An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Crohn’s disease: a clinical update
Hanan khalil.
- Author information
- Article notes
- Copyright and License information
Email: [email protected]
Issue date 2015 Nov.
Crohn’s disease is increasing in prevalence worldwide. It arises from a complex interplay between both genetic predisposition and environmental influence. A search of databases and clinical practice guidelines was performed to provide the most up-to-date evidence-based approach for diagnosing and managing patients with Crohn’s disease. No single gold standard investigation exists. Whilst full ileocolonoscopy with biopsies remains the mainstay for diagnosis, other less invasive imaging modalities are being actively considered in the workup, as well as the use of serological markers. Management should incorporate dietary and lifestyle modifications where necessary, the use of medications in induction and remission of disease, and consideration of surgical intervention where medical therapy has failed.
Keywords: Crohn’s disease, diagnosis, inflammatory bowel disease, investigations, management, colorectal cancer, risk factors
Introduction
Crohn’s disease (CD) is a chronic relapsing inflammatory bowel disease (IBD). It is characterized by a transmural granulomatous inflammation which can affect any part of the gastrointestinal tract, most commonly the ileum, colon or both [ Thia et al. 2010 ]. Its prevalence has continually increased over the past 50 years with the highest incidence being reported in northern Europe, the United Kingdom and North America [ Cosnes et al. 2011 ]. Despite biological treatment being associated with an improved health-related quality of life [ van der Have et al. 2014 ], patients still report significant impediment on lifestyle and daily activities during both flares and remissions [ Devlen et al. 2014 ]. The mortality amongst patients with CD has been persistently higher than the general population with a meta-analysis showing a pooled estimate for the standardized mortality ratio of 1.52 [ Canavan et al. 2007 ]. No statistically significant change has occurred for this estimate over the past 30 years and thus CD remains relevant to a broad spectrum of clinicians involved in the multidisciplinary care of affected patients.
This evidence-based review derives from a comprehensive search of several databases including: Ovid Medline, Cochrane library and PubMed. MeSH terms used were “Crohn disease”, “Inflammatory Bowel Diseases” with other search terms including “epidemiology”, “risk factors”, “diagnosis”, “investigations”, “management” and “colorectal cancer”.
Clinical features
CD is a clinical diagnosis formed by correlation of clinical signs and symptoms, objective data from imaging including endoscopic with histologic information as well as laboratory studies [ Baumgart and Sandborn, 2012 ]. Chronic diarrhoea, defined as a decrease in faecal consistency for more than 4 weeks [ Juckett and Trivedi, 2011 ], is the most common presenting symptom [ Sands, 2004 ]. Abdominal pain (70%), weight loss (60%) and blood, mucus or both in stools (40–50%) are also common findings in CD [ Lennard-Jones and Shivananda, 1997 ]. Extraintestinal manifestations affect approximately a third of patients with IBD [ Trost and McDonnell, 2005 ; Lourenco et al. 2010 ]. The most commonly observed extraintestinal manifestation is primary peripheral arthritis (33%); aphthous stomatitis, uveitis, erythema nodosum and ankylosing spondylitis can be seen whilst pyoderma gangrenosum, psoriasis and primary sclerosing cholangitis are relatively uncommon [ Bernstein et al. 2001 ; Vavricka et al. 2011 ]. Fistulae, a complication of CD, occurs in up to 35% of patients with CD, with perianal fistula occurring in 20% [ Schwartz et al. 2002 ]. These clinical features associated with disease activity were found to contribute to 37% of health-related quality-of-life (HRQOL) in a systematic review analysing determinants of HRQOL in CD [ van der Have et al. 2014 ]. According to a patient-reported qualitative analysis [ Devlen et al. 2014 ], there is impact on lifestyle in regards to taking regular medication, restricting diet and avoiding certain trigger foods, as well as impact on daily activities where patients report absence from employment or school during acute flares due to pain and fatigue.
Risk factors
CD has a peak age prevalence of 30–39 years old and gender influence differs in various demographics. In a Canadian and New Zealand population, females are 10–30% more likely to acquire the disease than males [ Bernstein et al. 2006 ; Gearry et al. 2006 ], whereas males with CD are reported as up to three times more likely in Japan and Korea [ Yao et al. 2000 ; Kim et al. 2015 ]. Although the exact aetiology remains unknown, it is a complex interaction between genetic predisposition, environmental risk factors and immune dysregulation to intestinal microbiota [ Sartor and Muehlbauer, 2007 ]. A co-twin British cohort study showed concordant monozygotic twins with CD had similar disease location, disease behaviour and a moderate agreement for age at diagnosis [ Ng et al. 2012 ]. This genetic influence is consistent with previous findings from another co-twin German study [ Spehlmann et al. 2008 ]. Familial aggregation has been shown with most children acquiring the disease at an earlier time in life compared with their parents [ Bengtson et al. 2009 ]. High prevalence has also been found amongst Jewish populations although varying prevalence in different geographic locations suggests the influence of environmental factors as well [ Fireman et al. 1989 ]. Other inflammatory diseases have been implicated with CD including asthma, psoriasis, pericarditis, ankylosing spondylitis, atopic dermatitis and primary sclerosing cholangitis [ Bernstein et al. 2005 ; Lees et al. 2011 ].
Moreover, environmental risk factors have attributed to the rising incidence of CD worldwide [ Thia et al. 2008 ]. Their impact tends to be most influential during childhood [ Feeney et al . 2002 . Smoking has been confirmed to influence the phenotype of CD [ Halfvarson et al. 2006 ; Bengtson et al. 2009 ; Ng et al. 2012 ] and a meta-analysis found that smoking increased the risk of CD by more than twice [ Calkins, 1989 ]. Previous history of symptomatic mumps [ Ng et al. 2012 ] and a high dietary intake of fats, polyunsaturated fatty acids, omega-6 fatty acids and meats have both been associated with an increased risk of CD, whilst a high fibre and fruit diet has been seen to be protective [ Hou et al. 2011 ]. The oral contraceptive pill has also been associated with the development of CD; a meta-analysis [ Cornish et al. 2008 ] assessing quantitative risk of the oral contraceptive pill (OCP) in the aetiology of CD found a pooled relative risk for women currently exposed to the OCP was 1.51 (95%CI 1.17–1.96, p = 0.002).
Diagnosis and investigations
No single definitive diagnostic investigation exists for the diagnosis of CD [ Dignass et al. 2010 ]. Full ileocolonoscopy with biopsies is currently the most widely used diagnostic investigation [ Baumgart and Sandborn, 2012 ]. This can demonstrate noncaseating granulomas, though may only be detected in up to 60% of resected specimens and even less so in biopsy samples [ Nikolaus and Schreiber, 2007 ]. Cross-sectional imaging studies have been increasingly involved in the diagnostic evaluation of CD. This includes ultrasonography (US), computed tomography (CT) and magnetic resonance imaging (MRI). A systematic review [ Panes et al. 2011 ] comparing the accuracy of each cross-sectional imaging modality in the diagnosis of CD found US had a sensitivity and specificity of 85% (95% CI 83–87%) and 98% (95% CI 95–99%), respectively. This was superior to MRI which had an overall sensitivity and specificity of 78% (95% CI 67–84%) and 85% (95% CI 76–90%) respectively. Another systematic review comparing the diagnostic accuracy of magnetic resonance enterography (MRE) and computed tomography (CTE) found MRE had a diagnostic yield comparable with CTE, but does not carry the same risks of radiation exposure, especially to younger patients [ Qiu et al. 2014 ].
A normal finding on ileocolonoscopy is not sufficient to exclude the diagnosis of CD as 27% of patients have disease localized to the terminal ileum [ Cleynen et al. 2013 ], which can prove difficult to diagnose [ Doherty et al. 2011 ]. Capsule endoscopy is a relatively new, simple, noninvasive imaging technique that is gaining recognition for small bowel exploration [ Munoz-Navas, 2009 ]. The investigation involves consumption of a disposable, small, wireless camera within a capsule which passes through the gastrointestinal tract allowing direct visualization of the mucosa [ Nakamura and Terano, 2008 ]. A meta-analysis comparing the diagnostic yield of capsule endoscopy to other imaging modalities found an increased diagnostic rate of 15% over colonoscopy with ileoscopy [ Dionisio et al. 2010 ].
Disease heterogeneity and atypical presentations of IBD have highlighted the need for new diagnostic tools in addition to ileocolonoscopy with biopsy and other imaging studies. This has led to research of serological markers with the two most intensively studied serological markers being atypical perinuclear anti-neutrophil cytoplasmic antibodies (pANCA) and anti Saccharomyces cerevisiae antibodies (ASCA) [ Bossuyt, 2006 ]. pANCA are antibodies formed against proteins in the nuclear lamina of neutrophils, whilst ASCA are antibodies against mannose epitopes from the yeast Saccharomyces cerevisiae [ van Schaik et al. 2013 ]. The generation of antibodies occurs most likely from abnormal response of mucosal immune system to commensal intestinal flora in genetically susceptible patients [ van Schaik et al. 2013 ]. A systematic review of the role of serological antibodies in diagnosing IBD found the most sensitive and specific test for CD was sera with positive ASCA and negative pANCA, 52–64% and 92–94%, respectively [ Prideaux et al. 2012 ]. The identification of these markers can help differentiate CD from ulcerative colitis where diagnosis remains ambiguous following clinical, histological and endoscopic grounds as well provide the opportunity for early intervention given their ability to predict development of CD [ van Schaik et al. 2013 ].
Decisions in the management of CD should always be made through discussion between the multidisciplinary team and patients themselves. Risk factors such as smoking should be ceased due to its harmful impact on the course of disease and potentially the OCP depending on the patient’s circumstances. A balanced diet of high fibre and fruits have been shown to be protective against CD and should be encouraged [ Hou et al . 2011 ].
Pharmacological management
Medications are intended to suppress the inflammatory response and a host of therapeutic agents are now available to treat CD [ Seow et al. 2008 ]. The conventional approach to managing active disease has been derived from progressive intensification of drug therapy, with the focus on inducing and maintaining clinical remission. However, there is evidence to suggest the use of aggressive treatment early to improve clinical outcomes in patients with risk factors predisposing to increased disease severity [ D’haens et al. 2014 ]. These include smoking [ Lees et al. 2011 ], initial requirement of steroid use, age below 40 years and presence of perianal disease [ Beaugerie et al. 2006 ]. Usually medications such as corticosteroids, budesonide or mesalazine are prescribed initially for induction of remission [ Dignass et al. 2010 ]. Anti-tumour necrosis factor (TNF) immunosuppressive therapies are also used in patients refractory to conventional therapy. Medical management of CD is reliant upon compliance and patient education is crucial, with patient age and follow up by a gastroenterologist being independently associated with nonadherence [ Zelante et al. 2014 ].
Corticosteroids are widely prescribed for the induction but not the maintenance of remission due to increasing resistance over time, patient dependence and adverse side effects with long-term use [ Kuenzig et al. 2014 ]. Budesonide is an alternate enteral glucocorticoid used for induction with limited systemic bioavailability due to extensive first-pass hepatic metabolism by cytochrome p-450 enzymes [ Kuenzig et al. 2014 ]. A systematic review evaluating use of budesonide for the induction of remission in CD showed budesonide to be significantly superior to placebo up to 8 weeks with a relative risk (RR) of 1.96 (95% CI, 1.19–3.23) [ Seow et al. 2008 ]. Although budesonide was shown to be inferior to conventional steroids for the induction of remission in active CD (RR 0.85; 95% CI 0.75–0.97), it had significantly fewer corticosteroid-related adverse events compared with those treated with conventional corticosteroids (RR 0.64; 95% CI 0.54–0.76) [ Seow et al. 2008 ].
5-Aminosalicylates also have a long established use in IBD [ Akobeng and Gardener, 2005 ], initially as sulfasalazine, a compound consisting of 5-aminosalicylic acid and sulfapyridine. More recently, 5-aminosalicylic acid has been isolated as a single agent being the active component without sulfapyridine, which was responsible for the majority of adverse side effects [ Ford et al. 2011 ]. However, a systematic review assessing the efficacy of 5-aminosalicylates in CD found insufficient evidence for their definitive use in either inducing remission or preventing relapse in CD [ Ford et al. 2011 ].
Purine antimetabolites azathioprine and 6-mercaptopurine have both been used in patients with active CD, although evidence has been conflicting and controversial. A Cochrane review found 48% of patients receiving antimetabolites achieved remission compared with 37% of placebo patients, with no statistically significant difference between the two groups [ Chande et al. 2013 ]. Evidence remains sparse in regards to whether purine analogues are superior to placebo for maintenance of surgically-induced remission in patients with CD [ Gordon et al. 2014 ]. Methotrexate, another antimetabolite with antagonistic action against folic acid, was superior to placebo in maintenance of remission at 40 weeks; 65% of patients receiving intramuscular methotrexate maintained remission compared to 39% in the placebo group (RR 1.67 95% CI 1.05–2.67) [ Patel et al. 2014 ].
Anti-TNF immunosuppressive therapies, most commonly infliximab and adalimumab, are another group of agents generally reserved for patients refractory to conventional therapies. A randomized controlled trial [ Colombel et al. 2010 ] evaluating the efficacy of infliximab monotherapy, azathioprine monotherapy and combination of both found 56.8% of patients receiving combination therapy achieved corticosteroid-free clinical remission at week 26 compared with 44.4% receiving infliximab alone ( p = 0.02) and 30.0% receiving azathioprine alone ( p < 0.001). Superiority of combination therapy compared with monotherapy of adalimumab remains unclear.
More recently, the novel agent vedolizumab, a monoclonal antibody targeting α4β7 [ Bryant et al. 2015 ], has shown efficacy in a randomized controlled trial for the induction of remission of CD compared with placebo (14.5% and 6.8%, respectively, p = 0.02) as well as maintenance of remission [ Sandborn et al. 2013 ]. Vedolizumab has also demonstrated similar efficacy to natalizumab, another anti-α4 integrin, although it does not pose the risk of progressive multifocal leukoencephalopathy as natalizumab does. However, a network meta-analysis found adalimumab to be superior to vedolizumab in maintenance of remission and its role in the stepwise management of CD still needs to be defined in further trials [ Hazlewood et al. 2015 ]. Ustekinumab, a monoclonal antibody against interleukin-12, has also demonstrated a role in maintenance of remission of CD [ Sandborn et al. 2012 ] and these biologic agents are likely to change the approach to medical management in the near future.
Surgical management
The majority of patients diagnosed with CD will have a surgical resection within 10 years of their diagnosis [ Bernell et al. 2000 ]. Surgical treatment is required for failed medical therapy, recurrent intestinal obstruction, malnutrition and for septic complications such as perforations and abscesses [ Dasari et al. 2011 ]. It has a role in limiting other complications including complex perianal disease and internal fistulas [ Baumgart and Sandborn, 2012 ] as well as improving quality of life [ Delaney et al. 2003 ]. However, the underlying pathology still persists resulting in high recurrence of disease, ranging from 28 to 45% at 5 years and 36 to 61% at 10 years [ Buisson et al. 2012 ]. Surgical admissions account for more than half of all hospitalizations and accounts for almost 40% of total financial costs to patients [ Cohen et al. 2000 ].
