An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager

Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Acute lymphoblastic leukaemia
Affiliations.
- 1 Department of Medicine and Surgery, University of Parma, Parma, Italy.
- 2 Translational Hematology and Chemogenomics (THEC), University of Parma, Parma, Italy.
- 3 Hematology and BMT Unit, Azienda Ospedaliero-Universitaria di Parma, Parma, Italy.
- 4 Shanghai Institute of Hematology, State Key Laboratory of Medical Genomics, National Research Center for Translational Medicine at Shanghai, Ruijin Hospital, Shanghai JiaoTong University School of Medicine, Shanghai, China.
- 5 Rutgers Cancer Institute of New Jersey, Rutgers Robert Wood Johnson Medical School, Rutgers University, New Brunswick, NJ, USA.
- 6 Department of Medicine, Hematology and Clinical Immunology, University of Perugia, Perugia, Italy.
- 7 Leukaemia Research Cytogenetics Group, Translational and Clinical Research Institute, Newcastle University Centre for Cancer, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, UK.
- 8 Department of Pathology, St. Jude Children's Research Hospital, Memphis, TN, USA.
- 9 Hôpital Saint-Louis, APHP, Institut de Recherche Saint-Louis, Université Paris Cité, Paris, France.
- 10 Children's Minnesota Cancer and Blood Disorders Program, Minneapolis, MN, USA.
- 11 Department of Medicine and Surgery, University of Parma, Parma, Italy. [email protected].
- 12 Translational Hematology and Chemogenomics (THEC), University of Parma, Parma, Italy. [email protected].
- 13 Hematology and BMT Unit, Azienda Ospedaliero-Universitaria di Parma, Parma, Italy. [email protected].
- PMID: 38871740
- DOI: 10.1038/s41572-024-00525-x
Acute lymphoblastic leukaemia (ALL) is a haematological malignancy characterized by the uncontrolled proliferation of immature lymphoid cells. Over past decades, significant progress has been made in understanding the biology of ALL, resulting in remarkable improvements in its diagnosis, treatment and monitoring. Since the advent of chemotherapy, ALL has been the platform to test for innovative approaches applicable to cancer in general. For example, the advent of omics medicine has led to a deeper understanding of the molecular and genetic features that underpin ALL. Innovations in genomic profiling techniques have identified specific genetic alterations and mutations that drive ALL, inspiring new therapies. Targeted agents, such as tyrosine kinase inhibitors and immunotherapies, have shown promising results in subgroups of patients while minimizing adverse effects. Furthermore, the development of chimeric antigen receptor T cell therapy represents a breakthrough in ALL treatment, resulting in remarkable responses and potential long-term remissions. Advances are not limited to treatment modalities alone. Measurable residual disease monitoring and ex vivo drug response profiling screening have provided earlier detection of disease relapse and identification of exceptional responders, enabling clinicians to adjust treatment strategies for individual patients. Decades of supportive and prophylactic care have improved the management of treatment-related complications, enhancing the quality of life for patients with ALL.
© 2024. Springer Nature Limited.
PubMed Disclaimer
Similar articles
- Novel Therapies in the Treatment of Adult Acute Lymphoblastic Leukemia. Gavralidis A, Brunner AM. Gavralidis A, et al. Curr Hematol Malig Rep. 2020 Aug;15(4):294-304. doi: 10.1007/s11899-020-00591-4. Curr Hematol Malig Rep. 2020. PMID: 32445026 Free PMC article. Review.
- Genomic characterization of paediatric acute lymphoblastic leukaemia: an opportunity for precision medicine therapeutics. Tasian SK, Hunger SP. Tasian SK, et al. Br J Haematol. 2017 Mar;176(6):867-882. doi: 10.1111/bjh.14474. Epub 2016 Dec 16. Br J Haematol. 2017. PMID: 27984637 Free PMC article. Review.
- Targeted therapy and immunotherapy for T cell acute lymphoblastic leukemia/lymphoma. Huang YH, Wan CL, Dai HP, Xue SL. Huang YH, et al. Ann Hematol. 2023 Aug;102(8):2001-2013. doi: 10.1007/s00277-023-05286-3. Epub 2023 May 25. Ann Hematol. 2023. PMID: 37227492 Review.
- Recent advances in the treatment of acute lymphoblastic leukemia. Rafei H, Kantarjian HM, Jabbour EJ. Rafei H, et al. Leuk Lymphoma. 2019 Nov;60(11):2606-2621. doi: 10.1080/10428194.2019.1605071. Epub 2019 May 16. Leuk Lymphoma. 2019. PMID: 31092071 Review.
- Pediatric acute lymphoblastic leukemia. Inaba H, Mullighan CG. Inaba H, et al. Haematologica. 2020 Nov 1;105(11):2524-2539. doi: 10.3324/haematol.2020.247031. Haematologica. 2020. PMID: 33054110 Free PMC article. Review.
- Risk factors in DUX4-positive childhood and adolescent B-cell acute lymphoblastic leukemia. Schinnerl D, Riebler M, Schumich A, Haslinger S, Bramböck A, Inthal A, Nykiel M, Maurer-Granofszky M, Haas OA, Pötschger U, Köhrer S, Nebral K, Dworzak MN, Attarbaschi A, Strehl S. Schinnerl D, et al. Blood Cancer J. 2024 Jul 22;14(1):119. doi: 10.1038/s41408-024-01099-3. Blood Cancer J. 2024. PMID: 39039054 Free PMC article.
- Zhang, N. et al. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 13, 82 (2023). - PubMed - PMC - DOI
- Yi, M., Zhou, L., Li, A., Luo, S. & Wu, K. Global burden and trend of acute lymphoblastic leukemia from 1990 to 2017. Aging 12, 22869–22891 (2020). - PubMed - PMC
- Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023). - PubMed - DOI
- Hunger, S. P. & Mullighan, C. G. Acute lymphoblastic leukemia in children. N. Engl. J. Med. 373, 1541–1552 (2015). - PubMed - DOI
- Malard, F. & Mohty, M. Acute lymphoblastic leukaemia. Lancet 395, 1146–1162 (2020). - PubMed - DOI
Publication types
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Nature Publishing Group
Miscellaneous
- NCI CPTAC Assay Portal

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Sign In to save searches and organize your favorite content.
- Not registered? Sign up
Recently viewed (0)
- Save Search
- Subscriptions
- Join E-mail List
Acute Lymphoblastic Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology
- Get Citation Alerts
- Download PDF to Print
The NCCN Guidelines for Acute Lymphoblastic Leukemia (ALL) focus on the classification of ALL subtypes based on immunophenotype and cytogenetic/molecular markers; risk assessment and stratification for risk-adapted therapy; treatment strategies for Philadelphia chromosome (Ph)-positive and Ph-negative ALL for both adolescent and young adult and adult patients; and supportive care considerations. Given the complexity of ALL treatment regimens and the required supportive care measures, the NCCN ALL Panel recommends that patients be treated at a specialized cancer center with expertise in the management of ALL This portion of the Guidelines focuses on the management of Ph-positive and Ph-negative ALL in adolescents and young adults, and management in relapsed settings.
Acute lymphoblastic lymphoma (ALL) is a heterogeneous hematologic disease characterized by the proliferation of immature lymphoid cells in the bone marrow, peripheral blood, and other organs. 1 The age-adjusted incidence rate of ALL in the United States is 1.8 per 100,000 individuals per year, 2 with approximately 5,690 new cases and 1,580 deaths estimated in 2021. 3 The median age at diagnosis for ALL is 17 years with 53.5% of patients diagnosed at younger than 20 years of age. 2 In contrast, 29.6% of cases are diagnosed at 45 years or older and only approximately 13.7% of patients are diagnosed at 65 years or older. 2 ALL represents 75%–80% of acute leukemias among children, making it the most common form of childhood leukemia; by contrast, ALL represents approximately 20% of all leukemias among adults. 1 , 4
Risk factors for developing ALL include older age (>70 years), exposure to chemotherapy or radiation therapy, and genetic disorders, particularly Down syndrome. 5 , 6 Although rare, other genetic conditions have been categorized as a risk factor for ALL and include Li–Fraumeni syndrome, 7 neurofibromatosis, 8 Klinefelter syndrome, 9 – 11 Fanconi anemia, 12 , 13 Shwachman-Diamond syndrome, 14 , 15 Bloom syndrome, 16 and ataxia telangiectasia. 17
The cure rates and survival outcomes for patients with ALL have improved dramatically over the past several decades, primarily among children. 18 Improvements are largely owed to advances in the understanding of the molecular genetics and pathogenesis of the disease, the incorporation of measurable residual disease (MRD) testing, the refinement of risk-adapted treatment algorithms, the advent of new targeted agents, and the use of allogeneic hematopoietic stem cell transplantation (HCT).
Analyses from the SEER database have shown improvements in survival for children and adolescent and young adult (AYA) patients with 5-year overall survival (OS) rates of 89% and 61%, respectively. 18 , 19 However, survival rates for adult patients remain low at approximately 20%–40%. 20 – 22 Survival rates are especially poor in older adult patients at approximately 20%. 21 , 23 , 24 Although the exact OS percentage can vary based on how the age range is defined for pediatric, AYA, and adult patients, the trend is nonetheless clear that OS decreases substantially with increased age. 21 The exception is infants younger than age 1, which is an age group that has not seen any improvement in survival over the last 30 years. The 5-year OS in this population is 55.8%. 18 Cure rates for AYAs with ALL remain suboptimal compared with those for children, although substantial improvements have been seen with the adoption of pediatric treatment regimens. 25 AYA patients represent a unique population, because they may receive treatment based on either a pediatric or an adult protocol, depending on local referral patterns and institutional practices. Favorable cytogenetic subtypes, such as ETV6-RUNX1 ALL and hyperdiploidy, occur less frequently among AYA patients compared with children, whereas the incidence of ALL in high-risk subgroups such as BCR-ABL (Ph-positive ALL) or with Ph-like ALL 26 is higher in AYA patients.
The initial workup for patients with ALL should include a thorough medical history and physical examination, along with laboratory and imaging studies (where applicable). Laboratory studies include a complete blood count (CBC) with platelets and differential, a blood chemistry profile, liver function tests, a disseminated intravascular coagulation panel (including measurements for d -dimer, fibrinogen, prothrombin time, and partial thromboplastin time), and a tumor lysis syndrome panel (including measurements for serum lactate dehydrogenase, uric acid, potassium, phosphate and calcium). Other recommended tests include hepatitis B/C, HIV, and cytomegalovirus antibody evaluations. Female patients should undergo pregnancy testing and all male patients should be evaluated for testicular involvement of disease, including a scrotal ultrasound as indicated; testicular involvement is especially common in cases of T-ALL. Fertility counseling and preservation options should be presented to all patients. CT scans of the neck, chest, abdomen, and pelvis with intravenous contrast are recommended as indicated by symptoms, and if any extramedullary involvement is suspected, a PET/CT may be considered for diagnosis and follow-up.
All patients should be evaluated for opportunistic infections as appropriate. In addition, an echocardiogram or multigated acquisition scan should be obtained for all patients due to the use of anthracyclines as the backbone of nearly all treatment regimens. Assessment of cardiac function is particularly important for patients with prior cardiac history, prior anthracycline exposure, or clinical symptoms suggestive of cardiac dysfunction, and for elderly patients. An early transplant evaluation and donor search should be strongly considered.
Appropriate imaging studies (eg, CT/MRI scan of the head with contrast) should be performed to detect meningeal disease, chloromas, or central nervous system (CNS) bleeding for patients with major neurologic signs or symptoms at diagnosis. CNS involvement should be evaluated through lumbar puncture at timing that is consistent with the treatment protocol. Pediatric-inspired regimens typically include lumbar puncture at diagnostic workup; the NCCN ALL Panel recommends that the first lumbar puncture be performed at the time of initial scheduled intrathecal therapy unless directed by symptoms to perform earlier.
It should be noted that the recommendations included in the guidelines represent a minimum set of workup considerations, and that other evaluations or testing may be needed based on clinical symptoms. Procurement of cells should be considered for purposes of future research (in accordance with institutional practices or policies).
Prognostic Factors and Risk Stratification
Various disease-related and patient-specific factors may have prognostic significance in patients with ALL. In particular, patient age, WBC count, immunophenotypic/cytogenetic subtype, presence of CNS disease, and response to induction/consolidation therapy have been identified as important factors in defining risk and assessing prognosis for both adult and childhood ALL.
- Prognostic Factors in AYA Patients With ALL
In 1993, a common set of risk criteria was established by the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) at an international conference hosted by the NCI. 27 In this system, two risk groups were designated: standard risk and high risk. Standard risk was assigned to patients age 1 to younger than 10 years of age and with a WBC count less than 50 × 10 9 cells/L, whereas all other patients with ALL, including T-ALL (regardless of age or WBC count), were considered high risk. 28 It should be noted that despite exclusion from this report, patients younger than age 1 should also be considered very high risk. 29 , 30 The POG and CCG have since merged to form the Children’s Oncology Group (COG) and subsequent risk assessment has produced additional risk factors, particularly in precursor B-ALL, to further refine therapy. Specifically, in B-ALL, a group identified as very high risk, was defined as patients with any of the following characteristics: t(9;22) chromosomal translocation (ie, Ph-positive ALL) and/or presence of BCR-ABL1 fusion protein; hypodiploidy (<44 chromosomes) 31 ; BCR-ABL1 –like or Ph-like ALL 32 ; iAMP21 30 ; or failure to achieve remission with induction therapy. 25 , 28 KMT2A rearrangements and a poor response to induction chemotherapy also recategorized patients into this group. 33 – 35 Conversely, criteria were refined for lower risk and included patients with hyperploidy, the t(12;21) chromosomal translocation ( ETV6-RUNX1 subtype), 36 or simultaneous trisomies of chromosomes 4, 10, and 17. 28 , 37 Presence of extramedullary disease and the early response to treatment also modified risk. Early marrow response to therapy was a strong positive prognostic factor while the presence of extramedullary disease at diagnosis was correlated with a poorer prognosis. Using the refined risk assessment, four risk categories for B-ALL, designated as low risk, standard risk, high risk, and very high risk, were identified encompassing 27%, 32%, 27%, and 4% of cases, respectively. 28
Risk stratification of T-ALL has been more difficult than in B-ALL. Although T-cell lineage has previously been considered a high-risk feature in ALL, modern treatment protocols have resulted in improved survival outcomes for these patients. The identification of genetic mutations and the use of targeted therapies may change the way T-ALL is treated and ultimately how these patients are assessed for risk.
Historically, the AYA population has been treated on either a pediatric or an adult ALL regimen, depending on referral patterns and the institution. In recent years, several retrospective studies from both the United States and Europe have shown that AYA patients (15–21 years of age) treated on a pediatric protocol have substantially improved event-free survival (EFS) compared with same-aged patients treated on adult ALL regimens. 25 , 38 Comparison of adult and pediatric protocols has shown that adults received lower doses of nonmyelosuppressive chemotherapy and less intense intrathecal chemotherapy regimens. 39 , 40 Adult protocols also are more likely to include allogeneic HCT compared with pediatric protocols, but the benefits of HCT in the AYA population have not been sufficiently studied, and the available data include conflicting findings. 41 – 45 There is clearly a significant difference between the way adults and pediatric patients are treated and this may be a variable in the treatment of AYA patients. Thus, the choice of initial treatment regimen can have a profound impact on overall clinical outcomes in AYA patients.
Despite improved outcomes for AYA patients treated on pediatric-inspired regimens versus adult ALL regimens, studies have shown poorer outcomes among patients in the AYA group compared with children younger than 10 years. 46 This may be attributed to factors that are based on biology and social differences. Compared with the pediatric population, AYA patients have a lower frequency of favorable chromosomal/cytogenetic abnormalities, such as hyperdiploidy or ETV6-RUNX1 , 47 and a greater incidence of poor-risk cytogenetics including Ph-positive ALL, Ph-like ALL, hypodiploidy, and complex karyotype, 48 and a higher incidence of ETP-ALL. 49 , 50 Furthermore, the positive prognostic values of the ETV6-RUNX1 mutation and hyperdiploidy are greater in the patients younger than 10 years, suggesting that the benefits decline with age. 48 The effects of treatment are also shown to be different in the AYA population compared with the pediatric population. In vitro studies showed that ALL cells from children older than 10 years are more resistant to chemotherapy compared with the cells from children younger than 10 years. 51 The COG AALL0232 study reported an initial delay in response to induction therapy in older AYA patients (aged 16–30 years) compared with younger patients (aged 1–15 years). 52 There was a statistically significant reduction in the number of patients in the older cohort who had negative end-induction MRD compared with the younger cohort (59% vs 74%; P <.0001) with fewer patients achieving M1 marrow on day 15 of induction (67% vs 80%, respectively; P =.0015). In addition to the biologic differences, the social component of treating AYA patients is important. Enrollment in clinical trials has been shown to improve patient outcomes 53 ; however, only 2% of AYA patients enroll in clinical trials compared with the 60% enrollment of pediatric patients. 54 Pediatric patients have been shown to be more compliant with treatment protocols compared with AYA patients, 55 which may be due to greater parental supervision of the treatment and better insurance. 56
- Prognostic Factors in Adults With ALL
Both age and initial WBC count have historically been considered clinically significant prognostic factors in the management of adult patients with ALL. 57 , 58 Early prospective multicenter studies defined values for older age (>35 years) and higher initial WBC count (>30 × 10 9 /L for B-cell lineage; >100 × 10 9 /L for T-cell lineage) that were predictive of significantly decreased remission duration. 59 , 60 Subsequent studies have confirmed the prognostic importance of these clinical parameters, although the cutoff values differed between studies. 57 , 58
In one of the largest studies to date (n=1521), conducted by the Medical Research Council (MRC) UKALL/ECOG, both age (>35 years) and WBC count (>30 × 10 9 /L for B-cell lineage; >100 × 10 9 /L for T-cell lineage) were found to be significant independent prognostic factors for decreased disease-free survival (DFS) and OS among patients with Ph-negative ALL; the independent prognostic value remained significant when these factors were evaluated as continuous variables in multivariate analysis. 61 All patients, regardless of Ph status, had received induction therapy followed by intensification (for patients with a complete response [CR] postinduction) with contemporary chemotherapy combination regimens. Patients with a CR after induction received allogeneic HCT (for patients <50 years of age and with HLA-compatible siblings), autologous HCT, or consolidation/maintenance treatment. Because Ph-positive ALL is associated with a very poor prognosis, patients with this subtype were assigned to undergo allogeneic HCT (including matched, unrelated donor [URD] HCT) when possible. The 5-year OS rate among patients with Ph-positive and Ph-negative disease was 25% and 41%, respectively. 61 Among patients with Ph-negative ALL, those older than 35 years or with elevated WBC count (>30 × 10 9 /L for B-cell lineage; >100 × 10 9 /L for T-cell lineage) at diagnosis were initially identified as high risk, whereas all others were classified as standard risk. The 5-year OS rates for the Ph-negative high-risk and standard-risk subgroups were 29% and 54%, respectively. 61 Further analysis of the Ph-negative population according to risk factors showed that patients could be categorized as low risk (no risk factors based on age or WBC count), intermediate risk (either age >35 years or elevated WBC count), or high risk (both age >35 years and elevated WBC count). The 5-year OS rates based on these risk categories were 55%, 34%, and 5%, respectively, suggesting that patients with Ph-negative ALL in the high-risk subgroup had even poorer survival outcomes than patients in the overall Ph-positive subgroup. 61
In a subsequent analysis from this MRC UKALL XII/ECOG E2993 study, cytogenetic data were evaluated in approximately 1,000 patients. 62 The analysis confirmed the negative prognostic impact of Ph-positive status compared with Ph-negative disease, with a significantly decreased 5-year EFS rate (16% vs 36%; P <.001, adjusted for age, gender, and WBC count) and OS rate (22% vs 41%; P <.001, adjusted for age, gender, and WBC count). Among patients with Ph-negative disease, the following cytogenetic subgroups had significantly decreased 5-year EFS (13%–24%) and OS rates (13%–28%) based on univariate analysis: t(4;11) KMT2A translocation, t(8;14), complex karyotype (≥5 chromosomal abnormalities), and low hypodiploidy (30–39 chromosomes)/near triploidy (60–78 chromosomes). 62 In contrast, del(9p) or high hyperdiploidy (51–65 chromosomes) was associated with more favorable 5-year EFS (49%–50%) and OS rates (53%–58%). 62 An earlier report of data from patients treated on the French ALL study group (LALA) protocols suggested that near triploidy (60–78 chromosomes) may be derived from duplication of hypodiploidy (30–39 chromosomes); both aneuploidies were associated with poor DFS and OS outcomes similar to that of patients with Ph-positive ALL. 63 Based on multivariate Cox regression analysis reported in the MRC UKALL XII/ECOG E2993 study, t(8;14), low hypodiploidy/near triploidy, and complex karyotype remained significant independent predictors for risk of relapse or death; the prognostic impact of these cytogenetic markers was independent of factors such as age, WBC count, or T-cell immunophenotype, and their significance was retained even after excluding patients who had undergone postinduction HCT. 62
The importance of cytogenetics as a prognostic factor for survival outcomes was shown in other studies, including the SWOG study conducted with 200 adult patients with ALL. 64 In this study, the prognostic impact of the different cytogenetic categories outweighed that of the more traditional factors, such as age and WBC count; in multivariate analysis for both relapse-free survival (RFS) and OS, cytogenetics remained a significant independent predictor of outcomes, whereas factors such as age and WBC count lost prognostic significance. 64 Moreover, the subgroup (n=19) of patients with “very high risk” cytogenetic features [identified based on outcomes from the MRC/ECOG study mentioned earlier: presence of t(4;11) KMT2A (MLL) translocation; t(8;14); complex karyotype; or low hypodiploidy] had substantially decreased 5-year RFS and OS rates (22%, for both endpoints). Analysis by ploidy status was not possible because only 2 patients were considered to have low hypodiploidy/near triploidy. The 5-year RFS and OS rates among patients with Ph-positive ALL (n=36) were 0% and 8%, respectively. 64
Management of Ph-Positive ALL
- Initial Treatment in AYA Patients With Ph-Positive ALL
Ph-positive ALL is rare in children with ALL, occurring in only approximately 3% of pediatric cases compared with 25% of adult cases. 65 The frequency of Ph-positive ALL among AYA patients ranges from 5% to 25% and increases with age, 62 , 66 although this subtype is still uncommon relative to the incidence in older adults. Historically, children and adolescents with Ph-positive disease had a poorer prognosis compared with patients with Ph-negative B-ALL. However, recent improvements in the treatment options are closing this gap.
Hematopoietic Cell Transplant
In a retrospective analysis of children with Ph-positive ALL treated between 1986 and 1996 (n=326) with intensive chemotherapy regimens with or without allogeneic HCT, the 7-year EFS and OS rates were 25% and 36%, respectively. This benefit with HCT versus chemotherapy alone was not observed with autologous HCT or with HCT from matched URDs. This study showed that allogeneic HCT from a matched related donor offered improvements in outcomes over chemotherapy alone.
In a subsequent analysis of outcomes in children with Ph-positive ALL treated between 1995 and 2005 but also without targeted tyrosine kinase inhibitors (TKIs), the 7-year EFS and OS rates were 32% and 45%, respectively. 67 Outcomes with allogeneic HCT from either matched related donors or URDs appeared similar, and HCT improved disease control over intensive chemotherapy alone. 67 Although this analysis showed an improved 7-year EFS rate, outcomes remained suboptimal in patients with Ph-positive ALL.
Allogeneic HCT has been considered the standard of care for AYA patients with Ph-positive ALL; however, its role has become less clear with the advent of BCR-ABL –targeted TKIs. Several studies evaluated the role of allogeneic HCT in the era of imatinib and whether imatinib-based therapies provided an additional benefit to HCT.
COG AALL-0031 Regimen
In a multicenter COG study (AALL-0031) of children and adolescents with high-risk ALL, the group of patients with Ph-positive ALL (n=92; aged 1–21 years) was treated with an intensive chemotherapy regimen combined with imatinib (340 mg/m 2 /day; given during postremission induction therapy and maintenance). 68 Among the cohort (n=44) who received continuous imatinib exposure (280 consecutive days before maintenance initiation), the 3-year EFS rate was 80.5% (95% CI, 64.5%–89.8%). This outcome compared favorably with that of a historical population of patients with Ph-positive ALL (n=120) treated on a POG protocol, which showed a 3-year EFS rate of only 35% ( P <.0001). 68 Moreover, the 3-year EFS rates were similar among the groups of patients who received chemotherapy combined with continuous imatinib (88%; n=25) or allogeneic HCT from a related donor (57%; n=21) or URD (72%; n=11). No major toxicities were found to be associated with the addition of imatinib to the intensive chemotherapy regimen. 68 Subsequent follow-up after 5 years confirmed these outcomes. 69 In a phase II single-arm COG trial (AALL-0622) of children and young adults with Ph-positive ALL (n=60; aged 1–30 years), imatinib was replaced with dasatinib on induction day 15 and combined with the same chemotherapy used in AALL-0031. 70 The 5-year OS and EFS rates (±standard deviation) were 86% ± 5% and 60% ± 7%, respectively, and outcomes were similar to those observed in AALL-0031. 70
The European intergroup study of postinduction treatment of Ph-chromosome positive ALL (EsPhALL) reported results of the randomized open-label trial designed to evaluate the safety and long-term efficacy of discontinuous postinduction imatinib plus chemotherapy with the Berlin-Frankfurt-Münster (BFM) backbone intensive treatment versus chemotherapy alone. 71 The study enrolled 108 patients with good risk and 70 patients with poor risk aged 1 to 18 years. Good-risk patients were randomized 1:1 and poor-risk patients were all assigned to receive chemotherapy plus imatinib. There was a trend toward improved 4-year DFS for patients with good risk who received imatinib plus chemotherapy versus those who received chemotherapy alone (72.9% vs 61.7%; P =.24). In the as-treated analysis, good-risk patients who received imatinib with chemotherapy had a 4-year EFS of 75.2% versus 55.9% in patients who did not receive imatinib ( P =.06). The incidence of serious adverse events was not statically different between the 2 groups ( P =.64). 71 Enrollment in this trial was stopped in 2009 following results of the COG AALL0031 study that demonstrated a benefit of continuous imatinib. The EsPhALL study was amended into a single-arm study to add continuous imatinib on induction day 15, with 97% of patients achieving first CR. 72 However, the 5-year EFS and OS rates (57% and 71.8%, respectively) were similar in cohorts that received discontinuous postinduction imatinib and continuous imatinib plus chemotherapy with the BFM backbone intensive treatment. 71 , 72 Additionally, a phase II trial evaluated the safety and efficacy of adding continuous dasatinib at day 15 to the intensive BFM regimen in pediatric patients with newly diagnosed Ph-positive ALL (n=109 enrolled; age range, 1–17 years). 73 The efficacy analysis included 104 patients, who all achieved CR; 15 of the patients received allogeneic HCT at first CR (CR1). An interim analysis showed a 3-year EFS of 66.0% (95% CI, 54.8%–75.0%) and a 3-year OS of 92.3% (95% CI, 85.2%–96.1). 73
Blinatumomab
Treatment of newly diagnosed Ph-positive ALL adults was evaluated in a phase 2 single-group trial using dasatinib chemotherapy-free induction followed by first-line consolidation therapy blinatumomab. 74 Sixty-three patients, aged 24 to 84 were enrolled. At the end of induction, 29% patients had a molecular response, which increased to 60% after 2 cycles of blinatumomab. ABL1 mutations occurred in 6 patients who had an increase in MRD, however were cleared on treatment with blinatumomab. Few toxic effects of grade 3 or higher were observed, with cytomegalovirus reactivation or infection occurring in 6 patients. As a result of high molecular response, overall and disease-free survival at a median follow-up of 18 months was achieved in 95% and 88% (95% CI, 90–100; 80–97) of patients, respectively. 74 Disease-free survival was lower in patients with IKZF1 deletions.
The safety and efficacy of blinatumomab in combination with TKIs has recently been evaluated in the treatment of Ph-positive ALL. 75 – 77 In a small retrospective study, adults with relapsed/refractory (R/R) Ph+ ALL (n=9) and chronic myeloid leukemia (CML) (n=3) for whom one line of chemotherapy and one class of TKIs had previously failed, were treated with the combination of blinatumomab and a TKI (ponatinib, dasatinib, or bosutinib). Of the 12 total patients, 75% (9/12) experienced complete molecular responses with no cardiovascular adverse events. 75
A recent single-arm phase II study exploring the chemotherapy-free combination of blinatumomab plus ponatinib in 28 patients with newly diagnosed or relapsed/refractory Ph-positive ALL showed an overall 95% response rate combined. 78 In the newly diagnosed cohort, a 1-year EFS and OS of 100% was reported, with no patients undergoing transplant. The 1-year EFS was 55% in the R/R cohort, with 88% OS reported. 78 Four patients (44%) with R/R disease underwent HSCT.
TKIs Combined With Hyper-CVAD
A phase II study at MD Anderson Cancer Center (MDACC) evaluated imatinib combined with the hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone) regimen in patients with previously untreated or minimally treated Ph+ ALL (n=54; median age, 51 years; range, 17–84 years); 14 patients underwent subsequent allogeneic HCT. 79 The 3-year OS rate with this regimen was 54%. Among the patients aged 40 years or younger (n=16), a strong trend was observed for OS benefit with allogeneic HCT (3-year OS rate, 90% vs 33%; P =.05). 79 Among patients aged 60 years or younger, no statistically significant difference was observed in the 3-year OS rate between patients who received HCT and those who did not (77% vs 57%).
Studies have shown the promising activity of other TKIs, including dasatinib and ponatinib when incorporated into frontline regimens for patients with ALL. In a phase II study from MDACC, dasatinib was combined with hyper-CVAD and subsequent maintenance therapy in patients with previously untreated Ph-positive ALL (n=35; median age, 53 years; range, 21–79 years; 31% were older than 60 years); 4 of the patients received allogeneic HCT in CR1. 80 The 2-year OS and EFS rates were 64% and 57%, respectively. The efficacy and safety of ponatinib combined with hyper-CVAD was examined in patients with Ph-positive ALL (n=37; aged ≥18 years; median age, 51 years; 12 patients were ≥60 years) in a phase II prospective trial. 81 Of the 32 patients with Ph-positive metaphases at the start of therapy, an overall complete cytogenetic response was observed in 32 patients (100%). By multiparametric flow cytometry, 35 of 37 patients (95%) had no MRD after a median of 3 weeks of therapy. 81 However, it is worth noting that only half of the patients 60 years or older were able to complete therapy with this regimen and were switched to alternate TKIs. The 2-year OS and EFS rates were 80% and 81%, respectively. A follow-up study (n=76; age ≥18 years; median age, 47 years) demonstrated long-term efficacy for ponatinib and hyper-CVAD with a 3-year EFS rate of 70%. 82
TKIs Combined With Multiagent Chemotherapy
In the phase II study from the Group for Research on Adult ALL (GRAALL; GRAAPH-2003), patients with previously untreated Ph-positive ALL (n=45; median age, 45 years; range, 16–59 years) received imatinib in combination with chemotherapy during either induction or consolidation therapy. 83 , 84 Patients in complete remission with a donor received allogeneic HCT (n=24), whereas those in complete remission with good molecular response but without a donor were eligible for autologous HCT (n=10). Nine patients did not receive HCT and were treated with imatinib-based maintenance therapy. The 4-year OS rate did not differ significantly for patients with a sibling donor compared with patients undergoing autologous HCT (76% vs 80%). The 4-year OS for patients who received only maintenance imatinib was 33%. 84 These data suggest that improved survival with imatinib-based therapy can be further enhanced by the addition of HCT.
In the subgroup of patients with Ph-positive ALL (n=94; median age, 47 years; range, 19–66 years) from the Northern Italy Leukemia Group study (NILG-09/00), outcomes were compared among patients who received chemotherapy with imatinib (n=59) or without imatinib (n=35), with or without subsequent HCT (allogeneic or autologous). 85 The patients who received imatinib (63% of eligible patients underwent allogeneic HCT) had significantly higher 5-year OS (38% vs 23%; P =.009) and DFS rates (39% vs 25%; P =.044) compared with those who did not receive imatinib (39% of eligible patients underwent allogeneic HCT). 85 The 5-year OS rates by treatment type were 47% for allogeneic HCT (n=45), 67% for autologous HCT (n=9), 30% for imatinib without HCT (n=15), and 7% for no imatinib and no HCT (n=13); the corresponding treatment-related mortality rates were 17%, 0%, 36%, and 23%, respectively. The 5-year relapse rates were 43%, 33%, 87%, and 100%, respectively. 85
The Japan Adult Leukemia Study Group (ALL-202) treated patients with Ph-positive ALL (n=100) with chemotherapy combined with imatinib administered during induction, consolidation, and maintenance phases. 86 , 87 An early analysis (n=80; median age, 48 years; range, 15–63 years) reported a 1-year OS rate of 73% among patients who underwent allogeneic HCT, compared with 85% for those who did not. 87 A subsequent analysis compared outcomes for the subgroup of patients who received allogeneic HCT at first CR in this study (n=51; median age, 38 years; range, 15–64 years) versus those for a historical cohort of patients who received allogeneic HCT without prior imatinib (n=122). 86 The 3-year OS (65% vs 44%; P =.015) and DFS rates (58% vs 37%; P =.039) were significantly higher among patients treated with imatinib compared with the historical cohort; the 3-year nonrelapse mortality rate was similar between cohorts (21% vs 28%, respectively). 86
A multicenter phase II study from the Adult Acute Lymphoblastic Leukemia Working Party of the Korean Society of Hematology investigated the effects of multiagent chemotherapy combined with nilotinib in patients with newly diagnosed Ph-positive ALL (n=90; median age, 47 years; range, 17–71 years). 88 Chemotherapy combined with nilotinib was administered during induction, consolidation, and maintenance phases. Of 90 evaluable patients, 82 (91%) experienced complete hematologic remission with a median time of 27 days (range, 13–72). The 2-year hematologic RFS and OS rates were both 72%. 88
In a phase II multicenter trial (CALGB 10701), patients with newly diagnosed Ph-positive ALL (n=64; median age, 60 years; range, 22–87 years) were treated with multiagent chemotherapy combined with dasatinib, and the efficacy of different postremission strategies were evaluated including allogeneic HCT, autologous HCT, and chemotherapy alone, followed by maintenance with dasatinib. 89 The CR rate was 97% with no induction deaths. With a median follow-up of 48 months for survivors, 3-year OS and DFS were 55% and 43%, respectively. 89 For patients who underwent consolidation with allogeneic HCT, autologous HCT, or chemotherapy, 3-year OS was 75%, 71%, and 55%, respectively, with median OS not reached for all groups. The 3-year DFS was 55%, 43%, and 46%, respectively. 89
- Treatment of Relapsed Ph-Positive ALL
The treatment of patients who experience relapse after initial therapy for ALL remains a challenge, because these patients have a very poor prognosis. Several large studies using conventional chemotherapy for relapsed adult patients have reported a median OS of 4.5 to 6 months, and a 5-year OS rate of 3%–10%. 90 – 93 One major factor associated with poorer survival outcomes after subsequent therapy for relapsed ALL is the duration of response to frontline treatment. In an analysis of data from the PETHEMA (Programa Español de Tratamientos en Hematologia) trials, patients with disease that relapsed more than 2 years after frontline therapy had significantly higher 5-year OS rates than the groups of patients who relapsed within 1 to 2 years or within 1 year of frontline therapy (31% vs 15% vs 2%; P <.001). 91 Similarly, in the MRC UKALL XII/ECOG E2993 trial, patients with disease that relapsed more than 2 years after initial diagnosis and frontline therapy had a significantly higher 5-year OS rate than those who relapsed within 2 years (11% vs 5%; P <.001). 90 In the pre-imatinib era, patients with Ph-positive ALL who relapsed after frontline therapy had dismal outcomes; subgroup data from the large, prospective trials LALA-94 and MRC UK XII/ECOG E2993 showed a median OS of 5 months and a 5-year OS rate of 3%–6% among patients subsequently treated for relapsed Ph-positive ALL. 90 , 92
Treatment options are extremely limited for patients with Ph-positive ALL who experience relapse after receiving consolidation with allogeneic HCT. Some investigators have reported on the feasibility of inducing a second molecular CR with dasatinib in those who have experienced an early relapse after first allogeneic HCT, which allowed for a second allogeneic HCT. 94 , 95 Studies that include donor lymphocyte infusion (DLI) to induce further graft-versus-leukemia effect in those who relapse after allogeneic HCT have reported little to no benefit, though it has been suggested that this is due to excessively high leukemic burden. 96 , 97 Indeed, published case reports have suggested that the use of DLI for residual disease or molecular relapse (as noted by levels of BCR-ABL fusion mRNA measured with PCR) after allogeneic HCT may eliminate residual leukemic clones and thereby prevent overt hematologic relapse. 98 – 100 Moreover, case reports have described using newer TKIs, such as dasatinib and nilotinib, along with DLI to manage relapse after allogeneic HCT. 101 , 102 Although these approaches are promising, only limited data are available. Evidence from prospective studies is needed to establish the role of DLI, with or without TKIs, in the treatment of relapsed disease.
Tyrosine Kinase Inhibitors
The emergence of resistance poses a challenge for patients relapsing after initial treatment with TKI-containing regimens. Point mutations within the ABL kinase domain and alternative signaling pathways mediated by the SRC family kinase have been implicated as mechanisms of resistance. 103 – 105 The former has been identified in a large proportion of patients who experience disease recurrence after imatinib-containing therapy. 106 , 107 Moreover, ABL kinase domain mutations may be present in a small group of imatinib-naïve patients even before initiation of any TKI therapy. 108 , 109
CNS relapse has been reported in both patients with disease responsive to imatinib therapy (isolated CNS relapse with CR in marrow) and patients with disease resistant to imatinib therapy. 110 – 113 The concentration of imatinib in the cerebrospinal fluid has been shown to be approximately 2 logs lower than that achieved in the blood, suggesting that this agent does not adequately penetrate the blood-brain barrier to ensure CNS coverage. 111 , 113 A study showed that among patients with ALL treated with imatinib and who did not receive routine prophylactic intrathecal therapy or cranial irradiation, 12% developed CNS leukemia. 112 Patients with imatinib-resistant disease who developed CNS disease rapidly died of progressive disease; conversely, patients with imatinib-sensitive disease who developed isolated CNS relapse could be successfully treated with intrathecal therapy with or without cranial irradiation. 110 , 112
Dasatinib and nilotinib are second-generation TKIs that have shown greater potency in inhibiting BCR-ABL compared with imatinib, and retention of antileukemic activity in cells with certain imatinib-resistant ABL mutations. 114 – 117 In addition, dasatinib has better CNS penetration than imatinib, and therefore may have advantages in preventing CNS relapse. Both TKIs have been evaluated as single-agent therapy in patients with Ph-positive ALL that is resistant to imatinib treatment. 118 – 120 A randomized phase III study examined the activity of dasatinib administered as once-daily (140 mg daily) versus twice-daily (70 mg twice daily) dosing in patients with Ph-positive leukemia resistant to imatinib 119 ; the once-daily dosing resulted in a higher response rate (major cytogenetic response) than the twice-daily dosing (70% vs 52%). Although the median OS was shorter with the once-daily dosing (6.5 vs 9 months), the median progression-free survival owas longer (4 vs 3 months). 119 These differences in outcomes between the dosing arms were not statistically significant.
Dasatinib in combination with the hyper-CVAD regimen (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) was investigated in a phase II trial that included patients with Ph-positive relapsed ALL (n=19) and lymphoid blast phase (BP) CML (n=15). 121 An overall response rate (ORR) of 91% was obtained with 26 patients (84%) experiencing complete cytogenetic remission, 13 patients (42%) having complete molecular response, and 11 patients (35%) having a major molecular response. There were 9 patients who went on to receive allogeneic HCT, including 2 patients with ALL. In the patients with relapsed ALL, 30% remained in complete remission at 3 years with a 3-year OS of 26%. At the median follow-up of 52 months (range, 45–59 months), 2 patients (11%) with ALL were still alive.
Bosutinib, a second-generation TKI that acts as a dual inhibitor of BCR-ABL and SRC family kinases, 122 , 123 was approved in September 2012 by the FDA for the treatment of chronic, accelerated phase, or BP Ph-positive CML in adult patients with resistance to prior TKI treatment based on an open-label, multicenter phase I/II trial. 123 Efficacy and safety analyses of bosutinib monotherapy included patients with advanced leukemia [accelerated phase CML (n=79), BP CML (n=64), or ALL (n=24)] who were previously treated with at least one TKI. 124 , 125 Of the 22 evaluable patients with ALL, 2 patients (9%) attained or maintained a confirmed overall hematologic response by 4 years. 124 Common overall treatment-related adverse events reported in patients with advanced leukemia included diarrhea (74%), nausea (48%), and vomiting (44%). 124 , 125
Ponatinib is a third-generation TKI that was initially approved by the FDA in December 2012 for the treatment of adult patients with chronic, AP, or BP Ph-positive CML or Ph-positive ALL, with resistance to prior therapy, and was added as a treatment option for R/R Ph-positive ALL in 2013. Though temporarily removed from the market in November 2013, ponatinib distribution resumed in December 2013 following revision to both the prescribing information and risk evaluation and mitigation strategies program to address the risk for serious cardiovascular adverse events. This TKI has been shown to inhibit both native and mutant forms of BCR-ABL (including those resulting from T315I mutation) in preclinical studies. 126 In a multicenter, open-label, phase II study (PACE trial; n=449), ponatinib showed substantial activity in patients with Ph-positive leukemias resistant or intolerant to second-generation TKIs. 127 Major hematologic response was observed in 41% of the subgroup with Ph-positive ALL (n=32). In the subset of patients with Ph-positive ALL with ABL T315I mutation (n=22), major hematologic response was observed in 36%. 127 Common overall treatment-related adverse events in the PACE trial included thrombocytopenia (37%), rash (34%), and dry skin (32%). Additionally, arterial thrombotic events were observed and 7.1% of patients experienced cardiovascular events, 127 though dose reduction may impart a lower risk.
Not all imatinib-resistant ABL mutations are susceptible to the newer TKIs. For instance, dasatinib is not as active against cells harboring the ABL mutations T315I, V299L, and F317L. 105 , 115 , 128 , 129 Thus, for patients with disease resistant to TKI therapy, it becomes important to identify potential ABL mutations that may underlie the observed resistance to treatment. A panel of experts from the European LeukemiaNet published recommendations for the analysis of ABL kinase domain mutations in patients with CML, and treatment options according to the presence of different ABL mutations. 130
In December 2014, the FDA approved blinatumomab for the treatment of relapsed or refractory Ph-negative precursor B-AL. In July 2017, blinatumomab received full approval from the FDA for the treatment of R/R precursor B-ALL (Ph-negative and Ph-positive). A follow-up, open-label, single-arm, multicenter, phase II study evaluated the efficacy and safety of blinatumomab in patients with R/R Ph-positive ALL who had progressed after imatinib and at least one second- or third-generation TKI (n=45). 131 During the first 2 cycles of blinatumomab, 36% achieved complete remission or complete remission with partial hematologic recovery, and 88% of these responders achieved a complete MRD response. 131 Notably, responses were independent of T315I mutation status.
Inotuzumab Ozogamicin
Inotuzumab ozogamicin (InO) is a calicheamicin-based antibody-drug conjugate targeting CD22. Following the generation of encouraging single-agent phase II data, 132 a randomized study was conducted comparing InO with standard intensive chemotherapy regimens in Ph-negative or Ph-positive ALL in first or second relapse, defined as >5% marrow blasts (n=326). Compared with standard therapy, InO produced a significantly higher CR/CRi rate (80.7% vs 29.4%; P <.001), and higher MRD-negative rates (78.4% vs 28.1%; P <.001). 133 Notably, responses were consistent across most subgroups, including those with high marrow burden, and those with Ph-positive leukemia. The overall incidence of severe adverse events was similar across treatment arms, with a higher incidence of hepatic veno-occlusive disease observed in the inotuzumab group, related in part to dual alkylator-based transplant conditioning administered in remission. These data translated into a significant benefit in the median duration of remission (4.6 vs 3.1 months; P =.03), median progression-free survival (5 vs 1.8 months; P <.001), and mean OS (13.9 vs 9.9 months; P =.005). 133 In August 2017, InO received full approval from the FDA for the treatment of R/R precursor B-ALL.
CAR T Cells
Currently, bone marrow transplant is the only cure for R/R ALL, but many patients are not eligible for transplant based on age or progression of the disease. The generation of CAR T cells to treat ALL represents a significant advance in the field and has shown significantly greater OS than current regimens. 134 The pretreatment of patients with CAR T cells has served as a bridge for transplant, and patients who were formerly unable to be transplanted due to poor remission status achieve a CR and ultimately proceed to transplantation. CAR T-cell therapy relies on the genetic manipulation of a patients’ T cells to engender a response against a leukemic cell-surface antigen, most commonly CD19 135 (see “Treatment of Relapsed Ph-Negative ALL” [page 1098] for a detailed discussion of CAR T cells). CAR T-cell therapy/tisagenlecleucel was recommended for accelerated approval by the FDA oncologic drug advisory committee in July 2017 and fully approved by the FDA in August 2017 for the treatment of patients up to age 25 years (aged <26 years) with R/R precursor B-ALL.
Management of Ph-Negative ALL
- Initial Treatment in AYA Patients With Ph-Negative ALL
The AYA population with ALL can pose a unique challenge given that patients may be treated with either a pediatric or an adult protocol, depending on local referral patterns and institutional practices. Retrospective analyses based on cooperative group studies from both the United States and Europe have consistently shown the superior outcomes for AYA patients (aged 15–21 years) treated on pediatric versus adult ALL regimens. In the AYA population, 5-year EFS rates ranged from 63% to 74% for patients treated on a pediatric study protocol versus 34%–49% for those receiving the adult protocol. 39 , 40 , 66 , 136 , 137 In a retrospective comparative study that analyzed outcomes of AYA patients (aged 16–20 years) treated on a pediatric CCG study protocol (n=197; median age, 16 years) versus an adult CALGB study protocol (n=124; median age, 19 years), patients treated on the pediatric regimen compared with those on the adult regimen had significantly improved 7-year EFS (63% vs 34%, respectively; P <.001) and OS (67% vs 46%, respectively; P <.001) rates. 66 Moreover, AYA patients treated on the adult protocol experienced a significantly higher rate of isolated CNS relapse at 7 years (11% vs 1%; P =.006). The substantial improvements in outcomes observed with the pediatric regimen in this study, and in the earlier retrospective analyses from other cooperative groups, may be largely attributed to the use of greater cumulative doses of drugs, such as corticosteroids (prednisone and/or dexamethasone), vincristine, and l -asparaginase, and to earlier, more frequent, and/or more intensive CNS-directed therapy compared with adult regimens. 66 Given the success seen with multiagent intensive chemotherapy regimens for pediatric patients with ALL, several clinical trials have evaluated pediatric-inspired regimens for the AYA patient population.
For AYA patients with Ph-negative ALL in first CR, allogeneic HCT may be considered for high-risk cases—particularly for patients with disease that is MRD positive any time after induction; or patients with elevated WBC counts; or patients with B-ALL and poor-risk cytogenetics (eg, hypodiploidy, KTM2A (MLL) rearrangement) at diagnosis. A large multicenter trial (LALA-94 study) evaluated the role of postinduction HCT as one of the study objectives in adolescent and adult ALL patients receiving therapy for previously untreated ALL (n=922; median age, 33 years; range, 15–55 years). 42 Patients were stratified into 4 risk groups: (1) Ph-negative standard-risk disease [defined as achievement of CR after 1 course of chemotherapy; absence of CNS disease; absence of t(4;11), t(1;19), or other 11q23 rearrangements; WBC count <30 × 10 9 /L]; (2) Ph-negative high-risk ALL (defined as patients with non–standard-risk disease and without CNS involvement); (3) Ph-positive ALL; and (4) evidence of CNS disease. After induction therapy, patients with Ph-negative high-risk ALL were eligible to undergo allogeneic HCT if a matched sibling donor was available; those without a sibling donor were randomized to undergo autologous HCT or chemotherapy alone. 42 Among the subgroup of patients with Ph-negative high-risk ALL (n=211), the 5-year DFS and OS rates were 30% (median, 16 months) and 38% (median, 29 months), respectively. Based on intent-to-treat analysis, outcomes in patients with Ph-negative high-risk ALL were similar for autologous HCT (n=70) and chemotherapy alone (n=59) in terms of median DFS (15 vs 11 months), median OS (28 vs 26 months), and 5-year OS rate (32% vs 21%). 42 Outcomes were improved in patients with Ph-negative high-risk ALL and those with CNS involvement allocated to allogeneic HCT. The median DFS was 21 months for these patients, and the median OS has not yet been reached; the 5-year OS rate was 51%. 42 Thus, it appears that in patients with Ph-negative high-risk disease, allogeneic HCT in first CR improved DFS outcomes, whereas autologous HCT did not result in significant benefit compared with chemotherapy alone.
