
AARON SAGUIL, MD, MPH, EDWIN A. FARNELL, IV, MD, AND TENEISHA S. JORDAN, MD
Am Fam Physician. 2022;106(2):173-183
Author disclosure: No relevant financial relationships.
Multiple sclerosis (MS) is a demyelinating disorder of the central nervous system and the most common cause of nontraumatic neurologic disability in young adults. Types of MS include relapsing-remitting (most common), secondary progressive, and primary progressive. Clinically isolated syndrome and radiologically isolated syndrome are additional categories for patients with findings concerning for MS who do not yet meet the diagnostic criteria for the disease. Symptoms of MS depend on the areas of neuronal involvement. Common symptoms include sensory disturbances, motor weakness, impaired gait, incoordination, optic neuritis, and Lhermitte sign. A patient history, neurologic examination, and application of the 2017 McDonald Criteria are needed to diagnose MS accurately. Patients with MS should be treated by a multidisciplinary team that may include physical and occupational therapists, speech and language therapists, mental health professionals, pharmacists, dietitians, neurologists, and family physicians. Steroids are the mainstay of treatment for the initial presentation of MS and relapses. Patients who do not adequately respond to steroids may benefit from plasmapheresis. Patients with MS who smoke tobacco should be strongly encouraged to quit. Disease-modifying therapy has been shown to slow disease progression and disability; options include injectable agents, infusions, and oral medications targeting different sites in the inflammatory pathway. Symptom-based care is important to address the bowel and bladder dysfunction, depression, fatigue, movement disorders, and pain that often complicate MS.
Multiple sclerosis (MS) is a demyelinating disorder of the central nervous system and the most common cause of nontraumatic neurologic disability in young adults. 1 Prevalence differs by latitude, with higher rates among those living further from the equator. The prevalence of MS is 40 per 100,000 people in Lubbock, Tex., compared with 191 per 100,000 people in Olmstead County, Minn. 2 An estimated 1 million people in the United States live with MS. 1 Risk factors include smoking and a history of infectious mononucleosis. Women are twice as likely as men to have MS, and there is a modest genetic influence. 3 , 4
A woman with MS diagnosed at 35 years of age has an average life expectancy of seven to eight years less than that of the general population. Because MS has a relatively high prevalence and patients have a long life span after diagnosis, many family physicians care for patients with the disease. 5

Pathophysiology
Types of MS include relapsing-remitting (RRMS; most common), secondary progressive, and primary progressive ( Table 1 6 – 13 ) . There are also classifications for people with first episodes concerning for MS who do not meet the diagnostic criteria for MS (clinically isolated syndrome) and those with incidental radiologic findings concerning for MS in the absence of clinical symptoms (radiologically isolated syndrome). 13
MS is characterized by focal areas of inflammation, demyelination, gliosis (proliferation and activation of glial cells), and degeneration (axonal loss) secondary to immune-mediated attacks. 10 There is debate about whether the inflammation leading to MS is initiated within or outside the central nervous system; however, T cells, B cells, macrophages (including central nervous system microglia), astrocytes, inflammatory mediators, and blood-brain barrier permeability are all involved in a response that is associated with myelin sheath destruction, axonal injury, and clinical symptoms. 4 , 10 , 14 – 16 In RRMS, clinical lesions may resolve through mechanisms such as axonal changes, neuroplasticity, and remyelination. 13 Progressive forms of MS are associated with cumulative axonal loss and increasing neurologic deficits. 10
Clinical Presentation
Symptoms and signs of MS depend on the areas of neuronal involvement 17 ( Table 2 1 , 18 – 22 ) . Common presenting symptoms include sensory disturbances, motor weakness, impaired gait, incoordination, optic neuritis (unilateral vision loss with pain worsened by extraocular movements), and Lhermitte sign (an electric shock–like sensation down the spine on neck flexion). 18 – 20 Other symptoms include urinary, bowel, and sexual dysfunction.
In RRMS, relapse symptoms evolve over days before partially or fully resolving, and patients are typically stable between acute exacerbations. Some symptoms, such as fatigue, can be persistent. 20 , 23
Multiple diseases may mimic MS clinically and radiologically ( Table 3 ) . 13 , 18 , 23 , 24 The differential diagnosis includes genetic, infectious, inflammatory, metabolic, and neoplastic processes. Psychiatric diseases, ingestions, and nutritional deficiencies may also be mistaken for MS. 13 , 18 , 23 , 24 Table 4 lists tests that may help differentiate MS from other diseases. 18
A patient history, neurologic examination, and application of the 2017 McDonald Criteria are needed to accurately diagnose MS ( Table 5 ) . 25 Diagnosis relies on the acute exacerbations of MS being disseminated in space and time ( Figure 1 18 ) . In cases where only part of the diagnostic criteria are met, magnetic resonance imaging (MRI) of the brain and spine may be used to confirm the presence of lesions consistent with MS ( Figure 2 , Figure 3 , and Figure 4 ) . 18 Cerebrospinal fluid assays demonstrating oligoclonal bands may also aid in meeting diagnostic criteria. 25
The diagnosis should be questioned if the patient has a family history of neurologic disorders other than MS, an abrupt or transient (less than 24 hours) presentation, progressive ataxia, cognitive dysfunction, other organ involvement, or nonspecific neurologic symptoms that are difficult to localize. 13 , 20 , 26
Patients with MS should be treated by a multidisciplinary team that may include physical and occupational therapists, speech and language therapists, mental health professionals, pharmacists, dietitians, neurologists, and family physicians. 27
INITIAL PRESENTATION AND ACUTE RELAPSES
Steroids are the mainstay of treatment for the initial presentation of MS and MS relapses. A Cochrane review and another systematic review and meta-analysis found no difference in effectiveness between intravenous and oral steroids for relapse recovery or MRI activity. 28 , 29 A higher dosage of steroids, such as 1,000 mg per day of methylprednisolone (intravenously or orally) for three days, is recommended. 30 , 31 Patients who do not have an adequate response to treatment with steroids may benefit from plasmapheresis. 30 , 32 A randomized controlled trial involving six plasmapheresis treatments in patients unresponsive to steroids found higher rates of complete recovery at one month than in those treated with placebo. 33
SMOKING CESSATION
Patients with MS who smoke tobacco should be strongly encouraged to quit. A cohort study found that each smoke-free year was associated with a decrease in disability progression. 34 A cross-sectional study found that each additional year of smoking accelerated the development of secondary progressive MS by 4.7% (95% CI, 2.3 to 7.2). 35
DISEASE-MODIFYING THERAPY
In patients with active MS, long-term disease-modifying therapy should be initiated to decrease new clinical attacks and radiographic lesions and delay disability progression. 36 , 37 There is disagreement about whether to use disease-modifying therapy in patients with clinically isolated syndrome. 36 – 38
Interferon beta-1b (Betaseron, Extavia) was the first disease-modifying therapy approved for use in 1993. Since then, multiple injectable agents, infusions, and oral medications such as monoclonal antibodies and other immunomodulatory medications targeting multiple steps in the MS inflammatory pathway have been approved by the U.S. Food and Drug Administration ( Table 6 ) . 13 , 37 – 39
The choice of initial disease-modifying therapy is dependent on patient preference, disease activity, potential adverse effects, and specialist input. All approved agents help prevent disease progression, with a relative risk of progression from 0.47 for mitoxantrone to 0.87 for interferon beta-1a (Avonex, Rebif). 40 For patients with less active disease, agents with a lower risk of adverse effects (e.g., cardiac arrhythmia, increased risk of malignancy, progressive multifocal leukoencephalopathy) are preferred at the cost of effectiveness. For patients with more active disease, effectiveness may be considered more important than avoiding adverse effects. Shared decision-making conversations should consider the availability of the medication options, route and frequency of administration, patient preferences regarding effectiveness vs. adverse effects, and the patient's ability to tolerate and comply with monitoring regimens. 36 , 37
For patients who have newly diagnosed RRMS with minimal symptoms and MRI burden of disease, an appropriate regimen may include a moderately effective agent such as interferon or glatiramer (Copaxone, Glatopa) to control disease activity while minimizing adverse effects. In patients with newly diagnosed, rapidly evolving RRMS, a highly effective agent such as alemtuzumab (Lemtrada), cladribine (Mavenclad), natalizumab (Tysabri), or ocrelizumab (Ocrevus) may be considered. A greater risk of debilitating adverse effects is weighed against a greater chance of controlling disease activity in this strategy. 38 Ocrelizumab is the only disease-modifying therapy currently approved by the U.S. Food and Drug Administration for primary progressive MS. 39
Medications should be continued for at least six months to allow time for benefits to occur. If the disease is not controlled by initial therapy, the patient should be offered a more effective medication, recognizing the increased potential for adverse effects. 37 , 38 It is appropriate to consider switching medications if adverse effects develop. 37
Once started, disease-modifying therapy is generally continued for the patient's lifetime; however, guidelines allow for exceptions. Discontinuation can be considered for patients with secondar y progressive MS who have a higher level of disability, are nonambulatory, and have not had a relapse in two years. Discontinuation can also be considered before conception for patients who want to become pregnant and have well-controlled MS. 37 , 38 During pregnancy, patients tend to have a lower risk of flare-ups, with overall better-controlled disease. 41
In addition to disease-modifying therapy, preliminary research suggests that hematopoietic stem cell transplantation may be a more effective alternative in preventing relapses and disability accumulation. 42
SYMPTOM-BASED CARE
In addition to treatment directed at acute relapses and disease progression, patients with MS require a comprehensive program that addresses overall wellness, symptom management, and comorbid mental health and physical conditions ( Table 7 ) . 13 , 22 , 38 , 43 – 85 A multidisciplinary approach is most effective for many symptoms. Physical activity has good evidence for improving walking ability (increased distance on six-minute walking test, faster times on 10-minute walking test), balance (as measured by the Berg Balance Scale), and depression (decreased scores on depression scales). 43 – 45 Pharmacotherapy used for symptoms associated with MS is often off-label and supported by low-quality evidence. A notable exception is dalfampridine extended-release (Ampyra), which has been approved by the U.S. Food and Drug Administration to improve walking in patients with MS. 86 Pain is treated with analgesics, neuromodulators, hydrotherapy, and sometimes cannabinoids. 49 , 82 , 84
More than one-half of patients with untreated RRMS transition to secondary progressive disease. 36 Greater disability and brain atrophy at the time of diagnosis, male sex, and older age are risk factors for progression to more significant functional limitations. 13 Disease-modifying therapy has been shown to alter the course of MS, decreasing the rate at which disability progresses, and is also associated with a lower likelihood of transitioning to progressive disease. 37 , 87
Many governments, nonprofit organizations, and websites provide information and support for individuals and families affected by MS ( eTable A ) .
This article updates previous articles on this topic by Saguil, et al. , 18 and Calabresi . 88
Data Sources: PubMed, the Cochrane Database of Systematic Reviews, Essential Evidence Plus, the National Institute for Health and Care Excellence (UK), and the European Committee for Treatment and Research in Multiple Sclerosis were searched for relevant articles and clinical practice guidelines. Key words included multiple sclerosis, demyelinating disorders, disease-modifying treatment, and others as directed by the search. Search dates: August 2021 and May 2022.
Editor's Note: Dr. Saguil is a contributing editor for AFP .
The views expressed in this article are those of the authors and do not reflect the official policy of the U.S. Army or the Uniformed Services University of the Health Sciences.
Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380-1390.e2.
Howard J, Trevick S, Younger DS. Epidemiology of multiple sclerosis. Neurol Clin. 2016;34(4):919-939.
Belbasis L, Bellou V, Evangelou E, et al. Environmental risk factors and multiple sclerosis: an umbrella review of systematic reviews and meta-analyses. Lancet Neurol. 2015;14(3):263-273.
Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. 2018;378(2):169-180.
Palmer AJ, van der Mei I, Taylor BV, et al. Modelling the impact of multiple sclerosis on life expectancy, quality-adjusted life years and total lifetime costs: evidence from Australia. Mult Scler. 2020;26(4):411-420.
Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286.
Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647-656.
Antel J, Antel S, Caramanos Z, et al. Primary progressive multiple sclerosis: part of the MS disease spectrum or separate disease entity?. Acta Neuropathol. 2012;123(5):627-638.
Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012;11(2):157-169.
Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545-558.
Lublin FD, Reingold SC National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Defining the clinical course of multiple sclerosis: results of an international survey. Neurology. 1996;46(4):907-911.
Koch-Henriksen N, Magyari M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat Rev Neurol. 2021;17(11):676-688.
Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet. 2018;391(10130):1622-1636.
Kutzelnigg A, Lassmann H. Pathology of multiple sclerosis and related inflammatory demyelinating diseases. Handb Clin Neurol. 2014;122:15-58.
Bø L, Vedeler CA, Nyland HI, et al. Subpial demyelination in the cerebral cortex of multiple sclerosis patients. J Neuropathol Exp Neurol. 2003;62(7):723-732.
Gilmore CP, Geurts JJ, Evangelou N, et al. Spinal cord grey matter lesions in multiple sclerosis detected by post-mortem high field MR imaging. Mult Scler. 2009;15(2):180-188.
Ledesma J, Puttagunta PP, Torabi S, et al. Presenting symptoms and disease severity in multiple sclerosis patients. Neurol Int. 2021;13(1):18-24.
Saguil A, Kane S, Farnell E. Multiple sclerosis: a primary care perspective. Am Fam Physician. 2014;90(9):644-652.
Colombo B, Martinelli Boneschi F, Rossi P, et al. MRI and motor evoked potential findings in nondisabled multiple sclerosis patients with and without symptoms of fatigue. J Neurol. 2000;247(7):506-509.
Brownlee WJ, Hardy TA, Fazekas F, et al. Diagnosis of multiple sclerosis: progress and challenges. Lancet. 2017;389(10076):1336-1346.
Nazari F, Shaygannejad V, Mohammadi Sichani M, et al. Sexual dysfunction in women with multiple sclerosis: prevalence and impact on quality of life. BMC Urol. 2020;20(1):15.
Amato MP, Langdon D, Montalban X, et al. Treatment of cognitive impairment in multiple sclerosis: position paper. J Neurol. 2013;260(6):1452-1468.
Gelfand JM. Multiple sclerosis: diagnosis, differential diagnosis, and clinical presentation. Handb Clin Neurol. 2014;122:269-290.
Ömerhoca S, Akkaş SY, İçen NK. Multiple sclerosis: diagnosis and differential diagnosis. Noro Psikiyatr Ars. 2018;55(suppl 1):S1-S9.
Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173.
Toledano M, Weinshenker BG, Solomon AJ. A clinical approach to the differential diagnosis of multiple sclerosis. Curr Neurol Neurosci Rep. 2015;15(8):57.
Kraft AK, Berger K. Quality of care for patients with multiple sclerosis—a review of existing quality indicators. Front Neurol. 2021;12:708723.
Burton JM, O'Connor PW, Hohol M, et al. Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev. 2012(12):CD006921.
Lattanzi S, Cagnetti C, Danni M, et al. Oral and intravenous steroids for multiple sclerosis relapse: a systematic review and meta-analysis. J Neurol. 2017;264(8):1697-1704.
Le Page E, Veillard D, Laplaud DA, et al.; COPOUSEP investigators; West Network for Excellence in Neuroscience. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomized, controlled, double-blind, non-inferiority trial [published correction appears in Lancet . 2016; 387(10016): 340]. Lancet. 2015;386(9997):974-981.
Smets I, Van Deun L, Bohyn C, et al.; Belgian Study Group for Multiple Sclerosis. Corticosteroids in the management of acute multiple sclerosis exacerbations. Acta Neurol Belg. 2017;117(3):623-633.
Cortese I, Chaudhry V, So YT, et al. Evidence-based guideline update: plasmapheresis in neurologic disorders: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76(3):294-300.
Brochet B, Deloire M, Germain C, et al. Double-blind, randomized controlled trial of therapeutic plasma exchanges vs. sham exchanges in moderate-to-severe relapses of multiple sclerosis. J Clin Apher. 2020;35(4):281-289.
Tanasescu R, Constantinescu CS, Tench CR, et al. Smoking cessation and the reduction of disability progression in multiple sclerosis: a cohort study. Nicotine Tob Res. 2018;20(5):589-595.
Ramanujam R, Hedström AK, Manouchehrinia A, et al. Effect of smoking cessation on multiple sclerosis prognosis. JAMA Neurol. 2015;72(10):1117-1123.
Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis [published correction appears in Mult Scler . 2020; 26(4): 517]. Mult Scler. 2018;24(2):96-120.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology [published correction appears in Neurology . 2019; 92(2): 112]. Neurology. 2018;90(17):777-788.
National Health Service England. Treatment algorithm for multiple sclerosis disease-modifying therapies. Updated March 8, 2019. Accessed November 23, 2021. https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2019/03/Treatment-Algorithm-for-Multiple-Sclerosis-Disease-Modifying-Therapies-08-03-2019-1.pdf
U.S. Food and Drug Administration. Drugs@FDA: FDA-approved drugs. Accessed November 23, 2021. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
Li H, Hu F, Zhang Y, et al. Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol. 2020;267(12):3489-3498.
Vukusic S, Michel L, Leguy S, et al. Pregnancy with multiple sclerosis. Rev Neurol (Paris). 2021;177(3):180-194.
Burt RK, Balabanov R, Burman J, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs. continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA. 2019;321(2):165-174.
National Institute for Health and Care Excellence. Multiple sclerosis in adults: management. Updated November 11, 2019. Accessed November 30, 2021. https://www.nice.org.uk/guidance/cg186/chapter/Recommendations#ms-symptom-management-and-rehabilitation
Haselkorn JK, Hughes C, Rae-Grant A, et al. Summary of comprehensive systematic review: rehabilitation in multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85(21):1896-1903.
Selph SS, Skelly AC, Wasson N, et al. Physical activity and the health of wheelchair users: a systematic review in multiple sclerosis, cerebral palsy, and spinal cord injury. Arch Phys Med Rehabil. 2021;102(12):2464-2481.e33.
Frohman TC, Castro W, Shah A, et al. Symptomatic therapy in multiple sclerosis. Ther Adv Neurol Disord. 2011;4(2):83-98.
Samkoff LM, Goodman AD. Symptomatic management in multiple sclerosis. Neurol Clin. 2011;29(2):449-463.
Filli L, Zörner B, Kapitza S, et al. Monitoring long-term efficacy of fampridine in gait-impaired patients with multiple sclerosis. Neurology. 2017;88(9):832-841.
Koppel BS, Brust JC, Fife T, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556-1563.
Herring MP, Puetz TW, O'Connor PJ, et al. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(2):101-111.
Rietberg MB, Brooks D, Uitdehaag BM, et al. Exercise therapy for multiple sclerosis. Cochrane Database Syst Rev. 2005(1):CD003980.
Nicholas RS, Friede T, Hollis S, et al. Anticholinergics for urinary symptoms in multiple sclerosis. Cochrane Database Syst Rev. 2009(1):CD004193.
Rosti-Otajärvi EM, Hämäläinen PI. Neuropsychological rehabilitation for multiple sclerosis. Cochrane Database Syst Rev. 2014(2):CD009131.
Coggrave M, Norton C, Cody JD. Management of faecal incontinence and constipation in adults with central neurological diseases. Cochrane Database Syst Rev. 2014(1):CD002115.
He D, Zhang Y, Dong S, et al. Pharmacological treatment for memory disorder in multiple sclerosis. Cochrane Database Syst Rev. 2013(12):CD008876.
Xiao Y, Wang J, Luo H. Sildenafil citrate for erectile dysfunction in patients with multiple sclerosis. Cochrane Database Syst Rev. 2012(4):CD009427.
Khan F, Turner-Stokes L, Ng L, et al. Multidisciplinary rehabilitation for adults with multiple sclerosis. Cochrane Database Syst Rev. 2007(2):CD006036.
Khan F, Ng L, Turner-Stokes L. Effectiveness of vocational rehabilitation intervention on the return to work and employment of persons with multiple sclerosis. Cochrane Database Syst Rev. 2009(1):CD007256.
Koch MW, Glazenborg A, Uyttenboogaart M, et al. Pharmacologic treatment of depression in multiple sclerosis. Cochrane Database Syst Rev. 2011(2):CD007295.
Mills RJ, Yap L, Young CA. Treatment for ataxia in multiple sclerosis. Cochrane Database Syst Rev. 2007(1):CD005029.
Shakespeare DT, Boggild M, Young C. Anti-spasticity agents for multiple sclerosis. Cochrane Database Syst Rev. 2001(4):CD001332.
Thomas PW, Thomas S, Hillier C, et al. Psychological interventions for multiple sclerosis. Cochrane Database Syst Rev. 2006(1):CD004431.
Silveira SL, Huynh T, Kidwell A, et al. Behavior change techniques in physical activity interventions for multiple sclerosis. Arch Phys Med Rehabil. 2021;102(9):1788-1800.
Molhemi F, Monjezi S, Mehravar M, et al. Effects of virtual reality vs. conventional balance training on balance and falls in people with multiple sclerosis: a randomized controlled trial. Arch Phys Med Rehabil. 2021;102(2):290-299.
Kim Y, Mehta T, Lai B, et al. Immediate and sustained effects of interventions for changing physical activity in people with multiple sclerosis: meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2020;101(8):1414-1436.
Lincoln NB, Bradshaw LE, Constantinescu CS, et al. Group cognitive rehabilitation to reduce the psychological impact of multiple sclerosis on quality of life: the CRAMMS RCT. Health Technol Assess. 2020;24(4):1-182.
Khan F, Amatya B. Rehabilitation in multiple sclerosis: a systematic review of systematic reviews. Arch Phys Med Rehabil. 2017;98(2):353-367.