Laparoscopy has been widely accepted in gastrointestinal surgery over open surgery in CD [ Duepree et al. 2002 ]. Whilst laparoscopy offers certain advantages of smaller abdominal wounds, lower risk of hernia and decreased rate of small bowel obstruction, there are concerns that occult segments of disease and severe strictures can be missed due to limited tactile ability [ Dasari et al. 2011 ]. However, a meta-analysis on perioperative complications and long-term outcomes between open surgery and laparoscopic surgery found a nonsignificant difference in rate of surgical recurrence and a decreased risk of perioperative complications in the laparoscopic group compared to the open surgery group (12% to 18%, RR = 0.71 CI = 0.58–0.86, p = 0.001) [ Patel et al. 2013 ]. The overall cost including hospital stay costs and costs associated with lost working days between laparoscopic-assisted bowel resection and open surgery was no different [ Scarpa et al. 2009 ]. Despite the evidence for the advantages of laparoscopic surgery, further randomized controlled trials with adequate follow up are required prior to firm recommendations being made [ Patel et al. 2013 ].
The association between CD and malignancy is well documented, although the risk of colorectal cancer is decreasing [ Soderlund et al. 2009 ]. A meta-analysis of population-based cohort studies found a pooled standardized incidence ratio (SIR) of 1.7 amongst patients with CD in the general population (95% CI 1.01–2.5) and a pooled SIR of 4.4 in referral centres (95% CI 1.5–7.2) [ Lutgens et al. 2013 ]. For patients with chronic perianal fistula, there is possibility of malignant transformation and it should not be overlooked by the treating clinician [ Thomas et al. 2010 ]. There is a lack of consensus in regards to the frequency of colonoscopic surveillance. The American Gastroenterological Association (AGA) recommends surveillance intervals of 1–3 years for a maximum of 8 years after diagnosis [ Farraye et al. 2010 ] whilst the British Society of Gastroenter-ology (BSG) recommends yearly, 3-yearly or 5-yearly intervals depending on risk factors after ten years [ Cairns et al. 2010 ]. Chromoendoscopy is also a relatively recent technique that uses topical application of dyes or pigments to improve detection of subtle mucosal aberrations compared with usual white light endoscopy. A meta-analysis showed that, although chromoendoscopy had a 7% increase in dysplasia detection (95% CI 3.2–11.3) compared with white light endoscopy, it is uncertain as to whether this confers a survival benefit in patients given most were low grade dysplasia [ Subramanian et al. 2011 ]. The SCENIC consensus statement (Surveillance for Colorectal Endoscopic Neoplasia Detection and Manage-ment in Inflammatory Bowel Disease Patients: International Consensus Recommendations) nevertheless recommends chromoendoscopy as the preferred technique for surveillance for dysplasia in IBD [ Laine et al. 2015 ].
There are still certain gaps in the evidence regarding the diagnosis and management of CD. Each patient must be assessed individually to determine which investigation is most appropriate, taking into consideration age, suspected location of disease, disease severity and likelihood of recurrence. Numerous diagnostic techniques, be it serological markers or imaging modalities, have assisted both diagnosis and monitoring of CD. Further research is needed to assess the efficacy of certain novel therapeutic agents including vedolizumab and ustekinumab, and their role in active CD compared with classic anti-TNF therapy.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Francis Ha, Faculty of Medicine, Nursing and Health Science, Monash University, Australia.
Hanan Khalil, School of Rural Health, Monash University, Ollerton Rd, Moe, Victoria, Australia.
- Akobeng A., Gardener E. (2005) Oral 5-aminosalicylic acid for maintenance of medically-induced remission in Crohn’s disease. Cochrane Database Syst Rev: Cd003715. [ DOI ] [ PubMed ] [ Google Scholar ]
- Baumgart D., Sandborn W. (2012) Crohn’s disease. Lancet 380: 1590–1605. [ DOI ] [ PubMed ] [ Google Scholar ]
- Beaugerie L., Seksik P., Nion-Larmurier I., Gendre J., Cosnes J. (2006) Predictors of Crohn’s disease. Gastroenterology 130: 650–656. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bengtson M., Solberg C., Aamodt G., Jahnsen J., Moum B., Sauar J., et al. (2009) Clustering in time of familial IBD separates ulcerative colitis from Crohn’s disease. Inflamm Bowel Dis 15: 1867–1874. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bernell O., Lapidus A., Hellers G. (2000) Risk factors for surgery and postoperative recurrence in Crohn’s disease. Ann Surg 231: 38–45. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bernstein C., Blanchard J., Rawsthorne P., Yu N. (2001) The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol 96: 1116–1122. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bernstein C., Wajda A., Blanchard J. (2005) The clustering of other chronic inflammatory diseases in inflammatory bowel disease: a population-based study. Gastroenterology 129: 827–836. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bernstein C., Wajda A., Svenson L., Mackenzie A., Koehoorn M., Jackson M., et al. (2006) The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am J Gastroenterol 101: 1559–1568. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bossuyt X. (2006) Serologic markers in inflammatory bowel disease. Clin Chem 52: 171–181. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bryant R., Sandborn W., Travis S. (2015) Introducing vedolizumab to Clinical practice: who, when, and how? J Crohns Colitis 9: 356–366. [ DOI ] [ PubMed ] [ Google Scholar ]
- Buisson A., Chevaux J., Allen P., Bommelaer G., Peyrin-Biroulet L. (2012) Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther 35: 625–633. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cairns S., Scholefield J., Steele R., Dunlop M., Thomas H., Evans G., et al. (2010) Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 59: 666–689. [ DOI ] [ PubMed ] [ Google Scholar ]
- Calkins B. (1989) A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci 34: 1841–1854. [ DOI ] [ PubMed ] [ Google Scholar ]
- Canavan C., Abrams K., Mayberry J. (2007) Meta-analysis: mortality in Crohn’s disease. Aliment Pharmacol Ther 25: 861–870. [ DOI ] [ PubMed ] [ Google Scholar ]
- Chande N., Tsoulis D., Macdonald J. (2013) Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 4: Cd000545. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cleynen I., Gonzalez J., Figueroa C., Franke A., McGovern D., Bortlik M., et al. (2013) Genetic factors conferring an increased susceptibility to develop Crohn’s disease also influence disease phenotype: results from the IBDCHIP European project. Gut 62: 1556–1565. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cohen R., Larson L., Roth J., Becker R., Mummert L. (2000) The cost of hospitalization in Crohn’s disease. Am J Gastroenterol 95: 524–530. [ DOI ] [ PubMed ] [ Google Scholar ]
- Colombel J., Sandborn W., Reinisch W., Mantzaris G., Kornbluth A., Rachmilewitz D., et al. (2010) Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 362: 1383–1395. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cornish J., Tan E., Simillis C., Clark S., Teare J., Tekkis P. (2008) The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol 103: 2394–2400. [ DOI ] [ PubMed ] [ Google Scholar ]
- Cosnes J., Gower-Rousseau C., Seksik P., Cortot A. (2011) Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140: 1785–1794. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dasari B., McKay D., Gardiner K. (2011) Laparoscopic versus open surgery for small bowel Crohn’s disease. Cochrane Database Syst Rev: Cd006956. [ DOI ] [ PubMed ] [ Google Scholar ]
- Delaney C., Kiran R., Senagore A., O’Brien-Ermlich B., Church J., Hull T., et al. (2003) Quality of life improves within 30 days of surgery for Crohn’s disease. J Am Coll Surg 196: 714–721. [ DOI ] [ PubMed ] [ Google Scholar ]
- Devlen J., Beusterien K., Yen L., Ahmed A., Cheifetz A., Moss A. (2014) The Burden of inflammatory bowel disease: a patient-reported qualitative analysis and development of a conceptual model. Inflamm Bowel Dis 20: 545–552. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- D’haens G., Sartor R., Silverberg M., Petersson J., Rutgeerts P. (2014) Future Directions in inflammatory bowel disease management. J Crohns Colitis 8: 726–734. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dignass A., Van Assche G., Lindsay J., Lemann M., Soderholm J., Colombel J., et al. (2010) The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis 4: 28–62. [ DOI ] [ PubMed ] [ Google Scholar ]
- Dionisio P., Gurudu S., Leighton J., Leontiadis G., Fleischer D., Hara A.et al. (2010) Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol 105: 1240–1248; quiz 1249. [ DOI ] [ PubMed ] [ Google Scholar ]
- Doherty G., Moss A., Cheifetz A. (2011) Capsule endoscopy for small-bowel evaluation in Crohn’s disease. Gastrointest Endosc 74: 167–175. [ DOI ] [ PubMed ] [ Google Scholar ]
- Duepree H., Senagore A., Delaney C., Brady K., Fazio V. (2002) Advantages of Laparoscopic resection for ileocecal Crohn’s disease. Dis Colon Rectum 45: 605–610. [ DOI ] [ PubMed ] [ Google Scholar ]
- Farraye F., Odze R., Eaden J., Itzkowitz S., McCabe R., Dassopoulos T., et al. (2010) AGA Medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 138: 738–745. [ DOI ] [ PubMed ] [ Google Scholar ]
- Feeney M. A., Murphy F., Clegg A. J., Trebble T. M., Sharer N. M., Snook J. A. (2002) A case-control study of childhood environmental risk factors for the development of inflammatory bowel disease. Eur J Gastroenterol Hepatol 14: 529–534. [ DOI ] [ PubMed ] [ Google Scholar ]
- Fireman Z., Grossman A., Lilos P., Eshchar Y., Theodor E., Gilat T. (1989) Epidemiology of Crohn’s disease in the Jewish Population of central Israel, 1970-1980. Am J Gastroenterol 84: 255–258. [ PubMed ] [ Google Scholar ]
- Ford A., Kane S., Khan K., Achkar J., Talley N., Marshall J., et al. (2011) Efficacy of 5-aminosalicylates in Crohn’s disease: systematic review and meta-analysis. Am J Gastroenterol 106: 617–629. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gearry R., Richardson A., Frampton C., Collett J., Burt M., Chapman B., et al. (2006) High incidence of Crohn’s disease in Canterbury, New Zealand: results of an epidemiologic study. Inflamm Bowel Dis 12: 936–943. [ DOI ] [ PubMed ] [ Google Scholar ]
- Gordon M., Taylor K., Akobeng A., Thomas A. (2014) Azathioprine and 6-Mercaptopurine for maintenance of surgically-induced remission in Crohn’s disease. Cochrane Database Syst Rev 8: Cd010233. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Halfvarson J., Jess T., Magnuson A., Montgomery S., Orholm M., Tysk C., et al. (2006) Environmental Factors in inflammatory bowel disease: a co-twin control study of a Swedish-Danish twin population. Inflamm Bowel Dis 12: 925–933. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hazlewood G., Rezaie A., Borman M., Panaccione R., Ghosh S., Seow C., et al. (2015) Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology 148: 344–354.e345; quiz e314–345. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hou J., Abraham B., El-Serag H. (2011) Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 106: 563–573. [ DOI ] [ PubMed ] [ Google Scholar ]
- Juckett G., Trivedi R. (2011) Evaluation of chronic diarrhea. Am Fam Physician 84: 1119–1126. [ PubMed ] [ Google Scholar ]
- Kim H., Hann H., Hong S., Kim K., Ahn I., Song J., et al. (2015) Incidence and natural course of inflammatory bowel disease in Korea, 2006–2012: a nationwide population-based study. Inflamm Bowel Dis 21: 623–630. [ DOI ] [ PubMed ] [ Google Scholar ]
- Kuenzig M., Rezaie A., Seow C., Otley A., Steinhart A., Griffiths A., et al. (2014) Budesonide for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 8: Cd002913. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Laine L., Kaltenbach T., Barkun A., McQuaid K., Subramanian V., Soetikno R. (2015) SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 148: 639–651.e628. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lees C., Barrett J., Parkes M., Satsangi J. (2011) New IBD genetics: common pathways with other diseases. Gut 60: 1739–1753. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lennard-Jones J., Shivananda S. (1997) Clinical uniformity of inflammatory bowel disease a presentation and during the first year of disease in the north and south of Europe. EC-IBD Study Group. Eur J Gastroenterol Hepatol 9: 353–359. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lourenco S., Hussein T., Bologna S., Sipahi A., Nico M. (2010) Oral manifestations of inflammatory bowel disease: a review based on the observation of six cases. J Eur Acad Dermatol Venereol 24: 204–207. [ DOI ] [ PubMed ] [ Google Scholar ]
- Lutgens M., Van Oijen M., Van Der Heijden G., Vleggaar F., Siersema P., Oldenburg B. (2013) Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 19: 789–799. [ DOI ] [ PubMed ] [ Google Scholar ]
- Munoz-Navas M. (2009) Capsule endoscopy. World J Gastroenterol 15: 1584–1586. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nakamura T., Terano A. (2008) Capsule endoscopy: past, present, and future. J Gastroenterol 43: 93–99. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ng S., Woodrow S., Patel N., Subhani J., Harbord M. (2012) Role of Genetic and environmental factors in British twins with inflammatory bowel disease. Inflamm Bowel Dis 18: 725–736. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nikolaus S., Schreiber S. (2007) Diagnostics of inflammatory bowel disease. Gastroenterology 133: 1670–1689. [ DOI ] [ PubMed ] [ Google Scholar ]
- Panes J., Bouzas R., Chaparro M., Garcia-Sanchez V., Gisbert J., Martinez De Guerenu B., et al. (2011) Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 34: 125–145. [ DOI ] [ PubMed ] [ Google Scholar ]
- Patel S., Patel S., Ramagopalan S., Ott M. (2013) Laparoscopic surgery for Crohn’s disease: a meta-analysis of perioperative complications and long term outcomes compared with open surgery. BMC Surg 13: 14. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Patel V., Wang Y., Macdonald J., McDonald J., Chande N. (2014) Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 8: Cd006884. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Prideaux L., De Cruz P., Ng S., Kamm M. (2012) Serological antibodies in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis 18: 1340–1355. [ DOI ] [ PubMed ] [ Google Scholar ]
- Qiu Y., Mao R., Chen B., Li X., He Y., Zeng Z., et al. (2014) Systematic review with meta-analysis: magnetic resonance enterography vs. computed tomography enterography for evaluating disease activity in small bowel Crohn’s disease. Aliment Pharmacol Ther 40: 134–146. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sandborn W., Feagan B., Rutgeerts P., Hanauer S., Colombel J., Sands B., et al. (2013) Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 369: 711–721. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sandborn W., Gasink C., Gao L., Blank M., Johanns J., Guzzo C., et al. (2012) Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 367: 1519–1528. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sands B. (2004) From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology 126: 1518–1532. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sartor R., Muehlbauer M. (2007) Microbial host interactions in IBD: Implications for pathogenesis and therapy. Curr Gastroenterol Rep 9: 497–507. [ DOI ] [ PubMed ] [ Google Scholar ]
- Scarpa M., Ruffolo C., Bassi D., Boetto R., D’inca R., Buda A., et al. (2009) Intestinal surgery for Crohn’s disease: predictors of recovery, quality of life, and costs. J Gastrointest Surg 13: 2128–2135. [ DOI ] [ PubMed ] [ Google Scholar ]
- Schwartz D., Loftus E., Jr., Tremaine W., Panaccione R., Harmsen W., Zinsmeister A., et al. (2002) The Natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology 122: 875–880. [ DOI ] [ PubMed ] [ Google Scholar ]
- Seow C., Benchimol E., Griffiths A., Otley A., Steinhart A. (2008) Budesonide for induction of remission in Crohn’s disease. Cochrane Database Syst Rev: Cd000296. [ DOI ] [ PubMed ] [ Google Scholar ]
