrogenation and purification. Doctoral thesis, Durham University. A study has been carried out into the reactions of ethanol over transition metal dehydrogenation catalysts, with particular emphasis on the reaction of ethanol to ethyl ethanoate. The reaction is of commercial interest, and the test work has been aimed at the development of a process that would yield ethyl ethanoate at commercially acceptable purity. Copper based catalysts have been shown to selectively promote the formation of ethyl ethanoate. Experimental work has been carried out to identify an optimised catalyst and reaction conditions for the ethanol to ethyl ethanoate reaction. A copper based catalyst that yields >95% selectivity to ethyl ethanoate, at >40% conversion of ethanol, has been identified. A purification scheme has been devised that incorporates selective hydrogenation using either nickel or ruthenium heterogeneous catalysts to remove aldehyde and ketone by-products. The purification scheme includes a novel distillation section. The catalyst system developed can be used to synthesise ethyl ethanoate at a purity of >99.98% from industrially available ethanol that contains up to 5% 2-propanol. A commercial plant producing 50,000 tonnes of ethyl ethanoate per annum, using the technology described in this thesis, has been in operation since April 2001.Four patents, based on the technology described in this thesis, have been applied for or granted. | Item Type: | Thesis (Doctoral) |
|---|
| Award: | Doctor of Philosophy |
|---|
| Thesis Date: | 2002 |
|---|
| Copyright: | Copyright of this thesis is held by the author |
|---|
| Deposited On: | 01 Aug 2012 11:40 |
|---|
 Quick links- Latest additions
- Browse by year
- Browse by department
- Deposit thesis
- Usage statistics
Prospective students - International students
- Research degrees
- Durham e-Theses
- Deposit Guide
Last Modified: Summer 2013 | Disclaimer | Trading name | Powered by EPrints 3 No notifications. Disclaimer: This dissertation has been written by a student and is not an example of our professional work, which you can see examples of here . View full disclaimer Any opinions, findings, conclusions, or recommendations expressed in this dissertation are those of the authors and do not necessarily reflect the views of UKDiss.com. Various Process Routes of the Production of Ethyl AcetateInfo: 10869 words (43 pages) Dissertation Published: 25th Oct 2021 Reference this Tagged: Chemistry Production of Ethyl Acetate: A feasibility study analysing various process routes of the production of ethyl acetate The following report will analyse various process routes for the production of ethyl acetate in terms of economic environmental, safety and technical aspects to ascertain a feasible method of producing 200 kilo tonnes of ethyl acetate per annum. Table of ContentsIntroduction, 1. technical feasibility. 1.1-Esterification 1.2-Tischenko Reaction 1.3-Dehydrogenation 1.4 Direct addition1.5 location viability, 1.6 feedstock ethics and security. 2-Enviromental and Safety Factors 2.3-Process Safety Culture Safety aspects of Process routesEnvironmental aspects, 3. economic feasibility. 3.1-Market Scope 3.2-Outside Battery Limit Costs 3.3 Engineering Costs3.4 contingency costs, 3.4 fixed capital investments, 3.5 cost of feedstock and selling prices of products, recommendations, conclusions. Ethyl acetate is a colourless organic compound having characteristic sweet smell. It is an eater of acetic acid and ethanol. It is used as a solvent in making of glues, tea and coffee, nail polish removers and also finds uses in cigarettes. On industrial scale it is generally synthesized using method of classic Fischer esterification reaction of ethanol and acetic acid. On laboratory scale it is used in column chromatography and extraction. The Design brief states that a new plant which produces 200 kilo tonnes per annum of ethyl acetate must be built. Firstly let’s look at the uses and properties of ethyl acetate which make it useful and in demand to be produced on a large scale. Ethyl acetate is a clear colourless liquid with a fruity odour ( National Centre for Biotechnology Information, 2017 ).Ethyl acetate is an environmentally friendly organic solvent used for the production of lacquers, enamels, inks, adhesives and pharmaceuticals, thus eliminating the use of aromatic compounds, in the working environment ( Inui et al, 2012 ). Ethyl acetate is widely used due to it being relatively low cost, environmentally friendly, low toxicity and having a relatively agreeable fruity odour. In 2008, the world market for ethyl acetate was estimated to be 2.5 million tons per annum ( Junge et al ). The chemical and physical properties of ethyl acetate are as follows: | | | | −84 °C | | | 76.5-77.5 °C | | | 897 kg/m | | | Liquid | | | C H O | | | 88.106 g/mol | ( National Center for Biotechnology Information, 2017) Ethyl acetate can also be seen in alcoholic beverages making industries. It is found in radishes, cereal crops, and wine, beer and fruit juices. It can also be used in preparing artificial essences. It also finds uses for colour and inks used to put a mark on fruit or vegetables. In the field of entomology, ethyl acetate is an effective asphyxiant for use in insect collecting and study. Market scope and future usage of ethyl acetate also reveals that it has a strong application scope in manufacturing process of artificial leather. According to recent forecasting report in 2024. Global artificial leather market size is poised to exceed USD 95 billion in 2024. Rise in leather accessories like purses, bags, covers, wallets and belts demand owing to availability of various fashion brands providing stylish and trendy accessories may drive industry growth. Stringent regulatory norms regarding natural leather production along with growing awareness for animal wellbeing is likely to contribute towards synthetic leather market size. Increasing trend for stylish seat and steering wheel cover, air bags and belts in automotive sector has propelled industry growth thereby promoting ethyl acetate demand. The North American region is growing significantly due to an increased consumption of ethyl acetate in decaffeination process to reduce caffeine content of coffee, tea leaves, and cocoa. It is predicted that growing investment in the end-use industries is likely to lead the market growth during the forecast period. The growing consumption of the product in healthcare, lipsticks, and ointments has propelled the growth in countries such as the U.S., Canada, and Mexico to achieve a stunning growth in the market as they provide quick evaporating nature, durable, and high standard performance to the product. Forecasting reports by different researches reveals that following is the global ethyl acetate capacity per region: 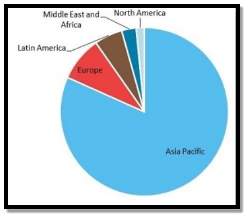 Riemenschneider, Wilhelm; Bolt, Hermann M. (2005) The design brief states that a plant producing 200 kilo tonnes of ethyl acetate per annum should be designed. At the preliminary stages of designing a chemical plant the choice of process route is the most vital decision. There are various ways of producing ethyl acetate on an industrial scale but in this study three of those will be analysed. They are as follows: The classical Fischer esterification process of acetic acid with ethanol (McMurry, 2000) This is the dimerization of acetaldehyde, in the presence of a metal catalyst (Inui et al., 2002) This is the addition of acetic acid to ethylene (Inui et al., 2001) The dehydrogenative dimerization of ethanol There are four main process routes used to produce ethyl acetate. They are: - Esterification (84%),
- Direct addition (6%),
- Ddehydrogenation (5%),
- Tischenko (4%)
When analysing these methods there are many factors which should be taken into consideration to come to a conclusion of the most feasible process according to the requirements of the design brief such as: - the feedstock or availability of raw material required,
- the yield or rate of reaction
- the operating conditions required
- environmental impact/carbon footprint of the process
The design brief also clearly states that ‘the process should be efficient in raw materials and energy usage, including the recovery of any by-products for sale’ so with this in mind the three methods of industrially producing ethyl acetate will be studied to ascertain the most feasible. 