Andreu-Caravaca L, Ramos-Campo DJ, Chung LH, et al. Dosage and effectiveness of aerobic training on cardiorespiratory fitness, functional capacity, balance, and fatigue in people with multiple sclerosis: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2021;102(9):1826-1839.
Tramontano M, Russo V, Spitoni GF, et al. Efficacy of vestibular rehabilitation in patients with neurologic disorders: a systematic review. Arch Phys Med Rehabil. 2021;102(7):1379-1389.
Abou L, Alluri A, Fliflet A, et al. Effectiveness of physical therapy interventions in reducing fear of falling among individuals with neurologic diseases: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2021;102(1):132-154.
Minden SL, Feinstein A, Kalb RC, et al.; Guideline Development Subcommittee of the American Academy of Neurology. Evidence-based guideline: assessment and management of psychiatric disorders in individuals with MS. Neurology. 2014;82(2):174-181.
Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. 2013;94(9):1800-1828.e3.
Amatya B, Khan F, La Mantia L, et al. Non pharmacological interventions for spasticity in multiple sclerosis. Cochrane Database Syst Rev. 2013(2):CD009974.
Phé V, Chartier-Kastler E, Panicker JN. Management of neurogenic bladder in patients with multiple sclerosis. Nat Rev Urol. 2016;13(5):275-288.
Van Der Walt A, Sung S, Spelman T, et al. A double-blind, randomized, controlled study of botulinum toxin type A in MS-related tremor. Neurology. 2012;79(1):92-99.
Oliveria SF, Rodriguez RL, Bowers D, et al. Safety and efficacy of dual-lead thalamic deep brain stimulation for patients with treatment-refractory multiple sclerosis tremor: a single-centre, randomised, single-blind, pilot trial. Lancet Neurol. 2017;16(9):691-700.
Motl RW, Sandroff BM, Kwakkel G, et al. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017;16(10):848-856.
Hempel S, Graham GD, Fu N, et al. A systematic review of the effects of modifiable risk factor interventions on the progression of multiple sclerosis. Mult Scler. 2017;23(4):513-524.
Ploughman M. A new era of multiple sclerosis rehabilitation: lessons from stroke. Lancet Neurol. 2017;16(10):768-769.
Boesen F, Nørgaard M, Trénel P, et al. Longer term effectiveness of inpatient multidisciplinary rehabilitation on health-related quality of life in MS patients: a pragmatic randomized controlled trial – The Danish MS Hospitals Rehabilitation Study. Mult Scler. 2018;24(3):340-349.
Abo Youssef N, Schneider MP, Mordasini L, et al. Cannabinoids for treating neurogenic lower urinary tract dysfunction in patients with multiple sclerosis: a systematic review and meta-analysis. BJU Int. 2017;119(4):515-521.
Thompson AJ, Toosy AT, Ciccarelli O. Pharmacological management of symptoms in multiple sclerosis: current approaches and future directions. Lancet Neurol. 2010;9(12):1182-1199.
Goverover Y, Chiaravalloti ND, O'Brien AR, et al. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
Castro-Sánchez AM, Matarán-Peñarrocha GA, Lara-Palomo I, et al. Hydrotherapy for the treatment of pain in people with multiple sclerosis: a randomized controlled trial. Evid Based Complement Alternat Med. 2012;2012:473963.
Yadav V, Bever C, Bowen J, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology. 2014;82(12):1083-1092.
Zhang E, Tian X, Li R, et al. Dalfampridine in the treatment of multiple sclerosis: a meta-analysis of randomised controlled trials. Orphanet J Rare Dis. 2021;16(1):87.
Iaffaldano P, Lucisano G, Patti F, et al.; Italian MS Register. Transition to secondary progression in relapsing-onset multiple sclerosis: definitions and risk factors. Mult Scler. 2021;27(3):430-438.
Calabresi PA. Diagnosis and management of multiple sclerosis. Am Fam Physician. 2004;70(10):1935-1944.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2022 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
The clinical manifestations of MS will be reviewed here. Other aspects of MS are discussed separately:
Pathogenesis and epidemiology of multiple sclerosis
Clinical presentation, course, and prognosis of multiple sclerosis in adults
Management of clinically and radiologically isolated syndromes suggestive of multiple sclerosis
- Patient Care & Health Information
- Diseases & Conditions
- Multiple sclerosis
Neurological exam
A complete neurological exam and medical history are needed to diagnose MS .
- Multiple sclerosis FAQs
Neurologist Oliver Tobin, M.B., B.Ch., B.A.O., Ph.D., answers the most frequently asked questions about multiple sclerosis.
So people who are overweight have a higher chance of developing MS and people who have MS who are overweight tend to have more active disease and a faster onset of progression. The main diet has been shown to be neuroprotective is the Mediterranean diet. This diet is high in fish, vegetables, and nuts, and low in red meat.
So this question comes up a lot because patients who have multiple sclerosis can sometimes get a transient worsening of their symptoms in heat or if they exercise strenuously. The important thing to note is that heat does not cause an MS attack or MS relapse. And so it's not dangerous. You're not doing any permanent damage if this occurs. Exercise is strongly recommended and is protective to the brain and spinal cord.
Scientists do not yet know which stem cells are beneficial in MS, what route to give them or what dose to give them or what frequency. So at the moment, stem cell treatments are not recommended outside of the context of a clinical trial.
Neuromyelitis optica spectrum disorder or NMOSD and MOG-associated disorder can give features similar to multiple sclerosis. These are more common in people of Asian or African-American ethnicity. And your doctor may recommend blood tests to exclude these disorders.
Well, the first drug approved by the FDA for treatment of multiple sclerosis was in 1993. Since then, over 20 drugs have become available for treatment of MS. And the potency of these drugs has increased over time to the point where we can almost completely suppress the inflammatory component of the disease. This would not be possible if patients like you did not enroll in research studies. There are many different types of research studies, not just drug trials, but also observational studies, as all of these enhance our understanding of the disease, hopefully to lead to even better cures for multiple sclerosis.
Well, the most important thing about having a diagnosis of multiple sclerosis is that you are at the center of your medical team. A comprehensive MS center is the best place for management of multiple sclerosis, and this typically includes physicians with expertise in multiple sclerosis, neurologists, but also urologists, physiatrists or physical medicine and rehabilitation providers, psychologists, and many other providers who have specialty interest in multiple sclerosis. Engaging this team around you and your particular needs will improve your outcomes over time.
There are no specific tests for MS . Instead, a diagnosis of multiple sclerosis often relies on ruling out other conditions that might produce similar signs and symptoms, known as a differential diagnosis.
Your doctor is likely to start with a thorough medical history and examination.
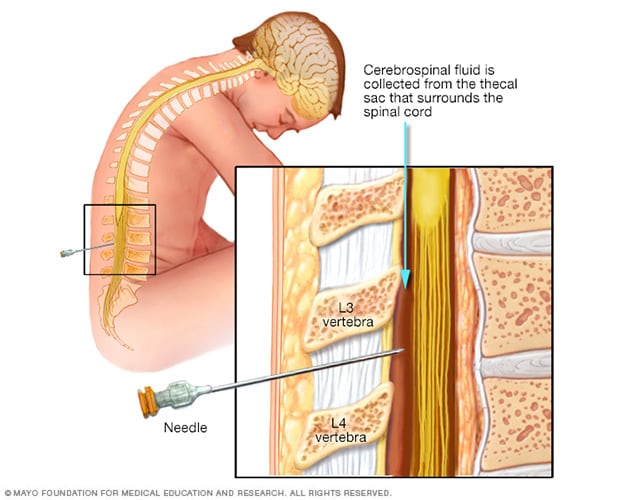
- Lumbar puncture (spinal tap)
During a lumbar puncture, also known as a spinal tap, you typically lie on your side with your knees drawn up to your chest. Then a needle is inserted into your spinal canal — in your lower back — to collect cerebrospinal fluid for testing.
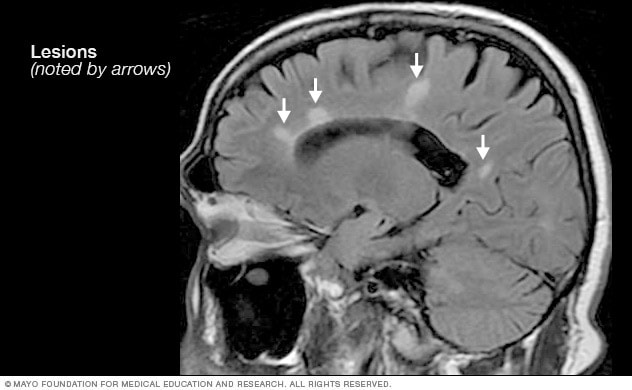
- MRI multiple sclerosis lesions
Brain MRI scan showing white lesions associated with multiple sclerosis.
Your doctor may then recommend:
- Blood tests, to help rule out other diseases with symptoms like MS . Tests to check for specific biomarkers associated with MS are currently under development and may also aid in diagnosing the disease.
- Spinal tap (lumbar puncture), in which a small sample of cerebrospinal fluid is removed from your spinal canal for laboratory analysis. This sample can show abnormalities in antibodies that are associated with MS . A spinal tap can also help rule out infections and other conditions with symptoms like MS . A new antibody test (for kappa free light chains) may be faster and less expensive than previous spinal fluid tests for multiple sclerosis.
- MRI, which can reveal areas of MS (lesions) on your brain, cervical and thoracic spinal cord. You may receive an intravenous injection of a contrast material to highlight lesions that indicate your disease is in an active phase.
- Evoked potential tests that record the electrical signals produced by your nervous system in response to stimuli may be done. An evoked potential test may use visual stimuli or electrical stimuli. In these tests, you watch a moving visual pattern, as short electrical impulses are applied to nerves in your legs or arms. Electrodes measure how quickly the information travels down your nerve pathways.
In most people with relapsing-remitting MS , the diagnosis is straightforward and based on a pattern of symptoms consistent with the disease and confirmed by brain imaging scans, such as an MRI.
Diagnosing MS can be more difficult in people with unusual symptoms or progressive disease. In these cases, further testing with spinal fluid analysis, evoked potentials and additional imaging may be needed.
Brain MRI is often used to help diagnose multiple sclerosis.
- Care at Mayo Clinic
Our caring team of Mayo Clinic experts can help you with your multiple sclerosis-related health concerns Start Here
More Information
Multiple sclerosis care at Mayo Clinic
- Explaining multiple sclerosis
There is no cure for multiple sclerosis. Treatment typically focuses on speeding recovery from attacks, reducing new radiographic and clinical relapses, slowing the progression of the disease, and managing MS symptoms. Some people have such mild symptoms that no treatment is necessary.

Multiple sclerosis research laboratory at Mayo Clinic
Treatments for MS attacks
- Corticosteroids, such as oral prednisone and intravenous methylprednisolone, are prescribed to reduce nerve inflammation. Side effects may include insomnia, increased blood pressure, increased blood glucose levels, mood swings and fluid retention.
- Plasma exchange (plasmapheresis). The liquid portion of part of your blood (plasma) is removed and separated from your blood cells. The blood cells are then mixed with a protein solution (albumin) and put back into your body. Plasma exchange may be used if your symptoms are new, severe and haven't responded to steroids.
Treatments to modify progression
There are several disease modifying therapies (DMTs) for relapsing-remitting MS . Some of these DMTs can be of benefit for secondary progressive MS , and one is available for primary progressive MS .
Much of the immune response associated with MS occurs in the early stages of the disease. Aggressive treatment with these medications as early as possible can lower the relapse rate, slow the formation of new lesions, and potentially reduce risk of brain atrophy and disability accumulation.
Many of the disease-modifying therapies used to treat MS carry significant health risks. Selecting the right therapy for you will depend on careful consideration of many factors, including duration and severity of disease, effectiveness of previous MS treatments, other health issues, cost, and child-bearing status.
Treatment options for relapsing-remitting MS include injectable, oral and infusions medications.
Injectable treatments include:
Interferon beta medications. These drugs used to be the most prescribed medications to treat MS . They work by interfering with diseases that attack the body and may decrease inflammation and increase nerve growth. They are injected under the skin or into muscle and can reduce the frequency and severity of relapses.
Side effects of interferons may include flu-like symptoms and injection-site reactions. You'll need blood tests to monitor your liver enzymes because liver damage is a possible side effect of interferon use. People taking interferons may develop neutralizing antibodies that can reduce drug effectiveness.
- Glatiramer acetate (Copaxone, Glatopa). This medication may help block your immune system's attack on myelin and must be injected beneath the skin. Side effects may include skin irritation at the injection site.
- Monoclonal antibodies. Ofatumumab (Kesimpta, Arzerra) targets cells that damage the nervous system. These cells are called B cells. Ofatumumab is given by an injection under the skin and can decrease multiple sclerosis brain lesions and worsening symptoms. Possible side effects are infections, local reactions to the injection and headaches.
Oral treatments include:
- Teriflunomide (Aubagio). This once-daily oral medication can reduce relapse rate. Teriflunomide can cause liver damage, hair loss and other side effects. This drug is associated with birth defects when taken by both men and women. Therefore, use contraception when taking this medication and for up to two years afterward. Couples who wish to become pregnant should talk to their doctor about ways to speed elimination of the drug from the body. This drug requires blood test monitoring on a regular basis.
- Dimethyl fumarate (Tecfidera). This twice-daily oral medication can reduce relapses. Side effects may include flushing, diarrhea, nausea and lowered white blood cell count. This drug requires blood test monitoring on a regular basis.
- Diroximel fumarate (Vumerity). This twice-daily capsule is similar to dimethyl fumarate but typically causes fewer side effects. It's approved for the treatment of relapsing forms of MS .
- Monomethyl fumarate (Bafiertam) was approved by the FDA as a delayed release medicine that has a slow and steady action. Because of its time release, it is hoped that side effects will be decreased. Possible side effects are flushing, liver injury, abdominal pain and infections.
Fingolimod (Gilenya). This once-daily oral medication reduces relapse rate.
You'll need to have your heart rate and blood pressure monitored for six hours after the first dose because your heart rate may be slowed. Other side effects include rare serious infections, headaches, high blood pressure and blurred vision.
- Siponimod (Mayzent). Research shows that this once-daily oral medication can reduce relapse rates and help slow progression of MS . It's also approved for secondary-progressive MS . Possible side effects include viral infections, liver problems and low white blood cell count. Other possible side effects include changes in heart rate, headaches and vision problems. Siponimod is harmful to a developing fetus, so women who may become pregnant should use contraception when taking this medication and for 10 days after stopping the medication. Some might need to have the heart rate and blood pressure monitored for six hours after the first dose. This drug requires blood test monitoring on a regular basis.
- Ozanimod (Zeposia). This oral medication decreases the relapse rate of multiple sclerosis and is given once a day. Possible side effects are an elevated blood pressure, infections and liver inflammation.
- Ponesimod (Ponvory). This oral medication is taken once a day with a gradually increasing dosing schedule. This medicine has a low relapse rate and has demonstrated fewer brain lesions than some other medications used to treat multiple sclerosis. The possible side effects are respiratory tract infections, high blood pressure, liver irritation and electrical problems in the heart that affect heart rate and rhythm.
- Cladribine (Mavenclad). This medication is generally prescribed as a second line treatment for those with relapsing-remitting MS . It was also approved for secondary-progressive MS . It is given in two treatment courses, spread over a two-week period, over the course of two years. Side effects include upper respiratory infections, headaches, tumors, serious infections and reduced levels of white blood cells. People who have active chronic infections or cancer should not take this drug, nor should women who are pregnant or breastfeeding. Men and women should use contraception when taking this medication and for the following six months. You may need monitoring with blood tests while taking cladribine.
Infusion treatments include:
Natalizumab (Tysabri). This is a monoclonal antibody that has been shown to decrease relapse rates and slow down the risk of disability.
This medication is designed to block the movement of potentially damaging immune cells from your bloodstream to your brain and spinal cord. It may be considered a first line treatment for some people with severe MS or as a second line treatment in others.
This medication increases the risk of a potentially serious viral infection of the brain called progressive multifocal leukoencephalopathy (PML) in people who are positive for antibodies to the causative agent of PML JC virus. People who don't have the antibodies have extremely low risk of PML .
Ocrelizumab (Ocrevus). This treatment reduces the relapse rate and the risk of disabling progression in relapsing-remitting multiple sclerosis. It also slows the progression of the primary-progressive form of multiple sclerosis.
This humanized monoclonal antibody medication is the only DMT approved by the FDA to treat both the relapse-remitting and primary-progressive forms of MS . Clinical trials showed that it reduced relapse rate in relapsing disease and slowed worsening of disability in both forms of the disease.
Ocrelizumab is given via an intravenous infusion by a medical professional. Infusion-related side effects may include irritation at the injection site, low blood pressure, a fever and nausea, among others. Some people may not be able to take ocrelizumab, including those with a hepatitis B infection. Ocrelizumab may also increase the risk of infections and some types of cancer, particularly breast cancer.
Alemtuzumab (Campath, Lemtrada). This treatment is a monoclonal antibody that decreases annual relapse rates and demonstrates MRI benefits.
This drug helps reduce relapses of MS by targeting a protein on the surface of immune cells and depleting white blood cells. This effect can limit potential nerve damage caused by the white blood cells. But it also increases the risk of infections and autoimmune disorders, including a high risk of thyroid autoimmune diseases and rare immune mediated kidney disease.
Treatment with alemtuzumab involves five consecutive days of drug infusions followed by another three days of infusions a year later. Infusion reactions are common with alemtuzumab.
The drug is only available from registered providers, and people treated with the drug must be registered in a special drug safety monitoring program. Alemtuzumab is usually recommended for those with aggressive MS or as second line treatment for patients who failed another MS medication.
Recent developments or emerging therapies
Bruton's tyrosine kinase (BTK) inhibitor is an emerging therapy being studied in relapsing-remitting multiple sclerosis and secondary-progressive multiple sclerosis. It works by mostly modulating B cells, which are immune cells in the central nervous system.
Stem cell transplantation destroys the immune system of someone with multiple sclerosis and then replaces it with transplanted healthy stem cells. Researchers are still investigating whether this therapy can decrease inflammation in people with multiple sclerosis and help to "reset" the immune system. Possible side effects are fever and infections.
Researchers are learning more about how existing disease modifying therapies work to lessen relapses and reduce multiple sclerosis-related lesions in the brain. Further studies will determine whether treatment can delay disability caused by the disease.
For primary-progressive MS , ocrelizumab (Ocrevus) is the only FDA-approved disease-modifying therapy (DMT). Those who receive this treatment are slightly less likely to progress than those who are untreated.
For secondary progressive MS , some might consider the use of FDA-approved disease modifying therapies such as ozanimod, siponimod and cladribine, which can potentially slow down disabilities.
Treatments for MS signs and symptoms

Physical therapy can build muscle strength and ease some of the symptoms of MS .
Therapy. A physical or occupational therapist can teach you stretching and strengthening exercises and show you how to use devices to make it easier to perform daily tasks.
Physical therapy along with the use of a mobility aid, when necessary, can also help manage leg weakness and other gait problems often associated with MS .
- Muscle relaxants. You may experience painful or uncontrollable muscle stiffness or spasms, particularly in your legs. Muscle relaxants such as baclofen (Lioresal, Gablofen), tizanidine (Zanaflex) and cyclobenzaprine may help. Onabotulinumtoxin A treatment is another option in those with spasticity.
- Medications to reduce fatigue. Amantadine (Gocovri, Osmolex), modafinil (Provigil) and methylphenidate (Ritalin) have been used to reduce MS -related fatigue. However, a recent study did not find amantadine, modafinil or methylphenidate to be superior to a placebo in improving MS -related fatigue and caused more frequent adverse events. Some drugs used to treat depression, including selective serotonin reuptake inhibitors, may be recommended.
- Medication to increase walking speed. Dalfampridine (Ampyra) may help to slightly increase walking speed in some people. Possible side effects are urinary tract infections, vertigo, insomnia and headaches. People with a history of seizures or kidney dysfunction should not take this medication.
- Other medications. Medications also may be prescribed for depression, pain, sexual dysfunction, insomnia, and bladder or bowel control problems that are associated with MS .
- Acetyl-L-carnitine: Can it relieve MS fatigue?
- Emerging treatments for multiple sclerosis
Clinical trials
Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition.
Lifestyle and home remedies
To help relieve the signs and symptoms of MS , try to:
- Get plenty of rest. Look at your sleep habits to make sure you're getting the best possible sleep. To make sure you're getting enough sleep, you may need to be evaluated — and possibly treated — for sleep disorders such as obstructive sleep apnea.
- Exercise. If you have mild to moderate MS , regular exercise can help improve your strength, muscle tone, balance and coordination. Swimming or other water exercises are good options if you have intolerance to heat. Other types of mild to moderate exercise recommended for people with MS include walking, stretching, low-impact aerobics, stationary bicycling, yoga and tai chi.
- Cool down. MS symptoms may worsen when the body temperature rises in some people with MS . Avoiding exposure to heat and using devices such as cooling scarves or vests can be helpful.
- Eat a balanced diet. Since there is little evidence to support a particular diet, experts recommend a generally healthy diet. Some research suggests that vitamin D may have potential benefit for people with MS .
- Relieve stress. Stress may trigger or worsen your signs and symptoms. Yoga, tai chi, massage, meditation or deep breathing may help.
- Exercise and multiple sclerosis
- Vitamin D and MS: Any connection?
- Vitamins for MS: Do supplements make a difference?
Alternative medicine
Many people with MS use a variety of alternative or complementary treatments or both to help manage their symptoms, such as fatigue and muscle pain.
Activities such as exercise, meditation, yoga, massage, eating a healthier diet, acupuncture and relaxation techniques may help boost overall mental and physical well-being in patients with MS .
According to guidelines from the American Academy of Neurology, research strongly indicates that oral cannabis extract (OCE) may improve symptoms of muscle spasticity and pain. There is a lack of evidence that cannabis in any other form is effective in managing other MS symptoms.