- Soderlund S., Brandt L., Lapidus A., Karlen P., Brostrom O., Lofberg R., et al. (2009) Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology 136: 1561–1567; quiz 1818–1569. [ DOI ] [ PubMed ] [ Google Scholar ]
- Spehlmann M., Begun A., Burghardt J., Lepage P., Raedler A., Schreiber S. (2008) Epidemiology of Inflammatory bowel disease in a German twin cohort: results of a nationwide study. Inflamm Bowel Dis 14: 968–976. [ DOI ] [ PubMed ] [ Google Scholar ]
- Subramanian V., Mannath J., Ragunath K., Hawkey C. (2011) Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther 33: 304–312. [ DOI ] [ PubMed ] [ Google Scholar ]
- Thia K., Loftus E., Jr., Sandborn W., Yang S. (2008) An Update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol 103: 3167–3182. [ DOI ] [ PubMed ] [ Google Scholar ]
- Thia K., Sandborn W., Harmsen W., Zinsmeister A., Loftus E., Jr. (2010) Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 139: 1147–1155. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thomas M., Bienkowski R., Vandermeer T., Trostle D., Cagir B. (2010) Malignant transformation in perianal fistulas of Crohn’s disease: a systematic review of literature. J Gastrointest Surg 14: 66–73. [ DOI ] [ PubMed ] [ Google Scholar ]
- Trost L., McDonnell J. (2005) Important cutaneous manifestations of inflammatory bowel disease. Postgrad Med J 81: 580–585. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Van der Have M., van der Aalst K., Kaptein A., Leenders M., Siersema P., Oldenburg B., et al. (2014) Determinants of health-related quality of life in Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis 8: 93–106. [ DOI ] [ PubMed ] [ Google Scholar ]
- Van Schaik F., Oldenburg B., Hart A., Siersema P., Lindgren S., Grip O., et al. (2013) Serological markers predict inflammatory bowel disease years before the diagnosis. Gut 62: 683–688. [ DOI ] [ PubMed ] [ Google Scholar ]
- Vavricka S., Brun L., Ballabeni P., Pittet V., Prinz Vavricka B., Zeitz J., et al. (2011) Frequency and Risk factors for extraintestinal manifestations in the Swiss Inflammatory Bowel Disease Cohort. Am J Gastroenterol 106: 110–119. [ DOI ] [ PubMed ] [ Google Scholar ]
- Yao T., Matsui T., Hiwatashi N. (2000) Crohn’s disease in Japan: diagnostic criteria and epidemiology. Dis Colon Rectum 43: S85–S93. [ DOI ] [ PubMed ] [ Google Scholar ]
- Zelante A., De Giorgi A., Borgoni R., Trevisani L., Gallerani M. (2014) Adherence to medical treatment in inflammatory bowel disease patients. Minerva Gastroenterol Dietol 60: 269–274. [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (392.8 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 02 April 2020
Crohn’s disease
- Giulia Roda 1 ,
- Siew Chien Ng 2 ,
- Paulo Gustavo Kotze 3 ,
- Marjorie Argollo 1 ,
- Remo Panaccione 4 ,
- Antonino Spinelli 5 , 6 ,
- Arthur Kaser 7 ,
- Laurent Peyrin-Biroulet 8 &
- Silvio Danese 1 , 6
Nature Reviews Disease Primers volume 6 , Article number: 22 ( 2020 ) Cite this article
37k Accesses
535 Citations
157 Altmetric
Metrics details
- Crohn's disease
- Gastrointestinal diseases
An Author Correction to this article was published on 19 June 2020
An Author Correction to this article was published on 20 May 2020
A Publisher Correction to this article was published on 06 April 2020
This article has been updated
Crohn’s disease is an inflammatory bowel disease that is characterized by chronic inflammation of any part of the gastrointestinal tract, has a progressive and destructive course and is increasing in incidence worldwide. Several factors have been implicated in the cause of Crohn’s disease, including a dysregulated immune system, an altered microbiota, genetic susceptibility and environmental factors, but the cause of the disease remains unknown. The onset of the disease at a young age in most cases necessitates prompt but long-term treatment to prevent disease flares and disease progression with intestinal complications. Thus, earlier, more aggressive treatment with biologic therapies or novel small molecules could profoundly change the natural history of the disease and decrease complications and the need for hospitalization and surgery. Although less invasive biomarkers are in development, diagnosis still relies on endoscopy and histological assessment of biopsy specimens. Crohn’s disease is a complex disease, and treatment should be personalized to address the underlying pathogenetic mechanism. In the future, disease management might rely on severity scores that incorporate prognostic factors, bowel damage assessment and non-invasive close monitoring of disease activity to reduce the severity of complications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Location is important: differentiation between ileal and colonic Crohn’s disease
Microscopic colitis
Ulcerative colitis
Change history, 19 june 2020.
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
20 May 2020
06 april 2020.
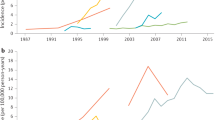
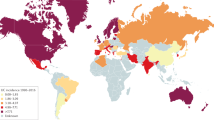
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390 , 2769–2778 (2018). This study provides a comprehensive analysis of the global IBD epidemiology .
Article Google Scholar
Torres, J., Mehandru, S., Colombel, J.-F. & Peyrin-Biroulet, L. Crohn’s disease. Lancet 389 , 1741–1755 (2017).
Article PubMed Google Scholar
Thia, K. T., Sandborn, W. J., Harmsen, W. S., Zinsmeister, A. R. & Loftus, E. V. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 139 , 1147–1155 (2010).
Fiorino, G., Bonifacio, C., Peyrin-Biroulet, L. & Danese, S. Preventing collateral damage in Crohn’s disease: the Lémann index. J. Crohns Colitis 10 , 495–500 (2016). This study clearly shows the importance of assessing bowel damage in a very early inflammatory stage of CD. The authors demonstrate that the presence of bowel damage in early CD is associated with a worse outcome .
Article PubMed PubMed Central Google Scholar
Zeng, Z. et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong province, China: a prospective population-based study. J. Gastroenterol. Hepatol. 28 , 1148–1153 (2013).
Zhao, J. et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of ‘western’ disease. Inflamm. Bowel Dis. 19 , 1839–1845 (2013).
PubMed Google Scholar
Ng, S. C. et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology 145 , 158–165.e2 (2013).
Kim, H. J. et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm. Bowel Dis. 21 , 623–630 (2015).
Park, S. H. et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong district of Seoul, Korea in 1986–2015. J. Crohns Colitis 13 , 1410–1417 (2019).
Ananthakrishnan, A. N. et al. Environmental triggers in IBD: a review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 15 , 39–49 (2018).
Bernstein, C. N. et al. Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm. Bowel Dis. 25 , 360–368 (2019).
Moradkhani, A., Beckman, L. J. & Tabibian, J. H. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J. Crohns Colitis 7 , 467–473 (2013).
Shah, S. C., Colombel, J.-F., Sands, B. E. & Narula, N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment. Pharmacol. Ther. 43 , 317–333 (2016).
Article CAS PubMed Google Scholar
Kaplan, G. G. & Ng, S. C. Globalisation of inflammatory bowel disease: perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 1 , 307–316 (2016).
Ng, S. C. et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 62 , 630–649 (2013).
Yen, H.-H. et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide population-based study. Intest. Res. 17 , 54–62 (2019).
Ng, S. C. et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm. Bowel Dis. 22 , 1954–1960 (2016).
Mansour-Ghanaei, F. et al. Epidemiologic features of inflammatory bowel disease in Guilan province, north of Iran, during 2002-2012. Middle East. J. Dig. Dis. 7 , 69–74 (2015).
PubMed PubMed Central Google Scholar
Linares de la Cal, J. A., Cantón, C., Hermida, C., Pérez-Miranda, M. & Maté-Jiménez, J. Estimated incidence of inflammatory bowel disease in Argentina and Panama (1987–1993). Rev. Esp. Enferm. Dig. 91 , 277–286 (1999).
CAS PubMed Google Scholar
Piovani, D. et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 157 , 647–659.e4 (2019).
Lakatos, P. L. et al. Is current smoking still an important environmental factor in inflammatory bowel diseases? Results from a population-based incident cohort. Inflamm. Bowel Dis. 19 , 1010–1017 (2013).
Kondo, K. et al. The association between environmental factors and the development of Crohn’s disease with focusing on passive smoking: a multicenter case-control study in Japan. PLoS One 14 , e0216429 (2019).
Article CAS PubMed PubMed Central Google Scholar
Ng, S. C. et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut 64 , 1063–1071 (2015).
Levine, A., Sigall Boneh, R. & Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 67 , 1726–1738 (2018).
Khalili, H. et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: results from two large prospective cohort studies. Gut https://doi.org/10.1136/gutjnl-2019-319505 (2020).
Ortizo, R. et al. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: a meta-analysis of case-controlled and cohort studies. Eur. J. Gastroenterol. Hepatol. 29 , 1064–1070 (2017).
Ananthakrishnan, A. N. et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann. Intern. Med. 156 , 350–359 (2012).
Moninuola, O. O., Milligan, W., Lochhead, P. & Khalili, H. Systematic review with meta-analysis: association between acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) and risk of Crohn’s disease and ulcerative colitis exacerbation. Aliment. Pharmacol. Ther. 47 , 1428–1439 (2018).
Ungaro, R. et al. Statins associated with decreased risk of new onset inflammatory bowel disease. Am. J. Gastroenterol. 111 , 1416–1423 (2016).
Green, N., Miller, T., Suskind, D. & Lee, D. A review of dietary therapy for IBD and a vision for the future. Nutrients 11 , E947 (2019).
Article PubMed CAS Google Scholar
Halfvarson, J., Bodin, L., Tysk, C., Lindberg, E. & Järnerot, G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology 124 , 1767–1773 (2003).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411 , 599–603 (2001).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411 , 603–606 (2001).
Yamazaki, K. et al. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn’s disease. Hum. Mol. Genet. 14 , 3499–3506 (2005).
Huang, H. et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 547 , 173–178 (2017).
Ellinghaus, D. et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 48 , 510–518 (2016).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491 , 119–124 (2012).
Ogura, Y. et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut 52 , 1591–1597 (2003).
Sidiq, T., Yoshihama, S., Downs, I. & Kobayashi, K. S. Nod2: a critical regulator of ileal microbiota and Crohn’s disease. Front. Immunol. 7 , 367 (2016).
Article PubMed PubMed Central CAS Google Scholar
Hampe, J. et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39 , 207–211 (2007).
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47 , 979–986 (2015).
Hong, M. et al. Immunochip meta-analysis of inflammatory bowel disease identifies three novel loci and four novel associations in previously reported loci. J. Crohns Colitis 12 , 730–741 (2018).
Zhu, L. et al. IL-10 and IL-10 receptor mutations in very early onset inflammatory bowel disease. Gastroenterology Res. 10 , 65–69 (2017).
Uniken Venema, W. T., Voskuil, M. D., Dijkstra, G., Weersma, R. K. & Festen, E. A. The genetic background of inflammatory bowel disease: from correlation to causality. J. Pathol. 241 , 146–158 (2017).
Cleynen, I. et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet 387 , 156–167 (2016).
Peterson, L. W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14 , 141–153 (2014).
Zeissig, S. et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56 , 61–72 (2007).
Weber, C. R., Nalle, S. C., Tretiakova, M., Rubin, D. T. & Turner, J. R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Invest. 88 , 1110–1120 (2008).
Odenwald, M. A. & Turner, J. R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 14 , 9–21 (2017).
Wehkamp, J. et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 53 , 1658–1664 (2004).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456 , 259–263 (2008).
Thachil, E. et al. Abnormal activation of autophagy-induced crinophagy in Paneth cells from patients with Crohn’s disease. Gastroenterology 142 , 1097–1099.e4 (2012).
Zhang, Q. et al. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat. Immunol. 16 , 918–926 (2015).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134 , 743–756 (2008).
Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503 , 272–276 (2013).
Tschurtschenthaler, M. et al. Defective ATG16L1-mediated removal of IRE1α drives Crohn’s disease-like ileitis. J. Exp. Med. 214 , 401–422 (2017).
Willson, T. A., Jurickova, I., Collins, M. & Denson, L. A. Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm. Bowel Dis. 19 , 512–525 (2013).
Diamanti, M. A. et al. IKKα controls ATG16L1 degradation to prevent ER stress during inflammation. J. Exp. Med. 214 , 423–437 (2017).
Zhou, C., Qiu, Y. & Yang, H. CD4CD8αα IELs: they have something to say. Front. Immunol. 10 , 2269 (2019).
Regner, E. H. et al. Functional intraepithelial lymphocyte changes in inflammatory bowel disease and spondyloarthritis have disease specific correlations with intestinal microbiota. Arthritis Res. Ther. 20 , 149 (2018).
Catalan-Serra, I., Sandvik, A. K., Bruland, T. & Andreu-Ballester, J. C. Gammadelta T cells in Crohn’s disease: a new player in the disease pathogenesis? J. Crohns Colitis 11 , 1135–1145 (2017).
Hosomi, S. et al. Intestinal epithelial cell endoplasmic reticulum stress promotes MULT1 up-regulation and NKG2D-mediated inflammation. J. Exp. Med. 214 , 2985–2997 (2017).
Allez, M., Skolnick, B. E., Wisniewska-Jarosinska, M., Petryka, R. & Overgaard, R. V. Anti-NKG2D monoclonal antibody (NNC0142-0002) in active Crohn’s disease: a randomised controlled trial. Gut 66 , 1918–1925 (2017).
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28 , 573–621 (2010).
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J. Med. 361 , 2066–2078 (2009).
Ouellette, A. J. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroenterol. 26 , 547–553 (2010).
de Souza, H. S. P. & Fiocchi, C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13 , 13–27 (2016).
Uhlig, H. H. & Powrie, F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu. Rev. Immunol. 36 , 755–781 (2018).
Pazmandi, J., Kalinichenko, A., Ardy, R. C. & Boztug, K. Early-onset inflammatory bowel disease as a model disease to identify key regulators of immune homeostasis mechanisms. Immunol. Rev. 287 , 162–185 (2019).
Cooney, R. et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 16 , 90–97 (2010).
Travassos, L. H. et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11 , 55–62 (2010).
Segal, A. W. The role of neutrophils in the pathogenesis of Crohn’s disease. Eur. J. Clin. Invest. 48 , e12983 (2018).
Geremia, A. & Arancibia-Cárcamo, C. V. Innate lymphoid cells in intestinal inflammation. Front. Immunol. 8 , 1296 (2017).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of CD127 + group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43 , 146–160 (2015).
van der Gracht, E., Zahner, S. & Kronenberg, M. When insult is added to injury: cross talk between ILCs and intestinal epithelium in IBD. Mediators Inflamm. 2016 , 9765238 (2016).
Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25 , 309–318 (2006).
Feagan, B. G. et al. Ustekinumab as Induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375 , 1946–1960 (2016).
Feagan, B. G. et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 389 , 1699–1709 (2017).
Sands, B. E. et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 153 , 77–86.e6 (2017).
Sarin, R., Wu, X. & Abraham, C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc. Natl Acad. Sci. USA 108 , 9560–9565 (2011).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314 , 1461–1463 (2006).
Fantini, M. C. et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology 136 , 1308–1316 (2009).
Lo Presti, A. et al. Fecal and mucosal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease. Front. Microbiol. 10 , 1655 (2019).
Vich Vila, A. et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl Med. 10 , eaap8914 (2018).