In the PETHEMA ALL-93 trial, adult patients with high-risk ALL [defined as having at least one of the following criteria: 30–50 years of age; WBC count ≥25 × 10 9 /L; presence of t(9;22), t(4;11), or other 11q rearrangements; and t(1;19)] received postremission induction therapy (n=222 eligible; median age, 27 years; range, 15–50 years) with allogeneic HCT (n=84; if matched related donor available), autologous HCT (n=50), or chemotherapy alone (n=48). 138 Based on intent-to-treat analysis of data from patients with Ph-negative high-risk disease, no significant advantage was observed in a donor versus no-donor comparison of median DFS (21 months vs 38 months), median OS (32 vs 67 months), 5-year DFS rate (37% vs 46%), or 5-year OS rate (40% vs 49%). In addition, when the analysis was conducted based on the actual postremission treatment received, no significant differences were noted between treatment arms for 5-year DFS rates (50% for allogeneic HCT; 55% for autologous HCT; and 54% for chemotherapy alone). 138
The role of allogeneic HCT in adults with ALL was also evaluated in the large multicenter MRC UKALL XII/ECOG E2993 study (n=1913; aged 15–59 years). 43 In this study, high risk was defined as 35 years of age or older; time to CR greater than 4 weeks from induction; elevated WBC counts (>30 × 10 9 /L for B-ALL; >100 × 10 9 /L for T-ALL); or the presence of Ph chromosome. All other patients were considered to be standard risk. Patients experiencing a remission with induction therapy were eligible to undergo allogeneic HCT if a matched sibling donor was available or, in the absence of a sibling donor, were randomized to undergo autologous HCT or chemotherapy. The 5-year OS rate was higher for patients randomized to chemotherapy alone compared with autologous HCT (46% vs 37%; P =.03). A donor versus no-donor comparison in all patients with Ph-negative ALL showed that the 5-year OS rate was significantly higher in the donor group than in the no-donor group (53% vs 45%; P =.01). This advantage in OS outcomes for the donor group was observed for patients with standard risk (62% vs 52%; P =.02) but not for those with Ph-negative high-risk disease (41% vs 35%). 43 This was partly because of the high rate of nonrelapse mortality observed with the donor group compared with the no-donor group in patients with high-risk disease (36% vs 14% at 2 years). Among patients with standard risk, the nonrelapse mortality rate at 2 years was 19.5% for the donor group and 7% for the no-donor group. Relapse rate was significantly lower in the donor group than in the no-donor group for both patients with standard risk (24% vs 49%; P <.001) and those with high risk (37% vs 63%; P <.001). 43 Nevertheless, the high nonrelapse mortality rate in the donor group among patients with high-risk disease seemed to diminish the advantage of reduced risk for relapse in this group. This study suggested that allogeneic HCT in first CR was beneficial in patients with standard-risk ALL.
The benefit of matched sibling allogeneic HCT in adult patients with standard-risk ALL was also reported by the HOVON cooperative group. In a donor versus no-donor analysis of patients with standard-risk ALL undergoing postremission therapy with matched sibling allogeneic HCT or autologous HCT, the donor arm was associated with a significantly reduced 5-year relapse rate (24% vs 55%; P <.001) and a higher 5-year DFS rate (60% vs 42%; P =.01) compared with the no-donor arm. 139 In the donor group, the nonrelapse mortality rate at 5 years was 16% and the 5-year OS rate was 69%. 139
As evidenced by the previously described studies, matched sibling HCT has been established as a valuable treatment strategy for patients with both standard and high-risk Ph-negative ALL, but subsequent studies have examined the role of URD transplants in high risk Ph-negative ALL. In a retrospective analysis of 169 patients who underwent URD HCT during first CR, 60 patients (36%) had one poor prognostic factor and 97 (57%) had multiple risk factors. The 5-year survival was 39%, which is higher than survival reported in studies of high-risk patients receiving chemotherapy alone. 140 The most significant percentage of treatment-related mortality occurred in patients who were given mismatched donors compared with partially or well-matched donors. There was no significant difference in outcome between older and younger patients, suggesting that URD transplants may be an option for older patients. In a follow-up retrospective study by the same group, RIC was evaluated to lower treatment-related mortality. 141 RIC conditioning most commonly comprised busulfan (9 mg/kg or less), melphalan (150 mg/m 2 ), low-dose total body irradiation (TBI) (less than 500 cGy single dose or less than 800 cGy fractionated), or fludarabine plus TBI of 200 cGy. RIC is more prominent in the treatment of older patients; therefore, the median age for patients receiving full-intensity (FI) conditioning was 28 years (range, 16–62 years), and for patients receiving RIC, the median age was 45 years (range, 17–66 years). Despite the variation in age, results from the study have shown no difference in relapse (35% vs 26%, P =.08) or in treatment-related mortality (FI, 33%; 95% CI, 31%–36% vs RIC, 32%; 95% CI, 23%–43%; P =.86) at 3 years. 141 The 3-year survival for HCT was similar after first CR (FI, 51%; 95% CI, 48%–55% vs RIC, 45%; 95% CI, 31–59%) and second CR (FI, 33%; 95% CI, 30%–37% vs RIC, 28%; 95% CI, 14%–44%). The DFS was similar in both groups following first CR (FI, 49%; 95% CI, 45%–53% vs RIC, 36%; 95% CI, 23%–51%) and in second CR (FI, 32%; 95% CI, 29%–36% vs RIC, 27%; 95% CI, 14%–43%). 141
A systematic review and meta-analysis of published randomized trials on postremission induction therapy in adults with ALL reported a significant reduction in all-cause mortality with allogeneic HCT in first CR (RR, 0.88; 95% CI, 0.80–0.97) compared with autologous HCT or chemotherapy. 142 A subgroup analysis showed a significant survival advantage with allogeneic HCT in standard-risk ALL, whereas a nonsignificant advantage was seen in high-risk ALL. 142 Autologous HCT in first remission was not shown to be beneficial relative to chemotherapy in several large studies and meta-analyses. 42 , 43 , 142 , 143
The CCG-1961 trial was a seminal study that allowed comparison of adult versus pediatric regimens in AYA patients. In an analysis of outcomes in children and AYA patients treated in the Dana-Farber Cancer Institute (DFCI) ALL Consortium Protocols (1991–2000), the 5-year EFS rate among younger AYA patients (age 15–18 years; n=51) was 78%, which was not significantly different from the EFS rates observed for children aged 10 to 15 years (77%; n=108) or those aged 1 to 10 years (85%; n=685). 144 The CCG 1961 study was designed to evaluate the benefit of augmented versus standard postinduction intensification therapy in children aged 1 to 9 years with high WBC counts (≥50 × 10 9 /L) or in older children and adolescents aged 10 to 21 years. 145 Patients were stratified by their initial response to induction therapy as either slow early responders (patients with >25% bone marrow blasts on day 7 of induction) or rapid early responders. Among the patients who were rapid early responders to induction (n=1,299), the augmented postinduction intensity arm was associated with significantly increased rates of 5-year EFS (81% vs 72%; P <.0001) and OS (89% vs 83%; P =.003) compared with the standard-intensity arm. 145 In the subgroup of AYA patients (age 16–21 years; n=262) from the CCG 1961 study treated with either augmented or standard-intensity regimens, the 5-year EFS and OS rates were 71.5% and 77.5%, respectively. 146 Among the AYA patients who were considered rapid early responders, the augmented-intensity (n=88) and standard-intensity (n=76) arms showed no statistically significant differences in rates of 5-year EFS (82% vs 67%, respectively) or OS (83% vs 76%, respectively). For the AYA patients who were considered slow early responders (all of whom received the augmented-intensity regimen), the 5-year EFS rate was 71%. 146
COG AALL0232
The AALL0232 trial enrolled 2,154 patients between the ages of 1 and 30 years who were diagnosed with high-risk B-ALL. 147 In this study, patients were randomly assigned to receive dexamethasone versus prednisone during induction and high-dose methotrexate versus Capizzi escalating-dose methotrexate plus pegaspargase (PEG) during interim maintenance 1. High-dose methotrexate showed improved 5-year EFS (80% vs 75%; P =.008) and OS (88.9% ± 1.2% vs 86.1% ± 1.4%; P =.25) rates compared with Capizzi escalating-dose methotrexate. No statistically significant difference was reported in the occurrence of mucositis, neurotoxicity, osteonecrosis, or other toxicities. The ALL0232 trial compared dexamethasone 10 mg/m 2 /day for 14 days to 60 mg/m 2 /day of prednisone for 28 days. Dexamethasone showed improved outcomes during induction in patients younger than 10 years of age; however, it was associated with a higher risk of osteonecrosis in patients aged 10 years or older. These data suggest that age may be an important factor for the selection of a corticosteroid. 147
PETHEMA ALL-96 Regimen
In the PETHEMA ALL-96 trial, adolescent (n=35; aged 15–18 years) and young adult (n=46; aged 19–30 years) patients with standard-risk Ph-negative ALL [defined as WBC count <30 × 10 9 /L; absence of t(9;22), t(1;19), t(4;11), or any other 11q23 rearrangements] received frontline therapy with a 5-drug induction regimen (vincristine, daunorubicin, prednisone, l -asparaginase, and cyclophosphamide), consolidation/reinduction, and maintenance, along with triple intrathecal therapy throughout the treatment period. 148 The 6-year EFS and OS rates for the entire patient cohort were 61% and 69%, respectively. No difference in EFS rate was observed between adolescents (60%; 95% CI, 43%–77%) and young adults (63%; 95% CI, 48%–78%); similarly, no significant difference was observed in OS for adolescents (77%; 95% CI, 63%–91%) versus young adults (63%; 95% CI, 46%–80%). 148 Based on multivariate regression analysis, slow response to induction therapy (defined as having >10% blast cells in the bone marrow aspirate performed on day 14 of treatment) was the only factor associated with a poor EFS (odds ratio [OR], 2.99; 95% CI, 1.25–7.17) and OS (OR, 3.26; 95% CI, 1.22–8.70). 148
DFCI ALL Regimen Based on DFCI Protocol 00-01
A multicenter phase II trial evaluated the pediatric-inspired regimen based on the DFCI Childhood ALL Consortium Protocol 00-01 in AYA and adult patients (aged 18–50 years) with previously untreated ALL; 20% of the patients in this study had Ph-positive disease. 149 The treatment regimen comprised induction (vincristine, doxorubicin, prednisone, l -asparaginase, and high-dose methotrexate), triple intrathecal therapy, intensification, and maintenance. Among the 75 patients with evaluable data, the estimated 2-year EFS and OS rates were 72.5% and 77%, respectively. 149 Adverse events included 1 death from sepsis (during induction), pancreatitis in 9 patients (12%; including 1 death), osteonecrosis in 2 patients (3%), thrombosis/embolism in 14 patients (19%), and neutropenic infection in 23 patients (31%). 149 After a median follow-up of 4.5 years, the 4-year DFS rate for patients with Ph-negative ALL (n=64) and those who achieved CR was 71% (95% CI, 58%–81%), and the 4-year OS rate for all patients with Ph-negative ALL was 70% (95% CI, 58%–79%). 150 A phase II successor trial was initiated to determine whether pegylated-asparaginase could be substituted for l -asparaginase in this regimen. 151 A high frequency of asparaginase toxicities precipitated reverting to l -asparaginase during induction and a dose-reduction of pegylated-asparaginase during consolidation. After 4 weeks, the CR rate was 89%, and with a median follow-up of 39 months, the estimated 3-year DFS and OS rates are 73% and 75%, respectively. 151 These data suggest that intensive pediatric regimens are feasible, with potential modifications, in young adults with previously untreated ALL; however, further follow-up data are needed to evaluate long-term survival outcomes.
GRAALL-2005 Regimen
The prospective phase II GRAALL-2003 study evaluated a pediatric-inspired regimen using intensified doses of vincristine, prednisone, and asparaginase for adolescents and adults with Ph-negative ALL (n=225; median age, 31 years; range, 15–60 years). 152 The induction regimen comprised vincristine, daunorubicin, prednisone, l -asparaginase, and cyclophosphamide. Patients with high-risk disease and donor availability were allowed to proceed to allogeneic HCT. The EFS and OS rates at 42 months were 55% and 60%, respectively. When data from patients who underwent transplantation at first CR were censored, the DFS rates at 42 months were 52% for patients with high-risk disease and 68% for patients with standard-risk disease (risk assignment based on GRAALL protocol); these DFS outcomes by risk groups were similar to outcomes using the MRC UKALL/ECOG definition for risk classification. 152 Advanced age was predictive of poorer survival outcomes on this study; the OS rate at 42 months was 41% for patients older than 45 years compared with 66% for those aged 45 years or younger. Moreover, compared with the younger cohort, patients older than 45 years had a higher cumulative incidence of therapy-related deaths (23% vs 5%) and deaths in first CR (22% vs 5%). 152 Thus, it seems that the benefit of this pediatric-inspired regimen outweighed the risks for therapy-related deaths only for those patients up to 45 years of age with Ph-negative ALL. The design of the GRAALL-2005 study was similar to the GRAALL-2003 trial, with the addition of randomized evaluation of hyperfractionated cyclophosphamide during induction and late intensification, as well as randomized evaluation of rituximab in patients with CD20-positive Ph-negative ALL (n=209; median age, approximately 40 years; range, 18–59 years). 153 The estimated 2-year EFS rate in the rituximab group was 65% (95% CI, 56%–75%) compared with the control group at 52% (95% CI, 43%–63%). After a median follow-up of 30 months, EFS was longer in the rituximab group than in the control group (HR, 0.66; 95% CI, 0.45–0.98; P =.04). 153
USC/MSKCC ALL Regimen Based on CCG-1882 Regimen
The USC ALL trial based on the pediatric CCG-1882 regimen has studied the regimen of daunorubicin, vincristine, prednisone, and methotrexate with augmented PEG in patients between the ages of 18 years and 60 years of age with newly diagnosed ALL (n=51). 154 , 155 The augmented arm included one long-lasting PEG dose in each cycle of the 6 total scheduled doses. Each dose of PEG (2000 IU/m 2 intravenous) was preceded with hydrocortisone for hypersensitivity prophylaxis followed by 1 to 2 weeks of oral steroids. Patients on this trial received a mean of 3.8 doses per patient with 45% of patients receiving all 6 doses, while 20% of patients discontinued treatment based on toxicity. The 7-year OS was 51% (58% of these patients were Ph-negative) and the 7-year DFS was 58%. The dose of PEG was lower than the FDA-approved dose of 2500 IU/m 2 and adjustments to the dosing interval were made to be greater than or equal to 4 weeks. This deviated from the pediatric protocol to account for the difference in drug enzymatic activity in adults. Study data suggest that adaptation of the pediatric regimen to the adult population may be feasible with modifications to reduce toxicity.
CALGB 10403 Regimen
A multicenter phase II Intergroup study (CALGB 10403) was conducted to evaluate a pediatric-inspired regimen in the treatment of AYA patients with Ph-negative ALL. One of the study objectives was to compare the outcomes of patients treated in this trial with those of a similar group of patients (in regard to age and disease characteristics) treated by pediatric oncologists in the COG trial (AALL-0232). The treatment protocol included a 4-drug induction regimen with intrathecal cytarabine and intrathecal methotrexate, consolidation, interim maintenance, delayed intensification, maintenance (for 2–3 years), and radiotherapy (for patients with testicular or CNS disease or those with T-ALL). Results from 295 evaluable patients (median age, 24 years; range 17–39 years) reported 2 postremission deaths and 3% overall treatment-related mortality. 156 The median EFS was 78.1 months (95% CI, 41.8 months to NR) and the 3-year EFS rate was 59% (95% CI, 54%–65%). The estimated 3-year OS rate was 73% (95% CI, 68%–78%). 156 It was also noted that postinduction MRD-positivity, Ph-like gene expression signatures and obesity were associated with worse treatment outcomes. 156
COG AALL0434 Regimen
Nelarabine is a nucleoside metabolic inhibitor and a prodrug of ara-G, approved for the treatment of patients with T-ALL with disease that has not responded to or that has relapsed after at least 2 chemotherapy regimens. The randomized phase III COG study (AALL0434) evaluated the safety of nelarabine as part of frontline therapy, using the augmented BFM chemotherapy regimen, with or without nelarabine, and showed that the toxicity profiles were similar between patients with high-risk T-ALL who received nelarabine (n=47) and those who did not (n=47). 157 No significant differences were observed in the occurrence of neurologic adverse events between these groups, including peripheral motor neuropathy, peripheral neuropathy, or CNS neurotoxicity. The incidence of adverse events such as febrile neutropenia and elevation of liver enzymes was also similar between treatment groups. These initial safety data suggest that nelarabine may be better tolerated in frontline regimens than in the R/R setting. 157
Results from the efficacy phase of this study evaluated data from 1,895 patients with newly diagnosed T-ALL and T-LL. 158 Patients were randomized to receive escalating dose methotrexate without leucovorin rescue and PEG or high-dose methotrexate with leucovorin rescue. Intermediate and high-risk patients with T-ALL and T-LL all received prophylactic or therapeutic cranial irradiation and were randomized into arms with or without nelarabine (650 mg/m 2 /day). The 4-year DFS rate for patients with T-ALL in the nelarabine arm (n=323) versus those who did not receive nelarabine (n=336) was 88.9%±2.2% and 83.3%±2.5%, respectively ( P =.0332). 158 Compared with the high-dose methotrexate and nelarabine arm, use of escalating-dose methotrexate and nelarabine appeared to enhance the 4-year DFS rates. 158 Another report from the COG AALL0434 study determined that compared with high-dose methotrexate, escalating-dose methotrexate combined with augmented BFM chemotherapy improves DFS and OS outcomes in patients with T-ALL. 159
A single-arm phase II study from the MDACC evaluated the efficacy of hyper-CVAD plus nelarabine as frontline therapy in adult patients with T-ALL (n=23). 160 With a median follow-up of 30.4 months (range, 2.4–69.2 months), the CR rate for patients with T-ALL was 89%; however, a trend for inferior DFS and OS was observed for patients with ETP ALL. 160 After a median follow-up of 42.5 months, the 3-year complete remission duration and OS rates were 66% (95% CI, 52%–77%) and 65% (95% CI, 51%–76%), respectively. 161 These studies suggest that for patients with T-ALL, the addition of nelarabine to frontline therapy may be a promising approach.
Hyper-CVAD With or Without Rituximab
The hyper-CVAD regimen constitutes another commonly used ALL treatment regimen for adult patients. A phase II study from MDACC evaluated hyper-CVAD in adolescents and adults with previously untreated ALL (n=288; median age, 40 years; range, 15–92 years; Ph-positive in 17%). 20 The median OS for all patients was 32 months and the 5-year OS rate was 38%, with a median follow-up of 63 months. Among the patients with Ph-negative ALL (n=234), the 5-year OS rate was 42%. 20 Among patients who experienced a CR (92% of all patients), the 5-year CR duration rate was 38%. 20 Death during induction therapy occurred in 5% of patients, and was more frequent among patients aged 60 years or older. The 5-year OS in patients aged 60 or older was 17%. 20 A subsequent retrospective review from the same institution suggested that this may be related to higher rates of death in remission (34%) relative to younger patients (7%). 162
Based on retrospective analyses of data from adults with B-ALL treated in clinical trials, CD20 positivity (generally defined as CD20 expression on >20% of blasts) was found to be associated with adverse outcomes measured by a higher cumulative incidence of relapse, decreased CR duration, or decreased survival. 163 , 164 Given the prognostic significance of CD20 expression in these patients, treatment regimens incorporating the CD20 monoclonal antibody rituximab have been evaluated. A phase II study from MDACC evaluated hyper-CVAD with or without rituximab in previously untreated patients with Ph-negative B-lineage ALL (n=282; median age, 41 years; range, 13–83 years). 165 Among the subgroup of patients with CD20-positive ALL who were treated with hyper-CVAD combined with rituximab, the 3-year CR duration and OS rates were 67% and 61%, respectively. In addition, among the younger patients (age <60 years) with CD20-positive disease, modified hyper-CVAD plus rituximab resulted in a significantly improved CR duration (70% vs 38%; P <.001) and OS rate (75% vs 47%; P =.003) compared with the standard hyper-CVAD regimen without rituximab. 165 No significant differences in outcomes with the addition of rituximab were noted for the subgroup of patients with CD20-negative disease. Notably, older patients (aged ≥60 years) with CD20-positive disease demonstrated higher rates of MRD negativity with the inclusion of rituximab; however, this did not translate into a survival benefit, again largely due to increased mortality in CR. It is worth noting that this high rate of death in CR for older patients may relate to anthracycline intensification as opposed to rituximab. 166
Linker 4-Drug Regimen
Linker et al 167 evaluated an intensified chemotherapy regimen that incorporated a 4-drug induction regimen (comprising vincristine, daunorubicin, prednisone, and asparaginase) with or without rituximab for CD-20 positive disease in adolescent and adult patients with ALL (n=84; Ph-positive in 16%; median age, 27 years; range, 16–59 years). The 5-year EFS and OS rates for all patients were 48% and 47%, respectively. Among the patients who experienced a CR (93% of all patients), the 5-year EFS rate was 52%. The 5-year EFS rate was 60% for the subgroup of patients without high-risk features (n=53). 167
Blinatumomab has shown promising clinical efficacy as a means of eradicating persistent MRD following upfront chemotherapy. In a multicenter, single-arm, phase II study, Topp et al 168 evaluated the efficacy of blinatumomab in MRD-positive patients with Ph-negative B-ALL (n=21; age range, 20–77 years). Patients were considered MRD-positive if they had never achieved MRD negativity before blinatumomab, or had experienced a hematologic CR with MRD ≥10 −4 . After blinatumomab treatment, 16 of 20 evaluable patients were determined to be MRD-negative at a detection threshold of 10 −4 . 168 After a median follow-up of 33 months, the hematologic RFS of the evaluable cohort was 61%. 169 Gökbuget et al 170 examined the efficacy of blinatumomab in an expanded cohort (n=116) using a higher threshold for MRD positivity (hematologic CR with MRD ≥10 −3 ). After one 28-day cycle of blinatumomab, 88 of 113 evaluable patients achieved a complete MRD response, and the RFS rate at 18 months was 54%. 170 In both of these trials, most patients achieving MRD negativity after blinatumomab proceeded to allogeneic HCT, establishing blinatumomab as an effective “bridge to transplant” in MRD-positive patients. Subsequent studies of blinatumomab evaluated its ability to induce CR (including rapid MRD-negative responses) in patients with R/R B-precursor ALL. 171 – 173 In March 2018, the FDA approved blinatumomab use for the treatment of adult and pediatric patients with B-cell precursor ALL in first or second CR with MRD defined as disease ≥0.1% (see “Treatment of Relapsed Ph-Negative ALL,” [next section] for discussion of studies related to blinatumomab use in R/R B-ALL).
- Treatment of Relapsed Ph-Negative ALL
Despite major advances in the treatment of childhood ALL, approximately 20% of pediatric patients experience relapse after initial CR to frontline treatment regimens. 174 – 176 Among those who experience relapse, only approximately 30% experience long-term remission with subsequent therapies. 177 – 179 Based on a retrospective analysis of historical data from COG studies (for patients enrolled between 1998 and 2002; n=9585), early relapse (<18 months from diagnosis) was associated with very poor outcomes, with an estimated 5-year survival (from time of relapse) of 21%. 174 For cases of isolated bone marrow relapse, the 5-year survival estimates among early (n=412), intermediate (n=324), and late (n=387) relapsing disease were 11.5%, 18.0%, and 43.5%, respectively ( P <.0001). Intermediate relapse was defined as relapse occurring between 18 and 36 months from time of diagnosis; late cases were defined as relapse occurring 36 months or more from time of diagnosis. For cases of isolated CNS relapse, the 5-year survival estimates among early (n=175), intermediate (n=180), and late (n=54) relapsing disease were 43.5%, 68.0%, and 78.0%, respectively ( P <.0001). 174 Based on multivariate analysis (adjusted for both timing and site of relapse), age (>10 years), presence of CNS disease at diagnosis, male gender, and T-cell lineage disease were found to be significant independent predictors of decreased survival after relapse. 174 In a separate analysis of data from one of the above COG studies (CCG-1952), the timing and site of first relapse were significantly predictive of EFS and OS outcomes, even among the patients with standard-risk ALL (n=347; based on NCI criteria: aged 1 to <10 years and WBC count <50 × 10 9 /L). 180 Early bone marrow relapse (duration of first CR <36 months) was associated with significantly shorter estimated 3-year EFS (30% vs 44.5%; P =.002) and OS (35% vs 58%; P =.001) rates compared with late bone marrow relapse. 180 Similarly, early isolated extramedullary relapse (duration of first CR <18 months) was associated with significantly shorter estimated 3-year EFS (37% vs 71%; P =.01) and OS (55% vs 81.5%; P =.039) rates compared with late extramedullary relapse. In a multivariate regression analysis, early bone marrow and extramedullary relapse were independent predictors of poorer EFS outcomes. 180
Data from patients with disease relapse after frontline therapy in the MRC UKALL XII/ECOG E2993 study and PETHEMA studies showed that the median OS after relapse was only 4.5 to 6 months; the 5-year OS rate was 7%–10%. 90 , 91 Approximately 20%–30% of patients experience a second CR with second-line therapies. 91 , 93 Factors predictive of more favorable outcomes after subsequent therapies included younger age and a first CR duration of more than 2 years. 91 , 181 Among younger patients (aged <30 years) whose disease relapsed after experiencing a first CR duration longer than 2 years with frontline treatment in PETHEMA trials, the 5-year OS rate from the time of first relapse was 38%. 91
HCT is the only potentially curative modality for R/R ALL. Based on findings from evidence-based review of the published literature, the American Society for Blood and Marrow Transplantation guidelines recommend HCT over chemotherapy alone for adult patients with ALL experiencing a second CR. 182 Several studies have shown that for AYA patients in second CR, allogeneic HCT may improve outcomes, particularly for patients who have early bone marrow relapse or have other high-risk factors. 178 , 179 , 183 Seemingly contradictory data were reported in the COG CCG-1952 study that showed prognosis after early bone marrow relapse in patients with standard-risk ALL (aged 1 to <10 years and WBC count <50 × 10 9 /L) remained poor with no apparent advantage of HCT, regardless of timing (ie, early or late) of bone marrow relapse. 180 However, data were not available on the conditioning regimen used for HCT in this study for comparison with other trials. The UKALLXII/ECOG2993 trial (n=609; age range, 15–60 years) examined the efficacy of transplantation after relapse in a subgroup of patients with relapsed ALL who had not received prior transplant. 90 Patients treated with HCT demonstrated a superior OS at 5 years compared with those treated with chemotherapy alone. 90 The CIBMTR group conducted an analysis of outcomes of patients with ALL (n=582; median age, 29 years; range, <1–60 years) who underwent transplant during relapse. 184 At 3 years, OS rates were 16% (95% CI, 13%–20%). 184 Response to salvage therapy prior to HCT may also predict outcome. One retrospective study has shown 3-year OS and EFS estimates of 69% and 62% (respectively) for patients in second or later MRD-negative remission at the time of HCT, similar to the outcomes of those who underwent HCT in MRD-negative first remission at the same center. 185
A component of the growing arsenal of immunotherapies for cancer treatment, blinatumomab is a bispecific anti-CD3/CD19 monoclonal antibody that showed high CR rates (69%; including rapid MRD-negative responses) in patients with R/R B-precursor ALL (n=25). 173 , 186 Blinatumomab was approved by the FDA based on data from a large phase II confirmatory study of 189 patients with R/R Ph-negative B-ALL that demonstrated a CR or CR with incomplete platelet recovery (CRp) in 43% of patients within the first 2 cycles of treatment. 172 , 187 In a follow-up prospective, multicenter, randomized, phase III trial, patients with R/R B-cell precursor ALL (n=405) were assigned to receive either blinatumomab (n=271) or standard chemotherapy (n=134). 171 The OS was longer in the blinatumomab group, with median OS at 7.7 months, compared with the standard chemotherapy group, with median OS at 4.0 months (95% CI, 0.55–0.93, P =.01). 171 Remission rates within 12 weeks after treatment initiation were significantly higher in the blinatumomab group than in the standard chemotherapy group with respect to both CR with full hematologic recovery (CR, 34% vs 16%; P <.001) and CR with full, partial, or incomplete hematologic recovery (CR, CRh, or CRi, 44% vs 25%; P <.001). 171 Of note, prespecified subgroup analyses of patients with high bone marrow count (≥50%) at relapse demonstrated lower blinatumomab-mediated median survival and remission rates. 171
There are significant and unique side effects to blinatumomab treatment compared with the current standard-of-care regimens. The most significant toxicities noted in clinical studies are CNS events and cytokine release syndrome (CRS). Neurologic toxicities have been reported in 50% of patients (median onset, 7 days) and grade 3 or higher neurologic toxicities, including encephalopathy, convulsions, and disorientation, have occurred in 15% of patients. 188 CRS typically occurs within the first 2 days after initiation of blinatumomab infusion. 188 Symptoms of CRS include pyrexia, headache, nausea, asthenia, hypotension, increased transaminases, and increased total bilirubin. The incidence of adverse events can be reduced with monitoring for early intervention at onset of symptoms. However, the serious nature of these events underscores the importance of receiving treatment in a specialized cancer center that has experience with blinatumomab.
Clinical studies described earlier include patients with relapsed or refractory Ph-positive and Ph-negative ALL. 132 , 133 For discussion of these studies, see “Treatment of Relapsed Ph-Positive ALL” (page 1091).
In a phase II study, the efficacy and safety of inotuzumab ozogamicin combined with low-intensity chemotherapy (mini-hyper-CVD) was evaluated in adults with R/R B-ALL (n=59; median age, 35 years; range, 18–87 years). 189 The response rate was 78%, with 35 of these patients achieving CR (59%). 189 The overall MRD negativity rate among responders was 82%. With a median follow-up of 24 months, the median RFS and OS were 8 and 11 months, respectively. The 1-year RFS and OS rates were 40% and 46%, respectively. When using this regimen, the risk of veno-occlusive disease should be considered in patients with previous liver damage and among transplant candidates. In this study, veno-occlusive disease occurred in 9 patients (15%). 189
In a subsequent report, to reduce the risk of veno-occlusive disease and improve outcomes, the investigators amended the protocol by lowering the weekly inotuzumab ozogamicin doses and including 4 cycles of blinatumomab in the consolidation phase. 190 In a cohort of adult patients with Ph-negative B-ALL treated in first relapse (n=48; median age, 39 years; range, 18–87 years), the rates of veno-occlusive disease prior to the protocol amendment and after the protocol amendment were 13% (n=5 of 38) and 0% (n=0 of 10), respectively. 190 In addition, based on propensity score matching, the combination of inotuzumab ozogamicin with mini-hyper-CVD with or without blinatumomab resulted in better outcomes than inotuzumab alone or intensive salvage chemotherapy. 190
One of the early treatments for patients with advanced ALL included adoptive cell therapy to induce a graft-versus-leukemia effect through allogeneic HCT or DLI. However, this method resulted in a significant risk of GVHD. To circumvent this issue, current advances are focused on the use of the patient’s own T cells to target the tumor. The generation of CAR T cells to treat ALL is a significant advancement in the field. 134 , 191 , 192 CAR T-cell therapy relies on the genetic manipulation of a patients’ T-cells to generate a response against a leukemic cell-surface antigen, most commonly CD19. 135 Briefly, T cells from the patient are harvested and engineered with a receptor that targets a cell surface tumor-specific antigen (eg, CD19 antigen on the surface of leukemic cells). The ability of CAR T cells to be reprogrammed to target any cell-surface antigen on leukemic cells is advantageous and avoids the issue of tumor evasion of the immune system via receptor downregulation. 135 The manufacture of CAR T cells requires ex vivo viral transduction, activation, and expansion over several days to produce a sufficient cell number to engender disease response. 193 Following infusion, debulking of tumors occurs in less than a week and these cells may remain in the body for extended periods of time to provide immunosurveillance against relapse.
There are several clinical trials using CAR T cells that differ in the receptor construct for patients with relapsed or refractory ALL. One of the first CAR constructs to be investigated, termed 19-28z—which links the CD19 binding receptor to the costimulatory protein CD28—demonstrated an overall CR in 14 of 16 patients with relapsed or refractory B-ALL after infusion with CAR T cells. 194 This average remission rate is significantly improved compared with the average remission rate for patients receiving standard-of-care chemotherapy following relapse (88% vs approximately 30%). 90 , 194 – 196 Furthermore, 7 of 16 patients were able to receive an allogeneic HCT, suggesting that CAR T cells may provide a bridge to transplant. 194 No relapse has been seen in patients who had allogeneic HCT (follow-up, 2–24 months); however, 2 deaths occurred from transplant complications. Follow-up data of adult patients enrolled on this trial (n=53) showed an 83% CR rate after the infusion and 32 patients achieved an MRD-negative CR. 197 At a median follow-up of 29 months (range, 1–65), the median OS was 12.9 months (95% CI, 8.7–23.4 months) and subsequent allogeneic HCT did not appear to improve survival. 197 In contrast, data in children and young adults treated on another clinical trial at the National Institutes of Health/National Cancer Institute with a similar CAR construct suggested consolidative allogeneic HCT post-CAR T-cell therapy might be associated with superior outcomes (2-year cumulative incidence of relapse posttransplant, 9.5%; 5-year event-free survival posttransplant, 62%). 198 The ZUMA-3 clinical trial phase II results using the CAR T-cell product KTE-X19 (brexucabtagene autoleucel) also showed promising efficacy in adults with R/R ALL, by demonstrating MRD-negative CR in 76% of patients, with a median duration of remission of 12.8 months, a median relapse-free survival of 11.6 months, and a median overall survival of 18.2 months. 199 KTE-X19 is currently being evaluated in children and young adults with R/R ALL.
Other CD19-targeted constructs have been investigated, some comprising an alternative costimulatory protein, 4-1BB have shown similar results to the 19-28z CAR T cells in terms of overall CR. 200 Relevant in this context are data from the ELIANA trial of CTL019 (tisagenlecleucel) in 75 children and young adults with R/R B-ALL, which demonstrated an overall remission rate of 81% within 3 months of infusion, all of which were notably MRD negative. 201 These results led to the approval of CTL019 by the FDA in August 2017 for the treatment of patients up to age 25 years (aged <26 years) with R/R precursor B-ALL. The efficacy of CTL019 in children and young adults with R/R B-ALL in the nontrial setting was recently confirmed using registry data from the Center for International Blood and Marrow Transplant Research (CIBMTR). This retrospective analysis showed morphologic CR in 85% of patients. 202 MRD negativity was reported in 99% of CR patients with available data. A comparable proportion of patients experienced durable responses at 12 months in the CIBMTR cohort compared with patients treated on the ELIANA clinical trial (61% and 67%, respectively). At the last update of the ELIANA data at the 2019 American Society of Transplantation and Cellular Therapy (ASTCT) Annual Meeting (median follow-up, 24 months), the median duration of remission and overall survival was not reached and the 24-month relapse-free survival probability in responders was 62%. Survival probability curves plateaued after 1 year. Consolidation with allo-HCT after CTL019 was reported in only 9% of CR patients. These updated results suggest treatment with CTL019 in children and young adults with R/R B-ALL could be curative in a subset of patients in the absence of consolidative allo-HCT. 203
As with blinatumomab, T-cell and CAR T-cell activation can be accompanied by severe CRS and neurologic toxicity (immune effector cell associated neurotoxicity syndrome or ICANS), as well as infectious risks—though treatment-related mortality remains low. 201 Although side effects from CAR T cells can be severe, they are reversible in most cases. CRS is clinically characterized by high fever, hypotension, tachycardia, and hypoxia; ICANS includes delirium, aphasia, headaches, tremor, focal deficits, and cerebral edema. Higher CRS and ICANS severity have been reported in B-ALL patients compared with non-Hodgkin lymphoma patients after CD19 CAR T-cell therapy. 204 It is recommended to evaluate CRS and ICANS severity using the ASTCT consensus criteria. 205 Tocilizumab (interleukin-6 receptor antagonist) and corticosteroids are the cornerstone of CRS and ICANS management. Expert consensus clinical guidelines were recently published by the Society of Immunotherapy of Cancer to guide toxicity management. 206
Nelarabine is a nucleoside analog that is currently approved for the treatment of patients with T-ALL who have unresponsive or relapsed disease after at least 2 chemotherapy regimens. A phase II study of nelarabine monotherapy in children and adolescents with R/R T-ALL or T-cell non-Hodgkin lymphoma (n=121) showed a 55% response rate among the subgroup with T-ALL with first bone marrow relapse (n=34) and a 27% response rate in the subgroup with a second or greater bone marrow relapse (n=36). 177 Major toxicities included grade 3 or higher neurologic (both peripheral and CNS) adverse events in 18% of patients. Nelarabine as single-agent therapy was also evaluated in adults with R/R T-ALL or T-cell lymphoblastic leukemia in a phase II study (n=39; median age, 34 years; range, 16–66 years; median 2 prior regimens; T-ALL, n=26). 207 The CR rate (including CRi) was 31%; an additional 10% of patients experienced a partial remission. The median DFS and OS were both 20 weeks and the 1-year OS rate was 28%. Grade 3 or 4 myelosuppression was common, but only one case of grade 4 CNS toxicity (reversible) was observed. 207
There are limited studies of nelarabine combination regimens in adults with R/R T-ALL. In a study by Commander et al, pediatric patients with R/R T-ALL (n=7; range, 1–19 years) were treated with nelarabine, etoposide and cyclophosphamide. 208 In addition, all patients received intrathecal prophylaxis with methotrexate or triple intrathecal therapy with methotrexate, cytarabine, and hydrocortisone. All patients experienced a CR after 1 or 2 courses of therapy. The most common adverse events attributed to nelarabine were grade 2 and 3 sensory and motor neuropathy and musculoskeletal pain. 208 In phase I of the NECTAR trial, pediatric patients with R/R T-ALL and T-LL (range, 1–21 years) were also treated with nelarabine, etoposide and cyclophosphamide. 209 Of nine evaluable T-ALL patients, there were 2 CRs, 1 partial CR, and 1 CR in the bone marrow/PR in an extramedullary site for a response rate of 44%. 209
Augmented Hyper-CVAD
A phase II study from the MDACC evaluated an augmented hyper-CVAD regimen (that incorporated asparaginase, intensified vincristine, and intensified dexamethasone) as therapy in adults with R/R ALL (n=90; median age, 34 years; range, 14–70 years; median 1 prior regimen). 210 Among evaluable patients (n=88), the CR rate was 47%; an additional 13% experienced a CRp and 5% experienced a partial remission. The 30-day mortality rate was 9% and median remission duration was 5 months. The median OS for all evaluable patients was 6.3 months; median OS was 10.2 months for patients who experienced a CR. In this study, 32% of patients were able to proceed to HCT. 210
Vincristine Sulfate Liposomal Injection
Vincristine sulfate liposome injection (VSLI) is a novel nanoparticle formulation of vincristine encapsulated in sphingomyelin and cholesterol liposomes; the liposome encapsulation prolongs the exposure of active drug in the circulation and may allow for delivery of increased doses of vincristine without increasing toxicities. 211 , 212 VSLI was evaluated in an open-label, multicenter, phase II study in adult patients with Ph-negative ALL (n=65; median age, 31 years; range, 19–83 years) in second or greater relapse, or with disease that progressed after 2 or more prior lines of therapy (RALLY study). 196 The CR (CR + CRi) rate with single-agent VSLI was 20%. The median duration of CR was 23 weeks (range, 5–66 weeks) and the median OS for all patients was 20 weeks (range, 2–94 weeks); median OS for patients achieving a CR was 7.7 months. 196 The incidence of early induction death (30-day mortality rate) was 12%. 196 These outcomes appeared favorable compared with published single-center historical data in patients with Ph-negative ALL treated with other agents at second relapse (n=56; CR rate, 4%; median OS, 7.5 weeks; early induction death, 30%). 196 , 213 The most common grade 3 or greater treatment-related toxicities with VSLI included neuropathy (23%), neutropenia (15%), and thrombocytopenia (6%). 196 Based on phase II data from the RALLY study, VSLI was given accelerated FDA approval in September 2012 for the treatment of adult patients with Ph-negative B-ALL in second or greater relapse. Confirmation of benefit from phase III studies is pending.
Clofarabine
Clofarabine is a nucleoside analog approved for the treatment of pediatric patients (aged 1–21 years) with ALL that is relapsed or refractory after at least 2 prior regimens. In a phase II study of single-agent clofarabine in heavily pretreated pediatric patients with R/R ALL (n=61; median age, 12 years; range, 1–20 years), the response rate (CR + CRp) was 20%. 214 Single-agent clofarabine in this setting was associated with severe liver toxicities (generally reversible) and frequent febrile episodes including grade 3 or 4 infections and febrile neutropenia. 214 Phase II studies evaluating the combination of clofarabine with cyclophosphamide and etoposide in pediatric patients with R/R ALL have resulted in response rates ranging from 44% to 52%. 215 , 216 This combination has been associated with prolonged and severe myelosuppression, febrile episodes, severe infections (including sepsis or septic shock), mucositis, and liver toxicities including fatal veno-occlusive disease (the latter occurring in the postallogeneic HCT setting). 215
There are limited studies of clofarabine combination regimens in adults with R/R disease. In a study by Miano et al, 217 pediatric patients with R/R ALL (n=24; median age, 7.6 years; range, 1–20 years) were treated with clofarabine, etoposide, and cyclophosphamide, and 42% (10 of 24) of patients responded to treatment, with a 24-month OS rate of 25%. 217 In a study from GRAALL, adult patients with R/R ALL (n=55) were treated with clofarabine in combination with conventional chemotherapy (cyclophosphamide [ENDEVOL cohort; median age, 53 years; range, 18–78 years], or a more intensive regimen with dexamethasone, mitoxantrone, etoposide, and PEG-asparaginase [VANDEVOL cohort; median age, 34 years; range, 19–67 years]). Patients in the ENDEVOL cohort achieved a CR of 50% (9 of 18) and patients in the VANDEVOL cohort yielded a CR rate of 41% (15 of 37); the median OS was 6.5 months after a median follow-up of 6 months. 218 The most common grade 3 or 4 toxicities included infection (58%) and liver toxicities (24%), with an early death rate of 11%. 218 Because the use of clofarabine-containing regimens require close monitoring and intensive supportive care measures, patients should only be treated in centers with expertise in the management of ALL, preferably in the context of a clinical trial.
MOpAD Regimen
A single-arm trial evaluating the efficacy of the MOAD regimen (methotrexate, vincristine, l -asparaginase, and dexamethasone) in newly diagnosed adults with ALL (n=55) demonstrated a CR rate of 76% with a median CR duration of over 12 months. 219 A phase II trial incorporated a new PEGylated formulation of l -asparaginase due to improved tolerability, 220 and examined the safety and efficacy of the MOpAD regimen (methotrexate, vincristine, PEG- l -asparaginase, and dexamethasone) in adults with relapsed or refractory ALL (n=37). 221 For patients with Ph-positive ALL, TKIs (ie, imatinib, dasatinib, nilotinib) were added to the regimen and if patients had CD20-positive B-ALL, rituximab was added to the regimen. The CR and ORR rates were 28% and 39%, respectively, with a median duration of response of 4.3 months. 221 Patients with Ph-positive ALL had CR and ORR rates of 50% and 67%, respectively. 221 This regimen may be considered in patients who have received a maximal dose of anthracycline and have cardiac dysfunction and limited performance status.
Studies evaluating other novel TKIs in targeting specific genetic subtypes have been evaluated for the treatment of R/R T-ALL disease. Although daratumumab has efficacy in its application for MRD, it has been reported to have potential preclinical benefit in T-ALL with positive CD38 expression. 222 The use of selective BCL2 inhibitor, venetoclax, has been retrospectively analyzed in the treatment of R/R T-ALL. In this analysis, 60% of patients receiving venetoclax plus various chemotherapeutic agents such as hyper-CVAD, nelarabine, or decitabine, achieved remission in marrow blasts, with a median overall survival of 7.7 months. 223 Proteasome inhibition with the use of bortezomib in combination with chemotherapeutic agents has been suggested to improved relapse response rates in T-ALL patients. In a phase II COG study, patients with ALL were treated with reinduction chemotherapy plus bortezomib. 224 Relapsed T-ALL patients showed a complete response rate of 68%, with end of induction MRD significantly predicting survival. 224
Jabbour EJ , Faderl S , Kantarjian HM . Adult acute lymphoblastic leukemia . Mayo Clin Proc 2005 ; 80 : 1517 – 1527 .
- Search Google Scholar
- Export Citation
Howlader NNA , Krapcho M , Miller D , et al. SEER Cancer Statistics Review 1975-2018 . Bethesda, MD : National Cancer Institute ; 2021 .
Siegel RL , Miller KD , Fuchs HE , et al. Cancer Statistics, 2021 . CA Cancer J Clin 2021 ; 71 : 7 – 33 .
Esparza SD , Sakamoto KM . Topics in pediatric leukemia--acute lymphoblastic leukemia . MedGenMed 2005 ; 7 : 23 .
Hasle H . Pattern of malignant disorders in individuals with Down’s syndrome . Lancet Oncol 2001 ; 2 : 429 – 436 .
Whitlock JA . Down syndrome and acute lymphoblastic leukaemia . Br J Haematol 2006 ; 135 : 595 – 602 .
Swaminathan M , Bannon SA , Routbort M , et al. Hematologic malignancies and Li-Fraumeni syndrome . Cold Spring Harb Mol Case Stud 2019 ; 5 : a003210 .
Stiller CA , Chessells JM , Fitchett M . Neurofibromatosis and childhood leukaemia/lymphoma: a population-based UKCCSG study . Br J Cancer 1994 ; 70 : 969 – 972 .
Shaw MP , Eden OB , Grace E , et al. Acute lymphoblastic leukemia and Klinefelter’s syndrome . Pediatr Hematol Oncol 1992 ; 9 : 81 – 85 .
Gürgey A , Kara A , Tuncer M , et al. Acute lymphoblastic leukemia associated with Klinefelter syndrome . Pediatr Hematol Oncol 1994 ; 11 : 227 – 229 .
Machatschek JN , Schrauder A , Helm F , et al. Acute lymphoblastic leukemia and Klinefelter syndrome in children: two cases and review of the literature . Pediatr Hematol Oncol 2004 ; 21 : 621 – 626 .
Flatt T , Neville K , Lewing K , et al. Successful treatment of fanconi anemia and T-cell acute lymphoblastic leukemia . Case Rep Hematol 2012 ; 2012 : 396395 .
Yetgin S , Tuncer M , Güler E , et al. Acute lymphoblastic leukemia in Fanconi’s anemia . Am J Hematol 1994 ; 45 : 94 .
Strevens MJ , Lilleyman JS , Williams RB . Shwachman’s syndrome and acute lymphoblastic leukaemia . BMJ 1978 ; 2 : 18 .
Woods WG , Roloff JS , Lukens JN , et al. The occurrence of leukemia in patients with the Shwachman syndrome . J Pediatr 1981 ; 99 : 425 – 428 .
Passarge E . Bloom’s syndrome: the German experience . Ann Genet 1991 ; 34 : 179 – 197 .
Taylor AM , Metcalfe JA , Thick J , et al. Leukemia and lymphoma in ataxia telangiectasia . Blood 1996 ; 87 : 423 – 438 .
Ma H , Sun H , Sun X . Survival improvement by decade of patients aged 0-14 years with acute lymphoblastic leukemia: a SEER analysis . Sci Rep 2014 ; 4 : 4227 .
Pulte D , Gondos A , Brenner H . Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century . Blood 2009 ; 113 : 1408 – 1411 .
Kantarjian H , Thomas D , O’Brien S , et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia . Cancer 2004 ; 101 : 2788 – 2801 .
Pulte D , Jansen L , Gondos A , et al. Survival of adults with acute lymphoblastic leukemia in Germany and the United States . PLoS One 2014 ; 9 : e85554 .
Sive JI , Buck G , Fielding A , et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial . Br J Haematol 2012 ; 157 : 463 – 471 .
Geyer MB , Hsu M , Devlin SM , et al. Overall survival among older US adults with ALL remains low despite modest improvement since 1980: SEER analysis . Blood 2017 ; 129 : 1878 – 1881 .
Wermann WK , Viardot A , Kayser S , et al. Comorbidities are frequent in older patients with de novo acute lymphoblastic leukemia (ALL) and correlate with induction mortality: analysis of more than 1200 patients from GMALL data bases . Blood 2018 ; 132 ( Supplement 1 ): 660 .
Stock W . Adolescents and young adults with acute lymphoblastic leukemia . Hematology (Am Soc Hematol Educ Program) 2010 ; 2010 : 21 – 29 .
Tasian SK , Loh ML , Hunger SP . Philadelphia chromosome-like acute lymphoblastic leukemia . Blood 2017 ; 130 : 2064 – 2072 .
Smith M , Arthur D , Camitta B , et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia . J Clin Oncol 1996 ; 14 : 18 – 24 .
Schultz KR , Pullen DJ , Sather HN , et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children’s Cancer Group (CCG) . Blood 2007 ; 109 : 926 – 935 .
Brown P , Pieters R , Biondi A . How I treat infant leukemia . Blood 2019 ; 133 : 205 – 214 .
Hunger SP , Loh ML , Whitlock JA , et al. Children’s Oncology Group’s 2013 blueprint for research: acute lymphoblastic leukemia . Pediatr Blood Cancer 2013 ; 60 : 957 – 963 .
Gadner H , Masera G , Schrappe M , et al. The Eighth International Childhood Acute Lymphoblastic Leukemia Workshop (‘Ponte di legno meeting’) report: Vienna, Austria, April 27-28, 2005 . Leukemia 2006 ; 20 : 9 – 17 .
Reshmi SC , Harvey RC , Roberts KG , et al. Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group . Blood 2017 ; 129 : 3352 – 3361 .
Behm FG , Raimondi SC , Frestedt JL , et al. Rearrangement of the MLL gene confers a poor prognosis in childhood acute lymphoblastic leukemia, regardless of presenting age . Blood 1996 ; 87 : 2870 – 2877 .
Pui CH , Chessells JM , Camitta B , et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements . Leukemia 2003 ; 17 : 700 – 706 .
Donadieu J , Auclerc MF , Baruchel A , et al. Prognostic study of continuous variables (white blood cell count, peripheral blast cell count, haemoglobin level, platelet count and age) in childhood acute lymphoblastic leukaemia. Analysis of a population of 1545 children treated by the French Acute Lymphoblastic Leukaemia Group (FRALLE) . Br J Cancer 2000 ; 83 : 1617 – 1622 .
Romana SP , Mauchauffé M , Le Coniat M , et al. The t(12;21) of acute lymphoblastic leukemia results in a tel-AML1 gene fusion . Blood 1995 ; 85 : 3662 – 3670 .
Sutcliffe MJ , Shuster JJ , Sather HN , et al. High concordance from independent studies by the Children’s Cancer Group (CCG) and Pediatric Oncology Group (POG) associating favorable prognosis with combined trisomies 4, 10, and 17 in children with NCI Standard-Risk B-precursor Acute Lymphoblastic Leukemia: a Children’s Oncology Group (COG) initiative . Leukemia 2005 ; 19 : 734 – 740 .
Seibel NL . Treatment of acute lymphoblastic leukemia in children and adolescents: peaks and pitfalls . Hematology (Am Soc Hematol Educ Program) 2008 ; 2008 : 374 – 380 .
