
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

What Is a Synthesis Reaction? Definition and Examples

A synthesis reaction is one of the four main types of chemical reactions , along with decomposition, single replacement , and double replacement reactions. Here is the synthesis reaction definition, examples of the reaction using elements and compounds, a look at how many reactants are involved, and how to recognize a synthesis reaction.
Synthesis Reaction Definition
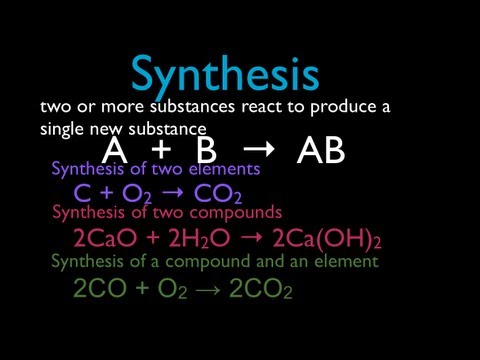
A synthesis reaction is a chemical reaction that combines two or more simple elements or compounds to form a more complex product . A + B → AB This type of reaction is also called a direct combination reaction or simply a combination reaction. It’s the type of reaction that forms compounds from their elements. Synthesis reactions also make large molecules from smaller ones. A synthesis reaction is the opposite of a decomposition reaction , which breaks complex molecules into simpler ones.
Synthesis Reaction Examples
There are many examples of synthesis reactions. Some involve elements. In others, an element reacts with a compound. In still other cases, compounds react with other compounds to form larger molecules.
Synthesis Reactions Between Elements
- Iron and sulfur react to form iron sulfide. 8 Fe + S 8 → 8 FeS
- Potassium and chlorine react to form potassium chloride. 2K (s) + Cl 2(g) → 2KCl (s)
- Iron and oxygen react to form rust. 4 Fe (s) + 3 O 2 (g) → 2 Fe 2 O 3 (s)
- Hydrogen reacts with oxygen to form water. 2 H 2 (g) + O 2 (g) → 2 H 2 O(g)
Synthesis Reactions Between an Element and a Compound
- Carbon monoxide reacts with oxygen to form carbon dioxide. 2 CO(g) + O 2 (g) → 2CO 2 (g)
- Nitric oxide reacts with oxygen to form nitrogen dioxide. 2NO + O 2 → 2NO 2
- CH 2 CH 2 (g) + Br 2 (ℓ) → CH 2 BrCH 2 Br
Synthesis Reactions Between Compounds
- Sulfur oxide reacts with water to form sulfuric acid. SO 3 (g) + H 2 O (l) → H 2 SO 4 (aq)
- Calcium oxide reacts with water to form calcium hydroxide. 2CaO (s) + 2H 2 O (l) → 2Ca(OH) 2 (aq)
- Iron oxide and sulfur oxide react to form iron sulfate. Fe 2 O 3 + 3SO 3 → Fe 2 (SO 4 ) 3
How Many Reactants Are There?
Usually, there are two reactants in a synthesis reaction. They could be two elements, an element and a compound, or two compounds. However, sometimes more reactants combine to form a product. Here are examples of synthesis reactions involving three reactants:
- Sodium carbonate reacts with water and carbon dioxide to form sodium bicarbonate. Na 2 CO 3 + H 2 O + CO 2 → 2NaHCO 3
- Nitrogen reacts with water and oxygen to form ammonium nitrate. 2N 2 (g) + 4H 2 O(g) + O 2 (g) → 2NH 4 NO 3 (s)
How to Recognize a Synthesis Reaction
The easiest way to recognize a synthesis reaction is to look for a reaction where multiple reactants produce a single product. However, sometimes a synthesis reaction equation includes multiple products and reactants. A good example is the overall reaction for photosynthesis, in which carbon dioxide and water combine to form glucose and oxygen. CO 2 + H 2 O → C 6 H 12 O 6 + O 2 But, even in this case, two simpler molecules react to form a more complex one. So, this is the key in synthesis reaction identification.
Some synthesis reactions form predictable products. If you recognize them, it’s easy to recognize the reaction type:
- Reacting two elements forms a binary compound. For example, hydrogen and oxygen react to form water.
- When two nonmetals react, more than one product is possible. For example, sulfur and oxygen react to form sulfur dioxide or sulfur trioxide.