Pascal, V. et al. A microbial signature for Crohn’s disease. Gut 66 , 813–822 (2017).
Palmela, C. et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 67 , 574–587 (2018).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105 , 16731–16736 (2008).
Barnich, N. & Darfeuille-Michaud, A. Adherent-invasive Escherichia coli and Crohn’s disease. Curr. Opin. Gastroenterol. 23 , 16–20 (2007).
Simpson, K. W. et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect. Immun. 74 , 4778–4792 (2006).
Yilmaz, B. et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat. Med. 25 , 323–336 (2019).
Libertucci, J. et al. Inflammation-related differences in mucosa-associated microbiota and intestinal barrier function in colonic Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 315 , G420–G431 (2018).
Vieira-Silva, S. et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat. Microbiol. 4 , 1826–1831 (2019).
Norman, J. M. et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160 , 447–460 (2015).
Pérez-Brocal, V. et al. Study of the viral and microbial communities associated with Crohn’s disease: a metagenomic approach. Clin. Transl. Gastroenterol. 4 , e36 (2013).
Imai, T. et al. Characterization of fungal dysbiosis in Japanese patients with inflammatory bowel disease. J. Gastroenterol. 54 , 149–159 (2019).
Feuerstein, J. D. & Cheifetz, A. S. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin. Proc. 92 , 1088–1103 (2017).
Gomollón, F. et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J. Crohns Colitis 11 , 3–25 (2017).
Kuriyama, M. et al. Specific gastroduodenoscopic findings in Crohn’s disease: comparison with findings in patients with ulcerative colitis and gastroesophageal reflux disease. Dig. Liver Dis. 40 , 468–475 (2008).
Sawczenko, A. & Sandhu, B. K. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch. Dis. Child. 88 , 995–1000 (2003).
Peyrin-Biroulet, L., Loftus, E. V., Colombel, J.-F. & Sandborn, W. J. The natural history of adult Crohn’s disease in population-based cohorts. Am. J. Gastroenterol. 105 , 289–297 (2010). This comprehensive article describes the natural history of CD .
Fiorino, G. et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn’s disease and its impact on disease outcome. J. Crohns Colitis 11 , 274–280 (2017).
Safroneeva, E. et al. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Aliment. Pharmacol. Ther. 42 , 977–989 (2015).
Peyrin-Biroulet, L. et al. Perianal Crohn’s disease findings other than fistulas in a population-based cohort. Inflamm. Bowel Dis. 18 , 43–48 (2012).
Ott, C. & Schölmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 10 , 585–595 (2013).
Park, S. H. et al. Update on the natural course of fistulizing perianal Crohn’s disease in a population-based cohort. Inflamm. Bowel Dis. 25 , 1054–1060 (2019).
Freeman, H. J. Natural history and long-term clinical course of Crohn’s disease. World J. Gastroenterol. 20 , 31–36 (2014).
Danese, S. et al. Development of red flags index for early referral of adults with symptoms and signs suggestive of Crohn’s disease: an IOIBD initiative. J. Crohns Colitis 9 , 601–606 (2015).
Vavricka, S. R. et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am. J. Gastroenterol. 106 , 110–119 (2011).
Jang, H.-J., Kang, B. & Choe, B.-H. The difference in extraintestinal manifestations of inflammatory bowel disease for children and adults. Transl. Pediatr. 8 , 4–15 (2019).
Peyrin-Biroulet, L., Loftus, E. V., Colombel, J.-F. & Sandborn, W. J. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn’s disease in population-based cohorts. Inflamm. Bowel Dis. 17 , 471–478 (2011). This comprehensive article describes long-term outcomes in patients with CD .
Pennazio, M. et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 47 , 352–376 (2015).
Koulaouzidis, A., Rondonotti, E. & Karargyris, A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J. Gastroenterol. 19 , 3726–3746 (2013).
Dionisio, P. M. et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am. J. Gastroenterol. 105 , 1240–1248 (2010).
Magro, F. et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohns Colitis 7 , 827–851 (2013).
Annese, V. et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohns Colitis 7 , 982–1018 (2013).
Tontini, G. E., Vecchi, M., Neurath, M. F. & Neumann, H. Advanced endoscopic imaging techniques in Crohn’s disease. J. Crohns Colitis 8 , 261–269 (2014).
Allocca, M., Fiorino, G. & Danese, S. Cross-sectional imaging modalities in Crohn’s disease. Dig. Dis. 31 , 199–201 (2013).
Chatu, S., Subramanian, V. & Pollok, R. C. G. Meta-analysis: diagnostic medical radiation exposure in inflammatory bowel disease. Aliment. Pharmacol. Ther. 35 , 529–539 (2012).
Horsthuis, K., Bipat, S., Bennink, R. J. & Stoker, J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 247 , 64–79 (2008).
Panés, J. et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment. Pharmacol. Ther. 34 , 125–145 (2011).
Sahni, V. A., Ahmad, R. & Burling, D. Which method is best for imaging of perianal fistula? Abdom. Imaging 33 , 26–30 (2008).
Allocca, M. et al. Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing Crohn’s disease and guiding clinical decision-making. J. Crohns Colitis 12 , 1280–1287 (2018).
Magro, F. et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohns Colitis 11 , 649–670 (2017).
Vermeire, S., Schreiber, S., Sandborn, W. J., Dubois, C. & Rutgeerts, P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin. Gastroenterol. Hepatol. 8 , 357–363 (2010).
Best, W. R. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw index. Inflamm. Bowel Dis. 12 , 304–310 (2006).
Mitsuyama, K. et al. Antibody markers in the diagnosis of inflammatory bowel disease. World J. Gastroenterol. 22 , 1304–1310 (2016).
Gu, P. et al. Serological, genetic and clinical associations with increased health-care resource utilization in inflammatory bowel disease. J. Dig. Dis. 19 , 15–23 (2018).
Plevy, S. et al. Combined serological, genetic, and inflammatory markers differentiate non-IBD, Crohn’s disease, and ulcerative colitis patients. Inflamm. Bowel Dis. 19 , 1139–1148 (2013).
Maaser, C. et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 13 , 144–164 (2019).
Vermeire, S., Van Assche, G. & Rutgeerts, P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm. Bowel Dis. 10 , 661–665 (2004).
Vermeire, S., Van Assche, G. & Rutgeerts, P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 55 , 426–431 (2006).
Solem, C. A. et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm. Bowel Dis. 11 , 707–712 (2005).
Cellier, C. et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 35 , 231–235 (1994).
Lakatos, P. L. et al. Serum lipopolysaccharide-binding protein and soluble CD14 are markers of disease activity in patients with Crohn’s disease. Inflamm. Bowel Dis. 17 , 767–777 (2011).
Kwon, J. H. et al. Disease phenotype, activity and clinical course prediction based on C-reactive protein levels at diagnosis in patients with Crohn’s disease: results from the CONNECT study. Gut Liver 10 , 595–603 (2016).
Carroccio, A. et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin. Chem. 49 , 861–867 (2003).
Diamanti, A. et al. Diagnostic work-up of inflammatory bowel disease in children: the role of calprotectin assay. Inflamm. Bowel Dis. 16 , 1926–1930 (2010).
Goutorbe, F. et al. Endoscopic factors influencing fecal calprotectin value in Crohn’s disease. J. Crohns Colitis 9 , 1113–1119 (2015).
van Rheenen, P. F., Van de Vijver, E. & Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 341 , c3369 (2010).
Suray, N. de et al. Close monitoring of CRP and fecal calprotectin is able to predict clinical relapse in patients with Crohn’s disease in remission after infliximab withdrawal. a sub-analysis of the Stori study. Gastroenterology 142 , S-149 (2012).
Orlando, A. et al. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn’s disease: a comparison with ultrasound. Eur. Rev. Med. Pharmacol. Sci. 10 , 17–22 (2006).
Guo, S. et al. A simple fecal bacterial marker panel for the diagnosis of Crohn’s disease. Front. Microbiol. 10 , 1306 (2019).
Marlicz, W., Skonieczna-Żydecka, K., Dabos, K. J., Łoniewski, I. & Koulaouzidis, A. Emerging concepts in non-invasive monitoring of Crohn’s disease. Ther. Adv. Gastroenterol. 11 , 1756284818769076 (2018).
Somineni, H. K. et al. Blood-derived DNA methylation signatures of Crohn’s disease and severity of intestinal inflammation. Gastroenterology 156 , 2254–2265.e3 (2019).
Leong, R. W. et al. Full-spectrum endoscopy improves surveillance for dysplasia in patients with inflammatory bowel diseases. Gastroenterology 152 , 1337–1344.e3 (2017).
Stidham, R. W. & Higgins, P. D. R. Colorectal cancer in inflammatory bowel disease. Clin. Colon. Rectal Surg. 31 , 168–178 (2018).
Tontini, G. E., Vecchi, M., Pastorelli, L., Neurath, M. F. & Neumann, H. Differential diagnosis in inflammatory bowel disease colitis: state of the art and future perspectives. World J. Gastroenterol. 21 , 21–46 (2015).
He, Y. et al. Development and validation of a novel diagnostic nomogram to differentiate between intestinal tuberculosis and Crohn’s disease: a 6-year prospective multicenter study. Am. J. Gastroenterol. 114 , 490–499 (2019).
Bae, J. H. et al. Development and validation of a novel prediction model for differential diagnosis between Crohn’s disease and intestinal tuberculosis. Inflamm. Bowel Dis. 23 , 1614–1623 (2017).
Lee, S. K., Kim, B. K., Kim, T. I. & Kim, W. H. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy 41 , 9–16 (2009).
Valenti, S., Gallizzi, R., De Vivo, D. & Romano, C. Intestinal Behçet and Crohn’s disease: two sides of the same coin. Pediatr. Rheumatol. Online J. 15 , 33 (2017).
Kedia, S. et al. Differentiating Crohn’s disease from intestinal tuberculosis. World J. Gastroenterol. 25 , 418–432 (2019).
Oliveira, S. B. & Monteiro, I. M. Diagnosis and management of inflammatory bowel disease in children. BMJ 357 , j2083 (2017).
Amre, D. K., Lu, S.-E., Costea, F. & Seidman, E. G. Utility of serological markers in predicting the early occurrence of complications and surgery in pediatric Crohn’s disease patients. Am. J. Gastroenterol. 101 , 645–652 (2006).
Gisbert, J. P., Marín, A. C. & Chaparro, M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment. Pharmacol. Ther. 42 , 391–405 (2015).
Peyrin-Biroulet, L. et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am. J. Gastroenterol. 110 , 1324–1338 (2015).
van Deen, W. K. et al. Value redefined for inflammatory bowel disease patients: a choice-based conjoint analysis of patients’ preferences. Qual. Life Res. 26 , 455–465 (2017).
Loy, L. et al. Detection and management of early stage inflammatory bowel disease: an update for clinicians. Expert Rev. Gastroenterol. Hepatol. 13 , 547–555 (2019).
Bewtra, M. et al. Inflammatory bowel disease patients’ willingness to accept medication risk to avoid future disease relapse. Am. J. Gastroenterol. 110 , 1675–1681 (2015).
Torres, J. et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J. Crohns Colitis 10 , 1385–1394 (2016).
Beaugerie, L., Seksik, P., Nion-Larmurier, I., Gendre, J.-P. & Cosnes, J. Predictors of Crohn’s disease. Gastroenterology 130 , 650–656 (2006).
Loly, C., Belaiche, J. & Louis, E. Predictors of severe Crohn’s disease. Scand. J. Gastroenterol. 43 , 948–954 (2008).
Beaugerie, L. & Sokol, H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J. Gastroenterol. 18 , 3806–3813 (2012).
Mao, R. et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm. Bowel Dis. 18 , 1894–1899 (2012).
Ghaly, S. et al. High vitamin D-binding protein concentration, low albumin, and mode of remission predict relapse in Crohn’s disease. Inflamm. Bowel Dis. 22 , 2456–2464 (2016).
Qin, G. et al. Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn’s Disease activity. Med. Sci. Monit. 22 , 4393–4400 (2016).
Allez, M. et al. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am. J. Gastroenterol. 97 , 947–953 (2002).
Nahon, S. et al. Diagnostic delay in a French cohort of Crohn’s disease patients. J. Crohns Colitis 8 , 964–969 (2014).
Maconi, G. et al. The impact of symptoms, irritable bowel syndrome pattern and diagnostic investigations on the diagnostic delay of Crohn’s disease: a prospective study. Dig. Liver Dis. 47 , 646–651 (2015).
Vavricka, S. R. et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm. Bowel Dis. 18 , 496–505 (2012).
Schoepfer, A. M. et al. Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am. J. Gastroenterol. 108 , 1744–1753 (2013).
Peyrin-Biroulet, L. et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: results of an international expert opinion process. Am. J. Gastroenterol. 107 , 1770–1776 (2012). This is the first description of early CD, a category of the disease defined by prognostic factors that predict a favourable response to early aggressive treatment .
Danese, S., Fiorino, G., Fernandes, C. & Peyrin-Biroulet, L. Catching the therapeutic window of opportunity in early Crohn’s disease. Curr. Drug Targets 15 , 1056–1063 (2014).
Høivik, M. L. et al. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN study. Gut 62 , 368–375 (2013).
Frøslie, K. F., Jahnsen, J., Moum, B. A., Vatn, M. H. & IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 133 , 412–422 (2007).
Colombel, J.-F. et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 390 , 2779–2789 (2018).
Peyrin-Biroulet, L. et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 63 , 88–95 (2014).
Louis, E. et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 142 , 63–70.e5 (2012).
Doherty, G. et al. European Crohn’s and Colitis Organisation topical review on treatment withdrawal [‘exit strategies’] in inflammatory bowel disease. J. Crohns Colitis 12 , 17–31 (2018).
Munkholm, P., Langholz, E., Davidsen, M. & Binder, V. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut 35 , 360–362 (1994).
Modigliani, R. et al. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Gastroenterology 98 , 811–818 (1990).
Lamb, C. A. et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68 , s1–s106 (2019).
Panés, J. et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology 145 , 766–774.e1 (2013).
Beaugerie, L. et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut 63 , 1416–1423 (2014).
Cosnes, J. et al. Early administration of azathioprine vs conventional management of Crohn’s disease: a randomized controlled trial. Gastroenterology 145 , 758–765.e2 (2013).
Chande, N., Townsend, C. M., Parker, C. E. & MacDonald, J. K. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 10 , CD000545 (2016).
Chatu, S., Subramanian, V., Saxena, S. & Pollok, R. C. G. The role of thiopurines in reducing the need for surgical resection in Crohn’s disease: a systematic review and meta-analysis. Am. J. Gastroenterol. 109 , 23–34 (2014).
Herfarth, H. H., Kappelman, M. D., Long, M. D. & Isaacs, K. L. Use of methotrexate in the treatment of inflammatory bowel diseases. Inflamm. Bowel Dis. 22 , 224–233 (2016).
Colombel, J. F. et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 362 , 1383–1395 (2010).
Dulai, P. S. et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am. J. Gastroenterol. 111 , 1147–1155 (2016).
Kariburyo, M. F., Xie, L., Teeple, A., Tan, H. & Ingham, M. Predicting pre-emptive discussions of biologic treatment: results from an openness and preference survey of inflammatory bowel disease patients and their prescribers. Adv. Ther. 34 , 1398–1410 (2017).
Sands, B. E. et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 381 , 1215–1226 (2019).