Boissel N , Auclerc M-F , Lhéritier V , et al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials . J Clin Oncol 2003 ; 21 : 774 – 780 .
Ramanujachar R , Richards S , Hann I , et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials . Pediatr Blood Cancer 2007 ; 48 : 254 – 261 .
Zhang MJ , Hoelzer D , Horowitz MM , et al. Long-term follow-up of adults with acute lymphoblastic leukemia in first remission treated with chemotherapy or bone marrow transplantation . Ann Intern Med 1995 ; 123 : 428 – 431 .
Thomas X , Boiron JM , Huguet F , et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial . J Clin Oncol 2004 ; 22 : 4075 – 4086 .
Goldstone AH , Richards SM , Lazarus HM , et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) . Blood 2008 ; 111 : 1827 – 1833 .
Vey N , Thomas X , Picard C , et al. Allogeneic stem cell transplantation improves the outcome of adults with t(1;19)/E2A-PBX1 and t(4;11)/MLL-AF4 positive B-cell acute lymphoblastic leukemia: results of the prospective multicenter LALA-94 study . Leukemia 2006 ; 20 : 2155 – 2161 .
Thiebaut A , Vernant JP , Degos L , et al. Adult acute lymphocytic leukemia study testing chemotherapy and autologous and allogeneic transplantation. A follow-up report of the French protocol LALA 87 . Hematol Oncol Clin North Am 2000 ; 14 : 1353 – 1366 ., x .
Nachman J . Clinical characteristics, biologic features and outcome for young adult patients with acute lymphoblastic leukaemia . Br J Haematol 2005 ; 130 : 166 – 173 .
Aguiar RC , Sohal J , van Rhee F , et al. TEL-AML1 fusion in acute lymphoblastic leukaemia of adults . Br J Haematol 1996 ; 95 : 673 – 677 .
Secker-Walker LM , Craig JM , Hawkins JM , et al. Philadelphia positive acute lymphoblastic leukemia in adults: age distribution, BCR breakpoint and prognostic significance . Leukemia 1991 ; 5 : 196 – 199 .
Coustan-Smith E , Mullighan CG , Onciu M , et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia . Lancet Oncol 2009 ; 10 : 147 – 156 .
Neumann M , Heesch S , Gökbuget N , et al. Clinical and molecular characterization of early T-cell precursor leukemia: a high-risk subgroup in adult T-ALL with a high frequency of FLT3 mutations . Blood Cancer J 2012 ; 2 : e55 .
Pieters R , Kaspers GJ , Klumper E , et al. Clinical relevance of in vitro drug resistance testing in childhood acute lymphoblastic leukemia: the state of the art . Med Pediatr Oncol 1994 ; 22 : 299 – 308 .
Raetz EA , Devidas M , Carroll AJ , et al. Cytogenetic and early-response characteristics of adolescents and young adults with acute lymphoblastic leukemia (ALL): a Children’s Oncology Group (COG) study [abstract] . J Clin Oncol 2010 ; 28 ( 15_suppl ): Abstract 9509 .
Bleyer A , Budd T , Montello M . Adolescents and young adults with cancer: the scope of the problem and criticality of clinical trials . Cancer 2006 ; 107 ( 7 , Suppl ) 1645 – 1655 .
Fern LA , Whelan JS . Recruitment of adolescents and young adults to cancer clinical trials--international comparisons, barriers, and implications . Semin Oncol 2010 ; 37 : e1 – e8 .
Schmiegelow K , Heyman M , Gustafsson G , et al. The degree of myelosuppression during maintenance therapy of adolescents with B-lineage intermediate risk acute lymphoblastic leukemia predicts risk of relapse . Leukemia 2010 ; 24 : 715 – 720 .
Martin S , Ulrich C , Munsell M , et al. Delays in cancer diagnosis in underinsured young adults and older adolescents . Oncologist 2007 ; 12 : 816 – 824 .
Bassan R , Hoelzer D . Modern therapy of acute lymphoblastic leukemia . J Clin Oncol 2011 ; 29 : 532 – 543 .
Gökbuget N , Hoelzer D . Treatment of adult acute lymphoblastic leukemia . Hematology (Am Soc Hematol Educ Program) 2006 ; 2006 : 133 – 141 .
Hoelzer D , Thiel E , Löffler H , et al. Intensified therapy in acute lymphoblastic and acute undifferentiated leukemia in adults . Blood 1984 ; 64 : 38 – 47 .
Hoelzer D , Thiel E , Löffler H , et al. Prognostic factors in a multicenter study for treatment of acute lymphoblastic leukemia in adults . Blood 1988 ; 71 : 123 – 131 .
Rowe JM , Buck G , Burnett AK , et al. Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993 . Blood 2005 ; 106 : 3760 – 3767 .
Moorman AV , Harrison CJ , Buck GAN , et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial . Blood 2007 ; 109 : 3189 – 3197 .
Charrin C , Thomas X , Ffrench M , et al. A report from the LALA-94 and LALA-SA groups on hypodiploidy with 30 to 39 chromosomes and near-triploidy: 2 possible expressions of a sole entity conferring poor prognosis in adult acute lymphoblastic leukemia (ALL) . Blood 2004 ; 104 : 2444 – 2451 .
Pullarkat V , Slovak ML , Kopecky KJ , et al. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study . Blood 2008 ; 111 : 2563 – 2572 .
Pui CH , Relling MV , Downing JR . Acute lymphoblastic leukemia . N Engl J Med 2004 ; 350 : 1535 – 1548 .
Stock W , La M , Sanford B , et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies . Blood 2008 ; 112 : 1646 – 1654 .
Aricò M , Schrappe M , Hunger SP , et al. Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005 . J Clin Oncol 2010 ; 28 : 4755 – 4761 .
Schultz KR , Bowman WP , Aledo A , et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study . J Clin Oncol 2009 ; 27 : 5175 – 5181 .
Schultz KR , Carroll A , Heerema NA , et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031 . Leukemia 2014 ; 28 : 1467 – 1471 .
Slayton WB , Schultz KR , Kairalla JA , et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with Philadelphia chromosome-positive acute lymphoblastic leukemia: results of Children’s Oncology Group Trial AALL0622 . J Clin Oncol 2018 ; 36 : 2306 – 2314 .
Biondi A , Schrappe M , De Lorenzo P , et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study . Lancet Oncol 2012 ; 13 : 936 – 945 .
Biondi A , Gandemer V , De Lorenzo P , et al. Imatinib treatment of paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL2010): a prospective, intergroup, open-label, single-arm clinical trial . Lancet Haematol 2018 ; 5 : e641 – e652 .
Hunger SP , Saha V , Devidas M , et al. CA180-372: an international collaborative phase 2 trial of dasatinib and chemotherapy in pediatric patients with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) . Blood 2017 ; 130 ( Suppl_1 ): 98 .
Foà R , Bassan R , Vitale A , et al. Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults . N Engl J Med 2020 ; 383 : 1613 – 1623 .
Assi R , Kantarjian H , Short NJ , et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed Philadelphia chromosome-positive leukemia . Clin Lymphoma Myeloma Leuk 2017 ; 17 : 897 – 901 .
Couturier MA , Thomas X , Raffoux E , et al. Blinatumomab + ponatinib for relapsed/refractory Philadelphia chromosome-positive acute lymphoblastic leukemia in adults . Leuk Lymphoma 2021 ; 62 : 620 – 629 .
King AC , Pappacena JJ , Tallman MS , et al. Blinatumomab administered concurrently with oral tyrosine kinase inhibitor therapy is a well-tolerated consolidation strategy and eradicates measurable residual disease in adults with Philadelphia chromosome positive acute lymphoblastic leukemia . Leuk Res 2019 ; 79 : 27 – 33 .
Short N , Kantarjian HM , Konopleva M , et al. Combination of ponatinib and blinatumomab in Philadelphia chromosome-positive acute lymphoblastic leukemia: early results from a phase II study [Abstract] . J Clin Oncol 2021 ; 39 ( Suppl_15 ): Abstract 7001 .
Thomas DA , O’Brien SM , Faderl S , et al. Long-term outcome after hyper-CVAD and imatinib (IM) for de novo or minimally treated Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph-ALL) . J Clin Oncol 2010 ; 28 ( 15_suppl ): 6506 .
Ravandi F , O’Brien S , Thomas D , et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia . Blood 2010 ; 116 : 2070 – 2077 .
Jabbour E , Kantarjian H , Ravandi F , et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study . Lancet Oncol 2015 ; 16 : 1547 – 1555 .
Jabbour E , Short NJ , Ravandi F , et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study . Lancet Haematol 2018 ; 5 : e618 – e627 .
de Labarthe A , Rousselot P , Huguet-Rigal F , et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study . Blood 2007 ; 109 : 1408 – 1413 .
Tanguy-Schmidt A , Rousselot P , Chalandon Y , et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study . Biol Blood Marrow Transplant 2013 ; 19 : 150 – 155 .
Bassan R , Rossi G , Pogliani EM , et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00 . J Clin Oncol 2010 ; 28 : 3644 – 3652 .
Mizuta S , Matsuo K , Yagasaki F , et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia . Leukemia 2011 ; 25 : 41 – 47 .
Yanada M , Takeuchi J , Sugiura I , et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group . J Clin Oncol 2006 ; 24 : 460 – 466 .
Kim DY , Joo YD , Lim SN , et al. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia . Blood 2015 ; 126 : 746 – 756 .
Wieduwilt MJ , Yin J , Wetzler M , et al. A phase II study of dasatinib and dexamethasone as primary therapy followed by transplantation for adults with newly diagnosed Ph/BCR-ABL1-positive acute lymphoblastic leukemia (Ph+ ALL): final results of Alliance/CALGB Study 10701 . Blood 2018 ; 132 ( Supplement 1 ): 309 .
Fielding AK , Richards SM , Chopra R , et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study . Blood 2007 ; 109 : 944 – 950 .
Oriol A , Vives S , Hernández-Rivas JM , et al. Outcome after relapse of acute lymphoblastic leukemia in adult patients included in four consecutive risk-adapted trials by the PETHEMA Study Group . Haematologica 2010 ; 95 : 589 – 596 .
Tavernier E , Boiron JM , Huguet F , et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial . Leukemia 2007 ; 21 : 1907 – 1914 .
Thomas DA , Kantarjian H , Smith TL , et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy . Cancer 1999 ; 86 : 1216 – 1230 .
Ishida Y , Terasako K , Oshima K , et al. Dasatinib followed by second allogeneic hematopoietic stem cell transplantation for relapse of Philadelphia chromosome-positive acute lymphoblastic leukemia after the first transplantation . Int J Hematol 2010 ; 92 : 542 – 546 .
Millot F , Cividin M , Brizard F , et al. Successful second allogeneic stem cell transplantation in second remission induced by dasatinib in a child with Philadelphia chromosome positive acute lymphoblastic leukemia . Pediatr Blood Cancer 2009 ; 52 : 891 – 892 .
Collins RH , Jr. , Goldstein S , Giralt S , et al. Donor leukocyte infusions in acute lymphocytic leukemia . Bone Marrow Transplant 2000 ; 26 : 511 – 516 .
Kolb HJ , Schattenberg A , Goldman JM , et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients . Blood 1995 ; 86 : 2041 – 2050 .
Keil F , Kalhs P , Haas OA , et al. Relapse of Philadelphia chromosome positive acute lymphoblastic leukaemia after marrow transplantation: sustained molecular remission after early and dose-escalating infusion of donor leucocytes . Br J Haematol 1997 ; 97 : 161 – 164 .
Matsue K , Tabayashi T , Yamada K , et al. Eradication of residual bcr-abl-positive clones by inducing graft-versus-host disease after allogeneic stem cell transplantation in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia . Bone Marrow Transplant 2002 ; 29 : 63 – 66 .
Yazaki M , Andoh M , Ito T , et al. Successful prevention of hematological relapse for a patient with Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic bone marrow transplantation by donor leukocyte infusion . Bone Marrow Transplant 1997 ; 19 : 393 – 394 .
Tiribelli M , Sperotto A , Candoni A , et al. Nilotinib and donor lymphocyte infusion in the treatment of Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) relapsing after allogeneic stem cell transplantation and resistant to imatinib . Leuk Res 2009 ; 33 : 174 – 177 .
Yoshimitsu M , Fujiwara H , Ozaki A , et al. Case of a patient with Philadelphia-chromosome-positive acute lymphoblastic leukemia relapsed after myeloablative allogeneic hematopoietic stem cell transplantation treated successfully with imatinib and sequential donor lymphocyte infusions . Int J Hematol 2008 ; 88 : 331 – 335 .
Branford S , Rudzki Z , Walsh S , et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance . Blood 2002 ; 99 : 3472 – 3475 .
Hu Y , Liu Y , Pelletier S , et al. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia . Nat Genet 2004 ; 36 : 453 – 461 .
Soverini S , Colarossi S , Gnani A , et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia . Clin Cancer Res 2006 ; 12 : 7374 – 7379 .
Hofmann WK , Jones LC , Lemp NA , et al. Ph(+) acute lymphoblastic leukemia resistant to the tyrosine kinase inhibitor STI571 has a unique BCR-ABL gene mutation . Blood 2002 ; 99 : 1860 – 1862 .
Jones D , Thomas D , Yin CC , et al. Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors . Cancer 2008 ; 113 : 985 – 994 .
Hofmann WK , Komor M , Wassmann B , et al. Presence of the BCR-ABL mutation Glu255Lys prior to STI571 (imatinib) treatment in patients with Ph+ acute lymphoblastic leukemia . Blood 2003 ; 102 : 659 – 661 .
Pfeifer H , Wassmann B , Pavlova A , et al. Kinase domain mutations of BCR-ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) . Blood 2007 ; 110 : 727 – 734 .
Bujassoum S , Rifkind J , Lipton JH . Isolated central nervous system relapse in lymphoid blast crisis chronic myeloid leukemia and acute lymphoblastic leukemia in patients on imatinib therapy . Leuk Lymphoma 2004 ; 45 : 401 – 403 .
Leis JF , Stepan DE , Curtin PT , et al. Central nervous system failure in patients with chronic myelogenous leukemia lymphoid blast crisis and Philadelphia chromosome positive acute lymphoblastic leukemia treated with imatinib (STI-571) . Leuk Lymphoma 2004 ; 45 : 695 – 698 .
Pfeifer H , Wassmann B , Hofmann WK , et al. Risk and prognosis of central nervous system leukemia in patients with Philadelphia chromosome-positive acute leukemias treated with imatinib mesylate . Clin Cancer Res 2003 ; 9 : 4674 – 4681 .
Takayama N , Sato N , O’Brien SG , et al. Imatinib mesylate has limited activity against the central nervous system involvement of Philadelphia chromosome-positive acute lymphoblastic leukaemia due to poor penetration into cerebrospinal fluid . Br J Haematol 2002 ; 119 : 106 – 108 .
Redaelli S , Piazza R , Rostagno R , et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants . J Clin Oncol 2009 ; 27 : 469 – 471 .
Shah NP , Tran C , Lee FY , et al. Overriding imatinib resistance with a novel ABL kinase inhibitor . Science 2004 ; 305 : 399 – 401 .
Talpaz M , Shah NP , Kantarjian H , et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias . N Engl J Med 2006 ; 354 : 2531 – 2541 .
Verstovsek S , Golemovic M , Kantarjian H , et al. AMN107, a novel aminopyrimidine inhibitor of p190 Bcr-Abl activation and of in vitro proliferation of Philadelphia-positive acute lymphoblastic leukemia cells . Cancer 2005 ; 104 : 1230 – 1236 .
Kantarjian H , Giles F , Wunderle L , et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL . N Engl J Med 2006 ; 354 : 2542 – 2551 .
Lilly MB , Ottmann OG , Shah NP , et al. Dasatinib 140 mg once daily versus 70 mg twice daily in patients with Ph-positive acute lymphoblastic leukemia who failed imatinib: results from a phase 3 study . Am J Hematol 2010 ; 85 : 164 – 170 .
Ottmann OG , Larson RA , Kantarjian HM , et al. Phase II study of nilotinib in patients with relapsed or refractory Philadelphia chromosome--positive acute lymphoblastic leukemia . Leukemia 2013 ; 27 : 1411 – 1413 .
Benjamini O , Dumlao TL , Kantarjian H , et al. Phase II trial of hyper CVAD and dasatinib in patients with relapsed Philadelphia chromosome positive acute lymphoblastic leukemia or blast phase chronic myeloid leukemia . Am J Hematol 2014 ; 89 : 282 – 287 .
Cortes JE , Kantarjian HM , Brümmendorf TH , et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib . Blood 2011 ; 118 : 4567 – 4576 .
Khoury HJ , Cortes JE , Kantarjian HM , et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure . Blood 2012 ; 119 : 3403 – 3412 .
Gambacorti-Passerini C , Kantarjian HM , Kim DW , et al. Long-term efficacy and safety of bosutinib in patients with advanced leukemia following resistance/intolerance to imatinib and other tyrosine kinase inhibitors . Am J Hematol 2015 ; 90 : 755 – 768 .
Kantarjian HM , Cortes JE , Kim DW , et al. Bosutinib safety and management of toxicity in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors . Blood 2014 ; 123 : 1309 – 1318 .
Cortes JE , Kantarjian H , Shah NP , et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias . N Engl J Med 2012 ; 367 : 2075 – 2088 .
Cortes JE , Kim DW , Pinilla-Ibarz J , et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias . N Engl J Med 2013 ; 369 : 1783 – 1796 .
Soverini S , Colarossi S , Gnani A , et al. Resistance to dasatinib in Philadelphia-positive leukemia patients and the presence or the selection of mutations at residues 315 and 317 in the BCR-ABL kinase domain . Haematologica 2007 ; 92 : 401 – 404 .
Soverini S , Martinelli G , Colarossi S , et al. Presence or the emergence of a F317L BCR-ABL mutation may be associated with resistance to dasatinib in Philadelphia chromosome-positive leukemia . J Clin Oncol 2006 ; 24 : e51 – e52 .
Soverini S , Hochhaus A , Nicolini FE , et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet . Blood 2011 ; 118 : 1208 – 1215 .
Martinelli G , Boissel N , Chevallier P , et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia following treatment with blinatumomab: results from a phase II, single-arm, multicenter study . J Clin Oncol 2017 ; 35 : 1795 – 1802 .
Kantarjian H , Thomas D , Jorgensen J , et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study . Lancet Oncol 2012 ; 13 : 403 – 411 .
Kantarjian HM , DeAngelo DJ , Stelljes M , et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia . N Engl J Med 2016 ; 375 : 740 – 753 .
Grupp SA , Kalos M , Barrett D , et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia . N Engl J Med 2013 ; 368 : 1509 – 1518 .
Sadelain M , Rivière I , Brentjens R . Targeting tumours with genetically enhanced T lymphocytes . Nat Rev Cancer 2003 ; 3 : 35 – 45 .
de Bont JM , Holt B , Dekker AW , et al. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands . Leukemia 2004 ; 18 : 2032 – 2035 .
Hallböök H , Gustafsson G , Smedmyr B , et al. Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol . Cancer 2006 ; 107 : 1551 – 1561 .
Ribera JM , Oriol A , Bethencourt C , et al. Comparison of intensive chemotherapy, allogeneic or autologous stem cell transplantation as post-remission treatment for adult patients with high-risk acute lymphoblastic leukemia. Results of the PETHEMA ALL-93 trial . Haematologica 2005 ; 90 : 1346 – 1356 .
Cornelissen JJ , van der Holt B , Verhoef GE , et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no-donor comparison . Blood 2009 ; 113 : 1375 – 1382 .
Marks DI , Pérez WS , He W , et al. Unrelated donor transplants in adults with Philadelphia-negative acute lymphoblastic leukemia in first complete remission . Blood 2008 ; 112 : 426 – 434 .
Marks DI , Wang T , Pérez WS , et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission . Blood 2010 ; 116 : 366 – 374 .
Ram R , Gafter-Gvili A , Vidal L , et al. Management of adult patients with acute lymphoblastic leukemia in first complete remission: systematic review and meta-analysis . Cancer 2010 ; 116 : 3447 – 3457 .
Yanada M , Matsuo K , Suzuki T , et al. Allogeneic hematopoietic stem cell transplantation as part of postremission therapy improves survival for adult patients with high-risk acute lymphoblastic leukemia: a metaanalysis . Cancer 2006 ; 106 : 2657 – 2663 .
Barry E , DeAngelo DJ , Neuberg D , et al. Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols . J Clin Oncol 2007 ; 25 : 813 – 819 .
Seibel NL , Steinherz PG , Sather HN , et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group . Blood 2008 ; 111 : 2548 – 2555 .
Nachman JB , La MK , Hunger SP , et al. Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the children’s oncology group . J Clin Oncol 2009 ; 27 : 5189 – 5194 .
Larsen EC , Devidas M , Chen S , et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group Study AALL0232 . J Clin Oncol 2016 ; 34 : 2380 – 2388 .
Ribera JM , Oriol A , Sanz MA , et al. Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96 . J Clin Oncol 2008 ; 26 : 1843 – 1849 .
DeAngelo DJ , Dahlberg S , Silverman LB , et al. A multicenter phase II study using a dose intensified pediatric regimen in adults with untreated acute lymphoblastic leukemia [abstract] . Blood 2007 ; 110 : Abstract 587 .
DeAngelo DJ , Stevenson KE , Dahlberg SE , et al. Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia . Leukemia 2015 ; 29 : 526 – 534 .
DeAngelo DJ , Stevenson K , Neuberg DS , et al. A multicenter phase II study using a dose intensified pegylated-asparaginase pediatric regimen in adults with untreated acute lymphoblastic leukemia: a DFCI ALL Consortium Trial . Blood 2015 ; 126 : 80 – 80 .
Huguet F , Leguay T , Raffoux E , et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study . J Clin Oncol 2009 ; 27 : 911 – 918 ; erratum in: J Clin Oncol 2009;27:2574 .
Maury S , Chevret S , Thomas X , et al. Rituximab in B-lineage adult acute lymphoblastic leukemia . N Engl J Med 2016 ; 375 : 1044 – 1053 .
Douer D , Aldoss I , Lunning MA , et al. Pharmacokinetics-based integration of multiple doses of intravenous pegaspargase in a pediatric regimen for adults with newly diagnosed acute lymphoblastic leukemia . J Clin Oncol 2014 ; 32 : 905 – 911 .
Geyer MB , Ritchie EK , Rao AV , et al. Pediatric-inspired chemotherapy incorporating pegaspargase is safe and results in high rates of minimal residual disease negativity in adults up to age 60 with Philadelphia chromosome-negative acute lymphoblastic leukemia . Haematologica 2020 ; 106 : 2086 – 2094 .
Stock W , Luger SM , Advani AS , et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403 . Blood 2019 ; 133 : 1548 – 1559 .
Winter SS , Dunsmore KP , Devidas M , et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0434 . Pediatr Blood Cancer 2015 ; 62 : 1176 – 1183 .
Dunsmore KP , Winter S , Devidas M , et al. COG AALL0434: a randomized trial testing nelarabine in newly diagnosed t-cell malignancy . J Clin Oncol 2018 ; 36 ( 15_suppl ): 10500 – 10500 .
Winter SS , Dunsmore KP , Devidas M , et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: results from the Children’s Oncology Group AALL0434 Methotrexate Randomization . J Clin Oncol 2018 ; 36 : 2926 – 2934 .
Jain P , Kantarjian H , Ravandi F , et al. The combination of hyper-CVAD plus nelarabine as frontline therapy in adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma: MD Anderson Cancer Center experience . Leukemia 2014 ; 28 : 973 – 975 .
Abaza Y , Kantarjian HM , Faderl S , et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma . Am J Hematol 2018 ; 93 : 91 – 99 .
O’Brien S , Thomas DA , Ravandi F , et al. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia . Cancer 2008 ; 113 : 2097 – 2101 .
Maury S , Huguet F , Leguay T , et al. Adverse prognostic significance of CD20 expression in adults with Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia . Haematologica 2010 ; 95 : 324 – 328 .
Thomas DA , O’Brien S , Jorgensen JL , et al. Prognostic significance of CD20 expression in adults with de novo precursor B-lineage acute lymphoblastic leukemia . Blood 2009 ; 113 : 6330 – 6337 .
Thomas DA , O’Brien S , Faderl S , et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia . J Clin Oncol 2010 ; 28 : 3880 – 3889 .
Thomas D , O’Brien S , Faderl S , et al. Anthracycline dose intensification in adult acute lymphoblastic leukemia: lack of benefit in the context of the fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen . Cancer 2010 ; 116 : 4580 – 4589 .
Linker C , Damon L , Ries C , et al. Intensified and shortened cyclical chemotherapy for adult acute lymphoblastic leukemia . J Clin Oncol 2002 ; 20 : 2464 – 2471 .
Topp MS , Kufer P , Gökbuget N , et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival . J Clin Oncol 2011 ; 29 : 2493 – 2498 .
Topp MS , Gökbuget N , Zugmaier G , et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL . Blood 2012 ; 120 : 5185 – 5187 .
Gökbuget N , Dombret H , Bonifacio M , et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia . Blood 2018 ; 131 : 1522 – 1531 .
Kantarjian H , Stein A , Gökbuget N , et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia . N Engl J Med 2017 ; 376 : 836 – 847 .
Topp MS , Gökbuget N , Stein AS , et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study . Lancet Oncol 2015 ; 16 : 57 – 66 .
Topp MS , Gökbuget N , Zugmaier G , et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia . J Clin Oncol 2014 ; 32 : 4134 – 4140 .
Nguyen K , Devidas M , Cheng SC , et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study . Leukemia 2008 ; 22 : 2142 – 2150 .
Pui CH , Evans WE . Acute lymphoblastic leukemia . N Engl J Med 1998 ; 339 : 605 – 615 .
Pui CH , Pei D , Sandlund JT , et al. Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia . Leukemia 2010 ; 24 : 371 – 382 .
Berg SL , Blaney SM , Devidas M , et al. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children’s Oncology Group . J Clin Oncol 2005 ; 23 : 3376 – 3382 .
Einsiedel HG , von Stackelberg A , Hartmann R , et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Münster Group 87 . J Clin Oncol 2005 ; 23 : 7942 – 7950 .
Tallen G , Ratei R , Mann G , et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90 . J Clin Oncol 2010 ; 28 : 2339 – 2347 .
Malempati S , Gaynon PS , Sather H , et al. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children’s Oncology Group study CCG-1952 . J Clin Oncol 2007 ; 25 : 5800 – 5807 .
Fielding AK , Rowe JM , Richards SM , et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993 . Blood 2009 ; 113 : 4489 – 4496 .
Hahn T , Wall D , Camitta B , et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in adults: an evidence-based review . Biol Blood Marrow Transplant 2006 ; 12 : 1 – 30 .
Eapen M , Raetz E , Zhang MJ , et al. Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children’s Oncology Group and the Center for International Blood and Marrow Transplant Research . Blood 2006 ; 107 : 4961 – 4967 .
Duval M , Klein JP , He W , et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure . J Clin Oncol 2010 ; 28 : 3730 – 3738 .
Cassaday RD , Alan Potts D , Jr. , Stevenson PA , et al. Evaluation of allogeneic transplantation in first or later minimal residual disease - negative remission following adult-inspired therapy for acute lymphoblastic leukemia . Leuk Lymphoma 2016 ; 57 : 2109 – 2118 .
Topp MS , Goekbuget N , Zugmaier G , et al. Anti-CD19 BiTE blinatumomab induces high complete remission rate in adult patients with relapsed B-precursor ALL: Updated results of an ongoing phase II trial [abstract] . Blood 2011 ; 118 : Abstract 252 .
Topp MS , Goekbuget N , Stein AS , et al. Confirmatory open-label, single-arm, multicenter phase 2 study of the BiTE antibody blinatumomab in patients (pts) with relapsed/refractory B-precursor acute lymphoblastic leukemia (r/r ALL) [abstract] . J Clin Oncol 2014 ; 32 ( 15_suppl ): Abstract 7005 .
U.S. Food and Drug Administration . Prescribing information. Blincyto® (blinatumomab) injection . 2014 . Accessed September 29, 2016. Available at :
Jabbour E , Ravandi F , Kebriaei P , et al. Salvage chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD for patients with relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia: a phase 2 clinical trial . JAMA Oncol 2018 ; 4 : 230 – 234 .
Jabbour E , Sasaki K , Ravandi F , et al. Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage . Cancer 2018 ; 124 : 4044 – 4055 .
Brentjens RJ , Davila ML , Riviere I , et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia . Sci Transl Med 2013 ; 5 : 177ra38 .
Kochenderfer JN , Dudley ME , Feldman SA , et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells . Blood 2012 ; 119 : 2709 – 2720 .
Hollyman D , Stefanski J , Przybylowski M , et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy . J Immunother 2009 ; 32 : 169 – 180 .
Davila ML , Riviere I , Wang X , et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia . Sci Transl Med 2014 ; 6 : 224ra25 .
Gökbuget N , Stanze D , Beck J , et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation . Blood 2012 ; 120 : 2032 – 2041 .
O’Brien S , Schiller G , Lister J , et al. High-dose vincristine sulfate liposome injection for advanced, relapsed, and refractory adult Philadelphia chromosome-negative acute lymphoblastic leukemia . J Clin Oncol 2013 ; 31 : 676 – 683 .
Park JH , Rivière I , Gonen M , et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia . N Engl J Med 2018 ; 378 : 449 – 459 .
Shah NN , Lee DW , Yates B , et al. Long-Term Follow-Up of CD19-CAR T-Cell therapy in children and young adults with B-ALL . J Clin Oncol 2021 ; 39 : 1650 – 1659 .
Shah BD , Ghobadi A , Oluwole OO , et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study . Lancet 2021 ; S0140-6736(21)01222-8 .
Grupp SA , Frey NV , Aplenc R , et al. T Cells engineered with a chimeric antigen receptor (CAR) targeting CD19 (CTL019) produce significant in vivo proliferation, complete responses and long-term persistence without GVHD in children and adults with relapsed, refractory ALL [abstract] . Blood 2013 ; 122 : Abstract 67 .
Maude SL , Laetsch TW , Buechner J , et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia . N Engl J Med 2018 ; 378 : 439 – 448 .
Pasquini MC , Hu ZH , Curran K , et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma . Blood Adv 2020 ; 4 : 5414 – 5424 .
Grupp SA , Maude SL , Rives S , et al. Tisagenlecleucel for the treatment of pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia: updated analysis of the ELIANA clinical trial . Biol Blood Marrow Transplant 2019 ; 25 : S126 – S127 .
Sheth VS , Gauthier J . Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL . Bone Marrow Transplant 2021 ; 56 : 552 – 566 .
Lee DW , Santomasso BD , Locke FL , et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells . Biol Blood Marrow Transplant 2019 ; 25 : 625 – 638 .
Maus MV , Alexander S , Bishop MR , et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune effector cell-related adverse events . J Immunother Cancer 2020 ; 8 : 8 .
DeAngelo DJ , Yu D , Johnson JL , et al. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801 . Blood 2007 ; 109 : 5136 – 5142 .
Commander LA , Seif AE , Insogna IG , et al. Salvage therapy with nelarabine, etoposide, and cyclophosphamide in relapsed/refractory paediatric T-cell lymphoblastic leukaemia and lymphoma . Br J Haematol 2010 ; 150 : 345 – 351 .
Whitlock J , dalla Pozza L , Goldberg JM , et al. Nelarabine in combination with etoposide and cyclophosphamide is active in first relapse of childhood T-acute lymphocytic leukemia (T-ALL) and T-lymphoblastic lymphoma (T-LL) . Blood 2014 ; 124 : 795 .
Faderl S , Thomas DA , O’Brien S , et al. Augmented hyper-CVAD based on dose-intensified vincristine, dexamethasone, and asparaginase in adult acute lymphoblastic leukemia salvage therapy . Clin Lymphoma Myeloma Leuk 2011 ; 11 : 54 – 59 .
Thomas DA , Kantarjian HM , Stock W , et al. Phase 1 multicenter study of vincristine sulfate liposomes injection and dexamethasone in adults with relapsed or refractory acute lymphoblastic leukemia . Cancer 2009 ; 115 : 5490 – 5498 .
Silverman JA , Reynolds L , Deitcher SR . Pharmacokinetics and pharmacodynamics of vincristine sulfate liposome injection (VSLI) in adults with acute lymphoblastic leukemia . J Clin Pharmacol 2013 ; 53 : 1139 – 1145 .
O’Brien S , Thomas D , Ravandi F , et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy . Cancer 2008 ; 113 : 3186 – 3191 .
Jeha S , Gaynon PS , Razzouk BI , et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia . J Clin Oncol 2006 ; 24 : 1917 – 1923 .
Hijiya N , Thomson B , Isakoff MS , et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia . Blood 2011 ; 118 : 6043 – 6049 .
Locatelli F , Testi AM , Bernardo ME , et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia . Br J Haematol 2009 ; 147 : 371 – 378 .
Miano M , Pistorio A , Putti MC , et al. Clofarabine, cyclophosphamide and etoposide for the treatment of relapsed or resistant acute leukemia in pediatric patients . Leuk Lymphoma 2012 ; 53 : 1693 – 1698 .
Pigneux A , Sauvezie M , Vey N , et al. Clofarabine combinations in adults with refractory/relapsed acute lymphoblastic leukemia (ALL): a GRAALL Report . Blood 2011 ; 118 : 2586 – 2586 .
Wiernik PH , Dutcher JP , Paietta E , et al. Long-term follow-up of treatment and potential cure of adult acute lymphocytic leukemia with MOAD: a non-anthracycline containing regimen . Leukemia 1993 ; 7 : 1236 – 1241 .
Wetzler M , Sanford BL , Kurtzberg J , et al. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511 . Blood 2007 ; 109 : 4164 – 4167 .
Kadia TM , Kantarjian HM , Thomas DA , et al. Phase II study of methotrexate, vincristine, pegylated-asparaginase, and dexamethasone (MOpAD) in patients with relapsed/refractory acute lymphoblastic leukemia . Am J Hematol 2015 ; 90 : 120 – 124 .
Bride KL , Vincent TL , Im SY , et al. Preclinical efficacy of daratumumab in T-cell acute lymphoblastic leukemia . Blood 2018 ; 131 : 995 – 999 .
Richard-Carpentier G , Jabbour E , Short NJ , et al. Clinical experience with venetoclax combined with chemotherapy for relapsed or refractory T-cell acute lymphoblastic leukemia . Clin Lymphoma Myeloma Leuk 2020 ; 20 : 212 – 218 .
Horton TM , Whitlock JA , Lu X , et al. Bortezomib reinduction chemotherapy in high-risk ALL in first relapse: a report from the Children’s Oncology Group . Br J Haematol 2019 ; 186 : 274 – 285 .
- NCCN CATEGORIES OF EVIDENCE AND CONSENSUS
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management of any patient with cancer is in a clinical trial. Participation in clinical trials is especially encouraged.
PLEASE NOTE
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of evidence and consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult the NCCN Guidelines is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient's care or treatment. The National Comprehensive Cancer Network® (NCCN®) makes no representations or warranties of any kind regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
The complete NCCN Guidelines for Acute Lymphoblastic Leukemia are not printed in this issue of JNCCN but can be accessed online at NCCN.org .
© National Comprehensive Cancer Network, Inc. 2021. All rights reserved. The NCCN Guidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN.
Disclosures for the NCCN Acute Lymphoblastic Leukemia Panel
At the beginning of each NCCN Guidelines Panel meeting, panel members review all potential conflicts of interest. NCCN, in keeping with its commitment to public transparency, publishes these disclosures for panel members, staff, and NCCN itself.
Individual disclosures for the NCCN Acute Lymphoblastic Leukemia Panel members can be found on page 1109. (The most recent version of these guidelines and accompanying disclosures are available at NCCN.org .)
The complete and most recent version of these guidelines is available free of charge at NCCN.org .
Individual Disclosures for the NCCN Acute Lymphoblastic Leukemia Panel

Journal of the National Comprehensive Cancer Network
Article Sections
- View raw image
- Download Powerpoint Slide
Article Information
- Get Permissions
- PubMed Citation
- Article by Patrick A. Brown
- Article by Bijal Shah
- Article by Anjali Advani
- Article by Patricia Aoun
- Article by Michael W. Boyer
- Article by Patrick W. Burke
- Article by Daniel J. DeAngelo
- Article by Shira Dinner
- Article by Amir T. Fathi
- Article by Jordan Gauthier
- Article by Nitin Jain
- Article by Suzanne Kirby
- Article by Michaela Liedtke
- Article by Mark Litzow
- Article by Aaron Logan
- Article by Selina Luger
- Article by Lori J. Maness
- Article by Stephanie Massaro
- Article by Ryan J. Mattison
- Article by William May
- Article by Olalekan Oluwole
- Article by Jae Park
- Article by Amanda Przespolewski
- Article by Sravanti Rangaraju
- Article by Jeffrey E. Rubnitz
- Article by Geoffrey L. Uy
- Article by Madhuri Vusirikala
- Article by Matthew Wieduwilt
- Article by Beth Lynn
- Article by Ryan A. Berardi
- Article by Deborah A. Freedman-Cass
- Article by Mallory Campbell
- Similar articles in PubMed
Google Scholar
Related articles.
| All Time | Past Year | Past 30 Days | |
|---|---|---|---|
| Abstract Views | 0 | 0 | 0 |
| Full Text Views | 33856 | 11781 | 685 |
| PDF Downloads | 29479 | 9967 | 574 |
| EPUB Downloads | 0 | 0 | 0 |
- Advertising
- Terms of Use
- Privacy Policy
- Permissions

© 2019-2024 National Comprehensive Cancer Network
Powered by:
- [185.80.151.41]
- 185.80.151.41
Character limit 500 /500
- Open access
- Published: 16 March 2023
The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades
- Elias Jabbour 1 ,
- Nicholas J. Short 1 ,
- Nitin Jain 1 ,
- Fadi G. Haddad 1 ,
- Mary Alma Welch 1 ,
- Farhad Ravandi 1 &
- Hagop Kantarjian 1
Journal of Hematology & Oncology volume 16 , Article number: 22 ( 2023 ) Cite this article
15k Accesses
33 Citations
10 Altmetric
Metrics details
Progress in the research and therapy of adult acute lymphoblastic leukemia (ALL) is accelerating. This analysis summarizes the data derived from the clinical trials conducted at MD Anderson between 1985 and 2022 across ALL subtypes. In Philadelphia chromosome-positive ALL, the addition of BCR::ABL1 tyrosine kinase inhibitors (TKIs) to intensive chemotherapy since 2000, improved outcomes. More recently, a chemotherapy-free regimen with blinatumomab and ponatinib resulted in a complete molecular remission rate of 85% and an estimated 3-year survival rate of 90%, potentially reducing the role of, and need for allogeneic stem cell transplantation (SCT) in remission. In younger patients with pre-B Philadelphia chromosome-negative ALL, the integration of blinatumomab and inotuzumab into the frontline therapy has improved the estimated 3-year survival rate to 85% across all risk categories. Our future strategy is to evaluate the early integration of both immunotherapy agents, inotuzumab and blinatumomab, with low-dose chemotherapy (dose-dense mini-Hyper-CVD-inotuzumab-blinatumomab) into the frontline setting followed by CAR T cells consolidation in high-risk patients, without any further maintenance therapy. In older patients, using less intensive chemotherapy (mini-Hyper-CVD) in combination with inotuzumab and blinatumomab has improved the 5-year survival rate to 50%. Among patients ≥ 65–70 years, the mortality in complete remission (CR) is still high and is multifactorial (old age, death in CR with infections, development of myelodysplastic syndrome or acute myeloid leukemia). A chemotherapy-free regimen with inotuzumab and blinatumomab is being investigated. The assessment of measurable residual disease (MRD) by next-generation sequencing (NGS) is superior to conventional assays, with early MRD negativity by NGS being associated with the best survival. We anticipate that the future therapy in B-ALL will involve less intensive and shorter chemotherapy regimens in combination with agents targeting CD19 (blinatumomab), CD20, and CD22 (inotuzumab). The optimal timing and use of CAR T cells therapy may be in the setting of minimal disease, and future trials will assess the role of CAR T cells as a consolidation among high-risk patients to replace allogeneic SCT. In summary, the management of ALL has witnessed significant progress during the past four decades. Novel combination regimens including newer-generation BCR::ABL1 TKIs and novel antibodies are questioning the need and duration of intensive chemotherapy and allogeneic SCT.
The management of adult acute lymphoblastic leukemia (ALL) is evolving rapidly. The classical adult ALL regimens deviated early on from the principles applied in pediatric regimens (shorter maintenance; reliance on allogeneic and autologous stem cell transplantation [SCT] in remission) and resulted in estimated 5-year survival rates of 30% to 35% [ 1 ]. In 1992, we developed the hyper-CVAD regimen (fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) in patients with newly diagnosed B-ALL [ 2 ]. This is a pediatric-inspired regimen that relegated asparaginase therapy to the consolidation phases (rather than during induction for active disease) in order to reduce the potential asparaginase-associated toxicities. This dose-intensive regimen resulted in improved outcome in adult B-ALL with a complete remission (CR) rate of 90 + % and a 5-year overall survival (OS) rate of 40%. This regimen incorporated central nervous system (CNS) prophylaxis with intrathecal (IT) chemotherapy consisting of cytarabine and methotrexate. It demonstrated the efficacy of IT chemotherapy in combination with systemic chemotherapy in reducing the risk of CNS recurrence, allowing the omission of cranial irradiation and associated adverse events [ 3 , 4 ]. To further improve outcomes, in 2000, we added rituximab as anti-CD20 therapy to hyper-CVAD based on the findings that CD20 expression (≥ 20%) on B-ALL blasts was associated with worse outcome [ 5 ]. The addition of rituximab improved the outcomes in patients with CD20-positive disease with 3-year OS rate of 50% overall and of 75% in younger patients < 60 years [ 6 ]. Hyper-CVAD was also combined with BCR::ABL1 tyrosine kinase inhibitors (TKI) in the frontline therapy of Philadelphia chromosome (Ph)-positive ALL and resulted in high CR and complete molecular response (CMR) rates, a higher proportion of patients bridged to allogeneic SCT, and a superior survival. Outcomes steadily improved with each newer generation of TKI, beginning with imatinib, then dasatinib, and most recently ponatinib [ 7 , 8 , 9 , 10 , 11 ].
Since early 2010, antibodies targeting CD19 and CD22 have been evaluated in relapsed/refractory (R/R) B-ALL [ 12 , 13 , 14 ], leading to the approval of these agents as monotherapy in the salvage setting [ 15 , 16 ]. These included blinatumomab, a CD19 targeting bispecific T cell engager (BiTE) approved in 2014, and inotuzumab, a CD22 targeting antibody–drug conjugate, approved in 2017 [ 15 , 16 ]. Therapeutic strategies have then moved to combination therapies in both the salvage and frontline settings, contributing to the improved survival in newly diagnosed B-ALL.
Cytogenetics play an important role in independent of molecular abnormalities [ 17 , 18 ]. High-risk cytogenetic features include KMT2A rearrangements, complex cytogenetics, and low hypodiploidy or near haploidy. Low hypodiploidy and complex cytogenetics are also associated with the presence of TP53 mutations. The presence of TP53 mutations, CRLF2 overexpression, and JAK2 mutation are particularly associated with a poor prognosis [ 19 ]. However, the introduction of novel agents and targeted antibodies such as blinatumomab and inotuzumab, combined with a better understanding of disease biology, has translated into a better survival [ 20 , 21 ]. We have recently reported on the results from the Surveillance, Epidemiology, and End Results (SEER) database that show an improvement in survival over the past five decades in adult ALL [ 22 ]. Data from MD Anderson show similar survival improvements over the past four decades in both B-ALL (Fig. 1 A) and T-ALL (Fig. 1 B).
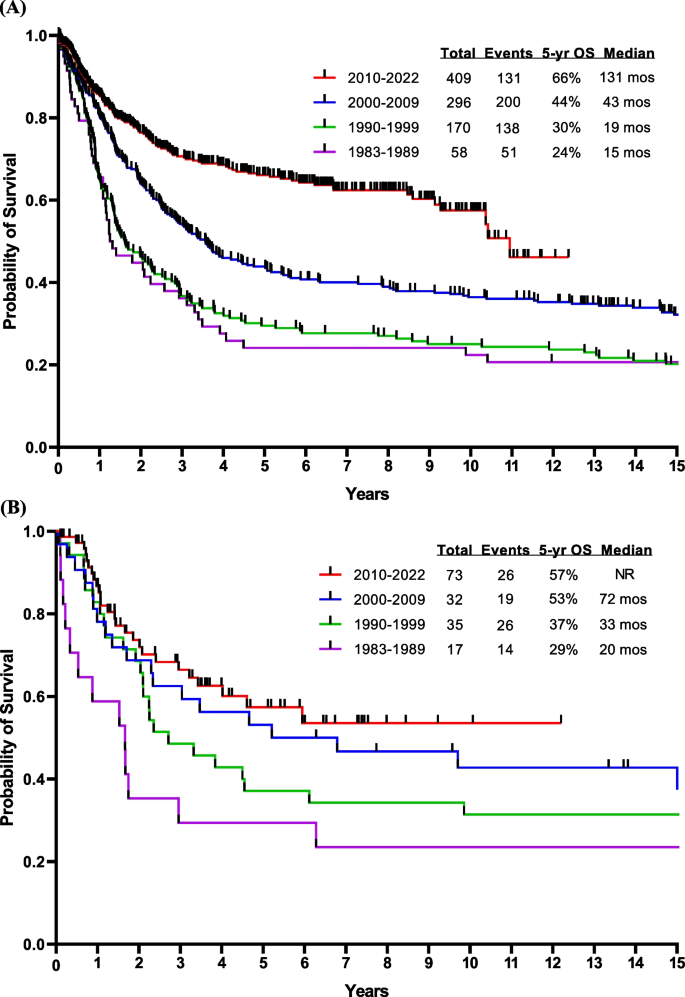
Overall survival by decade at MDACC of ( A ) B-ALL and ( B ) T-ALL. Abbreviations: mos, months; OS, overall survival
Race and ethnicity have been also shown to impact survival. In the SEER database analysis between 2000 and 2017, Hispanic and African American ethnicity were independent factors associated with worse outcome. The Ph-like ALL phenotype is more prevalent among Hispanic patients, which could explain the worse outcome observed in this population. Among African Americans, the higher incidence of comorbidities (metabolic syndrome and cardiovascular problems) and the poor financial status are the main factors associated with poor outcome [ 22 ].
Despite the efforts to develop new therapeutic strategies over the past 10 years and the number of clinical trials conducted, the evolution of ALL therapy and the adoption of new standard regimens remain slow (Fig. 2 ). Herein, we report the outcomes of patients with ALL treated at MD Anderson over the past four decades and discuss the results in relation to ongoing research and published data from outside our institution in ALL.
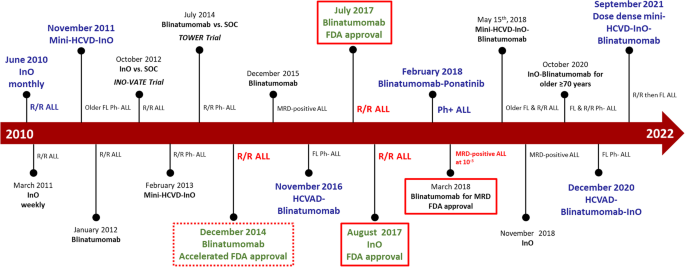
The evolving treatment landscape of B-ALL. Abbreviations: FL, frontline; InO, inotuzumab ozogamicin; MRD, measurable residual disease; Ph, Philadelphia chromosome; R/R, relapsed/refractory; SOC, standard of care
We reviewed the data derived from clinical trials conducted at MD Anderson between 1985 and 2022 across the subtypes of ALL. The results of these trials were reported in detail separately. We described the outcomes in these clinical trials including responses, survival, and rates of allogeneic SCT. Outcomes were stratified by time period, disease subtype, and patient age, highlighting the improvement in survival across different groups treated in a single cancer center. We then compared the outcomes to those published in the literature, in a descriptive fashion. The Kaplan–Meier method was used for survival analysis with the log-rank test.
Ph-positive ALL
Before 2000, the year when BCR::ABL1 TKIs were introduced into the treatment of Ph-positive ALL, the outcome of such patients was very poor. These patients had a 5-year OS rate of < 10% with intensive chemotherapy and 30–40% if they were able to undergo allogeneic SCT in first CR [ 23 , 24 , 25 ]. With the advent of regimens that combined chemotherapy with BCR::ABL1 TKIs (imatinib in 2000, dasatinib in 2006, ponatinib in 2010), the 5-year OS rates improved from < 10% to 50% (Fig. 3 A). The combination of hyper-CVAD and imatinib resulted in a CR rate of > 90% and a long-term OS rate of 40% [ 10 ]. The subsequent hyper-CVAD combination with dasatinib resulted in a CR rate of 96% and a 5-year OS rate of 46% [ 26 ].