- Alkali metals react with nonmetals to form ionic compounds. For example, sodium and chlorine form sodium chloride.
- Transition metals react with nonmetals to form more than one possible product. To predict the product, you need to know the oxidation state (charge) or the metallic cation.
- Nonmetal oxides react with water to form acids. For example sulfur dioxide reacts with water to make sulfurous acid.
- Metallic oxides react with water to form bases.
- Nonmetal oxides react with one another to form salts.
Related Posts
- Science, Tech, Math ›
- Chemistry ›
Synthesis Reaction Description Plus Examples
Two or more simple substances combine to form more complex products
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
While there are many types of chemical reactions, they all fall into at least one of four broad categories: synthesis reactions, decomposition reactions, single displacement reactions, and double displacement reactions.
A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The reactants may be elements or compounds, while the product is always a compound.
General Form of Synthesis Reactions
The general form of a synthesis reaction is:
A + B → AB
Examples of Synthesis Reactions
Here are some examples of synthesis reactions:
- Water: 2 H 2 (g) + O 2 (g) → 2 H 2 O(g)
- Carbon dioxide: 2 CO(g) + O 2 (g) → 2CO 2 (g)
- Ammonia: 3 H 2 (g) + N 2 (g) → 2 NH 3 (g)
- Aluminum oxide: 4 Al(s) + 3 O 2 (g) → 2 Al 2 O 3 (s)
- Iron sulfide: 8 Fe + S 8 → 8 FeS
- Potassium chloride: 2 K(s) + Cl 2 (g) → 2 KCl(s)
Recognizing Synthesis Reactions
The hallmark of a synthesis reaction is that a more complex product is formed from the reactants. One easy-to-recognize type of synthesis reaction occurs when two or more elements combine to form a compound. The other type of synthesis reaction happens when an element and a compound combine to form a new compound.
Basically, to identify this reaction, look for a product that contains all the reactant atoms. Be sure to count the number of atoms in both the reactants and the products. Sometimes when a chemical equation is written, "extra" information is given that might make it hard to recognize what is going on in a reaction . Counting numbers and types of atoms makes it easier to identify reaction types.
- Types of Chemical Reactions
- How to Balance Chemical Equations
- Examples of Physical Changes
- What Is Muriatic Acid? Facts and Uses
- Understanding Endothermic and Exothermic Reactions
- Chemical Properties of Matter
- 10 Examples of Heterogeneous and Homogeneous Mixtures
- What Is the Difference Between Oxidation and Reduction?
- Molarity Definition in Chemistry
- Examples of 10 Balanced Chemical Equations
- Chemistry Vocabulary Terms
- Actual Yield Definition (Chemistry)
- How to Balance Equations - Printable Worksheets
- What Happens to Candle Wax When a Candle Burns
- Fun and Interesting Chemistry Facts
- Back Titration Definition
What Is A Synthesis Reaction?

Did you eat a synthesis reaction for breakfast? It's highly likely if you consumed taurine, which is the result of an organic synthesis reaction and commonly found in milk and eggs. In chemistry, a synthesis reaction is when two or more chemicals combine and form a more complex product. You will also have more reactants than products since two or more chemical species combine to form one new larger compound.
What Happens in a Synthesis Reaction?
In a synthesis reaction, two or more chemical species combine, forming a more complex product in the reaction. It is also called a direct reaction and is one of the most common chemical reactions. When the two or more reactants combine they make a larger compound. A synthesis reaction is the opposite of a decomposition reaction, which is when the bonds are broken in a complex product, and it splits the product into its respective components or elements.
What Is the General Form of a Synthesis Reaction?
The word synthesis means to put together. When two or more products are put together it produces a new single product. The basic form of the chemical equation is written as:
What are Some Synthesis Reaction Examples?
Some synthesis reactions occur when burning various metals by adding oxygen to them. Here are some examples:
Magnesium + oxygen → magnesium oxide
Alternatively, in the chemical equation:
2Mg + O 2 → 2MgO
This synthesis reaction gives off a very bright light, so if you perform it, wear safety goggles and don't look directly at the light, or you can harm your eyes.
Aluminum + bromine → aluminum bromide
Or in the chemical equation:
2Al + 3Br 2 → 2AlBr 3
What Is a Synthesis Reaction in Organic Chemistry?