Vande Casteele, N. et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 64 , 1539–1545 (2015).
Nanda, K. S., Cheifetz, A. S. & Moss, A. C. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am. J. Gastroenterol. 108 , 40–47; quiz 48 (2013).
Seinen, M. L., De Boer, N. K. & van Bodegraven, A. A. Key insights from therapeutic drug monitoring in Crohn’s disease patients. Expert Opin. Drug Metab. Toxicol. 15 , 399–406 (2019).
Restellini, S., Khanna, R. & Afif, W. Therapeutic drug monitoring with ustekinumab and vedolizumab in inflammatory bowel disease. Inflamm. Bowel Dis. 24 , 2165–2172 (2018).
D’Amico, F., Fiorino, G., Furfaro, F., Allocca, M. & Danese, S. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin. Investig. Drugs 27 , 595–599 (2018).
Peyrin-Biroulet, L., Christopher, R., Behan, D. & Lassen, C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun. Rev. 16 , 495–503 (2017).
Ma, C., Jairath, V., Khanna, R. & Feagan, B. G. Investigational drugs in phase I and phase II clinical trials targeting interleukin 23 (IL23) for the treatment of Crohn’s disease. Expert Opin. Investig. Drugs 27 , 649–660 (2018).
Prideaux, L., Kamm, M. A., De Cruz, P. P., Chan, F. K. L. & Ng, S. C. Inflammatory bowel disease in Asia: a systematic review. J. Gastroenterol. Hepatol. 27 , 1266–1280 (2012).
Prideaux, L. et al. Comparison of clinical characteristics and management of inflammatory bowel disease in Hong Kong versus Melbourne. J. Gastroenterol. Hepatol. 27 , 919–927 (2012).
Gálvez, J. Role of Th17 cells in the pathogenesis of human IBD. ISRN Inflamm. 2014 , 928461 (2014).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61 , 1693–1700 (2012).
van der Giessen, J. et al. Modulation of cytokine patterns and microbiome during pregnancy in IBD. Gut 69 , 473–486 (2020).
van der Giessen, J., Huang, V. W., van der Woude, C. J. & Fuhler, G. M. Modulatory effects of pregnancy on inflammatory bowel disease. Clin. Transl. Gastroenterol. 10 , e00009 (2019).
Maunder, R. G., Cohen, Z., McLeod, R. S. & Greenberg, G. R. Effect of intervention in inflammatory bowel disease on health-related quality of life: a critical review. Dis. Colon Rectum 38 , 1147–1161 (1995).
Chen, X.-L. et al. Inflammatory bowel disease-specific health-related quality of life instruments: a systematic review of measurement properties. Health Qual. Life Outcomes 15 , 177 (2017).
Kaplan, G. G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12 , 720–727 (2015).
Cohen, R. D. The quality of life in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 16 , 1603–1609 (2002).
López Blanco, B., Moreno-Jiménez, B., Devesa Múgica, J. M. & Rodríguez Muñoz, A. Relationship between socio-demographic and clinical variables, and health-related quality of life in patients with inflammatory bowel disease. Rev. Esp. Enferm. Dig. 97 , 887–898 (2005). This study reveals the effect of IBD on QOL, which needs to be considered in clinical practice .
Blondel-Kucharski, F. et al. Health-related quality of life in Crohn’s disease: a prospective longitudinal study in 231 patients. Am. J. Gastroenterol. 96 , 2915–2920 (2001).
Andersson, P., Olaison, G., Bendtsen, P., Myrelid, P. & Sjödahl, R. Health related quality of life in Crohn’s proctocolitis does not differ from a general population when in remission. Colorectal Dis. 5 , 56–62 (2003).
Bernklev, T. et al. Course of disease, drug treatment and health-related quality of life in patients with inflammatory bowel disease 5 years after initial diagnosis. Eur. J. Gastroenterol. Hepatol. 17 , 1037–1045 (2005).
Casellas, F., López-Vivancos, J., Badia, X., Vilaseca, J. & Malagelada, J. R. Impact of surgery for Crohn’s disease on health-related quality of life. Am. J. Gastroenterol. 95 , 177–182 (2000).
Romberg-Camps, M. J. L. et al. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm. Bowel Dis. 16 , 2137–2147 (2010).
Schirbel, A. et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J. Gastroenterol. 16 , 3168–3177 (2010).
Katz, L. et al. Mechanisms of quality of life and social support in inflammatory bowel disease. J. Clin. Psychol. Med. Settings 23 , 88–98 (2016).
Reinink, A. R., Lee, T. C. & Higgins, P. D. R. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: a meta-analysis. Inflamm. Bowel Dis. 22 , 1859–1869 (2016).
Peyrin-Biroulet, L. et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin. Gastroenterol. Hepatol. 14 , 348–354.e17 (2016).
Pariente, B. et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm. Bowel Dis. 17 , 1415–1422 (2011).
Siegel, C. A. et al. Development of an index to define overall disease severity in IBD. Gut 67 , 244–254 (2018). This study discusses the development of severity indices to allow assessment of disease severity and bowel damage progression in the future .
Rieder, F. & Fiocchi, C. Intestinal fibrosis in inflammatory bowel disease - current knowledge and future perspectives. J. Crohns Colitis 2 , 279–290 (2008).
Burke, J. P. et al. Fibrogenesis in Crohn’s disease. Am. J. Gastroenterol. 102 , 439–448 (2007).
Allen, P. B., Gower-Rousseau, C., Danese, S. & Peyrin-Biroulet, L. Preventing disability in inflammatory bowel disease. Ther. Adv. Gastroenterol. 10 , 865–876 (2017).
McGovern, D. Personalized medicine in inflammatory bowel disease. Gastroenterol. Hepatol. 10 , 662–664 (2014).
Google Scholar
Siegel, C. A. et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment. Pharmacol. Ther. 43 , 262–271 (2016).
Download references
Acknowledgements
Work in the laboratory of A.K. is supported by the Wellcome Trust (Senior Investigator Award 106260/Z/14/Z) and the European Research Council (Consolidator Grant 648889).
Author information
Authors and affiliations.
IBD Center, Department of Gastroenterology, Humanitas Clinical and Research Center – IRCCS –, Rozzano, Italy
Giulia Roda, Marjorie Argollo & Silvio Danese
Department of Medicine and Therapeutics, Li Ka Shing Institute of Health Science, Chinese University of Hong Kong, Hong Kong, China
Siew Chien Ng
Colorectal Surgery Unit, Catholic University of Paraná, Curitiba, Brazil
Paulo Gustavo Kotze
Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, University of Calgary, Calgary, Alberta, Canada
Remo Panaccione
Humanitas Clinical and Research Center – IRCCS –, Rozzano, Italy
Antonino Spinelli
Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Italy
Antonino Spinelli & Silvio Danese
Division of Gastroenterology and Hepatology, Department of Medicine, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK
Arthur Kaser
Department of Gastroenterology, University Hospital of Nancy, Nancy, France
Laurent Peyrin-Biroulet
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (G.R., L.P.-B. and S.D.); Epidemiology (S.C.N.); Mechanisms/pathophysiology (A.K.); Diagnosis, screening and prevention (P.G.K. and M.A.); Management (R.P.); Quality of life (G.R., A.S., L.P.-B. and S.D.); Outlook (G.R., A.S., L.P.-B. and S.D.).
Corresponding author
Correspondence to Silvio Danese .
Ethics declarations
Competing interests.
S.D. has served as a speaker, consultant and advisory board member for Schering-Plough, AbbVie, Merck Sharp & Dohme, UCB Pharma, Ferring, Cellerix, Takeda Pharmaceutical Company, Nycomed, Pharmacosmos, Actelion, Alpha Wasserman, Genentech, Grünenthal, Pfizer, AstraZeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor, Johnson & Johnson and Nikkiso Europe GmbH. L.P.-B. has received consulting fees from AbbVie, Amgen, Biogaran, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Ferring, Genentech, HAC Pharma, Hospira, Index Pharmaceuticals, Janssen, Lilly, Merck, Mitsubishi, Norgine, Pfizer, Pharmacosmos, Pilege, Sandoz, Takeda, Therakos, Tillotts, UCB Pharma and Vifor and lecture fees from AbbVie, Ferring, HAC Pharma, Janssen, Merck, Mitsubishi, Norgine, Takeda, Therakos, Tillotts and Vifor. A.S. has acted as a consultant or speaker for Ethicon, Olympus, Frankenman, Transenterix (not active), Tigenyx, Pfizer, Takeda and Sandoz. P.G.K has been a lecturer for AbbVie, Janssen, Pfizer and Takeda and is a member of the advisory board of AbbVie, Pfizer and Takeda. A.K. has served as an adviser to Boehringer Ingelheim, Ferring, Genentech, GlaxoSmithKline, Gilead, Hospira, Janssen, Pfizer, and VHSquared. R.P. has received consultant and/or lecture fees from AbbVie, Amgen, AstraZeneca, Axcan Pharma (now Aptalis), Biogen Idec, Bristol-Myers Squibb, Centocor, ChemoCentryx, Eisai Medical Research Inc., Elan Pharmaceuticals, Ferring, Genentech, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Millennium Pharmaceuticals (now Takeda Oncology), Ocera Therapeutics Inc., Otsuka America Pharmaceutical, Pfizer, Shire Pharmaceuticals, Prometheus Laboratories, Schering-Plough Corporation, Synta Pharmaceuticals Corp., Teva, UCB Pharma and Warner Chilcott. S.C.N. has received consulting and speaker fees from AbbVie, Ferring, Janssen, Menarini and Takeda, has served as a scientific advisory board member for AbbVie, Ferring and Takeda and has received research grants from AbbVie, Ferring and Janssen. The other authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Disease Primers thanks B.D. Ye, A. Forbes, J. Gisbert, G. Monteleone, A. Regueiro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions

About this article
Cite this article.
Roda, G., Chien Ng, S., Kotze, P.G. et al. Crohn’s disease. Nat Rev Dis Primers 6 , 22 (2020). https://doi.org/10.1038/s41572-020-0156-2
Download citation
Accepted : 14 February 2020
Published : 02 April 2020
DOI : https://doi.org/10.1038/s41572-020-0156-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Anti-cdtb and anti-vinculin antibodies to diagnose irritable bowel syndrome in inflammatory bowel disease patients.
- Luisa Leite Barros
- Gabriela Leite
- Alberto Queiroz Farias
BMC Gastroenterology (2024)
Hyperbaric oxygen therapy ameliorates intestinal and systematic inflammation by modulating dysbiosis of the gut microbiota in Crohn’s disease
- Ruizheng Sun
- Xiaowei Liu
Journal of Translational Medicine (2024)
Risk factors for endoscopic postoperative recurrence in patients with Crohn’s Disease: a protocol for systematic review and meta-analysis
Efficacy and safety of stem cell therapy for crohn’s disease: a meta-analysis of randomized controlled trials.
- Yunfeng Qiu
- Changfeng Li
- Shihou Sheng
Stem Cell Research & Therapy (2024)
Increased CpG methylation at the CDH1 locus in inflamed ileal mucosa of patients with Crohn disease
- Charles de Ponthaud
- Solafah Abdalla
- Pierre Bougnères
Clinical Epigenetics (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

The state of the art on treatment of Crohn’s disease
Hai yun shi, siew chien ng.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Received 2018 May 18; Accepted 2018 May 20; Issue date 2018.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Crohn’s disease (CD) is a chronic, progressive, and destructive disease of the gastrointestinal tract. Although its incidence appears to be stable or decreasing in most countries in the North America and Europe, the incidence is rising rapidly in Asian countries. Immunomodulators and biologics are increasingly used to avoid long-term bowel damage and subsequent disability. Therapeutic drug monitoring facilitates optimizing thiopurines and anti-TNFs use. New biologic agents targeting various pathological pathways of CD are blooming in recent years, and the high cost of biologics and expiration of patents for several biologic agents have driven the utility of biosimilars for CD treatment. Here, the literature regarding the efficacy, safety, and withdrawal of the drugs, as well as the evolution of therapeutic targets will be reviewed.
Keywords: Crohn’s disease, Biologics, Mucosal healing, Target
Introduction
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic idiopathic inflammatory disorders of the gastrointestinal tract, resulting from a combination of genetic predisposition, environmental factors, and inappropriate immune response to the gut microbiota [ 1 ]. Since the recognition of the diseases, the incidence of IBD has increased substantially around the world [ 2 ]. During the 20th century, IBD prevalence reaches over 0.3% in North America, Oceania, and many countries in Europe [ 3 ]. At the turn of the 21st century, while IBD incidence appears to have plateaued in North America and Europe, the incidence of IBD continues to rise among newly industrialised countries in Asia, Africa, and South America [ 3 ]. The Asia–Pacific Crohn’s and Colitis Epidemiologic Study (ACCESS), a population-based cohort study of newly diagnosed patients with IBD (2011–2013) from 13 countries in the Asia–Pacific region, has reported that the overall incidence of IBD in Asia is 1.4 cases per 100,000 [ 4 ]. Although IBD incidence in Asia is still low at present, with a climbing incidence based on 60% of the global population ( https://www.statista.com/statistics/262881/global-population-by-continent/ ), the absolute number of IBD patients in Asia may catch up with that in the western world over the next decade [ 5 ]. In Japan, the prevalence of IBD is also rising based on the National Japan IBD Registry run by the Ministry of Health, Labour and Welfare [ 6 ]. Although UC is the predominant type of IBD in Asia, an increase in the incidence ratio of CD to UC over time has been reported [ 7 , 8 ]. Healthcare costs of CD are almost three times higher than that of UC [ 9 ].
Natural history
CD is a progressive and destructive disease, which may involve the whole gastrointestinal tract [ 10 ]. The majority (77–90%) of patients have a chronic intermittent course during 10 years after diagnosis [ 11 , 12 ]. In a regional inception cohort of 213 Danish CD patients with a follow-up period of at least 7 years, 15% of the patients presented with a worsening change in disease behaviour [ 13 ]. A meta-analysis of 198 population-based studies involving 211,563 CD patients showed that corticosteroids, immunomodulators, and biologics are used in 48–60, 32–60, and 6–20% of patients with CD, and the cumulative rates of surgery range from 29 to 49% across different populations [ 14 ]. In recent years, immunomodulators and biologics are increasingly used, which is accompanied by a persistent and significant drop of surgery rates for CD [ 15 , 16 ].
Evolution of endpoints and treat-to-target
Resolution of symptoms is the primary clinical target for CD treatment. Crohn’s Disease Activity Index (CDAI), which consists of eight variables including the number of liquid stools, the extent of abdominal pain, general well-being, the occurrence of extraintestinal symptoms, the need for antidiarrheal drugs, and the presence of abdominal masses, hematocrit, and body weight, is the standard and most widely used clinical index [ 17 , 18 ]. Clinical remission is defined as a CDAI score below 150, and clinical response is defined as a decrease of at least 100 points [ 19 ], although a lesser cutoff for response with a reduction of at least 70 points was used in some studies [ 20 ]. However, the target of symptom control does not appear to significantly alter the natural course of CD [ 10 , 21 ].