Daily intake of vitamin D3 of 2,000 to 5,000 international units daily is recommended in those with MS . The connection between vitamin D and MS is supported by the association with exposure to sunlight and the risk of MS .
Coping and support
Living with any chronic illness can be difficult. To manage the stress of living with MS , consider these suggestions:
- Maintain normal daily activities as best you can.
- Stay connected to friends and family.
- Continue to pursue hobbies that you enjoy and are able to do.
- Contact a support group, for yourself or for family members.
- Discuss your feelings and concerns about living with MS with your doctor or a counselor.
Preparing for your appointment
You may be referred to a doctor who specializes in disorders of the brain and nervous system (neurologist).
What you can do
- Write down your symptoms, including any that may seem unrelated to the reason why you scheduled the appointment.
- Make a list of all your medications, vitamins and supplements.
- Bring any clinical notes , scans, laboratory test results or other information from your primary care provider to your neurologist.
- Write down your key medical information, including other conditions.
- Write down key personal information, including any recent changes or stressors in your life.
- Write down questions to ask your doctor.
- Ask a relative or friend to accompany you, to help you remember what the doctor says.
What to expect from your doctor
Your doctor is likely to ask you questions. Being ready to answer them may reserve time to go over points you want to spend more time on. You may be asked:
- When did you begin experiencing symptoms?
- Have your symptoms been continuous or occasional?
- How severe are your symptoms?
- What, if anything, seems to improve your symptoms?
- What, if anything, appears to worsen your symptoms?
- Does anyone in your family have multiple sclerosis?
Questions to ask your doctor
- What's the most likely cause of my symptoms?
- What kinds of tests do I need? Do they require any special preparation?
- Is my condition likely temporary or chronic?
- Will my condition progress?
- What treatments are available?
- I have these other health conditions. How can I best manage them together?
In addition to the questions that you've prepared to ask your doctor, don't hesitate to ask other questions during your appointment.
- What is multiple sclerosis? National Multiple Sclerosis Society. https://www.nationalmssociety.org/What-is-MS. Accessed June 2, 2022.
- Daroff RB, et al. Multiple sclerosis and other inflammatory demyelinating diseases of the central nervous system. In: Bradley's Neurology in Clinical Practice. 7th ed. Philadelphia, Pa.: Elsevier Saunders; 2012. https://www.clinicalkey.com. Accessed June 2, 2022.
- Ferri FF. Multiple sclerosis. In: Ferri's Clinical Advisor 2019. Philadelphia, Pa.: Elsevier; 2019. https://www.clinicalkey.com. Accessed June 2, 2022.
- Olek MJ. Clinical presentation, course, and prognosis of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Wingerchuk DM (expert opinion). Mayo Clinic, Phoenix/Scottsdale, Ariz. Jan. 21, 2019.
- Ciccarelli O. Multiple sclerosis in 2018: New therapies and biomarkers. The Lancet. 2019; doi: 10.1016/S14744422 (18)30455-1.
- Keegan BM. Therapeutic decision making in a new drug era in multiple sclerosis. Seminars in Neurology. 2013; doi:10.1055/s0033-1345709.
- Goldman L, et al., eds. Multiple sclerosis and demyelinating conditions of the central nervous system. In: Goldman-Cecil Medicine. 25th ed. Philadelphia, Pa.: Saunders Elsevier; 2016. https://www.clinicalkey.com. Accessed Jun. 2, 2022.
- Lotze TE. Pathogenesis, clinical features, and diagnosis of pediatric multiple sclerosis. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Kantarci OH, et al. Novel immunomodulatory approaches for the management of multiple sclerosis. Clinical Pharmacology & Therapeutics. 2014; doi:10.1038/clpt.2013.196.
- Olek MJ. Disease-modifying treatment of relapsing-remitting multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Olek MJ, et al. Treatment of acute exacerbations of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Wingerchuk DM. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clinic Proceedings. 2014; doi:10.1016/j.mayocp.2013.11.002.
- Pizzorno JE, et al. Multiple sclerosis. In: Textbook of Natural Medicine. 4th ed. St. Louis, Mo.: Churchill Livingstone Elsevier; 2013. https://www.clinicalkey.com. Accessed June 2, 2022.
- Olek MJ, et al. Evaluation and diagnosis of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Gaetani L, et al. 2017 revisions of McDonald criteria shorten the time to diagnosis of multiple sclerosis in clinically isolated syndromes. Journal of Neurology. 2018;265:2684.
- http://onlinelibrary.wiley.com/doi/10.1002/ana.22366.
- Olek MJ, et al. Pathogenesis and epidemiology of multiple sclerosis.
- Ingram G, et al. Cannabis and multiple sclerosis. Practical Neurology. 2019; doi:10.1136/practneurol-2018-002137.
- Olek MJ, et al. Symptom management of multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed June 2, 2022.
- Yadav Y, et al. Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis. Neurology. 2014; doi: 10.1212/WNL.0000000000000250.
- Nimmagadda R. Allscripts EPSi. Mayo Clinic. April 22, 2022.
- National MS Society. Network of Pediatric MS Centers. https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS/Pediatric-MS/Care-for-Pediatric-MS. Accessed June 2, 2022.
- Rodriguez M. Plasmapheresis in acute episodes of fulminant CNS inflammatory demyelination. Neurology. 1993; doi:10.1212/wnl.43.6.1100.
- Deb C. CD8+ T cells cause disability and axon loss in a mouse model of multiple sclerosis. PLoS One. 2010; doi:101371/journal.pone.0012478.
- FDA approves new drug to treat multiple sclerosis. U.S. Food & Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm549325.htm. Accessed June 1, 2022.
- Keegan BM (expert opinion). Mayo Clinic, Rochester, Minn. January 15, 2019.
- FDA approves new oral drug to treat multiple sclerosis. U.S. Food and Drug Administration. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm634469.htm. Accessed June 2, 2022.
- Kappos L, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): A double-blind, randomized, phase 3 study. The Lancet. 2018; doi: 10.1016/S0140-6736(18)30475-6.
- Marin Collazo IV (expert opinion). Mayo Clinic, Rochester, Minn. April 2, 2019.
- AskMayoExpert. Multiple sclerosis. Mayo Clinic; 2020.
- AskMayoExpert. Medication monitoring guidelines. Mayo Clinic; 2020.
- Vumerity. National MS Society. https://www.nationalmssociety.org/Treating-MS/Medications/Vumerity. Accessed March 16, 2020.
- Gianfrancesco M, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obesity Research and Clinical Practice. 2014; doi.org/10.1016/j.orcp.2014.01.002.
- Pantavou KG, et al. Season of birth and multiple sclerosis: A systematic review and multivariate meta-analysis. Journal of Neurology. 2020; doi:10.1007/s00415019-09346-5.
- Cifu DX, et al., eds. Multiple sclerosis. In Braddom's Physical Medicine and Rehabilitation. 6th ed. Elsevier; 2021 https://www.clinicalkey.com. Accessed Jun. 2, 2022.
- Langer-Gould AM, et al. Racial and ethnic disparities in multiple sclerosis prevalence. Neurology. 2022; doi:10.1212/WNL.0000000000200151.
- Kasper LH, et al. Immunomodulatory activity of interferon-beta. Annals of Clinical and Translational Neurology. 2014; doi:10.1002/acn3.84.
- Goldschmidt CH, et al. Re-evaluating the use of IFN-B and relapsing multiple sclerosis: Safety, efficacy and place in therapy. Degenerative Neurological and Neuromuscular Disease. 2020; doi:10.2147/DNND.S224912.
- Kieseie BC. The mechanism of action of interferon-B in relapsing multiple sclerosis. Central Nervous System Drugs. 2011; doi:10.1007/s10067-008-0972-3.
- Betaseron. Bayer AG; 1993. www.bayer.com. Accessed Jun. 1, 2022.
- Hauser SL, et al. Ofatumumab versus teriflunomide in multiple sclerosis. The New England Journal of Medicine. 2020; doi:10.1056/NEJMoa1917246.
- Kesimpta. Novartis; 2020. www.novartis.com. Accessed Jun. 1, 2022.
- Marin Collazo V (expert opinion). Mayo Clinic. June 13, 2020.
- Olek MJ. Treatment of progressive multiple sclerosis in adults. https://www.uptodate.com/contents/search. Accessed Jun. 2, 2022.
- Wingerchuk DM, et al. Disease modifying therapies for relapsing multiple sclerosis. British Medical Journal. 2016; doi:10.1136/bmj.i3518.
- Saadeh RS, et al. CSF kappa free light chains: Cutoff validation for diagnosing multiple sclerosis. Mayo Clinic Proceedings. 2022; doi:10.1016/j.mayocp.2021.09.014.
- Goldschmidt C, et al. Advances in the treatment of multiple sclerosis. Neurologic Clinics. 2021; doi:10.1016/j.ncl.2020.09.002.
- Bafiertam. Banner Life Sciences LLC; 2013. www.bannerls.com. Accessed Jun. 1, 2022.
- Baliertam delayed release capsule. Banner Life Sciences LLC; 2013. www.bannerls.com. Accessed Jun. 1, 2022.
- Oral ponesimod versus teriflunomide in relapsing multiple sclerosis (OPTIMUM). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02425644. Accessed Jun. 2, 2022.
- Ponvory. Janssen Pharmaceuticals; 2021. www.janssen.com. Accessed Jun. 1, 2022.
- Torke S, et al. Inhibition of Bruton's tyrosine kinase as a novel therapeutic approach in multiple sclerosis. Expert Opinion on Investigational Drugs. 2020.
- Nash RA, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): A 3-year interim report. Journal of the American Medical Association Neurology. 2015; doi:10.1001/jamaneurol.2014.3780.
- Reston, et al. Autologous hematopoietic cell transplantation for multiple sclerosis: A systematic review. Multiple Sclerosis. 2011; doi:10,1177/1352458510383609.
- Petrou P, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. 2020; doi:10.1093/brain/awaa333.
- Liang J, et al. Allogenic mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Multiple Sclerosis. 2009; doi:10.1177/1352458509104590.
- Wingerchuk DM, et al. Multiple sclerosis: Current and emerging disease-modifying therapies and treatment strategies. Mayo Clinic Proceedings. 2014; doi:101016/j.mayocp.2013.11.002.
- Multiple sclerosis information page. National institute of neurological disorders and stroke. https://www.ninds.nih.gov/Disorders/All-Disorders/Multiple-Sclerosis-Information-Page. Accessed Jun. 2, 2022.
- Sadovnick AD. Genetic background of multiple sclerosis. Autoimmunity Reviews. 2012; doi:10.1016/j.autrev.2011.05.007.
- Demyelinating disease: What can you do about it?
- Infographic: Multiple Sclerosis
- Multiple sclerosis: Can it cause seizures?
- Myelin damage and the nervous system
- Physical therapy for multiple sclerosis
- What is multiple sclerosis? An expert explains
Associated Procedures
News from mayo clinic.
- Mayo Clinic Q&A podcast: Advances in managing MS July 12, 2022, 02:29 p.m. CDT
Products & Services
- A Book: Mayo Clinic Family Health Book, 5th Edition
- Newsletter: Mayo Clinic Health Letter — Digital Edition
Mayo Clinic in Rochester, Minnesota, Mayo Clinic in Phoenix/Scottsdale, Arizona, and Mayo Clinic in Jacksonville, Florida, have been ranked among the best Neurology & Neurosurgery hospitals in the nation for 2023-2024 by U.S. News & World Report.
- Symptoms & causes
- Diagnosis & treatment
- Doctors & departments
Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission.
- Opportunities
Mayo Clinic Press
Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press .
- Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence
- The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book
- Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance
- FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment
- Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
Multiple Sclerosis (MS)
- Pathophysiology |
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Prognosis |
- Key Points |
Multiple sclerosis (MS) is characterized by disseminated patches of demyelination in the brain and spinal cord. Common symptoms include visual and oculomotor abnormalities, paresthesias, weakness, spasticity, urinary dysfunction, and mild cognitive symptoms. Typically, neurologic deficits are multiple, with remissions and exacerbations gradually producing disability. Diagnosis requires clinical or MRI evidence of ≥ 2 characteristic neurologic lesions that are separated in both time and space (location in the central nervous system). Treatment includes corticosteroids for acute exacerbations, immunomodulatory medications to prevent exacerbations, and supportive measures.
(See also Overview of Demyelinating Disorders .)
Multiple sclerosis is believed to involve an immunologic mechanism. One postulated cause is infection by a latent virus (possibly a human herpesvirus such as Epstein-Barr virus ), which, when activated, triggers a secondary autoimmune response.
An increased incidence among certain families and presence of human leukocyte antigen (HLA) allotypes (HLA-DR2) suggests genetic susceptibility.
Age at onset ranges from 15 to 60 years, typically 20 to 40 years; women are affected somewhat more often.
Neuromyelitis optica spectrum disorder (Devic disease), previously considered a variant of MS, is now recognized as a separate disorder.
Pathophysiology of Multiple Sclerosis
Localized areas of demyelination (plaques) occur, with destruction of oligodendroglia, perivascular inflammation, and chemical changes in lipid and protein constituents of myelin in and around the plaques. Axonal damage is common, and neuronal cell bodies may also die or be damaged.
Fibrous gliosis develops in plaques that are disseminated throughout the central nervous system (CNS), primarily in white matter, particularly in the lateral and posterior columns (especially in the cervical regions), optic nerves, and periventricular areas. Tracts in the midbrain, pons, and cerebellum are also affected. Gray matter in the cerebrum and spinal cord can be affected but to a much lesser degree.
Symptoms and Signs of Multiple Sclerosis
Multiple sclerosis is characterized by varied CNS deficits, with remissions and recurring exacerbations. When MS is not treated with immunomodulating medications, exacerbations average about 1 every 2 years, but frequency varies greatly.
Although MS may progress and regress unpredictably, there are typical patterns of progression:
Relapsing-remitting pattern: Exacerbations alternate with remissions, when partial or full recovery occurs or symptoms are stable. Remissions may last months or years. Exacerbations can occur spontaneously or can be triggered by an infection such as influenza. Relapsing forms of MS include active secondary MS (defined as a clinical relapse or new lesion seen on an MRI scan of the brain or spinal cord).
Primary progressive pattern: The disease progresses gradually with no remissions, although there may be temporary plateaus during which the disease does not progress. Unlike in the relapsing-remitting pattern, there are no clear exacerbations.
Secondary progressive pattern: This pattern begins with relapses alternating with remissions (relapsing-remitting pattern), followed by gradual progression of the disease.
Progressive relapsing pattern: The disease progresses gradually, but progression is interrupted by sudden, clear relapses. This pattern is rare.
The most common initial symptoms of multiple sclerosis are the following:
Paresthesias in one or more extremities, in the trunk, or on one side of the face
Weakness or clumsiness of a leg or hand
Visual disturbances (eg, partial loss of vision and pain in one eye due to retrobulbar optic neuritis, diplopia due to internuclear ophthalmoplegia, scotomas)
Other common early symptoms of MS include slight stiffness or unusual fatigability of a limb, minor gait disturbances, vertigo, and mild affective disturbances; all usually indicate scattered CNS involvement and may be subtle. Most patients with MS have difficulty with bladder control (eg, frequency, urgency, hesitancy, incontinence , retention ). Fatigue is common. Excess heat (eg, warm weather, a hot bath, fever) may temporarily exacerbate symptoms and signs (Uhthoff phenomenon).
Mild cognitive symptoms are common. Apathy, poor judgment, or inattention may occur. Affective disturbances, including emotional lability, euphoria, or, most commonly, depression, are common. Depression may be reactive or partly due to cerebral lesions of MS. A few patients have seizures.
Cranial nerves
Unilateral or asymmetric optic neuritis and bilateral internuclear ophthalmoplegia are typical.
Central vision is affected more than peripheral vision.
Optic neuritis causes loss of vision (ranging from scotomas to blindness), eye pain during eye movement, and sometimes abnormal visual fields, a swollen optic disk, or a partial or complete afferent pupillary defect.
Internuclear ophthalmoplegia results if there is a lesion in the medial longitudinal fasciculus connecting the 3rd, 4th, and 6th nerve nuclei. During horizontal gaze, adduction of one eye is decreased, with nystagmus of the other (abducting) eye; convergence is intact. In MS, internuclear ophthalmoplegia is typically bilateral; unilateral internuclear ophthalmoplegia is often caused by ischemic stroke.
Rapid, small-amplitude eye oscillations in straight-ahead (primary) gaze (pendular nystagmus) are uncommon but characteristic of MS. Vertigo is common. Intermittent unilateral facial numbness or pain (resembling trigeminal neuralgia ), palsy, or spasm may occur. Mild dysarthria may occur, caused by bulbar weakness, cerebellar damage, or disturbance of cortical control. Other cranial nerve deficits are unusual but may occur secondary to brain stem injury.
Weakness is common. It usually reflects corticospinal tract damage in the spinal cord, affects the lower extremities preferentially, and is bilateral and spastic.
Deep tendon reflexes (eg, knee and ankle jerks) are usually increased, and an extensor plantar response ( Babinski sign ) and clonus are often present. Spastic paraparesis produces a stiff, imbalanced gait; in advanced cases, it may confine patients to a wheelchair. Painful flexor spasms in response to sensory stimuli (eg, bedclothes) may occur late. Cerebral or cervical spinal cord lesions may result in hemiparesis, which sometimes is the presenting symptom.
Reduced mobility increases the risk of osteoporosis.
In advanced MS, cerebellar ataxia plus spasticity may be severely disabling; other cerebellar manifestations include slurred speech, scanning speech (slow enunciation with a tendency to hesitate at the beginning of a word or syllable), and Charcot triad (intention tremor, scanning speech, and nystagmus).
Paresthesias and partial loss of any type of sensation are common and often localized (eg, to one or both hands or legs).
Various painful sensory disturbances (eg, burning or electric shocklike pains) can occur spontaneously or in response to touch, especially if the spinal cord is affected. An example is Lhermitte sign, an electric shocklike pain that radiates down the spine or into the legs or arms when the neck is flexed.
Objective sensory changes tend to be transient and difficult to demonstrate early in the disease.
Spinal cord
Involvement commonly causes bladder dysfunction (eg, urinary urgency or hesitancy, partial retention of urine, mild urinary incontinence). Constipation, erectile dysfunction in men, and genital anesthesia in women may occur. Frank urinary and fecal incontinence may occur in advanced MS.
Spinal cord lesions (plaques) are a common source of neuropathic pain.
Progressive myelopathy , a variant of MS, causes spinal cord motor weakness but no other deficits.
Diagnosis of Multiple Sclerosis
Clinical criteria
Brain and spinal MRI
Sometimes cerebrospinal fluid (CSF) IgG levels and evoked potentials
Multiple sclerosis is suspected in patients with optic neuritis , internuclear ophthalmoplegia , or other symptoms that suggest MS, particularly if deficits are multifocal or intermittent. If MS is suspected, brain MRI and spinal MRI are done.
MRI is the most sensitive imaging test for MS and can exclude other treatable disorders that may mimic MS, such as nondemyelinating lesions at the junction of the spinal cord and medulla (eg, subarachnoid cyst, foramen magnum tumors). Gadolinium-contrast enhancement can distinguish actively inflamed from older plaques. Also, higher-field MRI magnets (3 to 7 Tesla) can distinguish perivenular MS plaques from nonspecific white-matter lesions.
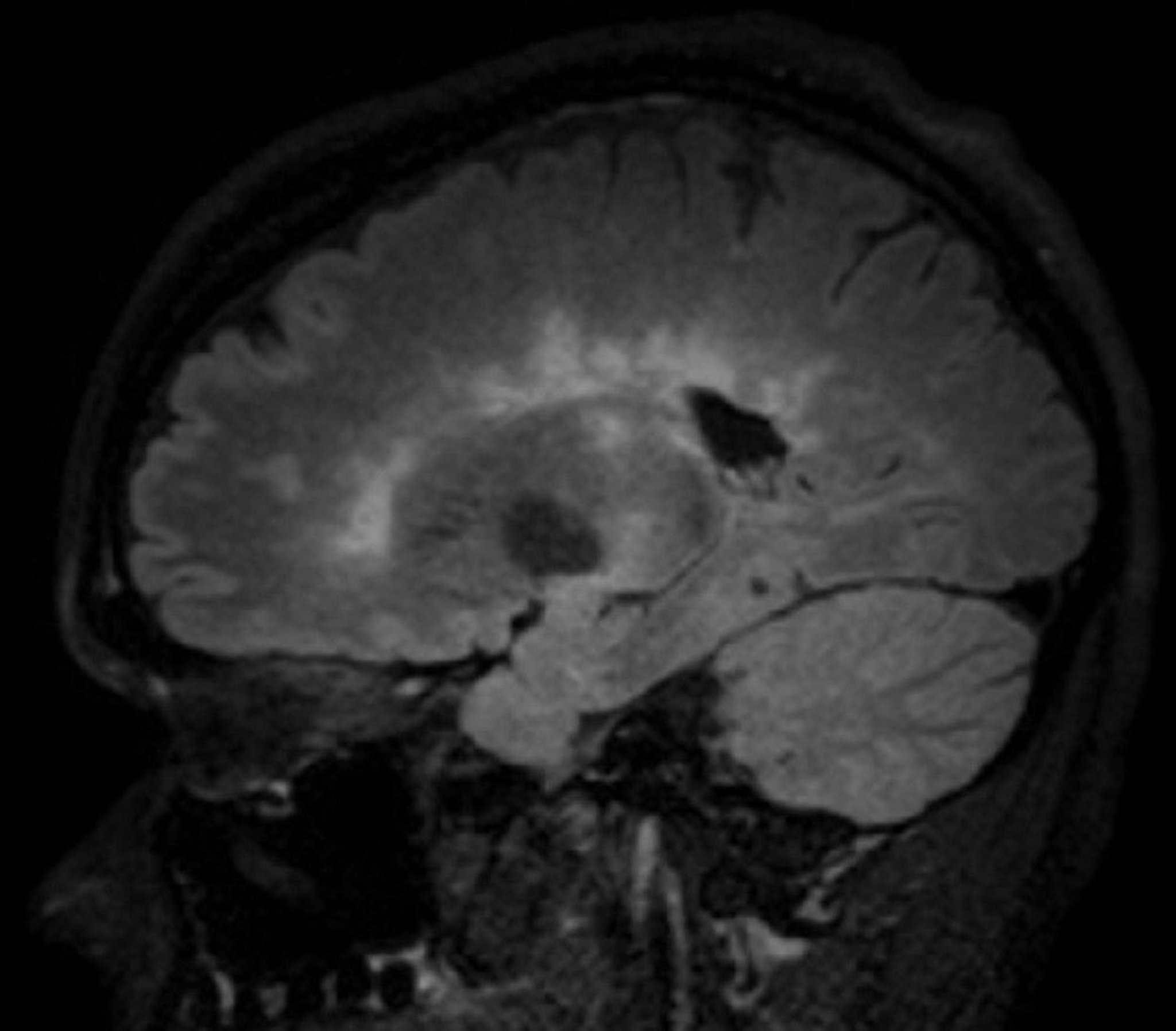
© 2017 Elliot K. Fishman, MD.