Organic synthesis reactions involve organic compounds. Organic molecules are more complex than their inorganic counterparts are. In many cases, because of the complexity, synthesis reactions of organic compounds require several steps one after the other to create a single product. This makes intermediate compounds for each step before the final single product.
For example, when water combines with ethyl leads it forms ethanol or:
CH 2 = CH 2 + HCl → CH 3 -CH 2 Cl
Other Considerations of a Synthesis Reaction
A synthesis reaction can occur when combining elements and producing a new compound, combining compounds to produce a new compound, or combining both elements and compounds to result in a new compound.
When a metal and non-metal are combined, they produce an ionic compound.
When two non-metals combine, they produce a covalent compound.
When combining metal oxide and water (both compounds), it produces a new compound of a metal hydroxide.
Non-metal and water combinations result in an oxy acid compound.
Metal oxides and carbon dioxide combined produce metal carbonates.
The combination of an element and a compound to produce a new compound can be seen in carbon dioxide. This is the product of carbon monoxide and oxygen, written in a chemical equation as:
2CO (g) + O 2 (g) → 2CO 2 (g)
- ThoughtCo.: Synthesis Reaction Definition and Examples
- Chemical Formula: Synthesis Reaction
- What Health: Taurine Overview
Cite This Article
Lougee, Mary. "What Is A Synthesis Reaction?" sciencing.com , https://www.sciencing.com/what-is-a-synthesis-reaction-13712164/. 19 May 2018.
Lougee, Mary. (2018, May 19). What Is A Synthesis Reaction?. sciencing.com . Retrieved from https://www.sciencing.com/what-is-a-synthesis-reaction-13712164/
Lougee, Mary. What Is A Synthesis Reaction? last modified August 30, 2022. https://www.sciencing.com/what-is-a-synthesis-reaction-13712164/
Recommended
Learning Materials
- Business Studies
- Combined Science
- Computer Science
- Engineering
- English Literature
- Environmental Science
- Human Geography
- Macroeconomics
- Microeconomics
- Synthesis Reaction
For millennia, all the way back to Ancient Egypt, alchemists have been attempting to synthesize gold from more common metals like lead. While their attempts always failed, the scientists of today have been able to do the impossible: make gold (though in a very, very expensive way).
Millions of flashcards designed to help you ace your studies
- Cell Biology
What is the basic structure for a synthesis reactions?
True or False: A synthesis can form an element or compound
True or False: Synthesis reactions only have 2 reactants
True or False: Synthesis reactions only make one (unique) product
Which of the following is NOT a synthesis reaction?
Which of the following is NOT possible for synthesis reaction?
Need help? Meet our AI Assistant

Need help with Synthesis Reaction? Ask our AI Assistant

Review generated flashcards
to start learning or create your own AI flashcards
Start learning or create your own AI flashcards

Vaia Editorial Team
Team Synthesis Reaction Teachers
- 6 minutes reading time
- Checked by Vaia Editorial Team
- Chemical Analysis
- Chemical Reactions
- Acid-Base Reactions
- Acid-Base Titration
- Bond Energy Calculations
- Decomposition Reaction
- Displacement Reactions
- Electrolysis Of Ionic Compounds
- Electrolysis of Aqueous Solutions
- Energy Changes
- Extracting Metals
- Extraction Of Aluminium
- Making Salts
- Net Ionic Equations
- Percent Composition
- Physical and Chemical Changes
- Precipitation Reaction
- Reactions of Acids
- Reactivity Series
- Redox Reactions
- Redox Titration
- Representing Chemical Reactions
- Single and Double Replacement Reactions
- Skeleton Equation
- Stoichiometric Calculations
- Stoichiometry
- Types of Chemical Reactions
- Chemistry Branches
- Inorganic Chemistry
- Ionic and Molecular Compounds
- Making Measurements
- Nuclear Chemistry
- Organic Chemistry
- Physical Chemistry
- The Earths Atmosphere
Jump to a key chapter

In 1980, Glenn Seaborg was able to "steal" protons and neutrons from bismuth, which did produce several thousand atoms of gold. However, this method was much too expensive for gold to be made this way.