Mucosal healing become the therapeutic goal in clinical practice, as emerging evidence shows that it is associated with a reduced risk of disease relapse [ 22 ], hospitalization, and surgery [ 23 , 24 ]. It has been noted that clinical symptoms as measured by CDAI have poor correlation with mucosal appearance in CD, as half of the patients in clinical remission have endoscopic inflammation, and 20% of patients with mucosal healing have persistent clinical symptoms [ 25 ]. The Crohn’s Disease Endoscopic Index of Severity (CDEIS) and Simple Endoscopic Score for Crohn’s Disease (SES-CD) are the most studies endoscopic scoring systems for CD [ 26 ]. CDEIS, respectively, scores the presence of deep ulceration, superficial ulceration, length of diseased mucosa, and length of the ulcerated surface in five segments (rectum, sigmoid and left colon, transverse colon, right colon, and ileum). The numbers are summed up and divided by the number of segments evaluated. Additional points are given to stenosis [ 27 ]. Endoscopic remission is defined as a CDEIS score of less than 3, and a decrease of over 5 points demonstrates endoscopic response [ 28 ]. However, the complexity in calculating the CDEIS score precludes its use in clinical practice [ 26 ]. SES-CD, which is scored based on four endoscopic variables (presence and size of ulcers, extent of ulcerated surface, extent of affected surface, and presence and type of narrowing) in the same five segments, provides a practical alternative to the CDEIS [ 29 ]. An SES-CD score below 2 is regarded as endoscopic remission [ 30 ]. Using relative change in CDEIS and SES-CD scores, a decrease from baseline of at least 50% has been used as the definition for endoscopic response [ 31 ]. The Rutgeerts score designed to assess post-operative recurrence, with a score of i2 and above indicating endoscopic recurrence [ 32 ]. Although the universal definition for mucosal healing is yet to be determined, the absence of ulceration at ileocolonoscopy has been adopted as the endoscopic endpoint for CD [ 28 , 30 ].
Given the transmural nature of the disease and inaccessible bowel regions through endoscopy in some cases, imaging modalities (ultrasonography, magnetic resonance imaging, and computed tomography enterography) have been used for assessing disease activity [ 33 ]. The most established tool for magnetic resonance imaging is the Magnetic Resonance Index of Activity, which scores wall thickness, relative contrast enhancement, mural edema, and ulcers in different segments of the bowel [ 34 , 35 ]. While resolution of lesions in imaging examinations has not been broadly accepted as a treatment goal, it has been proposed as a target when endoscopy cannot adequately evaluate inflammation [ 28 ].
A patient reported outcome (PRO), which is a measurement derived directly from a patient about any aspect of their health status, without interpretation of their response by a clinician or anyone else [ 36 ], has the potential to become a treatment endpoint for CD [ 37 ]. Several scales, such as Inflammatory Bowel Disease Questionnaire (IBDQ), Functional Assessment Chronic Illness Therapy-Fatigue (FACIT-F), and Work Productivity Activity Impairment Questionnaire (WPAI), have been used to assess patients’ perspectives towards the disease, although none of them was created according to the United States Food and Drug Administration (FDA) guidance for PRO development [ 37 ]. A 2-item interim PRO derived from CDAI has been developed, and resolution of abdominal pain and normalization of bowel habit are proposed to be the primary PRO for CD [ 28 ].
To overcome the drawbacks of the traditional progressive, stepwise management of CD for the avoidance of the long-term bowel damage and subsequent disability, a “treat-to-target” strategy has been advocated [ 38 ]. The principle of this strategy is to make adjustment of therapy according to regular assessment of disease activity using appropriate treatment targets [ 39 ]. A composite target of achieving resolution of abdominal pain and altered bowel habit, and ulceration at ileocolonoscopy as well has been proposed by the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) [ 28 ]. The recommended frequency for assessment is 6–9 months during the active phase [ 28 ].
Conventional medication
Aminosalicylates.
There is consensus that mesalazine has little role for either inducing remission or preventing relapse in CD [ 19 ]. Nonetheless, mesalazine and sulfasalazine can be considered in mild colonic CD with superficial lesions [ 40 , 41 ]. High-dose mesalazine is an option to prevent post-operative recurrence for patients with an isolated ileal resection, and sulfasalazine has beneficial effect on peripheral arthritis associated with CD [ 42 ].
Corticosteroids
Corticosteroids are the mainstay of therapy for inducing remission in CD [ 40 ]. Systemic corticosteroids are the first-line treatment to control active disease [ 19 , 41 ]. A recent network meta-analysis showed that corticosteroids have similar efficacy to high-dose budesonide (above 6 mg/d), but are more effective than high-dose mesalazine for induction of remission [ 43 ]. Budesonide is a synthetic steroid that has high topical glucocorticoid activity with a better safety profile compared to conventional corticosteroids, due to its relatively low bioavailability [ 44 ]. Therefore, it is recommended in preference to conventional corticosteroids in patients with mildly active localized terminal ileal or ileocecal disease [ 19 ].
Thiopurines
The European Crohn’s and Colitis Organization (ECCO) 2016 guideline advocates thiopurines as the first-line therapy for preventing disease relapse in patients who achieve remission with corticosteroids [ 41 ]. It has been confirmed by a meta-analysis that azathioprine (AZA) is superior to placebo for maintenance of remission, with a number needed to treat for an additional beneficial outcome of 9 [ 45 ]. The guideline also recommends thiopurines use to prevent post-operative recurrence in patients with higher risk for recurrence [ 42 ]. Thiopurine withdrawal is associated with a higher risk for relapse [ 46 ]. Randomized controlled trials showed that the relapse rates after stopping AZA ranged from 8 to 25% at 6 months, 16–53% at 12 months, 21–31% at 18 months, and 31% at 24 months, whereas the corresponding proportions were 0, 4–15, 8–12, and 15% in patients who continued on AZA [ 47 – 50 ]. AZA is converted to mercaptopurine (MP) within the thiopurine metabolic pathway [ 51 ]. MP is an alternative for AZA-intolerant patients, as MP is tolerated by over two-thirds of patients who are intolerant to AZA [ 52 ].
Adverse events of thiopurines lead to drug withdrawal in a significantly higher proportion of patients in randomized clinical trials [ 45 ], and the rate reaches up to one-third in some cohorts [ 53 , 54 ]. The main safety concerns related to thiopurine use are myelotoxicity (4–25%), hepatotoxicity (17%), pancreatitis (4–7%) [ 55 ], and an increased risk of various malignancies, including lymphoproliferative disorders, non-melanoma skin cancers (NMSC), myeloid disorders, and urinary tract cancers in a long run [ 19 , 56 – 59 ]. The France nationwide prospective observational cohort study—Cancers Et Surrisque Associé aux Maladies inflammatoires intestinales En France (CESAME) has reported an incidence of lymphoproliferative disorder as 0.90 per 1000 [95% confidence interval (CI) 0.50–1.49] patient-years among patients receiving thiopurines, with a multivariate-adjusted hazard ratio (HR) of 5.28 (2.01–13.9, p = 0.0007) over those who had never received thiopurines [ 56 ]. However, the absolute rate of lymphoma among patients taking thiopurines is low, and the increased risk seems to disappear after discontinuation of the therapy [ 60 ]. The CESAME cohort has also revealed that both ongoing thiopurine treatment (HR 5.9; 95% CI 2.1–16.4) and past thiopurine exposure (HR 3.9; 95% CI 1.3–12.1) are risk factors for NMSC [ 57 ]. Patients receiving thiopurines have a 2.8 higher risk for urinary tract cancer [ 59 ]. Past exposure to thiopurines is associated with a 7-fold increased risk of myeloid disorders [ 58 ]. Based on extrapolation from transplant data, it seems that the risks for malignancies begin to significantly accumulate after several years of with thiopurine therapy. It is reasonable to consider thiopurine withdrawal in selected patients after 3–4 years of treatment [ 61 ].
Thiopurines are metabolised through three competing pathways via three critical enzymes: hypoxanthine phosphoribosyl transferase (HPRT), thiopurine methyltransferase (TPMT), and xanthine oxidase (XO). MP is methylated to methylmercaptopurine (MMP) by TPMT in one pathway, while in another pathway, it undergoes metabolism to form thioguanine nucleotide (TGN), which is the main therapeutic metabolite of thiopurines [ 51 , 62 ]. As TPMT deficiency is known to be related to an increased risk of myelotoxicity, to assess TPMT levels prior to thiopurine therapy and adapt the dose accordingly are considered to be useful for the avoidance of serious myelosuppression [ 40 , 51 ]. Monitoring TGN and MMP levels can guide optimized therapy when an adequate response is not reached: patient education needs to be performed when TGN and MMP are undetectable, which indicates poor drug compliance; patients with low levels of both TGN and MMP require dose increase; allopurinol can be put on to improve the therapeutic efficacy of thiopurine in patients with a low TGN level and a high MMP level, which suggests thiopurine hypermethylation; and patients with TGN within a therapeutic range (235–450 pmol/8 × 10 8 red blood cells) and a normal level of MMP may be resistant to thiopurine therapy [ 63 ]. Recently, the presence of NUDT15 variant has been found to be prevalent amongst Asians and is associated with a higher risk of thiopurine-induced leucopenia [ 64 ].
Methotrexate
Guidelines recommend the use of methotrexate (MTX, 15 mg/week intramuscular) as an alternative to AZA for the maintenance of remission [ 41 ]. MTX is recommended in patients with steroid-dependent, steroid-refractory, or AZA-refractory CD, and is also indicated in patients intolerant to other immunomodulating agents [ 19 ]. The most common adverse effect of MTX is nausea (in up to 25% of treated patients) [ 65 ]. As MTX can include hepatotoxicity and bone-marrow suppression, full blood count and liver function monitoring are warranted [ 19 ]. Because of the potential teratogenicity, MTX is contraindicated during pregnancy [ 19 ].
Thalidomide
Refractory CD remains a clinical challenge in more than 20% of patients, despite the advances in biologic therapy [ 66 ]. A randomized controlled trial (RCT) of children and adolescents with refractory CD has shown a 46% clinical remission rate at 8 weeks in thalidomide-treated patients and longer duration of remission in responders [ 67 ]. Recent studies have found a clinical remission rate of around 50% at 6 months in patients with refractory CD on low-dose thalidomide (50–100 mg/d) [ 66 , 68 ]. A systematic review, of which 89% are CD patients, has reported an overall rate of sustained clinical remission on thalidomide of 72% at 1 year and an overall rate of complete endoscopic remission of 48% [ 69 ]. Lazzerini et al. [ 70 ] performed long-term follow-up to patients from two multicenter RCTs, and found mucosal healing and histologic healing achieved in 75 and 53%, respectively, among patients with clinical remission on thalidomide. However, due to the high frequency of major adverse effects, thalidomide should only be considered in short-term use among selected patients [ 69 ].
Anti-tumor necrosis factor agents and drug monitoring
The use of biologics in IBD started with the approval of infliximab, which is an anti-tumor necrosis factor (anti-TNF) agent in CD by the FDA in 1998 [ 71 ]. Since then, adalimumab and certolizumab have reached the marketplace for CD treatment [ 72 ]. The introduction of anti-TNF has revolutionised the therapeutic strategy of IBD, and its use continues to increase [ 15 ]. Well-designed RCTs have demonstrated the efficacy of anti-TNF agents in inducing and maintaining remission in CD [ 73 – 84 ]. A meta-analysis has confirmed the efficacy of all the anti-TNF agents for induction (vs. placebo: relative risks, RR 1.66, 95% CI 1.17–2.36) and maintenance (vs. placebo: RR 1.78, 95% CI 1.51–2.09) of clinical remission. For each anti-TNF agent, infliximab, adalimumab, and certolizumab result in a 3.70-fold (95% CI 0.87–15.80), 2.94-fold (95% CI 1.86–4.66), and 1.22-fold (95% CI 1.00–1.50) higher likelihood of inducing remission, and 1.86-fold (95% CI 1.21–2.86), 2.06-fold (95% CI 1.50–2.82) and 1.62-fold (95% CI 1.30–2.02) higher likelihood of maintaining remission, compared to placebo. There is no evidence of clinical superiority among anti-TNF agents [ 85 ]. In another meta-analysis, the pooled rate of mucosal healing is 29% in CD patients treated with anti-TNF (vs. 7% in the placebo arm) at weeks 10–12. Patients on anti-TNF are over three times more likely to achieve mucosal healing than those on placebo. The pooled rates of sustained mucosal healing at week 52–54 are 28 and 1% with anti-TNF or placebo, respectively, with an OR of 19.71 [ 86 ]. Anti-TNF agents significantly reduce the risk of hospitalization (OR 0.46, 95% CI 0.36–0.60) and surgery (OR 0.23, 95% CI 0.13–0.42) compared to placebo [ 87 ]. A network meta-analysis has shown that anti-TNF is superior to azathioprine in reducing the risk for both of the clinical relapse (RR, 0.11; 95% CI 0.01–0.40) and endoscopic recurrence (RR, 0.04; 95% CI 0.00–0.14) after surgery [ 88 ].
ECCO guidelines have swung strongly in favor of early anti-TNF use in CD therapy, especially in patients with high disease activity and features indicating unfavorable prognosis, including young-onset, extensive disease, early need for corticosteroids and perianal disease [ 19 ]. Anti-TNF based therapy is also recommended as prophylactic treatment after bowel resection in patients with at least one risk factor for recurrence [ 42 ]. Smoking, previous surgery, penetrating disease and perianal involvement are consistently considered as predictors for post-operative recurrence [ 42 , 71 ].
Up to 40% of patients show no clinical benefit (primary non-response) to anti-TNF therapy [ 89 ]. Secondary loss of response refers to the clinical situation, in which a patient has an initial response to anti-TNF, followed by a diminished or less durable response over time. In a recent meta-analysis, the pooled incidence of secondary loss of response with a median follow-up of 1-year is 33%, with an annual risk for loss of response as 21% per patient-year [ 90 ]. Immunogenicity failure, defined by the absence of disease improvement in the setting of low anti-TNF levels with high levels of anti-drug antibodies accounts for both of the primary non-response and secondary loss of response in anti-TNF therapy [ 89 ]. Combination therapy of an anti-TNF agent with immunomodulator is associated with a reduction in anti-drug antibody formation and better anti-TNF concentrations [ 91 ]. The serum levels of anti-TNF significantly correlate with clinical efficacy [ 92 ]. The Study of Biologic and Immunomodulator Naive Patient in Crohn’s Disease (SONIC) directly compared the efficacy of infliximab, azathioprine, and the two drugs combined in active CD patients who were naive to both drugs, and found that the combination therapy is superior to infliximab monotherapy in reducing remission (week 26: 56.8 vs. 44.4%; p = 0.02) [ 93 ]. In a network meta-analysis, the combination of infliximab and azathioprine shows a 98% probability of superiority to infliximab in maintaining remission in CD [ 94 ]. In another study, almost half of the IBD patients with anti-adalimumab antibodies and loss of response have sero-reversal of the antibodies, increase in drug trough levels and restoration of clinical response after the addition of a thiopurine or methotrexate [ 95 ]. However, the benefits of combination therapy in patients refractory to an immunomodulator before the initiation of anti-TNF is less clear [ 71 ]. A systematic review of 11 RCTs with the exclusion of studies involving only subjects naive to anti-TNF and immunomodulator therapy fails to demonstrate a superiority of combination therapy to anti-TNF monotherapy in both reducing and maintaining remission, although subgroup analysis shows the benefit of combination therapy in achieving clinical remission at week 24–30 in patients treated with infliximab who have not previously been exposed to immunomodulators [ 96 ].