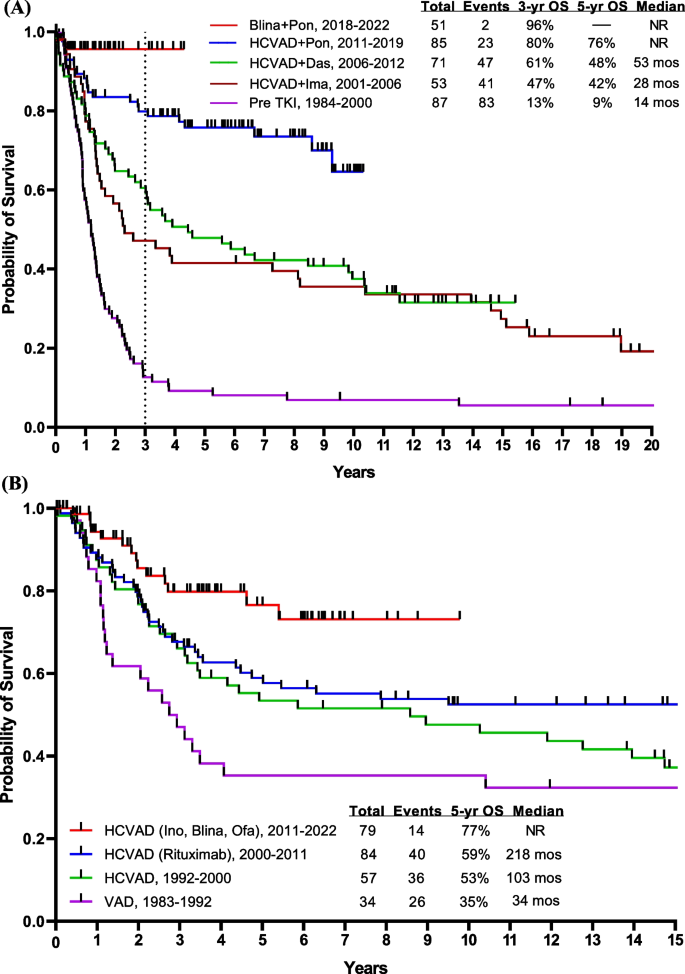
Overall survival by treatment era. A Ph-positive ALL, B AYA patients having Ph-negative ALL, C Ph-negative ALL < 60 years, and D Ph-negative ALL ≥ 60 years. Abbreviations: blina, blinatumomab; das, dasatinib; HCVAD, Hyper-CVAD regimen; ima, imatinib; ino; inotuzumab; NR, not reached; ofa, ofatumumab; OS, overall survival; pon, ponatinib; TKI, tyrosine kinase inhibitor; VAD, vincristine, adriamycin, dexamethasone; ven, venetoclax
The T315I mutation of the ABL1 kinase domain is highly resistant to imatinib and to second-generation TKIs and is detected in up to 75% of patients relapsing after first- and second-generation TKIs, with a higher incidence in older patients compared with younger adults [ 27 , 28 ]. Ponatinib, a potent pan-BCR::ABL1 inhibitor, suppresses the T315I mutation and eradicates molecular measurable residual disease (MRD) [ 29 ]. The combination of hyper-CVAD plus ponatinib investigated in 86 patients (median age, 46 years) with newly diagnosed Ph-positive ALL, produced a CMR rate of 74% at 3 months and a cumulative CMR rate of 86% [ 30 , 31 ]. After a median follow-up of 80 months, the 6-year OS rate for the entire cohort was 75%. An 8-month landmark analysis showed a trend for better OS in patients who did not undergo allogeneic SCT in the first remission. In the original protocol, ponatinib was administered at 45 mg daily [ 29 ]. After two patients had fatal ponatinib-associated cardiovascular toxicities, the protocol was amended to allow for a response-adjusted dosing of ponatinib (i.e., 45 mg in Course 1, 30 mg in CR, and 15 mg in CMR) [ 30 ]. This strategy reduced the risk of cardiovascular events, making the treatment more manageable; no further ponatinib-related mortality has been observed since then. For CNS prophylaxis, 12 IT chemotherapy doses were given, which reduced the rate of CNS relapses compared with 8 IT doses (6-year CNS relapse rate, 0% versus 13%) [ 32 , 33 ]. In a propensity-matched score analysis of hyper-CVAD plus ponatinib and hyper-CVAD plus dasatinib, ponatinib was associated with higher rates of CMR at 3 months and superior OS [ 34 ]. A meta-analysis of 26 clinical trials also confirmed the superiority of ponatinib over earlier-generation TKIs, with higher rates of CMR and survival [ 35 ]. Patients who achieve CMR at 3 months had superior OS compared with patients with detectable disease at 3 months (4-year OS rate, 66% versus 36%) [ 36 ]. Among patients who achieved CMR at 3 months, ponatinib therapy was independently associated with improved outcomes compared with dasatinib or imatinib, with better progression-free survival (PFS) and OS [ 37 ]. Therefore, patients who achieve early CMR at 3 months have an excellent survival, and allogeneic SCT may not be needed [ 36 , 37 , 38 ]. This is in contrast to studies done across the USA and Europe, where allogeneic SCT remains a standard of care in first CR.
Can we reduce or eliminate the need for intensive chemotherapy in Ph-positive ALL?
Several studies from Italy and Europe have reported on the efficacy of TKI-based regimens combined with low-dose chemotherapy or no chemotherapy [ 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 ]. They showed CMR rates of 20–40%, 3- to 5-year OS rates of 30–40%, and a high incidence of T315I mutations at relapse. Inotuzumab and blinatumomab had shown efficacy in R/R Ph-positive ALL, leading to the use of blinatumomab in combination with TKIs in the frontline setting [ 47 , 48 ]. Our group reported retrospectively on the safety and efficacy of a chemotherapy-free approach in Ph-positive ALL; a combination of blinatumomab plus TKIs resulted in a CMR rate of 75% and a 1-year OS rate of 73% [ 49 ]. The GIMEMA LAL2116 D-ALBA trial treated 63 patients (median age, 54 years) with dasatinib and the addition of blinatumomab 3 months into therapy. Overall, 60% of patients achieved a deep molecular response (41% CMR) after at least 2 courses of blinatumomab [ 50 ]. At a median follow-up of 40 months, the estimated 4-year OS and disease-free survival (DFS) rates were 78% and 75%, respectively. Fifty percent of patients received allogeneic SCT in CR. Nine patients relapsed: 4 hematologic relapses, 4 CNS relapses, and 1 nodal relapse [ 51 ]. At MD Anderson, we initiated a frontline regimen combining ponatinib and blinatumomab given together during induction, with continuation of blinatumomab for 5 total courses. Ponatinib 30 mg during induction-maintenance was reduced to 15 mg in CMR [ 52 ]. Among 44 patients treated in the frontline setting, the rates of CMR after one course and overall were 64% and 85%, respectively. Among 25 patients who had MRD assessment by next-generation sequencing (NGS), 22 (88%) had undetectable disease at a sensitivity level of 10 −6 . The estimated 3-year PFS and OS rates were both 95% (Fig. 3 A). Only one patient underwent allogeneic SCT in first remission due to persistently detectable BCR::ABL1 transcript levels < 0.05%. Thus, the future management of Ph-positive ALL may consist of chemotherapy-free and allogeneic SCT-sparing targeted therapies. Intensive chemotherapy may still be needed in some subsets: IKZF1 plus ( IKZF1 deletion plus additional genetic aberrations including CDKN2A , CDKN2B , or PAX5 ), mixed lineage dual lymphoid and myeloid clones, documentation of Philadelphia chromosome detected by FISH in differentiated myeloid cells. The decision of proceeding with allogeneic SCT depends on the early achievement of CMR within the first 3 months of therapy, which was shown to translate into improved OS. Furthermore, the recent introduction of NGS for the detection of MRD allows a more precise discrimination of patients between those who clear or not their leukemia blasts, at a sensitivity of 10 −6 . Patients who achieve MRD negativity by NGS could potentially be cured without the need for an allogeneic SCT and might potentially be candidates for treatment discontinuation [ 53 ].
Ph-negative adult pre-B ALL (age up to 60 years)
Adolescent and young adults (AYA; age 15–39 years) with ALL have traditionally been treated with pediatric-inspired regimens. The CALGB trial showed a 3-year survival rate of 73%. Hyper-CVAD-based therapy (also a pediatric-inspired regimen developed in 1992, and which delays asparaginase to later consolidations) showed equivalent results (Fig. 3 B). The addition of the anti-CD20 antibodies, rituximab in 2000 and ofatumumab in 2012, to hyper-CVAD has improved the survival [ 6 ]. A propensity-score matching analysis showed ofatumumab to result in better outcomes than rituximab, when added to hyper-CVAD [ 6 , 54 , 55 , 56 ]. Further improvement may be observed with the use of novel anti-CD20 agents such as bispecific anti-CD3/CD20 T cell engagers, which showed encouraging results in patients with non-Hodgkin lymphomas [ 57 , 58 ].
A better understanding of the B-ALL biology allows for better stratification of patients and for better subset-oriented therapies. For example, Ph-like ALL was recognized as a provisional entity by the World Health Organization (WHO) in 2016. It includes patients whose gene expression profile is similar to that of Ph-positive ALL but without the t(9;22) chromosomal abnormality [ 59 ]. The WHO 2022 classification and the International Consensus Classification recognized Ph-like ALL as having a wide variety of genetic lesions, alterations, and fusions, some of which could have therapeutic implications [ 60 , 61 ]. There are 2 major subtypes of Ph-like ALL: CRLF2 rearranged and non-CRLF2 rearranged. Flow cytometry and FISH are used to assess CRLF2 status in all Ph-negative B-ALL patients. Those who no rearrangement is present and/or CRLF2 protein expression is absent undergo Archer multiplex fusion assay and FISH for ABL1, ABL2, PDGFRB, JAK2, and EPOR to assess for multitude of fusions that have been described in non-CRLF2 Ph-like ALL [ 62 , 63 , 64 ]. Unfortunately, the gold standard for assessing Ph-like ALL, namely gene expression profile, is not commercially available for routine assessment at this time. Ph-like ALL occurs in 25% of adult pre-B ALL. Eighty percent of Ph-like ALL have CRLF2 overexpression, 50% of them have JAK mutations (adverse; may still require allogeneic SCT in first CR), and 20% have ABL1 or PDGFR translocations and are treated on the Ph-positive ALL protocols (not reported here). The Ph-like alterations involving CRLF2/JAK delineate a high-risk B-ALL requiring intensified therapy and upfront allogeneic SCT [ 65 , 66 ]. Patients with Ph-negative CRLF2/JAK Ph-like ALL have a disease that is more resistant to standard intensive chemotherapy, have high levels of persistent MRD positivity in CR and poor OS rates of 20–30%, and require more frequently allogeneic SCT to achieve long-term remission [ 54 , 66 ]. Blinatumomab was shown to be effective in the relapse setting of B-ALL [ 15 ], improving the MRD negativity rate as well as survival [ 15 , 67 ]. In a post hoc analysis from the phase III TOWER study, blinatumomab was effective in patients with and without Ph-like ALL, thereby negating the effect of Ph-like alterations [ 68 ]. The median OS was 7.9 months and 8.4 months, respectively, better than the survival observed with standard-of-care therapies [ 68 ]. The incorporation of blinatumomab into the frontline setting may negate the impact of the Ph-like phenotype through better MRD eradication, potentially eliminating the need for allogeneic SCT. At MD Anderson, we tested the efficacy of the frontline combination of Hyper-CVAD followed by blinatumomab and later (after 38 patients treated) the addition of lower-dose fractionated inotuzumab. The study aims were to improve efficacy, shorten the duration of intensive chemotherapy, and improve the treatment safety. With the amendment, we aimed to decrease the chemotherapy dose (inotuzumab given during even cycles with reduced-dose methotrexate at 500 mg/m 2 on Day 1 and cytarabine 1 g/m 2 every 12 h on Days 2–3), increase the rate of early MRD clearance by introducing inotuzumab, and improve survival [ 69 , 70 ]. In the initial cohort, 38 patients (median age, 37 years) were treated with Hyper-CVAD plus sequential blinatumomab (4 courses, with later additional 3 courses, 1 every 3 months during POMP [prednisone, vincristine, methotrexate, 6-mercaptopurine] maintenance). High-risk features such as TP53 mutation, CRLF2 positivity, KMT2A rearrangement, and JAK2 positivity were seen in 27%, 18%, 8%, and 5% of patients, respectively. The CR rate was 100% and the MRD negativity rate was 97%. The 3-year OS rate was 81%. Twenty-five patients (median age, 24 years) were treated after the amendment (which incorporated inotuzumab in Courses 2 and 4 of chemotherapy, and into 2 of the 4 later sequential blinatumomab courses); 15%, 15%, 10%, and 8% of patients had TP53 mutation, CRLF2 positivity, JAK2 positivity, and KMT2A rearrangement, respectively. The CR rate was 100% and the MRD negativity rate was 91%. No relapses or deaths have occurred among patients who received inotuzumab, and the estimated 1-year OS was 100%. Overall, the CR rate was 100% in the entire cohort regardless of the Ph-like phenotype, and the MRD negativity rate was 95%, with no early deaths observed. The 3-year OS was 84%, with most patients completing the therapy within 1.5 years (compared to 3 years with conventional Hyper-CVAD followed by maintenance with POMP). The addition of antibodies to Hyper-CVAD chemotherapy seems to overcome the negative impact of high-risk disease features. Nevertheless, longer follow-up is needed to better evaluate the impact of this combination on preventing late relapses and maintaining long-term remission and survival (Fig. 3 C). Other studies have also examined the role of adding blinatumomab to chemotherapy in patients with Ph-negative ALL, reporting similarly promising early results (Table 1 ) [ 71 , 72 , 73 ].
Older patients with B-ALL
Historically, older patients (≥ 55–60 years) with B-ALL have had poor outcomes with the standard intensive and dose-adjusted chemotherapy regimens, due a high incidence of adverse biologic features including adverse cytogenetics, TP53 mutations [ 22 , 74 , 75 , 76 , 77 ], and poor tolerance to intensive chemotherapy. The recent SEER ALL data showed a 5-year survival of 10–20% among patients 60 years and older [ 74 ]. The MD Anderson survival data for the same age group is shown in Fig. 3 D. The improved survival from 20% in the era of chemotherapy to 40–50% since 2010 is due to the use of targeted agents, namely inotuzumab and blinatumomab, in combination with low-intensity chemotherapy in the frontline setting. The novel targeted therapies are less myelosuppressive (inotuzumab) and more active than conventional chemotherapy, hence the rationale for the combined modality approach in the frontline setting. Since 2010, we have investigated the induction and consolidation regimen with mini-Hyper-CVD (dose-reduced hyperfractionated cyclophosphamide, vincristine, dexamethasone) and inotuzumab +/ blinatumomab in older patients with newly diagnosed Ph-negative B cell ALL. The maintenance phase consisted of monthly courses of POMP for up to 3 years initially, which was later shortened after the amendment of the study to 12 courses of POMP and 4 courses of blinatumomab (1 course of blinatumomab after 3 courses of POMP) [ 78 , 79 , 80 ]. An interim analysis of 80 patients treated (median age, 68 years) showed an overall response rate (ORR) of 99% (CR rate 89%), an MRD negativity rate of 80% after the first course and of 94% at any time, with no early (4-week) mortality. The 5-year OS rate was 47%, 57% in patients aged 60–69 years, and 28% in patients 70 years and older. Six (8%) patients developed hepatic sinusoidal obstruction syndrome (SOS), of whom one after allogeneic SCT [ 81 ]. In a propensity score matching analysis, the mini-Hyper-CVD-inotuzumab +/ blinatumomab therapy showed a survival advantage compared with dose-adjusted Hyper-CVAD (3-year OS rate 63% versus 34%) [ 78 ]. Still, the mortality rate in remission with mini-Hyper-CVD-inotuzumab +/ blinatumomab was high in patients ≥ 70 years old compared with patients aged 60–69 years (70% versus 35%). This necessitated the modification of the study to reduce/eliminate the chemotherapy and shift toward a chemotherapy-free regimen in patients ≥ 70 years old (inotuzumab and blinatumomab).
The German Multicenter Study Group on ALL (GMALL) evaluated the use of induction inotuzumab followed by chemotherapy in 45 patients 55 years and older (median age, 64 years) [ 82 ]. The rate of CR or CR with incomplete count recovery (CRi) was 100%, the MRD negativity rate was 74%, and the estimated 2-year survival rate was 77%. One patient had suspected SOS [ 82 ]. The EWALL-INO study also showed the efficacy of induction therapy with inotuzumab in combination with low-intensity chemotherapy in 115 older patients (median age, 69 years). Among evaluable patients, the ORR was 86%, the MRD negativity rate was 73%, and the 1-year OS rate was 79% [ 83 ]. The Southwest Oncology Group (SWOG) 1318 reported on their experience in 29 older patients treated with frontline blinatumomab followed by POMP maintenance [ 84 ]. The CR rate was 66%, the 3-year OS rate was 37% (Table 2 ). Several randomized trials will assess the efficacy of chemotherapy-free regimens with drugs such as inotuzumab and blinatumomab (or with minimal chemotherapy) in older Ph-negative pre-B ALL.
Mature B cell leukemia/Burkitt leukemia
Burkitt leukemia is a rare subtype of B cell ALL. The use of rituximab in combination with Hyper-CVAD improved survival in Burkitt leukemia. This was confirmed in a randomized French trial [ 85 , 86 ]. There has been a debate over whether Hyper-CVAD should remain the standard of care or be replaced by other less intensive regimens such as dose-adjusted EPOCH (DA-EPOCH; dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab). A study of 30 patients (median age, 33 years; age > 40 years, 40%) with Burkitt disease treated with either standard DA-EPOCH-R combination or lower-dose short-course EPOCH-R combination reported 7-year PFS and OS rates of 95–100% and 90–100%, respectively [ 87 ]. The majority of patients (90%) had low- and intermediate-risk disease. Recent data from a multicenter trial in 641 patients (median age, 47 years; range, 18 to 88 years) showed a promising outcome with DA-EPOCH [ 88 ]. The 3-year PFS and OS rates were 64% and 70%, respectively. In patients 40 years and younger, Hyper-CVAD therapy was more favorable than other regimens such as CODOX-M/IVAC (cyclophosphamide, doxorubicin, high-dose methotrexate/ifosfamide, etoposide, and high-dose cytarabine) and DA-EPOCH-R. However, in patients 60 years and older, Hyper-CVAD was associated with more toxicities [ 88 , 89 ]. Patients with CNS disease and marrow involvement had less favorable outcomes and may require an intensive approach such as Hyper-CVAD. Burkitt cells express bright CD20, CD19 and CD22 positivity. Our current strategy in Burkitt leukemia incorporates blinatumomab and inotuzumab with R-Hyper-CVAD in patients < 60 years and with R-mini-Hyper-CVD in older patients.
T cell ALL is a unique separate ALL entity composed of different immunophenotypic and biologic subsets: thymic (CD1a +), mature (surface CD3 +), early (both negative) and its subcategory of early T cell precursor-ALL (ETP-ALL; myeloid markers positive, CD1a negative, CD8 negative, CD5 < 75%). At the cytogenetic and molecular level, ETP-ALL resembles acute myeloid leukemia (AML) or a hybrid ALL-AML, is associated with a poor prognosis, may benefit from AML-type therapies (hypomethylating agents and venetoclax), and benefits from allogeneic SCT in CR [ 90 , 91 , 92 , 93 ]. Effective anti-T cell targeted therapies have been lacking so far and immune therapies lagging behind for the management of T-ALL (in contrast to pre-B ALL). Therefore, improving the outcome in T cell ALL and T cell acute lymphoblastic lymphoma (T-LBL) has been more difficult and has relied on modifications of standard chemotherapies (high-dose cytarabine, high-dose methotrexate, asparaginase, nelarabine). Adding nelarabine, a prodrug of 9-β-arabinofuranosylguanine (ara-G), to chemotherapy in a Children’s Oncology Group (COG) trial improved event-free survival (EFS) in T-ALL but not in T-LBL [ 94 ]. In this study, the absolute benefit from the addition of nelarabine varied according to the associated methotrexate schedule. The 5-year DFS rates were 91% and 87% with escalating-dose methotrexate without leucovorin rescue plus pegaspargase with and without nelarabine, respectively; and 86% and 78% with high-dose methotrexate with leucovorin rescue with and without nelarabine, respectively [ 94 ].
At MD Anderson, we added nelarabine to Hyper-CVAD frontline therapy and combined it with asparaginase. This combination was associated with a 5-year OS of 60% [ 95 ]. In a retrospective analysis, a noticeable difference in outcome was seen in ETP-ALL versus other T-ALL [ 96 ]. A landmark analysis at 3 and 6 months showed that the addition of nelarabine to Hyper-CVAD improved survival (compared with Hyper-CVAD alone) in non-ETP T-ALL but not in T-LBL. “Near-ETP” ALL shares a similar transcriptional profile to ETP-ALL but with similar survival to non-ETP-ALL [ 96 ].
Preclinical studies have shown that the BH3-mimetics, venetoclax and navitoclax, are active against B cell and T cell ALL, especially ETP-ALL [ 97 , 98 , 99 ]. Preliminary results of the combination of venetoclax with low-intensity chemotherapy in newly diagnosed older patients unfit for intensive chemotherapy showed objective response and MRD negativity rates of 91% and 100%, respectively [ 100 ]. At MD Anderson, venetoclax was added to frontline adult T cell ALL therapy with Hyper-CVAD-nelarabine-peg-asparaginase; 23% of patients enrolled on study had ETP-ALL. The ORR across all cohorts was 97%, with no early mortality. The median OS was 135 months (Fig. 4 ) [ 101 ]. The addition of venetoclax with dose adjustment might benefit selected patients.

Overall survival of T-ALL by type of therapy. Abbreviations: asp, asparaginase; mos, months; OS, overall survival; nel, nelarabine; ven, venetoclax
Chimeric antigen receptor (CAR) T cell therapy is also being developed for the management of T-ALL. However, harnessing and redirecting normal T cells to recognize and destroy malignant T cells is associated with significant challenges due to the shared surface antigens, including T cell aplasia, fratricide and product contamination. Various strategies and solutions have been proposed to overcome these challenges. Early preclinical and clinical studies have shown an antitumor activity of CAR T cells against T cell malignancies, which have led to the conception of different phase I/II trials of CAR T cell products targeting CD4, CD5, CD7, CD30, and TRBC1 [ 102 , 103 , 104 ]. Recently, naturally selected CD7 CAR-T cells were shown to be safe and effective among 53 patients with T-ALL ( n = 34) and T-LBL ( n = 18) including those with extramedullary disease and a history of prior allogeneic SCT. CR with MRD negativity was seen in 96% of the patients and CRS ≤ Grade 2 in 89%; the 18-month OS rate was 75% [ 105 ].
Prognostic impact of measurable residual disease
Measurable residual disease predicts outcome in ALL [ 106 , 107 ]. Persistence of MRD at the time of morphologic remission confers a poor prognosis, unless addressed [ 108 , 109 , 110 , 111 , 112 , 113 ]. In a meta-analysis, undetectable MRD was associated with a better outcome, with a hazard ratio of 0.28 for EFS and OS [ 106 ]. This translated into 10-year OS rates of 60% with undetectable MRD, and 15% with persistent MRD. Different techniques are currently used for the evaluation of MRD in patients with ALL. RT-PCR is a highly sensitive assay that is broadly applied in Ph-positive ALL for the detection of MRD by quantifying BCR::ABL1 mRNA transcripts. RT-PCR also allows for the quantification of MRD in Ph-negative ALL and T-ALL through allele-specific oligonucleotides able to detect unique sequences of the junctional regions of rearranged immunoglobulin IG or TCR genes [ 114 ]. So far, MRD in ALL has been assessed by multi-color flow cytometry (MFC) and RT-PCR assays [ 107 ]. We explored the role of NGS for the evaluation of MRD and compared the results from NGS with a sensitivity of 10 −6 , to MFC with a sensitivity of 10 −4 [ 115 ]. Thirty-eight percent of patients with MFC-negative MRD were positive by NGS. Patients with negative MRD status by NGS at CR had 5-year cumulative incidence of relapse and survival rates of 13% and 100%, respectively. This supports the strategy of incorporating NGS-based MRD assessment in the frontline setting, along with MFC. In future practice, NGS should be used from the beginning for more accurate MRD assessment in order to tailor therapy and improve outcome in ALL.
Therapeutic strategies for the eradication of measurable residual disease
In a European multicenter study, blinatumomab was evaluated among patients with B-ALL who achieve CR with persistent MRD. Among 113 evaluable patients, 88 (78%) converted to an MRD-negative status after one course of blinatumomab [ 67 ]. One hundred ten patients with Ph-negative ALL were evaluable for survival; 74 underwent allogeneic SCT. With a median follow-up of 53.1 months, the median OS was 36.5 months; the 4-year OS rate was 45%, 52% if MRD negativity was achieved. Continuous CR was noted in 40% of patients who underwent allogeneic SCT and 33% of patients who did not [ 116 ]. Favorable results were observed in our pilot study of 37 patients (median age, 43 years) with persistent MRD after first (73%) or second (27%) CR [ 117 ]. Patients received a median of 3 courses (range, 1 to 9 courses) of blinatumomab. Twenty-seven (73%) patients achieved complete MRD response within 2 courses of blinatumomab. Among the 19 patients with Ph-negative ALL, 83% achieved MRD negativity after a median of 41 days (range, 12 to 92 days). The overall 3-year OS rate was 67% [ 67 , 116 , 117 , 118 ]. The improvement in outcome observed in our study compared with the European BLAST trial could be explained by several factors: 1) the threshold of disease burden for eligibility in our study was ≥ 1 × 10 −4 compared with ≥ 1 × 10 −3 in the BLAST trial. Early intervention with blinatumomab at levels < 1 × 10 −3 was associated with favorable outcome with 3-year rates of relapse-free survival and OS of 83% and 77% versus 50% and 61% in patients treated with MRD ≥ 1 × 10 −3 . 2) In our study, patients received up to 5 blinatumomab courses (median 3 courses compared with 2 courses in the BLAST trial). 3) The flexibility for additional courses of blinatumomab for those unable to receive allogeneic SCT may have further improved the results.
Inotuzumab was also evaluated in 20 patients with MRD-positive pre-B ALL; 12 patients had Ph-positive ALL and 8 patients had Ph-negative ALL [ 119 ]. The median number of courses was 3 (range, 1 to 6 courses). Fourteen (70%) patients were in CR1, 11 (55%) had received prior blinatumomab and 4 (20%) had prior allogeneic SCT. MRD negativity was achieved in 12 (60%) patients, including 6 (75%) patients with Ph-negative ALL. One SOS occurred in this study [ 119 ].
Relapsed/refractory ALL (Table 3 )
Historically, R/R adult ALL carried a death sentence. With intensive chemotherapy, the CR rate was 30–50% and the median OS was 3–6 months. The 3-year survival rate was < 5–10%, even with allogeneic SCT in second CR [ 120 , 121 ]. While inotuzumab and blinatumomab were both superior to standard of care chemotherapy and approved for the treatment of R/R B-ALL, the median survival was only 7.7 months with either modality, and the long-term (at 2–3 years) OS was 25% or less [ 15 , 16 ]. At MD Anderson, we have investigated since 2010 a combination of low-intensity mini-Hyper-CVD chemotherapy with targeted therapies. Inotuzumab was added to mini-Hyper-CVD on Day 3 of each of the first 4 courses at 1.8 to 1.3 mg/m 2 for Course 1, followed by 1.3 to 1.0 mg/m 2 for subsequent courses. The protocol was later amended to lower and fractionate the inotuzumab dose into weekly doses (0.6 and 0.3 mg/m 2 during Course 1 and 0.3 and 0.3 mg/m 2 during subsequent courses) and to add sequential blinatumomab [ 122 , 123 , 124 ]. A total of 112 patients (67 without blinatumomab and 45 with blinatumomab) were treated; 71% were treated in Salvage 1 and 29% in Salvage 2 +. Overall, 93 (83%) patients responded, 70 (63%) of whom achieved CR. The overall MRD negativity rate among responders was 84%. Fifty-three (47%) patients were able to proceed to allogeneic SCT. The median OS was 17 months, significantly superior to historical results with inotuzumab. The 3-year OS rate was 41%. The addition of blinatumomab to mini-Hyper-CVD-inotuzumab in a sequential fashion, along with a lower dose of inotuzumab, improved outcomes. The median OS and 3-year OS were 14 months and 34% pre-amendment, compared with 37 months and 55% post-amendment, respectively. Better outcome was seen in Salvage 1 compared with Salvage 2 +, with 3-year OS rates of 51% versus 17%. No difference in survival was seen according to allogeneic SCT status in patients treated with this regimen. The median OS was 47 months, and 3-year OS rate was 55% in patients who underwent allogeneic SCT, compared with 31 months and 48% in those who did not, respectively. The rate of SOS also improved from 13% before the amendment to 2% after the amendment. These findings highlight the improved safety and efficacy with the addition of blinatumomab to mini-Hyper-CVD and with inotuzumab administered at lower weekly doses (together with ursodiol SOS prophylaxis). However, patients who fail to respond to blinatumomab- and inotuzumab-based combinations have dismal outcomes, with limited treatment options [ 125 ]. As blinatumomab and inotuzumab are being incorporated into the frontline setting, novel treatment strategies are needed for R/R patients such as CAR T cell therapies, venetoclax-based combinations, or other investigational drugs.
We are exploring the value of sequential CAR T cell therapies delivered at the time of CR1 or CR2. Currently, two CD19-directed CAR T cell products are approved for the management of ALL, tisagenlecleucel (Salvage 2 +; age < 26 years) and brexucabtagene autoleucel (R/R disease irrespective of salvage status; age ≥ 18 years) [ 126 , 127 ]. Among 97 patients (age < 26 years) enrolled on the ELIANA (tisagenlecleucel) trial, 65 (67%) achieved CR. Grade 3–4 cytokine release syndrome (CRS) was reported in 49% [ 128 ]. In a recent update, the 5-year EFS and OS rates among 66 patients treated with tisagenlecleucel were 42% and 55%, respectively [ 129 ]. The presence of detectable MRD by NGS at 3 months after tisagenlecleucel therapy independently predicted for worse EFS and OS by multivariate analysis [ 130 ]. Brexucabtagene autoleucel therapy (71 patients; median age 40 years; range, 28 to 52 years) resulted in an ORR of 55%, an MRD negativity of 97% among responders, and a median OS of 18.2 months. Grade ≥ 3 CRS was seen in 24% [ 127 ]. In a recent update, the median overall survival was 25.4 months among evaluable patients, with better survival observed in patients with lower disease burden [ 131 ]. Park and colleagues also reported better outcomes when CAR T cell therapy was offered to adult patients with low disease burden (< 5% bone marrow blasts; median EFS 10.6 months; median OS 20.1 months) compared to those with higher disease burden (≥ 5% bone marrow blasts or extramedullary disease; median OS 12.4 months; estimated 2-year OS around 10%) [ 132 ]. The Real World CAR Consortium data showed that the best outcome with CAR T cell therapy was in patients receiving therapy in low disease burden or no detectable disease, compared with high disease burden [ 133 ]. Despite the high remission rates observed with CD19 CAR T cells, relapses eventually occur in more than 50% of the patients, primarily driven by the downregulation or the loss of CD19 surface antigen expression [ 134 , 135 ]. CD22 is expressed on most B-ALL leukemic cells and is usually retained following CD19 loss. In a phase I trial, 21 children and young adults (17 of whom previously exposed to CD19 CAR T cells) were treated with CD22-targeted CAR T cells. Results showed a dose-dependent antileukemic activity, with a CR rate of 73% in patients receiving ≥ 1 × 10 6 /kg CD22 CAR T cells [ 136 ]. Another CD22 CAR T cell product was evaluated in a pediatric and adult population and resulted in a CR rate of 75% and MRD negativity of 56% [ 137 ]. Similar findings were observed with a novel CD22 CAR T cells product evaluated in 19 heavily pretreated children and young adults relapsing with CD19-negative B-ALL after treatment with CD19 CAR T cells. Of 17 patients infused, 13 (77%) achieved CR and 10 (59%) MRD negativity at day 28 of the infusion. A bispecific CAR product targeting CD19 and/or CD22 (CD19-22.BB.z-CAR) is also being evaluated in a phase I trial of patients with R/R B cell malignancies. Among 17 patients with R/R B-ALL (50% of relapses had low or absent CD19 expression), the ORR and MRD negativity rates were 88% and 100%, respectively [ 135 ]. Based on these observations, immunotherapy approaches including antibody–drug conjugates, BiTEs, and CAR T cell therapy, are complementary. We are currently exploring a consolidative approach with dose-dense mini-HCVD-inotuzumab-blinatumomab followed by CD19 CAR T cells.
Management of CNS relapses
The incidence of CNS leukemia in adults with ALL is around 5%–10% at diagnosis and 5% at relapse using current standard therapies [ 138 ]. Patients with CNS recurrence have a poor outcome, with an historical survival of less than 1 year [ 139 ]. The anti-CD19 and CD22 targeted therapies, blinatumomab and inotuzumab, do not cross the blood–brain barrier, and therefore, they are not recommended for treating active CNS relapses [ 140 , 141 ]. Treatment with CAR T cells has demonstrated its efficacy in patients with B-ALL and CNS relapses with or without bone marrow disease. In a retrospective analysis of 48 patients with R/R B-ALL, the administration of CD19 CAR T cells resulted in a remission rate of 85% in the CNS, and a cumulative incidence of CNS relapse of 11% at 12 months. Grade 3–4 neurotoxicity was reported in 23% of patients, with a higher frequency in those with a higher CNS disease burden before the CAR T cells infusion [ 142 ]. Preliminary findings from a post hoc analysis of pooled data from five clinical trials also support the use of CAR T cells in patients with CNS relapses. Treatment with tisagenlecleucel and huCART19 in 66 patients with CNS relapses resulted in a CR rate of 97% at Day 28; however, 42% of responders relapsed again in the CNS after CAR T cell infusion. The incidence of Grade 3–4 neurotoxicity and CRS was 11% and 29%, respectively [ 143 ].
Conclusions
In this analysis, we report our comprehensive data results from 1985 to 2022 in adults with ALL treated at our institution. Significant improvements have been documented in all subtypes of ALL since 2000 and 2010.
In Ph-positive ALL, therapy has shifted from intensive chemotherapy and allogeneic SCT before 2000 to regimens combining BCR::ABL1 TKIs with intensive or lower intensity chemotherapies followed by allogeneic SCT (2000–2010), the addition of ponatinib to intensive chemotherapy (2010–2018) demonstrating for the first time that allogeneic SCT may not be a requirement for a better cure, and finally the recent shift to (mostly) non-chemotherapy non-SCT targeted strategies with BCR::ABL1 TKIs and blinatumomab.
Similar improvements in outcomes were observed in younger patients using Hyper-CVAD in combination with antibodies targeting CD20 (rituximab, ofatumumab), CD19 (blinatumomab), and CD22 (inotuzumab). The recent trials incorporating blinatumomab and inotuzumab were associated with estimated 3-year OS rates above 80%. In older B-ALL, less intensive chemotherapy in combination with inotuzumab and blinatumomab almost doubled the 5-year survival rate (50% versus 25% with historical data of dose-adjusted Hyper-CVAD). Patients ≥ 70 years still have a poor outcome attributed to deaths in CR and the development of myelodysplastic syndrome and AML. In them, combinations of blinatumomab and inotuzumab with minimal chemotherapy might help [ 71 , 72 , 73 , 82 , 84 , 144 ].
The benefit observed with the addition of nelarabine in T-ALL is in line with the findings of the Children's Oncology Group (COG) trial, where the addition of nelarabine in non-ETP and non-lymphoblastic lymphoma has shown a survival benefit [ 94 ]. In the last update, further addition of asparaginase to the Hyper-CVAD regimen is favorable as well and may negate the poor baseline biological features and spare the need for allogeneic SCT as reported by Pui and colleagues (St Jude Total therapy XV) [ 145 ] and the Acute Leukemia Committee of the CIBMTR and the Dana Farber ALL Consortium, where younger adults (median age, 30 years; range, 18 to 50 years) with Ph-negative ALL treated with asparaginase-based therapy had lower treatment-related mortality rates, less relapses, and superior OS compared to allogeneic SCT [ 146 ]. Through BH3 profiling, Chongaile et al. have shown that ETP-ALL is BCL-2 dependent and is very sensitive to in vitro and in vivo treatment with venetoclax [ 147 ]. Furthermore, the combination of venetoclax, with low-dose navitoclax, a BCL-XL/BCL-2 inhibitor, and chemotherapy, was well tolerated and had promising efficacy in patients with relapsed/refractory disease [ 148 ]. Venetoclax with or without navitoclax combined with Hyper-CVAD (NCT03319901) or mini-Hyper-CVD (NCT03808610) are being evaluated in frontline T-ALL and relapsed/refractory Ph-negative ALL. Finally, the inhibition of LCK pathways may lead to a better outcome as well, and this has been shown in vitro where the LCK pathway activation was the driver of dasatinib sensitivity in some of the patients with T-ALL [ 149 ]. Currently, we are exploring the role of ponatinib in combination with mini-Hyper-CVD and venetoclax in T-ALL (NCT05268003).
The use of NGS for MRD assessment is superior to conventional MRD assays. Similar to the pediatric experience [ 150 ], we have shown that patients who achieve early MRD negativity by NGS have the best survival. No relapses were seen in our analysis; however, a longer follow-up with a larger cohort of patients is needed to confirm these findings. If confirmed, then the speed and depth of NGS response may help in the selection of the kinds of therapies needed and tailor the duration of therapy based on the duration of NGS MRD negative status. In Ph-positive ALL, the assessment of NGS MRD early on may allow designing trials that explore TKIs discontinuation, as is now practiced in chronic myeloid leukemia.
Novel bi-specific, tri-specific, and tetra-specific T cell engagers targeting CD19, CD20 and CD22 are under development. So far, CAR T cells have been assessed in the R/R B-ALL population, but their optimal use may be in the setting of minimal disease. Future trials at MD Anderson will assess the role of CAR T cells as a consolidation in frontline high-risk ALL and in second or later CRs. The MRD assessment by NGS post-CAR T cells will help identify patients who may not need allogeneic SCT.
In conclusion, the management of ALL is in the midst of a slow-motion therapeutic revolution. Novel combinations including second–third-generation BCR::ABL1 and novel antibodies are questioning the need and duration of intensive chemotherapy and allogeneic SCT. With these approaches, the outcomes in adult ALL may become potentially as favorable as those observed in pediatric ALL. Perhaps within the next 5 to 10 years, adult ALL therapy may change from the current prolonged intensive chemotherapy of 2–3 years to shorter and less intensive chemotherapy regimens (and perhaps no chemotherapy in Ph-positive and elderly ALL subsets) in combination with TKIs, targeted therapies directed at CD19, CD20 and CD22, BCL-2 inhibitors, and CAR T cells. While randomized controlled trials are useful, they may be outdated by the time the data mature, and this could represent an obstacle for the approval of new drugs. Rather, Bayesian design studies and prospective trials are encouraged. This strategy can help accelerate the development of new therapeutic strategies which might have the potential of improving disease outcomes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Acute lymphoblastic leukemia
Acute myeloid leukemia
Adolescent and young adults
Bispecific T cell engager
Chimeric antigen receptor
Complete molecular response
Central nervous system
Children’s Oncology Group
Complete remission
Complete remission with incomplete count recovery
Cytokine receptor-like factor 2
Cytokine release syndrome
Event-free survival
Early T cell precursor-ALL
German Multicenter Study Group on ALL
Intrathecal
Multi-color flow cytometry
Measurable residual disease
Next-generation sequencing
Overall response rate
Overall survival
Philadelphia chromosome
Progression-free survival
Relapsed/refractory
Stem cell transplantation
Surveillance, Epidemiology, and End Results
Sinusoidal Obstruction Syndrome
Southwest Oncology Group
T cell acute lymphoblastic lymphoma
Tyrosine kinase inhibitor
World Health Organization
Preti A, Kantarjian HM. Management of adult acute lymphocytic leukemia: present issues and key challenges. J Clin Oncol. 1994;12(6):1312–22.
Article CAS PubMed Google Scholar
Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18(3):547.
Pui C-H. Central nervous system disease in acute lymphoblastic leukemia: prophylaxis and treatment. Hematology. 2006;2006(1):142–6.
Article Google Scholar
Jabbour E, Thomas D, Cortes J, Kantarjian HM, O’Brien S. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia. Cancer. 2010;116(10):2290–300.
CAS PubMed Google Scholar
Kantarjian H, Andreeff M, Keating M, Kornblau S, Ferrajoli A, Verstovsek S, et al. Update of the modified hyper-CVAD regimen with or without rituximab in newly diagnosed adult acute lymphocytic leukemia (ALL). Blood. 2005;106(11):1831.
Thomas DA, O’Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3880–9.
Article CAS PubMed PubMed Central Google Scholar
Thomas DA, Kantarjian HM, Cortes J, Faderl S, Giles F, Garcia-Manero G, et al. Outcome with the hyper-CVAD and imatinib mesylate regimen as frontline therapy for adult philadelphia (Ph) positive acute lymphocytic leukemia (ALL). Blood. 2006;108(11):284.
Jabbour E, O’Brien SM, Tippett N, Alvarez J, Jain N, Jorgensen JL, et al. Long-term safety and efficacy of hyper-CVAD plus ponatinib as frontline therapy for adults with philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2019;134(Supplement_1):283.
Ravandi F, O’Brien S, Thomas D, Faderl S, Jones D, Garris R, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116(12):2070–7.
Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100(5):653–61.
Ravandi F, Othus M, O’Brien SM, Forman SJ, Ha CS, Wong JYC, et al. US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in Philadelphia chromosome positive ALL. Blood Adv. 2016;1(3):250–9.
Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66.
Topp MS, Gökbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–40.
Kantarjian H, Thomas D, Jorgensen J, Jabbour E, Kebriaei P, Rytting M, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13(4):403–11.
Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J-M, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836–47.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–53.
Mühlbacher V, Zenger M, Schnittger S, Weissmann S, Kunze F, Kohlmann A, et al. Acute lymphoblastic leukemia with low hypodiploid/near triploid karyotype is a specific clinical entity and exhibits a very highTP53mutation frequency of 93%. Genes Chromosom Cancer. 2014;53(6):524–36.
Article PubMed Google Scholar
Issa GC, Kantarjian HM, Yin CC, Qiao W, Ravandi F, Thomas D, et al. Prognostic impact of pretreatment cytogenetics in adult Philadelphia chromosome-negative acute lymphoblastic leukemia in the era of minimal residual disease. Cancer. 2017;123(3):459–67.
Moorman AV, Barretta E, Butler ER, Ward EJ, Twentyman K, Kirkwood AA, et al. Prognostic impact of chromosomal abnormalities and copy number alterations in adult B-cell precursor acute lymphoblastic leukaemia: a UKALL14 study. Leukemia. 2021;36(3):625–36.
Article PubMed PubMed Central Google Scholar
Short N, Kantarjian H, Ravandi F, Yilmaz M, Kadia T, Thompson P, et al. P371: hyper-CVAD with sequential blinatumomab, with or without inotuzumab ozogamicin, in adults with newly diagnosed philadelphia chromosome-negative B-cell acute lymphoblastic leukemia. HemaSphere. 2022;6:271–2.
Jabbour E, Short NJ, Jain N, Thompson PA, Kadia TM, Ferrajoli A, et al. Hyper-CVAD and sequential blinatumomab for newly diagnosed Philadelphia chromosome-negative B-cell acute lymphocytic leukaemia: a single-arm, single-centre, phase 2 trial. Lancet Haematol. 2022. https://doi.org/10.1016/S2352-3026(22)00285-X .
Sasaki K, Jabbour E, Short NJ, Jain N, Ravandi F, Pui CH, et al. Acute lymphoblastic leukemia: a population-based study of outcome in the United States based on the surveillance, epidemiology, and end results (SEER) database, 1980–2017. Am J Hematol. 2021;96(6):650–8.
Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X, et al. Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia–-results of the prospective multicenter LALA-94 trial. Blood. 2002;100(7):2357–66.
Thomas X, Thiebaut A, Olteanu N, Danaila C, Charrin C, Archimbaud E, et al. Philadelphia chromosome positive adult acute lymphoblastic leukemia: characteristics, prognostic factors and treatment outcome. Hematol Cell Ther. 1998;40(3):119–28.
Chao NJ, Blume KG, Forman SJ, Snyder DS. Long-term follow-up of allogeneic bone marrow recipients for Philadelphia chromosome-positive acute lymphoblastic leukemia [letter]. Blood. 1995;85(11):3353–4.
Ravandi F, O’Brien SM, Cortes JE, Thomas DM, Garris R, Faderl S, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121(23):4158–64.
Rousselot P, Coudé MM, Gokbuget N, Gambacorti Passerini C, Hayette S, Cayuela J-M, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774–82.
Nicolini FE, Mauro MJ, Martinelli G, Kim D-W, Soverini S, Müller MC, et al. Epidemiologic study on survival of chronic myeloid leukemia and Ph+ acute lymphoblastic leukemia patients with BCR-ABL T315I mutation. Blood. 2009;114(26):5271–8.
Jabbour E, Kantarjian H, Ravandi F, Thomas D, Huang X, Faderl S, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. Lancet Oncol. 2015;16(15):1547–55.
Jabbour E, Short NJ, Ravandi F, Huang X, Daver N, DiNardo CD, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. 2018;5(12):e618–27.
Kantarjian H, Short NJ, Jain N, Sasaki K, Huang X, Haddad FG, et al. Frontline combination of ponatinib and hyper-CVAD in Philadelphia chromosome-positive acute lymphoblastic leukemia: 80-months follow-up results. Am J Hematol. 2023. https://doi.org/10.1002/ajh.26816 .
Paul S, Sasaki K, Savoy JM, Dipippo A, Jammal N, Marx K, et al. Title: 12 versus 8 prophylactic intrathecal (IT) chemotherapy administration decrease incidence of central nervous system (CNS) relapse in patients (pts) with newly diagnosed philadelphia (Ph)-positive acute lymphocytic leukemia (ALL). Blood. 2019;134(Supplement_1):3810.
Paul S, Kantarjian H, Sasaki K, Marx K, Jain N, Savoy JM, et al. Intrathecal prophylaxis with 12 versus 8 administrations reduces the incidence of central nervous system relapse in patients with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia. Am J Hematol. 2022. https://doi.org/10.1002/ajh.26622 .
Sasaki K, Jabbour EJ, Ravandi F, Short NJ, Thomas DA, Garcia-Manero G, et al. Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2016;122(23):3650–6.
Jabbour E, DerSarkissian M, Duh MS, McCormick N, Cheng WY, McGarry LJ, et al. Efficacy of ponatinib versus earlier generation tyrosine kinase inhibitors for front-line treatment of newly diagnosed philadelphia-positive acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2018;18(4):257–65.
Short NJ, Jabbour E, Sasaki K, Patel K, O’Brien SM, Cortes JE, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128(4):504–7.
Sasaki K, Kantarjian HM, Short NJ, Samra B, Khoury JD, Kanagal Shamanna R, et al. Prognostic factors for progression in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia in complete molecular response within 3 months of therapy with tyrosine kinase inhibitors. Cancer. 2021;127(15):2648–56.
Ravandi F, Jorgensen JL, O’Brien SM, Jabbour E, Thomas DA, Borthakur G, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2016;172(3):392–400.
Chalandon Y, Thomas X, Hayette S, Cayuela J-M, Abbal C, Huguet F, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711–9.
Chiaretti S, Vitale A, Elia L, Fedullo AL, Albino S, Piciocchi A, et al. Multicenter total therapy gimema LAL 1509 protocol for de novo adult Ph+ acute lymphoblastic leukemia (ALL) patients. Updated results and refined genetic-based prognostic stratification. Blood. 2015;126(23):81.
Foà R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521–8.
Martinelli G, Papayannidis C, Piciocchi A, Robustelli V, Soverini S, Terragna C, et al. INCB84344–201: ponatinib and steroids in frontline therapy of unfit patients with Ph+ acute lymphoblastic leukemia. Blood Adv. 2021. https://doi.org/10.1182/bloodadvances.2021004821 .
Ottmann OG, Pfeifer H, Cayuela J-M, Spiekermann K, Jung W, Beck J, et al. Nilotinib (Tasigna®) and low intensity chemotherapy for first-line treatment of elderly patients with BCR-ABL1-positive acute lymphoblastic leukemia: final results of a prospective multicenter trial (EWALL-PH02). Blood. 2018;132(Supplement 1):31.
Rousselot P, Chalandon Y, Chevret S, Cayuela J-M, Huguet F, Chevallier P, et al. The omission of high-dose cytarabine during consolidation therapy of Ph-positive ALL patients treated with nilotinib and low-intensity chemotherapy results in an increased risk of relapses despite non-inferior levels of late BCR-ABL1 MRD response. First results of the randomized GRAAPH-2014 study. Blood. 2021;138:512.
Vignetti M, Fazi P, Cimino G, Martinelli G, Di Raimondo F, Ferrara F, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109(9):3676–8.
Yves Chalandon PR, Cayuela J-M, Thomas X, Clappier E, Havelange V, Chevallier P, Huguet F, Boissel N, Berthon C, Stüssi G, Maury S, Chantepie S, Pasquier F, Plantier I, Lhéritier V, Ifrah N, Chevret S, Dombret H. Nilotinib combined with lower-intensity chemotherapy for front-line treatment of younger adults with Ph-positive acute lymphoblastic leukemia: interim analysis of the GRAAPH-2014 trial. European Hematology Association.
Rambaldi A, Ribera JM, Kantarjian HM, Dombret H, Ottmann OG, Stein AS, et al. Blinatumomab compared with standard of care for the treatment of adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia. Cancer. 2019;126(2):304–10.
Stock W, Martinelli G, Stelljes M, DeAngelo DJ, Gökbuget N, Advani AS, et al. Efficacy of inotuzumab ozogamicin in patients with Philadelphia chromosome-positive relapsed/refractory acute lymphoblastic leukemia. Cancer. 2020;127(6):905–13.
Assi R, Kantarjian H, Short NJ, Daver N, Takahashi K, Garcia-Manero G, et al. Safety and efficacy of blinatumomab in combination with a tyrosine kinase inhibitor for the treatment of relapsed philadelphia chromosome-positive leukemia. Clin Lymphoma Myeloma Leuk. 2017;17(12):897–901.
Foà R, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo M-C, et al. Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383(17):1613–23.
Chiaretti S, Bassan R, Vitale A, Elias L, Piciocchi A, Viero P, et al. Forty months update of the GIMEMA LAL2116 (D-ALBA) protocol and ancillary LAL2217 study for newly diagnosed adult Ph+ ALL. Den Haag: European Hematology Association; 2022.
Book Google Scholar
Short NJ, Kantarjian HM, Konopleva M, Jain N, Huang X, Ravandi F, et al. Combination of ponatinib and blinatumomab in Philadelphia chromosome-positive acute lymphoblastic leukemia: early results from a phase II study. J Clin Oncol. 2021;39(15_suppl):7001.
Jabbour E, Haddad FG, Short NJ, Kantarjian H. Treatment of adults with philadelphia chromosome-positive acute lymphoblastic leukemia—from intensive chemotherapy combinations to chemotherapy-free regimens. JAMA Oncol. 2022. https://doi.org/10.1001/jamaoncol.2022.2398 .