1.1 EsterificationEsterification is a chemical reaction process between alcohol and carboxylic acid in the presence of catalyst that formed ester. This mixture converts to ester about 65% at room temperature. The commonly concentrated sulphuric acid is acting as an esterification catalyst to enhance the reaction. The sulphuric acid removes water to help shift the equilibrium towards forming more ester product. Water is a by- product and must be removed in order to get the equilibrium in the desired direction. This process is a simple process, well known reaction, and moderately exothermic where the heat or reaction is -0.0114kJ/mol with no danger of decomposition reaction. The optimum temperature for this reaction is in the range of 363 K – 400 K while the optimum pressure is in the range of 20 bar -40 bar. Esterification is the most common process route used in the production of ethyl acetate in industry. The fact that the esterification process is used to produce 84% of the ethyl acetate in industry gives a good indication of the feasibility of the process. Esterification reaction generally refers to the formation of esters by the interaction of alcohols and carboxylic acids. (Chidi and Peter, 2016) The overall esterification reaction of acetic acid with ethanol in the presence of concentrated H 2 SO 4 can be written as: Basic chemical equation for this chemical reaction may be of the form: 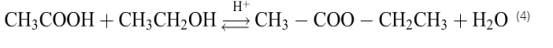 Ethanol and acetic acid together with crude ethyl acetate is fed into the reactor in the presence of concentrated sulphuric acid to produce ester and water. Then, the products are fed into the distillation column to separate water and ester (ethyl acetate). The bottom product of first distillation column is water and the overhead product is ethyl acetate. These parts are taken by dehydration and azeotropic distillation of ethyl acetate and water. The overhead product is passed to the decanter to separate the organic phase and aqueous phase. The upper layer known as organic phase while lower layer known as aqueous phase. Half of the organic phase is fed into the reactor and another potion of organic phased is passed into the second distillation column. The second column is a purify process where to give the pure ethyl acetate (bottom product). The top product is a mixture that consists of ethyl acetate, water and ethanol. This mixture is separated after cooling process and the light phase is fed back to second distillation column and the rest is transferred to the second decanter where its process is same as the first decanter to separate the organic and aqueous phase. It is a reversible process and does not proceed to any appreciable extent in the absence of catalysts or supercritical condition (Chidi and Peter, 2016). Hence, this reaction is catalysed by a strong acid usually sulphuric acid. Esterification is a very slow and highly reversible reaction. Therefore being a reversible reaction, equilibrium constant or the conversion of the reaction can generally be improved by the following methods (Beula &Sai, 2014): - using alcohol in large excess
- using a dehydrating agent,
- removal of water by physical means such as distillation
- addition of a catalyst
Equimolar amounts of acid and alcohol reach an equilibrium conversion of around 64% at 80 °C. To improve this temperature can be reduced however the equilibrium constant barely increases and the reaction rate is severely reduced increasing the required reactor volume (Santaella, 2015). Also, the use of excess amount of reactants will require the recovery and recycling of the excess amount used at some stage in the process. If it is in the case of excess alcohol being used then ternary separation of EtOH–EtAc–H 2 O is challenging because of the azeotropic behaviour of the mixture. When an excess of HAc is used, equipment has to be corrosion-resistant and it is necessary to accomplish HAc separation from water, which is also difficult and generally requires entrainer in distillation. (Santaella, 2015) To improve the rate of the reaction a higher temperature is required to increase the rate of the reaction. The following table shows that a higher conversion is reached quicker at a higher temperature: 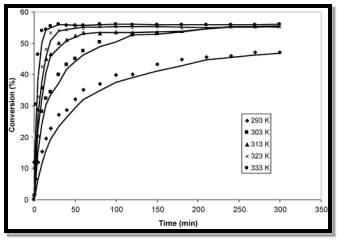 (Beula & Sai, 2014) Also, it is also important to note that this process uses fossil based raw materials which are limited in supply and of course contribute to global warming and greenhouse gases. For an esterification reaction of ethanol (82.2 mol %) and acetic acid (95.2 mol %) using reactive distillation and a thermodynamic model of (NRTL: HOC) gives an ethyl acetate product of (99.5% mol %) purity. This meets the design brief requirement of 98.8 mol% purity ethyl acetate (Beula &Sai, 2014). This shows that if the conditions are optimised then a highly concentrated product can be obtained, which is the objective. The process is also relatively efficient as the atom economy shows: 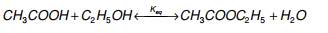 Atom economy=mass of atoms in desired productsmass of atoms in reactants 4453=83.2%(to 3sf) The atom economy of the reaction is 83%. This is relatively high meaning that there isn’t a lot of waste in converting from reactants to products in this process although other process routes are known to have a higher atom economy. Also the esterification process produces large amounts of water, unlike other processes which have toxic by-products, water is neither toxic nor difficult to handle. The water from the reaction can be disposed of in many ways, if there is a river, lake or sea then it can be pumped there or if there are nearby chemical plants which require water as part of their process then they can be recycled there. Another disadvantage is that concentrated sulphuric acid causes severe corrosion, many side reactions and causes a great deal of acid-containing wastewater. (Hua et al, 2015) Therefore corrosion resistant equipment is required. The catalyst is also homogenous so separation units are thus required for it to be removed from the product which thus means increased cost. Also, because this reaction does not tend to lead to formation of by-products which have boiling points close to that of ethyl acetate, recovery of substantially pure ethyl acetate from the esterification product mixture is usually not complicated by the presence of by-products (Colley et al, 2004) Evaluation of process This process is the most widely used in industry meaning that it must be feasible to produce on a large scale which is what is required from the design brief(200ktpa). Although there are disadvantages such as the use of sulphuric acid as a catalyst as it is corrosive and homogenous and the large amount of water by product which will have to disposed of in some manner. It also has many advantages; the feedstock required (acetic acid and ethanol) are widely available and relatively cheap. Also, if the required conditions are achieved then ethyl acetate of sufficient concentration can be produced (98.8%), again as per the design brief requirements. Therefore from a technical standpoint the esterification method is indeed feasible to produce ethyl acetate and other aspects of the process will be researched further to ascertain if it is feasible in other aspects. 1.2 Tischenko ReactionThe Tischenko reaction produces ethyl acetate by direct conversion of ethanol via acetaldehyde using a metal catalyst. Tishchenko’s reaction is a reaction that needs the presence of an alkoxide base while two equivalents of acetaldehyde is combining. This way is becoming commercial method of producing ethyl acetate in Europe since acetaldehyde become important intermediate on the basis of acetylene. Due to Tishchenko, the obtainable yield of ethyl acetate by adding aluminium ethoxide to acetaldehyde at -20°C is 61%. The Tischenko esterification is an efficient method for the production of esters from the corresponding aldehydes. The equation for the reaction is as follows:  For the process of Tishchenko’s reaction, acetaldehydes will be introduced to the catalyst solution continuously. The catalyst is first need to be prepared by dissolving granular Aluminium in an ethanol-ethyl acetate mixture in the presence of aluminium chloride and small amount of zinc chloride. In reactor, while acetaldehyde contact with the prepared catalyst, the ratio of the reaction partner must be adjust in order to obtain 98% transformation of acetaldehyde in one passage. A further 1.5% transformation is achieved in stirring vessels. Consecutively to make sure the reaction temperature is kept to 0°C, brine with normally -20°C will be used as the cooler. This reaction takes approximately 1 hour to completely mix before being transfer to residue separation. Next, separator is needed to remove the residue that contain in the mixture. The distillable products are removed by evaporation. For the economic issue, the residue is treated with water to regain ethanol. For the residual slurry, it can either be given to biological degradation plant or it can be burned together with other organic waste products. Subsequently, the distillable products need to be purifying in so that it can achieve commercial purity which is approximately 99.8%. Therefore, distillation column is used. For the 1st series of distillation column, light end are separated and this steam is further distilled to take non-converted acetaldehyde, which is returned to reactor. Then ethanol that contains ethyl acetate is separated for reuse in catalyst preparation. The bottom of 1st column give the high quality or grade of ethyl acetate that only will obtain at the head of the next column due to the need of separation of high boiling condensation products in mixture with ethyl acetate which will be remove at the bottom. In addition, further small column is needed to recover another part of pure ethyl acetate to isolate acetaldehyde diethyl acetal. Hence, after purification is done the recover product can used as an important intermediate or hydrolysed in an acid medium to give reusable acetaldehyde and ethanol. The Tischenko reaction is one of the main industrial processes for the manufacture of ethyl acetate and requires only acetaldehyde. (Hassan et al, 2012). The dimerization of acetaldehyde is carried out in the presence of aluminium or sodium alkoxide or of solid bases like alkaline oxides (Santacesaria, 2012) Acetaldehyde is the only feedstock required and it can be produced on a large scale industrially and is obtained by the large scale oxidation of ethylene. However, aldehyde is not available outside of a petrochemical industrial area. (Inui et al, 2002) This means there are huge restrictions in terms of a location for the plant as there are not many petrochemical industrial areas in many countries and it may not always be possible to build an ethyl acetate plant close to a petrochemical facility. Also, acetaldehyde is difficult to handle because of its toxicity (Gaspar et al, 2009). Acetaldehyde is an irritant of the skin, eyes, mucous membranes, and throat and is a stage 3 carcinogen (Leiber et al, 1988). This makes it very difficult to handle and great precaution must be taken to ensure that no one on the plant is exposed to acetaldehyde at any point without protection. The use of PPE such as safety helmets, gloves, eye protection, and safety footwear is essential for anyone that could possibly be exposed to acetaldehyde. Great care also must be taken in the transport of acetaldehyde to the plant and the toxicity of acetaldehyde means that corrosion resistant equipment will be required as to store the acetaldehyde as it is being transported as it is also highly corrosive. The tishchenko reaction has a high atom economy: Atom economy The atom economy = mass of atoms in desired productmass of atoms in reactants=4953=95.6% The use of aluminium or sodium alkoxide as a catalyst also improves the rate of reaction. However as with esterification there is again the need to separate the catalyst from the product and there is generation of a large amount of spent catalyst and waste water due to using homogeneous catalysts.