MS must be distinguished from the following:
Clinically isolated syndromes (consisting of only a single clinical manifestation typical of MS)
Radiologically isolated syndrome (MRI findings typical of MS that are incidentally noted in patients with no clinical manifestations)
MS can be distinguished because diagnosis of MS requires evidence of CNS lesions that are separated in both time and space (location in the CNS). For example, any of the following can indicate separation in time:
A history of exacerbations and remissions
MRI that shows simultaneous enhancing and nonenhancing lesions, even if patients are asymptomatic
A new lesion on a subsequent MRI in patients with a previous lesion
Separation (dissemination) in space can be established by finding lesions in ≥ 2 of the 5 following CNS areas typically affected by MS ( 1 ):
Periventricular: ≥ 3 lesions
Cortical/juxtacortical (white matter next to cortex and/or cortex): ≥ 1 lesions
Infratentorial: ≥ 1 lesions
Spinal cord: ≥ 1 lesions
Optic nerve: ≥ 1 lesions (either by MRI or clinical evaluation)
Additional testing
If MRI plus clinical findings are not diagnostic, additional testing may be necessary to objectively demonstrate separate neurologic abnormalities. Such testing may include evoked potentials and, occasionally, CSF examination or blood tests.
Evoked potentials (delays in electrical responses to sensory stimulation) are often more sensitive for MS than symptoms or signs. Visual evoked responses are sensitive and particularly helpful in patients with no confirmed cranial lesions (eg, those with lesions only in the spinal cord). Somatosensory evoked potentials and brain stem auditory evoked potentials are sometimes also measured.
CSF examination is being done less frequently (because the diagnosis can usually be based on MRI) but can be helpful if MRI plus clinical findings are inconclusive or if infection (eg, CNS Lyme disease <
Blood tests may be necessary. Sometimes systemic disorders (eg, SLE ) and infections (eg, Lyme disease ) can mimic MS and should be excluded with specific blood tests. Blood tests to measure an IgG antibody specific for neuromyelitis optica spectrum disorder (aquaporin-4 antibody [also known as NMO-IgG] and anti-MOG [myelin oligodendrocyte glycoprotein] antibodies) may be done to differentiate that disorder from MS.
Diagnosis reference
1. Filippi M, Rocca MA, Ciccarelli O, et al : MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol 15 (3):292–303, 2016. doi: 10.1016/S1474-4422(15)00393-2
Treatment of Multiple Sclerosis
Corticosteroids
Immunomodulators to prevent exacerbations and delay eventual disability
Supportive care
Goals for treatment of multiple sclerosis include the following:
Shortening acute exacerbations
Decreasing frequency of exacerbations
Relieving symptoms
Delaying disability, particularly maintaining the patient’s ability to walk
Treatment of exacerbations and relapses
Corticosteroids , given in brief courses, are used to treat acute onset of symptoms or exacerbations that cause objective deficits sufficient to impair function (eg, loss of vision, strength, or coordination); regimens include
1 , 2 ). Some evidence indicates that IV corticosteroids shorten acute exacerbations, slow progression, and improve MRI measures of disease.
If corticosteroids are ineffective in reducing the severity of an exacerbation, plasma exchange may be used. Plasma exchange can be used for any relapsing form of MS (relapsing-remitting, progressive relapsing, secondary progressive). It is not used for primary progressive MS.
Plasma exchange and hematopoietic stem cell transplantation may be somewhat useful for severe, intractable disease.
Disease-modifying therapies
For additional information, see Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis .
Common adverse effects of interferons include flu-like symptoms and depression (which tend to decrease over time), development of neutralizing antibodies after months of therapy, and cytopenias.
The following oral immunomodulatory medications can be used to treat relapsing forms of MS, including active secondary MS.
3 , 4 , 5 ).
Because most people are averse to self-injection, oral immunomodulatory medications are being increasingly used as first-line treatments for relapsing forms of MS.
Disease-modifying therapies can be used to treat relapsing forms of MS. There is no consensus regarding choice of disease-modifying immunomodulatory therapy. Many experts recommend patient education and shared decision-making, including when disease-modifying therapies are offered to patients who have > 1 lesion (seen on imaging) and a clinically isolated syndrome. If one medication is ineffective, a different one can be tried.
progressive multifocal leukoencephalopathy (PML).
Medications that increase the risk of PML include the following (in descending order of risk):
If any of these medications are used, consultation with a neurologist with training in MS is highly recommended. Before these medications are started, blood tests should be done to check for antibodies to JC virus (JCV), which causes PML. Based on the results, the following is done:
If results are positive, patients should be counseled about the risk of PML.
If results are negative, antibody tests should be done every 6 months as long as any of these medications is used because seroconversion is common.
If test results become positive, patients should be counseled again about the risk, and clinicians should consider switching to a medication without this risk.
If the high-risk medication is continued, MRI of the brain should be done about every 6 months.
Development of PML symptoms (eg, aphasia, change in mental status, hemianopia, ataxia) requires immediate brain MRI, with and without gadolinium. MRI can often distinguish PML from MS. After MRI, a lumbar puncture plasma exchange can be done to remove the medication quickly, and if immune reconstitution inflammatory syndrome (IRIS) develops, corticosteroids are given.
Pearls & Pitfalls
9 , 10 ). Treatments should be tailored to the patient and managed by MS specialists with expertise in their use.
If immunomodulatory medications are ineffective, monthly IV immune globulin may help.
Symptom control
Other treatments can be used to control specific symptoms:
Problems with gait
Painful paresthesias
Depression is treated with counseling and antidepressants .
Bladder dysfunction is treated based on its underlying mechanism.
Constipation may be treated with stool softeners or laxatives, taken regularly.
Tremor: 11 ).
Encouragement and reassurance help patients with multiple sclerosis.
Regular exercise (eg, stationary biking, treadmill, swimming, stretching, balance exercises), with or without physical therapy, is recommended, even for patients with advanced MS, because exercise conditions the heart and muscles, reduces spasticity, prevents contractures and falls, and has psychologic benefits.
Patients should maintain as normal and active a life as possible but should avoid overwork, fatigue, and exposure to excess heat. Cigarette smoking should be stopped.
Vaccination does not appear to increase risk of exacerbations.
Debilitated patients require measures to prevent pressure ulcers and urinary tract infections ; intermittent urinary self-catheterization may be necessary.

Treatment references
1. Le Page E, Veillard D, Laplaud DA, et al Lancet 386 (9997):974–981, 2015. doi: 10.1016/S0140-6736(15)61137-0
2. Burton JM, O'Connor PW, Hohol M, Beyene J : Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev 12:CD006921, 2012. doi: 10.1002/14651858.CD006921.pub3
3. Freedman MS, Devonshire V, Duquette P, et al : Treatment optimization in multiple sclerosis: Canadian MS working group recommendations. Can J Neurol Sci 47 (4):437–455, 2020. doi: 10.1017/cjn.2020.66 Epub 2020 Apr 6
4. Li H, Hu F, Zhang Y, Li K : Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing–remitting multiple sclerosis: A systematic review and network meta-analysis. J Neurol 267(12):3489-3498, 2020. doi: 10.1007/s00415-019-09395-w Epub 2019 May 25
5. Rae-Grant A, Day GS, Ruth Ann Marrie RA, et al : Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 90 (17):777–788, 2018. doi: 10.1212/WNL.0000000000005347
6. Hauser SL, Bar-Or A, Comi G, et al N Engl J Med 376 (3):221–234, 2017. doi: 10.1056/NEJMoa1601277
7. Hauser SL, Bar-Or A, Cohen JA, et al N Engl J Med 383 (6):546–557, 2020. doi: 10.1056/NEJMoa1917246
8. Granqvist M, Boremalm M , Poorghobad A, et al JAMA Neurol 75 (3):320–327, 2018. doi: 10.1001/jamaneurol.2017.4011
9. Casanova B, Quintanilla-Bordás C, Gascón F : Escalation vs. early intense therapy in multiple sclerosis. J Pers Med 12 (1):119, 2022. doi: 10.3390/jpm12010119
10. Simonsen CS, Flemmen HO, Broch, L, et al : Early high efficacy treatment in multiple sclerosis is the best predictor of future disease activity over 1 and 2 years in a Norwegian population-based registry. Front Neurol 12:693017, 2021. doi: 10.3389/fneur.2021.693017
11. Makhoul K, Ahdab R, Riachi N, et al : Tremor in multiple sclerosis-An overview and future perspectives. Brain Sci 10 (10):722, 2020. doi: 10.3390/brainsci10100722
12. Multiple Sclerosis Society of Canada Public Health Nutr (23) 7: 1278–1279, 2020.
Prognosis for Multiple Sclerosis
The course of multiple sclerosis is highly varied and unpredictable. In most patients, especially when MS begins with optic neuritis, remissions can last months to > 10 years.
Most patients (60 to 80% [ 1 ]) who initially have a clinically isolated syndrome eventually develop MS, with a second lesion becoming evident or MRI detecting a lesion, usually within 5 years after the initial symptoms begin. Treatment with disease-modifying therapies can delay this progression. If patients have a radiologically isolated syndrome without a history of a clinical episode consistent with demyelination, the risk of developing MS is 19 to 90%, depending on the patient's age and the presence of spinal cord or gadolinium-enhancing lesions ( 2 ).
If the initial brain or spinal MRI shows more extensive disease, patients may be at risk of earlier disability, as may patients who have motor, bowel, and/or bladder symptoms when they present or who have incomplete recovery during relapses. Some patients, such as men with onset in middle age and with frequent exacerbations, can become rapidly incapacitated. Cigarette smoking may accelerate disease progression.
Life span is shortened only in very severe cases.
Prognosis references
1. National Multiple Sclerosis Society : Clinically isolated syndrome (CIS). Accessed 5/1/23.
2. Lebrun-Frénay C, Rollot F, Mondot L, et al : Risk factors and time to clinical symptoms of multiple sclerosis among patients with radiologically isolated syndrome. JAMA Netw Open 4 (10):e2128271, 2021. doi: 10.1001/jamanetworkopen.2021.28271
Multiple sclerosis involves demyelination of the CNS; MS may progress unpredictably but has several typical patterns of progression.
The most common symptoms are paresthesias, weakness or clumsiness, and visual symptoms, but a wide variety of symptoms are possible.
MS is confirmed if MRI and clinical findings establish characteristic lesions that are separate in time and space; however, progression to MS is likely if patients have even a single characteristic clinical deficit or possibly a single radiologic lesion.
Treat patients with corticosteroids (for severe exacerbations) and immunomodulatory medications (to delay or prevent exacerbations).
Treat patients supportively, using medications to treat symptoms (eg, spasticity, painful paresthesias, depression, bladder dysfunction, fatigue, gait problems) when warranted.

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cold Spring Harb Perspect Med
- v.8(9); 2018 Sep
Clinical Course of Multiple Sclerosis
The 1996 originally established multiple sclerosis (MS) subtypes, based solely on clinical impression and consensus, were revised in 2013 to review potential imaging and biological correlates and to reflect recently identified clinical aspects of MS. As a result, potential new disease phenotypes, radiologically isolated syndrome, and clinically isolated syndrome were considered along with the addition of two new descriptor subtypes: activity and progression applied to relapsing remitting and progressive MS phenotypes. In this way, the description of an individual patient’s disease course is refined and provides temporal information about the ongoing disease process. There is still a lack of imaging and biological markers that would distinguish MS phenotypes and prognosticate the disease course on an individual patient’s level, creating a pressing need for large collaborative research efforts in this field.
Multiple sclerosis (MS), a chronic, inflammatory disease of the central nervous system (CNS) with hallmarks of demyelination and axonal degeneration, is characterized by heterogeneity in the symptoms, disease course, and outcomes ( Compston and Coles 2008 ). Typically affecting patients between 20 and 40 years of age, MS is a leading cause of disability in young adults in the United States and Europe ( Tullman 2013 ).
MS is considered to be an autoimmune disease mediated by autoreactive T helper (Th)1 and Th17 cells. Initial contact with a yet-unknown antigen leads to production of proinflammatory cytokines, interleukin (IL)-1, and interferon (IFN)-γ by Th1 cells and IL-17 by Th17 cells. Production of cytokines leads to further Th cell up-regulation, production of certain metalloproteinases, and destruction of the blood–brain barrier (BBB), allowing Th cells to migrate into the CNS. Recovery may be mediated by distinctive Th2 cell populations secreting IL-10 and -4 anti-inflammatory cytokines ( Dhib-Jalbut 2002 ; Sie et al. 2014 ). Although T cells have long been considered integral to MS pathogenesis, B-cell follicles and oligoclonal bands (OCBs) are also present in the MS CNS, and it is becoming increasingly clear that B cells contribute to MS through mechanisms more complex than previously appreciated ( Cross et al. 2001 ).
The first formally defined MS phenotypes, relapsing remitting MS (RRMS), primary progressive MS (PPMS), secondary progressive MS (SPMS), and progressive relapsing MS (PRMS), were proposed in 1996 by the U.S. National Multiple Sclerosis Society (NMSS) Advisory Committee on Clinical Trials in Multiple Sclerosis as a result of increased need for standardized terminology in the field. It was felt that lack of such terminology would be detrimental not only to clinical practice and communication among clinicians but also to future advances in clinical research. The unified terminology would ensure the proper design of clinical trials, ensure the homogeneity of population recruited, and thus provide the necessary groundwork for successful outcomes. However, the Committee also recognized the purely clinical nature of the phenotypes and acknowledged that this terminology might change over time ( Lublin and Reingold 1996 ).
The proposed nomenclature quickly became an inherent part of clinical and research practice and was partly instrumental in the approval process of new MS therapeutics. However, with the increased knowledge base of MS pathology, the limitations of purely clinical phenotypes, lacking imaging and biological correlates, became evident. In 2012, the Committee (sponsored by NMSS and the European Committee for Treatment and Research in MS) reexamined the original clinical phenotypes with a goal to provide improved terminology while incorporating imaging, fluid biomarkers, and other assays. The Committee recommended retention of the basics of the original 1996 MS phenotypes but provided enhanced characterization by introducing new descriptors of activity and progression. The Committee also reported on two new disease courses: radiologically isolated syndrome (RIS) and clinically isolated syndrome (CIS) ( Table 1 ) ( Lublin et al. 2014 ).
Changes in multiple sclerosis disease-course (or “type”) descriptions
Based on data from the National Multiple Sclerosis Society.
MRI, Magnetic resonance imaging.
a , Lublin et al. (2014) .
We will review currently recognized and new MS disease courses.
RADIOLOGICALLY ISOLATED SYNDROME
Although RIS is not considered a distinct MS phenotype ( Lublin et al. 2014 ), increasing frequency of this incidental magnetic resonance imaging (MRI) finding in patients has raised its awareness in the MS community.
The term RIS, first introduced in 2009 ( Okuda et al. 2009 ), identifies patients with incidentally found MRI abnormalities highly suggestive of demyelination in the absence of clinical signs or symptoms. The proposed diagnostic criteria used the original Barkhof criteria ( Barkhof et al. 1997 ) for dissemination in space- and lesion-specific morphologic features to enhance diagnostic certainty and eliminate patients with nonspecific white matter changes caused by other causative etiologies, such as vascular disease or migraines. As those patients are at increased risk of developing clinically definitive MS (CDMS) in the future, identifying baseline factors with prognostic relevance for future long-term outcomes is of high importance.
Few studies explored the natural disease course in RIS patients and attempted to identify risk factors for either clinical or radiological disease progression.
In 2009, Okuda reported outcomes in 44 RIS patients with regard to conversion to CIS or radiological progression. The study showed a 30% conversion rate to CIS or CDMS with a median time of 5.4 years to the event. Radiological progression was found in 59% of patients over a median time of 2.7 years. The presence of gadolinium-enhancing lesions on the baseline MRI substantially increased the risk for radiological progression (hazard ratio [HR] = 3.4) ( Okuda et al. 2009 ).
Another prospective study by French investigators also from 2009 studied outcomes in 70 RIS patients.
In agreement with the previous study, the investigators found a 37% CIS conversion rate among the subjects over a mean follow-up period of 5.2 years. The mean time to a second MRI was 6 months and radiological progression was found in 91% of patients, with 37% of patients developing gad-enhancing lesions. With regard to other baseline predictive factors, this study found a significant impact of positive OCBs or an increased immunoglobulin G (IgG) index but only when associated with ≥9 T2-hyperintense lesions on a first MRI ( Lebrun et al. 2009 ).
The largest study reporting on RIS disease course to date was a retrospective, multinational cohort study that in 2014 reported on 451 RIS subjects with the goal of estimating the 5-year risk of developing a first clinical event while also investigating the predictive validity of any associated demographic, clinical, and radiological risk factors. Similar to the two previous studies, the investigators found a 34% risk of first acute or progressive clinical event observed within 5 years. A unique observation in this study was that 9.6% of patients developed a progressive disease course from onset. This study also defined spinal cord lesions as the strongest predictive factor for future clinical events (HR = 3.08). Additional relevant factors were younger age and male gender. Risk also increased with the presence of multiple risk factors. Interestingly, factors known for their importance in CIS, such as gad-enhancing lesions, infratentorial lesions, and positive cerebrospinal fluid (CSF) profile were not predictive of first clinical event development. Bearing in mind that this study was not investigating the impact of treatment on the risk of first clinical event (16.2% of patients in the cohort were treated with disease-modifying treatments [DMTs]), the investigators found no benefit of DMT agents on extending the time period to the first event ( Okuda et al. 2014 ).
The importance of RIS and the need for accurate characterization of its course and associated risks is supported not only by the fact that a meaningful number of patients do convert to clinical MS but also by the concern for misdiagnosis and eventual exposure to treatment agents. Although still not yet recognized as a formal MS course, research efforts to validate the above-reported findings and to establish the role of DMT treatment in RIS are under way.
CLINICALLY ISOLATED SYNDROME
Although recognized for some time, the 2012 MS disease course nomenclature codified CIS as an established disease course.
The term CIS describes a first clinical event highly suggestive of demyelinating CNS disease but not yet meeting dissemination in time for diagnosis of CDMS. The presenting symptoms are usually monofocal, evolve acutely or subacutely over days to weeks, and involve optic nerve, spinal cord, brain stem, or cerebellum. Like other MS attacks, the episode is expected to last at least 24 hours and occurs in the absence of fever or infection ( Miller et al. 2012 ).
The CIS harbors a possibility of CDMS in the future. The relationship between CIS and MS was the subject of few observational studies that reported on a conversion rate to CDMS following different CIS events: optic neuritis, brain stem syndromes, and transverse myelitis. Despite the variations in the conversion rates observed (CDMS conversion after optic neuritis up to 85%, transverse myelitis up to 61%, and brain stem syndromes up to 60%), most likely related to geographical differences in the course of the disease and different follow-up periods, it is safe to conclude that CDMS risk rates are similar for all the CIS types ( Optic Neuritis Study Group 2008 ; Young et al. 2009 ; Tintore et al. 2010 ).
Similar to RIS, certain demographic and imaging characteristics influence the risk of clinically definite multiple sclerosis (CDMD) conversion. The presence and number of T2 white matter lesions and abnormal CSF profile, defined as an elevated IgG index or the presence of OCBs, are the two predictors most commonly used in clinical practice.
The majority of CIS patients (50%–70%) will have asymptomatic T2 white matter abnormalities on the baseline brain MRI consistent with demyelinating lesions. The predictive role of this finding with regard to CDMS conversion was reported in multiple observational long-term studies and showed up to 80% conversion rate in up to 20 years follow-up period. The risk of CDMS conversion is also correlated with the number of lesions ( Brex et al. 2002 ; Tintore et al. 2010 ; Miller et al. 2012 ).
Although the presence of abnormal CSF profile did not provide much added prognostic value to the presence of abnormal MRI in CIS patients, the predictive value further increased in patients with normal brain MRI, raising the risk to CDMS conversion from 4% to 23% ( Tintore et al. 2008 ).
RELAPSING REMITTING MS
The most common MS phenotype, found in about 85% of MS patients, RRMS, is characterized by alternating periods of neurological dysfunction—relapses and periods of relative clinical stability free of new neurological symptoms—remissions ( Fig. 1 ). The frequency of relapses can vary from patient to patient but generally does not exceed 1.5 per year. Various neurological symptoms, such as weakness, altered sensation, balance impairment, impairment of visual acuity, and color vision or double vision, can be present during the relapse, lasting at least 24 hours in the absence of infection or metabolic derangement. Relapses result in residual deficits in almost half of episodes, leading to stepwise accrual of impairment ( Lublin et al. 2003 ).