While he was able to make gold, he didn't "synthesize" it in the traditional sense. In this article, we will be learning all about the synthesis reaction . After reading this article, you'll see why the alchemists dream of synthesizing gold was always destined to fail.
- This article cover the topic synthesis reactions.
- First, we will define what a synthesis reaction is .
- Next, we will look at the basic chemical equation for a synthesis reaction.
- Then, we will look at some different examples of these reactions.
- After that, we will summarize the characteristics of the synthesis reaction.
- Lastly, we will compare and contrast these reactions with another type of reaction called single replacement.
Synthesis reaction definition
- A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new compound.
A key characteristic about synthesis reactions is that they only make one unique product. However, there can be multiple of that product produced.
As discussed in the intro, it is impossible to (traditionally) synthesis gold. Why? Because it is an element. When elements react, they will always form compounds.
There are ways for elements to combine to form other elements, but these methods are different from your typical reaction. For example, the sun fuses hydrogen nuclei into helium atoms by essentially smashing the nuclei together at very high temperatures.
Synthesis reaction chemical equation
All synthesis reactions follow this basic formula:
$$A + B \rightarrow AB$$
The basic structure is two (or more) elements and compounds combining to create one product.

Let's look at some examples, shall we?

Synthesis reaction examples
Let's have a look at some synthesis reaction examples to fully understand what synthesis reactions are and how they are represented.
First up, let's look at the synthesis of salt:
$$2Na_{(s)} + Cl_{2\,(g)} \rightarrow 2NaCl_{(s)}$$
As you can see, we have two elements (chlorine is naturally diatomic) forming one. Even though chlorine is a gas, it still forms a solid product. Synthesis reactions (and all reactions for that matter) don't always form a product of the same state as the reactants, so keep your eyes on the states listed in the equation.
Now let's look at a more complex example:
Here we have two compounds forming a product instead of elements. As long as one product is formed, it is a synthesis reaction, whether it is formed from elements or compounds.
$$CaO_{(s)} + H_2O_{(l)} \xrightarrow {heat} Ca(OH)_{2\,(aq)}$$
As a side not, the (aq) on Ca(OH) 2 means "aqueous". This means that the compound is dissolved in water. Usually, but not always, reactions that contain water will produce aqueous products.
Also, "heat" is written above the arrow to show heat is required for the reaction to proceed.
Let's look at one last example:
$$2N_{2\,(g)} + 4H_2O_{(l)} + O_{2\,(g)} \rightarrow 2NH_4NO_{3\,(s)}$$
The above reaction has 3 reactants instead of 2. While two-reactant synthesis reactions are more common, it's important to keep in mind that these reactions can have more than 2 reactants.
Characteristics of a synthesis reaction
Synthesis reactions are pretty easy to spot when you know what to look for. As a summary, here are the characteristics of a synthesis reaction:
- Have 2 or more reactants.
- Reactants can be elements and/or compounds.
- Reactants combine to form one compound.
- The compound formed is more complex than the reactant.
Synthesis reactions in Organic Chemistry
When you read up on synthesis reactions, you might encounter a different type used in Organic Chemistry .
In organic chemistry , a synthesis reaction involves taking one simple compound and transforming through a series of steps to get a much more complex compound. While they are similar, they have some key differences, such as the fact that Organic Synthesis reactions are multiple steps a nd that they can produce side products.
Synthesis reaction vs single replacement
Synthesis reactions can often be confused with another reaction called single replacement.
In a single-replacement reaction , an element will displace another element present in a compound, "swapping" with it.
A general single-replacement reaction looks like this:
$$A + BC \rightarrow AB + C$$
The lone element essentially "kicks out" the like element (i.e. metals swap with metals, non-metals swap with non-metals).
Below is an example:
Here the zinc is swapping with the copper (both are Transition Metals ).
Single replacement reactions are similar to synthesis reactions, since they involve 2 reactants coming together to form a new product. The key difference is that two products are formed instead of one. Also, this process involves swapping with synthesis involves only combining .
Synthesis Reaction - Key takeaways
Synthesis reactions are separate from single replacement reaction
Flashcards in Synthesis Reaction 6
$$Al + FeCl_2 \rightarrow AlCl_3 + Fe$$
2 elements form 1 compound
Learn with 6 Synthesis Reaction flashcards in the free Vaia app
We have 14,000 flashcards about Dynamic Landscapes.