(Yamamoto et al,2008) This would also mean more unit operations would be required which thus in turn makes the plant more expensive to install and maintain. Evaluation of Process The Tischenko process is advantageous as it requires only one feedstock and does not produce any toxic by-products. However the feedstock (acetaldehyde) is only available in areas where there is a petrochemical industry nearby. This restriction means that the available options for a location to build the plant are very limited and because these are less common in Europe due to Europe not being a large petrochemical producer and the fact that the ethyl acetate produced is required specifically for the European market means that that the only viable options would be to import from outside Europe or build the plant outside of Europe both of which would be very costly. For this reason, the tischenko process is not feasible for the requirements stated in the design brief and will not be looked into further. 1.3 DehydrogenationEthyl acetate can be produced by the dehydrogenative dimerization of ethanol. The synthesis route of ethyl acetate from ethanol dehydrogenation is an attractive process, because the process is relatively simple, non-corrosive and less toxic, and needs only one feedstock of ethanol. (Freitas et al,2014). There are two ways this can be done, oxidative and non-oxidative: - Oxidative- The oxidative dehydrogenative dimerization of ethanol is as follows:
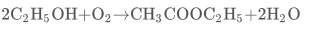 This process has several advantages as the raw products (ethanol and oxygen) are readily available and cheap and there are no harmful by-products formed as the only by-product formed is water. This process however has a risk of explosion due to a mixture of gaseous ethanol and oxygen in the reactor (Inui et al,2002) . This makes it unfeasible for the large scale production of ethyl acetate as it is highly hazardous and will not be considered any further. - Non-oxidative- The non-oxidative dehydrogenative dimerization of ethanol is as follows :
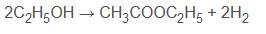 This is the main route for the dehydrogenative dimerization of ethanol used in industry. This process occurs by the removal of hydrogen in ethanol to produce an acetaldehyde, the aldehyde is then added to ethanol to form a hemiacetal which in turn is dehydrogenated to generate ethyl acetate (Inui et al,2002). The only feedstock for this process is ethanol. Ethanol is widely available; being the main orgonic feedstock in many processes, and is non-corrosive and non-toxic unlike the feedstock from other process routes such as the Tischenko reaction. Ethanol is also relatively cheap and the ethanol used in the process can be bio based ethanol which is more environmentally friendly as opposed to fossil based which requires the use of already dwindling fossil fuel reserves. This reaction is usually catalysed by a copper/copper based catalyst as Copper-based catalysts have been successfully employed for the selective conversion of ethanol to ethyl acetate. These catalysts are very efficient. The best results have been found by using a commercial copper/copper chromite catalyst, supported on alumina and containing barium chromite as promoter, operating at 220–240 °C, 20 bars and 98 g h mol −1 of ethanol contact time. In these conditions, a conversion of 65% with a selectivity to ethyl acetate of 98–99% has been obtained (Santacesaria et al,2011). This meets the design brief requirement of >98.8 mol% ethyl acetate. The catalyst for this reaction is also heterogenous, unlike other process routes such as the Tischenko route and esterification, so the catalyst can be easily separated from the required product. The reaction can also be made highly efficient by the recycling of unreacted reagents which increases the yield of ethyl acetate product. The atom economy of this reaction is also very high: mass of atoms in desired productmass of atoms in reactants=9094=95.7%(to 3sf) There is also a hydrogen gas by-product which is produced in this reaction. Hydrogen gas however, is useful and has many applications such as hydrogen fuel or in the Haber process to produce ammonia so it has monetary value and could be sold on, which is economically efficient. This however, requires the storage of the gas which is highly flammable and can cause explosions. The hydrogen gas produced can also be used as a carrier gas in the process (Santacesaria et al,2011). This process requires only one feedstock, ethanol which is readily available. The fact that bioethanol, a green environmentally friendly feedstock can be used also makes it highly attractive. This alternative makes this process highly sustainable as opposed to other process routes which require fossil based feedstock. It is also an efficient process which makes use of by-products. For these reasons the dehydrogenation route for producing ethyl acetate is feasible and other aspects of the process will be researched further to ascertain if it is feasible in other aspects. The equation for the direct addition of ethylene to acetic acid is as follows: 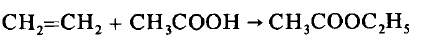 This process produces no by-products due to high atom efficiency when heterogeneous catalysts with high selectivity and activity are used, which makes it very attractive as this excludes the need to separate by products from the product and also saves economically on the unit operations required to facilitate this. As shown by the atom economy this reaction has high atom efficiency: mass of atoms in desired productmass of atoms in reactants=8888=100%(to 3sf) In a study by Yamamoto et al (2008) when a H 4 SiW 12 O 40 /SiO 2 was used a 99 mol% ethyl acetate product was produced. This meets the design brief requirement of >98.8 mol% ethyl acetate. The catalyst used for this reaction is also heterogenous so it is not required for the catalyst and product to be separated. The bulk of the reaction between ethylene and acetic acid occurs in the interlamellar spaces between the clay layers. This has been shown to be the case because collapsed catalysts with no interlamellar spaces have drastically reduced activity. In the conditions used in this work, the catalyst is stirred in liquid acetic acid containing dissolved ethylene which is in equilibrium with the gas and vapour phase. The acetic acid is believed to largely replace the bulk of intercalated water present in the fresh catalyst before the reaction commences. The ethylene molecules are then free to enter and leave the highly polarized interlamellar regions where reaction occurs. The feedstock required for this reaction is ethylene and acetic acid. Acetic acid is widely available and is relatively cheap. Ethylene however is difficult to source. It is usually produced in steam-cracking units from a range of petroleum-based feedstocks so is usually available in places where fracking takes place. In industry it is usually transported via pipeline to the plant. This places restrictions on a location for the plant as the location would have to have an already established ethylene transport network to connect to. In the UK there is one such network which is utilised by INEOS, Hull where ethylene is pumped via a pipeline from Grimsby. Also regarding the transport of ethylene to the plant it is important to consider that ethylene is extremely flammable and forms explosive mixtures with air and oxidising agents. It can also explode if heated under pressure. This process is highly efficient and produces no by products which are highly beneficial as there is no need to separate by-products from the product, a costly procedure which requires extra unit operations. The catalyst used is also heterogeneous so is easy to separate from the product. However one of the feedstock, ethylene is difficult to source and the choice of location is limited by the need for a local source of ethylene which is not available in many places. Despite this there are still many locations where it is still possible to source ethylene and where this is possible the process is still attractive. For these reasons, Direct addition is a feasible process to produce ethyl acetate on a large scale and other aspects of the process will be researched further to ascertain if it is feasible in other aspects. Choosing a location for a chemical plant is a very important decision and there are various factors which need to be considered when deciding on a location, such as availability of raw products, proximity to competitors, proximity to clients, labour needs and also economic factors such as local tax rates and local cost of labour. Three different possible locations for an ethyl acetate plant will be discussed to determine if they are feasible. Each of these will use a different process route. I have decided on these locations due to their suitability to the process route used for each one. Location 1- Brazil Process Route- Dehydrogenation The feedstock required for the production of ethyl acetate by dehydrogenation is ethanol. Ethanol