Therapeutic drug monitoring (TDM) refers to the evaluation of drug concentration and anti-drug antibodies during therapy. As the three possible causes of anti-TNF failure (mechanistic failure, nonimmune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure) present with different levels of serum drug concentration and anti-drug antibodies, TDM can be used to determine the reasons for drug failure and guide the optimization of treatment [ 97 ]. The American Gastroenterological Association Institute Guideline suggests reactive TDM in patients with suboptimal disease control on anti-TNF [ 98 ]. Secondary loss of response with therapeutic drug trough concentration is regarded as mechanistic failure, in which condition, patients may need to switch to a drug with an alternative mechanism of action, and add an immunomodulator if anti-drug antibodies are detected. The presence of sub-therapeutic drug trough level and undetectable anti-drug antibodies is considered as nonimmune-mediated pharmacokinetic failure, which requires dose escalation. The occurrence of immune-mediated pharmacokinetic failure is indicated when it shows sub-therapeutic drug trough concentration and detectable anti-drug antibodies, in which situation, patients may benefit from switching to a different anti-TNF agent or the addition of an immunomodulator if high level anti-drug antibodies are detected [ 97 ]. The suggested therapeutic drug trough concentrations for infliximab, adalimumab, and certolizumab are ≥ 5, ≥ 7.5 and ≥ 20 ug/ml, respectively, whereas the uniform thresholds for anti-drug antibody titers remain uncertain [ 98 ]. Reactive TDM has been proven more cost-effective than empiric dose escalation for secondary loss of response to anti-TNF treatment [ 99 , 100 ].
Anti-TNF therapy is relatively safe in CD treatment [ 101 ]. Meta-analyses showed no increased risk for serious infection of anti-TNF therapy as compared with placebo in CD [ 102 , 103 ]. However, the Crohn’s Therapy, Resource, Evaluation, and Assessment Tool (TREAT) Registry, reflecting over 13 years of real-world experience in CD treatment has revealed that compared to other-treatment-only, infliximab is associated with a higher rate of serious infection (2.15/100 vs. 0.86/100 patient-years) [ 104 ]. Anti-TNF therapy does not appear to increase the risk for malignancies and mortality [ 16 , 104 ]. However, its combination with immunomodulator significantly increases the risk of malignancies, including lymphoma and NMSC [ 105 , 106 ].
Relapse rate after anti-TNF withdrawal is between 30–40% at 1 year, and greater than 50% beyond 2 years [ 107 ]. As elevated biological markers (fecal calprotectin and C-reactive protein) and mucosal inflammation associate with higher risk of relapse after anti-TNF discontinuation, patients should achieve biological and endoscopic remission beyond be in clinical remission if anti-TNF withdrawal is considered [ 61 ]. Maintenance of immunomodulator treatment after anti-TNF withdrawal reduces the risk for relapse [ 41 , 61 ]. Retreatment with anti-TNF at relapse is effective with a success rate of 88% [ 107 ].
Other biologics
Vedolizumab, a monoclonal antibody to α 4 β 7 , was approved by the US FDA for the treatment of CD in 2014 [ 40 ]. It blocks the α 4 β 7 integrin on lymphocyte surface that facilitates trafficking of lymphocytes to the gut and the binding of the lymphocytes to gut-specific ligands known as mucosal address in cell adhesion molecule-1 [ 40 ]. GEMINI 2 study has evidenced the efficacy of vedolizumab in reducing and maintaining remission in CD. In this study, 14.5% of vedolizumab users achieved clinical remission (vs. 6.8% of those received placebo, p = 0.02) at week 6. Clinical remission was maintained over one year in 39 and 36.4% of patients on vedolizumab every 8 weeks and every 4 weeks, respectively (vs. 21.6% of those on placebo, p < 0.001 and p = 0.004 for the two vedolizumab arms, respectively, vs. placebo) [ 108 ]. The utility of vedolizumab as a “rescue” treatment after anti-TNF failed is supported by the GEMINI 3 study [ 109 ], and some cohort studies [ 110 – 112 ]. Vedolizumab can induce clinical remission in 20–30% of patients with anti-TNF refractory CD after 6 weeks [ 109 – 112 ]. According to the published data, Vedolizumab is well tolerated with a favorable safety profile [ 111 , 113 ].
Ustekinumab, an antibody to interleukin-12/23, was approved by the US FDA for the treatment of CD in 2016 [ 40 , 71 ]. Phase II and phase III trials have shown the superiority of ustekinumab over placebo for the induction and maintenance of clinical remission [ 114 ]. In UNITI-1 study, ustekinumab induced clinical remission in 18.5% of patients who failed or intolerant to anti-TNF at week 6 (vs. 8.9% of patients on placebo, – = 0.002) and in 20.9% of these patients at week 8 (vs. 7.3% of patients on placebo, p < 0.001) [ 115 ]. In UNITI-2 study, ustekinumab led to a higher rate of clinical remission at week 6 (34.9 vs. 17.7% in the placebo arm, p < 0.001) and week 8 (40.2 vs. 19.6% in the placebo arm, p < 0.001) in patients naive to anti-TNF [ 115 ]. IM-UNITI study showed that 2/3 of patients on ustekinumab had sustained clinical remission at week 44 (vs. 45.6% of those on placebo, p = 0.007) [ 115 ]. CD patients with psoriasis or those who develop anti-TNF induced psoriasis may be the ideal candidates for ustekinumab therapy [ 19 , 114 ]. Given a favorable safety profile, ustekinumab has potential to become a first-line biologic agent for CD, although much has to be learnt about its long-term safety [ 114 ]. Importantly, risk of tuberculosis (TB) is low with this drug, which may be useful in TB endemic regions [ 116 ].
Biosimilars
Healthcare costs for CD have shifted from hospitalization and surgery to expenditures associated with biologic agents, which account for 64% of the total costs [ 9 ]. The high cost of biologics and the expiration of patents for several biologics have triggered the development of biosimilar versions of these drugs. The approval of biosimilars follows an expedited process with indication extrapolation [ 117 ]. Extrapolated from the results of studied in rheumatoid arthritis, ankylosing spondylitis, or plaque psoriasis, several anti-TNF biosimilar agents have been approved for IBD treatment, and multiple additional agents are currently in development [ 117 , 118 ]. CT-P13, a biosimilar to infliximab is the first of these agents. Although none of the biosimilars were formally assessed in IBD, the observational “real-world” data so far have shown a comparative efficacy and safety profile of CT-P13 to its reference product infliximab [ 118 , 119 ]. The NOR-SWITCH study is a randomized controlled trial comparing patients who initiated and continued infliximab to those who initiated infliximab but then switched to CT-P13. The results support switching from infliximab to CT-P13 by showing a non-significant difference in disease worsening at 52 weeks between the two arms. However, it should be noted that more CD patients flared in the CT-P13 arm (36.5 vs. 21.2%), although it did not reach a statistical significance [ 120 ].
Conclusions
A combination of symptom control and mucosal healing has been proposed as the target for CD therapy. Corticosteroids and thiopurines remain as the mainstay of treatment, while anti-TNF agents are increasingly applied earlier in disease course during the recent two decades. Anti-TNF is recommended in patients with high risk for unfavorable prognosis. However, primary non-response or secondary loss of response to anti-TNF therapy occurs in a large proportion of patients, who may benefit from therapeutic drug monitoring. New biologics not only provide hope to patients refractory to anti-TNF, but also has potential to become first-line therapy in selected patients due to beneficial risk profile and long-term efficacy.
This work was funded by the National Natural Science Foundation of China (81702960) and Beijing Talents Fund (2017000021469G209).
Compliance with ethical standards
Conflicts of interest.
The authors declare that they have no competing interests.
- 1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46.e42–54.e42. doi: 10.1053/j.gastro.2011.10.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145:158.e2–165.e2. doi: 10.1053/j.gastro.2013.04.007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Kaplan GG, Jess T. The changing landscape of inflammatory bowel disease: east meets west. Gastroenterology. 2016;150:24–26. doi: 10.1053/j.gastro.2015.11.029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Asakura K, Nishiwaki Y, Inoue N, et al. Prevalence of ulcerative colitis and Crohn’s disease in Japan. J Gastroenterol. 2009;44:659–665. doi: 10.1007/s00535-009-0057-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Ng SC, Leung WK, Shi HY, et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm Bowel Dis. 2016;22:1954–1960. doi: 10.1097/MIB.0000000000000846. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986–2005: a KASID study. Inflamm Bowel Dis. 2008;14:542–549. doi: 10.1002/ibd.20310. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. van der Valk ME, Mangen M-JJ, Leenders M, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72–79. doi: 10.1136/gutjnl-2012-303376. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Peyrin-Biroulet L, Loftus EV, Jr, Colombel J-F, et al. The natural history of adult Crohn’s disease in population-based cohorts. Am j gastroenterol. 2010;105:289. doi: 10.1038/ajg.2009.579. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Wolters FL, Russel MG, Sijbrandij J, et al. Phenotype at diagnosis predicts recurrence rates in Crohn’s disease. Gut. 2006;55:1124–1130. doi: 10.1136/gut.2005.084061. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Solberg IC, Vatn MH, Høie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–1438. doi: 10.1016/j.cgh.2007.09.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Lo B, Vester-Andersen M, Vind I, et al. Changes in disease behaviour and location in patients with Crohn’s disease after seven years of follow-up: a Danish population-based inception cohort. J Crohn’s Colitis. 2018;12(3):265–272. doi: 10.1093/ecco-jcc/jjx138. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Shi HY, Levy AN, Trivedi HD, et al. Ethnicity influences phenotype and outcomes in inflammatory bowel disease: a systematic review and meta-analysis of population-based studies. Clin Gastroenterol Hepatol. 2018;16:190.e11–197e11. doi: 10.1016/j.cgh.2017.05.047. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut. 2014;63:1607–1616. doi: 10.1136/gutjnl-2013-305607. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Annese V, Duricova D, Gower-Rousseau C, et al. Impact of new treatments on hospitalisation, surgery, infection, and mortality in ibd: a focus paper by the epidemiology committee of ECCO. J Crohns Colitis. 2016;10:216–225. doi: 10.1093/ecco-jcc/jjv190. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn’s disease activity index: National Cooperative Crohn’s Disease Study. Gastroenterology. 1976;70:439–444. [ PubMed ] [ Google Scholar ]
- 18. Sandborn WJ, Feagan BG, Hanauer SB, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512–530. doi: 10.1053/gast.2002.31072. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J. Crohn’s Colitis. 2016;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Thia KT, Sandborn WJ, Lewis JD, et al. Defining the optimal response criteria for the Crohn’s disease activity index for induction studies in patients with mildly to moderately active Crohn’s disease. Am. J. gastroenterol. 2008;103:3123. doi: 10.1111/j.1572-0241.2008.02176.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Bernstein CN, Loftus EV, Jr, Ng SC, et al. Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61(4):622–629. doi: 10.1136/gutjnl-2011-301397. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–468. doi: 10.1053/j.gastro.2009.09.056. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. D’Haens G, Baert F, Van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. The Lancet. 2008;371:660–667. doi: 10.1016/S0140-6736(08)60304-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Reinink AR, Lee TC, Higgins PD. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: a meta-analysis. Inflamm Bowel Dis. 2016;22:1859–1869. doi: 10.1097/MIB.0000000000000816. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95. doi: 10.1136/gutjnl-2013-304984. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Buchner AM, Lichtenstein GR. How to assess and document endoscopies in ibd patients by including standard scoring systems. Inflamm Bowel Dis. 2016;22:1010–1019. doi: 10.1097/MIB.0000000000000649. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Mary J-Y, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes therapeutiques des affections inflammatoires du Tube Digestif (GETAID) Gut. 1989;30:983–989. doi: 10.1136/gut.30.7.983. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. doi: 10.1016/s0016-5107(04)01878-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Peyrin-Biroulet L, Panes J, Sandborn WJ, et al. defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol. 2016;14:348.e17–354.e17. doi: 10.1016/j.cgh.2015.06.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Ferrante M, Colombel JF, Sandborn WJ, et al. Validation of endoscopic activity scores in patients with Crohn’s disease based on a post hoc analysis of data from SONIC. Gastroenterology. 2013;145:978.e5–986.e5. doi: 10.1053/j.gastro.2013.08.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963. doi: 10.1016/0016-5085(90)90613-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Panes J, Bouhnik Y, Reinisch W, et al. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohn’s Colitis. 2013;7:556–585. doi: 10.1016/j.crohns.2013.02.020. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Rimola J, Rodríguez S, García-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–1120. doi: 10.1136/gut.2008.167957. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Rimola J, Ordás I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2010;17:1759–1768. doi: 10.1002/ibd.21551. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Health UDo, Evaluation HSFCfD Research et al. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4:1–20. doi: 10.1186/1477-7525-4-79. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Williet N, Sandborn WJ, Peyrin-Biroulet L. Patient-reported outcomes as primary end points in clinical trials of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1246.e6–1256.e6. doi: 10.1016/j.cgh.2014.02.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Bouguen G, Levesque BG, Feagan BG, et al. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:1042.e2–1050.e2. doi: 10.1016/j.cgh.2013.09.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Schipper LG, Van Hulst LT, Grol R, et al. Meta-analysis of tight control strategies in rheumatoid arthritis: protocolized treatment has additional value with respect to the clinical outcome. Rheumatology. 2010;49:2154–2164. doi: 10.1093/rheumatology/keq195. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Bernstein CN. Treatment of IBD: where we are and where we are going. Am J Gastroenterol. 2015;110:114–126. doi: 10.1038/ajg.2014.357. [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Ooi CJ, Makharia GK, Hilmi I, et al. Asia-Pacific consensus statements on Crohn’s disease. Part 2: management. J Gastroenterol Hepatol. 2016;31:56–68. doi: 10.1111/jgh.12958. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Gionchetti P, Dignass A, Danese S, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohn’s Colitis. 2016;11:135–149. doi: 10.1093/ecco-jcc/jjw169. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Coward S, Kuenzig ME, Hazlewood G, et al. Comparative effectiveness of mesalamine, sulfasalazine, corticosteroids, and budesonide for the induction of remission in Crohn’s disease: a Bayesian network meta-analysis. Inflamm Bowel Dis. 2017;23:461–472. doi: 10.1097/MIB.0000000000001023. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Nunes T, Barreiro-de Acosta M, Marin-Jimenez I, et al. Oral locally active steroids in inflammatory bowel disease. J Crohns Colitis. 2013;7:183–191. doi: 10.1016/j.crohns.2012.06.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Chande N, Patton PH, Tsoulis DJ, et al. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Libr. 2015 doi: 10.1002/14651858.CD000067.pub3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Torres J, Boyapati RK, Kennedy NA, et al. Systematic review of effects of withdrawal of immunomodulators or biologic agents from patients with inflammatory bowel disease. Gastroenterology. 2015;149:1716–1730. doi: 10.1053/j.gastro.2015.08.055. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. O’Donoghue D, Dawson A, Powell-Tuck J, et al. Double-blind withdrawal trial of azathioprine as maintenance treatment for Crohn’s disease. The Lancet. 1978;312:955–957. doi: 10.1016/s0140-6736(78)92524-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Vilien M, Dahlerup J, Munck L, et al. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn’s disease: increased relapse rate the following year. Aliment Pharmacol Ther. 2004;19:1147–1152. doi: 10.1111/j.1365-2036.2004.01944.