Already have an account? Log in
Frequently Asked Questions about Synthesis Reaction
What is the meaning of synthesis reaction?
A synthesis reaction is a chemical reaction in which two or more reactants combine to produce a single product:
A + B → AB
What is an example of a synthesis reaction?
An example of a synthesis reaction is the production of salt (NaCl) from sodium (Na) and chlorine (Cl):
2 Na (s) + Cl 2 (g) → 2NaCl (s)
How do you solve synthesis chemical equations?
A synthesis chemical equation always follows the same reaction structure, and thus always has the following general equation:
Sometimes AB can be represented as C (A + B → C), but it's the same concept: a synthesis chemical equation.
What are the characteristics of a synthesis reaction?
The characteristics of a synthesis reaction are:
Are synthesis reactions the same as a single replacements?
No, synthesis reactions and single replacement reactions are different types of reactions. Synthesis reactions consist of two or more reactants combining to produce a new product (A + B → C).
In the case of a single replacement, one of the reactants displaces an element in the other reactant, producing two products: the new compound with the replacement, and the element that was "kicked out" of the original reactant (A + BC → AB + C).
Test your knowledge with multiple choice flashcards
Join the Vaia App and learn efficiently with millions of flashcards and more!
That was a fantastic start, you can do better, sign up to create your own flashcards.
Access over 700 million learning materials
Study more efficiently with flashcards
Get better grades with AI
Already have an account? Log in
Keep learning, you are doing great.
Discover learning materials with the free Vaia app

Vaia is a globally recognized educational technology company, offering a holistic learning platform designed for students of all ages and educational levels. Our platform provides learning support for a wide range of subjects, including STEM, Social Sciences, and Languages and also helps students to successfully master various tests and exams worldwide, such as GCSE, A Level, SAT, ACT, Abitur, and more. We offer an extensive library of learning materials, including interactive flashcards, comprehensive textbook solutions, and detailed explanations. The cutting-edge technology and tools we provide help students create their own learning materials. StudySmarter’s content is not only expert-verified but also regularly updated to ensure accuracy and relevance.
Team Chemistry Teachers
Study anywhere. Anytime.Across all devices.
Create a free account to save this explanation..
Save explanations to your personalised space and access them anytime, anywhere!
By signing up, you agree to the Terms and Conditions and the Privacy Policy of Vaia.
Sign up to highlight and take notes. It’s 100% free.
Join over 22 million students in learning with our Vaia App
The first learning app that truly has everything you need to ace your exams in one place
- Flashcards & Quizzes
- AI Study Assistant
- Study Planner
- Smart Note-Taking
Privacy Overview
All Subjects
Physical Science
Study guides for every class, that actually explain what's on your next test, synthesis reaction, from class:.
A synthesis reaction is a type of chemical reaction where two or more reactants combine to form a single product. This process is fundamental in chemistry, as it illustrates how simpler substances can unite to create more complex compounds. Often represented as A + B → AB, synthesis reactions play a crucial role in the formation of various chemical compounds and are foundational in understanding other types of reactions.
congrats on reading the definition of Synthesis Reaction . now let's actually learn it.
5 Must Know Facts For Your Next Test
- Synthesis reactions are often exothermic, meaning they release energy in the form of heat when products are formed.
- These reactions are essential for the production of important compounds, such as water (H₂O) from hydrogen (H₂) and oxygen (O₂).
- Synthesis reactions can be affected by factors such as temperature, pressure, and concentration of reactants, influencing the rate and extent of the reaction.
- Inorganic synthesis reactions often occur when metals react with nonmetals to form ionic compounds.
- Biochemical synthesis reactions, such as those occurring during photosynthesis, demonstrate how living organisms use synthesis processes to create essential molecules like glucose.
Review Questions
- Synthesis reactions involve two or more reactants combining to form a single product, while decomposition reactions entail a single compound breaking down into multiple products. Together, they illustrate the dynamic nature of chemical processes. Synthesis reactions often create new compounds essential for various applications, whereas decomposition reactions can release energy and simpler substances necessary for further chemical transformations.