is produced in large amounts in Brazil from feedstock such as sugar cane which grows abundantly in Brazil. Brazil is the second largest producer of bioethanol worldwide. In 2008 its production reached 27.6 billion ( Waltar et al,2011) The Brazilian huge production of ethanol offers a good opportunity for the development of new processes or products using this alcohol as a raw material. (Gaspar et al) . The availability of cheap, abundant ethanol is beneficial from an economic perspective as the cost of feedstock is a large proportion of the costs of the plant. Brazil also has the necessary infrastructure and skills required to install and maintain a plant. A Brazilian solvents producer, Cloroetil already has a 50ktpa ethyl acetate plant in Brazil which also uses the dehydrogenation process route. However, the design brief states that the 200 kilo tonnes per annum of ethyl acetate are required for the European market. If it were too produced in Brazil then it would need to be imported from Brazil to Europe. This would incur the costs of shipping and various import taxes when shipping to the European Union. This would highly increase the costs and severely reduce the ability of the plant to make profits. For this reason it is not feasible for the plant to be located outside of the market it is intended for(Europe) so it is not feasible for the plant to be located in Brazil and other locations outside of Europe will not be considered from this point onwards on as well. Location 2-UK Process Route-Direct Addition The UK is an ideal location for the plant as it is located in Europe and the 200 kilo tonnes per year of ethyl acetate produced is required for the European Market. The direct addition process route requires ethylene and acetic acid as feedstock. Acetic acid is widely available and cheap in the UK (2.72£/KG)(ICIS,2017). Ethylene however is only available close to Fracking industries. In the UK ethylene production is mainly in Grangemouth, Mossmorran and Wilton. This means there are several available points from where ethylene can be sourced from and then pumped to the location of the plant. The UK is also a highly developed nation with minimal corruption so it would be relatively easy to install and maintain the plant. The UK also has a highly educated workforce so it would be possible to recruit suitable employees required to operate the plant. Bio based ethanol can be used as a feedstock in the esterification process. This is highly sustainable as it reduces the need for fossil fuel consumption. However bio ethanol is made from feedstock such as sugar cane, wheat, corn etc. which are valuable food sources. This ties in to the food vs fuel debate and whether it is morally acceptable to use scarce food resources for fuel when a portion of the world’s population is malnourished. Ethylene is mainly obtained by fracking. Fracking is a highly controversial process where drilling takes place 100’s of metres under the ground using chemicals. Fracking is a sensitive ethical issue as it said to be damaging to the local environment and ecosystem, is a cause of water contamination and is unhealthy for workers. AcetaldehydeAcetaldehyde is a carcinogen meaning that constant exposure to acetaldehyde could be a safety concern for employees. In the future, the company could also be held legally responsible and subject to financial penalties if employees do indeed develop cancer due to the exposure to acetaldehyde. 2 Enviromental and Safety FactorsIn the design and operation of a chemical plant, safety is of paramount importance. The fact that the plant will handle large amounts of hazardous chemicals means that there would be a serious risk to employees, the plant and to local residents and the environment if an accident were to occur. This means that the process safety management techniques employed throughout the process from start to finish should be effective and in line with local and international health and safety legislation. A HAZOP study is a highly disciplined procedure meant to identify how a process may deviate from its design intent. HAZOP studies rely on identifying and subsequently analysing possible scenarios that can cause accidents with different degrees of severity. (Dunjo et al, 2010). This prevention method has proved to be very effective as it is a systematic way of continuously looking for possible hazards and identifying and dealing with them before they progress to an accident. To implement a HAZOP study specifically for an ethyl acetate plant there would be certain things to consider such as the specific hazards at different stages of the process depending on the process route. For example, for a plant which produces ethyl acetate by the esterification process using sulphuric acid as a catalyst would have to consider the risk associated with the highly corrosive liquid and the dangers in the event of loss of containment and implement this in its HAZOP study. Hazard and Operability study (HAZOP) study is the most widely used and preferred approach in the chemical plant to accomplish hazard assignment qualitatively and quantitatively. This study is typically performed by a team of experts having specialized knowledge expertise in design, operation, and maintenance of the plant. The HAZOP team members examine the process P & ID systematically, identify every deviation from design intent in the plant using a “guideword ” approach; determine all possible abnormal causes and the adverse consequences of the deviation. – The present dissertation work has been carried out in Ethyl Acetate plant at Bharuch, where Ethyl Acetate is the main product. The HAZOP study is carried out from Ethanol Unloading to Reactor in Ethyl Acetate plant. Ethyl Acetate is a hazardous material in terms of toxicity, Flammable liquid and vapours, is harmful if swallowed or inhaled directly affect central nervous system, causes irritation to skin, eyes and respiratory tract. If it contacts with nitrates, strong oxidizers, strong alkalis, or strong acids may cause fire and explosions. The main raw materials such as Ethanol, Acetic Acid and catalyst PTSA (Paratoluene Sulfonic Acid) are used in the manufacturing process of Ethyl Acetate. The operating units involved for the production of Ethyl Acetate in the plant are Reactor, Reactive Distillation Column, Decanter, wash Column, final/ product column, Ethyl Acetate storage tank. Deviation in the process parameter (such as temperature, pressure, level, flow etc.) may lead to serious accident (over pressurization of tank, high pressure in the tank, occurrence of flammable condition etc.); therefore qualitative and manual HAZOP study has been carried out for a better performance of the process system as well as safety. The present HAZOP study shows the possible abnormal causes and consequence of hazard in the plant. A brief result and discussion is also performed based on the present HAZOP study and also preventive corrective measures – and recommendation have been made to improve the safety performance of the process and production line as well. As always for any plant implemented there is a competent body, which for the UK is the Health and Safety Executive (HSE) but for the UAE the government is the competent body and this can cause a lot of conflict and often introduce a lot of bribery and work that is not above board. The HSE have created a set of regulations, which reiterate safe working guidelines through a set of rules to avoid major disasters. Control of Major Accident Hazards (COMAH) regulations are used for businesses that present risk from carrying dangerous substances and an analysis of whether our future plant will need them implementing due to the dangerous substances carried. In accordance with the HSE any new entrant at risk of carry dangerous substances must check at what stage must they adhere to the regulations (Health and Safety Executive, 2016). COMAH is the Control of Major Accident Hazards (COMAH) Regulations ensuring that businesses “Take all necessary measures to prevent major accidents involving dangerous substances” and” Limit the consequences to people and the environment of any major accidents which do occur”(HSE,2017). They are regulations put in place in industry to ensure that safety incidents do not occur and if they do occur that they are managed as well as possible to ensure minimum damage to the plant, the environment and to staff. It also limits the ramifications of any large scale accidents which do manifest, through enforcing regulations on hazardous substances to protect people and the environment. The COMAH authority aims to safely deliver a regime that ensures that the people responsible for producing these risks, due to their line of work, do so by meeting their responsibilities and managing them efficiently and safely. Emergency preparations need to be in place, should anything go wrong and to reassure anyone who work or lives nearby somewhere that deals with hazardous substances that their safety is paramount to them. The COMAH authority also helps the industry to handle their activities in a safe manner and to understand that failures in doing so will affect the entire sector. The COMAH authority are fully committed to providing translucence in their decision making and operational functioning, so all policies and methodologies are clear and precise for everyone who are affected by them. It states that for liquefied flammable gas if more than 50 tonnes are being held then this constitutes a lower tier establishment, while if more than 200 tonnes are held this is would be a higher tiered establishments (Health and Safety Executive, 2013). In the circumstances of this study the basis given in the design brief was a production of 200 kilo tonnes per year. Regarding the classification of a higher tier COMAH site a boundary of 3.8-5.7 thousand