Jabbour E, Richard-Carpentier G, Sasaki Y, Konopleva M, Patel K, Roberts K, et al. Hyper-CVAD regimen in combination with ofatumumab as frontline therapy for adults with Philadelphia chromosome-negative B-cell acute lymphoblastic leukaemia: a single-arm, phase 2 trial. Lancet Haematol. 2020;7(7):e523–33.
Stock W, Luger SM, Advani AS, Yin J, Harvey RC, Mullighan CG, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–59.
Sasaki K, Kantarjian HM, Morita K, Short NJ, Konopleva M, Jain N, et al. Hyper-CVAD plus ofatumumab versus hyper-CVAD plus rituximab as frontline therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2021;127(18):3381–9.
Budde LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23(8):1055–65.
Bannerji R, Allan JN, Arnason JE, Brown JR, Advani R, Ansell SM, et al. Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-hodgkin lymphoma, including patients refractory to CAR T therapy. Blood. 2020;136(Supplement 1):42–3.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–19.
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International consensus classification of myeloid neoplasms and acute leukemia: integrating morphological, clinical, and genomic data. Blood. 2022. https://doi.org/10.1182/blood.2022015850 .
Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang Y-L, Pei D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–15.
Den Boer ML, van Slegtenhorst M, De Menezes RX, Cheok MH, Buijs-Gladdines JG, Peters ST, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10(2):125–34.
Mullighan CG, Su X, Zhang J, Radtke I, Phillips LAA, Miller CB, et al. Deletion of IKZF1and prognosis in acute lymphoblastic leukemia. N Engl J Med. 2009;360(5):470–80.
Roberts KG, Gu Z, Payne-Turner D, McCastlain K, Harvey RC, Chen IM, et al. High frequency and poor outcome of Philadelphia chromosome-like acute lymphoblastic leukemia in adults. J Clin Oncol. 2017;35(4):394–401.
Jain N, Roberts KG, Jabbour E, Patel K, Eterovic AK, Chen K, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood. 2017;129(5):572–81.
Bargou RC, Nagorsen D, Zugmaier G, Benjamin JE, Haddad V, Wessels H, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522–31.
Jabbour E, Patel K, Jain N, Duose D, Luthra R, Short NJ, et al. Impact of Philadelphia chromosome-like alterations on efficacy and safety of blinatumomab in adults with relapsed/refractory acute lymphoblastic leukemia: a post hoc analysis from the phase 3 TOWER study. Am J Hematol. 2021;96(10):E379–83.
Short NJ, Kantarjian HM, Ravandi F, Huang X, Ferrajoli A, Kadia TM, et al. Hyper-CVAD and sequential blinatumomab in adults with newly diagnosed philadelphia chromosome-negative B-cell acute lymphoblastic leukemia: results from a phase II study. Blood. 2020;136(Supplement 1):9–11.
Short NJ, Kantarjian HM, Ravandi F, Yilmaz M, Kadia TM, Thompson PA, et al. A phase II study of hyper-CVAD with sequential blinatumomab (Blina), with or without inotuzumab ozogamicin (INO), in adults with newly diagnosed B-cell acute lymphoblastic leukemia (ALL). J Clin Oncol. 2022;40(16_suppl):7034.
Fleming S, Reynolds J, Bajel A, Venn N, Kwan J, Moore J, et al. Sequential blinatumomab with reduced intensity chemotherapy in the treatment of older adults with newly diagnosed Ph negative B-precursor acute lymphoblastic leukemia-interim analysis of the australasian leukemia and lymphoma group ALL08 study. Blood. 2021;138(Supplement 1):1234.
Boissel N, Huguet F, Graux C, Hicheri Y, Chevallier P, Kim R, et al. Frontline consolidation with blinatumomab for high-risk philadelphia-negative acute lymphoblastic adult patients. Early results from the Graall-2014-QUEST Phase 2. Blood. 2021;138(Supplement 1):1232.
Bassan R, Chiaretti S, Starza ID, Spinelli O, Santoro A, Elia L, et al. Preliminary results of the GIMEMA LAL2317 sequential chemotherapy-blinatumomab front-line trial for newly diagnosed adult Ph-negative B-lineage ALL patients. Den Haag: European Hematology Association; 2021.
Google Scholar
Geyer MB, Hsu M, Devlin SM, Tallman MS, Douer D, Park JH. Overall survival among older US adults with ALL remains low despite modest improvement since 1980: SEER analysis. Blood. 2017;129(13):1878–81.
Li S, Molony JT, Chia V, Katz AJ. Patient characteristics and treatment patterns in elderly patients newly diagnosed with acute lymphoblastic leukemia (ALL) using 100% medicare ALL data. Blood. 2016;128(22):3981.
Goekbuget N, Beck J, Brueggemann M, Burmeister T, Buss EC, Frickhofen N, et al. Moderate intensive chemotherapy including CNS-prophylaxis with liposomal cytarabine is feasible and effective in older patients with Ph-negative acute lymphoblastic leukemia (ALL): results of a prospective trial from the german multicenter study group for adult ALL (GMALL). Blood. 2012;120(21):1493.
O’Brien S, Thomas DA, Ravandi F, Faderl S, Pierce S, Kantarjian H. Results of the hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone regimen in elderly patients with acute lymphocytic leukemia. Cancer. 2008;113(8):2097–101.
Jabbour EJ, Sasaki K, Ravandi F, Short NJ, Garcia-Manero G, Daver N, et al. Inotuzumab ozogamicin in combination with low‐intensity chemotherapy (mini‐HCVD) with or without blinatumomab versus standard intensive chemotherapy (HCVAD) as frontline therapy for older patients with Philadelphia chromosome‐negative acute lymphoblastic leukemia: a propensity score analysis. Cancer. 2019. https://doi.org/10.1002/cncr.32139 .
Short NJ, Kantarjian HM, Ravandi F, Huang X, Jain N, Kadia TM, et al. Reduced-intensity chemotherapy with mini-hyper-CVD plus inotuzumab ozogamicin, with or without blinatumomab, in older adults with newly diagnosed philadelphia chromosome-negative acute lymphoblastic leukemia: results from a phase II study. Blood. 2020;136(Supplement 1):15–7.
Kantarjian H, Ravandi F, Short NJ, Huang X, Jain N, Sasaki K, et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2018;19(2):240–8.
Macaron W, Kantarjian HM, Short NJ, Ravandi F, Jain N, Kadia TM, et al. Updated results from a phase II study of mini-hyper-CVD (mini-HCVD) plus inotuzumab ozogamicin (INO), with or without blinatumomab (Blina), in older adults with newly diagnosed Philadelphia chromosome (Ph)-negative B-cell acute lymphoblastic leukemia (ALL). J Clin Oncol. 2022;40(16_suppl):7011.
Stelljes M, Alakel N, Wäsch R, Scholl S, Nachtkamp K, Rank A, et al. Final induction therapy results of an open label phase ii study using inotuzumab ozogamicin for induction therapy, followed by a conventional chemotherapy based consolidation and maintenance therapy in patients aged 56 years and older with acute B-lymphoblastic leukemia (INITIAL-1 trial). Blood. 2021;138(Supplement 1):2300.
Chevallier P, Leguay T, Doubek M, Huguet F, Salek C, Cabannes A, et al. Fractionated inotuzumab ozogamicin combined with low-intensity chemotherapy provides very good outcome in older patients with newly diagnosed CD22+ Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia: first results from the EWALL-INO study. Blood. 2021;138(Supplement 1):511.
Advani AS, Moseley A, O’Dwyer KM, Wood BL, Fang M, Wieduwilt MJ, et al. SWOG 1318: a phase II trial of blinatumomab followed by POMP maintenance in older patients with newly diagnosed philadelphia chromosome-negative B-cell acute lymphoblastic leukemia. J Clin Oncol. 2022;40(14):1574–82.
Ribrag V, Koscielny S, Bosq J, Leguay T, Casasnovas O, Fornecker L-M, et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: a randomised, controlled, open-label, phase 3 trial. The Lancet. 2016;387(10036):2402–11.
Article CAS Google Scholar
Thomas DA, Faderl S, O’Brien S, Bueso-Ramos C, Cortes J, Garcia-Manero G, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106(7):1569–80.
Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM, Cole D, Grant C, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med. 2013;369(20):1915–25.
Evens AM, Danilov A, Jagadeesh D, Sperling A, Kim S-H, Vaca R, et al. Burkitt lymphoma in the modern era: real-world outcomes and prognostication across 30 US cancer centers. Blood. 2021;137(3):374–86.
Samra B, Khoury JD, Morita K, Ravandi F, Richard-Carpentier G, Short NJ, et al. Long term outcome of Hyper-CVAD-R for Burkitt leukemia/lymphoma and high-grade B-cell lymphoma: focus on CNS relapse. Blood Adv. 2021. https://doi.org/10.1182/bloodadvances.2021004427 .
Bond J, Graux C, Lhermitte L, Lara D, Cluzeau T, Leguay T, et al. Early response-based therapy stratification improves survival in adult early thymic precursor acute lymphoblastic leukemia: a group for research on adult acute lymphoblastic leukemia study. J Clin Oncol. 2017;35(23):2683–91.
Jain N, Lamb AV, O’Brien S, Ravandi F, Konopleva M, Jabbour E, et al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. 2016;127(15):1863–9.
Zhang J, Ding L, Holmfeldt L, Wu G, Heatley SL, Payne-Turner D, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–63.
Coustan-Smith E, Mullighan CG, Onciu M, Behm FG, Raimondi SC, Pei D, et al. Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol. 2009;10(2):147–56.
Dunsmore KP, Winter SS, Devidas M, Wood BL, Esiashvili N, Chen Z, et al. Children’s Oncology Group AALL0434: a phase III randomized clinical trial testing nelarabine in newly diagnosed T-cell acute lymphoblastic leukemia. J Clin Oncol. 2020;38(28):3282–93.
Maiti A, Jabbour E, Jain N, Borthakur G, Kadia TM, Burger JA, et al. Frontline HCVAD with nelarabine and peg-asparaginase in T-acute lymphoblastic leukemia (T-ALL) and T-lymphoblastic lymphoma (T-LBL): updated results of a phase II trial. Blood. 2020;136(Supplement 1):36–9.
Morita K, Jain N, Kantarjian H, Takahashi K, Fang H, Konopleva M, et al. Outcome of T-cell acute lymphoblastic leukemia/lymphoma: Focus on near-ETP phenotype and differential impact of nelarabine. Am J Hematol. 2021;96(5):589–98.
Frismantas V, Dobay MP, Rinaldi A, Tchinda J, Dunn SH, Kunz J, et al. Ex vivo drug response profiling detects recurrent sensitivity patterns in drug-resistant acute lymphoblastic leukemia. Blood. 2017;129(11):e26–37.
Khaw SL, Suryani S, Evans K, Richmond J, Robbins A, Kurmasheva RT, et al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood. 2016;128(10):1382–95.
Benito Juliana M, Godfrey L, Kojima K, Hogdal L, Wunderlich M, Geng H, et al. MLL-rearranged acute lymphoblastic leukemias activate BCL-2 through H3K79 methylation and are sensitive to the BCL-2-specific antagonist ABT-199. Cell Rep. 2015;13(12):2715–27.
Jain N, Stevenson KE, Winer ES, Garcia JS, Stone RM, Jabbour E, et al. A Multicenter phase I study combining venetoclax with mini-hyper-CVD in older adults with untreated and relapsed/refractory acute lymphoblastic leukemia. Blood. 2019;134(Supplement_1):3867.
Ravandi F, Jabbour E, Jain N, Kadia T, Gautam B, Konopleva M, et al. Incorporation of nelarabine (Nel), pegylated asparaginase (Peg) and venetoclax (Ven) in the frontline therapy of adult patients with T-acute lymphoblastic leukemia/T-lymphoblastic lymphoma (T-ALL/LBL). Den Haag: European Hematology Association; 2022.
Freiwan A, Zoine J, Crawford JC, Vaidya A, Schattgen SA, Myers J, et al. Engineering naturally occurring CD7 negative T cells for the immunotherapy of hematological malignancies. Blood. 2022. https://doi.org/10.1182/blood-2019-124903 .
Gomes-Silva D, Srinivasan M, Sharma S, Lee CM, Wagner DL, Davis TH, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130(3):285–96.
Fleischer LC, Spencer HT, Raikar SS. Targeting T cell malignancies using CAR-based immunotherapy: challenges and potential solutions. J Hematol Oncol. 2019;12(1):1–21.
Lu P, Liu Y, Yang J, Zhang X, Yang X, Wang H, et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: first-in-human phase I clinical trial. Blood. 2022. https://doi.org/10.1182/blood.2021014498 .
Berry DA, Zhou S, Higley H, Mukundan L, Fu S, Reaman GH, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia. JAMA Oncol. 2017;3(7):e170580.
Short NJ, Jabbour E, Albitar M, de Lima M, Gore L, Jorgensen J, et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: a consensus of North American experts. Am J Hematol. 2019;94(2):257–65.
Ribera J-M, Morgades M, Ciudad J, Montesinos P, Esteve J, Genescà E, et al. Chemotherapy or allogeneic transplantation in high-risk Philadelphia chromosome-negative adult lymphoblastic leukemia. Blood. 2021;137(14):1879–94.
Dhédin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V, et al. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486–96.
Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, et al. Different molecular levels of post-induction minimal residual disease may predict hematopoietic stem cell transplantation outcome in adult Philadelphia-negative acute lymphoblastic leukemia. Blood Cancer J. 2014;4(7):e225-e.
Brüggemann M, Raff T, Kneba M. Has MRD monitoring superseded other prognostic factors in adult ALL? Blood. 2012;120(23):4470–81.
Bassan R. Using minimal residual disease to improve treatment response definitions and hematopoietic cell transplantation strategy in acute leukemia. J Clin Oncol. 2016;34(4):300–2.
Yilmaz M, Kantarjian H, Wang X, Khoury JD, Ravandi F, Jorgensen J, et al. The early achievement of measurable residual disease negativity in the treatment of adults with Philadelphia-negative B-cell acute lymphoblastic leukemia is a strong predictor for survival. Am J Hematol. 2019;95(2):144–50.
Hein K, Short N, Jabbour E, Yilmaz M. Clinical value of measurable residual disease in acute lymphoblastic leukemia. Blood Lymphat Cancer: Targets Therapy. 2022;12:7–16.
Short NJ, Kantarjian HM, Patel K, Kornblau SM, Jorgensen JL, Wang SA, et al. Ultrasensitive next-generation sequencing-based measurable residual disease assessment in philadelphia chromosome-negative acute lymphoblastic leukemia after frontline therapy: correlation with flow cytometry and impact on clinical outcomes. Blood. 2020;136(Supplement 1):26–8.
Goekbuget N, Dombret H, Zugmaier G, Bonifacio M, Graux C, Faul C, et al. Blinatumomab for minimal residual disease (MRD) in adults with B-cell precursor acute lymphoblastic leukemia (BCP-ALL): median overall survival (OS) is not reached in complete MRD responders at a median follow-up of 53.1 months. Blood. 2018;132(Supplement 1):554.
Jabbour EJ, Short NJ, Jain N, Jammal N, Jorgensen J, Wang S, et al. Blinatumomab is associated with favorable outcomes in patients with B-cell lineage acute lymphoblastic leukemia and positive measurable residual disease at a threshold of 10–4 and higher. Am J Hematol. 2022. https://doi.org/10.1002/ajh.26634 .
Gökbuget N, Zugmaier G, Dombret H, Stein A, Bonifacio M, Graux C, et al. Curative outcomes following blinatumomab in adults with minimal residual disease B-cell precursor acute lymphoblastic leukemia. Leuk Lymphoma. 2020;61(11):2665–73.
Senapati J, Kantarjian H, Short N, Alvarado Y, Burger J, Jain N, et al. A phase II study of inotuzumab ozogamicin for the treatment of measurable residual disease-positive B-cell acute lymphoblastic leukemia. Den Haag: European Hematology Association; 2022.
Tavernier E, Boiron JM, Huguet F, Bradstock K, Vey N, Kovacsovics T, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21(9):1907–14.
Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2006;109(3):944–50.
Jabbour E, Sasaki K, Ravandi F, Huang X, Short NJ, Khouri M, et al. Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer. 2018;124(20):4044–55.
Jabbour E, Sasaki K, Short NJ, Ravandi F, Huang X, Khoury JD, et al. Long-term follow-up of salvage therapy using a combination of inotuzumab ozogamicin and mini-hyper-CVD with or without blinatumomab in relapsed/refractory Philadelphia chromosome-negative acute lymphoblastic leukemia. Cancer. 2021;127(12):2025–38.
Jabbour E, Ravandi F, Kebriaei P, Huang X, Short NJ, Thomas D, et al. Salvage chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD for patients with relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia. JAMA Oncol. 2018;4(2):230–4.
Short NJ, Macaron W, Konopleva M, Ravandi F, Jain N, Issa GC, et al. Dismal outcomes of patients with relapsed/refractory Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia after failure of both inotuzumab ozogamicin and blinatumomab. Am J Hematol. 2022;97(6):E201–4.
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–48.
Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. The Lancet. 2021;398(10299):491–502.
Grupp SA, Maude SL, Rives S, Baruchel A, Boyer MW, Bittencourt H, et al. Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory (R/R) acute lymphoblastic leukemia. Blood. 2018;132(Supplement 1):895.
Rives S, Maude SL, Hiramatsu H, Baruchel A, Bader P, Bittencourt H, et al. S112: Tisagenlecleucel in pediatric and young adult patients (Pts) with relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-All): final analyses from the Eliana study. HemaSphere. 2022;6:13–4.
Pulsipher MA, Han X, Maude SL, Laetsch TW, Qayed M, Rives S, et al. Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discov. 2022;3(1):66–81.
Shah BD, Ghobadi A, Oluwole OO, Logan A, Boissel N, Cassaday RD, et al. Two-year follow-up of KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in adult patients (Pts) with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL) in ZUMA-3. J Clin Oncol. 2022;40(16_suppl):7010.
Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–59.
Schultz LM, Baggott C, Prabhu S, Pacenta HL, Phillips CL, Rossoff J, et al. Disease burden affects outcomes in pediatric and young adult b-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J Clin Oncol. 2022;40(9):945–55.
Zhang Y, Li S, Wang Y, Lu Y, Xu Y, Rao Q, et al. A novel and efficient CD22 CAR-T therapy induced a robust antitumor effect in relapsed/refractory leukemia patients when combined with CD19 CAR-T treatment as a sequential therapy. Exp Hematol Oncol. 2022;11(1):1–18.
Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419–31.
Fry TJ, Shah NN, Orentas RJ, Stetler-Stevenson M, Yuan CM, Ramakrishna S, et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2017;24(1):20–8.
Jeyakumar N, Ramakrishna S, Frank MJ, Sahaf B, Feldman SA, Miklos DB, et al. CD22 CAR T cells demonstrate favorable safety profile and high response rates in pediatric and adult B-ALL: results of a phase 1b study. Blood. 2022;140(Supplement 1):2374–5.
Larson RA. Managing CNS disease in adults with acute lymphoblastic leukemia. Leuk Lymphoma. 2017;59(1):3–13.
Sancho J-M, Ribera J-M, Oriol A, Hernandez-Rivas J-M, Rivas C, Bethencourt C, et al. Central nervous system recurrence in adult patients with acute lymphoblastic leukemia. Cancer. 2006;106(12):2540–6.
McNeer JL, Rau RE, Gupta S, Maude SL, O’Brien MM. Cutting to the front of the line: immunotherapy for childhood acute lymphoblastic leukemia. Am Soc Clin Oncol Educ Book. 2020;40:e132–43.
Molina JC, Shah NN. CAR T cells better than BiTEs. Blood Adv. 2021;5(2):602–6.
Qi Y, Zhao M, Hu Y, Wang Y, Li P, Cao J, et al. Efficacy and safety of CD19-specific CAR T cell-based therapy in B-cell acute lymphoblastic leukemia patients with CNSL. Blood. 2022;139(23):3376–86.
Leahy AB, Newman H, Li Y, Liu H, Myers R, DiNofia A, et al. CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: a post-hoc analysis of pooled data from five clinical trials. The Lancet Haematology. 2021;8(10):e711–22.
Goekbuget N, Stoltefuß A, Topp M, Schwartz S, Renzelmann A, Faul C, et al. Dose reduced chemotherapy in sequence with blinatumomab for newly diagnosed older patients with B-precursor adult lymphoblastic leukemia (ALL): results of the ongoing GMALL bold trial. Blood. 2021;138(Supplement 1):3399.
Pui C-H, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–41.
Seftel MD, Neuberg D, Zhang M-J, Wang H-L, Ballen KK, Bergeron J, et al. Pediatric-inspired therapy compared to allografting for Philadelphia chromosome-negative adult ALL in first complete remission. Am J Hematol. 2016;91(3):322–9.
Chonghaile TN, Roderick JE, Glenfield C, Ryan J, Sallan SE, Silverman LB, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4(9):1074–87.
Pullarkat VA, Lacayo NJ, Jabbour E, Rubnitz JE, Bajel A, Laetsch TW, et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 2021;11(6):1440–53.
Gocho Y, Liu J, Hu J, Yang W, Dharia NV, Zhang J, et al. Network-based systems pharmacology reveals heterogeneity in LCK and BCL2 signaling and therapeutic sensitivity of T-cell acute lymphoblastic leukemia. Nat Cancer. 2021;2(3):284–99.
Wood B, Wu D, Crossley B, Dai Y, Williamson D, Gawad C, et al. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood. 2018;131(12):1350–9.
Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gökbuget N, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019;125(14):2474–87.
Download references
Acknowledgements
Not applicable.
No funding provided for this manuscript.
Author information
Authors and affiliations.
Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Boulevard, Unit 428, Houston, TX, 77030, USA
Elias Jabbour, Nicholas J. Short, Nitin Jain, Fadi G. Haddad, Mary Alma Welch, Farhad Ravandi & Hagop Kantarjian
You can also search for this author in PubMed Google Scholar
Contributions
EJ, FGH, HK collected and analyzed the data and wrote the first manuscript. NJS, MAW, NJ, and FR revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding author
Correspondence to Elias Jabbour .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
EJ received research grants from Abbvie, adaptive biotechnologies, Amgen, Pfizer, and Takeda; and consultancy fees from Abbvie, adaptive biotechnologies, Amgen, BMS, Genentech, Incyte, Novartis, Pfizer, and Takeda. NJS received research grants from Takeda Oncology, Astellas Pharma Inc., Xencor and Stemline Therapeutics; consultancy fees from Pfizer Inc. and Jazz Pharmaceuticals; and honoraria from Novartis, Amgen, Sanofi, and BeiGene. HK received research grants from AbbVie, Amgen, Ascentage, BMS, Daiichi-Sankyo, Immunogen, Jazz, Novartis, Pfizer; and honoraria from AbbVie, Amgen, Aptitude Health, Ascentage, Astellas Health, Astra Zeneca, Ipsen, Pharmaceuticals, KAHR Medical Ltd, NOVA Research, Novartis, Pfizer, Precision Biosciences, Taiho Pharmaceutical Canada. FGH, MAW, NJ, and FR declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Jabbour, E., Short, N.J., Jain, N. et al. The evolution of acute lymphoblastic leukemia research and therapy at MD Anderson over four decades. J Hematol Oncol 16 , 22 (2023). https://doi.org/10.1186/s13045-023-01409-5
Download citation
Received : 23 September 2022
Accepted : 09 February 2023
Published : 16 March 2023
DOI : https://doi.org/10.1186/s13045-023-01409-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Blinatumomab
- Chemotherapy-free
- Targeted therapies
- Tyrosine kinase inhibitors
Journal of Hematology & Oncology
ISSN: 1756-8722
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 30 June 2017
Acute lymphoblastic leukemia: a comprehensive review and 2017 update
- T Terwilliger 1 &
- M Abdul-Hay 1 , 2
Blood Cancer Journal volume 7 , page e577 ( 2017 ) Cite this article
133k Accesses
737 Citations
368 Altmetric
Metrics details
- Acute lymphocytic leukaemia
- Targeted therapies
Acute lymphoblastic leukemia (ALL) is the second most common acute leukemia in adults, with an incidence of over 6500 cases per year in the United States alone. The hallmark of ALL is chromosomal abnormalities and genetic alterations involved in differentiation and proliferation of lymphoid precursor cells. In adults, 75% of cases develop from precursors of the B-cell lineage, with the remainder of cases consisting of malignant T-cell precursors. Traditionally, risk stratification has been based on clinical factors such age, white blood cell count and response to chemotherapy; however, the identification of recurrent genetic alterations has helped refine individual prognosis and guide management. Despite advances in management, the backbone of therapy remains multi-agent chemotherapy with vincristine, corticosteroids and an anthracycline with allogeneic stem cell transplantation for eligible candidates. Elderly patients are often unable to tolerate such regimens and carry a particularly poor prognosis. Here, we review the major recent advances in the treatment of ALL.
Similar content being viewed by others
Acute lymphoblastic leukaemia


Updated risk-oriented strategy for acute lymphoblastic leukemia in adult patients 18–65 years: NILG ALL 10/07
Temporal changes in incidence of relapse and outcome after relapse of childhood acute lymphoblastic leukemia over three decades; a Nordic population-based cohort study
Introduction.
Acute lymphoblastic leukemia (ALL) is a malignant transformation and proliferation of lymphoid progenitor cells in the bone marrow, blood and extramedullary sites. While 80% of ALL occurs in children, it represents a devastating disease when it occurs in adults. Within the United States, the incidence of ALL is estimated at 1.6 per 100 000 population. 1 In 2016 alone, an estimated 6590 new cases were diagnosed, with over 1400 deaths due to ALL (American Cancer Society). The incidence of ALL follows a bimodal distribution, with the first peak occurring in childhood and a second peak occurring around the age of 50. 2 While dose-intensification strategies have led to a significant improvement in outcomes for pediatric patients, prognosis for the elderly remains very poor. Despite a high rate of response to induction chemotherapy, only 30–40% of adult patients with ALL will achieve long-term remission. 3
Pathophysiology
The pathogenesis of ALL involves the abnormal proliferation and differentiation of a clonal population of lymphoid cells. Studies in the pediatric population have identified genetic syndromes that predispose to a minority of cases of ALL, such as Down syndrome, Fanconi anemia, Bloom syndrome, ataxia telangiectasia and Nijmegen breakdown syndrome. 4 , 5 , 6 , 7 Other predisposing factors include exposure to ionizing radiation, pesticides, certain solvents or viruses such as Epstein-Barr Virus and Human Immunodeficiency Virus. 8 , 9 , 10 However, in the majority of cases, it appears as a de novo malignancy in previously healthy individuals. Chromosomal aberrations are the hallmark of ALL, but are not sufficient to generate leukemia. Characteristic translocations include t(12;21) [ ETV6-RUNX1 ], t(1;19) [ TCF3-PBX1 ], t(9;22) [ BCR-ABL1 ] and rearrangement of MLL . 11 More recently, a variant with a similar gene expression profile to (Philadelphia) Ph-positive ALL but without the BCR-ABL1 rearrangement has been identified. In more than 80% of cases of this so-called Ph-like ALL, the variant possesses deletions in key transcription factors involved in B-cell development including IKAROS family zinc finger 1 (IKZF1), transcription factor 3 (E2A), early B-cell factor 1 (EBF1) and paired box 5 (PAX5). 12 Similarly, kinase-activating mutations are seen in 90% of the Ph-like ALL. The most common of these include rearrangements involving ABL1, JAK2, PDGFRB, CRLF2 and EPOR, activating mutations of IL7R and FLT3 and deletion of SH2B3, which encodes the JAK2-negative regulator LNK. 13 This has significant therapeutic implications as it suggests that Ph-like ALL, which tends to carry a worse prognosis, may respond to kinase inhibitors. In fact, Roberts et al. 14 showed that cell lines and human leukemic cells expressing ABL1, ABL2, CSF1R and PDGFRB were sensitive in vitro and in vivo human xenograft models to second-generation TKIs (for example, dasatinib.); those with EPOR and JAK2 rearrangements were sensitive to JAK kinase inhibitors (for example, ruxolitinib); and those with ETV6-NTRK3 fusion were sensitive to ALK inhibitors crizotinib. Furthermore, Holmfeldt et al. 15 recently described the genetic basis of another subset with poor outcomes, hypodiploid ALL. In near-haploid (24–31 chromosomes) ALL, alterations in tyrosine kinase or Ras signaling was seen in 71% of cases and in IKAROS family zinc finger 3 (IKZF3) in 13% of cases. In contrast, low-hypodiploid (32–39 chromosomes) ALL, alterations in p53 (91%), IKZF2 (53%) and RB1 (41%) were more common. Both near-haploid and low-hypodiploid exhibited activation of Ras- and PI3K-signaling pathways, suggesting that these pathways may be a target for therapy in aggressive hypodiploid ALL. 15
Most of the clinical manifestations of ALL reflect the accumulation of malignant, poorly differentiated lymphoid cells within the bone marrow, peripheral blood, and, extramedullary sites. Presentation can be nonspecific, with a combination of constitutional symptoms and signs of bone marrow failure (anemia, thrombocytopenia, leukopenia). Common symptoms include ‘B symptoms’ (fever, weight loss, night sweats), easy bleeding or bruising, fatigue, dyspnea and infection. Involvement of extramedullary sites commonly occurs and can cause lymphadenopathy, splenomegaly or hepatomegaly in 20% of patients. 16 , 17 CNS involvement at time of diagnosis occurs in 5–8% of patients and present most commonly as cranial nerve deficits or meningismus. 3 T-cell ALL also may present with a mediastinal mass.
Diagnosis is established by the presence of 20% or more lymphoblasts in the bone marrow or peripheral blood. 16 Evaluation for morphology, flow cytometry, Immunophenotyping and cytogenetic testing is valuable both for confirming the diagnosis and risk stratification. Lumbar puncture with CSF analysis is standard of care at the time of diagnosis to evaluate for CNS involvement. If the CNS is involved, brain MRI should be performed. Other evaluation includes complete blood count with differential and smear to evaluate the other hematopoietic cell lines, coagulation profiles and serum chemistries. Baseline uric acid, calcium, phosphate and lactate dehydrogenase should be recorded to monitor for tumor lysis syndrome.
Classification
The first attempt at classifying ALL was the French American British (FAB) morphological criteria that divided ALL into 3 subtypes (L1, L2 and L3) based on cell size, cytoplasm, nucleoli, vacuolation and basophilia. 18 In 1997, the World Health Organization proposed a composite classification in attempt to account for morphology and cytogenetic profile of the leukemic blasts and identified three types of ALL: B lymphoblastic, T lymphoblastic and Burkitt-cell Leukemia. 19 Later revised in 2008, Burkitt-cell Leukemia was eliminated as it is no longer seen as a separate entity from Burkitt Lymphoma, and B-lymphoblastic leukemia was divided into two subtypes: B-ALL with recurrent genetic abnormalities and B-ALL not otherwise specified. B-ALL with recurrent genetic abnormalities is further delineated based on the specific chromosomal rearrangement present ( Table 1 ). 20 In 2016, two new provisional entities were added to the list of recurrent genetic abnormalities and the hypodiploid was redefined as either low hypodiploid or hypodiploid with TP53 mutations. 21 In adults, B-cell ALL accounts for ~75% of cases while T-cell ALL comprises the remaining cases.
Prognostic factors
Accurate assessment of prognosis is central to the management of ALL. Risk stratification allows the physician to determine the most appropriate initial treatment regimen as well as when to consider allogeneic stem cell transplantation (Allo-SCT). Historically, age and white blood cell count at the time of diagnosis have been used to risk stratify patients. Increasing age portends a worsening prognosis. Patients over the age of 60 have particularly poor outcomes, with only 10–15% long-term survival. 22 Age is at least in part a surrogate for other prognosticators as the elderly tend to have disease with intrinsic unfavorable biology (for example, Philadelphia chromosome positive, hypodiploidy and complex karyotype), more medical comorbidities and inability to tolerate standard chemotherapy regimens but helps guide therapy nonetheless. In the largest prospective trial to determine optimal treatment, MRC UKALL XII/ECOG E2993 found a significant difference of disease-free (DFS) and overall survival (OS) based on age using a cutoff of 35 in Ph-negative disease. 23 Similarly, they found an elevated white blood cell count at diagnosis, defined as >30 × 10 9 for B-ALL or >100 10 9 for T-ALL, was an independent prognostic factor for DFS and OS. On the basis of these results, Ph-negative disease could be categorized as low risk (no risk factors based on age or WBC count), intermediate risk (age >35 or elevated WBC count), or high risk (age >35 and elevated WBC count). The 5-year OS rates based on these risk categories were 55, 34 and 5%, respectively. 23
Although clinical factors play an important role in guiding therapy, cytogenetic changes have a significant role in risk determination. The cytogenetic aberration with the greatest impact on prognosis and treatment is the presence of the Philadelphia chromosome, t(9;22). The prevalence of t(9;22) in adult ALL can range from 15–50% and increases with age. 24 Ph-positivity has implications both in terms of prognosis and for treatment. Historically, Ph-positive ALL has a 1-year survival of around 10%. However, with the development of TKIs, survival has improved and thus the Ph-status of all patients must be obtained prior to starting therapy. Subsequent analysis of MRC UKALL XII/ECOG E2993, identified cytogenetic subgroups of Ph-negative disease with inferior outcomes. These included t(4;11), KMT2A translocation, t(8;14), complex karyotype ( ⩾ 5 chromosomal abnormalities) and low hypodiploidy (30–39 chromosomes)/near triploidy (60–78 chromosomes). In contrast, patients with hyperdiploidy and del(9p) had a significantly better outcome. 25 In a later study, the Southwest Oncology Group (SWOG) showed that among the 200 study patients, cytogenetic profile was a more important prognostic factor than age or WBC count. 26 More recently, a subset of high-risk ALL without t(9;22) has been identified with a genetic profile similar to that of Ph-positive ALL. This so called, Ph-like ALL has been associated with poor response to induction chemotherapy, elevated minimal residual disease and poor survival. 13 , 14 , 27
In addition to disease characteristics at the outset, it has long been recognized that response to initial therapy predicts outcome. Historically, treatment response was evaluated morphologically. Recently, it has become standard practice to evaluate patients for minimal residual disease (MRD) using molecular techniques such as flow cytometry and PCR. 28 Several studies have shown the importance of MRD in assigning risk. 29 , 30 , 31 , 32 , 33 , 34 Bruggemann et al. 29 re-stratified standard-risk patients to low risk, intermediate risk and high risk with relapse rates of 0%, 47% and 94%, respectively, based on the persistence of elevated MRD, defined as >10 −4 . In a multivariate analysis of 326 adolescent and adult patients with high-risk Ph-negative ALL treated in The Programa Espanol de Tratamientos en Hematologia (PETHEMA ALL-AR-03), Ribera et al. 35 showed that poor MRD clearance, defined as levels >1 × 10 −3 after induction and levels >5 × 10 −4 after early consolidation by flow cytometry, was the only significant prognostic factor for disease-free and overall survival.
On the basis of what is known about prognostic factors in adult ALL, the National Comprehensive Cancer Network (NCCN) has developed recommendations to approach risk stratification. 16 The National Cancer Institutes defines adolescent and young adults (AYA) to be those aged 15–39 years. The NCCN recognizes that AYA may benefit from treatment with pediatric-inspired regimens and thus are considered separately from adults >40 years. 36 , 37 Both age groups are then stratified into high-risk Ph-positive and standard-risk Ph-negative subgroups. The Ph-negative subgroup can further be categorized as high-risk based on the presence of MRD, elevated WBC (defined above) or unfavorable cytogenetics (defined above).
Established treatments
The structure of treatment of adult ALL has been adapted from pediatric protocols. Unfortunately, while long-term survival approaches 90% for standard-risk pediatric ALL, the success rate is much more modest in adults. Chemotherapy consists of induction, consolidation and long-term maintenance, with CNS prophylaxis given at intervals throughout therapy. The goal of induction therapy is to achieve complete remission and to restore normal hematopoiesis. The backbone of induction therapy typically includes vincristine, corticosteroids and an anthracycline. 38 , 39 In the Cancer and Leukemia Group B 8811 trial, Larsen et al. 40 achieved a complete response rate of 85% and a median survival of 36 months. The 4-week long induction schedule consists of cyclophosphamide on day 1, 3 consecutive days of daunorubicin, weekly vincristine, biweekly l -asparaginase and 3 weeks of prednisone. 40 Due to high induction-related mortality, one-third dose reductions of cyclophosphamide and daunorubicin were implemented for patients older than 60 and the duration of prednisone was shortened to 7 days in this age group. The role of L-asparaginase, while standard in pediatric protocols, is a challenge in adults at times due to the increased rate of adverse events. 41 In fact, in the UKALL 14 Trial, Patel et al. 42 , 43 demonstrated that asparaginase toxicity was the leading cause of induction-related mortality and the protocol was amended to omit asparaginase for patients over the age of 40. The MRC UKALL XII/ECOG 2993 23 regimen utilizes a similar structure to CALGB 8811. Induction is divided into two phases of four weeks. In contrast to CALGB 8811, cyclophosphamide is omitted in phase I of induction, but a single dose of intrathecal methotrexate is added for CNS prophylaxis. In phase II of induction, cyclophosphamide is introduced along with cytarabine, oral 6-mercaptopurine (6-MP), four additional intrathecal doses of methotrexate, and cranial radiation if CNS is positive. After induction therapy, patients received three cycles of intensification therapy of methotrexate with leucovorin rescue and l -asparaginase. Eligible patients with high-risk disease and a matched donor, then underwent Allo-SCT. All others were randomized to standard consolidation/maintenance or autologous stem cell transplant. This study yielded a complete response rate of 91% and an overall 5-year survival of 38%. 23
The Hyper-CVAD (HCVAD)/ Methotrexate-cytarabine regimen is utilizes an alternative structure to the approaches described above. It consists of four cycles of hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternated with four cycles of high dose cytarabine and methotrexate. 44 CNS prophylaxis with 4-16 doses of intrathecal chemotherapy depending on predetermined risk of CNS disease. HCVAD has demonstrated similar efficacy to the ECOG trial with a 92% complete response rate and 32% 5-year disease-free survival. 44 Several studies have suggested a benefit to using dexamethasone as opposed to prednisone due to the ability of dexamethasone to achieve higher concentrations in the CNS. Despite a reduction in CNS relapse and improved event-free survival, dexamethasone has increased risk of adverse events compared to prednisone. Since there have been no studies comparing overall survival, the benefit of one corticosteroid over the other has not been established. 45 , 46
After induction, eligible patients may go on to Allo-SCT while all others go on to intensification/consolidation and maintenance. 47 Consolidation varies in the different protocols, but generally utilize similar agents to induction and includes intrathecal chemotherapy and cranial radiation for CNS prophylaxis at times. Maintenance therapy consists of daily 6-MP, weekly methotrexate, and vincristine and a 5-day prednisone pulse every 3 months. Maintenance is administered for 2–3 years after induction, beyond which it has not been shown to have benefit. 17 , 47
Special consideration must be made in the treatment of Ph-positive ALL. Historically, Ph-positive ALL was a very bad player with 5-year survival ~5–20% and Allo-SCT being the only chance for cure. 48 , 49 Various studies have found that matched-sibling Allo-SCT may improve long-term survival to 35–55%, however, availability of matched donors represents a significant limitation. 49 , 50 , 51 The advent of TKIs marked a turning point in the treatment of Ph-positive ALL. Thomas et al. 52 , 53 showed that when added to traditional HCVAD, imatinib resulted in improvement in 3-year OS (54 vs 15%). Despite these promising results, some patients fails treatment due to resistance or relapse, particularly in the CNS where imatinib has limited penetration. 54 Second-generation ABL kinase inhibitor, dasatinib, was developed as a dual src/abl kinase inhibitor for chronic myeloid leukemia with a superior resistance profile to imatinib. Dasatinib was also shown to penetrate the blood-brain barrier and was effective at treating CNS disease in a mouse model and pediatric Ph-positive ALL. 55 In the first study of dasatinib in Ph-positive ALL, Ravandi et al. 56 found a CR rate of 96% when dasatinib was combined with HCVAD, and a 5-year OS of 46%. In a subsequent, multi-center trial HCVAD plus dasatinib achieve a 3-year OS of 71% in adult patients younger than 60. 57 In addition, prior resistance to imatinib did not preclude a response to dasatinib. 58 In addition, dasatinib was shown to be effective in inducing complete remission when used in combination with prednisone and intrathecal methotrexate. 59 In the GIMEMA LAL1205 study, 59 it was noted that the most common cause of relapse was a T315I mutation in the ABL kinase doman. Ponatinib, a third-generation TKI with the ability to inhibit most BCR-ABL1 kinase domain mutations, has recently gained approval for resistant Ph-positive ALL. The PACE trial 60 demonstrated the ability of ponatinib to generate a cytogenetic response in 47% of Ph-positve ALL patients after dasatinib failure. When compared head-to-head with dasatinib, ponatinib achieved significantly better 3-year EFS and OS when used as frontline therapy. 42 , 61 , 62 These data suggest that ponatinib may soon have a role in the frontline therapy of Ph-positive ALL.
Recent studies have suggested that the AYA population, defined as aged 15–39, may benefit from treatment on pediatric-inspired protocols. In an analysis of 262 AYA patients aged 16–21 on pediatric protocol CCG 1961, Nachman et al. 63 reported a 5-year EFS of 68%. Furthermore, patients in the study that were treated on augmented intensity therapy performed better. In a prospective study, Stock et al. 64 treated 317 patients aged 17–39 on Children’s Oncology Group AALL0232 protocol. Median EFS approached 60 months, which was statistically higher than the null hypothesis of 32 months. OS at 2-years was 78%. 64 Similarly, The Group for Research on Adult Acute Lymphoblastic Leukemia (GRAAL), compared 225 patients up to the age of 60 who were treated on pediatric-inspired regimen and historical data from 712 adults treated on standard adult regimen LALA-93. 36 They observed a significant improvement in CR, EFS and OS, which was most marked in patients younger than age of 45 years. In fact, in patients older than 45 years, there was a significantly higher rate of chemotherapy-related events compared to younger patients, suggesting that an age cutoff for pediatric-inspired regimens is appropriate. However still one of the adult regimens is still considered for AYA patients is HCVAD±rituximab. An MD Anderson Cancer Center study revealed no significant difference in CR rate or OS in AYA patients treated with HCVAD±rituximab vs an augmented-Berlin-Frankfurt-Munster regimen. 65
Refractory/relapsed disease
While 85–90% of patients go into remission after induction therapy, there are subsets that are refractory to induction therapy. In addition, a majority of patients that do achieve CR go on to relapse. Options of salvage therapy for relapsed/refractory (r/r) Ph-negative disease include augmented cytotoxic chemotherapy, reformulated single-agent chemotherapy and novel monoclonal antibodies. Augmented-HCVAD for salvage therapy was inspired by pediatric regimens that employ intensified doses of vincristine, corticosteroids and asparaginase in frontline therapy. Faderl et al. 66 treated 90 patients (median age 34) with relapsed or refractory disease with HCVAD in which the dosing of vincristine, dexamethasone and asparaginase where intensified as follows: vincristine 2 mg i.v. weekly on days 1, 8 and 15; dexamethasone 80 mg i.v. or orally (p.o.) on days 1–4 and 15–18, and pegaspargase 2500 units/m 2 i.v. on day 1 of the hyper-CVAD courses (1, 3, 5 and 7) and day 5 of the methotrexate/cytarabine courses (2, 4, 6 and 8). The majority of patients were in first salvage and ten patients were primary refractory, and patients with prior exposure to HCVAD were not excluded. Complete response was observed in 47% of the patients, with a median duration of 5 months. Median DFS and OS were 6.2 and 6 months respectively. 66 It was also noted that the addition of rituximab to HCVAD for B-ALL with high CD20 expression to improve the activity of this salvage regimen.
In patients with relapsed/refractory ALL, particularly those with multiple relapses, toxicity of multi-agent cytotoxic therapy may be limiting. Therefore, attempts have been made at salvage therapy with a single agent. In subgroup analysis of 70 patients receiving second salvage therapy with a single agent (most commonly vinorelbine (6), clofarabine (5), nelarabine (4) and topotecan (4)), only 3 achieved a complete response. 67 , 68 Vincristine sulfate liposomes injection (VSLI) was developed to overcome the dosing and pharmacokinetic limitations of nonliposomal vincristine (VCR). In a phase II study in adults with Ph-negative ALL in their second or greater relapse, VSLI was administered weekly at a dose of 2.25 mg/m 2 . 69 Of the 65 adults enrolled, 20% achieved complete response with a median duration of 23 weeks (range 5–66). Twelve patients were bridged to Allo-SCT, with five long-term survivors. 69 This study led to the accelerated approval of VSLI for salvage therapy in 2012. VSLI was well tolerated with a side effect profile similar to standard-formulation VCR, despite the massive cumulative doses of VCR achieved.
Despite the modest ability of cytotoxic chemotherapy to prolong survival, the only hope for long-term survival in these regimens remains Allo-SCT. However, recently novel monoclonal antibodies have transformed the landscape of salvage therapy by offering a chance at cure may be without Allo-SCT. The first of these is the bispecific anti-T-cell receptor/anti-CD19 antibody, blinatumomab. The proposed mechanism of action of blinatumomab is that it engages T cells to activate a B-cell specific inflammatory and cytolytic response. 70 Blinatumomab was first studied in patients with MRD positive ALL. In one trial, 80% of patients became MRD negative after the first cycle of blinatumomab, with 60% of patients remaining in CR at a median follow-up of 33 months. 71 Importantly, in a multi-center trial (BLAST), Gokbuget et al. 72 confirmed the ability of blinatumomab to eliminate MRD and showed no difference in OS or relapse-free survival (RFS) between patients who received Allo-SCT during the first CR (CR1) and those who did not. Based on these results, blinatumomab was studied for relapsed/refractory Ph-negative ALL. The landmark study was a multi-center, single-arm, open-label phase 2 trial in which 189 patients with primary refractory and relapsed ALL received single-agent therapy with blinatumomab. CR was achieved after 2 cycles in 43 with 82% achieving MRD negativity. The median response duration and the overall survival were 9 and 6 months, respectively. 73 Based on these results, blinatumomab was approved by the FDA for relapsed and refractory ALL in 2016. Subsequently, blinatumomab was compared to investigator’s choice of chemotherapy for r/r Ph-negative ALL in the phase 3 randomized trial (TOWER study). The blinatumomab study group ( n =271) had a median survival of 7.7 months (95% confidence interval (CI): 5.6, 9.6) versus 4.0 months (95% CI: 2.9, 5.3) for standard of care ( n =134) ( P =0.012, hazards ratio (HR), 0.71). 74 The study was terminated early for efficacy based on these results. Blinatumomab has also been investigated for r/r Ph-positive disease. In the ALCANTARA trial, standard dose blinatumomab was given for up to 5 cycles in 45 patients. CR was observed in 36 and 88% of whom were MRD negative, and with a median follow-up of 9 months, the median OS was 7.1 months. 75 Future investigation is planned for the frontline use of blinatumomab for Ph-positive ALL in conjunction with TKIs. 76 The toxicity profile of blinatumomab is acceptable. The most frequent adverse events include fever, chills, neutropenia, anemia and hypogammaglobulinemia. 3 More significant adverse events are rare, but include cytokine release syndrome, altered mental status and seizures. 73 Death from sepsis that is thought to be treatment-related has been reported.
Frontline therapy is the same for B-cell ALL and T-cell ALL. However, owing to different biology of the two subtypes, T-cell ALL is not amenable to salvage treatment with blinatumomab. Fortunately, alternative options for salvage therapy exist. Nelarabine is a T-cell specific purine nucleoside analog that is FDA approved for r/r T-cell ALL. Nelarabine accumulates in T cells at a high rate and incorporates into DNA causing an inhibition of DNA synthesis and subsequent apoptosis. 77 In a phase 2, open-label, multi-center trial, nelarabine was administered on alternate day schedule (days 1, 3 and 5) at 1.5 g/m 2 /day for r/r T-cell ALL. Cycles were repeated every 22 days. The rate of complete remission was 31% (95% CI, 17, 48%), the median DFS and OS were 20 weeks with a 1-year OS of 28%. 77 However, there is still more that needs to be done to achieve a better response and overall survival in patients with relapsed/refractory B- and T-cell ALL.
Future therapies
1-monoclonal antibodies, a-cd22-directed therapy.
CD22 is a B-lineage differentiation antigen expressed in B-cell ALL in 50–100% of adults and 90% of children. 78 , 79 , 80 Upon binding of an antibody, CD22 is rapidly internalized, thus making it an attractive target for delivering immunotoxin to leukemic cells. 81
Epratuzumab
Epratuzumab is an unconjugated monoclonal antibody targeting CD22 that has been studied in pediatric and adult relapsed/refractory ALL. Epratuzumab was evaluated in 15 pediatric patients as part of a salvage therapy regimen. The antibody was administered as a single-agent followed by the antibody in combination with standard re-induction chemotherapy. The treatment resulted in a CR in 9 of the patients, with 7 achieving complete MRD clearance at the end of re-induction. 82 A phase 2 study in adults with relapsed/refractory disease evaluated the addition of epratuzumab to clofaribine/cytarabine. The study demonstrated a superior response rate when compared to historical data of clofaribine/cytarabine alone. 83 More recently, epratuzumab conjugated to the topoisomerase I inhibitor, SN-38, has been shown to have activity against B-cell lymphoma and leukemia cell lines in in vitro and in vivo preclinical studies. 84
Inotuzumab ozogamicin
Inotuzumab ozogamicin (InO) is a monoclonal antibody against CD22 that is conjugated to calicheamicin, a potent cytotoxic compound that induces double-strand DNA breaks. 85 Upon internalization of the immunoconjugate, calicheamicin binds DNA and causes double-stranded DNA breaks, which induces apoptosis. Preclinical studies showed that calicheamicin conjugated to an anti-CD22 antibody resulted in potent cytotoxicity leading to regression of B-cell lymphoma and prevention of xenograft establishment at picomolar concentrations. 86 Phase 1 studies in non-hodgkin lymphoma (NHL) established a maximum tolerated dose of 1.8 mg/m 2 InO given intravenously every 3 to 4 weeks. 87 Subsequently, InO was studied in adults with relapsed/refractory ALL. 88 In this phase 2 trial, 90 patients were treated with either a single infusion every 3 to 4 weeks or weekly InO infusions. Cumulative doses were equivalent among the two treatment strategies. Overall response rate was 58%, with similar response between the two dosing schedules. Median survival was 6.2 months, with a non-significant benefit seen in weekly dosing. However, toxicity was greatly improved by weekly dosing, with a significant reduction in fever, hepatotoxicity and veno-occlusive disease. 89 A second phase 2 study of 35 patients with CD22+ ALL in second salvage or later showed similar complete response rate (66%) and median overall survival (7.4 months). 90 Based on these results, Kantarjian et al. 91 compared weekly dosing of InO to standard chemotherapy for relapsed/refractory ALL. The rate of complete remission was significantly higher in the InO group versus standard chemotherapy 80.7% (95% CI, 72.1–87.7) vs 29.4% (95% CI, 21.0–38.8), P <.001). 91 Progression-free survival (5 months vs 1.8 months) and overall survival (7.7 months vs 6.7 months) were also significantly prolonged with InO compared to standard chemotherapy. The most common adverse events of InO treatment included thrombocytopenia and neutropenia. Veno-occlusive liver disease occurred in 11% of patients treated with InO compared to 1% of those receiving standard chemotherapy. 91 Based on these results, InO was granted Breakthrough Therapy status by the FDA in 2015 and is a strong candidate for expedited approval for relapsed/refractory ALL.