- Synthesis reactions are critical in industrial applications, such as the production of fertilizers, plastics, and pharmaceuticals, where complex compounds are formed from simpler raw materials. In biological processes, they underpin vital functions like photosynthesis and cellular respiration. Understanding these reactions helps scientists develop methods for creating new materials and enhancing biochemical pathways that support life.
- Changes in temperature and pressure can significantly impact synthesis reactions by altering reaction rates and equilibria. For example, increasing temperature often speeds up the reaction rate due to higher kinetic energy among reactants, while increased pressure can favor the formation of products when gas reactants are involved. An example includes the synthesis of ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂) gases under high pressure and temperature conditions as outlined by the Haber process. This shows how conditions can be optimized to enhance product yield.
Related terms
Decomposition Reaction : A decomposition reaction is the opposite of a synthesis reaction, where a single compound breaks down into two or more simpler products.
Combustion Reaction : A combustion reaction is a chemical reaction that typically involves the rapid reaction of a substance with oxygen to produce heat and light, often resulting in the formation of water and carbon dioxide.
Single Replacement Reaction : A single replacement reaction involves an element reacting with a compound, where one element replaces another in that compound.
" Synthesis Reaction " also found in:
Subjects ( 5 ).
- AP Chemistry
- Anatomy and Physiology I
- Cell Biology
- Chemical Process Balances
- Intro to Chemical Engineering
© 2024 Fiveable Inc. All rights reserved.
Ap® and sat® are trademarks registered by the college board, which is not affiliated with, and does not endorse this website..
Synthesis Reactions — Definition & Examples - Expii
Synthesis reactions — definition & examples.
In a synthesis reaction, or combination reaction, simpler reactants combine to form more complex products. The general equation is A + B → AB.
Explanations (4)
Synthesis reactions.
What are Synthesis Reactions?
- Synthesis reactions are reactions that involve multiple reactants reacting to form one single product.
Example: A + B → AB
- Synthesis reactions are exothermic reactions. So, they release energy as heat or light .
They involve the formation of either ionic or covalent bonds. The formation of a bond releases energy and increases stability. In contrast, breaking bonds requires energy.

Image source: Caroline Monahan
Synthesis Reaction Practice Problem