Disease course of relapsing remitting multiple sclerosis (RRMS). (Reprinted, with permission, from the National Multiple Sclerosis Society.)
Pathologically, areas of inflammation rich in perivascular lymphocytic infiltrates with subsequent demyelination and axonal transection are the substrate of a relapse episode. Recovery of the symptoms during the relapse resolution suggests remyelination, which is most active during the early inflammatory phase of MS ( Compston and Coles 2008 ).
The magnitude of inflammatory pathology and frequency of relapses, most prominent in young adulthood, decreases with advanced disease and age. One of the natural disease history studies found a relapse rate of 0.54 associated with average disease duration of 16 years along with decreased duration of second remission from 71.32 months to 58.07 months ( Boiko et al. 2002 ).
Many potential relapse triggers have been investigated over the years to identify potential interventions to prevent an acute attack. Infections and stress, as well as pregnancy and their association with relapse, are the most relevant factors to everyday clinical practice.
Studies show an association between infections and increased relapse rate, prolonged relapse duration, and increased accumulation of disability; but no specific pathogen has yet been identified. Upper respiratory infections have been most often reported as potential triggers, but urinary tract infections and gastroenteritis are also associated with an increased relative risk for relapse ( Vollmer 2007 ). Although many patients report stressful events as a trigger for MS relapse, the review of literature by Mohr et al. (2004) did not find a strong causative relationship and proposed a rather additive role of stress to many other factors influencing relapse onset.
Pregnancy and associated biochemical changes are known to affect the relapse rate in MS. A study by the Pregnancy in Multiple Sclerosis Group showed a decreasing relapse rate during pregnancy, from 0.7 in the year before pregnancy to 0.5, and 0.6 and 0.2 during the subsequent trimesters. The relapse rate increased in the immediate postpartum period of 3 months to 1.2 before returning to 0.6 by the end of the postpartum year. This effect is believed to be caused by the natural immunotolerant effect of pregnancy, which is then lost at the time of delivery ( Confavreux et al. 1998 ).
Knowing that a significant number of the RRMS patients will eventually progress into SPMS; can relapse characteristics serve as a surrogate marker to predict the time of onset and degree of future progression? The research in this field showed conflicting results. Although several studies showed no effect of relapses, either before the progression or superimposed, on the severity of the progression, others clearly showed the opposite.
Using a natural history cohort, a study by Weinshenker et al. (1989) showed that the number of relapses within first 2 years of diagnosis did influence the median time of onset of the progressive disease. Another study, a systematic review of predictors for long-term disability identified the short interrelapse period after the first attack to be the strongest predictor for the time to onset of disability ( Langer-Gould et al. 2006 ). Work by Paz Soldan et al. (2015) also showed that pre- and postprogressive relapses independently accelerated the time to severe disability in progressive MS.
In concordance with these results, the study by French investigators showed slower onset of progression in patients with RRMS when compared with PPMS, but once the threshold of a progression was reached, both groups behaved in a similar fashion ( Confavreux et al. 2000 ).
In 2006, a large Canadian cohort study from London, Ontario, following more than 1000 patients with RRMS, did not confirm the relapse frequency as a substantial surrogate marker for the future progression. The investigators found no effect of the relapse rate on the slope of the progressive disease. The study showed dissociation between relapses and disease progression, evidenced by parallel progression of disability in both PPMS and SPMS with or without relapses. Using the same Canadian cohort in a subsequent study in 2009, the investigators found that frequent early attacks (first 2 years from onset) lead to earlier onset of progressive disease ( Kremenchutzky et al. 2006 ; Scalfari et al. 2013 ).
Differences in methodology and data interpretation are the possible reasons for such contradicting views on this topic; however, the importance of relapses on future disability should be recognized as it directly influences treatment strategies ( Lublin 2011 ).
SECONDARY PROGRESSIVE MS
The majority of untreated RRMS patients do eventually progress into SPMS, and research data suggest a median time to progressive phase of about 19 years after the onset of RRMS ( Rovaris et al. 2006 ). The diagnosis is most often established retrospectively, years after the actual progression started. On an individual patient level, it is difficult to determine when exactly in the disease course does the transition start, and patients as well as clinicians can encounter several years of diagnostic uncertainty. The most common reasons for this period of uncertainty, reported to last 2.9 ± 0.8 years on average in one examined population ( Katz Sand et al. 2014 ), are subtle and often fluctuating initial symptoms indicating early progression and reluctance to establish a diagnosis of progressive disease and thus increasing patient’s anxiety regarding prognosis and lack of approved therapies.
A few predictors of the conversion to SPMS have been identified, namely, higher age at RRMS onset was associated with earlier progression to SPMS, as well as male gender, albeit not consistently in all studies. Spinal cord symptoms and incomplete relapse recovery have also been shown to shorten the time to progression ( Rovaris et al. 2006 ).
Phenotypically, the course of SPMS is not uniform and consists of periods of progression with possible superimposed relapse activity but also periods of relatively stable disability ( Fig. 2 ). To date, there are no available imaging or immunological markers of progression, which is estimated based on clinical grounds over a period of at least 6 to 12 months.

Disease course of secondary progressive multiple sclerosis (SPMS). RRMS, Relapsing remitting multiple sclerosis; MRI, magnetic resonance imaging. (Reprinted, with permission, from the National Multiple Sclerosis Society.)
The pathology involved in SPMS is poorly understood and most likely complex, involving some degree of persistent inflammation, albeit to a lesser extent than in RRMS, combined with neurodegeneration caused by mitochondrial dysfunction and resultant axonal damage.
The peripheral innate immune system composed of the cells of myeloid origin, such as dendritic cells, macrophages, and natural killer cells, is now recognized to play an important role in mediating and regulating pathology in MS, notably in the progressive phase. A shift from adaptive to innate immunity has been proposed as a potential mechanism of progression. Recent research has shown changes in the cytokines (IL-12 and -18) and costimulatory molecules in the dendritic cells of patients transitioning from RRMS to SPMS. Additional processes leading to neuronal and oligodendrocyte cell death may include nitric oxide production and respiratory burst as well as secretion of soluble proteins (matrix metalloproteinases) with a direct effect on the BBB ( Weiner 2008 ).
The inflammatory changes present in progressive disease are thought to be compartmentalized within the CNS behind a closed or repaired BBB. This compartmentalized inflammation is the postulated driving force behind expansion of existing lesions and diffuse changes in normally appearing white matter. In addition, focal areas of inflammation can be also found within the meninges of progressive patients in lymphoid follicle-like structures containing dense clusters of B cells and plasma cells, possibly causing the higher degree of cortical pathology observed in progressive disease ( Lassmann et al. 2007 ).
The reorganization of voltage-gated sodium channels along demyelinated axons leading to increased energy requirements, failing ATP production, and accumulation of intracellular calcium resulting in mitochondrial dysfunction and axonal damage is a proposed mechanism explaining the role of mitochondria in neurodegeneration ( Su et al. 2009 ).
PRIMARY PROGRESSIVE MS
About 10%–20% of patients will develop this disease phenotype, characterized by the lack of initial RR phase and ongoing progression from the disease onset ( Compston and Coles 2008 ; Ransohoff et al. 2015 ). On an individual patient level, progression is not uniform throughout the course and superimposed relapses as well as periods of relative disease stability are possible ( Fig. 3 ).

Disease course of primary progressive multiple sclerosis (PPMS). MRI, Magnetic resonance imaging. (Reprinted, with permission, from the National Multiple Sclerosis Society.)
Increasing clinical, imaging, and genetic data suggest that PPMS is a part of the MS disease spectrum and any pathological differences from SPMS are relative rather than absolute. Natural history studies have shown that disability progresses in parallel in patients with PPMS and SPMS with or without relapses ( Kremenchutzky et al. 2006 ). The fact that ∼10% of RIS patients develop a PP disease course further supports the theory that the absence of RR phase in PPMS patients is potentially caused by clinically silent CNS lesions ( Okuda et al. 2014 ; Ransohoff et al. 2015 ).
Similar to SPMS, pathology in PPMS is complex and includes neurodegeneration occurring along with mild-to-moderate inflammation.
MODIFIERS OF ACTIVITY AND PROGRESSION IN MS
The addition of two new modifiers, activity and progression, to the established MS phenotypes, was proposed in the 2013 revision based on an increased understanding of the MS clinical course and the increasingly important role of MRI in clinical care and research. While the 1996 MS phenotypes provide a more static disease description, addition of the descriptors of activity and progression enhances characterization of the ongoing disease dynamic in a given time period and enhances prognostication, treatment decisions, and outcomes in clinical care as well as research.
Disease activity is defined as a clinical relapse or new MRI activity—presence of gad-enhancing lesions or new/enlarging T2 lesions. The descriptor of disease activity applies to relapsing and progressive MS patients. The Committee suggested yearly assessment of disease activity using the clinical examination in both RRMS and progressive MS patients. Annual assessment of MRI activity in relapsing patients was deemed satisfactory but no consensus on appropriate MRI frequency in progressive patients was reached. Because of the correlation between brain and spine MRI activity, annual spinal MRI surveillance scans to detect activity were not recommended unless there were new spinal symptoms present ( Bot and Barkhof 2009 ).
Inclusion of the new descriptive terminology then results in various MS phenotypes, such as RRMS-active, in RRMS patients with either clinical or MRI disease activity or PPMS–not active in PPMS patients with no acute attacks or MRI activity within a stated time period.
The descriptor of disease progression applies to either SPMS or PPMS patients. As progression in MS is not a uniform feature and patients can remain relatively stable over time, yearly assessment of progression is recommended. Owing to the lack of imaging or immunological biomarkers, the progression of the disease is determined on a clinical basis only, combining objective findings and patient-provided history. Again, combining features of activity and progression, various MS phenotypes can be observed, such as SPMS-active and progressing in SPMS patient with clinical or imaging activity and progression of the disability ( Table 1 ) ( Lublin et al. 2014 ).
The addition of the MS phenotypical modifiers of activity and progression is a first step toward more patient-specific terminology and subsequently toward more individualized care.
The original 1996 MS phenotypes were based on purely clinical grounds and clinical consensus and it was hoped that in the future, biomarkers, either imaging or biological, would be able to better support and define the phenotypes. Yet, to date, none of the many proposed candidates have made it into clinical practice. A review of biomarkers is beyond the scope of this article, but we will briefly review some of them with regard to the MS disease course.
The 2010 revision of McDonald diagnostic criteria enhanced the usage of MRIs in the diagnostic process and allowed for earlier RRMS diagnosis and treatment. The importance of an MRI in MS is indisputable but, to date, MRIs are not able to distinguish among MS disease courses.
It is clear that the presence of white matter lesions on MRIs in the correct clinical circumstances defines RIS and CIS disease phenotypes as well as transition from CIS to RRMS. Enhancing or new T2 lesions are now used as one of the markers of activity. However, in established MS, the conventional MRI metrics correlate only moderately with disability measures, causing the radiological and clinical paradox ( Filippi and Rocca 2011 ). This is caused by the relative lack of specificity that conventional MRI metrics have for the heterogeneous pathological substrates of the disease. Novel imaging techniques, such as magnetization transfer ratio (MTR), diffusion inversion recovery (DIR), and diffusion tensor imaging (DTI) have been expected to fill this knowledge gap.
Statistically significant differences in the degree of MTR reduction in T1 hypointense lesions have been reported among patients with RRMS and SPMS ( Filippi and Agosta 2007 ). Additionally, MTR changes found in normally appearing white matter and gray matter evolved in distinguished pattern among major MS phenotypes ( Filippi and Agosta 2010 ). The subtle DTI changes, specifically, increased mean diffusivity, can be found in some patients early before the formation of acute inflammatory lesions.
The introduction of DIR sequences enhanced the ability to detect cortical lesions. Cortical lesions are more frequently seen in SPMS patients than in RRMS or CIS patients, and association has been reported between these lesions and the progression of disability in specific MS phenotypes ( Filippi and Rocca 2011 ).
From the present and ongoing research, as well as our clinical experience, it is safe to say that only combining conventional and unconventional MRI techniques with different spectrums of specificity toward different processes might enhance our understanding of this complex disease and its course. Although this might be a feasible approach in clinical research, it is questionable whether the same is applicable to clinical care.
BIOLOGICAL BIOMARKERS
There are different requirements for biological biomarkers with regard to identifying an MS subtype (specific) or predicting an MS course (dynamically changing ahead, not after, the transition), but the common expectations are standardized analysis techniques, validation in large independent cohorts, and cost-effectiveness for clinical practice. The ideal biomarker should not be redundant in information already provided by MRI imaging, but rather additive, and provide a different spectrum of information.
Many promising biomarkers, either serum or CSF, have been identified but very few were also validated in at least two independent studies, and none of those have made it into clinical practice as yet.
The main areas where disease biomarkers could provide more information are identification of early MS and prediction of CIS to CDMD conversion, identification of MS subtypes and prognostication within and across the MS phenotypes, and response to therapy.
The following biomarkers showed promising results and were validated in at least one independent cohort for identification of early CIS to CDMS converters: CSF IgM OCBs, CSF C–X–C motif chemokine 13 (CXCL13), CSF chitinase-3-like protein 1 (CHI3L1), and CSF neurofilament light chain (NfL). Identification of MS subtypes, specifically identification of PPMS versus RRMS, was supported by the presence of serum microRNAs miR-223 and miR-15b. Decreased levels of CSF N -acetylaspartate (NAA) were found in patients with SPMS in comparison with RRMS and CIS. With regard to prognostication and intraphenotypical characterization of individual patients, CSF-restricted IgM OCBs were found in patients with high relapse rate and early progression to SPMS, elevated CSF CHI3L1 levels were associated with earlier progression to high Expanded Disability Status Scale (EDSS) scores in RRMS patients ( Teunissen et al. 2015 ).
Development of new biomarkers is a long process and large, collaborative studies are essential for the validation of results before any biomarker makes it into clinical practice. As optimal use of resources is an important factor in any health-care setting, we should consider the usage of information that any biomarker is able to provide and prioritize the most pressing clinical needs—progressive MS and prediction of treatment response.
CONCLUDING REMARKS
Our increased understanding of MS clinical course changed the view of the originally defined MS phenotypes and resulted in new MS subtypes with enhanced characterization of the individual patient disease course. This is the first step into personalized care in MS. New developments in the field of MRI and biomarkers will further pave the way toward a better future for our patients.
Editors: Howard L. Weiner and Vijay K. Kuchroo
Additional Perspectives on Multiple Sclerosis available at www.perspectivesinmedicine.org
- Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, Comi G, Ader HJ, Losseff N, Valk J. 1997. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis . Brain 120 : 2059–2069. [ PubMed ] [ Google Scholar ]
- Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D; University of British Columbia MS Clinic Neurologists. 2002. Early onset multiple sclerosis: A longitudinal study . Neurology 59 : 1006–1010. [ PubMed ] [ Google Scholar ]
- Bot JC, Barkhof F. 2009. Spinal-cord MRI in multiple sclerosis: Conventional and nonconventional MR techniques . Neuroimaging Clin N Am 19 : 81–99. [ PubMed ] [ Google Scholar ]
- Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. 2002. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis . N Engl J Med 346 : 158–164. [ PubMed ] [ Google Scholar ]
- Compston A, Coles A. 2008. Multiple sclerosis . Lancet 372 : 1502–1517. [ PubMed ] [ Google Scholar ]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. 1998. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group . N Engl J Med 339 : 285–291. [ PubMed ] [ Google Scholar ]
- Confavreux C, Vukusic S, Moreau T, Adeleine P. 2000. Relapses and progression of disability in multiple sclerosis . N Engl J Med 343 : 1430–1438. [ PubMed ] [ Google Scholar ]
- Cross AH, Trotter JL, Lyons J. 2001. B cells and antibodies in CNS demyelinating disease . J Neuroimmunol 112 : 1–14. [ PubMed ] [ Google Scholar ]
- Dhib-Jalbut S. 2002. Mechanisms of action of interferons and glatiramer acetate in multiple sclerosis . Neurology 58 : S3–S9. [ PubMed ] [ Google Scholar ]
- Filippi M, Agosta F. 2007. Magnetization transfer MRI in multiple sclerosis . J Neuroimaging 17 : 22S–26S. [ PubMed ] [ Google Scholar ]
- Filippi M, Agosta F. 2010. Imaging biomarkers in multiple sclerosis . J Magn Reson Imaging 31 : 770–788. [ PubMed ] [ Google Scholar ]
- Filippi M, Rocca MA. 2011. The role of magnetic resonance imaging in the study of multiple sclerosis: Diagnosis, prognosis and understanding disease pathophysiology . Acta Neurol Belg 111 : 89–98. [ PubMed ] [ Google Scholar ]
- Katz Sand I, Krieger S, Farrell C, Miller AE. 2014. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis . Mult Scler 20 : 1654–1657. [ PubMed ] [ Google Scholar ]
- Kremenchutzky M, Rice GP, Baskerville J, Wingerchuk DM, Ebers GC. 2006. The natural history of multiple sclerosis: A geographically based study 9: Observations on the progressive phase of the disease . Brain 129 : 584–594. [ PubMed ] [ Google Scholar ]
- Langer-Gould A, Popat RA, Huang SM, Cobb K, Fontoura P, Gould MK, Nelson LM. 2006. Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: A systematic review . Arch Neurol 63 : 1686–1691. [ PubMed ] [ Google Scholar ]
- Lassmann H, Bruck W, Lucchinetti CF. 2007. The immunopathology of multiple sclerosis: An overview . Brain Pathol 17 : 210–218. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lebrun C, Bensa C, Debouverie M, Wiertlevski S, Brassat D, de Seze J, Rumbach L, Pelletier J, Labauge P, Brochet B, et al. 2009. Association between clinical conversion to multiple sclerosis in radiologically isolated syndrome and magnetic resonance imaging, cerebrospinal fluid, and visual evoked potential: Follow-up of 70 patients . Arch Neurol 66 : 841–846. [ PubMed ] [ Google Scholar ]
- Lublin FD. 2011. Relapses do not matter in relation to long-term disability: No (they do) . Mult Scler 17 : 1415–1416. [ PubMed ] [ Google Scholar ]
- Lublin FD, Reingold SC. 1996. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis . Neurology 46 : 907–911. [ PubMed ] [ Google Scholar ]
- Lublin FD, Baier M, Cutter G. 2003. Effect of relapses on development of residual deficit in multiple sclerosis . Neurology 61 : 1528–1532. [ PubMed ] [ Google Scholar ]
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, et al. 2014. Defining the clinical course of multiple sclerosis: The 2013 revisions . Neurology 83 : 278–286. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller DH, Chard DT, Ciccarelli O. 2012. Clinically isolated syndromes . Lancet Neurol 11 : 157–169. [ PubMed ] [ Google Scholar ]
- Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. 2004. Association between stressful life events and exacerbation in multiple sclerosis: A meta-analysis . BMJ 328 : 731. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Okuda DT, Mowry EM, Beheshtian A, Waubant E, Baranzini SE, Goodin DS, Hauser SL, Pelletier D. 2009. Incidental MRI anomalies suggestive of multiple sclerosis: The radiologically isolated syndrome . Neurology 72 : 800–805. [ PubMed ] [ Google Scholar ]
- Okuda DT, Siva A, Kantarci O, Inglese M, Katz I, Tutuncu M, Keegan BM, Donlon S, Hua LH, Vidal-Jordana A, et al. 2014. Radiologically isolated syndrome: 5-year risk for an initial clinical event . PLoS ONE 9 : e90509. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Optic Neuritis Study Group. 2008. Multiple sclerosis risk after optic neuritis: Final optic neuritis treatment trial follow-up . Arch Neurol 65 : 727–732. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Paz Soldan MM, Novotna M, Abou Zeid N, Kale N, Tutuncu M, Crusan DJ, Atkinson EJ, Siva A, Keegan BM, Pirko I, et al. 2015. Relapses and disability accumulation in progressive multiple sclerosis . Neurology 84 : 81–88. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ransohoff RM, Hafler DA, Lucchinetti CF. 2015. Multiple sclerosis—A quiet revolution . Nat Rev Neurol 11 : 134–142. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rovaris M, Confavreux C, Furlan R, Kappos L, Comi G, Filippi M. 2006. Secondary progressive multiple sclerosis: Current knowledge and future challenges . Lancet Neurol 5 : 343–354. [ PubMed ] [ Google Scholar ]
- Scalfari A, Neuhaus A, Daumer M, Deluca GC, Muraro PA, Ebers GC. 2013. Early relapses, onset of progression, and late outcome in multiple sclerosis . JAMA Neurol 70 : 214–222. [ PubMed ] [ Google Scholar ]
- Sie C, Korn T, Mitsdoerffer M. 2014. Th17 cells in central nervous system autoimmunity . Exp Neurol 262 : 18–27. [ PubMed ] [ Google Scholar ]
- Su KG, Banker G, Bourdette D, Forte M. 2009. Axonal degeneration in multiple sclerosis: The mitochondrial hypothesis . Curr Neurol Neurosci Rep 9 : 411–417. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Teunissen CE, Malekzadeh A, Leurs C, Bridel C, Killestein J. 2015. Body fluid biomarkers for multiple sclerosis—The long road to clinical application . Nat Rev Neurol 11 : 585–596. [ PubMed ] [ Google Scholar ]
- Tintore M, Rovira A, Rio J, Tur C, Pelayo R, Nos C, Tellez N, Perkal H, Comabella M, Sastre-Garriga J, et al. 2008. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology 70 : 1079–1083. [ PubMed ] [ Google Scholar ]
- Tintore M, Rovira A, Arrambide G, Mitjana R, Rio J, Auger C, Nos C, Edo MC, Castillo J, Horga A, et al. 2010. Brainstem lesions in clinically isolated syndromes . Neurology 75 : 1933–1938. [ PubMed ] [ Google Scholar ]
- Tullman MJ. 2013. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis . Am J Manag Care 19 : S15–S20. [ PubMed ] [ Google Scholar ]
- Vollmer T. 2007. The natural history of relapses in multiple sclerosis . J Neurol Sci 256 : S5–S13. [ PubMed ] [ Google Scholar ]
- Weiner HL. 2008. A shift from adaptive to innate immunity: A potential mechanism of disease progression in multiple sclerosis . J Neurol 255 : 3–11. [ PubMed ] [ Google Scholar ]
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, Ebers GC. 1989. The natural history of multiple sclerosis: A geographically based study. 2: Predictive value of the early clinical course . Brain 112 : 1419–1428. [ PubMed ] [ Google Scholar ]
- Young J, Quinn S, Hurrell M, Taylor B. 2009. Clinically isolated acute transverse myelitis: Prognostic features and incidence . Mult Scler 15 : 1295–1302. [ PubMed ] [ Google Scholar ]
- Skip to content
- Accessibility help
Multiple sclerosis: When should I suspect that a person has multiple sclerosis?