tonnes per week or 549-824 tonnes per day (approximately) is applied; meaning if exceeding this amount would constitute a higher tier COMAH site. The amount of flammable ethanol held on our proposed site is 3-4 times that of the minimum boundary for a higher tier COMAH establishment, insinuating a higher tier classification. Implications from this would be Chemical Hazard Information and Packing for supply (CHIP) to be considered and followed due to the large amount of ethanol being held and transported. In addition a COMAH safety report must be submitted 3-6 months prior to operation start up. 2.3 Process Safety CultureAll the necessary process safety procedures and legislation could be in place but they would be ineffective if they are not adhered to and implemented at all levels of the plant at all times. The need for safety to be of paramount importance at all levels of a company is called process safety culture. Process safety culture is loosely used to describe the corporate atmosphere or culture in which safety is understood to be, and is accepted as, the number one priority (Cooper, 2000). If a process safety culture is adopted and instilled into the employees and management of the ethyl acetate plant then this would reduce the risk of a safety incident occurring. Esterification There are some health and safety issues that rise for the esterification process. Main issue is the use of the acid catalyst sulphuric acid due to its properties being corrosive. Sulphuric acid can cause serious burns if contact is made and could result in blinding if contact is made with the pupils, sulphuric acid also being a carcinogen and if breathed in damages could occur in the lungs. for reasons such as these extra safety precautions would have be taken to ignore any harmful incidents occurring such as to prevent contact with skin a lab coat and protective gloves may be worn with goggles to prevent any eye contact and also would be good if any respiratory protection can be worn to prevent inhalation. Acetic acid also has similar properties as it is also corrosive, hence similar precautions should be taken. In the case of environment, if sulphuric acid is released via spillage levels in the atmosphere could rise and could affect the crops and life of aquatic animals if contact is made decreasing the PH of water. Dehydrogenation In the production of ethyl acetate via ethanol dehydrogenation there are some safety concerns that rise, such as the huge amount of pure hydrogen that is produced as a by-product. To insure that these health and safety issues do not turn into incidents precautions must be put in order, due to hydrogen being flammable and have an explosive nature it would be required to have installed ventilators in order to prevent such an incident of a fire. also due to acetaldehyde being an intermediate product, precautions still need to be put in place as acetaldehyde is seen as highly toxic and is known to be a class 1 carcinogen, hence precautions such as minimum exposure of an outburst to be installed is preferred. Many of the potential safety risks in the esterification process stem from the reactants and catalyst involved as oppose to the process itself. Both Ethanol and Acetic Acid are flammable meaning that they should both be stored in appropriate containers preferably opaque and away from direct sunlight to minimise the potential fire risk posed. Acetic acid is also corrosive and capable of causing skin burns and eye damage therefore it is critical that those who come into contact with it are wearing appropriate PPE most importantly goggles to prevent eye damage and gloves and overalls to prevent the acid coming into contact with the skin. When considering the environmental impact of the three process routes analysed in this report it is important to consider if changes can be made to the process to improve its environmental feasibility. One possible way of doing this is to utilise bio based feedstock instead of fossil fuel based feedstock. By using bioethanol-based alternative processes that utilize bioethanol or bioethanol-derived chemicals such as acetic acid and ethylene as the initial raw materials rather than fossil based chemicals (Thuy et al, 2011) then the c02 emissions of the process can be reduced and this also prevents the use of limited fossil fuel supplies. The same study by (Thuy et al,2012) shows that the bio based esterification was the most cost effective and environmentally friendly when compared to fossil based esterification, bio based direct addition and bio based dehydrogenation. At this stage of the report there are still three feasible possible routes to produce the required 200kt pa of ethyl acetate. Even though there are various factors which need to be achieved to make the process feasible the bottom line is that to make the building and operating of the plant worthwhile the plant must make profit. The three feasible process routes will now be analysed economically taking into consideration factors such as costs, tax and revenues. 3.1 Market ScopeThe main use of ethyl acetate is in the manufacture of a variety of coating preparations, such as, epoxies, acrylics, urethanes, and vinyl’s. It is also used as a solvent in inks, adhesives, and a process solvent. The markets for construction, automobiles and solvents are all expected to increase worldwide which should in turn increase global demand for ethyl acetate. The volume of worldwide construction output is expected to grow by more than 70% by 2025. It is also used as a solvent in inks, adhesives, and a process solvent. These inks are mostly used in printing and food and drink packaging. As the world population explodes and more of the world’s population is lifted out of poverty to be able to afford a varied diet there will be a huge increase in demand in the food and drink industry. The demand from publications for the use of inks such as newspapers/magazines is expected to decrease as the demand for printed newspapers/magazines decreases as consumers are switching to digital publications. Global capacity for ethyl acetate exceeds demand. This means that there is an oversupply in the market forcing plants to work at less than the output they were built for. Inside Battery Limit Cost (ISBL)The inside battery limit cost is the cost of procuring and installing the process plant (Stevenson, 2017). This includes the cost of unit operations such as columns, vessels, reactors, heat exchangers, pumps. Also the cost of construction such as roads, foundations, the cost of building. However the cost of construction will not be included as an already established brownfield site as stated in the design brief. ISBL Cost of Dehydrogenation PlantA Bridgewater method will be used where Q >60,000: C=4320NQS0.675 (Sinnot et al,2008) Where N=Number of unit operations Q=Production Rate S= rate of conversion N=5(For dehydrogenation process) C=4320(5) (2000001)0.675 C= $81,780,356 C=$81,800,000(to 3 sf) Applying temporal conversion: Cost in Year A= Cost in yearB Cost index in Year ACost index in Year B) 566.6532.9) Cost in Year A=$869,524,054 Cost in Year A=$870,000,000(to 3sf) Convert to Pound Sterling (£) On a November 2017 basis: $1 USD=0.75 GBP (Bloomberg,2017) Therefore ISBL Cost in GBP (£) = ($869,524,054) (0.75) =£65214040.91 =£652,000,000 (to 3sf) ISBL Cost of Direct addition plant A Bridgewater method will be used again where Q >60,000: (Sinnot et al ,2008) N=8 (For Direct addition process) C=4320820000010.675 C=$130,848,570 C=$130,000,000(to 3 sf) Cost in Year A=$13912328.72 Cost in Year A=$139,000,000(to 3sf) Therefore ISBL Cost in GBP (£) = ($13912328) (0.75) =£10434246.54 =£104,000,000(to 3sf) ISBL Cost of Esterification ProcessN=9(For Esterification process) C=4320(6) (2000001)0.675 =4320620000010.675 C=$98136427.53 98136427.53 Cost in Year A=$104342465.5 Cost in Year A=$104,000,000(to 3sf) Therefore ISBL Cost in GBP (£) = ($104342465.5) (0.75) =£78,200,000 (to 3sf) 3.2 Outside Battery Limit CostsThe OSBL costs are the costs of infrastructure required by the new plant, either upgrading the supporting infrastructure or installation on a new site. The OSBL includes(Sinnot et al,2008): - Water supply, waste water treatment
- Labs, offices ,canteens
- Site security, fencing
- Workshop, maintenance facilities
The OSBL costs for each process are as follows: OSBL Cost for Direct addition ProcessOSBL cost=40% of ISBL Cost =0.4(10434246.54) =£4,170,000(to 3sf) OSBL Cost for Dehydrogenation Process=0.4(65214040.91) =£26,100,000(to 3sf) OSBL Cost for Esterification Process=0.4(78256849) =£31302739.6 =£31,300,000(to 3sf) The engineering costs are the costs incurred as a contractor (Stevenson,2017). They include the - Cost of design
- Cost of buying plant equipment
- Supervision of construction and service installation
- Consultancy Fees
For this project it will be assumed to be 20% (Sinnot et al, 2008). Engineering Cost of Direct addition plantEngineering Cost=0.2[ISBL Cost+OSBL Cost] Engineering Cost=0.2[ 10434246.54+4173698] Engineering Cost=£2921589 Engineering Cost=£2,920,000(to 3sf)  Engineering Cost of Dehydrogenation PlantEngineering Cost=0.2[ 65214040.91+26109616] Engineering Cost=£18264731 Engineering Cost=£ 18,200,000(to 3sf) Engineering Cost of Esterification PlantEngineering Cost=0.2[ 78256849+31302739.6] Engineering Cost=£21911917 Engineering Cost=£21,900,000 (to 3sf) Contingency Costs are a reservoir of cash which should be used in the event of unforeseen circumstances. They include: - Labour Dispute
- Price Changes
- Forex fluctuations
- Changes in Foreign Policy
- Political unrest