InO has also been studied in frontline therapy in combination with low-intensity HCVAD for elderly patients >60 years. 92 These patients are prone to adverse events from chemotherapy and have poorer outcomes than their younger counterparts. In attempt to reduce toxicity, doxorubicin was eliminated from induction therapy, and cyclophosphamide, prednisone, methotrexate and cytarabine were given at reduced doses. InO was given during each of the first four courses. The regimen was well tolerated and produced superior 1-year OS as compared to historical data among similar patient population (78 vs 60%). 92
Moxetumomab pasudodotox
A third anti-CD22 monoclonal antibody, moxetumomab, is currently in development for treatment of pediatric and adult ALL. Moxetumomab is a reformulation of an older study drug, BL22, which was composed the variable region (F v ) of an anti-CD22 monoclonal antibody fused to Pseudomonas aeruginosa exotoxin A. 93 BL22 was shown to be highly active against Hairy Cell Leukemia in a phase 2 trial. 94 In a phase 1 trial of children with relapsed/refractory ALL, BL22 was well tolerated and exhibited anti-leukemic activity at all doses, but clinical benefits were transient and modest. 95 Therefore, BL22 was reformulated as moxetumomab to contain a F v fragment with greater affinity for CD22. In phase 1 trials, moxetumomab showed an overall activity rate of 70% in children with relapsed/refractory ALL. 96 Enrollment is ongoing for a phase 1/2 trial of moxetumomab pasudodotox for treatment of relapsed/refractory ALL in adults. 97
Combotox is a combination immunotoxin that contains a 1:1 mixture of anti-CD19 and anti-CD22 antibodies, both conjugated to the cytotoxin deglycosylated ricin-A chain. In pediatric patients with relapsed/refractory ALL, combotox led to a CR in 3 of 17 patients. In addition, six additional patients experienced a >95% reduction in peripheral blasts. 98 In adults with relapsed/refractory disease, combotox led to reduction of peripheral blasts in all patients; however, a durable response was not seen as blast count rebounded quickly after the final dose of combotox. 99 A phase I trial is recruiting patients to evaluate combotox in combination with cytarabine for adults with relapsed/refractory ALL (NCT01408160).
CD20 is a B-lineage specific antigen expressed at nearly all stages of differentiation on the surface of both normal and malignant B-cells. Signaling through CD20 plays a role in cell cycle progression, differentiation pathways and regulation of apoptosis. CD20 is expressed in 40–50% of precursor lymphoblasts, and confers a worse prognosis. 100 Moreover, CD20-positive leukemia responds poorly to dose intensification, highlighting the need for targeted therapy. The addition of rituximab, a first-generation anti-CD20 monoclonal antibody, has improved outcomes in these patients, but resistance to rituximab represents a limitation to its use.
Ofatumumab is a second-generation anti-CD20 antibody with a distinct binding site from that of rituximab. Ofatumumab was first showed to have benefit in fludarabine-refractory chronic lymphocytic leukemia, irrespective of prior rituximab exposure. 101 Ofatumumab induces higher levels of complement-dependent cytotoxicity (CDC) and has a slower dissociation rate than rituximab, and thus holds promise for CD20+ lymphoid malignancies both as frontline therapy and as salvage for rituximab-refractory disease. 102 , 103 In a phase 2 study, ofatumumab was used in combination with HCVAD in patients with either newly diagnosed pre-B CD20+ ALL or those who had completed a single course of chemotherapy. In all study patients, CD20+ expression was >1%. 104 Ofatumumab was administered at a dose of 2 grams on days 1 and 11 of the first 4 cycles of induction therapy. All but one patient (98%) achieved CR after cycle 1 and 93% of patients were negative for MRD at end induction. The 3-year CR and OS rates were 78% and 68%, respectively. 105 This is similar to benefits seen when rituximab was used as frontline therapy in CD20+ ALL. 106 Ofatumumab represents a potential alternative frontline therapy for CD20+ pre-B-ALL and an option for patients who failed a rituximab-based regimen.
Obinutuzumab
Another novel anti-CD20 monoclonal antibody, obinutuzumab, has shown promise in preclinical trials for CD20-positive B-ALL. Obinutuzumab was engineered to have enhanced affinity for the FcγRIIIa receptor on effector cells and thus enhanced antibody-dependent cell-mediated cytotoxicity (ADCC). 107 This compromises the ability of obinutuzumab to activate complement and predictably, CDC was inferior to that of rituximab and ofatumumab in vitro . However, obinutuzumab induced direct cell death and ADCC more rapidly and effectively. When all three mechanisms of cell death were evaluated together in B-cell depletion assays, obinutuzumab was more effective than either rituximab or ofatumumab achieving higher maximal depletion and lower EC 50 . Furthermore, obinutuzumab was superior in inhibiting growth in NHL xenograft models. 107 Awasthi et al. 108 compared obinutuzumab to ritixumab in pre-B-ALL cell lines and found obinutuzumab to be superior in inducing cell death and ADCC. In a pre-B-ALL xenograft model, overall survival was improved with obinutuzumab compared to ritixumab. 108 In clinical trials, obinutuzumab has been added to chlorambucil for treatment of adults with CLL and shown to prolong progression-free survival and improve complete response rate when compared to rituximab and chlorambucil. 109 Taken together, these results suggest a role for obinutuzumab in CD20+ pre-B-ALL.
REGN1979 is a biallelic monoclonal antibody targeting CD20 and CD3. The theory of REGN1979 is similar to that of blinatumumab, to engage T cells and B-cells thus resulting in activation of T-cell immune response against B-cells. REGN1979 prevented the establishment of lymphoma xenografts and led to complete tumor regression in murine models. 110 In addition, in a primate model, REGN1979 led to a complete and durable depletion of B-cells. When compared to treatment with rituximab, treatment with REGN1979 led to significantly more profound depletion of B-cells. 110 The safety of REGN1979 was established in a phase 1 trial of 25 patients with NHL and CLL. Dose-dependent antitumor activity was observed. The most significant adverse events include cytokine release syndrome (CRS) and hypotension. 111 A phase 2 trial of REGN1979 in relapsed/refractory ALL is currently open for recruitment (NCT02651662).
CD19 is the most widely expressed B-lineage specific antigen, expressed during all stages of differentiation, but lost on maturation to plasma cells. CD19 serves as a co-receptor for the B-cell surface immunoglobulin and its activation triggers a phosphorylation cascade involving src-family kinases and PI3K as well as the activation of c-myc, leading to proliferation and differentiation. 112 , 113 , 114 CD19 is expressed in nearly all B-cell leukemias, and is rapidly internalized upon binding of an antibody, making it an ideal candidate for immunoconjugate therapy. 115
Coltuximab ravtansine (SAR3419)
Coltuximab ravtansine is an anti-CD19 humanized monoclonal antibody conjugated to a semisynthetic maytansinoid compound, an anti-tubulin molecule similar to vincristine. Maytansinoids are more potent than vinca alkaloids, and thus have been of limited use in systemic therapy due to unacceptable toxicity. 116 However, this potency makes them attractive candidates for targeted delivery. In preclinical studies, SAR3419 monotherapy delayed progression in pre-B-ALL xenografts and provided objective response. When used in combination with a chemotherapy regimen that mimicked pediatric induction protocols, SAR3419 was effective at prolonging the duration of remission. 117 SAR3419 was then evaluated in a Phase 1 clinical trial with CD19+ B-cell lymphoma. Dose-limiting toxicities were reversible blurred vision and neuropathy. A maximum tolerated dose (MTD) of 160 mg/m 2 administered once every three weeks was established. Reduction of tumor size was seen in 74% of patients, including 47% of patients with rituximab-resistant disease. 118 An initial phase 2 clinical trial was terminated early due to low response rate of 25%. 119
Denintuzumab mafodotin (SGN-CD19A)
A second anti-CD19 conjugated monoclonal antibody, denintuzumab mafodotin, is currently in development. In this case, the antibody is linked to the microtubule-disrupting agent monomethyl auristatin F (MMAF). In a phase 1 study of patients with relapsed/refractory B-ALL or aggressive B-cell lymphomas, a complete response rate of 35% was observed. 120 Dosing interval of 3 weeks was shown to be superior to weekly dosing. An MTD was identified at 5 mg/kg q3wk. Interestingly, among Ph-positive B-ALL, the response rate was 63%, leading to recruitment of Ph-positive patients for an expansion cohort. These results warrant further evaluation of denintuzumab mafodotin for relapsed/refractory ALL.
ADCT-402 is the newest anti-CD19 monoclonal antibody to enter development. It is a humanized monoclonal antibody conjugated to a pyrrolobenzodiazepine (PBD). PBDs are a class of natural antibiotics derived from actinomycetes bacteria that inhibit cell division by binding in the minor groove of DNA and cross-linking strands of DNA. In vivo studies show superior antitumor activity of ADCT-402 against CD19-positive lymphoma than maytansinoid or auristatin based therapy. 121 A phase 1 trial of ADCT-402 for relapsed/refractory ALL is underway (NCT02669264).
CD25 is a cell surface antigen and component of the Interleukin-2 receptor (IL-2 R) heterotrimer. 122 Binding of IL-2 R by its ligand activates JAK/STAT, MAP kinase and phosphoinositide 3-kinase (PI3K) signaling pathways, leading to cell proliferation. IL-2 R is rapidly recycled upon binding of its ligand. 123 The IL-2 R signaling pathway is particularly activated in T-cell immune response, and has thus been an attractive target for post-transplant immunosuppression. In some studies, CD25 expression has been as high as 30% of pre-B-ALL lymphoblasts, including 100% expression among the Ph-positive subset. 124
ADCT-301 is a monoclonal antibody against CD25 conjugated to a PBD. In preclinical studies, ADCT-301 has been shown to be potently cytotoxic to CD25-positive anaplastic large cell lymphoma and Hodgkin lymphoma cell lines. In vivo , ADCT-301 exhibited antitumor activity in xenograft and disseminated mouse models. 122 A phase 1 trial is recruiting participants for ADCT-301 in relapsed/refractory AML and ALL (NCT02588092).
2-Proteasome inhibitor (Bortezomib)
Bortezomib, a proteasome inhibitor, was first approved for the treatment of multiple myeloma. Preclinical studies have suggested a synergistic role of bortezomib with dexamethasone and additive effects to standard chemotherapy agents in acute leukemias. 125 As a single agent, bortezomib did not produce durable responses in patients with relapsed/refractory ALL, despite demonstrable proteasomal inhibition. 126 However, in a phase 2 study, bortezomib in combination with vincristine, dexamethasone, pegylated asparaginase and doxorubicin produced a response rate of 80% in children with relapsed/refractory pre-B-ALL. 127 In a recent phase 2 COG trial, re-induction chemotherapy plus bortezomib resulted in a complete response in 68% of children with relapsed pre-B-ALL. 128 Due to it’s ability to inhibit the NF-κB and NOTCH1 signaling pathways, bortezomib is being studied as frontline therapy in T-cell ALL. Recruitment is ongoing for a phase 3 trial of standard chemotherapy with or without bortezomib in children and young adults (age 2–30) with newly diagnosed T-cell ALL or T-cell lymphoblastic lymphoma (NCT02112916). In adults, recruitment has begun for a phase 2 trial of bortezomib with combination chemotherapy in relapsed/refractory ALL (NCT01769209).
3-JAK inhibitor (Ruxolitinib)
The JAK/STAT signaling pathway has been identified as a significant mechanism by which leukemic cells bypass normal growth and proliferation restrictions. 13 In particular, Ph-like ALL appears to be dependent on JAK signaling. The most common rearrangements in Ph-like ALL involve the transmembrane receptor CRLF2, which signals through downstream JAK kinases. Many cytokine receptors, including IL-7 R, act through JAK kinases as well. In addition, JAK1 and JAK2 mutations are found in approximately half of CRLF2-rearranged Ph-like ALL. 12 , 13 , 14 Preclinical studies have suggested benefit of ruxolitinib for the treatment of Ph-like ALL and CRLF2-rearranged ALL. 129 , 130 In addition, ruxolitinib inhibited tumor growth in in vitro and in vivo models of T-ALL with a gain of function in IL-7 R-alpha subunit. 131 A phase 2 trial of ruxolitinib with standard multi-agent chemotherapy is currently open for recruitment of children, adolescents and adults with newly diagnosed high-risk B-ALL with CRLF2 rearrangements (NCT02723994).
4-Hypomethylating agent (Decitabine)
DNA methylation is an important epigenetic modification that regulates gene expression. It has long been reported that DNA methylation may play a role in the development of ALL and that methylation status may be used as part of risk stratification. 132 , 133 , 134 , 135 Decitabine is a cytosine analog that inhibits DNA methyltransferase by targeting it for degredation, thus causing hypomethylation of key regulatory domains on DNA. This leads to differentiation and suppression of tumor growth. 136 Decitabine is currently approved for the treatment of myelodysplastic syndrome (MDS). In a case report, a young girl with her third-relapse of ALL was treated with a decitabine and dexamethasone regimen based on MDS dosing. She was able to undergo Allo-SCT after CR was achieved with re-induction therapy and remained in CR 8 months after transplant. 137 In a MD Anderson phase I trial of decitabine for relapsed/refractory ALL, decitabine was shown to have efficacy when used in combination with Hyper-CVAD for re-induction therapy. 138 In addition, decitabine monotherapy is well tolerated and thus offers a potential treatment option for relapsed disease in patients that cannot tolerate multi-agent chemotherapy. In a phase 2 study, decitabine and vorinostat (a histone deacetylase inhibitor) were given prior to vincristine, prednisone, PEG-L-asparaginase and doxorubicin for relapsed/refractory ALL. 139 Results were promising with a CR rate of 50% (95% CI, 15.7–84.3%) and the OR rate 75% (95% CI, 34.9–96.8%). Decitabine has also been studied in preclinical trials of early T-cell precursor ALL (ETP-ALL), where it has been shown to be synergistic to conventional chemotherapy. 140 Decitabine is currently being studied in the post-Allo-SCT setting (NCT02264873) and in combination with clofarabine, idarubicin and cytarabine for relapsed/refractory AML and ALL (NCT01794702).
5-PI3K/mTOR Inhibitors
The phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) and mammalian target of rapamycin (mTOR) pathways are shown to be constitutively activated in 50–75% of T-ALL. 141 Preclinical studies suggest that inhibition of the PI3K/AKT/mTOR pathways may be an effective treatment for T-ALL. 142 , 143 , 144 , 145 A dual PI3K/mTOR inhibitor, NVP-BEZ235, potently inhibited the proliferation ALL cells in vitro , causing G 0 /G 1 arrest. Moreover, inhibition of proliferation was synergistic when NVP-BEZ235 was combined with cytotoxic agents. 144 On the basis of this promising preclinical data, several clinical trials are underway to evaluate the use of mTOR and PI3K inhibitors in combination with multi-agent chemotherapy in the frontline and relapsed/refractory setting (NCT01756118, NCT02484430, NCT01523977, NCT01403415, NCT01614197 and NCT01184885).
6-Chimeric antigen receptor (CAR) T cells
Chimeric antigen receptor-modified (CAR) T cells are genetically engineered T cells that express the antigen-binding domain of an immunoglobulin linked via transmembrane domains to the intracellular T-cell receptor signaling moieties. 146 This allows the T cells to recognize unprocessed antigens and to be activated in a major histocompatibility complex (MHC)-independent manner. First generation CAR-Ts contain intracellular signaling moieties derived only from the T-cell receptor/CD3 complex. In contrast, second- and third-generation CAR-Ts include co-stimulatory signals in the CAR gene constructs. More recently, fourth-generation CAR-Ts have been engineered to include a cytokine-expressing cassette.
The process of CAR T-cell therapy involves collecting T cells, introducing the CAR construct, and then an autologous transplant of the modified T cells back into the patient. Options for gene delivery methods include viral vectors and RNA-based methods. 147 Using a viral vector has the benefit of inducing permanent gene expression and thus offering antitumor activity for as long as the transduced T cells persist. Theoretic risks of this method include malignant transformation of the engineered T cells if the CAR construct is inserted in such a way that it deregulates the expression of an oncogene. 148 Another method of gene delivery involves direct transfer of an mRNA construct through electroporation. 149 As no DNA is inserted into the genome of the T-cell, this eliminates the risk of malignant transformation. Given the high replicative potential of these T cells, this methods also offers the advantage of a profound antitumor response. 150 However, the effects of direct mRNA insertion are transient and antitumor activity rarely persists beyond 7 days. Preclinical studies have suggested a role for RNA-based methods with multiple infusions; however, all current clinical trials utilize a viral vector to deliver the CAR construct. 150
As mentioned above, CD19 is an ideal target for immunotherapy against B-cell ALL due to its near universal expression on B-lymphoblasts. In a pilot study at the Children’s Hospital of Philadelphia, Grupp et al. 151 treated 53 children with relapsed/refractory ALL with lymphocyte depleting chemotherapy followed by CD19-directed CAR-Ts. A CR was observed in 50 patients (94%), with a 12-month EFS rate of 45% (95% CI, 31–66%) and OS rate of 78% (95% CI, 67–91%). The CAR-Ts were persistent at 6 months in 68% of the patients. Nearly all of the patients developed cytokine release syndrome (CRS). The 15 patients in which CRS was severe were effectively treated with the anti-IL-6-receptor antibody, tocilizumab. 152 Important causes of treatment failure included the loss of circulating CAR-Ts and the expansion of a CD19-negative clone. CAR-Ts have also shown activity in adults with relapsed/refractory B-ALL. Davila et al. 153 treated 16 adults at Memorial Sloan Kettering Cancer Center (MSKCC) with conditioning chemotherapy followed by CD19-directed CAR T-cell infusion. CR was observed in 88% of patients, with a 1–3 month persistence of CAR-Ts. Lee et al. 154 reported a 66.7%% CR rate in a National Cancer Institute (NCI) intent-to-treat analysis of 20 children and young adults with ALL, with a median CAR-T persistence of 68 days. These data suggest a role for CAR-Ts in the treatment of relapsed/refractory ALL as a bridge to Allo-SCT or to produce durable remission. Limitations include the expansion of CD19-negative clones, the lack of long-term persistence of CAR-Ts after a single infusion, and the risk of CRS. Studies are ongoing to identify factors associated with the development of severe CRS and predict patients that would benefit from pretreatment. 155 , 156
Recently, the application of CAR-T cells has been expanded to CD22-positive B-ALL. Early preclinical studies have showed antitumor activity of CD22-directed CAR-Ts in in vitro and in vivo models that approximates that of CD19-directed CAR-Ts. 157 Based on these findings, phase 1 trials using CD22-directed CAR-Ts are in the recruiting stages (NCT02650414). Preliminary results of nine patients have demonstrated that therapy is well tolerated and produced a sustained remission at 3 months in all three patients treated with a dose level of 1 × 10 6 transduced T cells/kg. 158
Hematopoietic stem cell transplantation
After achieving complete response, treatment options include consolidation and maintenance chemotherapy or Allo-SCT for eligible patients. For high-risk patients and patients with relapsed/refractory disease, Allo-SCT has long been considered the standard of care and best chance for a durable response. While criteria differ between studies, in general high-risk disease is defined as Ph-positive ALL, elevated WBC count, CNS disease, high-risk gene rearrangements, or hypodiploidy. The LALA-94 and City of Hope and Stanford University series have shown a benefit of Allo-SCT over standard chemotherapy in these high-risk patients. 49 , 159 , 160 It is therefore recommended that all high-risk young adults with an available donor undergo Allo-SCT during their first CR (CR1). Recent studies have suggested that patients with ETP-ALL and Ph-like ALL be treated as high-risk and be offered Allo-SCT during CR1 as well. 161 , 162 The role of Allo-SCT in standard-risk adults is less clearly defined. In general, MRD has emerged as a prognostic marker that can restratify patients to high-risk, making them candidates for Allo-SCT. Studies 32 found that MRD-positivity is an independent risk factor for decreased relapse-free and overall survival. Subsequently, other studies 163 evaluated the risk factors in patients treated with Allo-SCT versus standard chemotherapy after CR1. In patients with positive MRD, Allo-SCT was associated with improved relapse-free survival. However, in patients with a complete MRD response, there was no survival benefit to Allo-SCT over standard chemotherapy. 163
Allo-SCT also should be considered in all patients that relapse, optimally after achieving a second CR (CR2). The LALA-94 trial showed a 5-year OS of 33% in patients who were able to undergo Allo-SCT during CR2 compared to 8% in patients who underwent Allo-SCT during active relapse. 164 Patients who are unable to achieve CR2 by conventional methods should be considered for clinical trials with novel agents as a bridge to Allo-SCT. In the MRC/ECOG 2993 study, 5-year survival was highest in the group receiving a sibling donor Allo-SCT compared to unmatched donor or chemotherapy alone (23%, 16% and 4%, respectively). 165
Acute lymphoblastic leukemia has been touted as a major success story in pediatric oncology through the implementation of dose-intensification chemotherapy and Allo-SCT. However, due to high-risk disease characteristics and significant toxicity associated with chemotherapy in adults, outcomes are far less encouraging. There remains much uncertainty about how best to treat adults with ALL, as some studies have shown benefit of pediatric-inspired regimens. However, not all adults are able to tolerate such dose intensification and the exact subset of patients who are likely to benefit has not clearly been defined. Furthermore, elderly patients are particularly susceptible to the dose-limiting toxicities of these agents and are often excluded from Allo-SCT on the basis of performance status and medical comorbidities. Novel targeted therapies offer the promise of effective anti-leukemic activity with reduced toxicity from off-target effects. Given the diverse molecular and genetic alterations occurring in ALL, it is unlikely that a single agent will be effective for all patients with ALL. However, with the ability to characterize the immunophenotype and genotype of each patient’s leukemia, targeted therapy can be expected to lead to improvements in remission and survival as part of individualized treatment strategies. The successes from tyrosine kinase inhibition in CML have been translated to Ph-positive ALL, and second and third generation TKIs are being studied for use in high-risk Ph-like disease. Other signaling pathways, such as PI3K/AKT/mTOR pathway, are also promising targets for small molecule inhibition. In addition to targeting intracellular pathways, monoclonal antibodies recognize cell surface antigens. Immunoconjugates, such as inotuzumab ozogamicin, bind to leukemic cells, are internalized and release a cytotoxin that kills the leukemic cell; whereas dual-specific antibodies, such as blinatumumab, cause the direct activation of T cells against blasts. CAR-Ts involve a similar mechanism, in which a patient’s own T cells are genetically programmed to recognize leukemic cells, inducing an anti-leukemic immune response. Finally, existing agents, such as bortezomib, decitabine and ruxolitinib that are well tolerated in the treatment of various malignancies are now being studied for application in ALL. As the role of these novel agents is further defined and integrated into new treatment strategies, adult ALL may follow pediatric ALL as a major success story in the near future.
National Cancer Institute. SEER cancer statistics review, 1975-2013:Leukemia, annual incidence rates (acute lymphocytic leukemia).
Paul S, Kantarjian H, Jabbour EJ . Adult Acute Lymphoblastic Leukemia. Mayo Clin Proc 2016; 91 : 1645–1666.
PubMed Google Scholar
Jabbour E, O'Brien S, Konopleva M, Kantarjian H . New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer 2015; 121 : 2517–2528.
Shah A, John BM, Sondhi V . Acute lymphoblastic leukemia with treatment—naive Fanconi anemia. Indian Pediatr 2013; 50 : 508–510.
German J . Bloom's syndrome. XX. The first 100 cancers. Cancer Genet Cytogenet 1997; 93 : 100–106.
CAS PubMed Google Scholar
Bielorai B, Fisher T, Waldman D, Lerenthal Y, Nissenkorn A, Tohami T et al . Acute lymphoblastic leukemia in early childhood as the presenting sign of ataxia-telangiectasia variant. Pediatr Hematol Oncol 2013; 30 : 574–582.
Chessells J, Harrison G, Richards S, Bailey C, Hill F, Gibson B et al . Down's syndrome and acute lymphoblastic leukaemia: clinical features and response to treatment. Arch Dis Child 2001; 85 : 321–325.
CAS PubMed PubMed Central Google Scholar
Spector LG, R J, Robison LL, Bhatia S . Epidemiology and Etiology Childhood Leukemias , 2nd edition. Cambridge University Press, pp 48–66.
Sehgal S, Mujtaba S, Gupta D, Aggarwal R, Marwaha RK . High incidence of Epstein Barr virus infection in childhood acute lymphocytic leukemia: a preliminary study. Indian J Pathol Microbiol 2010; 53 : 63–67.
Geriniere L, Bastion Y, Dumontet C, Salles G, Espinouse D, Coiffier B . Heterogeneity of acute lymphoblastic leukemia in HIV-seropositive patients. Ann Oncol 1994; 5 : 437–440.
Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin ML, Liu W, Zhang J et al . Rearrangement of CRLF2 in B-progenitor and down syndrome associated acute lymphoblastic leukemia. Nat Genet 2009; 41 : 1243–1246.
Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD et al . Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 2007; 446 : 758–764.
Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X et al . Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell 2012; 22 : 153–166.
Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D et al . Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 2014; 371 : 1005–1015.
PubMed PubMed Central Google Scholar
Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L et al . The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 2013; 45 : 242–252.
Alvarnas JC, Brown PA, Aoun P, Ballen KK, Barta SK, Borate U et al . Acute lymphoid leukemia (version 2.2015). Natl Comprehens Cancer Netw 2015; 13 : 1240–1279.
CAS Google Scholar
Jabbour EJ, Faderl S, Kantarjian HM . Adult acute lymphoblastic leukemia. Mayo Clin Proc 2005; 80 : 1517–1527.
Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR et al . Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol 1976; 33 : 451–458.
Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J et al . World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol 1999; 17 : 3835–3849.
Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al . The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114 : 937–951.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al . The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127 : 2391–2405.
Rowe JM . Prognostic factors in adult acute lymphoblastic leukaemia. Br J Haematol 2010; 150 : 389–405.
Rowe JM, Buck G, Burnett AK, Chopra R, Wiernik PH, Richards SM et al . Induction therapy for adults with acute lymphoblastic leukemia: results of more than 1500 patients from the international ALL trial: MRC UKALL XII/ECOG E2993. Blood 2005; 106 : 3760–3767.
Faderl, S HM Kantarjian, et al , T.U.o.T.M.D.A.C.C. Department of Leukemia, Houston, Texas, T.U.o.T.M.D.A.C.C. Department of Leukemia, P.O. Box 428, 1515 Holcombe Blvd., Houston, TX 77030, S. Jeha, T.U.o.T.M.D.A.C.C. Department of Leukemia, Houston, Texas, The biology and therapy of adult acute lymphoblastic leukemia. Cancer, 2017; 98 : 1337–1354.
Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M et al . Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood 2007; 109 : 3189–3197.
Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR . Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of Southwest Oncology Group 9400 study. Blood 2008; 111 : 2563–2572.
Hunger SP, Mullighan CG . Redefining ALL classification: toward detecting high-risk ALL and implementing precision medicine. Blood 2015; 125 : 3977–3987.
Dongen JJMv, v.d. Velden VHJ, Brüggemann M, Orfao A . Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood 2015; 125 : 3996–4009.
Bruggemann M, Raff T, Flohr T, Gokbuget N, Nakao M, Droese J et al . Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood 2006; 107 : 1116–1123.
Jacquy C, Delepaut B, Van Daele S, Vaerman JL, Zenebergh A, Brichard B et al . A prospective study of minimal residual disease in childhood B-lineage acute lymphoblastic leukaemia: MRD level at the end of induction is a strong predictive factor of relapse. Br J Haematol 1997; 98 : 140–146.
Sutton R, Venn NC, Tolisano J, Bahar AY, Giles JE, Ashton LJ et al . Clinical significance of minimal residual disease at day 15 and at the end of therapy in childhood acute lymphoblastic leukaemia. Br J Haematol 2009; 146 : 292–299.
Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B et al . Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood 2009; 113 : 4153–4162.
Campana D . Minimal residual disease in acute lymphoblastic leukemia. Semin Hematol 2009; 46 : 100–106.
Raff T, Gökbuget N, Lüschen S, Reutzel R, Ritgen M, Irmer S et al . Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood 2007; 109 : 910–915.
Ribera JM, Oriol A, Morgades M, Montesinos P, Sarra J, Gonzalez-Campos J et al . Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: final results of the PETHEMA ALL-AR-03 trial. J Clin Oncol 2014; 32 : 1595–1604.
Huguet F, Leguay T, Raffoux E, Thomas X, Beldjord K, Delabesse E et al . Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol 2009; 27 : 911–918.
Stock W, La M, Sanford B, Bloomfield CD, Vardiman JW, Gaynon P et al . What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood 2008; 112 : 1646–1654.
Scavino HF, George JN, Sears DA . Remission induction in adult acute lymphocytic leukemia. Use of vincristine and prednisone alone. Cancer 1976; 38 : 672–677.
Gottlieb A, Weinberg V, Ellison R, Henderson E, Terebelo H, Rafla S et al . Efficacy of daunorubicin in the therapy of adult acute lymphocytic leukemia: a prospective randomized trial by cancer and leukemia group B. Blood 1984; 64 : 267–274.
Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P et al . A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood 1995; 85 : 2025–2037.
Nagura E, Kimura K, Yamada K, Ota K, Maekawa T, Takaku F et al . Nation-wide randomized comparative study of doxorubicin, vincristine and prednisolone combination therapy with and without L-asparaginase for adult acute lymphoblastic leukemia. Cancer Chemother Pharmacol 1994; 33 : 359–365.
Short NJ, Jabbour E, Sasaki K, Patel K, O'Brien SM, Cortes JE et al . Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2016; 128 : 504–507.
Patel B, Kirkwood A, Dey A, Rowntree C, McMillan A, Marks D et al . Feasibility Of pegylated-asparaginase (PEG-ASP) during induction in adults with acute lymphoblastic leukaemia (ALL): results from the UK Phase 3 Multicentre Trial UKALL 14. 2013.
Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M et al . Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000; 18 : 547–561.
Hurwitz CA, Silverman LB, Schorin MA, Clavell LA, Dalton VK, Glick KM et al . Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia. Cancer 2000; 88 : 1964–1969.
Jones B, Freeman AI, Shuster JJ, Jacquillat C, Weil M, Pochedly C et al . Lower incidence of meningeal leukemia when prednisone is replaced by dexamethasone in the treatment of acute lymphocytic leukemia. Med Pediatr Oncol 1991; 19 : 269–275.
Narayanan S, Shami PJ . Treatment of acute lymphoblastic leukemia in adults. Crit Rev Oncol Hematol 2012; 81 : 94–102.
Faderl S, Kantarjian HM, Thomas DA, Cortes J, Giles F, Pierce S et al . Outcome of Philadelphia chromosome-positive adult acute lymphoblastic leukemia. Leuk Lymphoma 2000; 36 : 263–273.
Dombret H, Gabert J, Boiron JM, Rigal-Huguet F, Blaise D, Thomas X et al . Outcome of treatment in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia—results of the prospective multicenter LALA-94 trial. Blood 2002; 100 : 2357–2366.
Laport GG, Alvarnas JC, Palmer JM, Snyder DS, Slovak ML, Cherry AM et al . Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20-year experience with the fractionated total body irradiation-etoposide regimen. Blood 2008; 112 : 903–909.
Fielding AK, Rowe JM, Richards SM, Buck G, Moorman AV, Durrant IJ et al . Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood 2009; 113 : 4489–4496.
Thomas DA, Faderl S, Ravandi Kashani F, Wierda WG, Andreeff M, Garris RS et al . Long-term outcome after hyper-CVAD and imatinib (IM) for de novo or minimally treated Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph-ALL)[abstract]. 2010; 28(15 s):abstr 8506:[Available from http://meetinglibrary.asco.org/content/52502-74 .
Thomas DA, Faderl S, Cortes J, O'Brien S, Giles FJ, Kornblau SM et al . Treatment of Philadelphia chromosome–positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood 2004; 103 : 4396–4407.
Jones D, Thomas D, Yin CC, O'Brien S, Cortes JE, Jabbour E et al . Kinase domain point mutations in Philadelphia chromosome-positive acute lymphoblastic leukemia emerge after therapy with BCR-ABL kinase inhibitors. Cancer 2008; 113 : 985–994.
Porkka K, Koskenvesa P, Lundan T, Rimpilainen J, Mustjoki S, Smykla R et al . Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood 2008; 112 : 1005–1012.
Ravandi F, O'Brien SM, Cortes JE, Thomas DM, Garris R, Faderl S et al . Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 2015; 121 : 4158–4164.
Ravandi F, Othus M, O'Brien S, Forman SJ, Ha CS, Wong JYC et al . Multi-Center US Intergroup Study of Intensive Chemotherapy Plus Dasatinib Followed By Allogeneic Stem Cell Transplant in Patients with Philadelphia Chromosome Positive Acute Lymphoblastic Leukemia Younger Than 60 . 2015.
Ottmann O, Dombret H, Martinelli G, Simonsson B, Guilhot F, Larson RA et al . Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood 2007; 110 : 2309–2315.
Foà R, Vitale A, Vignetti M, Meloni G, Guarini A, Propris MSD et al . Dasatinib as first-line treatment for adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia. Blood 2011; 118 : 6521–6528.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C et al . A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013; 369 : 1783–1796.
Sasaki K, Jabbour EJ, Ravandi F, Short NJ, Thomas DA, Garcia-Manero G et al . Hyper-CVAD plus ponatinib versus hyper-CVAD plus dasatinib as frontline therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: A propensity score analysis. Cancer 2016; 122 : 3650–3656.
Sasaki Koji, Ravandi Farhad, Thomas Deborah A, Cortes Jorge E, Pemmaraju Naveen, Kadia Tapan M et al . Updated results from phase II study of combination of hyper-CVAD (HCVAD) with ponatinib in frontline therapy of patients (pts) with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL)[abstract]. 2016; 34 s; abstr 7036: Available from http://meetinglibrary.asco.org/content/167324-176 .
Nachman J, Siebel N, Sather H, Steinherz P, DeLaat C, Fryer D et al . Outcome for Adolescent and Young Adults 16–21 years of age (AYA) with Acute Lymphoblastic Leukemia (ALL) Treated on the Children’ s Cancer Group (CCG) 1961 Study . 2004.
Stock W, Luger SM, Advani AS, Geyer S, Harvey RC, Mullighan CG et al . Favorable Outcomes for Older Adolescents and Young Adults (AYA) with Acute Lymphoblastic Leukemia (ALL): Early Results of US Intergroup Trial C10403 , 2014.
Rytting ME, Jabbour EJ, Jorgensen JL, Ravandi F, Franklin AR, Kadia TM et al . Final results of a single institution experience with a pediatric-based regimen, the augmented Berlin-Frankfurt-Munster, in adolescents and young adults with acute lymphoblastic leukemia, and comparison to the hyper-CVAD regimen. Am J Hematol 2016; 91 : 819–823.
Faderl S, Thomas DA, O'Brien S, Ravandi F, Garcia-Manero G, Borthakur G et al . Augmented hyper-CVAD based on dose-intensified vincristine, dexamethasone, and asparaginase in adult acute lymphoblastic leukemia salvage therapy. Clin Lymphoma Myeloma Leuk 2011; 11 : 54–59.
O’Brien S, Thomas D, Ravandi F, Faderl S, Cortes J, Borthakur G et al . Outcome of Adults With Acute Lymphocytic Leukemia After Second Salvage Therapy. Cancer 2008; 113 : 3186–3191.
Deitcher OR, O'Brien S, Deitcher SR, Thomas DA, Kantarjian HM . Single-Agent Vincristine Sulfate Liposomes Injection (Marqibo) Compared to Historical Single-Agent Therapy for Adults with Advanced, Relapsed and/or Refractory Philadelphia Chromosome Negative Acute Lymphoblastic Leukemia , 2011.
O'Brien S, Schiller G, Lister J, Damon L, Goldberg S, Aulitzky W et al . High-Dose Vincristine Sulfate Liposome Injection for Advanced, Relapsed, and Refractory Adult Philadelphia Chromosome–Negative Acute Lymphoblastic Leukemia. in J Clin Oncol 2013; 31 : 676–683.
Nagorsen D, Kufer P, Baeuerle PA, Bargou R . Blinatumomab: a historical perspective. Pharmacol Ther 2012; 136 : 334–342.
Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S et al . Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 2011; 29 : 2493–2498.
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C et al . Long-Term Outcomes after Blinatumomab Treatment: Follow-up of a Phase 2 Study in Patients (Pts) with Minimal Residual Disease (MRD) Positive B-Cell Precursor Acute Lymphoblastic Leukemia (ALL). Blood 2015; 126 : 680–680.
Google Scholar
Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC et al . Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol 2015; 16 : 57–66.
Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J-M et al . Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. NEJM 2017; 376 : 836–847.
Martinelli G, Dombret H, Chevallier P, Ottmann OG, Goekbuget N, Topp MS et al . Complete Molecular and Hematologic Response in Adult Patients with Relapsed/Refractory (R/R) Philadelphia Chromosome-Positive B-Precursor Acute Lymphoblastic Leukemia (ALL) Following Treatment with Blinatumomab: Results from a Phase 2 Single-Arm, Multicenter Study (ALCANTARA)[Abstract]. Blood 2015; 126 : 679.
D-ALBA Frontline Sequential Dasatinib and Blinatumomab in Adult Philadelphia Positive Acute Lymphoblastic Leukemia - Full Text View - ClinicalTrials.gov. 2017; Available at: https://clinicaltrials.gov/ct2/show/NCT02744768 .
DeAngelo DJ, Yu D, Johnson JL, Coutre SE, Stone RM, Stopeck AT et al . Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood 2007; 109 : 5136–5142.
Shah NN, Stevenson MS, Yuan CM, Richards K, Delbrook C, Kreitman RJ et al . Characterization of CD22 expression in acute lymphoblastic leukemia. Pediatr Blood Cancer 2015; 62 : 964–969.
Piccaluga PP, Arpinati M, Candoni A, Laterza C, Paolini S, Gazzola A et al . Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk Lymphoma 2011; 52 : 325–327.
Chevallier P, Robillard N, Houille G, Ayari S, Guillaume T, Delaunay J et al . Simultaneous study of five candidate target antigens (CD20, CD22, CD33, CD52, HER2) for antibody-based immunotherapy in B-ALL: a monocentric study of 44 cases in Leukemia 2009; England pp 806–807.
Carnahan J, Wang P, Kendall R, Chen C, Hu S, Boone T et al . Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clin Cancer Res 2003; 9 (10 Pt 2): 3982s–3990ss.
Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA et al . Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group Pilot Study. J Clin Oncol 2008; 26 : 3756–3762.
Advani AS, McDonough S, Coutre S, Wood B, Radich J, Mims M et al . SWOG S0910: a phase 2 trial of clofarabine/cytarabine/epratuzumab for relapsed/refractory acute lymphocytic leukaemia. Br J Haematol 2014; 165 : 504–509.
Sharkey RM, Govindan SV, Cardillo TM, Goldenberg DM . Epratuzumab–SN-38: A New Antibody–Drug Conjugate for the Therapy of Hematologic Malignancies. Mol Cancer Ther 2012; 11 : 224–234.
Hinman LM, Hamann PR, Wallace R, Menendez AT, Durr FE, Upeslacis J . Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res 1993; 53 : 3336–3342.
DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L et al . Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 2004; 103 : 1807–1814.
Advani A, Coiffier B, Czuczman MS, Dreyling M, Foran J, Gine E et al . Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study. J Clin Oncol 2010; 28 : 2085–2093.
Kantarjian H, Thomas D, Jorgensen J, Jabbour E, Kebriaei P, Rytting M et al . Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol 2012; 13 : 403–411.
Kantarjian H, Thomas D, Jorgensen J, Kebriaei P, Jabbour E, Rytting M et al . Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer 2013; 119 : 2728–2736.
Advani AS, Stein AS, Kantarjian HM, Shustov AR, DeAngelo DJ, Ananthakrishnan R et al . A Phase II Study of Weekly Inotuzumab Ozogamicin (InO) in Adult Patients with CD22-Positive Acute Lymphoblastic Leukemia (ALL) in Second or Later Salvage. Blood 2014; 124 : 2255.
Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W et al . Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med 2016; 375 : 740–753.
Jabbour E, O’Brien S, Thomas D, Sasaki K, Garcia-Manero G, Ravandi F et al . Inotuzumab Ozogamicin (IO) in Combination with Low-Intensity Chemotherapy (mini-hyper-CVD) as Frontline Therapy for Older Patients (pts) and as Salvage Therapy for Adult with Relapsed/Refractory (R/R) Acute Lymphoblastic Leukemia (ALL). Clin Lymphoma Myeloma Leuk 2015, 15.
Kreitman RJ, Pastan I . Antibody Fusion Proteins: Anti-CD22 Recombinant Immunotoxin Moxetumomab Pasudotox. Clin Cancer Res 2011; 17 : 6398–6405.
Kreitman RJ, Stetler-Stevenson M, Margulies I, Noel P, FitzGerald DJP, Wilson WH et al . Phase II Trial of Recombinant Immunotoxin RFB4(dsFv)-PE38 (BL22) in Patients With Hairy Cell Leukemia. J Clin Oncol 2009; 27 : 2983–2990.
Wayne AS, Kreitman RJ, Findley HW, Lew G, Delbrook C, Steinberg SM et al . Anti-CD22 Immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-Positive Hematologic Malignancies of Childhood: Preclinical Studies and Phase I Clinical Trial. Clin Cancer Res 2010; 16 : 1894–1903.
Wayne AS, Bhojwani D, Silverman LB, Richards K, Stetler-Stevenson M, Shah NN et al . A Novel Anti-CD22 Immunotoxin, Moxetumomab Pasudotox: Phase I Study in Pediatric Acute Lymphoblastic Leukemia (ALL). Blood 2011; 118 : 248.
Ravandi F, Kantarjian HM, Goswami T, Wang F, Ibrahim R . Design Of a Phase 1/2 Study Of Moxetumomab Pasudotox In Adult Patients With Relapsed and/Or Refractory Acute Lymphoblastic Leukemia (ALL). Blood 2013; 122 : 5021.
Herrera L, Bostrom B, Gore L, Sandler E, Lew G, Schlegel PG et al . A phase 1 study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2009; 31 : 936–941.
Schindler J, Gajavelli S, Ravandi F, Shen Y, Parekh S, Braunchweig I et al . A Phase I Study of a Combination of anti-CD19 and anti-CD22 Immunotoxins (Combotox) in Adult Patients with Refractory B-Lineage Acute Lymphoblastic Leukaemia. Br J Haematol 2011; 154 : 471–476.
Thomas DA, O'Brien S, Jorgensen JL, Cortes J, Faderl S, Garcia-Manero G et al . Prognostic significance of CD20 expression in adults with de novo precursor B-lineage acute lymphoblastic leukemia. Blood 13 : 6330–6337.
Wierda WG, Padmanabhan S, Chan GW, Gupta IV, Lisby S, Österborg A et al . Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood 2011; 118 : 5126–5129.
Teeling JL, French RR, Cragg MS, v.d. Brakel J, Pluyter M, Huang H et al . Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 2004; 104 : 1793–1800.
Pawluczkowycz AW, Beurskens FJ, Beum PV, Lindorfer MA, v.d. Winkel JGJ, Parren PWHI et al . Binding of Submaximal C1q Promotes Complement-Dependent Cytotoxicity (CDC) of B Cells Opsonized with Anti-CD20 mAbs Ofatumumab (OFA) or Rituximab (RTX): Considerably Higher Levels of CDC Are Induced by OFA than by RTX. J Immunol 2009; 183 : 749–758.
Jabbour E, Hagop K, Thomas D, Garcia-Manero G, Hoehn D, Garris R et al . Phase II Study Of The Hyper-CVAD Regimen In Combination With Ofatumumab As Frontline Therapy For Adults With CD-20 Positive Acute Lymphoblastic Leukemia (ALL). Blood 2013; 122 : 2664.
Sasaki K, Kantarjian HM, Ravandi F, Daver N, Kadia TM, Khouri RB et al . Frontline Ofatumumab in Combination with Hyper-CVAD for Adult Patients with CD-20 Positive Acute Lymphoblastic Leukemia (ALL): Interim Result of a Phase II Clinical Trial. Poster presented at the American Society of Hematology 58th Annual Meeting and Exposition . 2 December 2016, San Diego, CA, USA, 2016.
Thomas DA, O'Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Wierda W et al . Chemoimmunotherapy With a Modified Hyper-CVAD and Rituximab Regimen Improves Outcome in De Novo Philadelphia Chromosome–Negative Precursor B-Lineage Acute Lymphoblastic Leukemia. J Clin Oncol 2010; 28 : 3880–3889.
Herter S, Herting F, Mundigl O, Waldhauer I, Weinzierl T, Fauti T et al . Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 2013; 12 : 2031–2042.
Awasthi A, Ayello J, Van de Ven C, Elmacken M, Sabulski A, Barth MJ et al . Obinutuzumab (GA101) compared to rituximab significantly enhances cell death and antibody-dependent cytotoxicity and improves overall survival against CD20(+) rituximab-sensitive/-resistant Burkitt lymphoma (BL) and precursor B-acute lymphoblastic leukaemia (pre-B-ALL): potential targeted therapy in patients with poor risk CD20(+) BL and pre-B-ALL. Br J Haematol 2015; 171 : 763–775.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM et al . Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N Engl J Med 2014; 370 : 1101–1110.
Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD et al . A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys. Scientific Reports, Published online 2015; 5 : 17943, 2015.
Bannerji R, Brown JR, Advani RH, Arnason J, O'Brien SM, Allan JN et al . Phase 1 Study of REGN1979, an Anti-CD20 x Anti-CD3 Bispecific Monoclonal Antibody, in Patients with CD20+ B-Cell Malignancies Previously Treated with CD20-Directed Antibody Therapy. Blood 2016; 128 : 621.
Fujimoto M, Poe JC, Jansen PJ, Sato S, Tedder TF . CD19 amplifies B lymphocyte signal transduction by regulating Src-family protein tyrosine kinase activation. J Immunol 1999; 162 : 7088–7094.
Otero DC, Omori SA, Rickert RC . CD19-dependent Activation of Akt Kinase in B-lymphocytes. J Biol Chem 2001; 276 : 1474–1478.
Chung EY, Psathas JN, Yu D, Li Y, Weiss MJ, Thomas-Tikhonenko A . CD19 is a major B cell receptor-independent activator of MYC-driven B-lymphomagenesis. J Clin Invest 2012; 122 : 2257–2266.
Ning BT, Tang YM, Chen YH, Shen HQ, Qian BQ . Comparison between CD19 and CD20 expression patterns on acute leukemic cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2005; 13 : 943–947.
Widdison Wayne C, Wilhelm Sharon D, Cavanagh Emily E, Whiteman Kathleen R, Leece Barbara A, Kovtun Yelena et al . Semisynthetic Maytansine Analogues for the Targeted Treatment of Cancer. J Med Chem 2006; 49 : 4392–4408.
Carol H, Szymanska B, Evans K, Boehm I, Houghton PJ, Smith MA et al . The Anti-CD19 Antibody–Drug Conjugate SAR3419 Prevents Hematolymphoid Relapse Postinduction Therapy in Preclinical Models of Pediatric Acute Lymphoblastic Leukemia. Clin Cancer Res 2013; 19 : 1795–1805.
Younes A, Kim S, Romaguera J, Copeland A, Farial Sdc, Kwak LW et al . Phase I Multidose-Escalation Study of the Anti-CD19 Maytansinoid Immunoconjugate SAR3419 Administered by Intravenous Infusion Every 3 Weeks to Patients With Relapsed/Refractory B-Cell Lymphoma. Blood 2009; 114 : 585.
Kantarjian HM, Lioure B, Kim SK, Atallah E, Leguay T, Kelly K et al . A Phase II Study of Coltuximab Ravtansine (SAR3419) Monotherapy in Patients With Relapsed or Refractory Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk 2016; 16 : 139–145.
Fathi AT, Borate U, DeAngelo DJ, O'Brien MM, Trippett T, Shah BD et al . A Phase 1 Study of Denintuzumab Mafodotin (SGN-CD19A) in Adults with Relapsed or Refractory B-Lineage Acute Leukemia (B-ALL) and Highly Aggressive Lymphoma. Blood 2015; 126 : 1328.
Zammarchi F, Williams DG, Adams L, Havenith K, Chivers S, D'Hooge F et al . Pre-Clinical Development of Adct-402, a Novel Pyrrolobenzodiazepine (PBD)-Based Antibody Drug Conjugate (ADC) Targeting CD19-Expressing B-Cell Malignancies. Blood 2015; 126 : 1564.
Flynn MJ, Pv Berkel, Zammarchi F, Levy J-N, Tiberghien A, Masterson LA et al . Pre-Clinical Activity of Adct-301, a Novel Pyrrolobenzodiazepine (PBD) Dimer-Containing Antibody Drug Conjugate (ADC) Targeting CD25-Expressing Hematological Malignancies. Blood 2014; 124 : 4491.