Last revised in March 2024
When should I suspect that a person has multiple sclerosis?
- About 0.5% of adults with MS first develop symptoms aged 60 years or older — older age at onset is associated with a progressive course.
- A history of previous neurological symptoms.
- Symptoms that evolve over more than 24 hours, may persist over several days or weeks and then improve.
- Loss or reduction of vision in one eye with painful eye movements.
- Ascending sensory disturbance and/or weakness.
- Balance or gait problems, unsteadiness, or clumsiness.
- Altered sensation radiating down the back and sometimes into the limbs on neck flexion (Lhermitte's symptom).
- Optic neuritis is the initial presentation in about 20–30% of people with MS.
- The person may describe partial or total unilateral visual loss developing over a few days, pain behind the eye (in particular on eye movement) and/or loss of colour discrimination (particularly reds).
- Fundoscopy is often normal but the disc may appear pale or swollen. There may be paradoxical dilation of the pupil when light is rapidly shifted from the unaffected eye to the affected eye (relative afferent pupillary defect).
- Optic neuritis may be bilateral, but if this occurs extra vigilance is needed to rule out neuromyelitis optica which is often confused with MS and needs urgent treatment.
- May present with sensory symptoms (such as paraesthesia) or motor symptoms (such as weakness) below the level of the inflammation that typically develop over hours or days.
- Some people describe a tight band sensation around the trunk at the level of the inflammation, or a shock-like sensation radiating down the spine induced by neck flexion (Lhermitte’s phenomena).
- There may be urinary symptoms such as urgency, frequency, or retention.
- Examination may reveal focal muscle weakness and reduced sensation below the affected spinal level. Muscle tone is initially reduced.
- Symptoms and signs may be symmetrical or asymmetrical, and tend to reflect a partial myelitis that only affects a part of the spinal cord — symptoms and signs similar to a full spinal cord transection are rare.
- These may include ataxia, vertigo, clumsiness, and dysmetria (as demonstrated by abnormalities with finger-to-nose testing and walking heel to toe).
- Eye movement abnormalities that can cause diplopia, oscillopsia (a sensation of movement of the vision), nystagmus, and internuclear ophthalmoplegia (inability to adduct one eye and nystagmus in the abducting eye on oculomotor examination).
- Bulbar muscle problems resulting in dysarthria or dysphagia.
- The person's main symptoms are fatigue, depression, or dizziness unless they have a history or evidence of focal neurological symptoms or signs.
Basis for recommendation
The information on the possible presentations of multiple sclerosis is based on the National Institute for Health and Care Excellence (NICE) guideline Multiple sclerosis in adults: management [ NICE, 2022 ] and expert opinion in review articles [ Reich, 2018 ; Thompson, 2018 ; Wallin, 2019 ; BMJ Best Practice, 2021 ].
The content on the NICE Clinical Knowledge Summaries site (CKS) is the copyright of Clarity Informatics Limited (trading as Agilio Software Primary Care) . By using CKS, you agree to the licence set out in the CKS End User Licence Agreement .
Lectures: Clinical Presentation Introduction Initial Symptoms Ongoing Symptoms Clinical Cases Introduction It is important to note that patients with MS have subjective complaints and objective signs that frequently are not attributable to one specific lesion in the CNS. It is usually possible to distinguish at least two or more separate foci of involvement based on the clinical assessment of the patient. Multiple Sclerosis most often is characterized by episodes of neurological dysfunction followed by periods of stabilization or partial to complete remission of symptoms. These symptoms (relapses or exacerbations) can appear over a few hours or days, can be gradually worsening over a period of a few weeks, or sometimes can present themselves acutely. Depending on a course and a subtype of the disease, these symptoms will either persist or slowly resolve over weeks or months and may even culminate as a complete remissions. A relapsing-remitting pattern is the most common and is characteristic for this disease . Initial Symptoms Certain signs and symptoms are more common in the early stages of the disease. Patients may be complaining of double or blurred vision, numbness, weakness in one or two extremities, instability in walking, tremors and problems with bladder control, heat intolerance. As is well known, sensory exam is the most difficult one to perform reliably and accurately in evaluation of patients with neurologic complaints. However, certain distributions of sensory problems can be suspicious for early MS. Among those are: - ascending numbness starting in the feet; - bilateral hand numbness; - hemiparesthesia; - dysesthesia in one of the above distributions; - generalized heat intolerance Objectively the most common sensory findings in the"numb" areas are dorsal column signs, such as reduction of vibration, proprioception and stereognosis, rather than problems with spinothalamic tract. Usually double vision in MS patients results from a unilateral or bilateral partial of complete internuclear ophthalmoplegia . VI nerve paresis and palsy also have been described as presenting symptoms of MS. III and IV nerves palsy are rather uncommon. Optic Neuritis is a frequent presenting symptom of MS. It is characterized by blurred vision, a change in color perception, visual field defect i.e.,. Central scotoma, and possible headaches and retro-orbital pain precipitated by eye movements. These symptoms may require neuro-ophthalmologic evaluation, MRI imaging and Visual Evoked Potential studies to establish a degree of optic nerve function. Motor weakness often is accompanied by upper motor neuron signs, such as mild spasticity, hyperreflexia, and pathologic signs. The most common initial presentation is paraparesis, but weakness can be also found in just one extremity (monoparesis) or all four extremities (quadriparesis). Ongoing Symptoms and Signs As the disease progresses, the original signs and symptoms may worsen, and the new ones may appear. The most common symptoms and signs include: Motor system: -weakness (variable severity mono- and paraparesis, hemiparesis, quadriparesis) -increased spasticity resulting in spastic gait -pathologic signs (Babinski's, Chaddock's, Hoffmann, Oppenheim's, etc.) -dysarthria Cerebellar signs: -incoordination (dysdiadochokinesia, problems with heel-to-shin test) -slowing of rapid repeating movements -cerebellar ataxia (ataxic gait) -scanning speech -loss of balance Sensory systems: -Lhermitte's sign -dysesthetic pain -paresthesia -numbness -dorsal column signs (i.e.,. severe decrease or loss of vibratory sense and proprioception, positive Romberg's test) Urinary incontinence, incomplete emptying, increased frequency of urination. All of these problems may result in urinary tract infections. Optic disc pallor, atrophy, blurred vision, diplopia, nystagmus, oscillopsia, intranuclear ophthalmoplegia, central scotomas or other visual field defects Cognitive and emotional abnormalities (emotional lability, depression, anxiety) Fatigue Sexual dysfunction At this stage in the disease, uncommon but important problems may include bowel incontinence, difficulty swallowing, seizures, trigeminal neuralgia, dystonia, hearing loss, and facial nerve (Bell's) palsy. All of the above-mentioned symptoms can be precipitated by heat, i.e.,. being in a hot, humid environment, or taking a hot bath. Clinical Cases Case 1 History Case 1 Questions Case 2: History Case 2: Questions Case 3 History Case 3 Questions © John W.Rose, M.D., Maria Houtchens, MSIII, Sharon G. Lynch, M.D.
Clinical presentation and diagnosis of multiple sclerosis
Affiliation.
- 1 Leeds Centre for Neurosciences, Leeds, UK [email protected].
- PMID: 32675142
- PMCID: PMC7385797
- DOI: 10.7861/clinmed.2020-0292
The diagnosis of multiple sclerosis (MS) is through clinical assessment and supported by investigations. There is no single accurate and reliable diagnostic test. MS is a disease of young adults with a female predominance. There are characteristic clinical presentations based on the areas of the central nervous system involved, for example optic nerve, brainstem and spinal cord. The main pattern of MS at onset is relapsing-remitting with clinical attacks of neurological dysfunction lasting at least 24 hours. The differential diagnosis includes other inflammatory central nervous system disorders. Magnetic resonance imaging of the brain and lumbar puncture are the key investigations. New diagnostic criteria have been developed to allow an earlier diagnosis and thus access to effective disease modifying treatments.
Keywords: MS; Multiple sclerosis; neurology.
© Royal College of Physicians 2020. All rights reserved.
- Diagnosis, Differential
- Magnetic Resonance Imaging
- Multiple Sclerosis* / diagnosis
- Spinal Cord
- Young Adult
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 20 May 2024
Inflammatory and neurodegenerative serum protein biomarkers increase sensitivity to detect clinical and radiographic disease activity in multiple sclerosis
- Tanuja Chitnis ORCID: orcid.org/0000-0002-9897-4422 1 ,
- Ferhan Qureshi ORCID: orcid.org/0000-0002-5158-0485 2 ,
- Victor M. Gehman 2 ,
- Michael Becich ORCID: orcid.org/0000-0003-4687-8751 2 ,
- Riley Bove ORCID: orcid.org/0000-0002-2034-8800 3 ,
- Bruce A. C. Cree 3 ,
- Refujia Gomez 3 ,
- Stephen L. Hauser ORCID: orcid.org/0000-0002-4932-4001 3 ,
- Roland G. Henry ORCID: orcid.org/0000-0002-8232-7562 3 ,
- Amal Katrib 2 ,
- Hrishikesh Lokhande 1 ,
- Anu Paul 1 ,
- Stacy J. Caillier 3 ,
- Adam Santaniello ORCID: orcid.org/0000-0002-9816-5932 3 ,
- Neda Sattarnezhad 1 ,
- Shrishti Saxena 1 ,
- Howard Weiner ORCID: orcid.org/0000-0003-0203-9681 1 ,
- Hajime Yano 1 &
- Sergio E. Baranzini ORCID: orcid.org/0000-0003-0067-194X 3
Nature Communications volume 15 , Article number: 4297 ( 2024 ) Cite this article
1019 Accesses
4 Altmetric
Metrics details
- Diagnostic markers
- Multiple sclerosis
The multifaceted nature of multiple sclerosis requires quantitative biomarkers that can provide insights related to diverse physiological pathways. To this end, proteomic analysis of deeply-phenotyped serum samples, biological pathway modeling, and network analysis were performed to elucidate inflammatory and neurodegenerative processes, identifying sensitive biomarkers of multiple sclerosis disease activity. Here, we evaluated the concentrations of > 1400 serum proteins in 630 samples from three multiple sclerosis cohorts for association with clinical and radiographic new disease activity. Twenty proteins were associated with increased clinical and radiographic multiple sclerosis disease activity for inclusion in a custom assay panel. Serum neurofilament light chain showed the strongest univariate correlation with gadolinium lesion activity, clinical relapse status, and annualized relapse rate. Multivariate modeling outperformed univariate for all endpoints. A comprehensive biomarker panel including the twenty proteins identified in this study could serve to characterize disease activity for a patient with multiple sclerosis.
Similar content being viewed by others
Proteomics reveal biomarkers for diagnosis, disease activity and long-term disability outcomes in multiple sclerosis

An autoantibody signature predictive for multiple sclerosis
Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis
Introduction.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system, with a variable presentation and heterogenous disease course 1 , 2 . While the exact pathophysiology of MS remains elusive, inflammatory and degenerative processes are believed to play a role 3 , 4 , 5 . Identifying disease-specific biomarker sets may assist in predicting diverse disease courses, classifying patients to high versus low risk for disease activity (DA) and progression (DP) and may also provide insights into mechanisms of new inflammatory DA 6 , 7 . Multivariate models reflecting multiple biological pathways involved in the complex pathophysiology of MS will likely increase the predictive accuracy of these biomarkers 6 .
Most studies have focused primarily on neurofilament light chain in blood serum (sNfL) as a biomarker in MS. Concentration of sNfL has been associated with neurodegeneration in MS and correlates with manifestations of DA, including the presence of gadolinium-enhancing (Gd+) lesions and clinical relapse 6 , 7 , 8 , 9 , 10 , 11 . For example, in a recent study of over 500 samples, a significant elevation in sNfL was observed after a clinical relapse only when associated with a Gd+ lesion 12 . In the 3 months after a Gd+ lesion, an average 35% elevation in sNfL ( p < 0.0001) was reported when compared with samples from patients in remission 12 . Similarly, an average 32.3% elevation in sNfL was observed at the time of, or prior to a Gd+ lesion ( p = 0.002) versus remission 12 . However, the observed increase in sNfL levels during a relapse has limited sensitivity and specificity and is of the same magnitude as the group-level coefficient of variation seen across populations of healthy individuals, thus rendering this metric insufficient for clinical decision-making 12 , 13 .
The MS relapse disease process includes both an inciting inflammatory peripheral and central immune activation, with subsequent central nervous system damage in the form of myelin and neuronal degradation 1 , as evidenced by immunological studies, and response to specific immune cell–targeting disease-modifying therapies. Moreover, MS genetic susceptibility studies demonstrate involvement of T and B cell–associated genes 14 . Thus, including additional inflammatory and neurodegenerative protein biomarkers, can offer deeper insights and reveal stronger correlations to clinical and radiographic DA than sNfL alone.
In the search for more specific and comprehensive sets of markers, several cytokines, chemokines, and other immune-related molecules have been associated with DA in patients with MS. For example, baseline levels of cerebrospinal fluid (CSF) proteins CXCL13, CXCL12, IFN γ , TNF, sCD163, LIGHT, and APRIL have been associated with evident DA compared with no evidence of DA (NEDA) in patients with MS 15 . CSF levels of glial biomarkers, including glial fibrillary acidic protein (GFAP) and Chitinase 3-like-1 (CHI3L1 or YKL-40), have been associated not only with DA but also with disability 16 , 17 . Masvekar et al. found that the additive model of IL12p40 and CHI3L1 correlated with new MS lesion activity 18 . MS disease severity has been associated with alterations in proteins reflecting astrocyte (MMP7, SERPINA3, GZMA, and CLIC1) and microglial activation (DSG2 and TNFRSF25) 19 .
To provide insights and identify sensitive biomarkers of new MS DA, both radiographic (new Gd+ lesions) and clinical (relapses), we evaluated over 1400 serum proteins in over 600 samples from three MS cohorts. Biological pathway modeling and network analysis were performed to ensure comprehensive representation of MS neurophysiology and to gain insights into the inflammatory, immune, and neurodegenerative process in MS.
Results from this cross-sectional study are divided into the following three sections: protein (i.e., feature) selection analysis, univariate analysis of each endpoint outlined in the Methods, and multivariate modeling of each endpoint.
Twenty proteins associated with disease activity were selected for the custom assay panel (CAP)
The final list of CAP proteins was selected by examining the univariate and multivariate associations of 1411 proteins with our three endpoints, constrained by a number of analytic and operational considerations. A detailed discussion of the process for arriving at this final list can be found in the Protein feature-selection section (and Table 1 ). GFAP was not one of the original proteins described in the Methods section. It was added to our panel after much of the development work described in this report was completed because of its strong association with several DA- and DP-related endpoints. Therefore, it was not part of the analysis. The remaining 20 proteins in Table 1 were carried forward.
Univariate analysis identified several individual proteins significantly associated with Gd lesion regression, Gd lesion classification, and annualized relapse rate (ARR)
As a precursor to a formal univariate analysis of each endpoint, we grouped samples by label and represented the results as box plots to look for qualitative trends in concentration for each class at a population level. Gd lesion samples were separated into groups for zero, one, two, and three or more Gd+ lesions (Fig. 1 ). Purely binary endpoints include clinical relapse status (CRS, quiescence vs. exacerbation) and low versus high annualized relapse rate (ARR, ≤ 0.2/year for low and ≥ 1.0/year for high) were split according to positive and negative labels (Fig. 2 ).
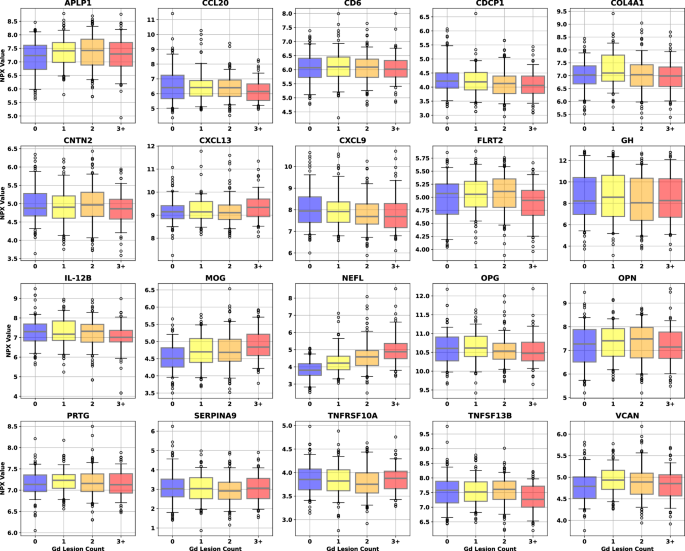
Different color boxes correspond to the lesion count in that population of samples (blue for zero lesions, yellow for one, orange for two, and red for three or more). Sample counts for each lesion bin are 138 for 0 lesions, 126 for 1, 148 for 2, and 89 for 3 or more. The black line through each box shows the median (50 th percentile) of the population. The height of each box shows the interquartile range (25 th –75 th percentile). The whiskers show the central 90% of the distribution (5 th –95 th percentile). The 5% of outliers furthest from the median are drawn as open black circles. Source data are provided as a Source Data file. CAP custom assay panel, Gd gadolinium, NPX normalized protein expression.
a Univariate box plots for the CAP proteins separation of samples taken during quiescence (remission, blue boxes, 64 samples) or exacerbation (relapse, red boxes, 60 samples). b Box plots of the univariate separation of CAP proteins for low ( ≤ 0.2/year, blue boxes, 148 samples) and high ( ≥ 1.0/year, red boxes, 13 samples) ARR. The black line through each box shows the median (50 th percentile) of the population. The height of each box shows the interquartile range (25th–75th percentile). The whiskers show the central 90% of the distribution (5th–95th percentile). The 5% of outliers furthest from the median are drawn as open black circles. Source data are provided as a Source Data file. ARR annualized relapse rate, CAP custom assay panel.
To quantitatively investigate the univariate significance, Spearman’s correlation and Student’s t test were used. The former checked for correlation between protein concentration and lesion count. The latter checked for differences in protein concentration means in the binary endpoints, univariate separation of samples associated with no Gd lesions from those associated with ≥ 1, as well as CRS and ARR status. Spearman’s correlation and Student’s t test were computed for each protein in Fig. 3 to assess the relationship between protein concentration and all endpoints.
Top: Spearman’s ⍴ correlation between NPX concentration and Gd lesion count (green bars, left axis) and Student’s t statistic (two-sided) for separation of samples associated with zero lesions from those with one or more by NPX concentration (purple bars, right axis) for each protein. Bottom: Student’s t statistic (two-sided) for separation of samples associated with clinically inactive from those with clinically active disease state (left axis) and those associated with low from high ARR (right axis) by NPX concentration. Bars corresponding to statistical tests showing a p -value > 0.05 have been drawn in a lighter shade of the same color to denote their lack of statistical significance. Source data are provided as a Source Data file. ARR annualized relapse rate, Gd gadolinium, NPX normalized protein expression.
Examination of the bar charts in Fig. 3 allowed us to check the directionality and significance of all univariate statistical tests. Of particular interest were proteins showing consistency between the direction of the correlation/separation for all endpoints. Agreement between correlation and separation was particularly important for Gd lesion count and presence, since these two endpoints are not independent of each other. Proteins showing a test statistic with opposite signs between the two Gd tests did not pass the significance threshold. Furthermore, only CDCP1 shows a difference between the Gd endpoints and the other two, with negative correlation/separation for Gd lesions, but positive mean shift with clinically active disease state (CDCP1 shows no significant relationship to our ARR endpoint).
The proteins passing the significance threshold in each of the univariate analyses were (with test p-values): Gd lesion regression: (NfL [3.3 × 10 −34 ], MOG [1.6 × 10 −7 ], CDCP1 [5.0 × 10 −3 ], CXCL9 [8.4 × 10 −3 ], IL-12B [2.4 × 10 −2 ], TNFSF13B [2.7 × 10 −2 ], OPG [2.8 × 10 −2 ], CCL20 [4.2 × 10 −2 ]); Gd lesion classification: (NfL [1.0 × 10 −19 ], MOG [1.9 × 10 −6 ], APLP1 [2.3 × 10 −3 ], VCAN [9.9 × 10 −3 ], CDCP1 [1.8 × 10 −2 ], CXCL9 [4.7 × 10 −2 ]); CRS: (NfL [5.0 × 10 −5 ], GH [4.4 × 10 −3 ], SERPINA9 [5.3 × 10 −3 ], FLRT2 [6.6 × 10 −3 ], CDCP1 [1.6 × 10 −2 ], PRTG [4.2 × 10 −2 ]); ARR: NfL (7.9 × 10 −4 ).