Contingency Cost of Direct Addition PlantContingency Cost=0.1(ISBL Cost+OSBL Cost) Contingency Cost=0.1(10434246.54+4173698) Contingency Cost=£1462294.5 Contingency Cost=£1,460,000(to 3sf) Contingency Cost of Dehydrogenation PlantContingency Cost=0.1(65214040.91+26109616) Contingency Cost=£9132365.5 Contingency Cost=£9,130,000(to 3sf) Contingency Cost of Esterification PlantContingency Cost=0.1(78256849+31302739.6) Contingency Cost=£10955958.5 Contingency Cost=£11,000,000(to 3sf) The fixed capital investments are the overall cost of setting up the plant. This includes the ISBL costs, OSBL cost, Engineering Costs and Contingency cost. Essentially it is the investment required. Fixed Capital Investment for Direct addition plantFixed Capital Investment=ISBL +OSBL +Engineering Costs+Contingency Costs Fixed Capital Investment=10434246.54+4173698+ 1462294.5+2921589 Fixed Capital Investment=£18991828 Fixed Capital Investment=£19,000,000(to 3 sf) Fixed Capital Investment for Dehydrogenation PlantFixed Capital Investment= 65214040.91+26109616+ 9132365.5+ 18264731 Fixed Capital Investment=£118702753 Fixed Capital Investment=£119,000,000(to 3sf) Fixed Capital Investment for Esterification PlantFixed Capital Investment=78256849+31302739.6+ 10955958.5+21911917 Fixed Capital Investment=£142427464 Fixed Capital Investment=£142,000,000(to 3sf) Using the equation: Mass of Required Feedstock, X=Mass of Ethyl Acetate requiredMr of Ethyl AcetateMr of X Sales of Ethyl acetate 200000 tonnes=203,209,382kg Mr of Ethyl Acetate=88 gmol Market Price of ethyl acetate: £1.05/kg Therefore Overall Sales of ethyl acetate are: £213369851 Improvements in the production of ethyl acetate have dealt mainly with finding alternative reaction processes to produce ethyl acetate such as by dehydrogenation of ethanol or liquid phase oxidation of n-butane. Other improvements have involved different catalysts or the use of different unit operations such as the use of membrane separation techniques or advanced distillation techniques. However, little attention has been paid with regard to less costly non-chemical methods to accelerate or improve the production of ethyl acetate. Consequently, there is a need for alternative methods for accelerating production of ethyl acetate. The direct addition process is a very sustainable, highly efficient process and produces no by-products. However the feedstock required (ethylene) is not easily available however this can be managed as in the UK for instance there are various places where it is produced where it can be shipped from. The esterification method is also a highly efficient process and is the most widely used in industry indicating that it is highly feasible. However the catalyst used is hard to manage and also the catalyst is homogenous and must be removed which is difficult but is manageable. Other advantages of using esterification process may include the chemicals used and by-products released. These both are nontoxic towards the environment, as compared to ester synthesis via acyl chlorides. The reaction can also be carried out with a simple laboratory setup without need for stringent safety precautions or access to less common synthesis reagents. As a result, the reaction tends towards the more stable species (the ester), which is helpful when the reagents contain multiple reactive sites. This design appears to be highly profitable, but further analysis should be performed to discover how profitable the plant is when certain assumptions are not made. Before this project is fully invested in, there are multiple experiments and investigations into assumptions that need to be performed to determine the effect of these assumptions on profitability. To begin with, one of the main assumptions made in this project is that there is no pressure drop in the reactor. This is highly unlikely to be true, however. Even when tested in the laboratory reactor there was a small pressure drop, which while assumed to be negligible, it is possible that with design scale up the pressure drop is no longer negligible. Experiments on pressure drop across the reactor must be done before the final decision to invest in this project is made. Another assumption made in this design that must be further investigated is that the catalyst is fully active until the moment that it needs to be changed. Catalyst deactivation over time was not taken into account in this analysis but is likely to be occurring and may affect the rates of reaction. It is possible that deactivation over time will cause the rates of reaction to slow as it approaches time to replace the catalyst. This slowing of the reaction rates could potentially not only cause less product to be made, but also cause the composition into the distillation columns to drastically differ leading to failure of one of the columns. The final main assumption made in this initial design was that the heat exchanger system was not interconnected. This is something that could likely increase the profitability of the plant since the use of one fluid to heat or cool another fluid would decrease the use of a utility to perform the same job. By designing a system to handle this, it is likely that the profitability of the plant will increase. Overall I would recommend the use of Esterification or Direct Addition as process routes the mass production of ethyl acetate for the reasons given above. After analysing the various process routes and understanding the various advantages and disadvantages of each process in the report above there are various recommendations to be made. Firstly the Tischenko reaction was found to be highly unfeasible as the feedstock required for the process is only available from petrochemical industries so must be located close to petrochemical sites. This is highly unsuitable as they are very difficult to locate and it is not guaranteed that the feedstock will always be available. An assessment of different intensified processes for the production of ethyl acetate through Fisher esterification has been carried out. The analysis allowed selecting reliable thermodynamic models to describe the multicomponent phase equilibria involved in the process, as well as a homogeneous kinetic model that correctly describes the reaction rate. The thermodynamic and kinetic models selected together with commercial specifications of raw materials and products were implemented for the simulation of different intensified processes proposed in the literature. Nielsen, M., Junge, H., Kammer, A. and Beller, M., 2012. Towards a Green Process for Bulk‐Scale Synthesis of Ethyl Acetate: Efficient Acceptorless Dehydrogenation of Ethanol. Angewandte Chemie International Edition , 51 (23), pp.5711-5713. Inui, K., Kurabayashi, T. and Sato, S., 2002. Direct synthesis of ethyl acetate from ethanol carried out under pressure. Journal of Catalysis , 212 (2), pp.207-215. Inui, K., Kurabayashi, T. and Sato, S., 2002. Direct synthesis of ethyl acetate from ethanol over Cu-Zn-Zr-Al-O catalyst. Applied Catalysis A: General , 237 (1), pp.53-61. McMurry, J. 2000. Organic Chemistry, 5th Edition, Brooks/Cole, p. 855 Peter, Chidi, C.P., 2016. Kinetics and Mechanism of Ethyl Acetate Production Using Eco-Benign Solid Catalyst. Journal of Physical Chemistry & Biophysics, 6(3), 18-24. Hua, Y.H., 2015. Synthesis of ethyl acetate catalyzed by (NH4)6[MnMo9O32].8H2O with Waugh structure. Journal of Chemical and Pharmaceutical Research , 7(10), 445-448. Hassan, A., Bagherzadeh, E., Anthony, R.G., Borsinger, G. and Hassan, A., HRD Corporation, 2011. Method of producing ethyl acetate . U.S. Patent 8,080,684. Freitas, I.C., Damyanova, S., Oliveira, D.C., Marques, C.M.P. and Bueno, J.M.C., 2014. Effect of Cu content on the surface and catalytic properties of Cu/ZrO 2 catalyst for ethanol dehydrogenation. Journal of Molecular Catalysis A: Chemical , 381 , pp.26-37. Colley, S.W., Fawcett, C.R., Rathmell, C. and Tuck, M.W.M., Davy Process Technology Limited, 2004. Process for the preparation of ethyl acetate . U.S. Patent 6,809,217. Gaspar, A.B., Esteves, A.M.L., Mendes, F.M.T., Barbosa, F.G. and Appel, L.G., 2009. Chemicals from ethanol—The ethyl acetate one-pot synthesis. Applied Catalysis A: General , 363 (1), pp.109-114. Walter, A., Dolzan, P., Quilodrán, O., de Oliveira, J.G., da Silva, C., Piacente, F. and Segerstedt, A., 2011. Sustainability assessment of bio-ethanol production in Brazil considering land use change, GHG emissions and socio-economic aspects. Energy Policy , 39 (10), pp.5703-5716. Dunjó, J., Fthenakis, V., Vílchez, J.A. and Arnaldos, J., 2010. Hazard and operability (HAZOP) analysis. A literature review. Journal of hazardous materials , 173 (1), pp.19-32. Cooper Ph. D, M.D., 2000. Towards a model of safety culture. Safety science , 36 (2), pp.111-136. Thuy, H., Nguyen, T., Kikuchi, Y., Sugiyama, H., Noda, M. and Hirao, M., 2011. Techno‐economic and environmental assessment of bioethanol‐based chemical process: A case study on ethyl acetate. Environmental Progress & Sustainable Energy , 30 (4), pp.675-684. Santacesaria, E., Carotenuto, G., Tesser, R. and Di Serio, M., 2012. Ethanol dehydrogenation to ethyl acetate by using copper and copper chromite catalysts. Chemical Engineering Journal , 179 , pp.209-220. Leiber, C.S., 1988. Metabolic effects of acetaldehyde.. Biochem Soc Trans., 16(3), 241-7. Yamamoto, Y., Hatanaka, S., Tsuji, K., Tsuneyama, K., Ohnishi, R., Imai, H., Kamiya, Y. and Okuhara, T., 2008. Direct addition of acetic acid to ethylene to form ethyl acetate in the presence of H 4 SiW 12 O 40/SiO 2. Applied Catalysis A: General , 344 (1), pp.55-60. Beula,Sai, C,P.S, 2017. Kinetics of Esterification of Acetic Acid and Ethanol with a Homogeneous Acid Catalyst. Indian Chemical Engineer, 57(2), 177-96. ICIS. 2017. Indicative Chemical Prices A-Z. [ONLINE] Available at: https://www.icis.com/chemicals/channel-info-chemicals-a-z/ . [Accessed 30 November 2017]. Dutia, Pankaj (August 10, 2004). “Ethyl Acetate: A Techno-Commercial Profile” .Chemical Weekly: 184. Hse.gov.uk. (2017). HSE: Control of Major Accident Hazards (COMAH). [online] Available at: http://www.hse.gov.uk/comah/ [Accessed 30 Nov. 2017]. Bloomberg. 2017. GBP/USD. [ONLINE] Available at: https://www.bloomberg.com/quote/GBPUSD:CUR. [Accessed 28 November 2017]. Cite This WorkTo export a reference to this article please select a referencing stye below: Related Services  - Dissertation Writing Service