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Lémann M, Mary J-Y, Colombel J-F, et al. A randomized, double-blind, controlled withdrawal trial in Crohn’s disease patients in long-term remission on azathioprine. Gastroenterology. 2005;128:1812–1818. doi: 10.1053/j.gastro.2005.03.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Wenzl HH, Primas C, Novacek G, et al. Withdrawal of long-term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn’s disease. Dig Dis Sci. 2015;60:1414–1423. doi: 10.1007/s10620-014-3419-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Warner B, Johnston E, Arenas-Hernandez M, et al. A practical guide to thiopurine prescribing and monitoring in IBD. Frontline Gastroenterol. 2018;9:10–15. doi: 10.1136/flgastro-2016-100738. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Kennedy NA, Rhatigan E, Arnott ID, et al. A trial of mercaptopurine is a safe strategy in patients with inflammatory bowel disease intolerant to azathioprine: an observational study, systematic review and meta-analysis. Aliment Pharmacol Ther. 2013;38:1255–1266. doi: 10.1111/apt.12511. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Jharap B, Seinen ML, De Boer N, et al. Thiopurine therapy in inflammatory bowel disease patients: Analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541–1549. doi: 10.1002/ibd.21221. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Moran GW, Dubeau M-F, Kaplan GG, et al. Clinical predictors of thiopurine-related adverse events in Crohn’s disease. World J Gastroenterol WJG. 2015;21:7795. doi: 10.3748/wjg.v21.i25.7795. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Goldberg R, Irving PM. Toxicity and response to thiopurines in patients with inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2015;9:891–900. doi: 10.1586/17474124.2015.1039987. [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. The Lancet. 2009;374:1617–1625. doi: 10.1016/S0140-6736(09)61302-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141:1621.e5–1628.e2. doi: 10.1053/j.gastro.2011.06.050. [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Lopez A, Mounier M, Bouvier A-M, et al. Increased risk of acute myeloid leukemias and myelodysplastic syndromes in patients who received thiopurine treatment for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2014;12:1324–1329. doi: 10.1016/j.cgh.2014.02.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Bourrier A, Carrat F, Colombel JF, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther. 2016;43:252–261. doi: 10.1111/apt.13466. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol. 2015;13:847.e4–858.e2. doi: 10.1016/j.cgh.2014.05.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Doherty G, Katsanos KH, Burisch J, et al. European Crohn’s and colitis organisation topical review on treatment withdrawal [‘exit strategies’] in inflammatory bowel disease. J Crohn’s Colitis. 2017;12:17–31. doi: 10.1093/ecco-jcc/jjx101. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Gonzalez-Lama Y, Gisbert JP. Monitoring thiopurine metabolites in inflammatory bowel disease. Frontline Gastroenterol. 2016;7:301–307. doi: 10.1136/flgastro-2015-100681. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Goel RM, Blaker P, Mentzer A, et al. Optimizing the use of thiopurines in inflammatory bowel disease. Ther Adv Chronic Dis. 2015;6:138–146. doi: 10.1177/2040622315579063. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Yang S-K, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. 2014;46:1017. doi: 10.1038/ng.3060. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Herfarth HH, Kappelman MD, Long MD, et al. Use of methotrexate in the treatment of inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:224–233. doi: 10.1097/MIB.0000000000000589. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Simon M, Pariente B, Lambert J, et al. Long-term outcomes of thalidomide therapy for adults with refractory Crohn’s disease. Clin Gastroenterol Hepatol. 2016;14:966e2–972.e2. doi: 10.1016/j.cgh.2015.10.034. [ DOI ] [ PubMed ] [ Google Scholar ]
- 67. Lazzerini M, Martelossi S, Magazzu G, et al. Effect of thalidomide on clinical remission in children and adolescents with refractory Crohn disease: a randomized clinical trial. JAMA. 2013;310:2164–2173. doi: 10.1001/jama.2013.280777. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. He Y, Mao R, Chen F, et al. Thalidomide induces clinical remission and mucosal healing in adults with active Crohn’s disease: a prospective open-label study. Therap adv gastroenterol. 2017;10:397–406. doi: 10.1177/1756283X17698910. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Bramuzzo M, Ventura A, Martelossi S, et al. Thalidomide for inflammatory bowel disease: Systematic review. Med (Baltimore) 2016;95:e4239. doi: 10.1097/MD.0000000000004239. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Lazzerini M, Villanacci V, Pellegrin MC, et al. Endoscopic and Histologic Healing in Children With Inflammatory Bowel Diseases Treated With Thalidomide. Clin Gastroenterol Hepatol. 2017;15:1382.e1–1389.e1. doi: 10.1016/j.cgh.2017.02.029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ. 2017;357:j2505. doi: 10.1136/bmj.j2505. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369:754–762. doi: 10.1056/NEJMct1209614. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn’s disease. N Engl J Med. 1997;337:1029–1036. doi: 10.1056/NEJM199710093371502. [ DOI ] [ PubMed ] [ Google Scholar ]
- 74. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti–tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I Trial. Gastroenterology. 2006;130:323–333. doi: 10.1053/j.gastro.2005.11.030. [ DOI ] [ PubMed ] [ Google Scholar ]
- 75. Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. doi: 10.7326/0003-4819-146-12-200706190-00159. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. Watanabe M, Hibi T, Lomax KG, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. J Crohn’s Colitis. 2012;6:160–173. doi: 10.1016/j.crohns.2011.07.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Schreiber S, Rutgeerts P, Fedorak RN, et al. A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129:807–818. doi: 10.1053/j.gastro.2005.06.064. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. doi: 10.1056/NEJMoa067594. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Sandborn WJ, Schreiber S, Feagan BG, et al. Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol. 2011;9:670.e3–678.e3. doi: 10.1016/j.cgh.2011.04.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. The Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Rutgeerts P, Feagan BG, Lichtenstein GR, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126:402–413. doi: 10.1053/j.gastro.2003.11.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 82. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250. doi: 10.1056/NEJMoa062897. [ DOI ] [ PubMed ] [ Google Scholar ]
- 85. Stidham R, Lee T, Higgins P, et al. Systematic review with network meta-analysis: the efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment Pharmacol Ther. 2014;39:1349–1362. doi: 10.1111/apt.12749. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Cholapranee A, Hazlewood G, Kaplan G, et al. Systematic review with meta-analysis: comparative efficacy of biologics for induction and maintenance of mucosal healing in Crohn’s disease and ulcerative colitis controlled trials. Aliment Pharmacol Ther. 2017;45:1291–1302. doi: 10.1111/apt.14030. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Mao EJ, Hazlewood GS, Kaplan GG, et al. Systematic review with meta-analysis: comparative efficacy of immunosuppressants and biologics for reducing hospitalisation and surgery in Crohn’s disease and ulcerative colitis. Aliment Pharmacol Ther. 2017;45:3–13. doi: 10.1111/apt.13847. [ DOI ] [ PubMed ] [ Google Scholar ]
- 88. Singh S, Garg SK, Pardi DS, et al. Comparative efficacy of pharmacologic interventions in preventing relapse of Crohn’s disease after surgery: a systematic review and network meta-analysis. Gastroenterology. 2015;148:64.e2–76.e2. doi: 10.1053/j.gastro.2014.09.031. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Ding N, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease—algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51. doi: 10.1111/apt.13445. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Qiu Y, B-l Chen, Mao R, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J Gastroenterol. 2017;52:535–554. doi: 10.1007/s00535-017-1324-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol. 2015;12:537. doi: 10.1038/nrgastro.2015.135. [ DOI ] [ PubMed ] [ Google Scholar ]
- 92. Moore C, Corbett G, Moss AC. Systematic review and meta-analysis: serum infliximab levels during maintenance therapy and outcomes in inflammatory bowel disease. J Crohn’s Colitis. 2016;10:619–625. doi: 10.1093/ecco-jcc/jjw007. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [ DOI ] [ PubMed ] [ Google Scholar ]
- 94. Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology. 2015;148:344.e5–354.e5. doi: 10.1053/j.gastro.2014.10.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Ungar B, Kopylov U, Engel T, et al. Addition of an immunomodulator can reverse antibody formation and loss of response in patients treated with adalimumab. Aliment Pharmacol Ther. 2017;45:276–282. doi: 10.1111/apt.13862. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Jones JL, Kaplan GG, Peyrin-Biroulet L, et al. Effects of concomitant immunomodulator therapy on efficacy and safety of anti-tumor necrosis factor therapy for Crohn’s disease: a meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2015;3(13):2233–40. doi: 10.1016/j.cgh.2015.06.034. [ DOI ] [ PubMed ] [ Google Scholar ]
- 97. Casteele NV, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017;153:835.e6–857.e6. doi: 10.1053/j.gastro.2017.07.031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 98. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–834. doi: 10.1053/j.gastro.2017.07.032. [ DOI ] [ PubMed ] [ Google Scholar ]
- 99. Velayos FS, Kahn JG, Sandborn WJ, et al. A test-based strategy is more cost effective than empiric dose escalation for patients with Crohn’s disease who lose responsiveness to infliximab. Clin Gastroenterol Hepatol. 2013;11:654–666. doi: 10.1016/j.cgh.2012.12.035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 100. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut. 2014;63(6):919–927. doi: 10.1136/gutjnl-2013-305279. [ DOI ] [ PubMed ] [ Google Scholar ]
- 101. Papamichael K, Mantzaris GJ, Peyrin-Biroulet L. A safety assessment of anti-tumor necrosis factor alpha therapy for treatment of Crohn’s disease. Expert Opin Drug Saf. 2016;15:493–501. doi: 10.1517/14740338.2016.1145653. [ DOI ] [ PubMed ] [ Google Scholar ]
- 102. Peyrin-Biroulet L, Deltenre P, de Suray N, et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–653. doi: 10.1016/j.cgh.2008.03.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 103. Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. A pooled analysis of infections, malignancy, and mortality in infliximab-and immunomodulator-treated adult patients with inflammatory bowel disease. Am J gastroenterol. 2012;107:1051. doi: 10.1038/ajg.2012.89. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 104. Lichtenstein GR, Feagan BG, Cohen RD, et al. Infliximab for Crohn’s disease: more than 13 years of real-world experience. Inflamm Bowel Dis. 2018;24:490–501. doi: 10.1093/ibd/izx072. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 105. Siegel CA, Marden SM, Persing SM, et al. Risk of lymphoma associated with combination anti–tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874–881. doi: 10.1016/j.cgh.2009.01.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 106. Osterman MT, Sandborn WJ, Colombel J-F, et al. Increased risk of malignancy with adalimumab combination therapy, compared with monotherapy, for Crohn’s disease. Gastroenterology. 2014;146:941.e2–949.e2. doi: 10.1053/j.gastro.2013.12.025. [ DOI ] [ PubMed ] [ Google Scholar ]
- 107. Kennedy NA, Warner B, Johnston EL, et al. Relapse after withdrawal from anti-TNF therapy for inflammatory bowel disease: an observational study, plus systematic review and meta-analysis. Aliment Pharmacol Ther. 2016;43:910–923. doi: 10.1111/apt.13547. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 108. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [ DOI ] [ PubMed ] [ Google Scholar ]
- 109. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618.e3–627.e2. doi: 10.1053/j.gastro.2014.05.008. [ DOI ] [ PubMed ] [ Google Scholar ]
- 110. Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis. 2015;21:2879–2885. doi: 10.1097/MIB.0000000000000561. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 111. Amiot A, Grimaud J-C, Peyrin-Biroulet L, et al. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14:1593.e2–1601.e2. doi: 10.1016/j.cgh.2016.02.016. [ DOI ] [ PubMed ] [ Google Scholar ]
- 112. Baumgart D, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice–a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090–1102. doi: 10.1111/apt.13594. [ DOI ] [ PubMed ] [ Google Scholar ]
- 113. Bonovas S, Fiorino G, Allocca M, et al. Biologic Therapies and Risk of Infection and Malignancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Network Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1385.e10–1397.e10. doi: 10.1016/j.cgh.2016.04.039. [ DOI ] [ PubMed ] [ Google Scholar ]
- 114. Deepak P, Sandborn WJ. Ustekinumab and Anti-Interleukin-23 Agents in Crohn’s Disease. Gastroenterol Clin North Am. 2017;46:603–626. doi: 10.1016/j.gtc.2017.05.013. [ DOI ] [ PubMed ] [ Google Scholar ]
- 115. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. doi: 10.1056/NEJMoa1602773. [ DOI ] [ PubMed ] [ Google Scholar ]
- 116. Tsai TF, Ho V, Song M, et al. The safety of ustekinumab treatment in patients with moderate-to-severe psoriasis and latent tuberculosis infection. Br J Dermatol. 2012;167:1145–1152. doi: 10.1111/j.1365-2133.2012.11142.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 117. Ben-Horin S, Vande Casteele N, Schreiber S, et al. Biosimilars in Inflammatory Bowel Disease: Facts and Fears of Extrapolation. Clin Gastroenterol Hepatol. 2016;14:1685–1696. doi: 10.1016/j.cgh.2016.05.023. [ DOI ] [ PubMed ] [ Google Scholar ]
- 118. Scott FI, Lichtenstein GR. Biosimilars in the Treatment of Inflammatory Bowel Disease: Supporting Evidence in 2017. Curr Treat Options Gastroenterol. 2018;16(1):147–167. doi: 10.1007/s11938-018-0177-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 119. Fiorino G, Manetti N, Armuzzi A, et al. The PROSIT-BIO cohort: a prospective observational study of patients with inflammatory bowel disease treated with infliximab biosimilar. Inflamm Bowel Dis. 2017;23:233–243. doi: 10.1097/MIB.0000000000000995. [ DOI ] [ PubMed ] [ Google Scholar ]
- 120. Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. The Lancet. 2017;389:2304–2316. doi: 10.1016/S0140-6736(17)30068-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (447.2 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Crohn's disease
Affiliations.
- 1 Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York City, NY, USA.
- 2 Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York City, NY, USA. Electronic address: [email protected].
- 3 Department of Gastroenterology, University Hospital of Nancy-Brabois, Vandœuvre-lès-Nancy, France.
- PMID: 27914655
- DOI: 10.1016/S0140-6736(16)31711-1
Crohn's disease is a chronic inflammatory disease of the gastrointestinal tract, with increasing incidence worldwide. Crohn's disease might result from a complex interplay between genetic susceptibility, environmental factors, and altered gut microbiota, leading to dysregulated innate and adaptive immune responses. The typical clinical scenario is a young patient presenting with abdominal pain, chronic diarrhoea, weight loss, and fatigue. Assessment of disease extent and of prognostic factors for complications is paramount to guide therapeutic decisions. Current strategies aim for deep and long-lasting remission, with the goal of preventing complications, such as surgery, and blocking disease progression. Central to these strategies is the introduction of early immunosuppression or combination therapy with biologicals in high-risk patients, combined with a tight and frequent control of inflammation, and adjustment of therapy on the basis of that assessment (treat to target strategy). The therapeutic armamentarium for Crohn's disease is expanding, and therefore the need to develop biomarkers that can predict response to therapies will become increasingly important for personalised medicine decisions in the near future. In this Seminar, we provide a physician-oriented overview of Crohn's disease in adults, ranging from epidemiology and cause to clinical diagnosis, natural history, patient stratification and clinical management, and ending with an overview of emerging therapies and future directions for research.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Publication types
- Biological Products / therapeutic use
- Crohn Disease / diagnosis*
- Crohn Disease / etiology
- Crohn Disease / therapy*
- Disease Management
- Disease Progression
- Drug Monitoring / methods
- Remission Induction
- Risk Factors
- Biological Products

IMAGES
VIDEO