Multivariate models significantly outperform univariate models with NfL emerging as the strongest biomarker
Forward selection curves, regression, and classification Gd lesion analysis, as well as the classification of CRS and AAR are shown in Fig. 4 .
The points represent the mean over the bootstrap splits and the shaded region represents the standard deviation. The protein features selected for each of the multivariate analyses were Gd lesion regression (NfL, GH, IL-12B, CNTN2, MOG, TNFSF13B), Gd lesion classification (NfL, CNTN2, TNFRSF10A, CXCL13, TNFSF13B), Clinical relapse status (NfL, SERPINA9, TNFSF13B, FLRT2), and annualized relapse rate (NfL, OPG, CD6). Note that the regression analysis was clipped at a lesion count of five (only 5.6% of our samples had more than five lesions, making any model behavior above that range unreliable). Source data are provided as a Source Data file. AUROC area under the receiver operator characteristic, ARR annualized relapse rate, CRS clinical relapse status, Gd gadolinium, GFS greedy forward selection, NfL neurofilament light chain.
Multivariate model performance for all four analyses, along with permutation-based feature importance were plotted in Fig. 5 . Results for the Gd lesion regression analysis were presented as a two-dimensional histogram depicting the distribution of actual versus predicted lesion count for the regression analysis, and as receiver operating characteristic (ROC) curves for the classification analysis. The central line in each ROC band represented the mean of the ROC across all bootstraps; band width was the uncertainty in that mean. The regression analysis panel of Fig. 5 also included the best fit line through the results drawn in solid black with the root mean square error as a shaded gray region. Perfect agreement (actual = predicted) was drawn for reference in dashed gray. For the classification analysis panels, we included the following two feature sets in addition to the greedy forward selection (GFS) proteins for each endpoint: NfL only, and all features except NfL. Feature importance was estimated by randomly permuting the concentration values of each protein individually across samples and checking the performance decrease of the model for each bootstrap built on the GFS features.
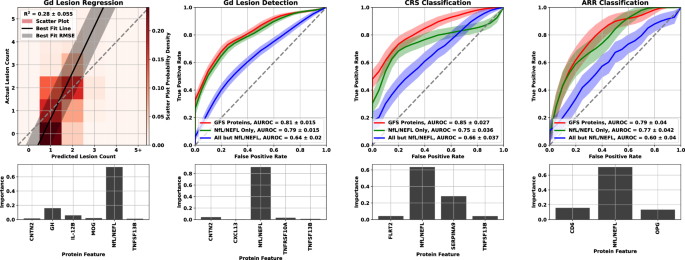
For Gd lesion count estimation, the R 2 is reported as the mean and standard deviation across the bootstrap splits. A heat map of the scatter plot probability density is represented by lighter to darker shades of red. The black line is the best fit to the scatter plot of actual vs. predicted lesion counts, and the gray shaded region is the RMSE. For comparison, we also include a dashed line for perfect agreement (actual equals predicted). ROC curves for the three classification analyses are represented as a solid line for the mean and shaded region for the standard deviation across all bootstrap splits using the following colors: red represents the model built with the greedy forward selection proteins, green represents the model built with NfL/NEFL only, and blue represents the model built with every protein but NfL/NEFL. Each analysis was plotted with the model performance plot above a feature importance bar graph for the GFS proteins. Source data are provided as a Source Data file. ARR annualized relapse rate, CRS clinical relapse status, Gd gadolinium, GFS greedy forward selection, R 2 square of Pearson’s correlation coefficient.
For the Gd lesion detection endpoint, we quantified model performance in three different ways. General DA (GDA) was used for separation of samples with no Gd lesions from those with any positive count of lesions, Subtle DA (SDA) was used for separation of samples with no Gd lesions from those with only one lesion, and Extreme DA (EDA) was used for separation of samples with no Gd lesions from those with three or more lesions.
While we only trained lesion detection models on GDA, we included model performance metrics for the SDA and EDA endpoints as well in our discussion of Gd lesion detection. Additionally, we checked Gd lesion regression performance on models constructed from both NfL only and all proteins except NfL for comparison to the classification endpoint. The GFS feature models performed as follows: the square of Pearson’s correlation coefficient (R 2 ) = 0.280 ± 0.027 for Gd lesion count regression, area under the receiver operator characteristic (AUROC) = 0.813 ± 0.015 for GDA classification, AUROC = 0.845 ± 0.026 for CRS classification, and AUROC = 0.803 ± 0.039 for ARR status classification. We tabulated the performance of all multivariate analyses in Table 2 .
Comparison of the actual versus estimated Gd lesion count (left panel, Fig. 5 ) showed that the regression model slightly overpredicts Gd lesion count for samples with no lesions and underpredicts it for those with five or more, while the model’s accuracy for lesion counts of 1-2 anchored the performance of the model. While regression models performed acceptably, results from the classification analysis were considerably stronger. For all Gd lesion analyses, NfL had the largest effect (Table 3 ; Fig. 5 ). NfL performance as the strongest biomarker was further reflected in the feature importance plots at the two left bottom panels of Fig. 5 . However, there is an incremental improvement in performance between NfL only and the GFS proteins, particularly in the Gd lesion regression analysis. Furthermore, the models using all proteins except NfL were significantly better than random chance. There is clearly signaling for this endpoint in the other proteins that is drowned out by the performance of NfL for predicting Gd lesions.
While NfL was the strongest performer for CRS and ARR as well, its lead over the other proteins was considerably attenuated. GFS protein performance for CRS outperformed all endpoints except EDA, and achieved similar ARR results to that of GDA. Multivariate separation of samples by CRS and ARR was significantly better than chance and significantly better than univariate NfL. Consistent with the Gd lesion analysis, multivariate models trained on all features except NfL displayed an AUROC significantly better than random chance.
Biological context of protein-endpoint associations reveals heterogeneous pathophysiology and complex molecular crosstalk
The biological context of the 21 proteins listed in Table 1 was analyzed in the following two ways: direct spatial, functional, and gene expression correlations to a curated set of open-source databases, and expanded graph techniques using the Scalable Precision Medicine Open Knowledge Engine (SPOKE).
For the direct correlation analysis, protein concentration values were associated with data from the Human Protein Atlas 20 (aggregated lymphoid tissue, peripheral blood immune cell type, and brain region proteomic data) as well as the Allen Brain Atlas 21 (brain structure, cell type, and transcriptomic data). Protein-protein interaction modeling was performed by inputting proteins into STRING 22 for network construction. Physically and functionally associated proteins that exhibited a minimum interaction score of 0.7 (high confidence) were classified as interacting with each CAP protein. Markov Clustering 23 , 24 was used to detect distinct subgraphs of interconnected proteins. Topological surveillance and centrality metric calculations were performed in Cytoscape 25 . Enrichr 26 was leveraged to functionally annotate protein subgraphs.
Comparative proteomic analysis across human organs, tissues, and cell types helped home in on MS-relevant information by isolating organ-specific blood analytes to address the blood’s pervasive nature. This revealed the MS-specific locality of CAP proteins, facilitating downstream directionality assessment and revealing a rich repertoire of cell types related to MS. Constitutive expression of these proteins helped to facilitate downstream mechanistic modeling. The resultant mapping of the 21 CAP proteins onto 10 biological hallmarks of MS is presented in the left panel of Fig. 6 . The 10 hallmarks were each subdivided into two to three related biological processes. CAP proteins were then sorted into these processes based on their correlations with the databases and tools mentioned.
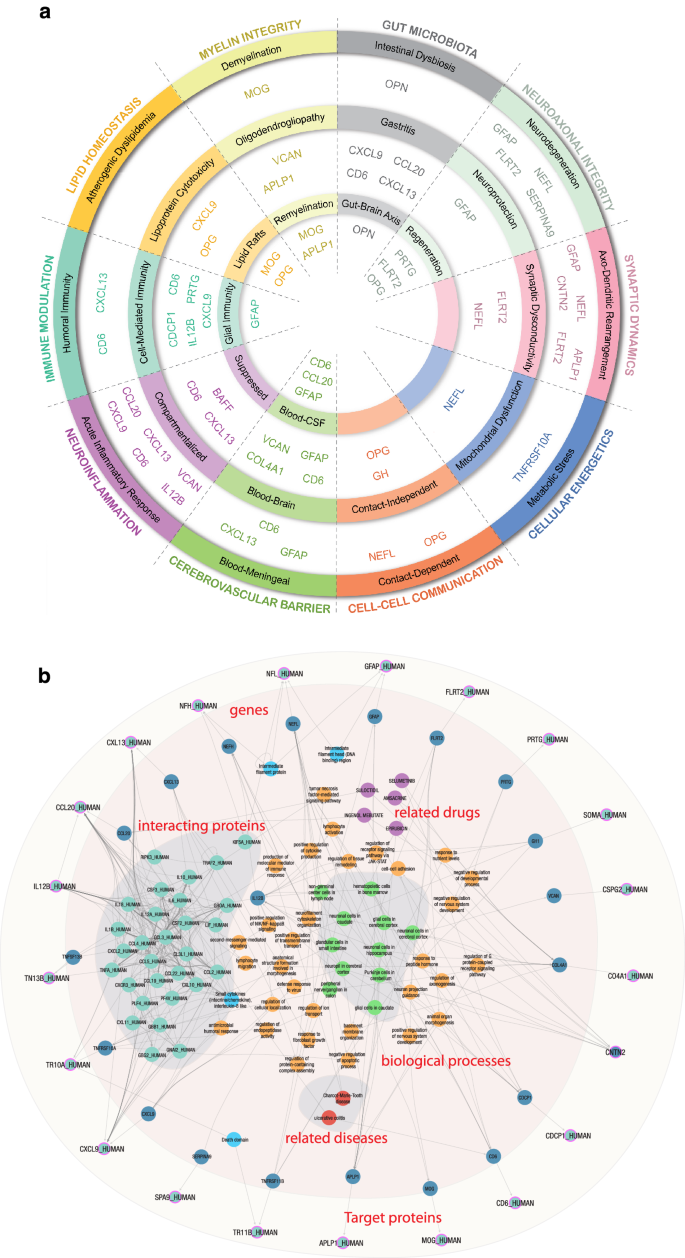
a CAP proteins sorted into 10 MS hallmarks, categorically grouped by color, representing associated biological processes using each protein’s correlation to spatial, functional, and gene expression data. b SPOKE graph visualization of biological neighborhood of CAP proteins. Using proteins as inputs (light blue circles with purple borders) results in a fully connected module including encoding genes (dark blue circles), directly interacting proteins (teal circles), their domains (sky blue circles), biological processes (orange circles) and a short list of related diseases (red circles). CAP custom assay panel, MS multiple sclerosis, SPOKE Scalable Precision Medicine Open Knowledge Engine.
The SPOKE Neighborhood Explorer ( https://spoke.rbvi.ucsf.edu ) allows targeted exploration of any component of the SPOKE knowledge graph. SPOKE is a comprehensive graph with millions of biomedical concepts and has been previously utilized for drug repurposing 27 , to conduct genetic analyses 28 , and for clinical predictions 29 . SPOKE has integrated data from close to 40 databases, including ChEMBL, OMIM, LINCS, and the Human Protein Atlas, among others. As such, SPOKE contains all human genes and proteins, more than a million pharmaceutical compounds, more than 7000 diseases, and a comprehensive representation of signaling and metabolic pathways. Further, biological interpretation of the 21 proteins also included spatial expression profiling, Protein-Protein interaction modeling, and Gene Set Enrichment. The SPOKE knowledge graph for the CAP proteins is shown in the right panel of Fig. 6 .
We evaluated multivariate analyses of blood serum biomarkers from three independent cohorts and identified twenty proteins that were strongly associated with increased clinical and radiographic activity of MS. To quantify MS activity, we focused principally on the presence of Gd+ lesions compared with patients lacking such lesions. We also examined the following two clinical measures of MS relapse activity: clinician assessment of relapse state at or near the time of blood draw, and the ARRs in the time leading up to the draw.
Protein feature-selection processes can inherently introduce bias. To minimize bias, we deliberately attempted to balance weight and normalization strategies. For example, we weighted each feature importance by the AUROC of the model in which it appeared. When AUROC is calculated on the same data on which the model was trained, it tends to bias the result in favor of proteins that perform well in large models. Larger models are more prone to overfitting noise in the training data, which will lead to systematically higher AUROC values. Also, normalizing the vector of feature importance values for each model to 1 tends to favor proteins that perform better in models with only a few features. Since the total importance score for models with fewer features is split among fewer proteins compared with ones with larger feature sets, the values themselves will be numerically higher.
There are two points that strongly favor multivariate modeling over univariate NfL regarding the Gd endpoint. First, the optimized multivariate model significantly outperformed univariate NfL in every framing of the problem of using serum protein chemistry to predict Gd lesion activity. Second, we find statistically significant performance in a multivariate modeling even when the model ignores NfL concentrations. Both statements hold for an endpoint that should be most advantageous to the performance of univariate NfL (Gd lesions), and the difference was even larger for the clinical relapse endpoints. Furthermore, univariate NfL lacks specificity, as NfL levels are known to be elevated for neurodegenerative conditions other than MS 30 .
NfL showed the strongest univariate correlation with the radiographic and clinical measures of DA examined in this study. Furthermore, multivariate techniques showed increased performance compared with NfL alone for all analyses of all endpoints. Similarly, multivariate models without NfL were still significantly more performant than chance for all endpoints, suggesting biological signal from the other CAP proteins as well. The inclusion of additional inflammatory and neurodegenerative protein biomarkers can provide deeper insights and reveal stronger correlations to clinical and radiographic DA than NfL individually. Cytokines, chemokines, and other immune-related molecules have consistently been associated with DA in patients with MS and they constitute an attractive target for interrogation in biological samples from patients at different stages of their disease course. Additionally, measuring protein concentration has several advantages over transcriptional profiling, including higher stability and more straightforward biological interpretation of the results. A biological pathway-centered approach using a subset of those shown in the left panel of Fig. 6 is likely to be a successful strategy for planning future investigation.
In addition to overall efficacy of the models used to examine the endpoints in this study, information from a broader panel of serum biomarkers allowed for insights into the pathophysiology of new Gd lesions and clinical relapse. This includes the identification of chemokines that are significantly altered by DA mediated by inflammation or immune response. A multi-protein panel like the one developed in this study has the capability to capture the state of a patient’s MS from multiple angles, allowing for a fuller picture of their pathophysiology.
This study leveraged three large, well-characterized cohorts of patients with MS, and evaluated well over 1000 protein biomarkers using highly sensitive assays and applied network modeling to the findings to provide insights into MS. We used a systems biology framework to contextualize the mechanism-of-action of selected serum protein biomarkers with respect to MS DA. Through a complementary integration of machine learning and functional network analysis, we were able to shed light on the heterogeneous pathophysiological underpinnings of MS and unveil the orchestrated crosstalk between the various molecular facets of the disease. The analysis presented here was, however, purely cross-sectional. Large-scale longitudinal studies will be necessary to better understand MS and its evolution over time to unlock the potential of personalized MS care.
The end goal of the research program, the beginning of which is presented in this article, is a MS DA test that is fully validated in a clinical trial. Such a test would have tremendous clinical utility for many issues in MS care, including identification of active relapse, prediction of impending relapse, confirmation of NEDA status, assessment of patient-specific longitudinal changes relative to previous tests, and response to disease-modifying therapies (DMT). MS relapses can be quite subtle, especially early in the disease course, and can be easily confused with recurrences of symptoms in the setting of stressors (pseudo-flares) or conditions other than MS. This can lead to either the misattribution of an unrelated symptom to an MS relapse or to an early relapse being misattributed to some other clinical event. An inexpensive, clinically simple, precise, repeatable test for MS relapses would go a long way toward reducing both types of errors. Response to relapses often includes an escalation in DMT or steroid doses, both of which can have side effects. A test like the one we describe here has the potential to also serve as a leading indicator of impending relapses ahead of clinical presentation. This capability would need to be tested and validated clinically to quantify the scale of such a lead time. NEDA is the clinical gold standard for MS care. A NEDA designation indicates the patient’s clinical and radiographic activity are held in check. A test like this one could alert a patient’s MS care team to otherwise subclinical (or sub-radiographic) DA. An underlying truth beneath many scientific discoveries is that differential measurements tend to be simpler and more accurate than absolute ones. This is likely true of quantitative DA measurements as well. Some absolute level of DA score would be instructive to both patient and clinician, but deviations from baseline levels for an individual patient would be more so.
The ability to quantify the level of DA with a test like the one proposed could serve as an endpoint for clinical trials of DMT drugs and help clinicians to evaluate the efficacy of a DMT for a particular patient in less time than it would take for clinical or radiographic evidence to present. This would greatly enhance the quality of life and increase the health span of patients with MS and offer insight into patient adherence to their treatment plan.
Analytical validation of this DA panel will be followed by clinical validation studies to verify association with DA endpoints (primarily Gd lesions) in multiple independent cohorts 31 . Expansion of the test’s clinical utility will be investigated with future studies to evaluate biomarker correlations with endpoints associated with MS DP, therapy selection, and differential diagnosis.
Ethics statement
The study protocol and study procedures were approved by institutional review boards and independent ethics committees at each study site. The University of California San Francisco Institutional Review Board granted ethical approval for the Expression, Proteomics, Imaging, Clinical at UCSF (EPIC) cohort. Mass General Brigham Human Research Committee granted ethical approval for the Comprehensive Longitudinal Investigation of MS at Brigham and Women’s Hospital (CLIMB) cohort. Western Institutional Review Board, Copernicus Group IRB, Sheperd Center Research Review Committee, Institutional Review Board for Human Research at St. Joseph’s Hospital and Medical Center, University of Massachusetts Medical School Committee for the Protection of Human Subjects in Research, Ohio State University Biomedical Institutional Review Board, Beth Israel Deaconess Medical Center Committee on Clinical Investigations, Johns Hopkins Medicine Office of Human Subjects Research Institutional Review Board, and the Southwestern Medical Center Institutional Review Board all granted ethical approval for the Accelerated Cure Project (ACP) cohort.
Serum samples were obtained from a subset of three deeply phenotyped cohorts were analyzed for protein levels and associated with clinical and radiographic endpoints and a subset of the associated clinical manifest to select features for inclusion in a custom assay panel and used in a subsequent cross-sectional analysis. The three endpoints in this study were: presence of Gd lesions for samples (defined as samples for which the blood draw was performed within 30 days of a contrast-enhancing magnetic resonance imaging, with the count of Gd lesions determined by a neuroradiologist), clinically active MS versus clinically inactive MS samples (defined as samples for which the blood draw was performed during a state of active relapse or inactive remission as defined by a clinician), and high versus low ARR samples (defined as those for which the blood draw was performed, and corresponding ARR-derived binary labels were determined (high: ≥ 1.0 and low: ≤ 0.2 relapses per year).
These three endpoints were taken from three different cohorts of patients and samples. All three cohorts are much larger than the subsets analyzed in this cross-sectional study. Samples were chosen to balance occupancy across the Gd lesion and CRS endpoints (ARR represented more of an opportunistic endpoint). The three cohorts contributing samples to this study were CLIMB (CLIMB endpoints: radiographically defined relapse status using Gd lesions [primary], ARR [secondary]), EPIC (EPIC endpoint: radiographically defined relapse status using Gd lesions), ACP (ACP endpoint: clinically defined relapse status - active versus inactive).
A total of 506 samples from CLIMB and EPIC were included in the Gd lesion analysis. One hundred and twenty-four samples from ACP contributed to the CRS endpoint. A subset of 161 of the CLIMB samples, for which ARR was available, were used for that analysis. A detailed summary of the salient demographic and clinical features from each cohort is presented in Table 3 .
Application of selected proteins to study endpoints
After selecting the top 20 performing proteins (20, not 21 because one spot in the panel was saved for a desired protein being added to the Olink platform–see Results for further discussion), we next evaluated their performance in the clinically relevant endpoints in an independent statistical analysis. The Gd lesion endpoint was investigated using both a classification and a regression approach. Specifically, a logistic regression model was trained to classify each serum sample as being associated with some positive number of Gd lesions or not, and a Poisson regression model was trained to estimate the number of Gd lesions associated with each sample. Investigation of the CRS involved the analysis of blood serum taken from patients during a clinically active relapse (exacerbation) or during a period of clinical stability, or inactive status (quiescence), following an approach similar to that taken for lesion presence classification. Finally, we addressed the ARR endpoint by considering the question of whether each sample had ARR < 0.2 relapses per year (low) or >1.0 relapse per year (high). Recognizing that the modest sample size and low positivity rate of ARR (6.9%, or 13 out of 188 samples) impact the power of this analysis, the same overall strategy as the two previous binary endpoints was used.
Due to the finite timescale on which brain lesions will be enhanced under Gd contrast, we precleaned our Gd lesion data by discarding all serum samples drawn more than 30 days from (before or after) the magnetic resonance imaging from which the lesion count was extracted. We accounted for batch-to-batch variability in relative quantitation by measuring bridge normalization samples across all assays. Intra- and inter-assay percent coefficients of variation are reported in Table 4 .
The univariate analysis of all three endpoints was performed using the bridge-normalized NPX values described above. The multivariate analysis for each endpoint used those same NPX values after correction for the following clinical variables: age, sex, and MS disease duration. An ordinary least squares linear regression model was fitted to the batch-normalized NPX values of the Gd-negative samples for each protein using the clinical variables as features. This estimate was then subtracted from all sample NPX values to make the demographic/clinical correction. The residual from this correction process was used as features in multivariate modeling.