 - Dissertation Proposal Writing Service
 - Topics and Titles Writing Service
Related Content Content relating to: "Chemistry" Chemistry is a science involving the study of the elements and matter at the atomic and molecular level including their composition, structure, properties, behaviour, and how they react or combine. Related Articles  Application of Heisenberg’s S Matrix Programme to Rainbows Application of Heisenberg’s S Matrix Programme to rainbows, supernumerary rainbows and interference effects in the angular scattering of chemical reactions.... Analysis of Denitrification Methods in Various Environments and Its Effectiveness This paper aims to identify and propose various denitrifying methods that can be employed, with a focus on the biological methods, and the environments that they can be applied to, to improve the efficiency or Nr removal.... Fipronil Contamination Prevention FOODBORNE OUTBREAK: FIPRONIL Fipronil (5 – amino -1- [ 2, 6 – dichloro- 4 (trifluoromethyl) phenyl] 4 [(trifluoromethyl) sulfinyl] 1H- pyrazole – 3 – carbonitrile) is considered a... DMCA / Removal RequestIf you are the original writer of this dissertation and no longer wish to have your work published on the UKDiss.com website then please: Our academic writing and marking services can help you! - Marking Service
- Samples of our Service
- Full Service Portfolio
Study Resources Free resources to assist you with your university studies! - Dissertation Examples
- Dissertation Proposal Examples
- Example Dissertation Titles
- Example Dissertation Topics
- How to Write a Dissertation
- Free Resources Index
- International
- Education Jobs
- Schools directory
- Resources Education Jobs Schools directory News Search
 Unit 4 Assignment B - ethyl ethanoateSubject: Chemistry Age range: 16 - 18 Resource type: Lesson (complete)  Last updated 18 May 2021 - Share through email
- Share through twitter
- Share through linkedin
- Share through facebook
- Share through pinterest
 A PowerPoint lesson for Unit 4 assignment B preparation of an organic liquid. Includes lesson plan, helpful videos, pre-practical questions and starter activity worksheets (with teacher answers). I was observed in this lesson and obtained good feedback. Includes a worksheet to assist with ideas and a starting point for research tasks/discussion around P4. Other details: While students are refluxing for 30min, you could get them to look up literature values for the boiling point of the ester (so they know the temperature it should come out at during distillation) and research factors that affect purity and the effect that impurities will have on the boiling point. What are possible impurities and where may they come from etc. If you have efficient, confident students, this full lesson with the 3 stage practical including activities will take 3 hours. If your students are less confident, I recommend 4 hours. **note - **recommended videos included are from the RSC written lesson plan is mine, however the template is from ‘teacher toolkit’ :) Tes paid licence How can I reuse this? Your rating is required to reflect your happiness. It's good to leave some feedback. Something went wrong, please try again later. This resource hasn't been reviewed yet To ensure quality for our reviews, only customers who have purchased this resource can review it Report this resource to let us know if it violates our terms and conditions. Our customer service team will review your report and will be in touch. Not quite what you were looking for? Search by keyword to find the right resource:ethyl ethanoate Quick ReplyRelated discussions. - how is ethyl ethanoate manufactured industrially
- Applied Science Unit 4
- ethyl ethanoate comparison between lab and industry manufacture
- Chemistry question help
- Industrial ethyl ethanoate
- assignment assistance
- Esterification
- AQA AS Chemistry 2017 Paper 1 Titration MCQ Q16
- chemistry pH question HELP
- Can someone please help me with this question about buffers?
- year 13 alevel chemistry
- Esterification- Evaluation Error
- Please help
- Kc Titration Question
- Organic Chemistry help
- A Level Chemistry Organic Help
- acids and bases HELP
- chemistry paper 2 higher edexel
- OCR chemistry weak acid and base question
Last reply 5 days ago Last reply 1 week ago Last reply 2 weeks ago Last reply 3 weeks ago Last reply 4 weeks ago Last reply 1 month ago Posted 1 month ago Last reply 2 months ago Last reply 3 months ago Articles for you Finding a university place in Ucas Clearing 2024: 10 top tips to help you get ready Top 10 tips for Ucas Clearing 2024  Bringing business people into the classroom: what students learn from industry professionals  Will artificial intelligence put legal graduates out of work? Ethanoic Acid & Esterification Reactions ( Cambridge O Level Chemistry )Revision note.  Physics Lead Formation of Ethanoic AcidMaking carboxylic acids. - Oxidation by fermentation
- Using oxidising agents
- The microbial oxidation (fermentation) of ethanol will produce a weak solution of vinegar (ethanoic acid)
- This occurs when a bottle of wine is opened as bacteria in the air (acetobacter) will use atmospheric oxygen from air to oxidise the ethanol in the wine
C 2 H 5 OH (aq) + O 2 (g) → CH 3 COOH (aq)+ H 2 O (l) - The acidic, vinegary taste of wine which has been left open for several days is due to the presence of ethanoic acid
- Alternatively, oxidising agent potassium manganate(VII) can be used
- This involves heating ethanol with acidified potassium manganate(VII) in the presence of an acid
- The heating is performed under reflux which involves heating the reaction mixture in a vessel with a condenser attached to the top
- The condenser prevents the volatile alcohol from escaping the reaction vessel as alcohols have low boiling points
- The equation for the reaction is:
CH 3 CH 2 OH (aq) + [O] → CH 3 COOH (aq) + H 2 O (l) - The solution will change from purple to colourless
 Diagram showing the experimental setup for the oxidation with KMnO 4 using reflux apparatus Esterification- Alcohols and carboxylic acids react to make esters in esterification reactions
- Esters are compounds with the functional group R-COO-R
- Esters are sweet-smelling oily liquids used in food flavourings and perfumes
- Ethanoic acid will react with ethanol in the presence of concentrated sulfuric acid (catalyst) to form ethyl ethanoate:
CH 3 COOH (aq) + C 2 H 5 OH (aq) ⇌ CH 3 COOC 2 H 5 (aq) + H 2 O (l)  Diagram showing the formation of ethyl ethanoate - For more information on how esters are named, see our revision note on Naming Esters
You've read 0 of your 0 free revision notesGet unlimited access. to absolutely everything: - Downloadable PDFs
- Unlimited Revision Notes
- Topic Questions
- Past Papers
- Model Answers
- Videos (Maths and Science)
Join the 100,000 + Students that ❤️ Save My Examsthe (exam) results speak for themselves: Did this page help you? Author: CarolineCaroline graduated from the University of Nottingham with a degree in Chemistry and Molecular Physics. She spent several years working as an Industrial Chemist in the automotive industry before retraining to teach. Caroline has over 12 years of experience teaching GCSE and A-level chemistry and physics. She is passionate about creating high-quality resources to help students achieve their full potential.  Is Decaffeinated Coffee Bad for You?Some worry that a chemical used to strip caffeine from coffee beans can increase the risk of cancer. Experts explain if you should be concerned. Credit... Eric Helgas for The New York Times Supported by  By Katie Mogg Q: I enjoy coffee but dislike how caffeine makes me feel, so I drink decaffeinated coffee. Are there any health risks? Drinking coffee is a key component of many people’s morning routine, offering energy along with an earthy aroma and nutty flavor. But indulging in a cup can bring unwelcome effects. An eight-ounce brew can contain between 80 and 100 milligrams of caffeine, which can also cause jitters, anxiety and trouble falling asleep. “For some people it’s, ‘I want to be able to drink coffee in the afternoon because I really like the taste, but I don’t want to be up all night,’” said Eric Brenner, the assistant director of the Center for Coffee Research and Education at Texas A&M University. Decaffeinated coffee, generally stripped of at least 97 percent of its caffeine , is a tasty alternative. But some health advocacy organizations have raised concerns about a chemical used in the decaffeination process because it may raise the risk of some cancers. Should you worry? Here’s what experts say you should know. How is coffee decaffeinated?There are several ways to make decaffeinated coffee , but two common methods use the chemicals methylene chloride or ethyl acetate to extract and dissolve caffeine from coffee beans. We are having trouble retrieving the article content. Please enable JavaScript in your browser settings. Thank you for your patience while we verify access. If you are in Reader mode please exit and log into your Times account, or subscribe for all of The Times. Thank you for your patience while we verify access. Already a subscriber? Log in . Want all of The Times? Subscribe . Advertisement  |
IMAGES
VIDEO
COMMENTS
Ethyl acetate is a moderately polar solvent that has the advantages of being volatile, relatively non-toxic, and non-hygroscopic. Ethyl Acetate is an organic compound which also known as, ethyl ethanoate, commonly abbreviated EtOAc or EA. Below is the table of Ethyl Acetate general data and physical properties: FORMULA.