Which of the following are synthesis reactions?
2S + 3O2 → 2SO3
HCl + NaOH → NaCl + H2O
MgO + CO2 → MgCO3
CO2 + H2O → H2CO3
2Ni2O3 → 4Ni + 3O2
Related Lessons
(Video) Synthesis Reactions: Chemical Reaction (5 of 11) Synthesis Reactions, an Explanation
By Step by Step Science
In this video, you will learn that a synthesis reaction occurs when two or more compounds or elements react with each other to form a singular compound. You will also learn different examples of synthesis reactions in this video.
Synthesis Reactions: What are synthesis reactions?
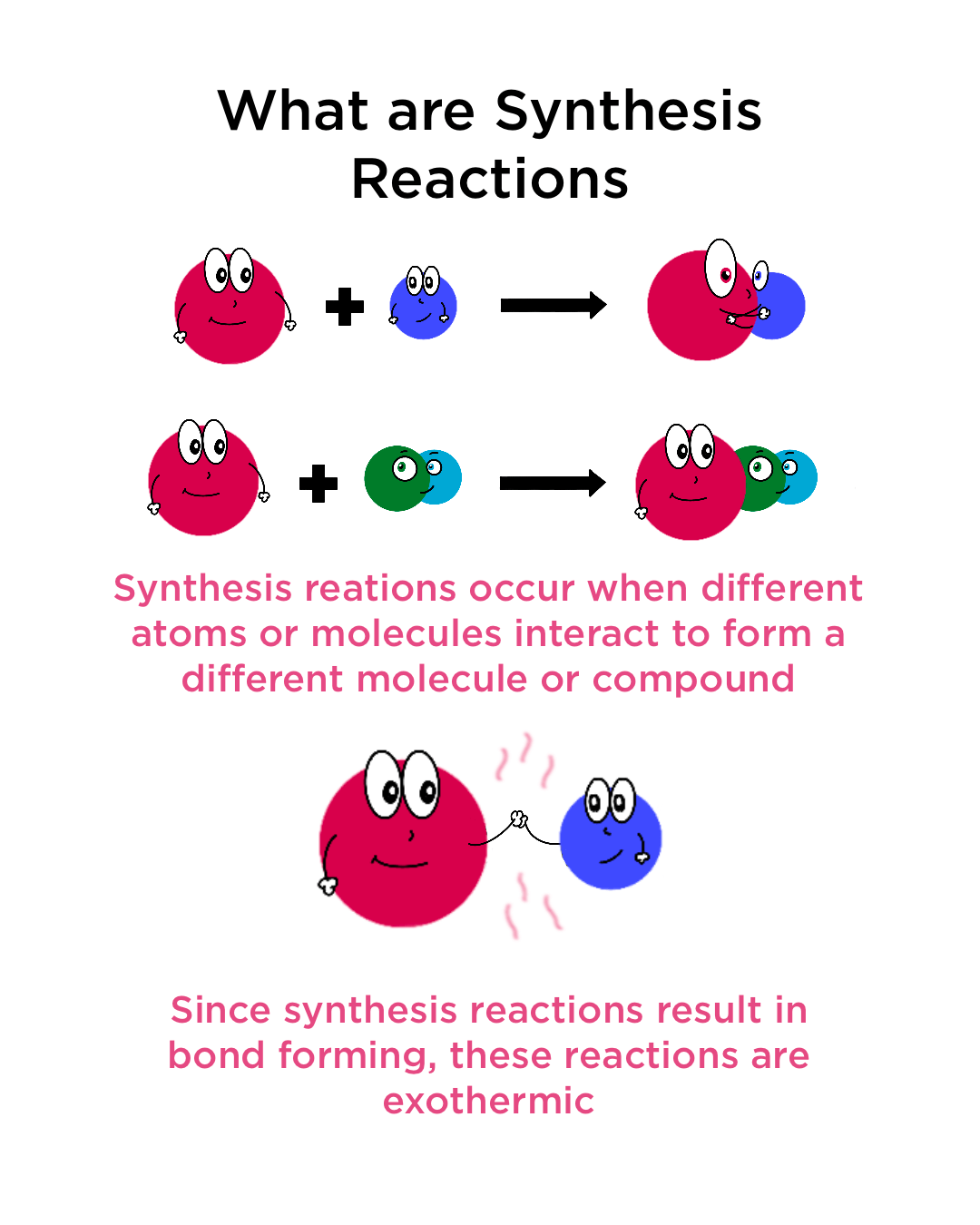
Image Source: Leo Dong
What is Chemical Synthesis like for Professional Chemists?
In general chemistry, synthesis reactions are the least complicated chemical reaction. You take two reactants and make one product. A generic balanced chemical equation would be A+B→AB. Unfortunately, in the real world, chemical synthesis is rarely so straightforward.
I remember a nightmare scenario from my organic chemistry class. I had one question left on our online homework. I was so close to finishing! I clicked the next button and saw the final problem. It was a fifteen-step synthesis! When you're learning organic chemistry, that's not a short puzzle. Often in chemical synthesis, there are many steps. Why? There are a couple of possible reasons. Sometimes, the reactants that produce an easy synthesis reaction are expensive. So, you might have to start with cheap reactants and build up your molecules. Other times, the reaction intermediates may be unstable. They only form during a series of chemical reactions. So, again we have to build up our reactants.
Planning a Chemical Synthesis
Chemical synthesis is the heart of applied chemistry . The chemist works to develop a procedure to produce their desired compound. What's the result? They construct the reaction mechanism , piece by piece. Along the way, they often have to manipulate specific steps to achieve their goals. They use their knowledge of chemistry principles to influence the reaction.
How would a chemist go about planning a synthesis? Most often, they start at the final product and work backward. Each step focuses on changing one specific bond or group of atoms . In organic chemistry, a compound's reactivity comes from functional groups. They are groupings of atoms with set chemical properties . For example, a carbon - oxygen - hydrogen set is the alcohol group. Because oxygen is bound to hydrogen, it is always a polar group . The oxygen-hydrogen bond is also a weak acid . So, we might manipulate it with a strong base . Other chemists, like inorganic chemists, often focus on individual atoms. But they also have to consider the reactivity of the element .