Throughout each stage of the multivariate modeling process, an ensemble of bootstrap simulations was generated to control for overfitting. The data for each endpoint was randomly split into train (two-thirds) and test (one-third) subsets 1000 times. A different model of the same configuration was then trained on each bootstrap split, and model performance was taken to be the mean across the ensemble (with uncertainty parameterized by the square root of the variance). We chose bootstrap simulations instead of k-fold cross validation because the particular random split in the cross validation could have small effects on the outcome of some of the modeling efforts. The large number of random splits generated in the bootstrap process smoothed contributions from the small number of outlier samples in our data.
Both the regression and classification analyses were performed on an optimized subset of the entire panel chosen using GFS of protein features, with R 2 and AUROC, respectively, as the target metric and average over the ensemble of bootstrap splits. GFS starts with the top-performing univariate protein then checks each of the remaining features to construct the most performant two-protein model. With that in hand, each of the remaining proteins is checked in turn to see which combination provides the best three-protein performance. The process continues until all available proteins are included in the model. Optimal model size is then determined as the feature set size where performance is no longer significantly improved by adding an additional protein feature. In general, this function reaches a plateau value of optimal performance and then turns over as extra features are added that contribute only noise to the model. This is often a global maximum value of all possible combinations of features but was taken for the purposes of our study as the optimal model size and performance.
Statistics and reproducibility
Analytical methods.
For the purposes of the screening studies, up to 1411 proteins were measured using two separate immunoassay platforms. The first panel of 1196 proteins were analyzed using Proximity Extension Assay technology on the Olink™ Platform 32 . Protein concentrations were reported as NPX values (normalized protein expression), which provide expression levels relative to the other samples included on the plate and within the batch. An additional panel of 215 proteins were analyzed using xMAP® technology immunoassays at Myriad RBM, Inc. (RBM). Absolute protein concentrations from the RBM platform were determined using calibrated standard curves.
Exploratory data analysis was conducted to filter noise, reduce dimensionality, and avoid collinearity. Univariate significance was combined with multivariate importance from models generated with randomly selected combinations of different numbers of proteins to select features for inclusion into the custom assay panel. Biomarkers selected as features in the panel were investigated for relevance and interactions using biological network models. A 21-plex custom assay panel was then manufactured and analytically validated 33 to establish the following specifications and parameters: accuracy, precision, sensitivity, specificity, reference ranges, stability (reagents and samples), diurnal variation, drug interference, and assay robustness. The custom assay panel has been manufactured to include calibrators to report results in absolute concentration and a fit-for-purpose analytical validation has been performed. All samples previously run in the biomarker screening studies were reanalyzed using the custom assay panel.
Biostatistical methods
The program outlined in this report can be organized into the following two phases: the feature selection of the final 21 protein analytes and the studies that use those proteins as features to examine the three study endpoints. The former balanced information about all three endpoints to select a final ensemble of proteins for use as features in a more detailed cross-sectional analysis of all three endpoints in the latter phase. All analyses were performed in the python 34 programming language (version 3.11.7), making use of the SciPy 35 (version 1.11.4) and Scikit-Learn 36 (version 1.2.2) packages for statistical tests and machine learning models respectively.
Protein feature selection
The protein feature selection phase of this analysis preceded and was completely independent of the work reported in the Results section of this article. That analysis used only the 20 proteins chosen in the protein feature selection exercise described in this section. We followed two parallel tracks to identify the proteins most strongly associated with the three study endpoints.
First, we looked at the univariate correlation between each of 1411 proteins (1196 on the Olink platform and 215 from RBM). Gd lesions were treated as a binary variable used to classify samples based on their presence or absence (i.e., zero lesions vs. one or more). This allowed us to treat this endpoint consistently with the other two, which are inherently binary in nature. We computed the AUROC for separating the positive from negative samples for all three endpoints for all 1411 proteins and ranked them in decreasing order for each, paying special attention to analytes that showed a strong association with more than one endpoint.
To avoid biasing our multivariate feature selection process toward proteins that performed well in one but not another model architecture (e.g., tree-based vs. linear models), we investigated each of the following model types: logistic regression, support vector classifiers, and random forest classifiers. Furthermore, we did not have an a priori estimate of the optimal number of features in each model, so we tested models with 3–21 proteins in steps of three. Because of its strong univariate performance in our data and its well-established association with many aspects of MS pathophysiology in the published literature, we used NfL (NfL is referred to by its gene name NEFL on the Olink platform, so we used the two names interchangeably in the analysis of these data) as the seed for multivariate feature selection analyses. We then randomly selected proteins in addition to NfL to fill out the set number of features (3–21, by threes) and used them to construct models of all three architecture types. This was repeated 100,000 times for each combination of model size and architecture. All models were trained on the entire dataset and evaluated for efficacy against the endpoint under consideration by calculating AUROC. After generating this ensemble of models, we extracted feature importance values from each one and tracked the feature importance for each protein over all the models in which it appeared.
In logistic regression models, feature importance was taken to be the variance-normalized absolute value of each feature coefficient. Protein concentration was expressed in NPX for this study, which is analogous to the log of the absolute concentration of each protein. The variance across all samples therefore did not numerically vary as widely across protein as it would if we were using linear concentration values. In support vector classifiers, we took feature importance to be the feature coefficient absolute value. We used a linear kernel for our support vector classifier models so that feature coefficients were well defined. In random forest classifier models, we simply used the Gini coefficients attached to the model.
Feature importance vectors were all normalized to unit sum for each model. When averaging the feature importance value for each protein across all models in which it appeared, we weighted each importance by the AUROC of its model so that proteins appearing in highly efficacious models would be favored over ones in models with poor performance. We then ranked each protein by its average AUROC-weighted feature importance so that we could quantify the multivariate performance of each protein separately from its univariate performance. We selected the top performers integrated across each endpoint’s univariate and multivariate lists. The univariate and multivariate feature importance was then weighed along with more operational/analytical constraints (e.g., analyte precision and stability, confounding temporal or behavioral dependencies, association with biologically interesting physiological pathways) in the choice of the 20 proteins included in the assay. The final list of selected proteins is compiled in Table 1 of this report.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are openly available in GitHub at https://github.com/vmgehman/infl-neurodeg-bmkr-ms . Source data are provided with this paper.
Code availability
The code that supports the findings of this study is openly available in GitHub at https://github.com/vmgehman/infl-neurodeg-bmkr-ms .
Baecher-Allan, C., Kaskow, B. J. & Weiner, H. L. Multiple sclerosis: mechanisms and immunotherapy. Neuron 97 , 742–768 (2018).
Article CAS PubMed Google Scholar
Reich, D. S., Lucchinetti, C. F. & Calabresi, P. A. Multiple sclerosis. N. Engl. J. Med. 378 , 169–180 (2018).
Article CAS PubMed PubMed Central Google Scholar
Chitnis, T. & Weiner, H. L. CNS inflammation and neurodegeneration. J. Clin. Invest 127 , 3577–3587 (2017).
Article PubMed PubMed Central Google Scholar
Macaron, G. & Ontaneda, D. Diagnosis and management of progressive multiple sclerosis. Biomedicines 7 , 56 (2019).
Wingerchuk, D. M. & Carter, J. L. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin. Proc. 89 , 225–240 (2014).
Article PubMed Google Scholar
Barro, C. et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 141 , 2382–2391 (2018).
Comabella, M. & Montalban, X. Body fluid biomarkers in multiple sclerosis. Lancet Neurol. 13 , 113–126 (2014).
Disanto, G. et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 81 , 857–870 (2017).
Mellergård, J. et al. Cerebrospinal fluid levels of neurofilament and tau correlate with brain atrophy in natalizumab-treated multiple sclerosis. Eur. J. Neurol. 24 , 112–121 (2017).
Novakova, L. et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 89 , 2230–2237 (2017).
Varhaug, K. N. et al. Neurofilament light chain predicts disease activity in relapsing-remitting MS. Neurol. Neuroimmunol. Neuroinflamm. 5 , e422 (2018).
Rosso, M. et al. Temporal association of sNfL and gad-enhancing lesions in multiple sclerosis. Ann. Clin. Transl. Neurol. 7 , 945–955 (2020).
Hviid, C. V. B., Madsen, A. T. & Winther-Larsen, A. Biological variation of serum neurofilament light chain. Clin. Chem. Lab. Med. 60 , 569–575 (2021).
Sawcer, S. et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476 , 214–219 (2011).
Article ADS CAS PubMed PubMed Central Google Scholar
Magliozzi, R. et al. The CSF profile linked to cortical damage predicts multiple sclerosis activity. Ann. Neurol. 88 , 562–573 (2020).
Comabella, M. et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain 133 , 1082–1093 (2010).
Modvig, S. et al. Cerebrospinal fluid levels of chitinase 3-like 1 and neurofilament light chain predict multiple sclerosis development and disability after optic neuritis. Mult. Scler. 21 , 1761–1770 (2015).
Masvekar, R., Phillips, J., Komori, M., Wu, T. & Bielekova, B. Cerebrospinal fluid biomarkers of myeloid and glial cell activation are correlated with multiple sclerosis lesional inflammatory activity. Front. Neurosci. 15 , 649876 (2021).
Masvekar, R. et al. Cerebrospinal fluid biomarkers link toxic astrogliosis and microglial activation to multiple sclerosis severity. Mult. Scler. Relat. Disord. 28 , 34–43 (2019).
Karlsson, M. et al. A single-cell type transcriptomics map of human tissues. Sci. Adv. 7 , eabh2169 (2021).
Allen Institute for Brain Science. Allen Cell Types Database—Human Morphology Electrophysiology. (2015).
Szklarczyk, D. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 47 , D607–D613 (2019).
Enright, A. J., Van Dongen, S. & Ouzounis, C. A. An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res. 30 , 1575–1584 (2002).
Van Dongen S. Graph clustering by flow simulation. PhD thesis, Center for Math and Computer Science (CWI) , (2000).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13 , 2498–2504 (2003).
Kuleshov, M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44 , W90–W97 (2016).
Himmelstein, D. S. et al. Systematic integration of biomedical knowledge prioritizes drugs for repurposing. Elife 6 , e26726 (2017).
Himmelstein, D. S. & Baranzini, S. E. Heterogeneous network edge prediction: a data integration approach to prioritize disease-associated genes. PLoS Comput. Biol. 11 , e1004259 (2015).
Article ADS PubMed PubMed Central Google Scholar
Nelson, C. A., Bove, R., Butte, A. J. & Baranzini, S. E. Embedding electronic health records onto a knowledge network recognizes prodromal features of multiple sclerosis and predicts diagnosis. J. Am. Med. Inform. Assoc. 29 , 424–434 (2022).
Article PubMed Central Google Scholar
Varhaug, K. N., Torkildsen, Ø., Myhr, K. M. & Vedeler, C. A. Neurofilament light chain as a biomarker in multiple sclerosis. Front. Neurol. 10 , 338 (2019).
Chitnis, T. et al. Clinical validation of a multi-protein, serum-based assay for disease activity assessments in multiple sclerosis. Clin. Immunol. 253 , 109688 (2023).
Slenter, D. N. et al. WikiPathways: a multifaceted pathway database bridging metabolomics to other omics research. Nucleic Acids Res. 46 , D661–D667 (2018).
Qureshi, F. et al. Analytical validation of a multi-protein, serum-based assay for disease activity assessments in multiple sclerosis. Proteomics. Clin. Appl. 17 , e2200018 (2023).
Van Rossum G., Drake F. L. Python 3 Reference Manual (2009).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17 , 261–272 (2020).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12 , 2825–2830 (2011).
MathSciNet Google Scholar
Download references
Acknowledgements
Editorial assistance was provided by Maureen Wallace-Nadolski, PhD, of The Lockwood Group (Stamford, CT) and was supported by funding from Octave Bioscience, Inc (Menlo Park, CA).
Author information
Authors and affiliations.
Brigham and Women’s Hospital, Boston, MA, USA
Tanuja Chitnis, Hrishikesh Lokhande, Anu Paul, Neda Sattarnezhad, Shrishti Saxena, Howard Weiner & Hajime Yano
Octave Bioscience, Inc, Menlo Park, CA, USA
Ferhan Qureshi, Victor M. Gehman, Michael Becich & Amal Katrib
Department of Neurology. Weill Institute for Neurosciences. University of California San Francisco, San Francisco, CA, USA
Riley Bove, Bruce A. C. Cree, Refujia Gomez, Stephen L. Hauser, Roland G. Henry, Stacy J. Caillier, Adam Santaniello & Sergio E. Baranzini
You can also search for this author in PubMed Google Scholar
Contributions
T.C., F.Q., V.M.G, M.B., R.B, B.A.C.C., R.G., S.L.H., R.G.H, A.K., H.L., A.P., S.J.C., A.S., N.S., S.S., H.W., H.Y., and S.E.B. were involved in the conception and design of the study, data acquisition, and data analysis. V.M.G., M.B., A.K., H.L., and S.E.B. conducted the statistical analysis. T.C., F.Q., V.M.G., M.B., and S.E.B. drafted the manuscript. T.C., F.Q., V.M.G, M.B., R.B, B.A.C.C., R.G., S.L.H., R.G.H, A.K., H.L., A.P., S.J.C., A.S., N.S., S.S., H.W., H.Y., and S.E.B. critically reviewed the manuscript and approved the submitted version.
Corresponding author
Correspondence to Ferhan Qureshi .
Ethics declarations
Competing interests.
The authors declare the following competing interests: T.C. has received compensation for consulting from Biogen, Novartis Pharmaceuticals, Roche Genentech, and Sanofi Genzyme, and received research support from the National Institutes of Health, National MS Society, US Department of Defense, EMD Serono, I-Mab Biopharma, Novartis Pharmaceuticals, Octave Bioscience, Inc, Roche Genentech, and Tiziana Life Sciences. V.M.G., M.B., and A.K. were employees of Octave Bioscience, Inc at the time the study was completed. R.B. is funded by the NMSS Harry Weaver Award, NIH, DOD, NSF, as well as Biogen, Novartis, and Roche Genentech. She has received personal fees for consulting from Alexion, EMD Serono, Horizon, Janssen, Sanofi-Genzyme, and TG Therapeutics. B.A.C.C. has received personal compensation for consulting from Alexion, Atara, Autobahn, Avotres, Biogen, Boston Pharma, EMD Serono, Gossamer Bio, Hexal/Sandoz, Horizon, Immunic AG, Neuron23, Novartis, Sanofi, Siemens, and TG Therapeutics, and received research support from Genentech. S.L.H. currently serves on the scientific advisory board of Accure, Alector, and Annexon; board of directors of Neurona; and has previously consulted for BD, Moderna, and NGM Bio. Dr. Hauser also has received travel reimbursement and writing support from F. Hoffmann-La Roche and Novartis AG for anti-CD20 therapy-related meetings and presentations and is supported by grants from the NIH/NINDS (R35NS111644). R.G. H. has received fees for consultation from Roche/Genentech, Novartis, Neuron23, QIA Consulting, and research funding from Roche/Genentech and Atara. H.L. has received research support from the US Department of Defense and Octave Bioscience, Inc. A.P. is currently an employee of Moderna Therapeutics. F.Q. is an employee of Octave Bioscience, Inc. N.S. has received the Sylvia Lawry Physician Fellowship Award from the National MS Society. She has also received compensation for consulting from EMD Serono. H.W. has received research support from the Department of Defense, Genentech, Inc., National Institutes of Health, National Multiple Sclerosis Society, Novartis, and Sanofi Genzyme. He has received compensation for consulting from Genentech, Inc., IM Therapeutics, IMAB Biopharma, MedDay Pharmaceuticals, Tiziana Life Sciences, and vTv Therapeutics. S.E.B. is co-Founder of Mate Bioservices. H.Y., R.G., S.S., S.J.C., and A.S. declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Cheng You, Bernd Zolitschka and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Chitnis, T., Qureshi, F., Gehman, V.M. et al. Inflammatory and neurodegenerative serum protein biomarkers increase sensitivity to detect clinical and radiographic disease activity in multiple sclerosis. Nat Commun 15 , 4297 (2024). https://doi.org/10.1038/s41467-024-48602-9
Download citation
Received : 16 June 2023
Accepted : 07 May 2024
Published : 20 May 2024
DOI : https://doi.org/10.1038/s41467-024-48602-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO
COMMENTS
The diagnosis of multiple sclerosis (MS) is through clinical assessment and supported by investigations. There is no single accurate and reliable diagnostic test. ... with a presentation other than a typical clinically isolated syndrome, or with atypical features. If imaging or other tests (eg CSF) are undertaken and are negative, caution needs ...
People with multiple sclerosis may also develop: Muscle stiffness or spasms. Severe weakness or paralysis, typically in the legs. Problems with bladder, bowel or sexual function. Cognitive problems, like forgetfulness or word finding difficulties. Mood problems, such as depression, anxiety or mood swings.
Multiple sclerosis (MS) is an immune-mediated inflammatory disease that attacks myelinated axons in the central nervous system, destroying the myelin and the axon in variable degrees and producing significant physical disability within 20-25 years in more than 30% of patients. The hallmark of MS is symptomatic episodes that occur months or ye...
Multiple sclerosis (MS) is the most common immune-mediated inflammatory demyelinating disease of the central nervous system. MS is characterized pathologically by multifocal areas of demyelination with loss of oligodendrocytes and astroglial scarring. Axonal injury is also a prominent pathologic feature, especially in the later stages.
Clinical presentation, course, and prognosis of multiple sclerosis in adults. Literature review current through: Jan 2024. This topic last updated: Aug 30, 2023. Multiple sclerosis (MS) is the most common immune-mediated inflammatory demyelinating disease of the central nervous system. The onset and phenotypes of MS will be reviewed here.
Multiple sclerosis (MS) is a demyelinating disorder of the central nervous system and the most common cause of nontraumatic neurologic disability in young adults. Types of MS include relapsing ...
The most common immune-mediated inflammatory demyelinating disease of the central nervous system is multiple sclerosis (MS). The clinical manifestations of MS will be reviewed here. Other aspects of MS are discussed separately: Pathogenesis and epidemiology of multiple sclerosis. Clinical presentation, course, and prognosis of multiple ...
Multiple sclerosis affects more than 2 million people worldwide and is currently incurable. ... Typical syndromes at presentation ... probably because of the heterogeneous presentation and course ...
Multiple sclerosis (MS) is the most common disabling neurological disease of young adults with symptom onset generally occurring between the ages of 20 to 40 years. In MS, the immune system cells that normally protect us from viruses, bacteria, and unhealthy cells mistakenly attack myelin in the central nervous system (brain, optic nerves, and spinal cord).
The diagnosis of multiple sclerosis (MS) is through clinical assessment and supported by investigations. There is no single accurate and reliable diagnostic test. MS is a disease of young adults with a female predominance. There are characteristic clinical presentations based on the areas of the central nervous system involved, for example ...
The diagnosis of multiple sclerosis (MS) is through clinical assessment and supported by investigations. There is no single accurate and reliable diagnostic test. MS is a disease of young adults with a female predominance. There are characteristic clinical presentations based on the areas of the central nervous system involved, for example optic nerve, brainstem and spinal cord. The main ...
There is no cure for multiple sclerosis. Treatment typically focuses on speeding recovery from attacks, reducing new radiographic and clinical relapses, slowing the progression of the disease, and managing MS symptoms. Some people have such mild symptoms that no treatment is necessary. Multiple sclerosis research laboratory at Mayo Clinic.
Reviewed/Revised May 2023. Multiple sclerosis (MS) is characterized by disseminated patches of demyelination in the brain and spinal cord. Common symptoms include visual and oculomotor abnormalities, paresthesias, weakness, spasticity, urinary dysfunction, and mild cognitive symptoms. Typically, neurologic deficits are multiple, with remissions ...
Multiple sclerosis (MS) is an autoimmune inflammatory disorder that affects more than 900,000 Americans. Patient presentations vary widely; therefore, symptom recognition and an understanding of diagnostic criteria are critical in providing timely patient referrals. This article describes recognition and diagnosis of MS using the updated 2017 ...
Multiple sclerosis (MS), a chronic, inflammatory disease of the central nervous system (CNS) with hallmarks of demyelination and axonal degeneration, is characterized by heterogeneity in the symptoms, disease course, and outcomes ( Compston and Coles 2008 ). Typically affecting patients between 20 and 40 years of age, MS is a leading cause of ...
Multiple sclerosis (MS) typically presents between 20-50 years of age. About 0.5% of adults with MS first develop symptoms aged 60 years or older — older age at onset is associated with a progressive course. The person may have: A history of previous neurological symptoms. Symptoms that evolve over more than 24 hours, may persist over ...
Typical syndromes at presentation include, but are not limited to, monocular visual loss due to optic neuritis, limb weakness ... cost of multiple sclerosis in the United States is approximately ...
Introduction. Multiple Sclerosis (MS) is a chronic inflammatory, demyelinating, and neurodegenerative disorder of the central nervous system (CNS) that affects the white and grey matter of the brain, spinal cord, and optic nerve. MS is one of the most common causes of non-traumatic disability among young and middle-aged adults.
Initial Symptoms. Certain signs and symptoms are more common in the early stages of the disease. Patients may be complaining of double or blurred vision, numbness, weakness in one or two extremities, instability in walking, tremors and problems with bladder control, heat intolerance. As is well known, sensory exam is the most difficult one to ...
The diagnosis of multiple sclerosis (MS) is through clinical assessment and supported by investigations. There is no single accurate and reliable diagnostic test. MS is a disease of young adults with a female predominance. There are characteristic clinical presentations based on the areas of the central nervous system involved, for example ...
The initial presentation of multiple sclerosis varies according to both the location of lesions and the type of . symptom onset (relapsing or progressive). Patients can ... yield in patients with presentations typical of multiple sclerosis.17 Non-specific antibodies are frequently detected that might not be clinically relevant, with low-titre ...
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system, with a variable presentation and heterogenous disease course 1,2.While the exact ...