Flynn MJ, Berkel PHv, Zammarchi F, Tyrer PC, Akarca AU, Janghra N et al . Pre-Clinical Activity of Adct-301, a Novel Pyrrolobenzodiazepine (PBD) Dimer-Containing Antibody Drug Conjugate (ADC) Targeting CD25-Expressing Hematological Malignancies. Blood 2014; 124 : 4491.
Owaidah TM, Rawas FI, Al Khayatt MF, Elkum NB . Expression of CD66c and CD25 in acute lymphoblastic leukemia as a predictor of the presence of BCR/ABL rearrangement. Hematol Oncol Stem Cell Ther 2008; 1 : 34–37.
Horton TM, Gannavarapu A, Blaney SM, D'Argenio DZ, Plon SE, Berg SL . Bortezomib interactions with chemotherapy agents in acute leukemia in vitro . Cancer Chemother Pharmacol 2006; 58 : 13–23.
Cortes J, Thomas D, Koller C, Giles F, Estey E, Faderl S et al . Phase I Study of Bortezomib in Refractory or Relapsed Acute Leukemias. Clin Cancer Res 2004; 10 : 3371–3376.
Messinger YH, Gaynon PS, Sposto R, v.d. Giessen J, Eckroth E, Malvar J et al . Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood 2012; 120 : 285–290.
Horton Terzah M, O'Brien Maureen Megan, Borowitz Michael J, Devidas Meenakshi, Raetz Elizabeth A, Brown Patrick Andrew et al . Bortezomib reinduction therapy to improve response rates in pediatric ALL in first relapse: A Childrenâ?'s Oncology Group (COG) study (AALL07P1) [Abstract]. 2013; Available at: http://meetinglibrary.asco.org/content/111816-132 .
Maude SL, Tasian SK, Vincent T, Hall JW, Sheen C, Roberts KG et al . Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood 2012; 120 : 3510–3518.
Tasian SK, Doral MY, Borowitz MJ, Wood BL, Chen IM, Harvey RC et al . Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia. Blood 2012; 120 : 833–842.
Senkevitch E, Hixon J, Andrews C, Barata JT, Li W, Durum S . The JAK Inhibitor Ruxolitinib Is Effective in Treating T Cell Acute Lymphoblastic Leukemia with Gain of Function Mutations in IL-7R Alpha. Blood 2015; 126 : 1330.
Nordlund J, Milani L, Lundmark A, Lonnerholm G, Syvanen AC . DNA methylation analysis of bone marrow cells at diagnosis of acute lymphoblastic leukemia and at remission. PLoS One 2012; 7 : e34513.
Nordlund J, Backlin CL, Zachariadis V, Cavelier L, Dahlberg J, Ofverholm I et al . DNA methylation-based subtype prediction for pediatric acute lymphoblastic leukemia. Clin Epigenetics 2015; 7 : 11.
Chatterton Z, Morenos L, Mechinaud F, Ashley DM, Craig JM, Sexton-Oates A et al . Epigenetic deregulation in pediatric acute lymphoblastic leukemia. Epigenetics 2014; 9 : 459–467.
Milani L, Lundmark A, Kiialainen A, Nordlund J, Flaegstad T, Forestier E et al . DNA methylation for subtype classification and prediction of treatment outcome in patients with childhood acute lymphoblastic leukemia. Blood 2010; 115 : 1214–1225.
Issa J-PJ . DNA Methylation as a Therapeutic Target in Cancer. Clin Cancer Res 2007; 13 : 1634–1637.
Yánez L, Bermúdez A, Richard C, Bureo E, Iriondo A . Successful induction therapy with decitabine in refractory childhood acute lymphoblastic leukemia. Leukemia 2009; 23 : 1342–1343.
Benton CB, Thomas DA, Yang H, Ravandi F, Rytting M, O’Brien S et al . Safety and clinical activity of 5-aza-2’-deoxycytidine (decitabine) with or without Hyper-CVAD in relapsed/refractory acute lymphocytic leukaemia. Br J Haematol 2014; 167 : 356–365.
Burke MJ, Lamba JK, Pounds S, Cao X, Ghodke-Puranik Y, Lindgren BR et al . A therapeutic trial of decitabine and vorinostat in combination with chemotherapy for relapsed/refractory acute lymphoblastic leukemia. Am J Hematol 2014; 89 : 889–895.
Lu BY, Thanawala SU, Zochowski KC, Burke MJ, Carroll WL, Bhatla T . Decitabine enhances chemosensitivity of early T-cell precursor-acute lymphoblastic leukemia cell lines and patient-derived samples. Leuk Lymphoma 2016; 57 : 1938–1941.
Zhao WL . Targeted therapy in T-cell malignancies: dysregulation of the cellular signaling pathways. Leukemia 2010; 24 : 13–21.
Chiarini F, Fala F, Tazzari PL, Ricci F, Astolfi A, Pession A et al . Dual inhibition of class IA phosphatidylinositol 3-kinase and mammalian target of rapamycin as a new therapeutic option for T-cell acute lymphoblastic leukemia. Cancer Res 2009; 69 : 3520–3528.
Bressanin D, Evangelisti C, Ricci F, Tabellini G, Chiarini F, Tazzari PL et al . Harnessing the PI3K/Akt/mTOR pathway in T-cell acute lymphoblastic leukemia: Eliminating activity by targeting at different levels. Oncotarget 2012; 3 : 811–823.
Schult C, Dahlhaus M, Glass A, Fischer K, Lange S, Freund M et al . The dual kinase inhibitor NVP-BEZ235 in combination with cytotoxic drugs exerts anti-proliferative activity towards acute lymphoblastic leukemia cells. Anticancer Res 2012; 32 : 463–474.
Saunders P, Cisterne A, Weiss J, Bradstock KF, Bendall LJ . The mammalian target of rapamycin inhibitor RAD001 (everolimus) synergizes with chemotherapeutic agents, ionizing radiation and proteasome inhibitors in pre-B acute lymphocytic leukemia. Haematologica 2011; 96 : 69–77.
Dai H, Wang Y, Lu X, Han W . Chimeric Antigen Receptors Modified T-Cells for Cancer Therapy. Natl Cancer Inst 2016; 108 : pii djv439.
Maude SL, Teachey DT, Porter DL, Grupp SA . CD19-targeted chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Blood 2015; 125 : 4017–4023.
Suerth JD, Schambach A, Baum C . Genetic modification of lymphocytes by retrovirus-based vectors. Curr Opin Immunol 2012; 24 : 598–608.
Riet T, Holzinger A, Dorrie J, Schaft N, Schuler G, Abken H . Nonviral RNA transfection to transiently modify T cells with chimeric antigen receptors for adoptive therapy. Methods Mol Biol 2013; 969 : 187–201.
Barrett DM, Liu X, Jiang S, June CH, Grupp SA, Zhao Y . Regimen-specific effects of RNA-modified chimeric antigen receptor T cells in mice with advanced leukemia. Hum Gene Ther 2013; 24 : 717–727.
Grupp SA, Maude SL, Shaw PA, Aplenc R, Barrett DM, Callahan C et al . Durable Remissions in Children with Relapsed/Refractory ALL Treated with T Cells Engineered with a CD19-Targeted Chimeric Antigen Receptor (CTL019). Blood 2015; 126 : 681–681.
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ et al . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371 : 1507–1517.
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K et al . Efficacy and Toxicity Management of 19-28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med 2014; 6 : 224ra25.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA et al . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385 : 517–528.
Maude SL, Barrett D, Teachey DT, Grupp. SA . Managing Cytokine Release Syndrome Associated With Novel T Cell-Engaging Therapies. Cancer J 2014; 20 : 119–122.
Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M et al . Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014; 124 : 188–195.
Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH et al . Anti-CD22–chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 2013, 1165–1174.
Shah NN, Stetler-Stevenson M, Yuan CM, Shalabi H, Yates B, Delbrook C et al . Minimal Residual Disease Negative Complete Remissions Following Anti-CD22 Chimeric Antigen Receptor (CAR) in Children and Young Adults with Relapsed/Refractory Acute Lymphoblastic Leukemia (ALL). Blood 2016; 128 : 650.
Jamieson CH, Amylon MD, Wong RM, Blume KG . Allogeneic hematopoietic cell transplantation for patients with high-risk acute lymphoblastic leukemia in first or second complete remission using fractionated total-body irradiation and high-dose etoposide: a 15-year experience. Exp Hematol 2003; 31 : 981–986.
Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N et al . Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol 2004; 22 : 4075–4086.
Jain N, Lamb AV, O'Brien S, Ravandi F, Konopleva M, Jabbour E et al . Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood 2016; 127 : 1863–1869.
Gokbuget N, Kneba M, Raff T, Trautmann H, Bartram CR, Arnold R et al . Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 2012; 120 : 1868–1876.
Dhedin N, Huynh A, Maury S, Tabrizi R, Beldjord K, Asnafi V et al . Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood 2015; 125 : 2486–2496, quiz 2586.
Tavernier E, Boiron JM, Huguet F, Bradstock K, Vey N, Kovacsovics T et al . Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia 2007; 21 : 1907–1914.
Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G et al . Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007; 109 : 944–950.
Download references
Author information
Authors and affiliations.
New York University School of Medicine, New York, USA
T Terwilliger & M Abdul-Hay
Department of Hematology, New York University Perlmutter Cancer Center, New York, USA
M Abdul-Hay
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to M Abdul-Hay .
Ethics declarations
Competing interests.
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
Reprints and permissions
About this article
Cite this article.
Terwilliger, T., Abdul-Hay, M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 7 , e577 (2017). https://doi.org/10.1038/bcj.2017.53
Download citation
Received : 28 March 2017
Accepted : 21 April 2017
Published : 30 June 2017
Issue Date : June 2017
DOI : https://doi.org/10.1038/bcj.2017.53
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Targeting ferroptosis for leukemia therapy: exploring novel strategies from its mechanisms and role in leukemia based on nanotechnology.
- Muhammad Hossein Ashoub
- Razieh Razavi
- Mahnaz Amiri
European Journal of Medical Research (2024)
Catechin-Induced changes in PODXL, DNMTs, and miRNA expression in Nalm6 cells: an integrated in silico and in vitro approach
- Alireza Keyhani
- Reza Vahidi
BMC Complementary Medicine and Therapies (2024)
Exploring the potential of IL-10 for risk assessment and early intervention in pediatric ALL
- Roqaia E. Radwan
- Ahmad Darwish
- Wafaa M. El-kholy
BMC Cancer (2024)
Nutritional interventions in children with acute lymphoblastic leukemia undergoing antineoplastic treatment: a systematic review
- Alan E. Guzmán-León
- Jessica Avila-Prado
- Veronica Lopez-Teros
BMC Nutrition (2024)
Cardiotoxic profiles of CAR-T therapy and bispecific T-cell engagers in hematological cancers
- Badri Karthikeyan
- Sunitha Shyam Sunder
- Umesh C. Sharma
Communications Medicine (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
A deep learning-based approach for the diagnosis of acute lymphoblastic leukemia.

1. Introduction
- We proposed the Multi-Attention EfficientNetB3 and EfficientNetV2S models to distinguish the ALL (unhealthy cells) and hem (healthy cells) in this article;
- We simply modified the last block of both models and added the Multi-Attention Layers in both models. After including this Multi-Attention mechanism not only reduces the model’s complexities but also generalizes its network quite well;
- We added a crop function to reduce the unwanted part of the image;
- To address the issue of unbalanced data, we also applied the augmentation technique to expand the dataset;
- Our Multi-Attention EfficientNetV2S and EfficientNetB3 models achieved the 99.73% and 99.25% accuracy, respectively, on the test dataset for ALL and hem cells;
- We also compared our model to other CNN models that were previously used for the detection of normal cells and cancerous cells from blood smear images but our Multi-Attention EfficientNetV2S and EfficientNetB3 models provided a higher classification accuracy.
2. Related Work
3. methods and materials, multi-attention mechanism, 4. results and discussion.
- The learning rate hyperparameter determines how much change will be made to the network’s weights after each backpropagation pass. We set a learning rate of 0.001 for both models. The learning rate is reduced to a 0.5 factor if the monitor value does not improve;
- Epochs are set to 20 for both efficietNetB3 and efficientNetV2S;
- The batch size is set to 16 for both models;
- The patience parameter is set to 1 and the stop patience parameter is set to 3;
- Both models are saved with the highest accuracy in the validation set;
- Adamax optimizer is used for training purposes with extension of Adam that try to combine the best part of the RMSProp and momentum optimizer. In some scenarios, the Adamax optimizer provides the better optimization than the Adam optimizer;
- Categorical cross-entropy is used to calculate the loss during training that is well-suited for the categorical problem;
- We added an additional batch norm [ 43 ] layer before fully connected layers;
- The TensorFlow [ 44 ] framework and Python 3.7 were used to implement the experiments;
5. Grad-Cam Analysis
6. conclusions and future work, author contributions, acknowledgments, conflicts of interest.
- Das, P.K.; Meher, S. An efficient deep Convolutional Neural Network based detection and classification of Acute Lymphoblastic Leukemia. Expert Syst. Appl. 2021 , 183 , 115311. [ Google Scholar ] [ CrossRef ]
- Sahlol, A.T.; Kollmannsberger, P.; Ewees, A.A. Efficient Classification of White Blood Cell Leukemia with Improved Swarm Optimization of Deep Features. Sci. Rep. 2020 , 10 , 2536. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Alagu, S.; Bagan, K. Chronological Sine Cosine Algorithm Based Deep CNN for Acute Lymphocytic Leukemia Detection. Available online: https://www.researchgate.net/publication/353659892 (accessed on 4 April 2022).
- Rehman, A.; Abbas, N.; Saba, T.; Rahman, S.I.U.; Mehmood, Z.; Kolivand, H. Classification of acute lymphoblastic leukemia using deep learning. Microsc. Res. Tech. 2018 , 81 , 1310–1317. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Key Statistics for Acute Lymphocytic Leukemia. Available online: https://www.cancer.org/cancer/acute-lymphocytic-leukemia/about/key-statistics (accessed on 4 April 2022).
- American Cancer Society What’s New in Acute Lymphocytic Leukemia (ALL) Research? Available online: https://www.cancer.org/cancer/acute-lymphocytic-leukemia/about/new-research.html (accessed on 4 April 2022).
- Chang, J.H.; Poppe, M.M.; Hua, C.; Marcus, K.J.; Esiashvili, N. Acute lymphoblastic leukemia. Pediatr. Blood Cancer 2021 , 68 , e28371. [ Google Scholar ] [ CrossRef ]
- Cho, P.; Dash, S.; Tsaris, A.; Yoon, H.-J. Image transformers for classifying acute lymphoblastic leukemia. In Proceedings of the Medical Imaging 2022: Computer-Aided Diagnosis, San Diego, CA, USA, 4 April 2022. [ Google Scholar ] [ CrossRef ]
- Kasani, P.H.; Park, S.-W.; Jang, J.-W. An Aggregated-Based Deep Learning Method for Leukemic B-lymphoblast Classification. Diagnostics 2020 , 10 , 1064. [ Google Scholar ] [ CrossRef ]
- Papiththira, S.; Kokul, T. Melanoma Skin Cancer Detection Using EfficientNet and Channel Attention Module. In Proceedings of the International Conference on Industrial and Information Systems (ICIIS), Kandy, Sri Lanka, 9–11 December 2021; pp. 227–232. [ Google Scholar ] [ CrossRef ]
- Claro, M.; Vogado, L.; Veras, R.; Santana, A.; Tavares, J.; Santos, J.; Machado, V.M. Convolution Neural Network Models for Acute Leukemia Diagnosis. In Proceedings of the 2020 International Conference on Systems, Signals and Image Processing (IWSSIP), Niteroi, Brazil, 1–3 July 2020; pp. 63–68. [ Google Scholar ] [ CrossRef ]
- Duong, L.T.; Nguyen, P.T.; Di Sipio, C.; Di Ruscio, D. Automated fruit recognition using EfficientNet and MixNet. Comput. Electron. Agric. 2020 , 171 , 105326. [ Google Scholar ] [ CrossRef ]
- Ali, S.; Javaid, N.; Javeed, D.; Ahmad, I.; Ali, A.; Badamasi, U.M. A Blockchain-Based Secure Data Storage and Trading Model for Wireless Sensor Networks. In International Conference on Advanced Information Networking and Applications ; Springer: Cham, Switzerland; Caserta, Italy, 2020; pp. 499–511. [ Google Scholar ] [ CrossRef ]
- Raza, A.; Ayub, H.; Khan, J.A.; Ahmad, I.; Salama, A.S.; Daradkeh, Y.I.; Javeed, D.; Rehman, A.U.; Hamam, H. A Hybrid Deep Learning-Based Approach for Brain Tumor Classification. Electronics 2022 , 11 , 1146. [ Google Scholar ] [ CrossRef ]
- Javeed, D.; Gao, T.; Khan, M. SDN-Enabled Hybrid DL-Driven Framework for the Detection of Emerging Cyber Threats in IoT. Electronics 2021 , 10 , 918. [ Google Scholar ] [ CrossRef ]
- Al Razib, M.; Javeed, D.; Taimoor Khanet, M.; Alkanhel, R.; Ali Muthanna, M.S. Cyber Threats Detection in Smart Environments Using SDN-Enabled DNN-LSTM Hybrid Framework. IEEE Access 2022 , 10 , 53015–53026. [ Google Scholar ] [ CrossRef ]
- Javeed, D.; Gao, T.; Khan, M.T.; Shoukat, D. A Hybrid Intelligent Framework to Combat Sophisticated Threats in Secure Industries. Sensors 2022 , 22 , 1582. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ahmad, I.; Liu, Y.; Javeed, D.; Ahmad, S. A decision-making technique for solving order allocation problem using a genetic algorithm. IOP Conf. Ser. Mater. Sci. Eng. 2020 , 853 , 012054. [ Google Scholar ] [ CrossRef ]
- Javeed, D.; Gao, T.; Khan, M.; Ahmad, I. A Hybrid Deep Learning-Driven SDN Enabled Mechanism for Secure Communication in Internet of Things (IoT). Sensors 2021 , 21 , 4884. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Ullah, M.Z.; Zheng, Y.; Song, J.; Aslam, S.; Xu, C.; Kiazolu, G.D.; Wang, L. An Attention-Based Convolutional Neural Network for Acute Lymphoblastic Leukemia Classification. Appl. Sci. 2021 , 11 , 10662. [ Google Scholar ] [ CrossRef ]
- Xu, C.; Xu, L.; Ohorodnyk, P.; Roth, M.; Chen, B.; Li, S. Contrast agent-free synthesis and segmentation of ischemic heart disease images using progressive sequential causal GANs. Med. Image Anal. 2020 , 62 , 101668. [ Google Scholar ] [ CrossRef ]
- Gao, Z.; Wang, X.; Sun, S.; Wu, D.; Bai, J.; Yin, Y.; Liu, X.; Zhang, H.; de Albuquerque, V.H.C. Learning physical properties in complex visual scenes: An intelligent machine for perceiving blood flow dynamics from static CT angiography imaging. Neural Netw. 2020 , 123 , 82–93. [ Google Scholar ] [ CrossRef ]
- Gao, Z.; Chung, J.; Abdelrazek, M.; Leung, S.; Hau, W.K.; Xian, Z.; Zhang, H.; Li, S. Privileged Modality Distillation for Vessel Border Detection in Intracoronary Imaging. IEEE Trans. Med. Imaging 2019 , 39 , 1524–1534. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Jiang, Z.; Dong, Z.; Wang, L.; Jiang, W. Method for Diagnosis of Acute Lymphoblastic Leukemia Based on ViT-CNN Ensemble Model. Comput. Intell. Neurosci. 2021 , 2021 , 7529893. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Alagu, S.; Bagan, K. A Novel Segmentation Approach for Acute Lymphocytic Leukemia Detection Using Deep Learning. 2021. Available online: https://www.researchgate.net/publication/353659988 (accessed on 3 September 2022).
- Alagu, S. Automatic Detection of Acute Lymphoblastic Leukemia Using UNET Based Segmentation and Statistical Analysis of Fused Deep Features. Appl. Artif. Intell. 2021 , 35 , 1952–1969. [ Google Scholar ] [ CrossRef ]
- Genovese, A.; Hosseini, M.S.; Piuri, V.; Plataniotis, K.N.; Scotti, F. Acute Lymphoblastic Leukemia Detection Based on Adaptive Unsharpening and deep Learning. In Proceedings of the ICASSP 2021–2021 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Toronto, ON, Canada, 6–11 June 2021; pp. 1205–1209. [ Google Scholar ]
- Shafique, S.; Tehsin, S. Acute Lymphoblastic Leukemia Detection and Classification of Its Subtypes Using Pretrained Deep Convolutional Neural Networks. Technol. Cancer Res. Treat. 2018 , 17 , 1–7. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Loey, M.; Naman, M.; Zayed, H. Deep Transfer Learning in Diagnosing Leukemia in Blood Cells. Computers 2020 , 9 , 29. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ghaderzadeh, M.; Hosseini, A.; Asadi, F.; Abolghasemi, H.; Bashash, D.; Roshanpoor, A. Automated Detection Model in Classification of B-Lymphoblast Cells from Normal B-Lymphoid Precursors in Blood Smear Microscopic Images Based on the Majority Voting Technique. Sci. Program. 2022 , 2022 , 4801671. [ Google Scholar ] [ CrossRef ]
- Genovese, A.; Hosseini, M.S.; Piuri, V.; Plataniotis, K.N.; Scotti, F. Histopathological Transfer Learning for Acute Lymphoblastic Leukemia Detection. In Proceedings of the 2021 IEEE International Conference on Computational Intelligence and Virtual Environments for Measurement Systems and Applications (CIVEMSA), Di Milano, Italy, 1 September 2021; pp. 1–6. [ Google Scholar ] [ CrossRef ]
- Gebremeskel, K.D.; Kwa, T.C.; Raj, K.H.; Zewdie, G.A.; Shenkute, T.Y.; Maleko, W.A. Automatic Early Detection and Classification of Leukemia from Microscopic Blood Image. AbyssiniaJ. Sci. Technol. 2021 , 3 , 1–10. Available online: https://journals.wu.edu.et/index.php/ajec/article/view/160 (accessed on 20 April 2022).
- Kandhari, R.; Bhan, A.; Bhatnagar, P.; Goyal, A. Computer based diagnosis of Leukemia in blood smear images. In Proceedings of the 2021 Third International Conference on Intelligent Communication Technologies and Virtual Mobile Networks (ICICV), Tirunelveli, India, 4–6 February 2021; pp. 1462–1466. [ Google Scholar ] [ CrossRef ]
- Bodzas, A.; Kodytek, P.; Zidek, J. Automated Detection of Acute Lymphoblastic Leukemia From Microscopic Images Based on Human Visual Perception. Front. Bioeng. Biotechnol. 2020 , 8 , 1005. [ Google Scholar ] [ CrossRef ]
- Chen, Y.-M.; Chou, F.-I.; Ho, W.-H.; Tsai, J.-T. Classifying microscopic images as acute lymphoblastic leukemia by Resnet ensemble model and Taguchi method. BMC Bioinform. 2021 , 22 , 615. [ Google Scholar ] [ CrossRef ]
- Tan, M.; Le, Q.V. EfficientNet: Rethinking model scaling for convolutional neural networks. In Proceedings of the International Conference on Machine Learning, Long Beach, CA, USA, 11 September 2019; pp. 10691–10700. [ Google Scholar ]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L. MobileNetV2: Inverted residuals and linear bottlenecks. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Salt Lake City, UT, USA, 18–23 June 2018; pp. 4510–4520. [ Google Scholar ] [ CrossRef ]
- Tan, M.; Le, Q.V. EfficientNetV2: Smaller Models and Faster Training. In Proceedings of the 38th International Conference on Machine Learning, Virtual, CA, USA, 23 June 2021; Available online: http://arxiv.org/abs/2104.00298 (accessed on 6 June 2022).
- Woo, S.; Park, J.; Lee, J.Y.; Kweon, I.S. Cbam: Convolutional block attention module. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018; pp. 3–19. [ Google Scholar ] [ CrossRef ]
- Ahmad, I.; Wang, X.; Zhu, M.; Wang, C.; Pi, Y.; Khan, J.; Li, G. EEG-Based Epileptic Seizure Detection via Machine/Deep Learning Approaches: A Systematic Review. Comput. Intell. Neurosci. 2022 , 2022 , 6486570. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Mondal, C.; Hasan, K.; Ahmad, M.; Awal, A.; Jawad, T.; Dutta, A.; Islam, R.; Moni, M.A. Ensemble of Convolutional Neural Networks to diagnose Acute Lymphoblastic Leukemia from microscopic images. Inform. Med. Unlocked 2021 , 27 , 100794. [ Google Scholar ] [ CrossRef ]
- Khan, T.U. Internet of Things (IOT) systems and its security challenges. Int. J. Adv. Res. Comput. Eng. Technol. (IJARCET) 2019 , 8 , 12. [ Google Scholar ]
- Ahmad, I.; Ullah, I.; Khan, W.U.; Ur Rehman, A.; Adrees, M.S.; Saleem, M.Q.; Shafiq, M. Efficient algorithms for E-healthcare to solve multiobject fuse detection problem. J. Healthc. Eng. 2021 , 2021 , 9500304. [ Google Scholar ] [ CrossRef ]
- Abaddi, M.; Barham, P.; Chen, J.; Chen, Z.; Davis, A.; Dean, J.; Devin, M.; Ghemawat, S.; Irving, G.; Isard, M.; et al. TensorFlow: A system for large-scale machine learning. In Proceedings of the 12th USENIX Symposium on Operating Systems Design and Implementation, Savannah, GA, USA, 2 November 2016; pp. 265–283. [ Google Scholar ]
Click here to enlarge figure
| Model | Accuracy % | Precision % | Sensitivity % | Specificity % | F1-Score |
|---|---|---|---|---|---|
| EfficientNetV2S | 99.73 | 99.85 | 99.60 | 99.85 | 99.72 |
| EfficientNetB3 | 99.25 | 99.00 | 99.50 | 99.00 | 99.25 |
| Ref | Year | Methods | Accuracy |
|---|---|---|---|
| [ ] | 2021 | VGG16 + ECA module | 91% |
| [ ] | 2021 | EfficientNetB0 | 95.18% |
| [ ] | 2021 | Vision Transformer | 98.90% |
| [ ] | 2020 | NasNetLarge + VGG19 | 96.58% |
| [ ] | 2022 | Ensemble model based on majority voting technique | 98.50% |
| [ ] | 2021 | VIT-CNN Ensemble Model (EfficientNetB0 + Vision Transformer) | 99.03% |
| Proposed | 2022 | Multi-Attention EfficientNetB3 | 99.25% |
| Multi-Attention EfficientNetV2S | 99.73% |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
Share and Cite
Saeed, A.; Shoukat, S.; Shehzad, K.; Ahmad, I.; Eshmawi, A.A.; Amin, A.H.; Tag-Eldin, E. A Deep Learning-Based Approach for the Diagnosis of Acute Lymphoblastic Leukemia. Electronics 2022 , 11 , 3168. https://doi.org/10.3390/electronics11193168
Saeed A, Shoukat S, Shehzad K, Ahmad I, Eshmawi AA, Amin AH, Tag-Eldin E. A Deep Learning-Based Approach for the Diagnosis of Acute Lymphoblastic Leukemia. Electronics . 2022; 11(19):3168. https://doi.org/10.3390/electronics11193168
Saeed, Adnan, Shifa Shoukat, Khurram Shehzad, Ijaz Ahmad, Ala’ Abdulmajid Eshmawi, Ali H. Amin, and Elsayed Tag-Eldin. 2022. "A Deep Learning-Based Approach for the Diagnosis of Acute Lymphoblastic Leukemia" Electronics 11, no. 19: 3168. https://doi.org/10.3390/electronics11193168
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
Recent advancements in biomarkers, therapeutics, and associated challenges in acute myeloid leukemia
- Published: 29 August 2024
Cite this article

- Suresh Kumar Prajapati 1 ,
- Neha Kumari 2 ,
- Doulat Bhowmik 2 &
- Reeshu Gupta ORCID: orcid.org/0000-0002-1743-4388 1 , 2
Acute myeloid leukemia (AML) is a common type of leukemia that has a high mortality rate. The reasons for high mortality in patients with AML are therapeutic resistance, limited ability to predict duration of response, and likelihood of cancer relapse. Biomarkers, such as leukemic stem cell biomarkers, circulatory biomarkers, measurable residual disease biomarkers, and molecular biomarkers, are used for prognosis, diagnosis, and targeted killing to selectively eliminate AML cells. They also play an indispensable role in providing therapeutic resistance to patients with AML. Therefore, targeting these biomarkers will improve the outcome of AML patients. However, identifying biomarkers that can differentiate between treatment-responsive and non-responsive AML patients remains a challenge. This review discusses recent advancements in AML biomarkers, promising therapeutics, and associated challenges in the treatment of AML.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data availability
No datasets were generated or analysed during the current study.
Ding G, Zhang (2017) The biomarkers of leukemia stem cells in acute myeloid leukemia. Stem Cell Investig 4:19
Article PubMed PubMed Central Google Scholar
Greim et al (2014) The bone marrow niche, stem cells, and leukemia: impact of drugs, chemicals, and the environment. Ann N Y Acad Sci 1310(1):7–31
Article CAS PubMed PubMed Central Google Scholar
Thakral G, Khan (2022) Leukemic stem cell signatures in Acute myeloid leukemia- targeting the guardians with novel approaches. Stem Cell Rev Rep 18(5):1756–1773
Article CAS PubMed Google Scholar
Murone et al (2017) The multi-kinase inhibitor Debio 0617B reduces maintenance and self-renewal of primary human AML CD34(+) Stem/Progenitor cells. Mol Cancer Ther 16(8):1497–1510
Al-Mawali G, Lewis (2016) Immunoprofiling of leukemic stem cells CD34+/CD38-/CD123 + delineate FLT3/ITD-positive clones. J Hematol Oncol 9(1):61
Horna Z, Sotomayor L, Moscinski (2014) Diagnostic immunophenotype of acute promyelocytic leukemia before and early during therapy with all-trans retinoic acid. Am J Clin Pathol 142(4):546–552
Donato et al (2023) Retained functional normal and preleukemic HSCs at diagnosis are associated with good prognosis in DNMT3AmutNPM1mut AMLs. Blood Adv 7(6):1011–1018
Schuurhuis et al (2013) Normal hematopoietic stem cells within the AML bone marrow have a distinct and higher ALDH activity level than co-existing leukemic stem cells. PLoS ONE 8(11):e78897
Agerstam et al (2015) Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia. Proc Natl Acad Sci U S A 112(34):10786–10791
Chiesa et al (2023) Base-edited CAR7 T cells for relapsed T-Cell Acute Lymphoblastic Leukemia. N Engl J Med 389(10):899–910
Kersten et al (2016) CD45RA, a specific marker for leukaemia stem cell sub-populations in acute myeloid leukaemia. Br J Haematol 173(2):219–235
Chung SS (2014) CD99 is a therapeutic target on disease stem cells in acute myeloid leukemia and the myelodysplastic syndromes. Blood 124:3760
Article Google Scholar
van Rhenen et al (2007) The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood 110(7):2659–2666
Article PubMed Google Scholar
Jan et al (2011) Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl Acad Sci U S A 108(12):5009–5014
Terwijn et al (2009) Interleukin-2 receptor alpha-chain (CD25) expression on leukaemic blasts is predictive for outcome and level of residual disease in AML. Eur J Cancer 45(9):1692–1699
Majeti (2011) Monoclonal antibody therapy directed against human acute myeloid leukemia stem cells. Oncogene 30(9):1009–1019
Mohd Amin et al (2023) ENPP4 and HOXA3 as potential leukaemia stem cell markers in acute myeloid leukaemia. Malays J Pathol 45(1):65–76
CAS PubMed Google Scholar
Wang D, Wang (2020) ENPP4 overexpression is associated with no recovery from Barrett’s esophagus. Int J Clin Exp Pathol 13(12):2927–2936
CAS PubMed PubMed Central Google Scholar
Paczulla et al (2019) Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature 572(7768):254–259
Herrmann et al (2020) Delineation of target expression profiles in CD34+/CD38- and CD34+/CD38 + stem and progenitor cells in AML and CML. Blood Adv 4(20):5118–5132
Riether et al (2020) Targeting CD70 with cusatuzumab eliminates acute myeloid leukemia stem cells in patients treated with hypomethylating agents. Nat Med 26(9):1459–1467
Wang H, Chen (2017) Understanding of leukemic stem cells and their clinical implications. Mol Cancer 16(1):2
Barreto et al (2022) Leukemic stem cell: a Mini-review on clinical perspectives. Front Oncol 12:931050
Nomdedeu et al (2011) Immunophenotype of acute myeloid leukemia with NPM mutations: prognostic impact of the leukemic compartment size. Leuk Res 35(2):163–168
Aparna SK (2018) Aberrant phenotypes in acute myeloid leukemia in India. Int J Adv Med 5(2):361–365
Gholami et al (2014) The expression analysis of LATS2 gene in de novo AML patients. Med Oncol 31(5):961
Thakral et al (2023) Integrated single-cell transcriptome analysis of CD34 + enriched leukemic stem cells revealed intra- and inter-patient transcriptional heterogeneity in pediatric acute myeloid leukemia. Ann Hematol 102(1):73–87
Majeti et al (2009) CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138(2):286–299
Pearce et al (2005) Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells 23(6):752–760
Zeijlemaker et al (2016) A simple one-tube assay for immunophenotypical quantification of leukemic stem cells in acute myeloid leukemia. Leukemia 30(2):439–446
Ehninger et al (2014) Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J 4(6):e218
Willier et al (2021) CLEC12A and CD33 coexpression as a preferential target for pediatric AML combinatorial immunotherapy. Blood 137(8):1037–1049
Jung D, Gentles M, Feinberg (2015) An LSC epigenetic signature is largely mutation independent and implicates the HOXA cluster in AML pathogenesis. Nat Commun 6:8489
Shin (2022) Human acute myeloid leukemia stem cells: evolution of concept. Blood Res 57(S1):67–74
Liu et al (2019) MiR-29b/Sp1/FUT4 axis modulates the malignancy of leukemia stem cells by regulating fucosylation via Wnt/beta-catenin pathway in acute myeloid leukemia. J Exp Clin Cancer Res 38(1):200
Singh T, Gupta (2021) Regulatory noncoding RNAs: potential biomarkers and therapeutic targets in acute myeloid leukemia. Am J Blood Res 11(5):504–519
Bhattacharya, Gutti (2022) Non-coding RNAs: are they the protagonist or antagonist in the regulation of leukemia? Am J Transl Res 14(3):1406–1432
Abdelhafiz E, Saber G, Hamdy (2020) Low expression of miR-204 is associated with expression of CD34 and poor performance status in denovo AML. Int J Lab Hematol 42(3):263–269
Li et al (2018) Upregulated microRNA-146a expression induced by granulocyte colony-stimulating factor enhanced low-dosage chemotherapy response in aged acute myeloid leukemia patients. Exp Hematol 68:66–79e3
Seipel M, Wiedemann B, Pabst (2020) MN1, FOXP1 and hsa-miR-181a-5p as prognostic markers in acute myeloid leukemia patients treated with intensive induction chemotherapy and autologous stem cell transplantation. Leuk Res 89:106296
de Leeuw et al (2014) Attenuation of microRNA-126 expression that drives CD34 + 38- stem/progenitor cells in acute myeloid leukemia leads to tumor eradication. Cancer Res 74(7):2094–2105
Wu et al (2022) Exosomes from bone marrow mesenchymal stem cells decrease chemosensitivity of acute myeloid leukemia cells via delivering miR-10a. Biochem Biophys Res Commun 622:149–156
Ye et al (2023) DNA hypermethylation-induced miR-182 silence targets BCL2 and HOXA9 to facilitate the self-renewal of leukemia stem cell, accelerate acute myeloid leukemia progression, and determine the sensitivity of BCL2 inhibitor venetoclax. Theranostics 13(1):77–94
Spinello et al (2011) MicroRNA-146a and AMD3100, two ways to control CXCR4 expression in acute myeloid leukemias. Blood Cancer J 1(6):e26
Chavali F, Chavali (2011) Cis-regulation of microRNA expression by scaffold/matrix-attachment regions. Nucleic Acids Res 39(16):6908–6918
Liu et al (2016) miR-181a promotes G1/S transition and cell proliferation in pediatric acute myeloid leukemia by targeting ATM. J Cancer Res Clin Oncol 142(1):77–87
De Luca et al (2017) Knockdown of miR-128a induces Lin28a expression and reverts myeloid differentiation blockage in acute myeloid leukemia. Cell Death Dis 8(6):e2849
Smith et al (2020) Comprehensive Transcriptome Profiling of Cryptic CBFA2T3-GLIS2 Fusion-positive AML defines Novel Therapeutic options: a COG and TARGET Pediatric AML Study. Clin Cancer Res 26(3):726–737
Masetti et al (2013) CBFA2T3-GLIS2 fusion transcript is a novel common feature in pediatric, cytogenetically normal AML, not restricted to FAB M7 subtype. Blood 121(17):3469–3472
Hao, Shao (2015) HOTAIR is upregulated in acute myeloid leukemia and that indicates a poor prognosis. Int J Clin Exp Pathol 8(6):7223–7228
Neaga et al (2020) MicroRNAs Associated with a good prognosis of Acute myeloid leukemia and their effect on macrophage polarization. Front Immunol 11:582915
Cheng et al (2021) Human mesenchymal stem cells derived exosomes inhibit the growth of acute myeloid leukemia cells via regulating miR-23b-5p/TRIM14 pathway. Mol Med 27(1):128
Jiang et al (2022) Bone mesenchymal stem cell-derived exosomal microRNA-7-5p inhibits progression of acute myeloid leukemia by targeting OSBPL11. J Nanobiotechnol 20(1):29
Article CAS Google Scholar
Ji et al (2021) Small-sized extracellular vesicles (EVs) derived from acute myeloid leukemia bone marrow mesenchymal stem cells transfer miR-26a-5p to promote acute myeloid leukemia cell proliferation, migration, and invasion. Hum Cell 34(3):965–976
Ge et al (2023) miR-30e-5p regulates leukemia stem cell self-renewal through the Cyb561/ROS signaling pathway. Haematologica
Zhang et al (2020) Exosomes derived from human bone marrow mesenchymal stem cells transfer mir-222-3p to suppress acute myeloid leukemia cell proliferation by targeting IRF2/INPP4B. Mol Cell Probes 51:101513
Chen et al (2021) microRNA-1246-containing extracellular vesicles from acute myeloid leukemia cells promote the survival of leukemia stem cells via the LRIG1-meditated STAT3 pathway. Aging 13(10):13644–13662
Shang M, Wu, Xiao (2021) Downregulation of circ_0012152 inhibits proliferation and induces apoptosis in acute myeloid leukemia cells through the miR-625-5p/SOX12 axis. Hematol Oncol 39(4):539–548
Hu et al (2020) MicroRNA-34a-mediated death of acute myeloid leukemia stem cells through apoptosis induction and exosome shedding inhibition via histone deacetylase 2 targeting. IUBMB Life 72(7):1481–1490
Mammoli et al (2019) Physiological expression of miR-130a during differentiation of CD34(+) human hematopoietic stem cells results in the inhibition of monocyte differentiation. Exp Cell Res 382(1):111445
Crisafulli et al (2019) MicroRNA-127-3p controls murine hematopoietic stem cell maintenance by limiting differentiation. Haematologica 104(9):1744–1755
Zhang et al (2020) Mir-221-3p delivered by BMMSC-Derived microvesicles promotes the development of Acute Myelocytic Leukemia. Front Bioeng Biotechnol 8:81
Krivdova et al (2022) Identification of the global miR-130a targetome reveals a role for TBL1XR1 in hematopoietic stem cell self-renewal and t(8;21) AML. Cell Rep 38(10):110481
Xu et al (2020) MicroRNAs of bone marrow mesenchymal stem cell-derived exosomes regulate acute myeloid leukemia cell proliferation and apoptosis. Chin Med J (Engl) 133(23):2829–2839
Luo et al (2019) HOTTIP lncRNA promotes hematopoietic Stem Cell Self-Renewal leading to AML-like Disease in mice. Cancer Cell 36(6):645–659e8
Gao et al (2018) Long noncoding RNA HOTAIR promotes the self-renewal of leukemia stem cells through epigenetic silencing of p15. Exp Hematol 67:32-40 e3
Cui et al (2021) Long non-coding RNA LINC00152 regulates Self-Renewal of Leukemia Stem cells and induces chemo-resistance in Acute myeloid leukemia. Front Oncol 11:694021
Wu C, Huo S, Wang (2023) Alternative splicing of HOXB-AS3 underlie the promoting effect of nuclear m6A reader YTHDC1 on the self-renewal of leukemic stem cells in acute myeloid leukemia. Int J Biol Macromol 237:123990
Feng S, Xu, He, He, Ge (2022) Expression of CD123 related Long non-coding RNA in Acute myeloid leukemia bone marrow mononuclear cells and its clinical significance. Cell Mol Biol (Noisy-le-grand). 68(6):140–147
Chen F, Zhu C, Liu, and, Zhou (2020) LncRNA MAGI2-AS3 inhibits the self-renewal of leukaemic stem cells by promoting TET2-dependent DNA demethylation of the LRIG1 promoter in acute myeloid leukaemia. RNA Biol 17(6):784–793
Garzon et al (2008) MicroRNA signatures associated with cytogenetics and prognosis in acute myeloid leukemia. Blood 111(6):3183–3189
Dai et al (2014) MicroRNA let-7f is down-regulated in patients with refractory acute myeloid leukemia and is involved in chemotherapy resistance of adriamycin-resistant leukemic cells. Leuk Lymphoma 55(7):1645–1648
Zhao L, Torrance, Bader (2013) TP53-independent function of miR-34a via HDAC1 and p21(CIP1/WAF1). Mol Ther 21(9):1678–1686
Di Martino et al (2014) In vivo activity of miR-34a mimics delivered by stable nucleic acid lipid particles (SNALPs) against multiple myeloma. PLoS ONE 9(2):e90005
Liu P, Chandrasekara S, Dear (2016) Epigenetic regulation of miRNA-124 and multiple downstream targets is associated with treatment response in myeloid malignancies. Oncol Lett 12(3):2175–2180
Ufkin P, Yang D, Duarte, and, Sathyanarayana (2014) miR-125a regulates cell cycle, proliferation, and apoptosis by targeting the ErbB pathway in acute myeloid leukemia. Leuk Res 38(3):402–410
Osiriphan I, Anuchapreeda K, Duangmano (2024) MicroRNA–223 overexpression suppresses protein kinase C epsilon expression in human leukemia stem cell–like KG–1a cells. Mol Clin Oncol 21(1):48
Issa B, Winkler S, Heckl, and, Klusmann (2023) Preclinical testing of miRNA-193b-3p mimic in acute myeloid leukemias. Leukemia 37(7):1583–1587
Wallace, Connell O (2017) MicroRNAs and acute myeloid leukemia: therapeutic implications and emerging concepts. Blood 130(11):1290–1301
Zhang et al (2019) Clinical resistance to crenolanib in acute myeloid leukemia due to diverse molecular mechanisms. Nat Commun 10(1):244
Lang D, Bullinger, and, Frick (2023) Mechanisms of resistance to small molecules in Acute myeloid leukemia. Cancers (Basel), 15(18)
Lindblad et al (2016) Aberrant activation of the PI3K/mTOR pathway promotes resistance to sorafenib in AML. Oncogene 35(39):5119–5131
McMahon et al (2019) Clonal selection with RAS Pathway Activation mediates secondary clinical resistance to selective FLT3 inhibition in Acute myeloid leukemia. Cancer Discov 9(8):1050–1063
Rummelt et al (2021) Activating JAK-mutations confer resistance to FLT3 kinase inhibitors in FLT3-ITD positive AML in vitro and in vivo. Leukemia 35(7):2017–2029
Patel W, Shen S, Cooper, and, Smithgall (2019) Expression of myeloid src-family kinases is associated with poor prognosis in AML and influences Flt3-ITD kinase inhibitor acquired resistance. PLoS ONE 14(12):e0225887
Choe et al (2020) Molecular mechanisms mediating relapse following ivosidenib monotherapy in IDH1-mutant relapsed or refractory AML. Blood Adv 4(9):1894–1905
Quek et al (2018) Clonal heterogeneity of acute myeloid leukemia treated with the IDH2 inhibitor enasidenib. Nat Med 24(8):1167–1177
Wang et al (2021) Leukemia stemness and co-occurring mutations drive resistance to IDH inhibitors in acute myeloid leukemia. Nat Commun 12(1):2607
Memoona, Khan (2018) 3 ISOFORMS OF PML RARα IN ACUTE PROMYELOCYTIC LEUKAEMIA. Pak Armed Forces Med J 68(6):1677–1682
Sazawal S (2009) Haematological and molecular profile of acute myelogenous leukaemia in India. Indian J Med Res 129(3):256–261
Chen, Li, Guo, Cheng, Wei (2023) Oridonin synergistically enhances the Pro-apoptotic Effect of Venetoclax on Acute myeloid leukemia cells by inhibiting AKT Signaling. Front Biosci (Landmark Ed) 28(9):195
Ebner et al (2023) ABCC1 and glutathione metabolism limit the efficacy of BCL-2 inhibitors in acute myeloid leukemia. Nat Commun 14(1):5709
Ren et al (2023) Targeting cytohesin-1 suppresses acute myeloid leukemia progression and overcomes resistance to ABT-199. Acta Pharmacol Sin
Google Scholar
Gaur et al (2023) Novel covalent CDK7 inhibitor potently induces apoptosis in acute myeloid leukemia and synergizes with Venetoclax. J Exp Clin Cancer Res 42(1):186
Hua et al (2023) Metformin sensitizes AML cells to venetoclax through endoplasmic reticulum stress-CHOP pathway. Br J Haematol 202(5):971–984
Wang et al (2023) Dexamethasone enhances venetoclax-induced apoptosis in acute myeloid leukemia cells. Med Oncol 40(7):193
Zhou et al (2023) Epigenetic silencing of ZCCHC10 by the lncRNA SNHG1 promotes progression and venetoclax resistance of acute myeloid leukemia. Int J Oncol, 62(5)
Jang et al (2023) DRP1 inhibition enhances Venetoclax-Induced mitochondrial apoptosis in TP53-Mutated Acute myeloid leukemia cells through BAX/BAK activation. Cancers (Basel), 15(3)
Zhao et al (2023) A novel Mcl-1 inhibitor synergizes with venetoclax to induce apoptosis in cancer cells. Mol Med 29(1):10
Satta et al (2023) Dual mTORC1/2 inhibition synergistically enhances AML Cell death in combination with the BCL2 antagonist Venetoclax. Clin Cancer Res 29(7):1332–1343
Zhai et al (2023) Lisaftoclax in Combination with Alrizomadlin overcomes Venetoclax Resistance in Acute myeloid leukemia and Acute Lymphoblastic Leukemia: Preclinical studies. Clin Cancer Res 29(1):183–196
Wang et al (2022) Palbociclib promotes the antitumor activity of Venetoclax plus Azacitidine against acute myeloid leukemia. Biomed Pharmacother 153:113527
Chiou W, Lee, and, Chang (2023) BCL2L1 inhibitor A-1331852 inhibits MCL1 transcription and triggers apoptosis in acute myeloid leukemia cells. Biochem Pharmacol 215:115738
Ma et al (2023) Blockade of de novo pyrimidine biosynthesis triggers autophagic degradation of oncoprotein FLT3-ITD in acute myeloid leukemia. Oncogene
Wang et al (2023) Targeting C/EBPalpha overcomes primary resistance and improves the efficacy of FLT3 inhibitors in acute myeloid leukaemia. Nat Commun 14(1):1882
Xu et al (2023) 2-Aminopyrimidine derivatives as selective dual inhibitors of JAK2 and FLT3 for the treatment of acute myeloid leukemia. Bioorg Chem 134:106442
Jiang et al (2023) Dual inhibition of CHK1/FLT3 enhances cytotoxicity and overcomes adaptive and acquired resistance in FLT3-ITD acute myeloid leukemia. Leukemia 37(3):539–549
Yang, Friedman (2023) Combination strategies to overcome drug resistance in FLT(+) acute myeloid leukaemia. Cancer Cell Int 23(1):161
Darici et al (2023) Improved efficacy of quizartinib in combination therapy with PI3K inhibition in primary FLT3-ITD AML cells. Adv Biol Regul 89:100974
Zhang W, Wang, and, Shen (2023) Sitravatinib as a potent FLT3 inhibitor can overcome gilteritinib resistance in acute myeloid leukemia. Biomark Res 11(1):8
Choi et al (2023) PLM-101 is a novel and potent FLT3/RET inhibitor with less adverse effects in the treatment of acute myeloid leukemia. Biomed Pharmacother 165:115066
Zhou et al (2023) HDAC11 mediates the ubiquitin-dependent degradation of p53 and inhibits the anti-leukemia effect of PD0166285. Med Oncol 40(11):325
Zhang et al (2023) Concomitant targeting of FLT3 and BTK overcomes FLT3 inhibitor resistance in acute myeloid leukemia through the inhibition of autophagy. Haematologica 108(6):1500–1514
Qian et al (2023) ACC010, a novel BRD4 inhibitor, synergized with homoharringtonine in acute myeloid leukemia with FLT3-ITD. Mol Oncol 17(7):1402–1418
Sun et al (2023) A selective Nano cell cycle checkpoint inhibitor overcomes Leukemia Chemoresistance. Small 19(25):e2300736
Astolfi et al (2022) From Serendipity to Rational Identification of the 5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidin-4(3H)-one Core as a New Chemotype of AKT1 Inhibitors for Acute Myeloid Leukemia. Pharmaceutics 14(11)
Shang et al (2023) Nuclear factor Nrf2 promotes glycosidase OGG1 expression by activating the AKT pathway to enhance leukemia cell resistance to cytarabine. J Biol Chem 299(1):102798
Li X, Lu C, Guan, and, Li (2022) Exosomal miR92a promotes Cytarabine Resistance in Myelodysplastic syndromes by activating Wnt/beta-catenin Signal Pathway. Biomolecules, 12(10)
Zhang et al (2023) A small molecule BCL6 inhibitor as chemosensitizers in acute myeloid leukemia. Biomed Pharmacother 166:115358
Li et al (2023) Reciprocal regulation of TWIST1 and OGT determines the decitabine efficacy in MDS/AML. Cell Commun Signal 21(1):255
Yabushita et al (2023) Mitotic perturbation is a key mechanism of action of decitabine in myeloid tumor treatment. Cell Rep 42(9):113098
Lei et al (2023) Transfer of mir-4755-5p through extracellular vesicles and particles induces decitabine resistance in recipient cells by targeting CDKN2B. Mol Carcinog 62(6):743–753
Chen et al (2024) Overcoming adaptive resistance in AML by synergistically targeting FOXO3A-GNG7-mTOR axis with FOXO3A inhibitor Gardenoside and rapamycin. Genes Dis 11(1):397–412
Liu et al (2022) Targeting STAT5 signaling overcomes resistance to IDH inhibitors in Acute myeloid leukemia through suppression of stemness. Cancer Res 82(23):4325–4339
Dai et al (2023) Targeting HDAC3 to overcome the resistance to ATRA or arsenic in acute promyelocytic leukemia through ubiquitination and degradation of PML-RARalpha. Cell Death Differ 30(5):1320–1333
Peterlin et al (2023) Tocilizumab in combination with a standard induction chemotherapy in acute myeloid leukaemia patients (TOCILAM study): a single-centre, single-arm, phase 1 trial. EClinicalMedicine 64:102254
Senapati et al (2023) A phase I study of Milademetan (DS3032b) in combination with low dose cytarabine with or without venetoclax in acute myeloid leukemia: clinical safety, efficacy, and correlative analysis. Blood Cancer J 13(1):101
Carter et al (2023) Acquired resistance to venetoclax plus azacitidine in acute myeloid leukemia: in vitro models and mechanisms. Biochem Pharmacol 216:115759
Weidenauer et al (2023) The ribosomal protein S6 kinase alpha-1 (RPS6KA1) induces resistance to venetoclax/azacitidine in acute myeloid leukemia. Leukemia 37(8):1611–1625
Romine et al (2023) BET inhibitors rescue anti-PD1 resistance by enhancing TCF7 accessibility in leukemia-derived terminally exhausted CD8(+) T cells. Leukemia 37(3):580–592
Petersdorf et al (2013) A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 121(24):4854–4860
Lowenberg et al (2009) High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 361(13):1235–1248
Burnett et al (2015) A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 125(25):3878–3885
Srinivasan S (2022) Pediatric Acute myeloid leukemia in India: a systematic review. Ind J Med Paediatr Oncol 43:342–348
Pigneux et al (2010) Addition of lomustine to idarubicin and cytarabine improves the outcome of elderly patients with de novo acute myeloid leukemia: a report from the GOELAMS. J Clin Oncol 28(18):3028–3034
Farooq M, Farooq K, Mir (2019) FLAG vs FLAG-IDA: outcomes in relapsed/refractory acute leukemias. Cancer Chemother Pharmacol 83(6):1191–1193
Meillon-Garcia, Demichelis-Gomez (2020) Access to Therapy for Acute myeloid leukemia in the developing world: barriers and solutions. Curr Oncol Rep 22(12):125
Kayal S (2019) Induction related mortality in Acute myeloid leukemia: Multivariate Model of Predictive score from the Indian Acute Leukemia Research Database (INwARD) of the Hematology Cancer Consortium (HCC). Blood 134:2615
Hafez S, Bilal H, Shalaby (2019) Early deaths in Pediatric Acute Leukemia: a major challenge in developing countries. J Pediatr Hematol Oncol 41(4):261–266
Kapoor R (2018) Hematological Cancer Consortium: Multi-center Acute myeloid leukemia Registry Data from India. Blood 132:4006
Morris et al (2013) Re-induction therapy decisions based on day 14 bone marrow biopsy in acute myeloid leukemia. Leuk Res 37(1):28–31
Terry S, Estey, and, Lim (2017) Day 14 bone marrow examination in the management of acute myeloid leukemia. Am J Hematol 92(10):1079–1084
Vakiti A (2024) Acute myeloid leukemia. StatPearls Publishing, ed. StatPearls
Tallman G, Rowe (2005) Drug therapy for acute myeloid leukemia. Blood 106(4):1154–1163
Stone et al (2017) Midostaurin plus chemotherapy for Acute myeloid leukemia with a FLT3 mutation. N Engl J Med 377(5):454–464
Wang et al (2023) Discovery of 4-(4-aminophenyl)-6-phenylisoxazolo[3,4-b]pyridine-3-amine derivatives as novel FLT3 covalent inhibitors for the intervention of acute myeloid leukemia. Drug Dev Res 84(2):296–311
Ishii, Yano (2022) New therapeutic strategies for adult Acute Myeloid Leukemia. Cancers (Basel), 14(11)
Tung S, Chiang Z, Stehr (2021) Accurate detection and quantification of FLT3 Internal Tandem duplications in Clinical Hybrid capture next-generation sequencing data. J Mol Diagn 23(10):1404–1413
Daver S, Russell, and, Levis (2019) Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33(2):299–312
Cortes et al (2019) Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 20(7):984–997
Erba et al (2023) Quizartinib plus chemotherapy in newly diagnosed patients with FLT3-internal-tandem-duplication-positive acute myeloid leukaemia (QuANTUM-First): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 401(10388):1571–1583
Perl et al (2017) Selective inhibition of FLT3 by gilteritinib in relapsed or refractory acute myeloid leukaemia: a multicentre, first-in-human, open-label, phase 1–2 study. Lancet Oncol 18(8):1061–1075
Cortes et al (2013) Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J Clin Oncol 31(29):3681–3687
Wang et al (2022) Phase 3 trial of gilteritinib plus azacitidine vs azacitidine for newly diagnosed FLT3mut + AML ineligible for intensive chemotherapy. Blood 140(17):1845–1857
Levis et al (2024) Gilteritinib as post-transplant maintenance for AML with Internal Tandem Duplication Mutation of FLT3. J Clin Oncol 42(15):1766–1775
Sasaki et al (2019) Sorafenib plus intensive chemotherapy improves survival in patients with newly diagnosed, FLT3-internal tandem duplication mutation-positive acute myeloid leukemia. Cancer 125(21):3755–3766
Perl et al (2019) Gilteritinib or Chemotherapy for relapsed or refractory FLT3-Mutated AML. N Engl J Med 381(18):1728–1740
Tiong, Wei (2019) New drugs creating new challenges in acute myeloid leukemia. Genes Chromosomes Cancer 58(12):903–914
DiNardo et al (2018) Durable remissions with Ivosidenib in IDH1-Mutated relapsed or refractory AML. N Engl J Med 378(25):2386–2398
Stein et al (2017) Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130(6):722–731
Takahashi (2023) Is induction of Hypomethylation with Ivosidenib and 5-Azacitidine curative regimen against IDH1-Mutated Acute myeloid leukemia? Anticancer Agents Med Chem 23(8):864–866
DiNardo et al (2021) Mutant isocitrate dehydrogenase 1 inhibitor Ivosidenib in Combination with azacitidine for newly diagnosed Acute myeloid leukemia. J Clin Oncol 39(1):57–65
Fruchtman A, Waksal B, Mascarenhas (2024) Management of isocitrate dehydrogenase 1/2 mutated acute myeloid leukemia. Leukemia 38(5):927–935
Issa, DiNardo (2021) Acute myeloid leukemia with IDH1 and IDH2 mutations: 2021 treatment algorithm. Blood Cancer J 11(6):107
Roboz et al (2020) Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 135(7):463–471
Fathi et al (2018) Differentiation syndrome Associated with Enasidenib, a selective inhibitor of mutant isocitrate dehydrogenase 2: analysis of a phase 1/2 study. JAMA Oncol 4(8):1106–1110
Konopleva et al (2016) Efficacy and Biological correlates of response in a phase II study of Venetoclax Monotherapy in patients with Acute Myelogenous Leukemia. Cancer Discov 6(10):1106–1117
Othman T, Moskoff A, Jonas (2021) An evaluation of venetoclax in combination with azacitidine, decitabine, or low-dose cytarabine as therapy for acute myeloid leukemia. Expert Rev Hematol 14(5):407–417
Garciaz et al (2022) Azacitidine Plus Venetoclax for the treatment of relapsed and newly diagnosed Acute myeloid leukemia patients. Cancers (Basel), 14(8)
Wei et al (2022) Long-term follow-up of VIALE-C in patients with untreated AML ineligible for intensive chemotherapy. Blood 140(25):2754–2756
DiNardo et al (2020) 10-day decitabine with venetoclax for newly diagnosed intensive chemotherapy ineligible, and relapsed or refractory acute myeloid leukaemia: a single-centre, phase 2 trial. Lancet Haematol 7(10):e724–e736
Wolach O (2021) Real world prospective observational Multicenter Trial of Venetoclax-based therapy for patients with AML Reveals Unique Patterns of Patient Selection and Treatment Utilization - revive study. Blood, 138(1)
Burnett, Stone (2020) AML: New drugs but New challenges. Clin Lymphoma Myeloma Leuk 20(6):341–350
Abaza MM, Garcia (2024) Advancements and challenges in the treatment of AML. Am Soc Clin Oncol Educ Book 44(3):e438662
Juarez-Salcedo (2019) Desai, and Dalia, Venetoclax: evidence to date and clinical potential . Drugs Context 8:212574
Feldman et al (2011) First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol 29(8):979–985
Lancet et al (2021) CPX-351 versus 7 + 3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol 8(7):e481–e491
Chiche et al (2021) Real-life experience with CPX-351 and impact on the outcome of high-risk AML patients: a multicentric French cohort. Blood Adv 5(1):176–184
Guolo F (2023) Optimal duration of CPX-351 treatment and best timing for consolidation with allogeneic stem cell transplantation: evidence from a large real-world Italian study. Blood 142(1):731
Daver W (2020) Pollyea, Fathi, Vyas, and DiNardo, New directions for emerging therapies in acute myeloid leukemia: the next chapter . Blood Cancer J 10(10):107
Wei et al (2020) Oral azacitidine maintenance therapy for Acute myeloid leukemia in First Remission. N Engl J Med 383(26):2526–2537
Huls et al (2019) Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood 133(13):1457–1464
Yang H, Sanchez-Gonzalez K, Garcia-Manero (2005) Antileukemia activity of the combination of 5-aza-2’-deoxycytidine with valproic acid. Leuk Res 29(7):739–748
Moreno V, Badar (2022) Clinical utility of Azacitidine in the management of Acute myeloid leukemia: update on patient selection and reported outcomes. Cancer Manag Res 14:3527–3538
de Lima et al (2018) CC-486 maintenance after stem cell transplantation in patients with Acute myeloid leukemia or myelodysplastic syndromes. Biol Blood Marrow Transpl 24(10):2017–2024
Kittai et al (2021) NEDD8-activating enzyme inhibition induces cell cycle arrest and anaphase catastrophe in malignant T-cells. Oncotarget 12(20):2068–2074
Swords et al (2015) Pevonedistat (MLN4924), a first-in-class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br J Haematol 169(4):534–543
Neeraj, Yadav (2020) STUDY OF SERUM BETA CATENIN LEVELS IN ACUTE MYELOID LEUKEMIA (AML) PATIENTS. Int J Adv Res (IJAR) 8(3):188–194
Cluzeau et al (2021) Eprenetapopt Plus Azacitidine in TP53-Mutated myelodysplastic syndromes and Acute myeloid leukemia: a phase II study by the Groupe Francophone Des Myelodysplasies (GFM). J Clin Oncol 39(14):1575–1583
Oka et al (2004) Induction of WT1 (Wilms’ tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proc Natl Acad Sci U S A 101(38):13885–13890
Dohner et al (2022) Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 140(12):1345–1377
Bhattacharya (2022) Acute myeloid leukaemia – newer drugs in the pipelines: Acute myeloid leukaemia – newer drugs in the pipelines. Annals Trop Med Public Health 1(1):1–3
Zeidan et al (2020) A phase ib study of Onvansertib, a novel oral PLK1 inhibitor, in combination therapy for patients with relapsed or refractory Acute Myeloid Leukemia. Clin Cancer Res 26(23):6132–6140
Morales et al (2023) Posttranslational splicing modifications as a key mechanism in cytarabine resistance in acute myeloid leukemia. Leukemia 37(8):1649–1659
Li et al (2022) Jiyuan oridonin A induces differentiation of acute myeloid leukemia cells including leukemic stem-like cells. Front Pharmacol 13:1001552
Erba HP (2022) Update on a phase 1/2 first-in-human study of the Menin-KMT2A (MLL) inhibitor ziftomenib (KO-539) in patients with relapsed or refractory Acute Myeloid Leukemia. Blood 140:153–156
Uy et al (2021) Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 137(6):751–762
Barrett, Blanc L (2010) Immunotherapy prospects for acute myeloid leukaemia. Clin Exp Immunol 161(2):223–232
Pawlitzky I (2022) Trial in Progress: a phase I/II study to assess Safety, Tolerability and preliminary efficacy of Bexmarilimab in Combination with Standard of Care in patients with hematological malignancies (BEXMAB). Blood 140:11751–11752
Dohner et al (2021) Adjunctive volasertib in patients with Acute myeloid leukemia not eligible for standard induction therapy: a Randomized, Phase 3 Trial. Hemasphere 5(8):e617
Mehta P (2015) High dose (18 g/m2) versus low dose (12 g/m2) cytosine arabinoside as consolidation for acute myeloid leukemia: a phase 3 study: an interim analysis. Ann Oncol 26(1):85
Zhou, Chng (2014) Identification and targeting leukemia stem cells: the path to the cure for acute myeloid leukemia. World J Stem Cells 6(4):473–484
Chauhan et al (2022) Real-world challenges in the management of acute myeloid leukemia: a single-center experience from North India. Ann Hematol 101(6):1261–1273
Kulkarni, George (2019) Access to hematopoietic stem-cell transplantation in India. J Postgrad Med 65(1):1–4
Philip et al (2015) Acute myeloid leukaemia: challenges and real world data from India. Br J Haematol 170(1):110–117
Saikia (2020) Challenges in managing acute leukemia in India. Cancer Res Stat Treat 3:645–646
John K, Smith R, Tauro (2021) The long shadow of socioeconomic deprivation over the modern management of acute myeloid leukemia: time to unravel the challenges. Blood Cancer J 11(8):141
Sudha, Sinha (2019) Treatment adherence and abandonment in acute myeloid leukemia in pediatric patients at a low-resource cancer center in India. Indian J Med Pediatr Oncol 40(4):501–506
Singh S (2022) Real World Data on Unique challenges and outcomes of older patients with AML from Resource Limited settings: Indian Acute Leukemia Research Database (INwARD) of the Hematology Cancer Consortium (HCC). Blood 140:6096–6098
Heuser et al (2021) Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood 138(26):2753–2767
Download references
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and affiliations.