Performing the Lab Work
In college, I had a friend that was doing a research project. One of the steps in his synthesis formed a hydrate . But, before he could proceed to the next step, he needed to remove the water. So, his compound had to sit in a chemical oven for twelve hours. He didn't get it in until after his afternoon classes. So, he had to return to the lab at 3:00 am to take it out! In chemical synthesis, the lab work is often the most challenging part. Why? A chemical reaction gets influenced by so many factors! We must consider temperature , pressure , pH , time, and other factors. Often we have to manipulate many factors along the way.
Sometimes, we use chemical manipulation . For example, maybe step seven of a fifteen-step reaction is the rate-limiting step . What could we do? We might try to develop a catalyst . Remember, they lower a reaction's activation energy and improve its rate . Sometimes, we can research the published work of other chemists for possible catalysts. Other times, we may need to use theoretical modeling to develop a catalyst. Sometimes, we even have to synthesize the catalyst!
Other times, we need to use physical manipulations. For example, one of our steps could be an equilibrium reaction. But we want it to go to completion. How do we manipulate the equilibrium? We take advantage of Le' Chatlier's principle . If the reaction is endothermic, we could apply heat. What if it's exothermic? We could cool it. Sometimes we can physically separate our products. By removing the products, we alter the equilibrium.
What are Your Results?
Chemistry is complex. For example, sometimes, a reaction can produce multiple products. So, even if we successfully implement our plan, we may not get our desired result. Why? We also have to worry about the percent yield . Part of planning the mechanism is calculating the theoretical yield . But often, one of the steps will act as a limiting reagent . So, very rarely are the theoretical and actual yields equivalent. Sometimes, experimental errors mean we don't produce our desired amount.
Synthesis Reactions
Synthesis reactions, also known as combination reactions, are chemical reactions in which two or more reactants combine to form a single product. This type of reaction is represented by the general equation: A + B → AB
Formation of a new substance: Synthesis reactions result in creating a new compound or molecule from simpler reactants.
Energy absorption: These reactions often require an input of energy, typically in the form of heat or light, to initiate the reaction.
Common examples of synthesis reactions include the formation of water (2H 2 + O 2 → 2H 2 O), the synthesis of ammonia (3H₂ + N₂ → 2NH₃), and photosynthesis (6CO 2 + 6H 2 O → C 6 H 12 O 6 + 6O 2 + 6H 2 ).

COMMENTS
A synthesis reaction or direct combination reaction reacts two or more simple elements or compounds to form a more complex product. A synthesis reaction is one of the four main types of chemical reactions, along with decomposition, single replacement, and double replacement reactions.
A synthesis reaction or direct combination reaction is one of the most common types of chemical reaction. In a synthesis reaction, two or more chemical species combine to form a more complex product: A + B → AB.
A synthesis reaction or direct combination reaction is a type of chemical reaction in which two or more simple substances combine to form a more complex product. The reactants may be elements or compounds, while the product is always a compound.
In a synthesis reaction, two or more chemical species combine, forming a more complex product in the reaction. It is also called a direct reaction and is one of the most common chemical reactions. When the two or more reactants combine they make a larger compound. A synthesis reaction is the opposite of a decomposition reaction, which is when ...
A synthesis reaction occurs when two or more reactants combine to form a single product. This type of reaction is represented by the general equation: A + B → AB. An example of a synthesis reaction is the combination of sodium (Na) and chlorine (Cl) to produce sodium chloride (NaCl).
A synthesis reaction (also called a combination reaction) is a reaction where two or more elements or compounds combine to form a new compound. A key characteristic about synthesis reactions is that they only make one unique product. However, there can be multiple of that product produced.
A synthesis reaction is a type of chemical reaction where two or more reactants combine to form a single product. This process is fundamental in chemistry, as it illustrates how simpler substances can unite to create more complex compounds.
What are Synthesis Reactions? Synthesis reactions are reactions that involve multiple reactants reacting to form one single product. Example: A + B → AB . Synthesis reactions are exothermic reactions. So, they release energy as heat or light. They involve the formation of either ionic or covalent bonds. The formation of a bond releases energy ...
Synthesis reactions (also called combination reactions) are the simplest type of chemical reaction. In a synthesis reaction two or more substances undergo a chemical reaction producing a new substance.
Synthesis reactions, also known as combination reactions, are chemical reactions in which two or more reactants combine to form a single product. This type of reaction is represented by the general equation: A + B → AB.