Firstly the concentrations of Ethyl Acetate used were 0.01M and 0.02M which means that for 0.01M of Ethyl Acetate was obtained by diluting 0.49cm3 of pure Ethyl Acetate and 0.02M was obtained by diluting 0.92cm3 of pure Ethyl Acetate. By comparing the graphs (Fig 3 and 4) above, the reaction (0.02M) was the fastest.
A comparison of the different processes to manufacture ethyl ethanoate in the laboratory and in industry. This document compares the similarities and differences between both ways of manufacturing. It also looks at the amount of yield produced by the processes.
Abundance acid and alcohol both dissolve and are tucked securely away under the ester layer. Little esters like ethyl ethanoate smell like ordinary natural solvents (ethyl ethanoate is a common solvent in, for example, glues). As the esters get greater, the smells tend towards artificial fruit flavouring - "pear drops", for example.
A common ester - ethyl ethanoate. The most commonly discussed ester is ethyl ethanoate. In this case, the hydrogen in the -COOH group has been replaced by an ethyl group. The formula for ethyl ethanoate is: Notice that the ester is named the opposite way around from the way the formula is written. The "ethanoate" bit comes from ethanoic acid.
Methods for Making Ethyl Ethanoate. Industrial method Ethyl ethanoate is made by esterification. A mixture of sulfuric acid, acetic acid and ethanol are heated and poured into an esterifying column. The mixture that evaporates first goes into a second column. 85% of ethyl acetate is separated.
The mechanism for the formation of ethyl ethanoate. A reminder of the facts. Ethanoic acid reacts with ethanol in the presence of concentrated sulphuric acid as a catalyst to produce the ester, ethyl ethanoate. The reaction is slow and reversible. To reduce the chances of the reverse reaction happening, the ester is distilled off as soon as it ...
The preparation of ethyl ethanoate. Method: A mixture of ethanoic acid, ethanol and concentrated sulfuric acid is gently heated by either a water bath or an electric heater (ethanol is flammable, so a Bunsen can't be used!) ... IGCSE, A Level and IB teacher for more than 30 years in the UK as well as overseas, and has also been an examiner ...
The catalyst system developed can be used to synthesise ethyl ethanoate at a purity of >99.98% from industrially available ethanol that contains up to 5% 2-propanol. A commercial plant producing 50,000 tonnes of ethyl ethanoate per annum, using the technology described in this thesis, has been in operation since April 2001.Four patents, based ...
Location 1- Brazil Process Route- Dehydrogenation. The feedstock required for the production of ethyl acetate by dehydrogenation is ethanol. Ethanol is produced in large amounts in Brazil from feedstock such as sugar cane which grows abundantly in Brazil. Brazil is the second largest producer of bioethanol worldwide.
Introduction - Manufacture of an organic solid in industry. The main process in an industry is, ethanoic acid, toluene and 2-hydroxbenzoic acid combined and then put through a reactor, water is then added, cooling water is added, this is then filtered, the solids are washed, this is then dried, which is then filtered, packaged and aspirin is ...
Introduction. Ethyl ethanoate is used in various industrial applications such as in paints as a hardener, adhesives, paint and coating additives, degreasing solvents, active agents, processing aids and plasticisers. At a. lower purity, it can be used in printing and pharmaceuticals. It is also used in coating formulations for.
Diagram showing the formation of ethyl ethanoate. During this esterification reaction, a molecule of water is also produced. Naming Esters. An ester is made from an alcohol and carboxylic acid; The first part of the name indicates the length of the carbon chain in the alcohol, and it ends with the letters '- yl' ...
Calculate the relative molecular mass of ethyl ethanoate. The reaction between ethanoic acid and ethanol to form ethyl ethanoate and another product is a reversible reaction. Give the sign that is used in reactions to show that a reaction is reversible. The reaction between ethanoic acid and ethanol will reach dynamic equilibrium.
pdf, 950.04 KB. A PowerPoint lesson for Unit 4 assignment B preparation of an organic liquid. Includes lesson plan, helpful videos, pre-practical questions and starter activity worksheets (with teacher answers). I was observed in this lesson and obtained good feedback. Includes a worksheet to assist with ideas and a starting point for research ...
Ethyl acetate is a moderately polar solvent that has the advantages of b Ethyl acetate is the most popular ester from ethanol and acetic acid. It is manufactured on a large scale for use as a solvent.
Here is an example of a hydrolysis reaction, where an Ester compound (Ethyl Ethanoate), is split by water in the presence of an acid catalyst, into ethanoic acid and ethanol. The reaction involves the splitting of the Ester bond (-COO) in Ethyl Ethanoate, to give the two compounds that the Ester was made from.
I have a 1500 word essay due in seven hours but I've only written 800 words. Israel vs Palestine and in-group bias; Civil Service Fast Stream 2024 - Applicants thread; USA Applicant: What do I put in Access Validating Agency; John Locke Essay Competition 2023 [Official thread] Hamas attacks Israel: Israel to launch ground offense in Gaza
Ethanoic acid will react with ethanol in the presence of concentrated sulfuric acid (catalyst) to form ethyl ethanoate: CH 3 COOH (aq) + C 2 H 5 OH (aq) ⇌ CH 3 COOC 2 H 5 (aq) + H 2 O (l) Diagram showing the formation of ethyl ethanoate. For more information on how esters are named, see our revision note on Naming Esters
Ethyl acetate, the other chemical used to decaffeinate coffee, also doesn't warrant much concern, Mr. Brenner said. It naturally occurs in some fruits, such as kiwi and guava, and is used in ...
Research the industrial production and testing of ethyl ethanoate and describe the scale, equipment, testing and the raw materials used to produce ethyl ethanoate in industry. The main method of industrial production to produce ethanol ethanoate involves the process esterification of ethanol with acetic acid. However, some is produced by the ...
re the laboratory and industrial manufacture and testing of an organic liquid. B.P3 Prepare and test the purity of an organic liquid and draw conclusions. B.P4 Describe the industrial manufacture and testing of an organic liquid