Research and Development Cell, Parul Institute of Applied Sciences, Parul University, Vadodara, 391760, India
Suresh Kumar Prajapati & Reeshu Gupta
Parul Institute of Applied Sciences, Parul University, Vadodara, 380060, India
Neha Kumari, Doulat Bhowmik & Reeshu Gupta
You can also search for this author in PubMed Google Scholar
Contributions
SKP. study concept, wrote the main manuscript. RG. Editing and review, supervision. NK and DB. Prepared tables.
Corresponding author
Correspondence to Reeshu Gupta .
Ethics declarations
Ethical approval.
Ethics approval is not applicable.
Consent to participate
No original data from new patients were collected, consent to participate is not applicable.
Consent for publication
Consent for publication is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Prajapati, S.K., Kumari, N., Bhowmik, D. et al. Recent advancements in biomarkers, therapeutics, and associated challenges in acute myeloid leukemia. Ann Hematol (2024). https://doi.org/10.1007/s00277-024-05963-x
Download citation
Received : 22 June 2024
Accepted : 19 August 2024
Published : 29 August 2024
DOI : https://doi.org/10.1007/s00277-024-05963-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Acute myeloid leukemia
- Leukemia stem cells
- Therapeutic resistance
- Clinical trials
- Find a journal
- Publish with us
- Track your research
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Adithya Chennamadhavuni ; Varun Lyengar ; Shiva Kumar R. Mukkamalla ; Alex Shimanovsky .
Affiliations
Last Update: January 17, 2023 .
- Continuing Education Activity
Leukemia is a heterogeneous group of hematologic malignancies that arise from the dysfunctional proliferation of developing leukocytes. It is classified as either acute or chronic based on the rapidity of proliferation and as myelocytic or lymphocytic based on the cell of origin. Treatment depends on the type of leukemia but generally involves chemotherapy. Multiple genetic and environmental risk factors are identified in the development of leukemia. According to the Surveillance, Epidemiology, and End Results (SEER) database, there are 61,090 estimated new cases of leukemia in 2021, accounting for 3.2% of all new cancer cases, making leukemia the 10th most common cancer in the United States. This activity describes the evaluation and management of leukemia and reviews the role of the interprofessional team in improving care for patients with this condition.
- Identify the epidemiology of leukemia.
- Review the appropriate evaluation of leukemia.
- Outline the management options available for leukemia.
- Describe interprofessional team strategies for improving care coordination and communication when treating patients with leukemia.
- Introduction
The production of abnormal leukocytes defines leukemia as either a primary or secondary process. They can be classified as acute or chronic based on the rapidity of proliferation and myeloid or lymphoid based on the cell of origin. Predominant subtypes are acute myeloid leukemia (AML) and chronic myeloid leukemia (CML), involving the myeloid lineage; acute lymphoblastic leukemia (ALL); and chronic lymphocytic leukemia (CLL), involving the lymphoid chain. Other less common variants, such as mature B-cell and T-cell leukemias, and NK cell-related leukemias, to name a few, arise from mature white blood cells. However, with the advent of next-generation sequencing (NGS) and the identification of various biomarkers, the World Health Organization (WHO) classification was updated in 2016, bringing multiple changes to the traditional classification for acute leukemias and myeloid neoplasms. [1] GLOBOCAN, a global observatory for cancer trends, showed a global incidence of 474,519 cases, with 67,784 in North America. The Age-Standardized Rates are around 11 per 100,000, with a mortality rate of approximately 3.2. [2]
Many genetic risk factors have been identified, such as Klinefelter and Down syndromes, ataxia telangiectasia, Bloom syndrome, and telomeropathies such as Fanconi anemia, dyskeratosis congenita, and Shwachman-Diamond syndrome; germline mutations in RUNX1, CEBPA, to name a few. Viral infections associated with Epstein Barr virus, human T-lymphotropic virus, ionizing radiation exposure, radiation therapy, environmental exposure with benzene, smoking history, history of chemotherapy with alkylating agents, and topoisomerase II agents have also been implicated in the development of acute leukemias. Symptoms are nonspecific and can include fever, fatigue, weight loss, bone pain, bruising, or bleeding. Definitive diagnoses often require bone marrow biopsy, the results of which help the hematologists and stem cell transplant physicians regarding the selection of treatment options ranging from chemotherapy to allogeneic stem cell transplantation. The prognosis is variable depending on the leukemia subtype in question.
Acute vs. chronic myeloid leukemia: Blasts, which are immature and dysfunctional cells, normally make up 1% to 5% of marrow cells. Acute leukemias are characterized by greater than 20% blasts in the peripheral blood smear or on bone marrow leading to a more rapid onset of symptoms. In contrast, chronic leukemia has less than 20% blasts with a relatively chronic onset of symptoms. The accelerated/blast phase is a transformation of chronic myeloid leukemia into an acute phase with a significantly higher degree of blasts. [1] [3] [4]
As such, the four major subtypes of leukemia are:
- Acute lymphoblastic leukemia (ALL): ALL is seen in patients with the blastic transformation of B and T cells. It is the most common leukemia in the pediatric population, accounting for up to 80% of cases in this group vs. 20% of cases in adults. Treatment among adolescents and young adults is predominantly inspired by pediatric regimens with better survival rates.
- Acute myelogenous leukemia (AML): AML is characterized by greater than 20% myeloid blasts and is the most common acute leukemia in adults. It is the most aggressive cancer with a variable prognosis depending upon the molecular subtypes.
- Chronic lymphocytic leukemia (CLL): CLL occurs from the proliferation of monoclonal lymphoid cells. Most cases occur in people between the ages of 60 and 70. CLL is considered an indolent disease, for the most part, meaning not all patients with a diagnosis will need to start treatment until symptomatic from the disease.
- Chronic myelogenous leukemia (CML): CML typically arises from reciprocal translocation and fusion of BCR on chromosome 22 and ABL1 on chromosome 9, resulting in dysregulated tyrosine kinase on chromosome 22 called the Philadelphia (Ph) chromosome. This, in turn, causes a monoclonal population of dysfunctional granulocytes, predominantly neutrophils, basophils, and eosinophils. [1] [3] [5] [6]
Multiple genetic and environmental risk factors are identified in the development of leukemia.
- Exposure to ionizing radiation is associated with an increased risk of multiple leukemia subtypes. [7] [8]
- Exposure to benzene is a risk factor for leukemia in adults, particularly AML. [9]
- Previous exposure to chemotherapy, especially alkylating agents and topoisomerase II inhibitors, increases the risk for acute leukemia later in life. [7] [8]
- A history of any hematologic malignancy is a risk factor for subsequently developing another subtype of leukemia. [10]
- Viral infections (e.g., human T-cell leukemia virus, Epstein Barr virus) are linked with subtypes of ALL. [11]
- Several genetic syndromes (e.g., Down syndrome, Fanconi anemia, Bloom syndrome, Li-Fraumeni syndrome) are associated with an increased risk of AML and ALL. [12]
- Epidemiology
GLOBOCAN, which is a global observatory for cancer trends, showed a global incidence of 474,519 cases, with 67,784 in North America. The age-standardized rates are around 11 per 100,000, with a mortality rate of about 3.2. ALL and AML, which are important diseases in both childhood and adulthood, have bimodal age distributions, with CML and CLL mostly in the older age groups. According to the Surveillance, Epidemiology, and End Results (SEER) database, there are 61,090 estimated new cases of leukemia in 2021, accounting for 3.2% of all new cancer cases, making leukemia the 10th most common cancer in the United States. Estimated deaths are about 23,660, which comprises 3.9% of all cancer deaths. Since 2006, the incidence of the disease has increased by an average of 0.6% per year, while the mortality has decreased by an annual average of 1.5%. [6] [7]
- Pathophysiology
Leukemia occurs due to the malignant transformation of pluripotent (i.e., it can give rise to both myeloid and lymphoid precursors) hematopoietic stem cells. Rarely, it can also involve a more committed stem cell with limited self-renewal capacity. In acute leukemias, these malignant cells are generally immature, poorly differentiated, abnormal leukocytes (blasts) that can either be lymphoblasts or myeloblasts. These blasts can undergo clonal expansion and proliferation, leading to replacement and interference with the development and function of normal blood cells, leading to clinical symptoms.
Acute Leukemia
In ALL, chromosomal translocation or abnormal chromosome numbers can lead to mutations in precursor lymphoid cells leading to lymphoblasts. Common mutations include t(12;21) and t(9;22). In AML, chromosomal translocations, rearrangements, and gain or loss of chromosomes can lead to mutations and abnormal production of myeloblasts. One important translocation is t(15;17), which leads to the fusion of retinoic acid receptor alpha (RARA) and a promyelocytic leukemia transcription factor (PML). This leads to the development of acute promyelocytic leukemia, which can present with hallmarks of disseminated intravascular coagulation and need emergent treatment with all-trans retinoic acid.
Chronic Leukemia
Chromosomal abnormalities in hematopoietic stem cells that are precursors to leucocytes are the most common cause of chronic leukemia. Examples of abnormalities are deletions, translocations, or extra chromosomes. In CML, mutations mainly affect granulocytes (most commonly the t(9;22) translocation), and in CLL, they primarily affect lymphocytes (especially B lymphocytes). Unlike acute leukemias, in chronic leukemias, cells are partially mature. These partially mature cells do not function effectively and divide too quickly. They accumulate in the peripheral blood and lymphoid organs, which can lead to anemia and thrombocytopenia, and leukopenia.
- Histopathology
In acute leukemia, the peripheral blood or bone marrow is characterized by more than 20% blasts. However, regardless of the blast percentage, patients with t(8;21)(q22;q22), RUNX1-RUNX1T1, inv(16)(p13.1q22) or t(16;16)(p13.1;q22), CBFB-MYH11 or t(15;17)(q24.1;q21.1), PML-RARA, are considered and treated as acute leukemia. There is usually increased cellularity noted on bone marrow biopsy that is packed with blasts and a variable number of granulocytic or monocytic cells and erythroid precursors. Traditional markers included in the evaluation are CD7, CD11b, CD13, CD14, CD15, CD16, CD33, CD34, CD45, CD56, CD117, HLA-DR. Also, either peripheral smear or bone marrow aspirate is sent for a mutation panel of multiple genes with therapeutic and prognostic implications, such as ASXL1, CEBPA, DNMT3A, FLT3, IDH1, IDH2, NPM1, RUNX1, and TP53, to mention a few.
There is also increased bone marrow cellularity in ALL, composed of B and T lymphoblasts (with small nucleoli, dispersed chromatin, cleaved and irregular nuclei with undetectable cytoplasm). Common T-cell lymphoid immunostains include TdT, CD2, CD3, CD5, and CD7. Common B-cell lymphoid immunostains include HLA-DR, CD10, CD19, CD22, CD79a, PAX5, and CD20. There should not be any myeloid markers, such as myeloperoxidase (MPO), to confirm the diagnosis of the pure lymphoid lineage. Mixed phenotype acute leukemia (MPAL) has both myeloid and lymphoid markers but is a rare entity. Cytogenetics evaluation for Ph chromosome status and Ph-like translocation is a must as newer therapeutic agents are now incorporated into treatment algorithms. [13]
The white blood cell count in chronic leukemia is often elevated, with a smear suggestive of significant left shift/granulocyte predominance. Such a picture is commonly seen during the acute illness phase, but if such a picture persists upon repeat labs, CML should be evaluated. In CML, the translocation t(9;22) can be diagnosed by fluorescence in-situ hybridization (FISH) on peripheral blood. Bone marrow biopsy is not necessary for diagnosis, but if done, it will usually show 100% cellular marrow with increased granulocyte precursors, basophils, eosinophils, and occasional monocytes.
In CLL, the white cell count is elevated, with mostly CD5+ and CD23+ B-lymphocytes. The clonal lymphocyte population has to be greater than 5,000/mcL for diagnosis. If the clonal lymphocyte population is less than 5,000/mcL, the entity is termed monoclonal B cell lymphocytosis of undetermined significance. Flow cytometry is often diagnostic. Patients would need evaluation for del(17p) and TP53 mutation status, immunoglobulin heavy chain variable region (IGHV) gene mutation status, del(11q), del(13q), and trisomy 12 evaluation, which can help in selecting appropriate treatment regimens. [14]
- History and Physical
Acute leukemia tends to present non-specifically, although the most common presenting features include fever, lethargy, and bleeding. Hepatosplenomegaly, lymphadenopathy, and musculoskeletal symptoms (especially involving the spine and long bones) can also be clues to the diagnosis. Adults may also have more prominent anemia-related symptoms, such as shortness of breath, or symptoms related to thrombocytopenia, such as excessive bruising or increased bleeding tendency. Patients with acute promyelocytic leukemia (APL), which is associated with disseminated intravascular coagulation-type symptoms, can present with mucosal bleeding, including gum bleeds, nosebleeds, or menorrhagia.
Chronic leukemia subtypes occur almost exclusively in adults. Many patients are asymptomatic at the time of diagnosis, identified only incidentally after marked leukocytosis is discovered on a complete blood count (CBC) performed for another reason. Hepatosplenomegaly and lymphadenopathy can be appreciated in some cases, while bleeding and bruising are less common, presenting features relative to acute leukemia subtypes. [15]
The workup of leukemia is very involved, and multiple tests are needed to confirm a diagnosis and, subsequently, to stage the disease. Helpful initial studies include a CBC, comprehensive metabolic panel, liver function tests (LFT), and coagulation panel, which are often followed by a peripheral blood smear evaluation and a bone marrow biopsy and aspiration.
On rare occasions, leukemia can be diagnosed on histology alone. For example, AML is characterized by the presence of Auer rods (red-staining, needle-like bodies seen in the cytoplasm of myeloblasts) on a peripheral smear. In most other cases, more detailed analyses with flow cytometry, cytogenetics, and FISH testing are required to distinguish between subtypes. [16]
A bone marrow aspiration and biopsy are often required for the diagnosis of acute leukemias. For chronic leukemias, peripheral blood evaluation is often enough, and an invasive bone marrow biopsy may not be needed. For example, CML can be diagnosed by looking for BCR-ABL fusion protein on peripheral blood FISH analysis. CLL can be diagnosed by looking for a monoclonal B-cell population through peripheral blood flow cytometry.
- Treatment / Management
Patients with leukemia should be referred to a hematologist-oncologist to initiate treatment. Therapy varies significantly based on the leukemia subtype and patient factors (e.g., age, comorbid conditions). Acute leukemias are treated predominantly as an in-patient needing significant support, frequent monitoring of vitals, and assessment for opportunistic infections and electrolyte imbalances. The predominant challenge at the time of diagnosis of acute myeloid leukemia is to identify the possibility of APL, which has a significantly different treatment compared to the rest of AML.
APL: APL patients typically present with bleeding diathesis with increased coagulation parameters (elevated PT, aPTT) and low fibrinogen. Peripheral smear shows a predominance of myeloid blasts with Auer rods. It is important to start the treatment with ATRA (all-trans-retinoic acid) when APL is suspected rather than awaiting confirmatory tests with FISH. ATRA advances arrested promyeloblasts into becoming mature granulocytes which can result in differentiation syndrome. [17] Differentiation syndrome is seen during 48 hours of ATRA initiation to even three weeks from starting therapy for APL. Patients have a fever, respiratory distress with acute pulmonary infiltration on imaging, and capillary leak resulting in edema. It can mimic sepsis, resulting in delaying the treatment with dexamethasone. The commonly accepted starting dosage is 10mg every 12 hours till improvement in symptoms and counts. [18] Other significant complication with ATRA includes raised intracranial pressure leading to headaches and significant vision changes from papilledema.
Specific treatment for APL depends on whether the patient is at low or intermediate risk (also known as standard risk) with a WBC count <10,000/ mcL or high risk with a WBC count >10,000/ mcL. Low or intermediate-risk APL is further differentiated by platelets above or below 40,000/mcL.
- Standard-risk APL: Patients respond well to ATRA and arsenic trioxide (ATO) with lesser complications during induction and recovery without needing an allogeneic stem cell transplant (SCT). During the utilization of ATO, patients need to be monitored for electrolyte changes closely and electrocardiogram for QTc prolongation changes(Framingham formula). [19]
- High-risk APL: Along with ATRA + ATO, high-risk patients achieve better responses with the addition of idarubicin. [20] Recent studies have included CD33-targeted drug conjugate, gemtuzumab ozogamicin (GO), during the induction therapy combined with ATRA + ATO. [21]
APL patients have better overall survival and prognosis than other types of AML without needing a transplant.
AML: Standard therapy for AML is well known as the '7+3' regimen, which includes a 7-day course of cytarabine continuous infusion with a 3-day course of an anthracycline (either daunorubicin or idarubicin). With the advent of cytogenetics and NGS testing, patients are now being risk-stratified based on the molecular markers resulting in prognostic and therapeutic implications. [22] The outline of therapy based on the risk status per ELN (European LeukemiaNet) is as follows
Standard 7+3 regimen with/without GO.[23] This chemo regimen can be attempted in patients > 60 years old if they have good tolerability as determined by performance status.
ALL is divided into B or T lymphocyte variants based on the lymphoblast origin and the presence of >20% lymphoblasts in peripheral smear or BM. The presence or absence of the Ph chromosome is the most important molecular marker leading to therapeutic implications in treating ALL.
Combination of chemotherapy with oral tyrosine kinase inhibitor (TKI) favorably 2nd generation and beyond such as dasatinib, ponatinib, bosutinib, nilotinib, and imatinib. Not all the TKI combinations have data with chemotherapy agents, and the availability (more...)
The overall outcome depends upon the patient's response to induction therapy and the presence or absence of MRD (minimal residual disease) needing further therapies and BMT.
CML: CML is one of the first cancers revolutionized by utilizing targeted therapy with Ph chromosome targeting TKIs. Patients have a significant response to TKIs, negating the need for acute chemotherapy unless they are in an accelerated phase/blast crisis. A patient's risk can be assessed based on multiple available calculators such as the Sokal score, EUTOS Score, and EUTOS long-term survival score (ELTS). [36] [37] [38] For patients having high-risk disease, second-generation (nilotinib, dasatinib, and bosutinib) TKIs are utilized as first-line therapy to achieve the therapy milestones faster with deeper responses. [39] For low and intermediate-risk patients, imatinib can be initiated as first-line therapy. However, there is no significant difference in overall survival based on the generation of the TKI used.
Major milestones after initiation of TKI include:
- At 3 months: BCR-ABL1 [International Scale (IS)] at ≤10 percent and/or ≤35% Ph-positive metaphase cells
- At 6 months: BCR-ABL1 (IS) at ≤1 percent or/and 0 % Ph-positive metaphase cells
- At 1 year : BCR-ABL1 (IS) ≤0.1 percent
Patients need to be monitored for resistance mutations, predominantly T315I mutation, for which ponatinib, asciminib, and omacetaxine are approved. [40] [41] Patients might continue to develop resistance to multiple TKIs for whom SCT can be attempted.
CLL: CLL runs its course in a more indolent fashion than all the other leukemic subtypes, with the patient's lifespan minimally impacted by the disease. Patients do not benefit from early treatment unless they meet the criteria for therapy. Patients with a rapid doubling time of lymphocytes, worsening cytopenias, increasing spleen size causing abdominal discomfort, and significant B symptoms (fatigue, night sweats, and weight loss) benefit from treatment. The most important determinant in treating CLL is knowing the IGVH mutation status and the presence of del17p and TP53 mutation. t(11:14) is often obtained to rule out mantle cell lymphoma.
For patients with IGVH mutation who have a relatively good prognosis, chemotherapy with FCR (fludarabine, cyclophosphamide, rituximab) [42] or BR (bendamustine, rituximab) [43] can be attempted as patients would be able to achieve prolonged disease-free survival for over ten years. For high-risk patients with del17p / TP53 mutation, patients benefit significantly from targeted therapy with venetoclax (BCL-2 inhibitor) or Bruton's tyrosine kinase (BTK) inhibitors (ibrutinib, acalabrutinib), either as a single agent or in combination with rituximab or obinutuzumab. [44] [45] [46] [47] Older patients with comorbidities tolerate BTK inhibitors better.
Rarely do patients with CLL/SLL who have a dormant course present with acute aggressive lymphadenopathy. They need an urgent lymph node or bone marrow biopsy to rule out Richter transformation into aggressive diffuse large B cell lymphoma and rarely Hodgkin lymphoma or T cell lymphomas.
- Differential Diagnosis
The differential diagnosis is broad because leukemia is a broad diagnosis with non-specific symptoms. One must rule out infection, drug effects, vitamin/micronutrient deficiencies, and other myelodysplastic disorders that can cause abnormalities in blood cell lines.
Consider the following when seeing abnormalities in the blood count:
- B12 and folate deficiencies
- Copper deficiencies
- Viral infections (e.g., HIV, cytomegalovirus, Epstein-Barr virus)
- Drugs (chemotherapeutic agents, valproic acid, ganciclovir, mycophenolate mofetil)
- Autoimmune conditions (e.g., systemic lupus erythematosus)
Long-term survival with leukemia varies tremendously based on leukemia subtype, cytogenetic and molecular findings, patient age, and comorbid conditions. Broadly, leukemia's 5-year cancer survival rate increased from 33% in 1975 to 59% in 2005. [6]
- Complications
Tumor Lysis Syndrome (TLS)
TLS is a complication of chemotherapy that can result when tumor cells die quickly. The widespread cellular destruction releases intracellular contents into the bloodstream overwhelming the kidneys and resulting in dangerously high serum levels of potassium, phosphorus, and uric acid. [48] Patients need aggressive hydration, frequent lab monitoring, and management of hyperuricemia with allopurinol and rasburicase. Hyperkalemia and hypocalcemia can lead to significant cardiac toxicity requiring urgent correction.
Disseminated Intravascular Coagulation (DIC)
DIC is a complication of leukemia itself in which the proteins that control the blood clotting process become dysfunctional, leading to both thrombosis and hemorrhage. DIC is often associated with acute promyelocytic leukemia but can be seen in other subtypes of leukemia as well. [16] . Frequent lab monitoring with active replacement of fibrinogen with cryoprecipitate is vital to the patient's survival.
Immunosuppression from chemotherapy, stem cell transplantation, or leukemia itself increases the risk of dangerous infections. Fever with neutropenia in an immunosuppressed patient should prompt an immediate evaluation for infection source and the initiation of broad-spectrum antibiotic therapy. [49]
Survivors of leukemia are at an increased risk of subsequent cancers. For example, the Childhood Cancer Survivor Study demonstrated that the 30-year cumulative incidence of any cancer after leukemia was 5.6%; the median time to occurrence of the subsequent cancer was nine years. The most common second neoplasms in childhood leukemia survivors are different subtypes of leukemia or lymphoma. [10]
- Deterrence and Patient Education
Leukemia is the production of abnormal white blood cells from bone marrow and lymphatic tissues. Excess production of such white blood cells affects the production of normal blood cells, which are essential to fight infections, carry oxygen, and help clot blood. Such abnormal cell production can be fast, making it acute leukemia or a relatively slower process leading to chronic leukemia. Common symptoms include recurrent infections, weight loss, fatigue, fevers, abdominal pain, and bleeding. Multiple types of leukemias are present, and they require evaluation by a hematologist for further guidance on treatment.
- Enhancing Healthcare Team Outcomes
Acute and chronic leukemias are heterogeneous hematologic diseases with complex diagnostic and therapeutic implications requiring an interprofessional healthcare team. The involvement of healthcare professionals from across specialties and disciplines - clinicians, nurses, specialists (especially hematologists and oncologists), nurses, pharmacists, nutritionists, etc. - is needed to achieve effective management, mitigate adverse events, and ensure their quality of life.
The patient's initial encounter will often be with their family clinician, who runs initial bloodwork and other tests, but specialist input is an absolute necessity if there are any indications that leukemia is the diagnosis. Specialists will primarily guide the therapy regimen, but nursing will play a pivotal role in assisting in the evaluation, coordinating activities between specialists, and providing patient counseling. A specialized oncology pharmacist is a valuable asset to the team. Their consultation can help guide chemotherapy, appropriate dosing medication reconciliation, and medication counseling for patients, especially regarding adverse events. All interprofessional team members must maintain meticulous records on all interactions and interventions with the patient; this is part of communicating all patient data to the rest of the team. Everyone involved in care must keep other team members informed of any changes in the patient's condition as appropriate and include the patient in all care decisions, answering questions and offering counsel. Patient-centered communication and shared decision-making are integral to successful patient outcomes in the interprofessional team model. [Level 5]
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Blast crisis, Leukemia, CML Contributed by Centers of Disease Control and Prevention (Public Domain)
Hairy cell leukemia Image courtesy S Bhimji MD
Chronic Myeloid Leukemia. Micrograph of a bone marrow biopsy of chronic myeloid leukemia showing hypercellularity and expansion of immature granulocytic paratrabecular cuff. Contributed by R Eden, DO
Image of leukemia cutis & Immunophenotyping of leukemia cutis using CD markers Contributed by Shabir Bhimji, MD
Simplified hematopoiesis Contributed By A. Rad and M. Häggström. (CC-BY-SA 3.0 license https://creativecommons.org/licenses/by-sa/3.0/deed.en_US)
Disclosure: Adithya Chennamadhavuni declares no relevant financial relationships with ineligible companies.
Disclosure: Varun Lyengar declares no relevant financial relationships with ineligible companies.
Disclosure: Shiva Kumar Mukkamalla declares no relevant financial relationships with ineligible companies.
Disclosure: Alex Shimanovsky declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Chennamadhavuni A, Lyengar V, Mukkamalla SKR, et al. Leukemia. [Updated 2023 Jan 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- Review Targeted chronic myeloid leukemia therapy: seeking a cure. [J Manag Care Pharm. 2007] Review Targeted chronic myeloid leukemia therapy: seeking a cure. Fausel C. J Manag Care Pharm. 2007 Oct; 13(8 Suppl A):8-12.
- The receptor tyrosine kinase p185HER2 is expressed on a subset of B-lymphoid blasts from patients with acute lymphoblastic leukemia and chronic myelogenous leukemia. [Blood. 1995] The receptor tyrosine kinase p185HER2 is expressed on a subset of B-lymphoid blasts from patients with acute lymphoblastic leukemia and chronic myelogenous leukemia. Bühring HJ, Sures I, Jallal B, Weiss FU, Busch FW, Ludwig WD, Handgretinger R, Waller HD, Ullrich A. Blood. 1995 Sep 1; 86(5):1916-23.
- Physician Education: Myelodysplastic Syndrome. [Oncologist. 1996] Physician Education: Myelodysplastic Syndrome. Yoshida Y. Oncologist. 1996; 1(4):284-287.
- Erythroid variant evolving from chronic myeloid leukemia resistant to multiple tyrosine kinase inhibitors: a rare case report. [Diagn Pathol. 2024] Erythroid variant evolving from chronic myeloid leukemia resistant to multiple tyrosine kinase inhibitors: a rare case report. Lee MH, Song A, Li JY. Diagn Pathol. 2024 Jan 24; 19(1):21. Epub 2024 Jan 24.
- Review Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. [Drugs. 2003] Review Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Plosker GL, Figgitt DP. Drugs. 2003; 63(8):803-43.
Recent Activity
- Leukemia - StatPearls Leukemia - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

IMAGES
VIDEO
COMMENTS
OS at 2-years was 78%. 64 Similarly, The Group for Research on Adult Acute Lymphoblastic Leukemia (GRAAL), compared 225 patients up to the age of 60 who were treated on pediatric-inspired regimen and historical data from 712 adults treated on standard adult regimen LALA-93. 36 They observed a significant improvement in CR, EFS and OS, which was ...
Abstract. Acute lymphoblastic leukaemia develops in both children and adults, with a peak incidence between 1 year and 4 years. Most acute lymphoblastic leukaemia arises in healthy individuals, and predisposing factors such as inherited genetic susceptibility or environmental exposure have been identified in only a few patients.
Introduction. Acute lymphoblastic leukaemia (ALL) is the most common childhood malignancy, a leading cause of death from disease in children and teens (<19 years), and an important cause of cancer ...
Acute lymphoblastic leukaemia develops in both children and adults, with a peak incidence between 1 year and 4 years. Most acute lymphoblastic leukaemia arises in healthy individuals, and predisposing factors such as inherited genetic susceptibility or environmental exposure have been identified in only a few patients. It is characterised by chromosomal abnormalities and genetic alterations ...
Acute lymphoblastic leukaemia (ALL) is the most common childhood malignancy, a leading cause of death from disease in children and teens (<19 years), and an important cause of cancer-related death in
Abstract. Acute lymphoblastic leukaemia (ALL) is a haematological malignancy characterized by the uncontrolled proliferation of immature lymphoid cells. Over past decades, significant progress has been made in understanding the biology of ALL, resulting in remarkable improvements in its diagnosis, treatment and monitoring.
Acute lymphoblastic leukemia (ALL) is characterized by chromosomal translocations and somatic mutations that lead to leukemogenesis. The incorporation of pediatric-type regimens has improved survival in young adults, and the incorporation of tyrosine kinase inhibitors for patients with Philadelphia chromosome-positive disease has led to further improvements in outcomes. However, older ...
Overview. Acute lymphoblastic lymphoma (ALL) is a heterogeneous hematologic disease characterized by the proliferation of immature lymphoid cells in the bone marrow, peripheral blood, and other organs. 1 The age-adjusted incidence rate of ALL in the United States is 1.8 per 100,000 individuals per year, 2 with approximately 5,690 new cases and 1,580 deaths estimated in 2021. 3 The median age ...
Progress in the research and therapy of adult acute lymphoblastic leukemia (ALL) is accelerating. This analysis summarizes the data derived from the clinical trials conducted at MD Anderson between 1985 and 2022 across ALL subtypes. In Philadelphia chromosome-positive ALL, the addition of BCR::ABL1 tyrosine kinase inhibitors (TKIs) to intensive chemotherapy since 2000, improved outcomes. More ...
Introduction. Acute lymphoblastic leukemia (ALL) is a hematologic malignancy arising from precursors of the lymphoid lineage. It has a bimodal distribution, with the first peak occurring at ~5 years of age (80% of cases) and the second peak occurring around the age of 50 (20% of cases). 1 In adults, precursor B-cell ALL (B-ALL) accounts for ~75% of cases and precursor T-cell ALL (T-ALL ...
We studied 400 adult patients with ALL treated at the University of Chicago, Moffitt Cancer Center, and City of Hope between 2014 and 2022 (Fig 1A).Genetic profiling of pre-treatment bone marrow samples was performed with a comprehensive panel covering 147 myeloid and lymphoid genes.
Acute lymphoblastic leukemia blasts are typically detected in CSF by morphologic evaluation of cytospin samples. Flow cytometric analysis of CSF improved ALL blast detection, and positive results were associated with a higher incidence of any relapse, 124 although another study failed to show such an association. 125.
OS at 2-years was 78%. 64 Similarly, The Group for Research on Adult Acute Lymphoblastic Leukemia (GRAAL), compared 225 patients up to the age of 60 who were treated on pediatric-inspired regimen ...
Leukemia is a deadly disease caused by the overproduction of immature white blood cells (WBS) in the bone marrow. If leukemia is detected at the initial stages, the chances of recovery are better. Typically, morphological analysis for the identification of acute lymphoblastic leukemia (ALL) is performed manually on blood cells by skilled medical personnel, which has several disadvantages ...
Progress in treatment and in the understanding of disease biology has been continuous in acute lymphoblastic leukemia (ALL) over the last 50 years. At pres ... Recent translational research findings and potentials are highlighted in the review series "acute lymphoblastic leukemia," which we launch in this issue ... In a last paper of the ...
B-Lineage Acute Lymphoblastic Leukaemia (B-ALL), a prevalent childhood white blood cancer happens owing to the excessive growth of while blood cells (WBC) or blasts in the bone marrow. B-ALL is diagnosed using preliminary tests of the counts of blood cells followed by their morphological, cytochemical and immunophenotypic evaluation.
Florent Malard, Mohamad Mohty. Acute lymphoblastic leukaemia develops in both children and adults, with a peak incidence between 1 year and 4 years. Most acute lymphoblastic leukaemia arises in healthy individuals, and predisposing factors such as inherited genetic susceptibility or environmental exposure have been identified in only a few ...
A phase 2 study of ruxolitinib with chemotherapy in children with Philadelphia chromosome-like acute lymphoblastic leukemia (AALL1521/INCB18424-269): biologic characteristics and minimal residual disease response of patients with non-CRLF2- rearranged JAK pathway alterations.
Acute lymphoblastic leukemia (ALL) is the most frequently diagnosed cancer in the pediatric age group, amounting to approximately 25-30% of all childhood malignant disorders. The annual incidence of acute lymphoblastic leukemia in the United States is approximately 4.6 cases per 100,000 between the ages 0-14 years, with a peak incidence at ...
Acute lymphoblastic leukemia (ALL) is the most common malignancy in children and adolescents with an incidence of 1-5 per 100,000 per year, more than 60% of which are children or young adults [Citation 1, Citation 2].In recent decades, the survival rates are >90% in children [Citation 3], which is largely attributable to improvements in chemotherapy scheduling and dosing, supportive care and ...
T-cell acute lymphoblastic leukemia (T-ALL) is an aggressive hematological disease characterized by the abnormal proliferation of immature T cells in bone marrow and lymphoid tissues .The incidence of T-ALL accounts for approximately 15% of pediatric ALL cases, whereas it accounts for 25% of adult ALL cases .To date, although the prognosis of T-ALL patients has been significantly improved due ...
All research articles are scrutinized, and the quality of the papers relevant to each research subject is determined . The authors read and analyse each of the selected articles by hand. ... Liu Y, Long F (2019) Acute lymphoblastic leukemia cells image analysis with deep bagging ensemble learning. In ISBI 2019 C-NMC challenge: classification in ...
Acute lymphocytic leukemia (ALL) is a malignancy of B or T lymphoblasts characterized by uncontrolled proliferation of abnormal, immature lymphocytes and their progenitors, which ultimately leads to the replacement of bone marrow elements and other lymphoid organs resulting in a typical disease pattern characteristic of acute lymphocytic leukemia. ALL accounts for approximately 2 percent of ...
This study reports a positive association between protracted low dose exposure to ionising radiation and mortality due to some haematological malignancies. Given the relatively low doses typically accrued by workers in this study (16 mGy average cumulative red bone marrow dose) the radiation attributable absolute risk of leukaemia mortality in this population is low (one excess death in 10 000 ...
Acute lymphoblastic leukaemia (ALL) is seen in both children and adults, but its incidence peaks between ages 2 and 5 years. The causation of ALL is considered to be multi-factorial, including exogenous or endogenous exposures, genetic susceptibility, and chance. The survival rate of paediatric ALL has improved to approximately 90% in recent ...
Acute myeloid leukemia (AML) is a common type of leukemia that has a high mortality rate. The reasons for high mortality in patients with AML are therapeutic resistance, limited ability to predict duration of response, and likelihood of cancer relapse. Biomarkers, such as leukemic stem cell biomarkers, circulatory biomarkers, measurable residual disease biomarkers, and molecular biomarkers ...
The production of abnormal leukocytes defines leukemia as either a primary or secondary process. They can be classified as acute or chronic based on the rapidity of proliferation and myeloid or lymphoid based on the cell of origin. Predominant subtypes are acute myeloid leukemia (AML) and chronic myeloid leukemia (CML), involving the myeloid lineage; acute lymphoblastic leukemia (ALL); and ...